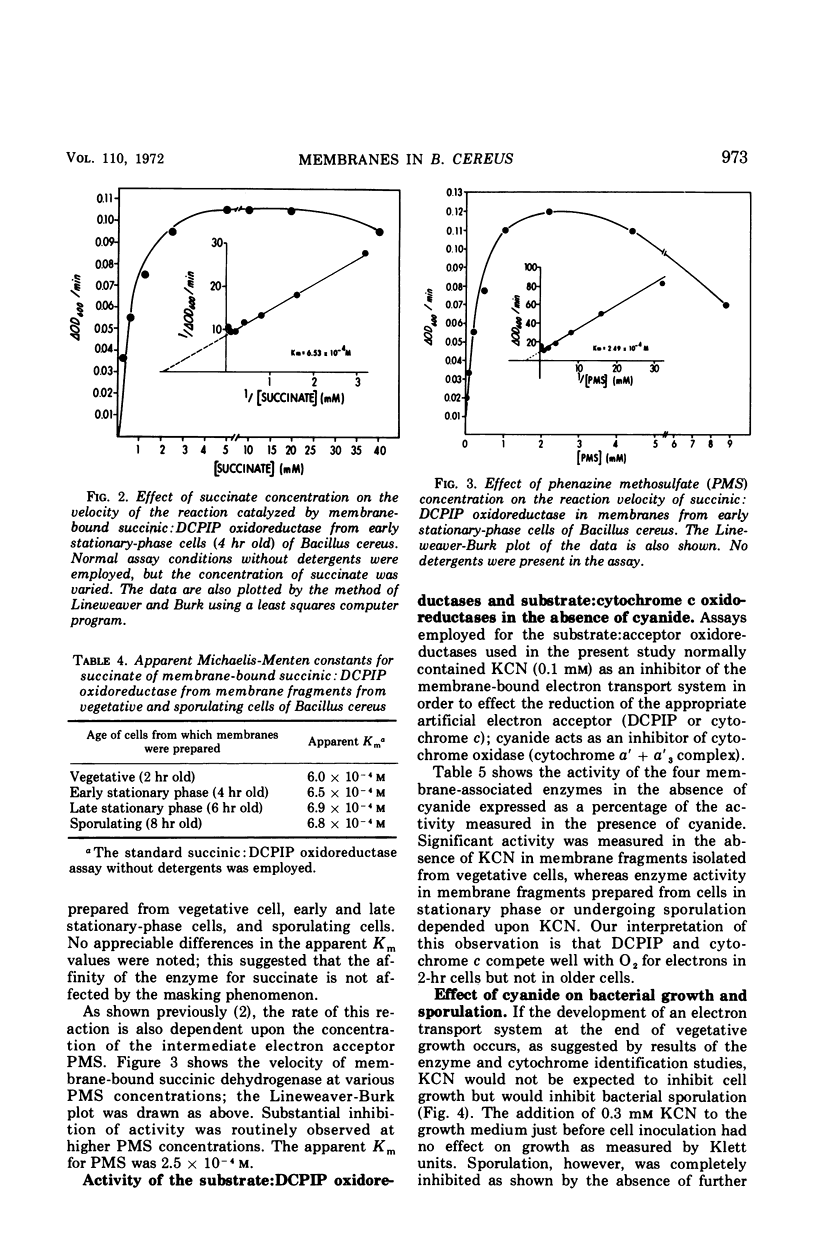
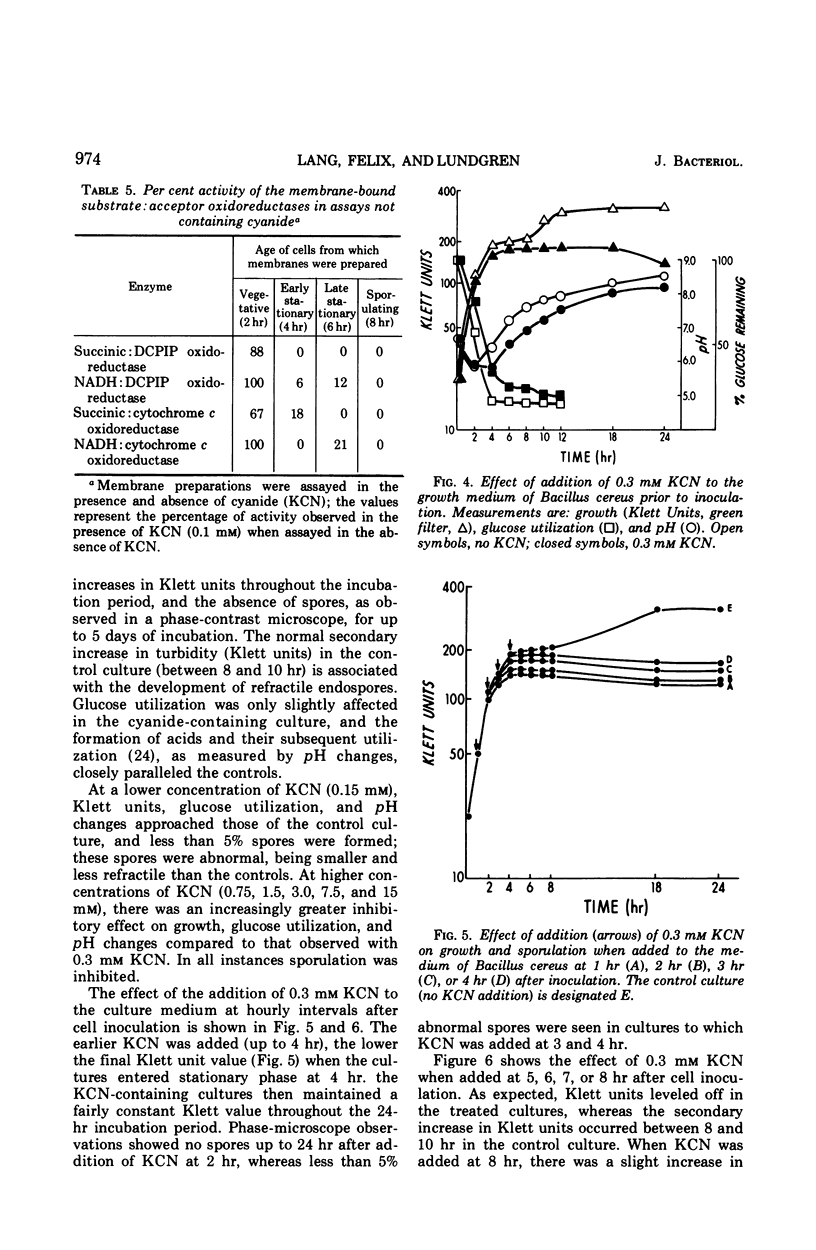
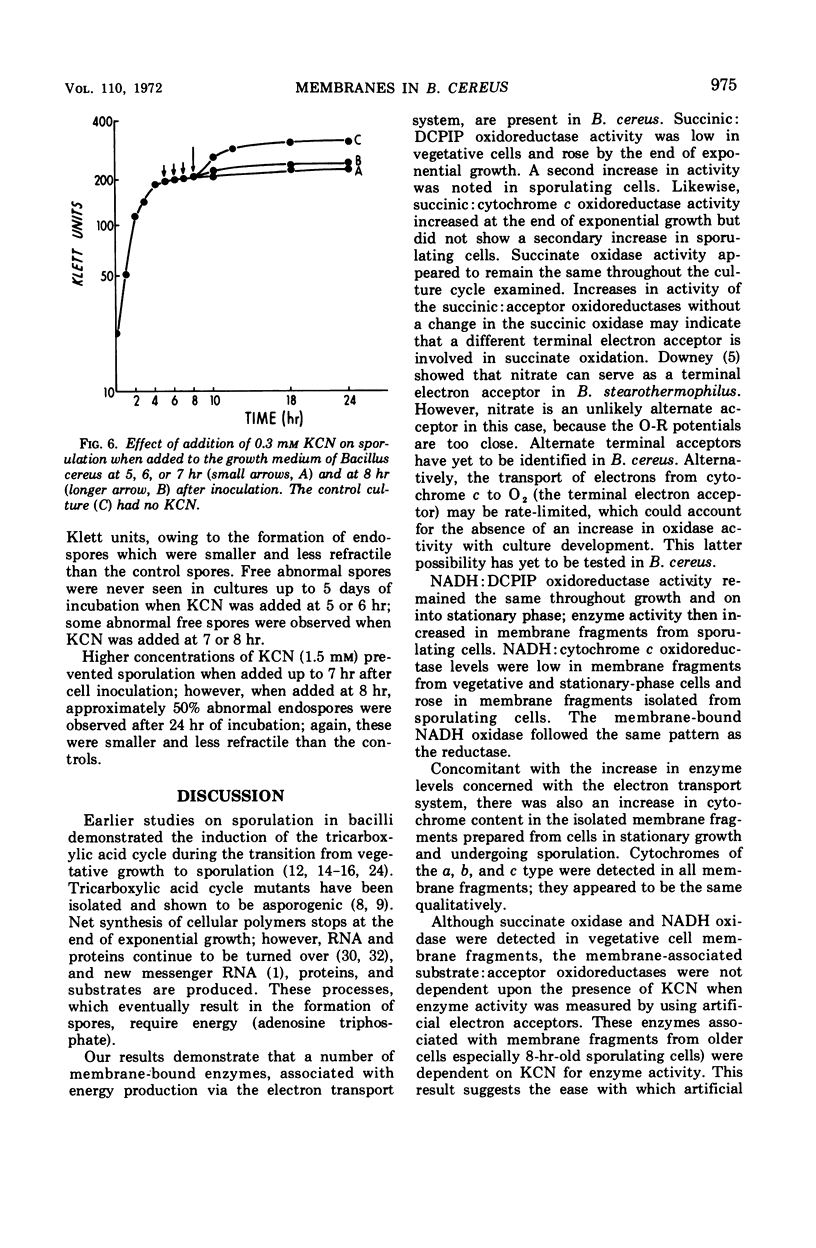
Abstract
Bulk membrane fragments were prepared from cells of Bacillus cereus ATCC 4342 harvested at different stages of growth and sporulation and examined for enzymes involved in electron transport functions. The presence of succinate: DCPIP oxidoreductase (EC 1.3.99.1), succinate: cytochrome c oxidoreductase (EC 1.3.2.1), NADH:DCPIP oxidoreductase (EC 1.6.99.1), NADH:cytochrome c oxidoreductase (EC 1.6.2.1), succinate oxidase [succinate: (O2) oxidoreductase, EC 1.3.3.1], and NADH oxidase [NADH:(O2) oxidoreductase, EC 1.6.3.1] were demonstrated in membrane fragments from vegetative cells, early and late stationary-phase cells, and in cells undergoing sporulation. During the transition from a vegetative cell to a spore, there was a significant increase in the levels of enzymes associated with energy production via the electron transport system. Cytochromes of the a, b, and c type were detected in all membrane preparations; however, there was a marked increase in the level of cytochromes by the end of vegetative growth which remained throughout sporulation; there were no qualitative changes in the cytochromes throughout growth and sporulation. Sporulation was inhibited by cyanide, stressing the significance of the electron transport system. Enzyme activities were partially masked in washed membrane fragments; however, unmasking (stimulation) was achieved by sodium deoxycholate, sodium dodecyl sulfate, or Triton X-100. The degree of enzyme masking was less in vegetative cell membrane