Abstract
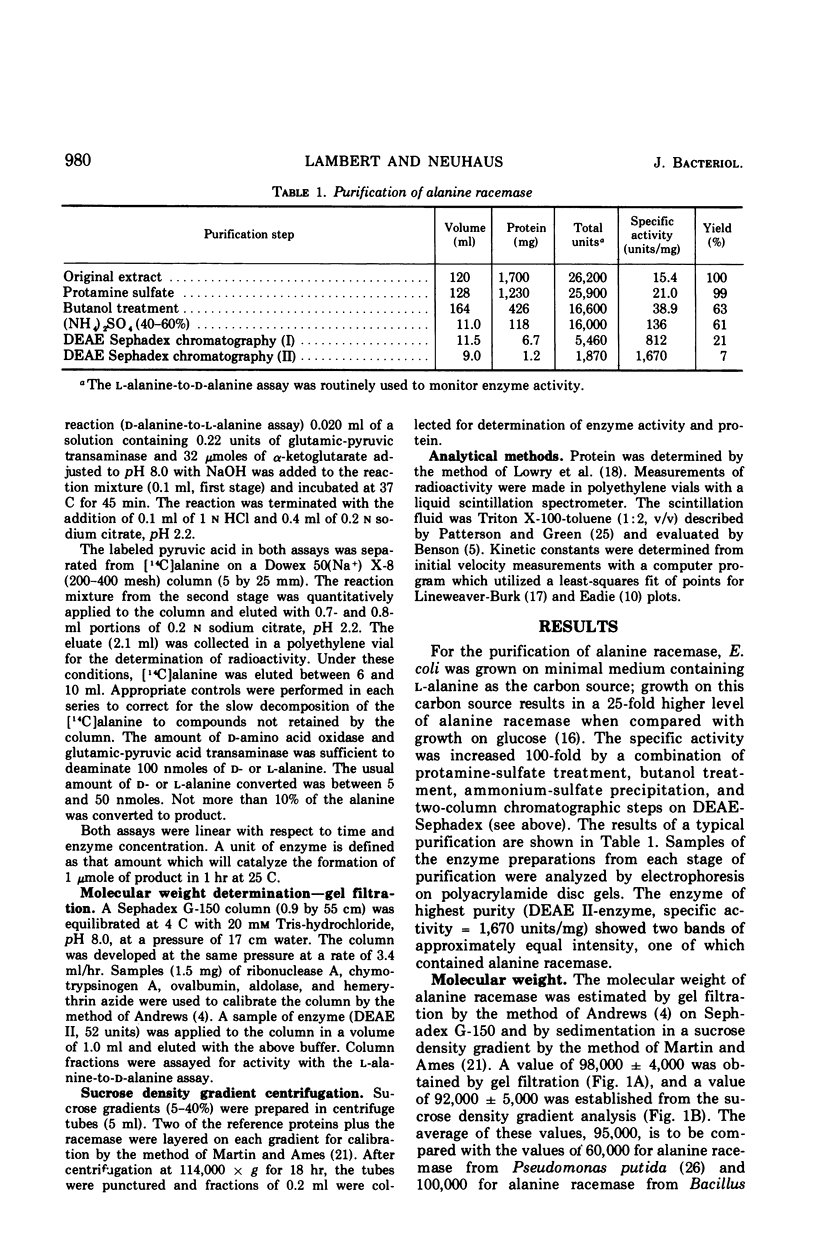
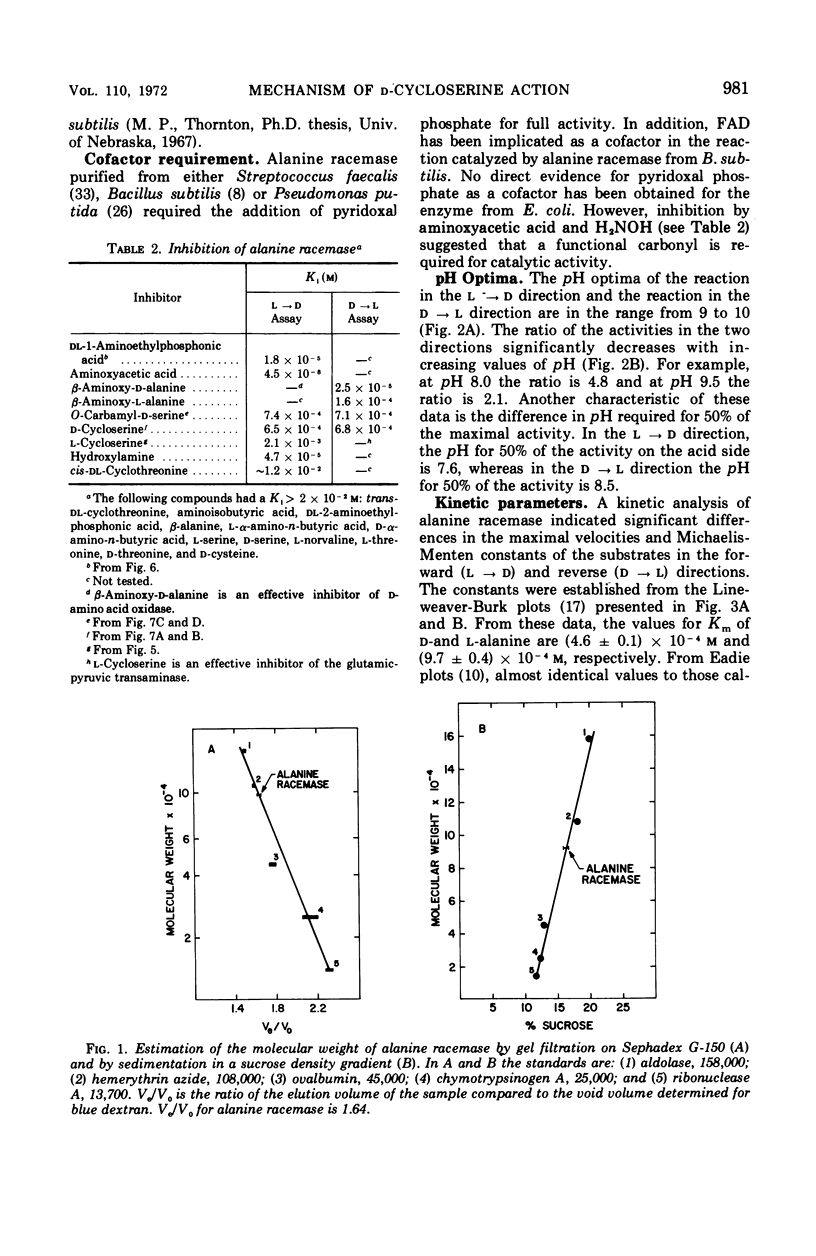
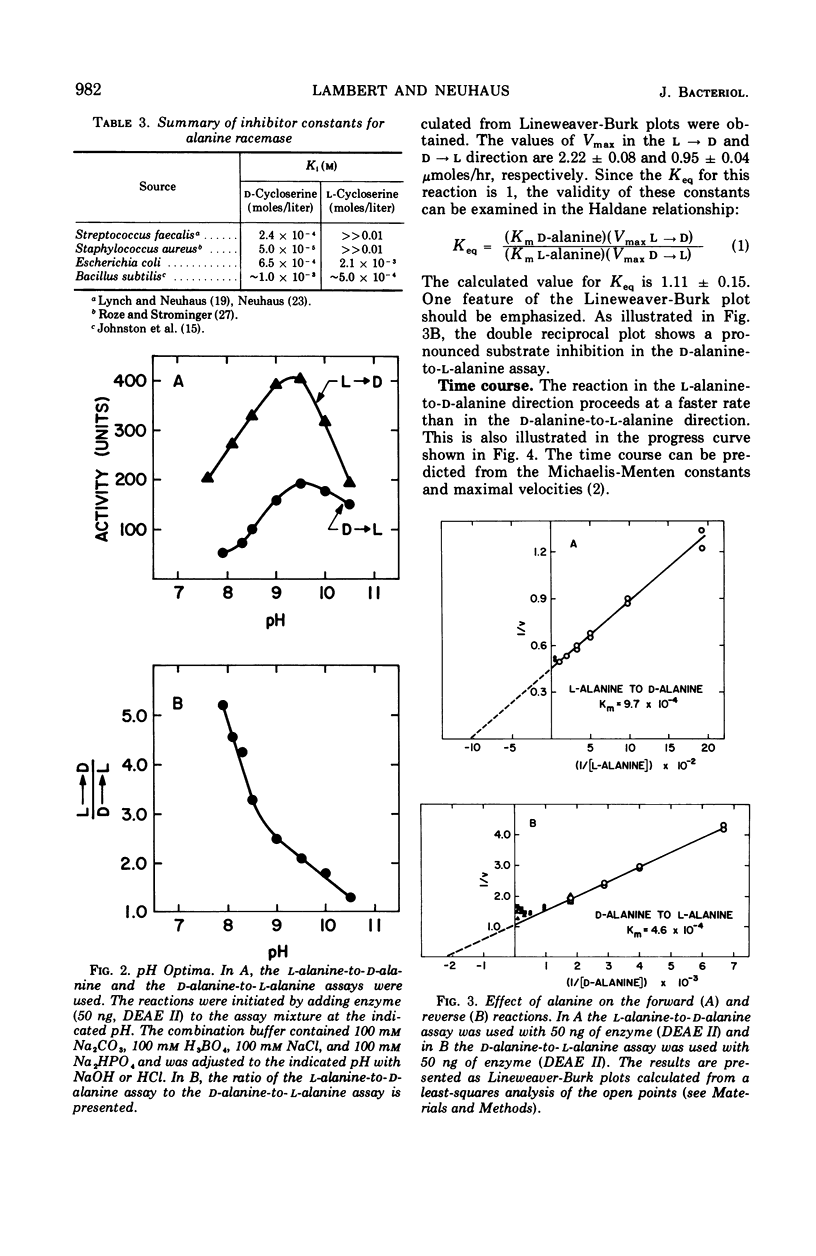
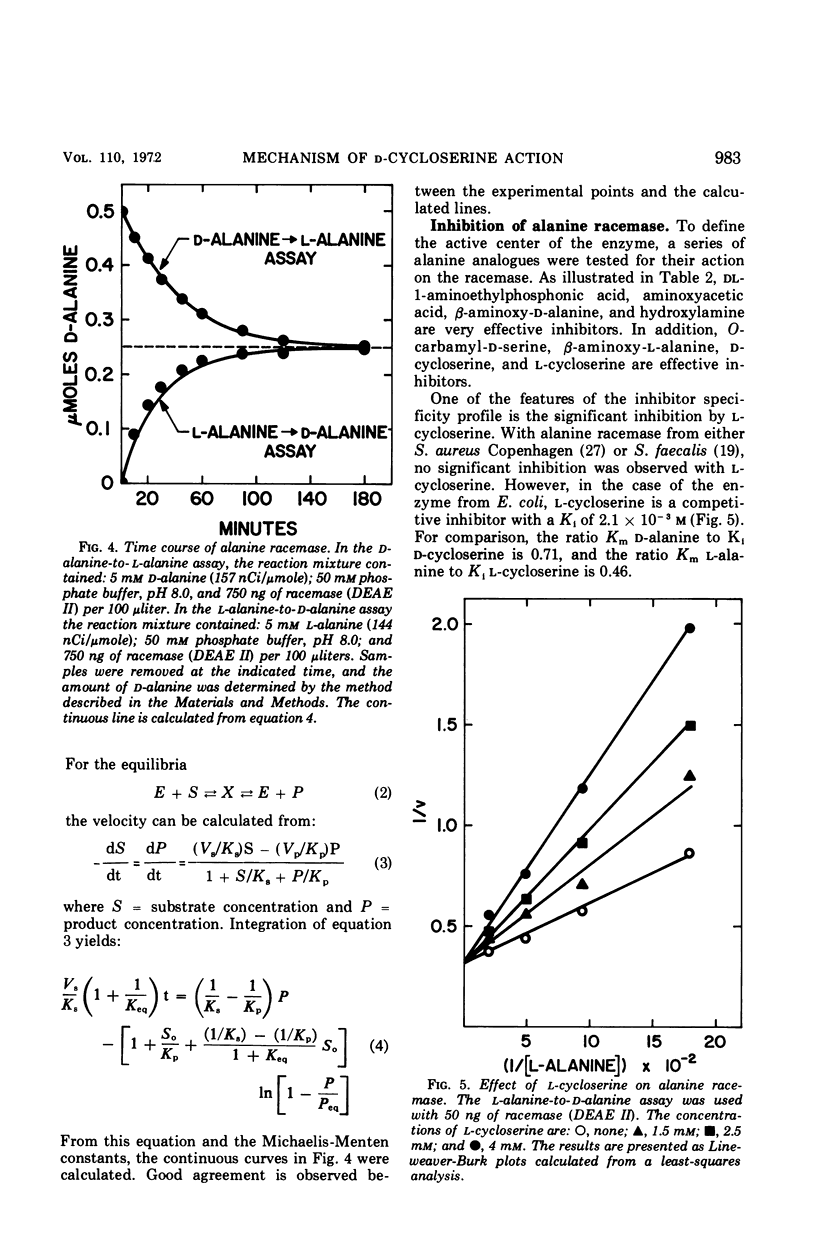
The antibiotic d-cycloserine is an effective inhibitor of alanine racemase. The lack of inhibition by l-cycloserine of alanine racemase from Staphylococcus aureus led Roze and Strominger to formulate the cycloserine hypothesis. This hypothesis states that d-cycloserine has the conformation required of the substrates on the enzyme surface and that l-cycloserine cannot have this conformation. Alanine racemase from Escherichia coli W has been examined to establish whether these observations are a general feature of all alanine racemases. The enzyme (molecular weight = 95,000) has Michaelis-Menten constants of 4.6 × 10−4m and 