Abstract
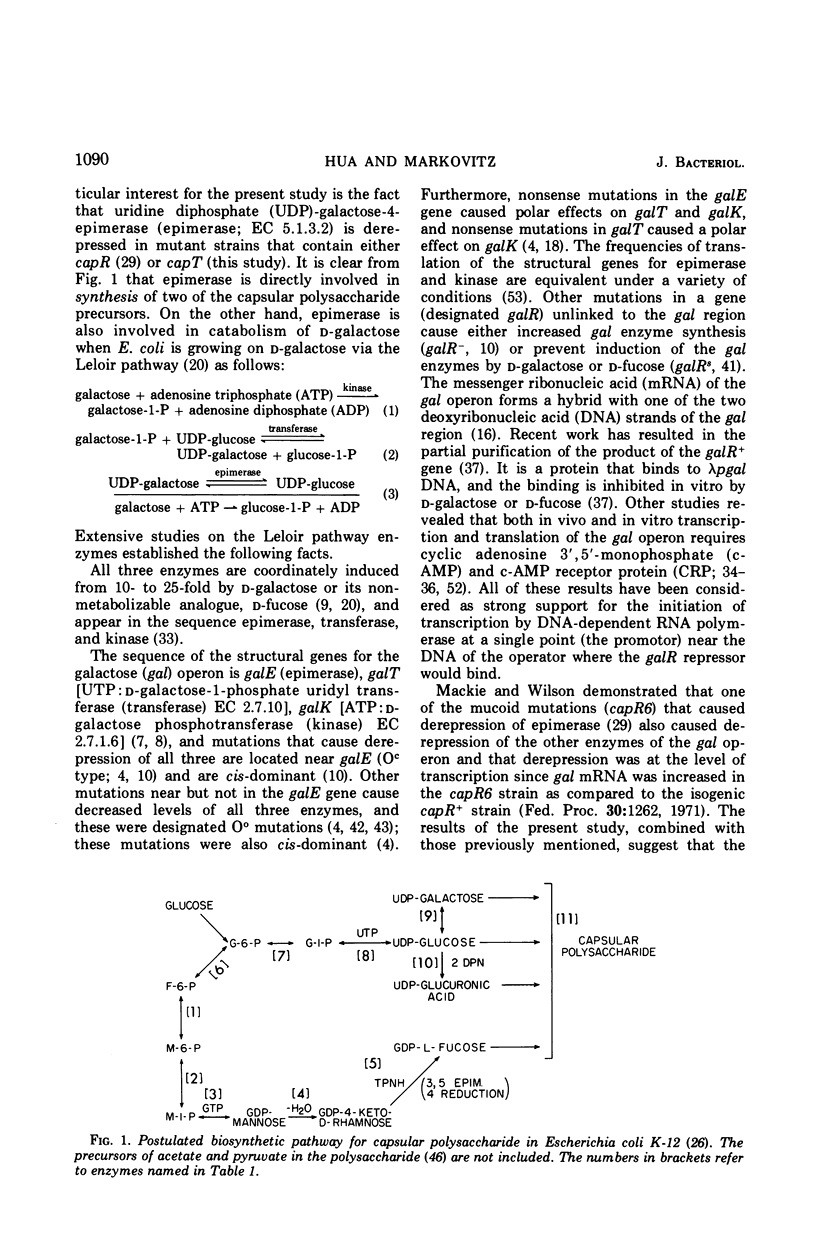
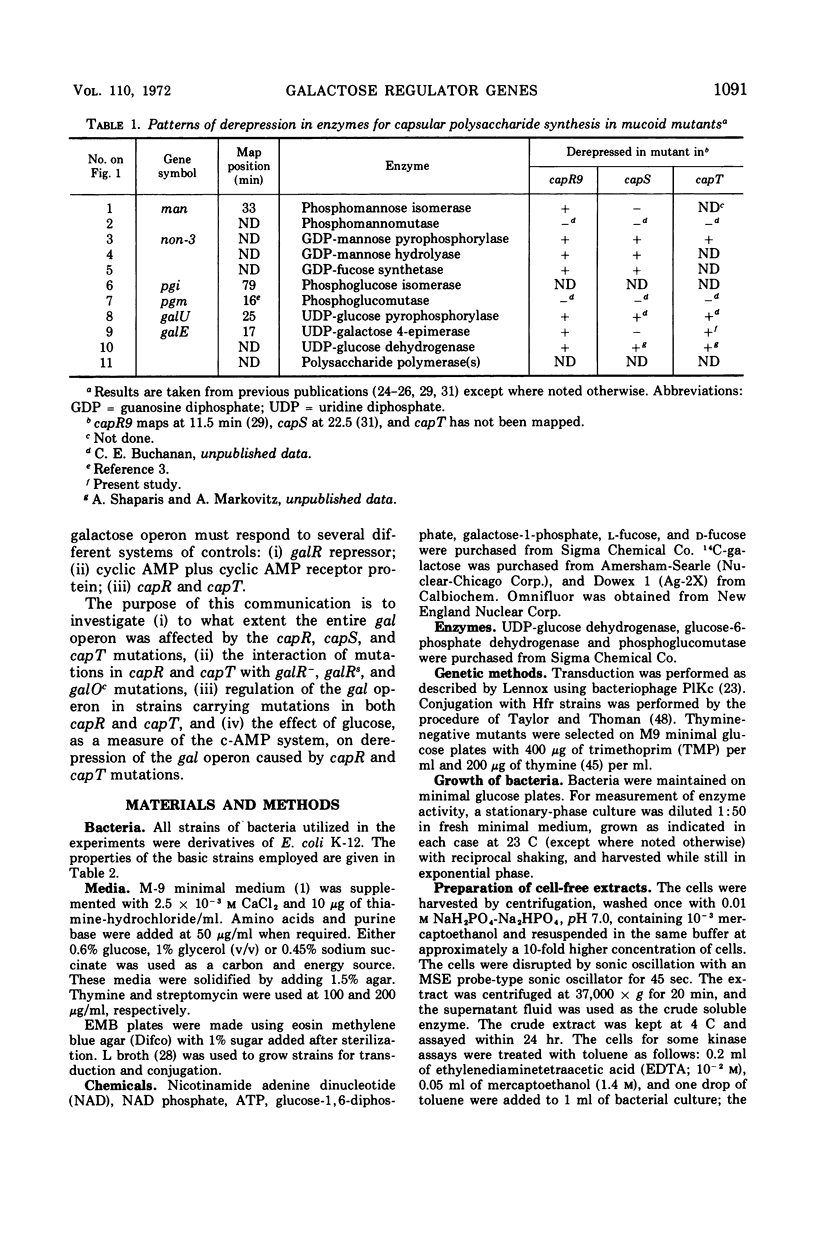
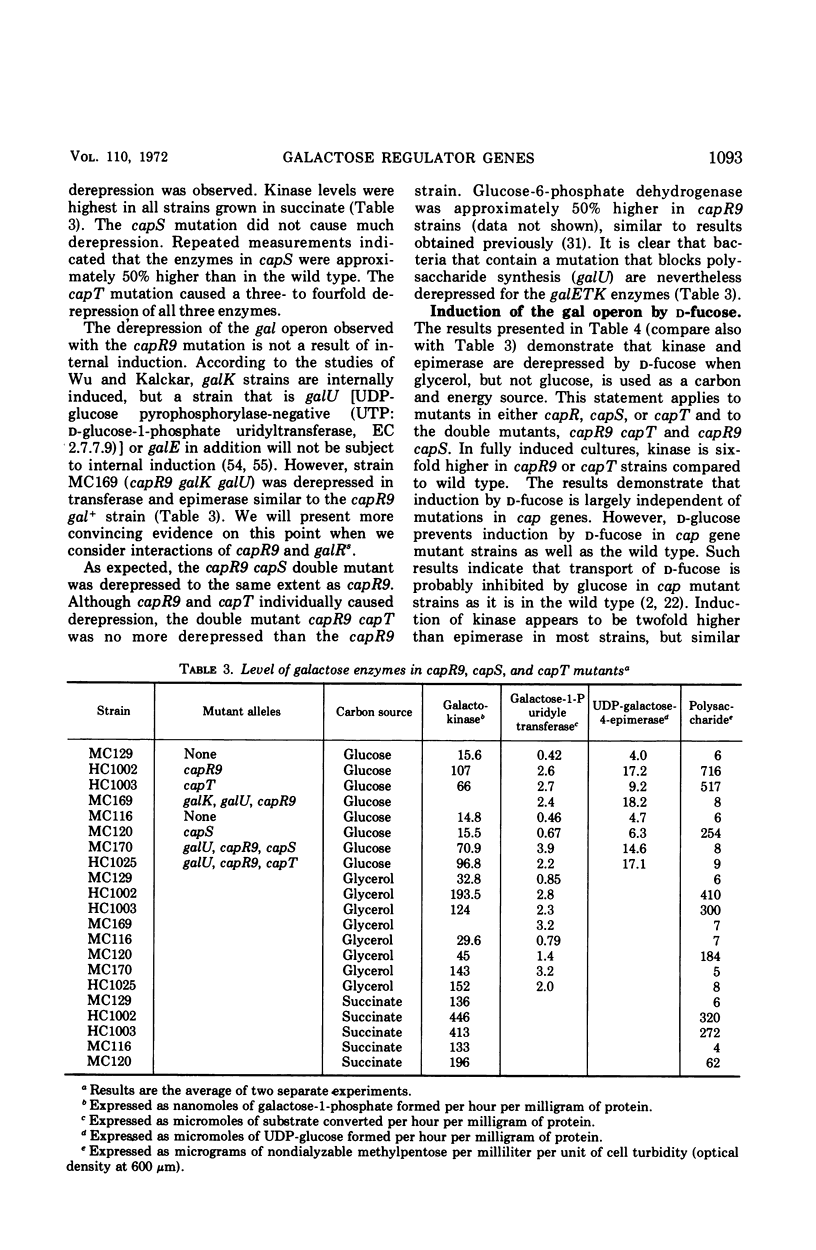
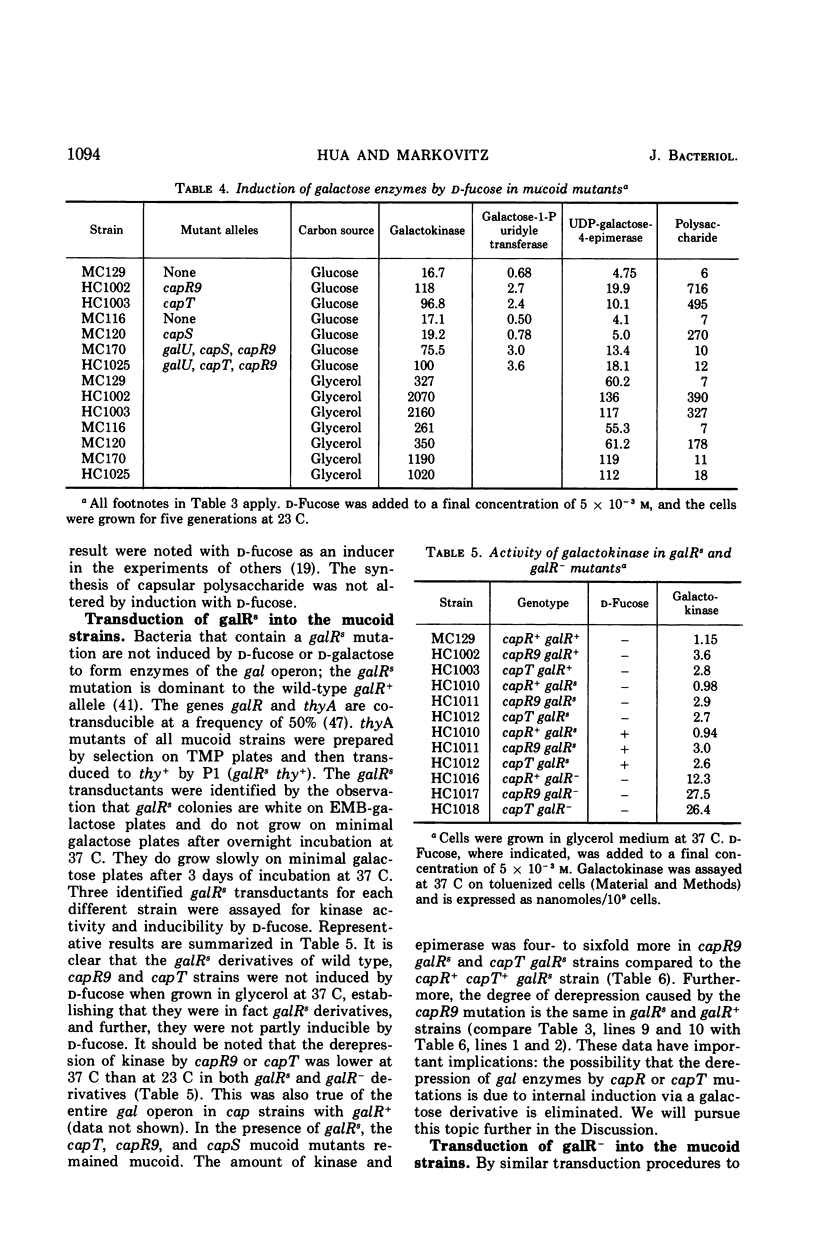
Previous studies showed that nonsense mutations in either of two genes (capR or capS) or an undefined mutation in a third gene (capT) led to pleiotropic effects: (i) increased capsular polysaccharide synthesis (mucoid phenotype); (ii) increased synthesis of enzymes specified by at least four spatially separated operons involved in synthesis of capsular polysaccharide including the product of the galE gene, UDP-galactose-4-epimerase (EC 5.1.3.2) in capR mutants. The present study demonstrated that the entire galactose (gal) operon (galE, galT, and galK) is derepressed by mutations in either the capR or the capT genes, but not by mutation in capS. Double mutants (capR9 capT) were no more derepressed than the capR9 mutant, indicating that capR9 and capT regulate the gal operon via a common pathway. Isogenic double mutants containing either galR+, galR−, galRs, or galOc in combination with either capR+ or capR9 were prepared and analyzed for enzymes of the gal operon. The results demonstrated that capR9 caused derepression as compared to capR+ in all of the combinations. Strains with a galRs mutation are not induced, for the gal operon, by any galactose compound including d-fucose, and this was confirmed in the present study using d-fucose. Nevertheless, the derepression of galRs capR9 compared to galRs capR+ was four- to sixfold. The same derepression was observed when galR+capR9 was compared to galR+capR+. The data eliminate the explanation that internal induction of the gal operon by a galactose derivative was causing increased gal operon enzyme synthesis in capR or capT mutants. Furthermore, the same data suggest that the galR