Abstract
Bcl-2, which can both reduce apoptosis and retard cell cycle entry, is thought to have important roles in hematopoiesis. To evaluate the impact of its ubiquitous overexpression within this system, we targeted expression of the human bcl-2 gene in mice by using the promoter of the vav gene, which is active throughout this compartment but rarely outside it. The vav-bcl-2 transgene was expressed in essentially all nucleated cells of hematopoietic tissues but not notably in nonhematopoietic tissues. Presumably because of enhanced cell survival, the mice displayed increases in myeloid cells as well as a marked elevation in B and T lymphocytes. The spleen was enlarged and the lymphoid follicles expanded. Although total thymic cellularity was normal, T cell development was altered: cells at the very immature and most mature stages were increased, whereas those at the intermediate stage were decreased. Unexpectedly, blood platelets were reduced by half, suggesting that their production from megakaryocytes is regulated by the Bcl-2 family. Colony formation by myeloid progenitor cells in vitro remained cytokine dependent, and the frequency of most progenitor and preprogenitor cells was normal. Macrophage progenitors were less frequent and yielded smaller colonies, however, perhaps reflecting inhibitory effects of Bcl-2 on cell cycling in specific lineages. After irradiation or factor deprivation, Bcl-2 markedly enhanced clonogenic survival of all tested progenitor and preprogenitor cells. Thus, Bcl-2 has multiple effects on the hematopoietic system. These mice should help to further clarify the role of apoptosis in the development and homeostasis of this compartment.
Keywords: apoptosis, transgenic mice, homeostasis
Cellular attrition through programmed cell death, apoptosis, is prominent in the development and homeostasis of the entire hematopoietic system. Its critical role is evident from the short lifespan of the neutrophil (1) and the minute proportion of nascent T lymphocytes that ever leave the thymus (2). Moreover, one recognized critical function of the hemopoietins is to maintain cell survival, and the sensitivity of this compartment to cytotoxic agents such as radiation is well known. It is therefore important to establish how apoptosis is controlled within this system.
As reviewed recently (3), the major regulators of apoptosis include members of the Bcl-2 family of cytoplasmic proteins. Indeed, the bcl-2 gene, identified via its frequent translocation in follicular lymphoma (reviewed by Cory in ref. 4), became the first recognized mediator of cell survival when it was found to permit the survival of cytokine-deprived hematopoietic cells (5). Several more recently discovered related proteins, including Bcl-x, Mcl-1, A1, and Bcl-w, also counter apoptosis, whereas other family members instead favor cell death. These proteins act upstream of the proteases (caspases) that dismantle the cell, although how they control caspase activation remains uncertain (3). In addition to their antiapoptotic role, the pro-survival proteins have an independent function that influences cell cycling: under suboptimal growth conditions, for example, cells expressing Bcl-2 leave the cycle more readily and re-enter it more slowly (6–8).
Critical roles in hematopoiesis have been demonstrated by gene disruption for three of the pro-survival proteins: Bcl-2 itself is required for maintenance of a functional lymphoid compartment (9, 10), Bcl-x for survival of maturing erythroid cells (11), and A1 for neutrophils (12). Moreover, several of these proteins may be mediators of cytokine-regulated survival signals (3). Indeed, bcl-2 transgenes rescued macrophage production in mice deficient in macrophage colony-stimulating factor (M-CSF) (13) and T lymphopoiesis in mice deficient in IL-7 signaling (14–17).
The impact of bcl-2 transgenes has been evaluated extensively in B and T lymphocytes (18–21), but studies on wider effects of bcl-2 expression in this compartment have been more limited (13, 15, 22–25). Widespread effects were obtained with a transgene driven by an MHC H-2Kb promoter (24), but some of those effects may be attributable to the nonhematopoietic expression also observed with this promoter (see Discussion).
Recently we have developed a transgenic vector for targeting expression specifically to the hematopoietic compartment by exploiting the promoter region of the vav gene, which is expressed in virtually all hematopoietic cells but very few others (26–29). In initial studies (30), expression of a bacterial (lacZ) reporter gene was confined to lymphocytes and was sporadically silenced, but these limitations proved to be caused by the prokaryotic reporter. With a human cell surface reporter (hCD4), nonhematopoietic expression appeared negligible and the vav promoter was active in virtually all the nucleated cells of hematopoietic tissues (31), including all tested types of clonogenic progenitor cells.
To explore further the impact of Bcl-2 on hematopoiesis, we have generated vav-bcl-2 transgenic mice and evaluated the consequences of constitutive Bcl-2 expression within the hematopoietic compartment. The results show how Bcl-2 influences homeostasis in this system, the number and activity of clonogenic progenitor cells, and the responses of those cells to factor deprivation and γ-irradiation.
Materials and Methods
Generation of Transgene and Transgenic Mice.
The coding region of human bcl-2 cDNA amplified with Pfu polymerase was inserted between an intron from the potent SRα expression vector and the simian virus 40 (SV40) late region polyadenylation signal in a cassette bounded by Flp recognition sequences (31). This cassette then was used to replace the hCD4 cassette in the HS21/45 vav-hCD4 transgene construct (31). The vav-bcl-2 transgene was excised, purified, and introduced into the inbred C57BL/6J mouse genome by pronuclear microinjection as described (31, 32). Transgenic pups were identified by PCR on tail DNA, by using primers specific to the SV40 pA sequence.