9.7 × 10−4m for d- and l-alanine, respectively. The ratio of Vmax in the d- to l-direction is 2.3. The equilibrium constant calculated from the Haldane relationship is 1.11 ± 0.15. Both d- and l-cycloserine are competitive inhibitors with constants (Ki) of 6.5 × 10−4m and 2.1 × 10−3m, respectively. The ratio of Kmd-alanine to Kid-cycloserine is 0.71, and the ratio of Kml-alanine to Kil-cycloserine is 0.46. Since l-cycloserine is an effective inhibitor, it is concluded that the cycloserine hypothesis does not apply to the enzyme from E. coli W.
Full text
PDF

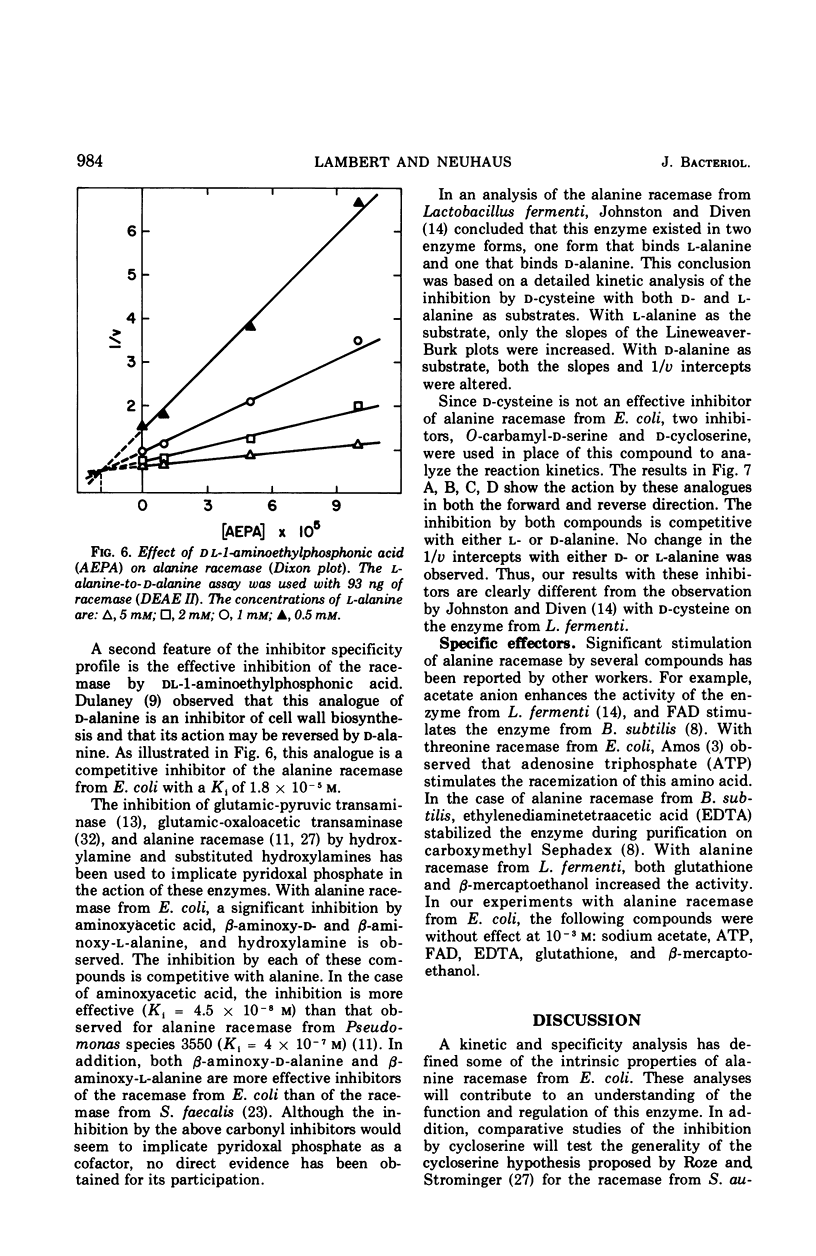
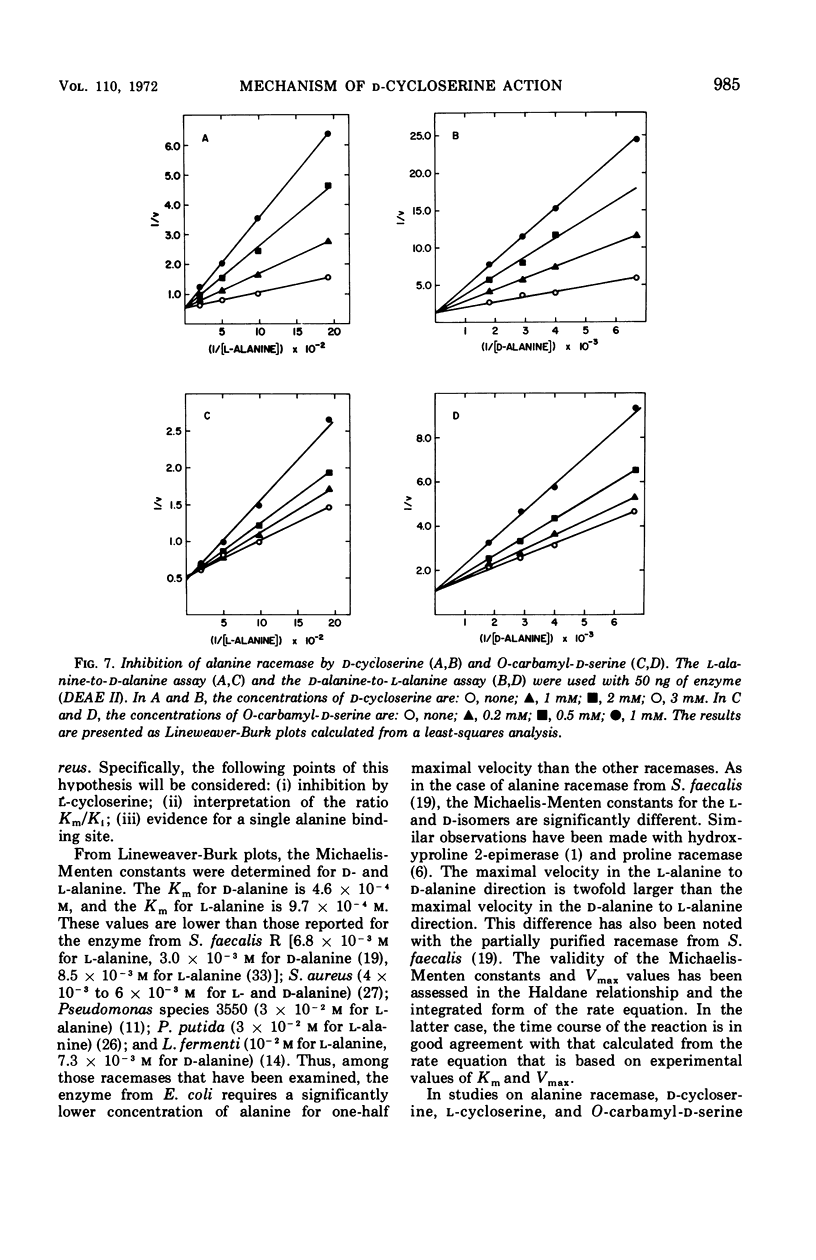


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS E., NORTON I. L. PURIFICATION AND PROPERTIES OF INDUCIBLE HYDROXYPROLINE 2-EPIMERASE FROM PSEUDOMONAS. J