and capR genes are acting independently to derepress the gal operon. A modified model for the structure of the gal operon is proposed to explain these results. The new feature of the model is that two operator sites are suggested, one to combine with the galR repressor and one to combine with the capR repressor.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER H. I., HARDIGREE A. A. POSTIRRADIATION GROWTH, DIVISION, AND RECOVERY IN BACTERIA. Radiat Res. 1965 May;25:92–102. [PubMed] [Google Scholar]
- ADLER J., KAISER A. D. Mapping of the galactose genes of Escherichia coli by transduction with phage P1. Virology. 1963 Feb;19:117–126. doi: 10.1016/0042-6822(63)90001-1. [DOI] [PubMed] [Google Scholar]
- ADLER J., TEMPLETON B. THE AMOUNT OF GALACTOSE GENETIC MATERIAL IN LAMBDA-DG BACTERIOPHAGE WITH DIFFERENT DENSITIES. J Mol Biol. 1963 Dec;7:710–720. doi: 10.1016/s0022-2836(63)80118-7. [DOI] [PubMed] [Google Scholar]
- Adhya S. L., Shapiro J. A. The galactose operon of E. coli K-12. I. Structural and pleiotropic mutations of the operon. Genetics. 1969 Jun;62(2):231–247. doi: 10.1093/genetics/62.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhya S., Echols H. Glucose effect and the galactose enzymes of Escherichia coli: correlation between glucose inhibition of induction and inducer transport. J Bacteriol. 1966 Sep;92(3):601–608. doi: 10.1128/jb.92.3.601-608.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhya S., Schwartz M. Phosphoglucomutase mutants of Escherichia coli K-12. J Bacteriol. 1971 Nov;108(2):621–626. doi: 10.1128/jb.108.2.621-626.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTIN G. M'ECANISMES R'EGULATEURS DANS LA BIOSYNTH'ESE DES ENZYMES DU M'ETABOLISME DU GALACTOSE CHEZ ESCHERICHIA COLI K12. I. LA BIOSYNTH'ESE INDUITE DE LA GALACTOKINASE ET L'INDUCTION SIMULTAN'EE DE LA S'EQUENCE ENZYMATIQUE. J Mol Biol. 1963 Aug;7:164–182. doi: 10.1016/s0022-2836(63)80044-3. [DOI] [PubMed] [Google Scholar]