Flow Cytometric Analyses.
For cell composition analysis, blood and tissues were taken from mice at 7–20 weeks of age. To detect Bcl-2 in blood leukocytes, paraformaldehyde-fixed, Saponin-permeabilized cells were stained with Bcl-2–100 mAb (33) and then goat anti-mouse IgG1-PE (Southern Biotechnology Associates), and analyzed in a FACScan (Becton Dickinson) as described (20). Single cell suspensions from femoral bone marrow, thymus, spleen, and mesenteric lymph nodes, and peritoneal cells were analyzed similarly. Cell lineage analysis was by flow cytometry of surface immunofluorescence as described (20, 31).
Immunoblotting.
On sacrifice, mice were perfused with PBS to displace circulating leukocytes and serum. Snap-frozen tissues were homogenized at 4°C in buffer (20 mM Tris⋅HCl, pH 7.4/1 mM EGTA/135 mM NaCl/1.5 mM MgCl2/10% glycerol/1% Triton X-100) containing Complete Protease Inhibitor (Boehringer Mannheim). Proteins in postnuclear supernatant were resolved by SDS/4–20% PAGE (10 μg of protein per lane), transferred to nitrocellulose membranes (Hybond-C Extra, Amersham Pharmacia), and blotted with anti-human Bcl-2 mAb Bcl-2/100/D5 (NovoCastra, Newcastle, U.K.), anti-human Vav mAb (Upstate Biotechnology, Lake Placid, NY), or anti-Hsp-70 mAb N6 (the kind gift of Robyn Anderson, Peter MacCallum Cancer Institute, East Melbourne), followed by horseradish peroxidase-conjugated sheep anti-mouse IgG (Silenus, Melbourne) and chemiluminescence reagents (Amersham Pharmacia).
In Vitro Culture and Survival Assays of Bone Marrow Progenitor Cells.
Bone marrow was flushed from both femurs of vav-bcl-2 transgenic mice (6–8 weeks old) and cytocentrifuge preparations stained with May-Grunwald-Giemsa to enumerate the different cell types. Cells were cultured with recombinant growth factors to stimulate colony formation as described (31, 34). The stimuli included recombinant mouse M-CSF (10 ng), IL-3 (10 ng), granulocyte/macrophage-CSF (10 ng), stem cell factor (SCF, 100 ng), Flk ligand (FL, 100 ng) plus leukemia inhibitory factor (10 ng), or human granulocyte-CSF (10 ng).
For colony assays, 50,000 nucleated cells were cultured in semisolid medium as described (34), and colony formation was scored after 7 days. The dried cultures were stained, first for acetylcholinesterase activity, then with Luxol fast blue and finally with hematoxylin, and scored microscopically to verify colony counts and to determine the cell composition of each colony. To assess survival of progenitor cells on factor deprivation, femoral bone marrow cells were cultured in the absence of stimulus for various times, then factor was added and colonies were scored after an additional 7-day incubation. Radiation sensitivity of the progenitors was determined by subjecting marrow cells in vitro to measured doses of γ-irradiation (from 60Co at 360 cGy/min), culturing a fixed volume of the cell suspension with various stimuli and enumerating the resulting colonies as above.
Results
Expression of vav-bcl-2 Transgene in Hematopoietic Tissues.
The mouse vav locus contains five hematopoietic-specific DNase I-hypersensitive sites in the chromatin surrounding its promoter (30). Our previous work showed that a transgenic vector bearing the two proximal upstream sites and the two intron sites targeted hCD4 expression to the hematopoietic system as effectively as one that also included the distal upstream site HS3 (30, 31). The equivalent vav-bcl-2 transgene used here contained those four site regions, with a human bcl-2 cDNA replacing the coding portion of vav exon 1.
We generated 58 primary vav-bcl-2 transgenic mice and tested their blood leukocytes for intracellular human Bcl-2. As found with vav-hCD4 transgenes, the vav-bcl-2 transgene was active in the great majority of the animals (46 of 58; 79%). Breeding strains were established from three of these, and the transgenic progeny displayed human Bcl-2 in all of their nucleated blood cells (e.g., Fig. 1A).
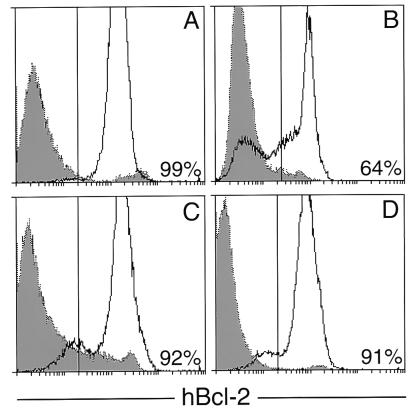
Figure 1.
Flow cytometric analysis of hBcl-2 in hematopoietic tissues. Transgenic profiles (unbroken lines) are superimposed on those from nontransgenic littermates (dotted lines and shaded). Peripheral blood leukocytes from a typical vav-bcl-2 progeny mouse (A) and an Eμ-bcl-2-36 mouse (B); vav-bcl-2 bone marrow (C) and (D) spleen. The proportion of cells with transgene-associated fluorescence above that from the nontransgenic littermate is given.