Biol Chem. 1964 May;239:1525–1535. [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale G. J., Abeles R. H. Purification and mechanism of action of proline racemase. Biochemistry. 1968 Nov;7(11):3970–3978. doi: 10.1021/bi00851a026. [DOI] [PubMed] [Google Scholar]
- DIVEN W. F., SCHOLZ J. J., JOHNSTON R. B. PURIFICATION AND PROPERTIES OF THE ALANINE RACEMASE FROM BACILLUS SUBTILIS. Biochim Biophys Acta. 1964 May 4;85:322–332. doi: 10.1016/0926-6569(64)90253-6. [DOI] [PubMed] [Google Scholar]
- Dulaney E. L. 1-aminoethylphosphonic acid, an inhibitor of bacterial cell wall synthesis. J Antibiot (Tokyo) 1970 Nov;23(11):567–568. doi: 10.7164/antibiotics.23.567. [DOI] [PubMed] [Google Scholar]
- Free C. A., Julius M., Arnow P., Barry G. T. Inhibition of alanine racemase by aminoxyacetic acid. Biochim Biophys Acta. 1967;146(2):608–610. doi: 10.1016/0005-2744(67)90252-5. [DOI] [PubMed] [Google Scholar]
- HOPPER S., SEGAL H. L. Kinetic studies of rat liver glutamicalanine transaminase. J Biol Chem. 1962 Oct;237:3189–3195. [PubMed] [Google Scholar]
- Johnston M. M., Diven W. F. Studies on amino acid racemases. I. Partial purification and properties of the alanine racemase from Lactobacillus fermenti. J Biol Chem. 1969 Oct 10;244(19):5414–5420. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambert M. P., Neuhaus F. C. Factors affecting the level of alanine racemase in Escherichia coli. J Bacteriol. 1972 Mar;109(3):1156–1161. doi: 10.1128/jb.109.3.1156-1161.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. L., Neuhaus F. C. On the mechanism of action of the antibiotic O-carbamyld-serine in Streptococcus faecalis. J Bacteriol. 