fragments than in membranes prepared from stationary-phase or sporulating cells. Results indicate the development of a membrane-bound electron transport system in B. cereus by the end of growth and prior to sporulation, which results in an increased masking of a number of enzymes associated with the terminal respiratory system of the cell.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARONSON A. I. CHARACTERIZATION OF MESSENGER RNA IN SPORULATING BACILLUS CEREUS. J Mol Biol. 1965 Mar;11:576–588. doi: 10.1016/s0022-2836(65)80012-2. [DOI] [PubMed] [Google Scholar]
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- DOI R. H., HALVORSON H. Comparison of electron transport systems in vegetative cells and spores of Bacillus cereus. J Bacteriol. 1961 Jan;81:51–58. doi: 10.1128/jb.81.1.51-58.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. C., Yu L., Wolin M. J. Masking of Bacillus megaterium KM membrane reduced nicotinamide adenine dinucleotide oxidase and solubilization studies. J Bacteriol. 1970 Apr;102(1):161–171. doi: 10.1128/jb.102.1.161-171.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdman D. L., Himmelreich N. G., Dyadyusha G. P. The enzyme activity in a detergent-treated sarcolemma of skeletal muscles. Biochim Biophys Acta. 1970 Dec 1;219(2):372–378. doi: 10.1016/0005-2736(70)90214-2. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Fortnagel P. Analysis of sporulation mutants. I. Response of uracil incorporation to carbon sources, and other mutant properties. J Bacteriol. 1967 Dec;94(6):1957–1969. doi: 10.1128/jb.94.6.1957-1969.