The vav promoter appears to give a high level of expression per cell. The level of Bcl-2 in the blood leukocytes of vav-bcl-2 mice was similar to or higher than that driven in lymphocytes by the Ig Eμ enhancer (19, 20), shown in Fig. 1B. The vav-bcl-2 transgene was also highly expressed in the vast majority, if not all, of the cells from transgenic bone marrow and spleen (Fig. 1 C and D) and from thymus, mesenteric lymph node and peritoneal cavity (not shown). The level in the thymus was an order of magnitude higher than that in an Eμ-bcl-2 strain (A. Strasser, personal communication). Thus, the vav promoter region may be even more potent than this well-characterized Ig regulatory element (35).
vav-bcl-2 Expression Restricted to Hematopoietic Tissues.
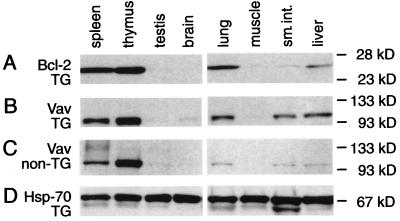
With vav-hCD4 mice, transgene expression appeared to be confined largely, if not entirely, to hematopoietic tissues (31). To assess whether that held for vav-bcl-2 mice, lysates from various organs were immunoblotted with the mAb specific for human Bcl-2, as well as a Vav antibody. In transgenic tissues (Fig. 2A), hBcl-2 was abundant in the spleen and thymus, but none was evident in testis, brain, or muscle, nor in kidney (not shown). Although testis is known to contain a distinct Vav isoform (36), it would be undetectable by the Vav antibody used here, and no testicular hBcl-2 expression would be expected, because that isoform arises from a promoter not present in our transgene. Some hBcl-2 was present in lung and liver and a trace in small intestine, but these organs normally contain some hematopoietic cells, and the vav-bcl-2 mice often showed focal accumulations of lymphocytes in lung and liver (see below). In keeping with that notion, the levels of Vav detected in the transgenic lung, liver, and small intestine (Fig. 2B) were higher than those in the corresponding nontransgenic tissues (Fig. 2C), and that also held for mouse Ig (data not shown). Overall, these results are consistent with other evidence (see Introduction) that expression of vav and of vav-based vectors is very largely confined to the hematopoietic compartment.
Figure 2.
Immunoblots revealing hBcl-2 and Vav in lysates of various organs from vav-bcl-2 (TG) and nontransgenic littermate mice. (A) hBcl-2 in transgenic mouse tissues. (B) Vav in transgenic tissues. (C) Vav in control tissues. (D) Hsp-70 as a control for loading and protein integrity.
Increased Abundance of Several Hematopoietic Cell Types.
The peripheral blood of vav-bcl-2 mice differed in several respects from that of nontransgenic control mice. Table 1 presents the data for vav-bcl-2 strain 69, but similar results were obtained with two other strains (45 and 68). Lymphocytes were increased about 15-fold and monocytes 7-fold. A small apparent rise in neutrophils was not statistically significant, and these cells did not exhibit the nuclear hypersegmentation indicative of an increased lifespan. Eosinophil numbers were unchanged. Hematocrit values were near normal, and no nucleated red cells appeared in the blood. Unexpectedly, however, platelet numbers in vav-bcl-2 mice of all three strains were consistently only 50–60% of those in the control mice (P < 0.01) (see Discussion).
Table 1.
Altered tissue composition in vav-bcl-2 mice
| Tissue | Nucleated cells
|
||
|---|---|---|---|
| Control | vav-bcl-2 | Ratioa | |
| Bloodb | 4.0 ± 2.6 | 48 ± 24 | 12** |
| Lymphocytes | 2.9 ± 1.7 | 45 ± 23 | 16** |
| Monocytes | 0.30 ± 0.28 | 2.1 ± 1.7 | 7.0** |
| Neutrophils | 0.67 ± 0.61 | 1.3 ± 1.9 | 1.9 |
| Eosinophils | 0.07 ± 0.11 | 0.05 ± 0.20 | 0.7 |
| Platelets | 1,020 ± 230 | 510 ± 140 | 0.5** |
| Hematocrit, % | 45 ± 1.3 | 43 ± 2.1 | 0.96* |
| Bone marrowc | 40 ± 4 | 45 ± 3 | 1.1 |
| Lymphocytes | 8.5 ± 3.3 | 20 ± 6 | 2.4* |
| Monocytes | 3.2 ± 1.0 | 3.6 ± 0.8 | 1.1 |
| Blasts | 1.6 ± 0.6 | 1.8 ± 1.4 | 1.1 |
| Myelocytes | 3.9 ± 1.9 | 2.2 ± 1.1 | 0.6 |
| Neutrophils | 13 ± 2 | 13 ± 6 | 1.0 |
| Eosinophils | 1.4 ± 1.1 | 0.8 ± 0.3 | 0.6 |
| Erythroid cells | 8.1 ± 0.7 | 4.2 ± 2.1 | 0.5* |
| Spleenc | 220 ± 40 | 1,400 ± 700 | 6.4** |
| Lymphocytes | 190 ± 30 | 1,300 ± 600 | 6.8** |
| blasts | 6 ± 2 | 44 ± 18 | 7.3** |
| Neutrophils | 7 ± 5 | 30 ± 16 | 4.3** |
| Erythroid cells | 4 ± 2 | 77 ± 65 | 19** |
| Peritoneal cavityc | 4.7 ± 1.9 | 20 ± 3 | 4.3** |
| Lymphocytes | 0.8 ± 0.4 | 10 ± 2 | 13* |
| Macrophages | 3.8 ± 1.7 | 9.8 ± 0.9 | 2.6** |
| Neutrophils | 0.01 ± 0.02 | 0.07 ± 0.12 | 7.0 |
a Ratio of vav-bcl-2 to nontransgenic values; those where P < 0.05 or P < 0.01 are marked with one or two asterisks, respectively.