- BUTTIN G. M'ECANISMES R'EGULATEURS DANS LA BIOSYNTH'ESE DES ENZYMES DU M'ETABOLISME DU GALACTOSE CHEZ ESCHERICHIA COLI K12. II. LE D'ETERMINISME G'EN'ETIQUE DE LA R'EGULATION. J Mol Biol. 1963 Aug;7:183–205. doi: 10.1016/s0022-2836(63)80045-5. [DOI] [PubMed] [Google Scholar]
- Donch J., Greenberg J. Genetic analysis of lon mutants of strain K-12 of Escherichia coli. Mol Gen Genet. 1968;103(2):105–115. doi: 10.1007/BF00427138. [DOI] [PubMed] [Google Scholar]
- GOEBEL W. F. Colanic acid. Proc Natl Acad Sci U S A. 1963 Apr;49:464–471. doi: 10.1073/pnas.49.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W. D., Sutherland I. W., Wilkimson J. F. Control of colanic acid synthesis. J Bacteriol. 1970 Jul;103(1):89–96. doi: 10.1128/jb.103.1.89-96.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W. D., Sutherland I. W., Wilkinson J. F. Exopolysaccharide colanic acid and its occurrence in the Enterobacteriaceae. J Bacteriol. 1969 Dec;100(3):1187–1193. doi: 10.1128/jb.100.3.1187-1193.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A., Tabaczyński M., Szybalski W. Orientation of transcription for the galactose operon as determined by hybridization of gal mRNA with the separated DNA strands of coliphages lambda-dg. J Mol Biol. 1968 Jul 14;35(1):207–213. doi: 10.1016/s0022-2836(68)80048-8. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan E., Saedler H., Lengeler J., Starlinger P. Changes in the specific activities of the galactose enzymes in E. coli under different growth conditions. Mol Gen Genet. 1967;100(2):203–209. doi: 10.1007/BF00333606. [DOI] [PubMed] [Google Scholar]