1966 Jan;91(1):449–460. doi: 10.1128/jb.91.1.449-460.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARR A. G., WILSON P. W. The alanine racemase of Brucella abortus. Arch Biochem Biophys. 1954 Apr;49(2):424–433. doi: 10.1016/0003-9861(54)90211-8. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- NEUHAUS F. C., LYNCH J. L. THE ENZYMATIC SYNTHESIS OF D-ALANYL-D-ALANINE. 3. ON THE INHIBITION OF D-ALANYL-D-ALANINE SYNTHETASE BY THE ANTIBIOTIC D-CYCLOSERINE. Biochemistry. 1964 Apr;3:471–480. doi: 10.1021/bi00892a001. [DOI] [PubMed] [Google Scholar]
- Neuhaus F. C. Selective inhibition of enzymes utilizing alanine in the biosynthesis of peptidoglycan. Antimicrob Agents Chemother (Bethesda) 1967;7:304–313. [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Rosso G., Takashima K., Adams E. Coenzyme content of purified alanine racemase from Pseudomonas. Biochem Biophys Res Commun. 1969 Jan 6;34(1):134–140. doi: 10.1016/0006-291x(69)90539-7. [DOI] [PubMed] [Google Scholar]
- STEWART B. T., HALVORSON H. O. Studies on the spores of aerobic bacteria. I. The occurrence of alanine racemase. J Bacteriol. 1953 Feb;65(2):160–166. doi: 10.1128/jb.65.2.160-166.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VELICK S. F., VAVRA J. A kinetic and equilibrium analysis of the glutamic oxaloacetate transaminase mechanism. J Biol Chem. 1962 Jul;237:2109–2122. [PubMed] [Google Scholar]
- WOOD W. A., GUNSALUS I. C. D-Alanine formation; a racemase in Streptococcus faecalis. J Biol Chem. 1951 May;190(1):403–416. [PubMed] [Google Scholar]