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN M., BLUMENTHAL H. J. CHANGES IN TERMINAL RESPIRATORY PATHWAYS OF INTACT CELLS OF BACILLUS CEREUS AT VARIOUS STAGES OF DEVELOPMENT. J Bacteriol. 1964 Feb;87:387–390. doi: 10.1128/jb.87.2.387-390.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN M., BLUMENTHAL H. J. PATHWAYS OF GLUCOSE CATABOLISM IN BACILLUS CEREUS. J Bacteriol. 1964 Feb;87:377–386. doi: 10.1128/jb.87.2.377-386.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLLAKOTA K. G., HALVORSON H. O. Biochemical changes occurring during sporulation of Bacillus cereus. Inhibition of sporulation by alpha-picolinic acid. J Bacteriol. 1960 Jan;79:1–8. doi: 10.1128/jb.79.1.1-8.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HACHISUKA Y., ASANO N., KANEKO M., KANBE T. Evolution of respiratory enzyme system during germination of Bacillus subtilis. Science. 1956 Jul 27;124(3213):174–175. doi: 10.1126/science.124.3213.174. [DOI] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. BIOCHEMISTRY OF SPORULATION. II. ENZYMATIC CHANGES DURING SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Jul;86:45–50. doi: 10.1128/jb.86.1.45-50.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. Biochemistry of sporulation. I. Metabolism of acetate by vegetative and sporulating cells. J Bacteriol. 1963 Feb;85:451–460. doi: 10.1128/jb.85.2.451-460.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING T. E. RECONSTITUTION OF RESPIRATORY CHAIN ENZYME SYSTEMS. XI. USE OF ARTIFICIAL ELECTRON ACCEPTORS IN THE ASSAY OF SUCCINATE-DEHYDROGENATING ENZYMES. J Biol Chem. 1963 Dec;238:4032–4036. [PubMed] [Google Scholar]