- Jordan E., Saedler H. Polarity of amber mutations and suppressed amber mutations in the galactose operon of E. coli. Mol Gen Genet. 1967;100(3):283–295. doi: 10.1007/BF00381824. [DOI] [PubMed] [Google Scholar]
- Kalckar H. M., Kurahashi K., Jordan E. HEREDITARY DEFECTS IN GALACTOSE METABOLISM IN ESCHERICHIA COLI MUTANTS, I. DETERMINATION OF ENZYME ACTIVITIES. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1776–1786. doi: 10.1073/pnas.45.12.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., Markovitz A. Induction of capsular polysaccharide synthesis by rho-fluorophenylalanine in Escherichia coli wild type and strains with altered phenylalanyl soluble ribonucleic acid synthetase. J Bacteriol. 1967 Feb;93(2):584–591. doi: 10.1128/jb.93.2.584-591.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- Lengeler J. Untersuchungen zum Glukose-Effekt bei der Synthese der Galaktose-Enzyme von Escherichia coli. Z Vererbungsl. 1966;98(3):203–229. [PubMed] [Google Scholar]
- Lieberman M. M., Buchanan C. E., Markovitz A. Derepression of GDP-alpha-mannose and UDP-glucose pyrophosphorylases by a regulator gene mutation; episomal dominance in partial diploids. Proc Natl Acad Sci U S A. 1970 Mar;65(3):625–632. doi: 10.1073/pnas.65.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. M., Markovitz A. Depression of guanosine diphosphate-mannose pyrophosphorylase by mutations in two different regulator genes involved in capsular polysaccharide synthesis in Escherichia coli K-12. J Bacteriol. 1970 Mar;101(3):965–972. doi: 10.1128/jb.101.3.965-972.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M. M., Shaparis A., Markovitz A. Control of uridine diphosphate-glucose dehydrogenase synthesis and uridine diphosphate-glucuronic acid accumulation by a regulator gene mutation in Escherichia coli K-12. J Bacteriol. 1970 Mar;101(3):959–964. doi: 10.1128/jb.101.3.959-964.