b Nucleated cells (mean ± SD × 103 cells/μl) from 10 nontransgenic and 14 transgenic mice.
c Nucleated cells (mean ± SD × 106 cells) per femur, per spleen, and per peritoneal cavity, respectively, from three nontransgenic and three transgenic mice.
Because the three vav-bcl-2 strains gave very similar blood profiles, strain 69 was chosen for subsequent studies. In the bone marrow, all cell types had normal morphology and the total nucleated cell content was not significantly increased (Table 1). The major marrow abnormality was a greater than 2-fold increase in lymphocytes, accompanied by fewer nucleated erythroid cells. Flow cytometric analysis revealed that the rise in bone marrow lymphocytes mainly reflected a 4-fold increase in B220+ IgM+ B cells. However, the proportion of immature (B220+, IgM−) B-lineage cells also was elevated by 40% (P < 0.01). In contrast, the proportions of Gr1+ granulocytes and Ter119+ erythroid cells in the marrow were lower, whereas the frequency of Mac1+ cells was unaltered.
The total cellularity of the spleen was increased 6-fold (Table 1), as reflected in a higher spleen weight: 267 ± 48 mg (n = 12) versus 80 ± 12 mg (n = 10), P < 0.01. By cell morphology there were major increases in the lymphoid, myeloid, and erythroid populations. Flow cytometric analysis of spleen cells revealed no significant differences in the proportions of any cell type examined (not shown). Hence, given the marked rise in total cellularity, the splenic content of B lymphoid, myeloid, and erythroid cells, as well as T lymphocytes of both the helper and cytotoxic sets had risen 4- to 6-fold.
Similarly, the cellularity of the peritoneal cavity was increased 4-fold, with a more than 10-fold rise in lymphocytes and a 2-fold increase in macrophages (Table 1). An abnormality of the peritoneal macrophage population was that 5–15% of the cells were multinucleated, containing up to four separate nuclei. Multinucleate macrophages with this morphology can arise by cell fusion (37).
Altered T Cell Development.
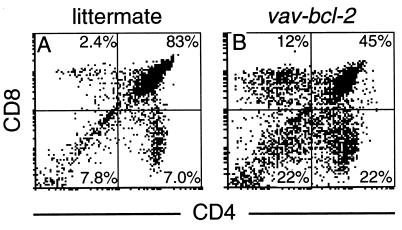
The vav-bcl-2 thymus revealed a marked alteration in T lymphocyte development, even though its weight was normal: 56 ± 19 mg in 12 vav-bcl-2 mice versus 53 ± 14 mg in 10 nontransgenic littermates. When thymocytes were subdivided by their expression of CD4 and CD8 (Fig. 3 A and B), it became evident that a marked decrease in the double-positive (CD4+ CD8+) population (from 84% in the nontransgenic mice to 44% in vav-bcl-2 mice) was balanced by rises in all three other subsets: the more immature, double-negative (CD4− CD8−) population, as well as both of the mature subsets, the CD4+ and CD8+ single-positive cells (Fig. 3 A and B). The CD8+ rise was greater; the ratio of CD4+ to CD8+ thymocytes was 1.6 in the transgenic mice and 3.2 in the control animals. All these differences were very reproducible (P < 0.01).
Figure 3.
Flow cytometric analysis of the cellular composition of the thymus from a nontransgenic mouse (A) or a vav-bcl-2 transgenic littermate (B) showing thymocytes stained for CD4 and CD8. The proportion of cells within each quadrant is given as the percentage of total cells analyzed.
Changes in Tissue Architecture.
The most striking histological abnormalities appeared in the spleen. The enlarged spleen showed increased numbers of lymphoid follicles, with grossly expanded germinal centers that often occupied almost the entire follicle. In these germinal centers, mitotic cells appeared to be normal in frequency, but apoptotic cells were less frequent than in control spleens (data not shown). Phagocytic macrophages were not prominent in the vav-bcl-2 spleen, and the red pulp areas appeared normal in cellular composition.
Other hematopoietic organs were less perturbed. The lymph nodes of vav-bcl-2 mice were slightly enlarged, but there were no abnormal accumulations of macrophages or neutrophils. The bone marrow was unremarkable except for the marked excess of lymphocytes; megakaryocytes appeared to be present in normal numbers. The thymus was of normal morphology with well-defined cortex and medulla. No histological abnormalities were observed in nonhematopoietic organs, including gonads, kidney, bladder, gut, heart, pancreas, and skeletal muscle. The liver and lung often showed portal and perivascular foci of lymphocytes. High-power cell counts revealed no marked increase in the ratio of Kupffer cells to liver parenchymal cells.
Progenitor Cells of vav-bcl-2 Mice.