- Klofat W., Picciolo G., Chappelle E. W., Freese E. Production of adenosine triphosphate in normal cells and sporulation mutants of Bacillus subtilis. J Biol Chem. 1969 Jun 25;244(12):3270–3276. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang D. R., Lundgren D. G. Lipid composition of Bacillus cereus during growth and sporulation. J Bacteriol. 1970 Feb;101(2):483–489. doi: 10.1128/jb.101.2.483-489.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennarz W. J., Talamo B. The chemical characterization and enzymatic synthesis of mannolipids in Micrococcus lysodeikticus. J Biol Chem. 1966 Jun 10;241(11):2707–2719. [PubMed] [Google Scholar]
- NAKATA H. M., HALVORSON H. O. Biochemical changes occurring during growth and sporulation of Bacillus cereus. J Bacteriol. 1960 Dec;80:801–810. doi: 10.1128/jb.80.6.801-810.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H., Lennarz W. J. Studies on the membranes of bacilli. I. Phospholipid biosynthesis. J Biol Chem. 1971 Feb 25;246(4):1062–1072. [PubMed] [Google Scholar]
- Pollock J. J., Linder R., Salton M. R. Characterization of the membrane-bound succinic dehydrogenase of Micrococcus lysodeikticus. J Bacteriol. 1971 Jul;107(1):230–238. doi: 10.1128/jb.107.1.230-238.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R. Bacterial membranes. CRC Crit Rev Microbiol. 1971 May;1(1):161–197. doi: 10.3109/10408417109104480. [DOI] [PubMed] [Google Scholar]
- Tochikubo K. Changes in terminal respiratory pathways of Bacillus subtilis during germination, outgrowth and vegetative growth. J Bacteriol. 1971 Nov;108(2):652–661. doi: 10.1128/jb.108.2.652-661.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