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKOVITZ A. REGULATORY MECHANISMS FOR SYNTHESIS OF CAPSULAR POLYSACCHARIDE IN MUCOID MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1964 Feb;51:239–246. doi: 10.1073/pnas.51.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz A., Baker B. Suppression of radiation sensitivity and capsular polysaccharide synthesis in Escherichia coli K-12 by ochre suppressors. J Bacteriol. 1967 Aug;94(2):388–395. doi: 10.1128/jb.94.2.388-395.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz A., Lieberman M. M., Rosenbaum N. Derepression of phosphomannose isomerase by regulator gene mutations involved in capsular polysaccharide synthesis in Escherichia coli K-12. J Bacteriol. 1967 Nov;94(5):1497–1501. doi: 10.1128/jb.94.5.1497-1501.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz A., Rosenbaum N. A regulator gene that is dominant on an episome and recessive on a chromosome. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1084–1091. doi: 10.1073/pnas.54.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis G., Starlinger P. Sequential appearance of the galactose enzymes in E. coli. Mol Gen Genet. 1967;100(2):210–215. doi: 10.1007/BF00333607. [DOI] [PubMed] [Google Scholar]
- Miller Z., Varmus H. E., Parks J. S., Perlman R. L., Pastan I. Regulation of gal messenger ribonucleic acid synthesis in Escherichia coli by 3',5'-cyclic adenosine monophosphate. J Biol Chem. 1971 May 10;246(9):2898–2903. [PubMed] [Google Scholar]
- Parks J. S., Gottesman M., Perlman R. L., Pastan I. Regulation of galactokinase synthesis by cyclic adenosine 3',5'-monophosphate in cell-free extracts of Escherichia coli. J Biol Chem. 1971 Apr 25;246(8):2419–2424. [PubMed] [Google Scholar]