It was conceivable that constitutive Bcl-2 expression might enhance the number or clonogenicity of hematopoietic progenitor cells, lead to larger colonies, or even allow colony formation in the absence of cytokines. To address these possibilities, transgenic and control bone marrow cells were cultured in agar by using six different stimuli (see Materials and Methods). Four of these (granulocyte/macrophage-CSF, granulocyte-CSF, IL-3, and M-CSF) stimulate colony formation by lineage-committed progenitor cells, whereas the other two (SCF and FL plus leukemia inhibitory factor) also reveal more ancestral preprogenitor cells by stimulating the formation of blast colonies composed of lineage-committed progenitor cells.
It was noteworthy that Bcl-2 did not allow colony formation in the absence of growth factors, and in the supplemented cultures, the range of colony types and their cellular composition were very similar to those generated in parallel cultures of control marrow cells (data not shown). Furthermore, the frequency of granulocyte, eosinophil, and blast progenitors was normal. A consistent and significant reduction was observed, however, in the frequency of macrophage progenitor cells (P < 0.05). The deficit in macrophage colonies was greatest (4-fold) when M-CSF was the stimulus; a smaller reduction (2- to 3-fold) was observed with granulocyte/macrophage-CSF and IL-3 stimuli. There was also a marginal deficit in megakaryocyte colonies arising from IL-3-stimulated transgenic marrow cells (8 ± 2 versus 15 ± 6 from littermate marrow), but the animal-to-animal variation precluded any firm conclusion. Similar assays for progenitor cells in the spleen revealed comparable low frequencies in transgenic and control mice (data not shown).
The macrophage and granulocytic colonies developing in cultures of vav-bcl-2 marrow cells were normal in shape. No unusual migration of colony cells, which might have suggested prolonged survival of mature colony cells with continuing centrifugal migration, was observed. Although some fusion of macrophages can occur in aging macrophage colonies, the numbers of such cells seen in vav-bcl-2 macrophage colonies was not unusual.
The number of cells present in 7-day colonies probably is influenced by cell death as well as cell proliferation. To determine whether Bcl-2 affected colony size, we counted the cells in sequential macrophage colonies from stained intact cultures stimulated by granulocyte/macrophage-CSF. The macrophage colonies formed by vav-bcl-2 cells contained less than half as many cells as those produced by control cells: 153 ± 85 (n = 13) versus 368 ± 410 (n = 15), P < 0.01. Because colonies of both genotypes appeared healthy, the smaller colony size may have been caused by a slower rate of proliferation of the vav-bcl-2 cells. However, the number of cells in granulocyte colonies stimulated by granulocyte-CSF did not differ significantly: 179 ± 174 for 23 vav-bcl-2 colonies versus 234 ± 166 for 29 controls.
The blast colonies stimulated by FL plus leukemia inhibitory factor were affected by Bcl-2. As previously noted (38), blast colonies formed by normal cells in response to this stimulus characteristically exhibit a high content of apoptotic cells. Most such blast colonies formed by vav-bcl-2 cells were of larger size and often contained no apoptotic cells.
Clonogenic Survival of Progenitors Deprived of Cytokines.
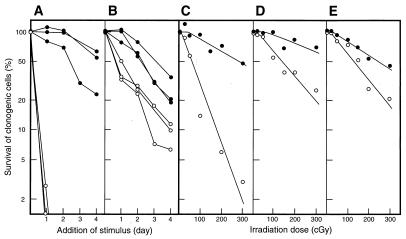
We investigated how Bcl-2 affected the survival in vitro of progenitor cells and preprogenitors (blast colony-forming cells) deprived of growth factors. After culture for increasing periods without added factors, various cytokines were provided and colonies were scored 7 days later. As shown in the examples in Fig. 4, preprogenitors and granulocyte progenitors from vav-bcl-2 marrow had a marked survival advantage over their normal counterparts. The most striking advantage appeared with preprogenitor cells (Fig. 4A). In the absence of added factor, SCF-responsive preprogenitors from control mice exhibited a half-life of only 6 ± 0 h. In sharp contrast, that of preprogenitors from vav-bcl-2 bone marrow was 88 ± 18 h. Similarly, for the IL-3-responsive preprogenitor cells, the half-life was extended by Bcl-2 from 6 ± 0 h to 71 ± 2 h.
Figure 4.
Survival of clonogenic bone marrow progenitors in vitro on delayed addition of SCF or after γ-irradiation. Survival of (A) SCF-responsive preprogenitors and (B) granulocyte progenitors. Three mice of each type were sampled. Survival after γ-irradiation of (C) preprogenitors responsive to FL, (D) granulocyte colony-forming cells responsive to SCF, and (E) macrophage colony-forming cells responsive to M-CSF. Vav-bcl-2 and nontransgenic littermate data are shown as ● and ○, respectively.
Bcl-2 also rendered lineage-committed progenitor cells more refractory to factor deprivation. The SCF-responsive granulocyte progenitors from vav-bcl-2 mice exhibited a half-life of 59 ± 13 h versus 26 ± 4 h for control cells (Fig. 4B). A large increase in survival also was observed with IL-3-responsive granulocyte progenitor cells (from 25 ± 3 to 81 ± 14 h), but the values for the M-CSF-responsive macrophage-committed progenitors rose only slightly (from 39 to 46 h).
