Abstract
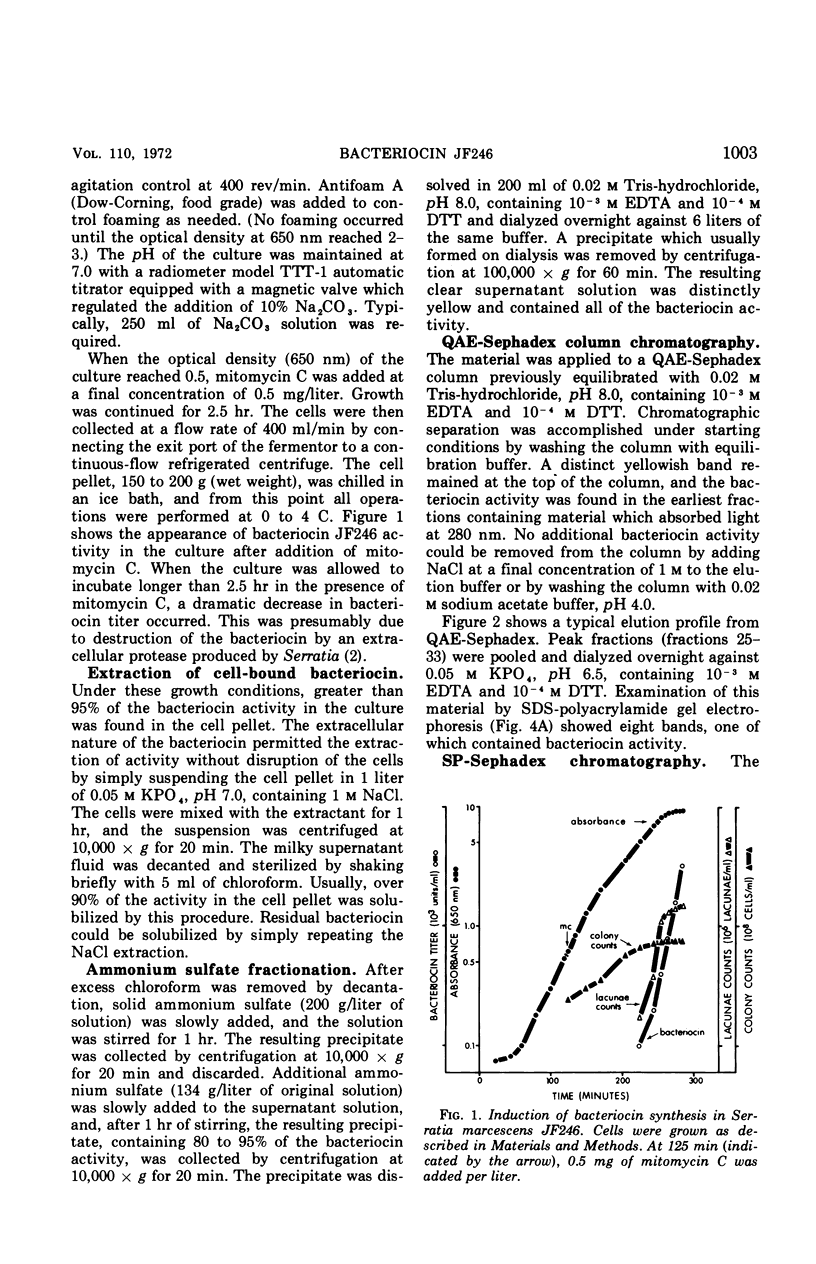
Bacteriocin JF246 has been purified from mitomycin C-induced Serratia marcescens cells by salt extraction, ammonium sulfate fractionation, and chromatography on QAE-Sephadex and SP-Sephadex. The purified material is homogeneous on polyacrylamide gel electrophoresis in the presence of 2% sodium dodecyl sulfate or 6 m urea. In the absence of these agents, the bacteriocin associates into aggregates which can be dissociated with 0.4 m NaCl. The bacteriocin is probably composed of a single subunit with a molecular weight of 64,000 daltons. Analytical studies show the bacteriocin to be essentially protein in nature containing less than one residue of glucose or phosphorus per 64,000 daltons.