- Parks J. S., Gottesman M., Shimada K., Weisberg R. A., Perlman R. L., Pastan I. Isolation of the gal repressor. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1891–1895. doi: 10.1073/pnas.68.8.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt L., Kaiser A. D. Control of lambda repressor synthesis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2185–2189. doi: 10.1073/pnas.68.9.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff W. S., Miller J. H., Scaife J. G., Beckwith J. R. A mechanism for repressor action. J Mol Biol. 1969 Jul 14;43(1):201–213. doi: 10.1016/0022-2836(69)90089-8. [DOI] [PubMed] [Google Scholar]
- SHERMAN J. R., ADLER J. Galactokinse from Escherichia coli. J Biol Chem. 1963 Mar;238:873–878. [PubMed] [Google Scholar]
- STACEY K. A., SIMSON E. IMPROVED METHOD FOR THE ISOLATION OF THYMINE-REQUIRING MUTANTS OF ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:554–555. doi: 10.1128/jb.90.2.554-555.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saedler H., Gullon A., Fiethen L., Starlinger P. Negative control of the galactose operon in E. coli. Mol Gen Genet. 1968;102(1):79–88. doi: 10.1007/BF00341872. [DOI] [PubMed] [Google Scholar]
- Saedler H., Starlinger P. 0 degree mutations in the galactose operon in E. coli. I. Genetic characterization. Mol Gen Genet. 1967;100(2):178–189. doi: 10.1007/BF00333604. [DOI] [PubMed] [Google Scholar]
- Saedler H., Starlinger P. 0 degree mutations in the galactose operon in E. coli. II. Physiological characterization. Mol Gen Genet. 1967;100(2):190–202. doi: 10.1007/BF00333605. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W. Structural studies on colanic acid, the common exopolysaccharide found in the enterobacteriaceae, by partial acid hydrolysis. Oligosaccharides from colanic acid. Biochem J. 1969 Dec;115(5):935–945. doi: 10.1042/bj1150935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR A. L., THOMAN M. S. THE GENETIC MAP OF ESCHERICHIA COLI K-12. Genetics. 