The survival curves of the vav-bcl-2 cells had notable shoulders. This result may mean that Bcl-2-expressing cells are initially very effective at correcting abnormalities arising from the factor deprivation.
Protection of Progenitor Cells Against Irradiation.
To assess survival in response to a different cellular insult, we evaluated the radiosensitivity of progenitor cells by clonogenic assays on γ-irradiated marrow cells (Fig. 4 C–E). Each type of normal preprogenitor and progenitor cell exhibited a distinctive sensitivity to radiation, reflected in differing values for D37 (radiation dose yielding 37% survival). The most sensitive were the preprogenitor cells responding to FL plus leukemia inhibitory factor (D37 = 50–80 cGy), followed by the preprogenitor cells responding to SCF (D37 = 110–140 cGy), whereas the granulocyte-committed progenitor cells that respond to SCF and the macrophage-committed progenitor cells stimulated by M-CSF were the most resistant, having D37 values of 190 and 175 cGy, respectively.
In sharp contrast, the corresponding vav-bcl-2 preprogenitor and progenitor cells were much less sensitive, with D37 values exceeding 300 cGy for each (Fig. 4). Although the sensitivity of control and vav-bcl-2 cells was slightly higher in some of the six experiments than in that shown, the relative radiosensitivity of the different clonogenic cell types was maintained, and the control and vav-bcl-2 cells always differed by the same magnitude.
The survival curves for irradiated vav-bcl-2 cells displayed a more pronounced shoulder than those of control cells. Because the shoulder usually is thought to reflect the capacity of cells to repair radiation-induced damage, vav-bcl-2 cells appear to be more effective in that repair process than control cells.
Discussion
As we found with vav-hCD4 transgenes (31), the vav promoter region targeted expression of hBcl-2 throughout the hematopoietic compartment. Essentially all the nucleated cells in the hematopoietic tissues of vav-bcl-2 transgenic mice expressed a high level of Bcl-2 (Fig. 1), and the enhanced clonogenic survival of progenitor cells (see below) confirmed that the transgene also was expressed well in very immature cell types. In contrast, immunoblots with various nonhematopoietic tissues revealed either no hBcl-2 or levels that could be accounted for by resident Vav-expressing hematopoietic cells (Fig. 2). These findings reinforce the view (31, 39) that the vav transgenic vector will be valuable for addressing many issues concerning hematopoiesis.
Constitutive Bcl-2 expression, reflecting its pro-survival activity, caused a marked rise in the number of nonlymphoid as well as lymphoid cells. Monocytes were elevated in the blood, macrophages in the peritoneal cavity and neutrophils in the spleen. The increase in erythroid cells in the spleen may reflect displacement of erythropoiesis from the marrow by excess B-lymphoid cells. The effects in the B-lymphoid lineage were similar to those reported for bcl-2 transgenes driven by Ig regulatory elements (18, 19). Mature B cells were increased about 5-fold, whereas preB cells increased only slightly.
In the T lineage, the results reported here diverge somewhat from those with bcl-2 transgenes expressed selectively in that compartment (20, 21), probably because the vav-driven expression is higher and extends over more differentiation stages. In the periphery, both the helper (CD4+) and the cytotoxic (CD8+) populations were increased. In the thymus, T cell development was clearly disturbed (Fig. 3), as also reported for lck-bcl-2 mice (21). The increases in cells at both the early CD4−CD8− stage and the mature single-positive stages can be ascribed to enhanced cell survival, but the basis for the decrease at the predominant CD4+CD8+ intermediate stage is less clear. It might reflect either the ability of Bcl-2 to retard cell cycle entry (6–8) or a negative feedback process mediated by the increased numbers of mature T cells.
Some of our findings differ somewhat from previous reports. A MRP8-BCL-2 transgene, which was expressed specifically in certain myeloid cells, did not produce an increase in neutrophils (22), although another strain with higher Bcl-2 expression was later shown to have elevated numbers of splenic neutrophils (13). With the vav-bcl-2 mice, the several-fold increases in monocytes/macrophages in the blood and peritoneal cavity, together with the increased total numbers of myeloid cells in the spleen, show that Bcl-2 can perturb myeloid homeostasis in vivo. Although some results obtained with H2K-BCL-2 mice (24), such as the increase in total spleen cells, were similar to those found here, the reported 2-fold increase in total thymocytes is at variance with both our results and those reported with other bcl-2 transgenes expressed well in the thymus (20, 21). Because the H-2Kb promoter is also active in most nonhematopoietic cell types, the thymic expansion provoked by H2K-BCL-2 could be an indirect consequence of effects on thymic support cells, an interpretation mentioned in that study (24). This possibility illustrates the advantage of a vector with hematopoietic specificity.
Because monocytes and macrophages were more numerous in vav-bcl-2 mice, we were somewhat surprised to find that the numbers of both macrophage progenitors and cells per macrophage colony were reduced. Perhaps any decreased production of macrophages in vivo is more than balanced by their increased survival. The lower numbers of macrophage progenitors and their reduced cell production may be related to the ability of Bcl-2 to promote exit from the cell cycle or to retard re-entry into cycle (6–8). It seems relevant that this effect of Bcl-2, which can be distinguished genetically from its pro-survival action (40), has been observed in differentiating HL-60 promyelocytic cells (7). At present, however, it is unclear why this inhibitory effect was observed in macrophage but not granulocyte precursors.