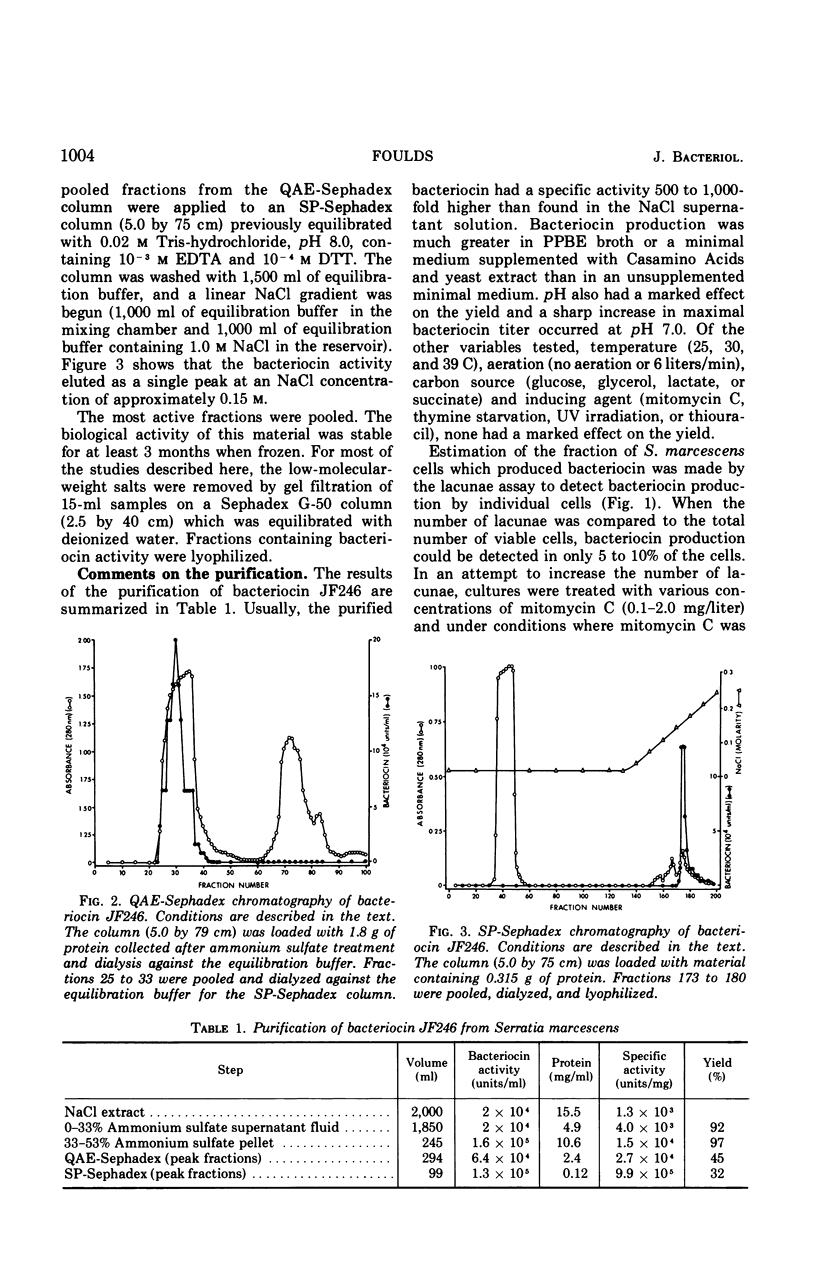
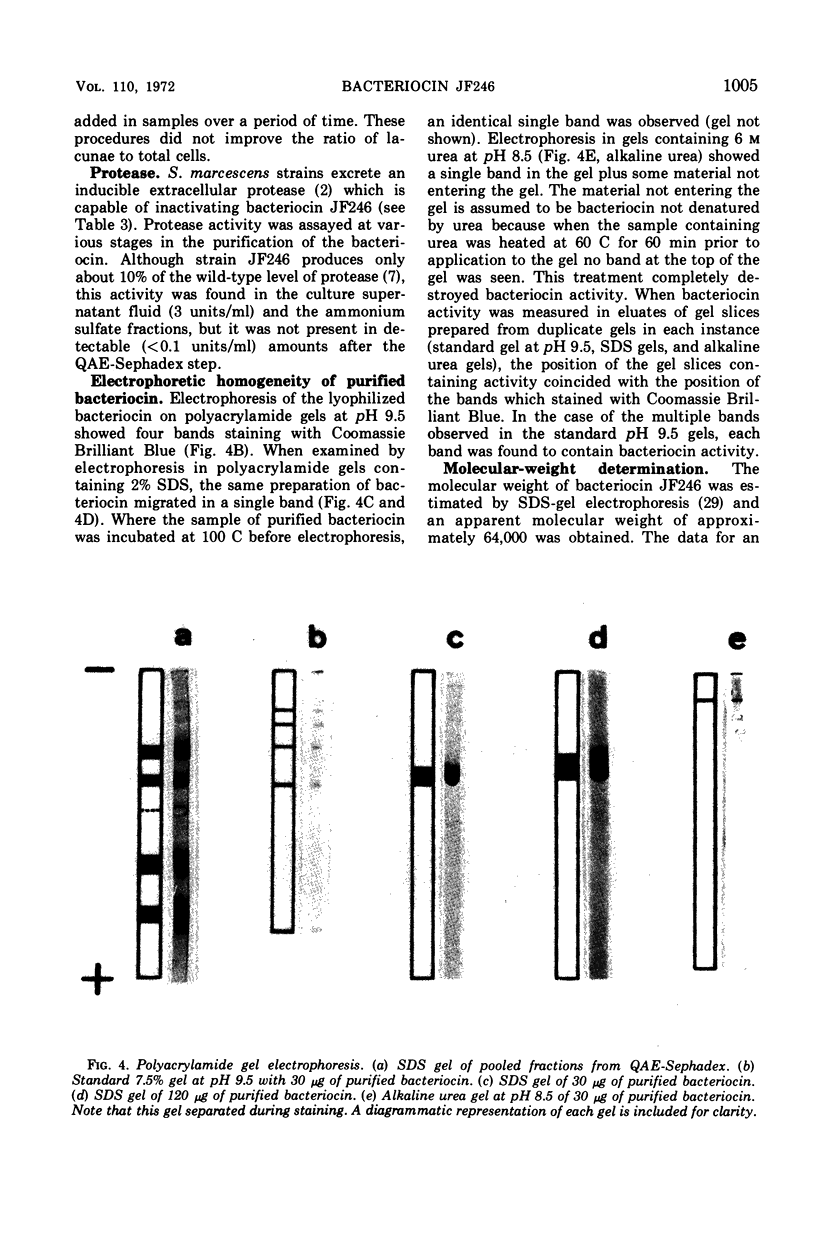
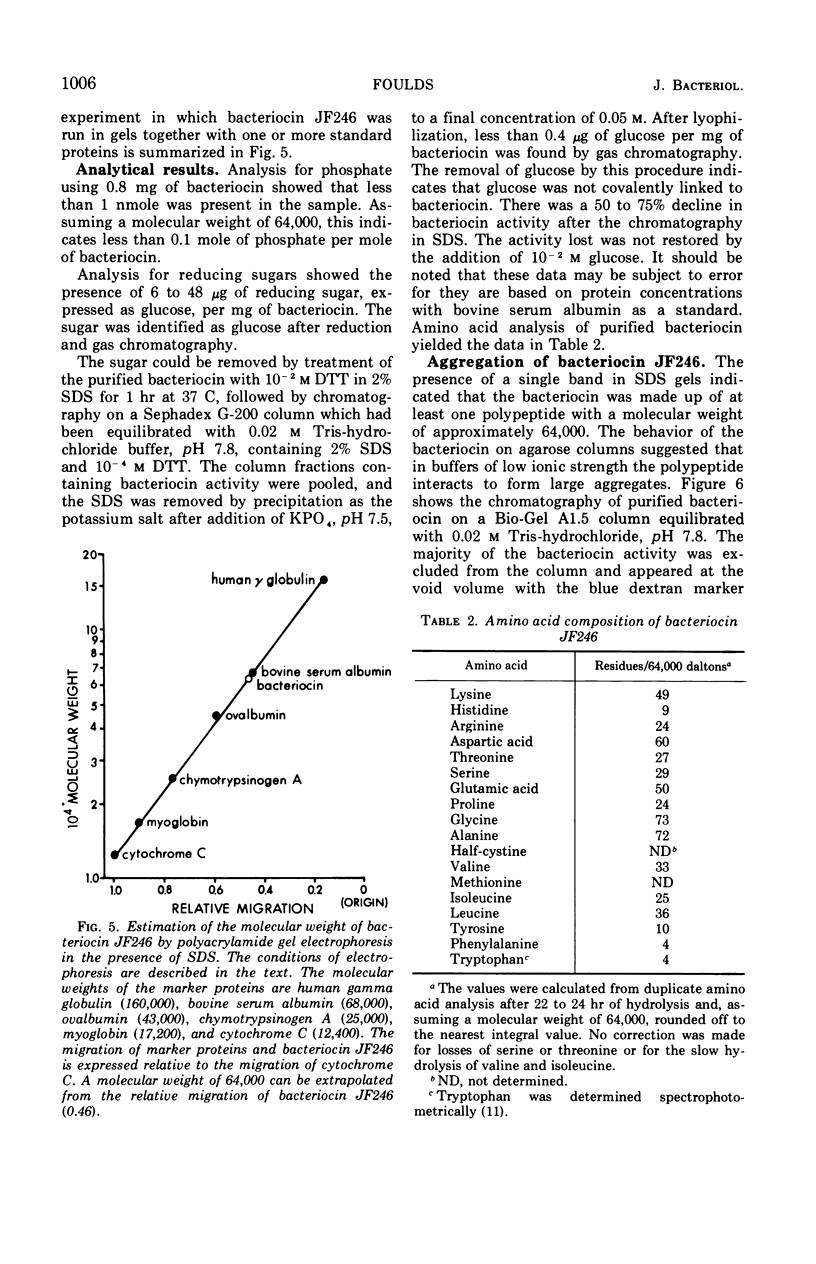
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- CASTANEDA-AGULLO M. Studies on the biosynthesis of extracellular proteases by bacteria. I. Serratia marcescens, synthetic and gelatin media. J Gen Physiol. 1956 Jan 20;39(3):369–375. doi: 10.1085/jgp.39.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandeu J. P., Barbu E. Etude comparée de quelques colicines. C R Acad Sci Hebd Seances Acad Sci D. 1968 Feb 5;266(6):634–636. [PubMed] [Google Scholar]
- Eidels L., Osborn M. J. Lipopolysaccharide and aldoheptose biosynthesis in transketolase mutants of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1673–1677. doi: 10.1073/pnas.68.8.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J. D., Shemin D. Concomitant synthesis of bacteriocin and bacteriocin inactivator from Serratia marcescens. J Bacteriol. 1969 Sep;99(3):661–666. doi: 10.1128/jb.99.3.661-666.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J. D., Shemin D. Properties and characteristics of a bacteriocin from Serratia marcescens. J Bacteriol. 1969 Sep;99(3):655–660. doi: 10.1128/jb.99.3.655-660.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J. Mode of action of a bacteriocin from Serratia marcescens. J Bacteriol. 