1964 Oct;50:659–677. doi: 10.1093/genetics/50.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uretz R. B., Markovitz A. Dominance of ultraviolet radiation resistance in partial diploids of Escherichia coli K-12. J Bacteriol. 1969 Nov;100(2):1118–1120. doi: 10.1128/jb.100.2.1118-1120.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Perlman R. L., Pastan I. Regulation of lac messenger ribonucleic acid synthesis by cyclic adenosine 3',5'-monophosphate and glucose. J Biol Chem. 1970 May 10;245(9):2259–2267. [PubMed] [Google Scholar]
- Varmus H. E., Perlman R. L., Pastan I. Regulation of lac transcription in Escherichia coli by cyclic adenosine 3',5'-monophosphate. Studies with deoxyribonucleic acid-ribonucleic acid hybridization and hybridization competition. J Biol Chem. 1970 Dec 10;245(23):6366–6372. [PubMed] [Google Scholar]
- Wetekam W., Staack K., Ehring R. DNA-dependent in vitro synthesis of enzymes of the galactose operon of Escherichia coli. Mol Gen Genet. 1971;112(1):14–27. doi: 10.1007/BF00266928. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Hogness D. S. The enzymes of the galactose operon in Escherichia coli. IV. The frequencies of translation of the terminal cistrons in the operon. J Biol Chem. 1969 Apr 25;244(8):2143–2148. [PubMed] [Google Scholar]
- Wu H. C., Kalckar H. M. Endogenous induction of the galactose operon in Escherichia coli K12. Proc Natl Acad Sci U S A. 1966 Mar;55(3):622–629. doi: 10.1073/pnas.55.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C. Role of the galactose transport system in the establishment of endogenous induction of the galactose operon in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):213–223. doi: 10.1016/0022-2836(67)90327-0. [DOI] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]