Unexpectedly, vav-bcl-2 mice of all three strains tested had only ≈50% of the normal concentration of platelets in their blood. In principle, the reduction could reflect reduced megakaryocyte production or a decrease in their breakdown to release platelets. We did not find a statistically significant difference in the frequency of megakaryocyte progenitors in bone marrow. We therefore favor the hypothesis that the release of platelets from megakaryocytes involves a mechanism normally regulated by the Bcl-2 family. Platelet release may be associated with apoptosis of the megakaryocyte (41). The simplest possibility therefore would be that megakaryocyte dissolution involves an apoptotic process mediated by the caspases, such as caspase-9, controlled by the Bcl-2 family.
This notion is supported by recent evidence (42) that platelet levels are reduced to a similar extent in mice that lack the gene for Bim, a negative regulator of Bcl-2-like pro-survival members of the Bcl-2 family (43, 44). Indeed, the hematopoietic phenotype of bim−/− mice is remarkably similar to that of the vav-bcl-2 mice. They exhibit comparable increases in lymphoid and myeloid cells and a similar distortion of T cell populations in the thymus (42). The congruence of these findings argues that all the effects reported here are related to normal regulatory steps in homeostasis controlled by the Bcl-2 family.
Bcl-2 strikingly enhanced the clonogenic survival of progenitor and preprogenitor cells, both in response to factor deprivation and irradiation (Fig. 4). Although substantial enhancements were found with all types of these precursor cells, the most marked were with the preprogenitors responsive to SCF (or IL-3). Whether the greater effects observed in the earliest cells reflect higher expression of the vav transgene in that subcompartment or more potent Bcl-2 action is not known. These findings complement and extend observations that H2K-bcl-2 mice have reduced radiosensitivity because of augmented survival of various hematopoietic cell types, including certain primitive ones (24).
Although the vav-bcl-2 mice showed effects in myeloid cells, the largest alterations appeared in the lymphoid lineages. Why might this be? A partial answer might be simply that the level of Bcl-2 in these mice is somewhat lower in the myeloid cells than in the lymphocytes. A more interesting possibility is that the physiological regulation of apoptosis in the nonlymphoid lineages mainly involves pathways that bypass control by the Bcl-2 family. Cell death can be triggered by the “death receptors” of the tumor necrosis factor receptor family such as CD95 (45), and their engagement seems to activate caspases directly, in a manner independent of Bcl-2 control (46). Recently the conjunction of Bcl-2 overexpression in myeloid cells with loss of CD95 has been reported to promote the development of myeloid leukemia (25). Hence, the physiological control of myeloid cell numbers may be regulated both by Bcl-2 family members and an independent pathway from receptors for cytotoxic ligands.
Acknowledgments
We are grateful to Leonie Gibson and Sandra Mifsud for technical assistance, Adrian Mifsud and Jodie de Winter for animal husbandry, Dr. Andreas Strasser for antibodies and discussions, and Jeanette Birtles for preparation of the manuscript. This work was supported by grants from the National Health and Medical Research Council, Canberra (Reg. Key 973002) and the U.S. National Cancer Institute (CA12421).
Footnotes
Abbreviations: CSF, colony-stimulating factor; FL, Flk ligand; M-CSF, macrophage CSF; SCF, stem cell factor.
References
- 1.Lord B I, Molineux G, Pojda Z, Souza L M, Mermod J-J, Dexter T M. Blood. 1991;77:2154–2159. [PubMed] [Google Scholar]
- 2.Egerton M, Scollay R, Shortman K. Proc Natl Acad Sci USA. 1990;87:2579–2582. doi: 10.1073/pnas.87.7.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams J M, Cory S. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 4.Cory S. Annu Rev Immunol. 1995;13:513–543. doi: 10.1146/annurev.iy.13.040195.002501. [DOI] [PubMed] [Google Scholar]
- 5.Vaux D L, Cory S, Adams J M. Nature (London) 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 6.O'Reilly L A, Huang D C S, Strasser A. EMBO J. 1996;15:6979–6990. [PMC free article] [PubMed] [Google Scholar]
- 7.Vairo G, Innes K M, Adams J M. Oncogene. 1996;13:1511–1519. [PubMed] [Google Scholar]
- 8.Linette G P, Li Y, Roth K, Korsmeyer S J. Proc Natl Acad Sci USA. 1996;93:9545–9552. doi: 10.1073/pnas.93.18.9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veis D J, Sorenson C M, Shutter J R, Korsmeyer S J. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama K-I, Nakayama K, Izumi N, Kulda K, Shinkai Y, Louie M C, Fields L E, Lucas P J, Stewart V, Alt F W, et al. Science. 1993;261:1884–1888. doi: 10.1126/science.8372353. [DOI] [PubMed] [Google Scholar]