1971 Sep;107(3):833–839. doi: 10.1128/jb.107.3.833-839.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMON Y., PERON Y. [Study of the bacteriocinogenic property in the genus Serratia]. Ann Inst Pasteur (Paris) 1961 Jun;100:818–821. [PubMed] [Google Scholar]
- Herschman H. R., Helinski D. R. Comparative study of the events associated with colicin induction. J Bacteriol. 1967 Sep;94(3):691–699. doi: 10.1128/jb.94.3.691-699.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R., Helinski D. R. Purification and characterization of colicin E2 and colicin E3. J Biol Chem. 1967 Nov 25;242(22):5360–5368. [PubMed] [Google Scholar]
- Ishii S. I., Nishi Y., Egami F. The fine structure of a pyocin. J Mol Biol. 1965 Sep;13(2):428–431. doi: 10.1016/s0022-2836(65)80107-3. [DOI] [PubMed] [Google Scholar]
- JACOB F., SIMINOVITCH L., WOLLMAN E. Sur la biosynthèse d'une colicine et sur son mode d'action. Ann Inst Pasteur (Paris) 1952 Sep;83(3):295–315. [PubMed] [Google Scholar]
- Jesaitis M. A. The nature of colicin K from Proteus mirabilis. J Exp Med. 1970 May 1;131(5):1016–1038. doi: 10.1084/jem.131.5.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konisky J., Richards F. M. Characterization of colicin Ia and colicin Ib. Purification and some physical properties. J Biol Chem. 1970 Jun 10;245(11):2972–2978. [PubMed] [Google Scholar]
- Kunugita K., Matsuhashi M. Purification and properties of colicin K. J Bacteriol. 1970 Nov;104(2):1017–1019. doi: 10.1128/jb.104.2.1017-1019.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levisohn R., Konisky J., Nomura M. Interaction of colicins with bacterial cells. IV. Immunity breakdown studied with colicins Ia and Ib. J Bacteriol. 1968 Sep;96(3):811–821. doi: 10.1128/jb.96.3.811-821.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- OZEKI H., STOCKER B. A., DE MARGERIE H. Production of colicine by single bacteria. Nature. 1959 Aug 1;184:337–339. doi: 10.1038/184337a0. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- REEVES P. THE BACTERIOCINS. Bacteriol Rev. 1965 Mar;29:24–45. doi: 10.1128/br.29.1.24-45.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDOVAL H. K., REILLY H. C., TANDLER B. COLICIN 15: POSSIBLY A DEFECTIVE BACTERIOPHAGE. Nature. 1965 Jan 30;205:522–523. doi: 10.1038/205522a0. [DOI] [PubMed] [Google Scholar]
- Schwartz S. A., Helinski D. R. Purification and characterization of colicin E1. J Biol Chem. 1971 Oct 25;246(20):6318–6327. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]