- 11.Motoyama N, Kimura T, Takahashi T, Watanabe T, Nakano T. J Exp Med. 1999;189:1691–1698. doi: 10.1084/jem.189.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamasaki A, Sendo F, Nakayama K, Ishida N, Negishi I, Nakayama K-I, Hatakeyama S. J Exp Med. 1998;188:1985–1992. doi: 10.1084/jem.188.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagasse E, Weissman I L. Cell. 1997;89:1021–1031. doi: 10.1016/s0092-8674(00)80290-1. [DOI] [PubMed] [Google Scholar]
- 14.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman I L. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 15.Kondo M, Akashi K, Domen J, Sugamura K, Weissman I L. Immunity. 1997;7:155–162. doi: 10.1016/s1074-7613(00)80518-x. [DOI] [PubMed] [Google Scholar]
- 16.Maraskovsky E, O'Reilly L A, Teepe M, Corcoran L M, Peschon J J, Strasser A. Cell. 1997;89:1011–1019. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 17.Maraskovsky E, Peschon J J, McKenna H, Teepe M, Strasser A. Int Immunol. 1998;10:1367–1375. doi: 10.1093/intimm/10.9.1367. [DOI] [PubMed] [Google Scholar]
- 18.McDonnell T J, Deane N, Platt F M, Nuñez G, Jaeger U, McKearn J P, Korsmeyer S J. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 19.Strasser A, Whittingham S, Vaux D L, Bath M L, Adams J M, Cory S, Harris A W. Proc Natl Acad Sci USA. 1991;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strasser A, Harris A W, Cory S. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 21.Sentman C L, Shutter J R, Hockenbery D, Kanagawa O, Korsmeyer S J. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- 22.Lagasse E, Weissman I L. J Exp Med. 1994;179:1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lacronique V, Varlet P, Mayeux P, Porteu A, Gisselbrecht S, Kahn A, Lacombe C. Blood. 1997;90:3050–3056. [PubMed] [Google Scholar]
- 24.Domen J, Gandy K L, Weissman I L. Blood. 1998;91:2272–2282. [PubMed] [Google Scholar]
- 25.Traver D, Akashi K, Weissman I L, Lagasse E. Immunity. 1998;9:47–57. doi: 10.1016/s1074-7613(00)80587-7. [DOI] [PubMed] [Google Scholar]
- 26.Katzav S, Martin Zanca D, Barbacid M. EMBO J. 1989;8:2283–2290. doi: 10.1002/j.1460-2075.1989.tb08354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams J M, Houston H, Allen J, Lints T, Harvey R. Oncogene. 1992;7:611–618. [PubMed] [Google Scholar]
- 28.Coppola J, Bryant S, Koda T, Conway D, Barbacid M. Cell Growth Differ. 1991;2:95–105. [PubMed] [Google Scholar]
- 29.Bustelo X R, Rubin S D, Suen K-L, Carrasco D, Barbacid M. Cell Growth Differ. 1993;4:297–308. [PubMed] [Google Scholar]
- 30.Ogilvy S, Elefanty A G, Visvader J, Bath M L, Harris A W, Adams J M. Blood. 1998;91:419–430. [PubMed] [Google Scholar]
- 31.Ogilvy S, Metcalf D, Gibson L, Bath M L, Harris A W, Adams J M. Blood. 1999;94:1855–1863. [PubMed] [Google Scholar]
- 32.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. [Google Scholar]
- 33.Pezzella F, Tse A G D, Cordell J L, Pulford K A F, Gatter K C, Mason D Y. Am J Pathol. 1990;137:225–232. [PMC free article] [PubMed] [Google Scholar]
- 34.Metcalf D. Clonal Cultures of Haemopoietic Cells: Techniques and Applications. Amsterdam: Elsevier; 1984. [Google Scholar]
- 35.Adams J M, Cory S. Science. 1991;254:1161–1167. doi: 10.1126/science.1957168. [DOI] [PubMed] [Google Scholar]
- 36.Okumura K, Kaneko Y, Nonoguchi K, Nishiyama H, Yokoi H, Higuchi T, Itoh K, Yoshida O, Miki T, Fujita J. Oncogene. 1997;14:713–720. doi: 10.1038/sj.onc.1200878. [DOI] [PubMed] [Google Scholar]
- 37.Metcalf D, Elliott M J, Nicola N A. J Exp Med. 1992;175:877–884. doi: 10.1084/jem.175.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metcalf D. Proc Natl Acad Sci USA. 1997;94:11552–11556. doi: 10.1073/pnas.94.21.11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams J M, Harris A W, Strasser A, Ogilvy S, Cory S. Oncogene. 1999;18:5268–5277. doi: 10.1038/sj.onc.1202997. [DOI] [PubMed] [Google Scholar]
- 40.Huang D C S, O'Reilly L A, Strasser A, Cory S. EMBO J. 1997;16:4628–4638. doi: 10.1093/emboj/16.15.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zauli G, Vitale M, Falcieri E, Gibellini D, Bassini A, Celeghini C, Columbaro M, Capitani S. Blood. 1997;90:2234–2243. [PubMed] [Google Scholar]
- 42.Bouillet, P., Metcalf, D., Huang, D. C. S., Tarlinton, D. M., Kay, T. W. H., Köntgen, F., Adams, J. M. & Strasser, A. (1999) Science, in press. [DOI] [PubMed]
- 43.O'Connor L, Strasser A, O'Reilly L A, Hausmann G, Adams J M, Cory S, Huang D C S. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puthalakath H, Huang D C S, O'Reilly L A, King S M, Strasser A. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- 45.Ashkenazi A, Dixit V M. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 46.Strasser A, Harris A W, Huang D C S, Krammer P H, Cory S. EMBO J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]