Abstract
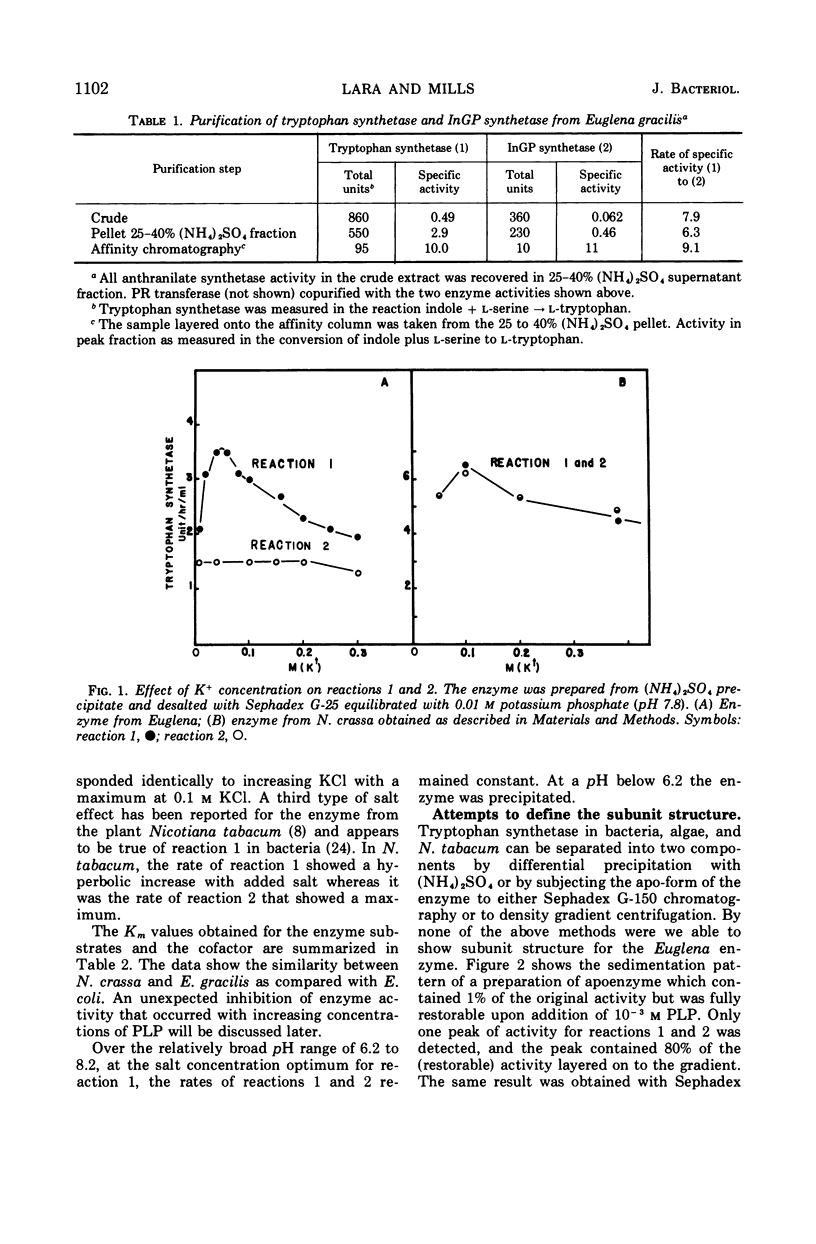
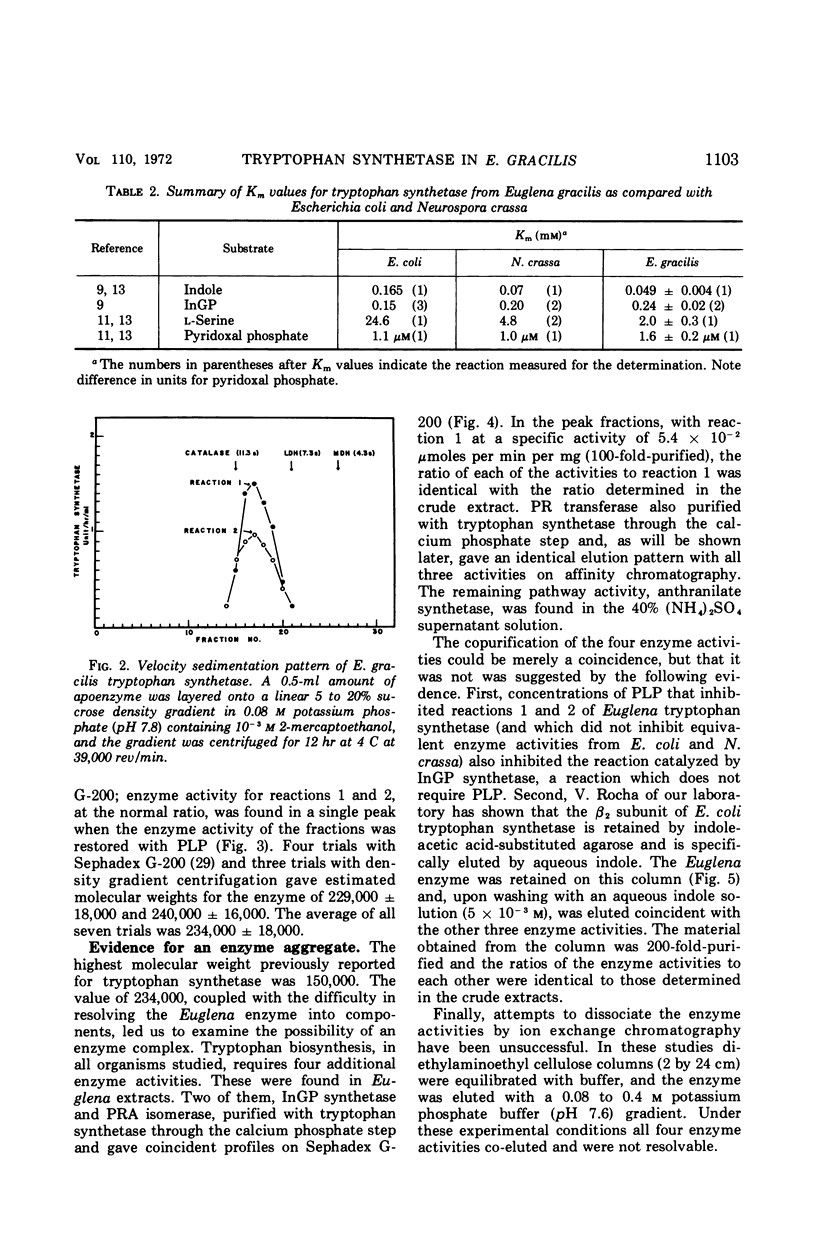
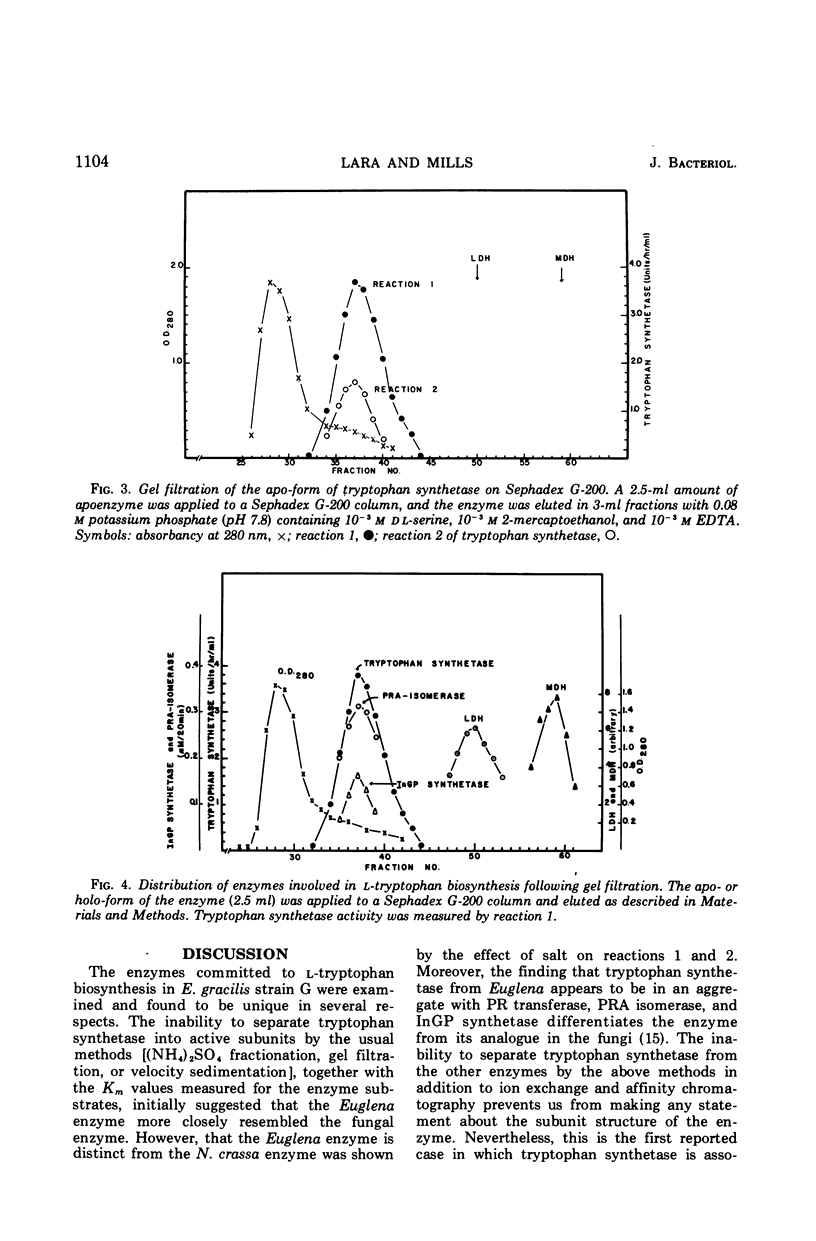
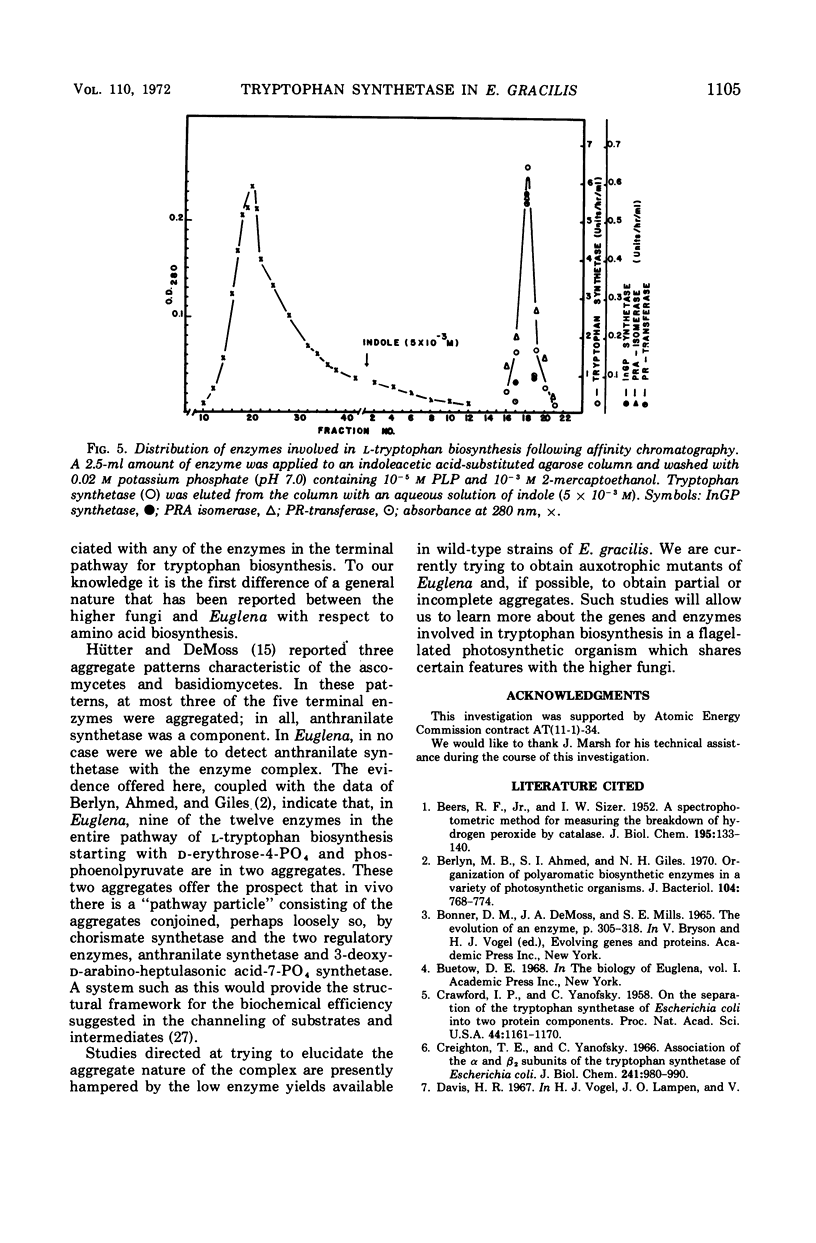
The five enzyme activities in the synthesis of l-tryptophan have been obtained in extracts of Euglena gracilis. One of these, tryptophan synthetase, has been studied in detail. The general catalytic properties of tryptophan synthetase, including the range of reactions catalyzed and its substrate and cofactor affinities, are similar to those reported for other organisms. The Euglena enzyme has two properties never previously observed for tryptophan synthetase. First, the rate of catalysis of the conversion of indole-glycerol phosphate to l-tryptophan remained at its maximal value and was unaffected by the ionic environment up to 0.3 m KCl. In contrast, the conversion of indole to tryptophan showed a sharp maximum at 0.08 m KCl. Second, the enzyme is a component of a complex that includes every enzyme in the pathway committed to tryptophan biosynthesis with the exception of anthranilate synthetase, the regulatory enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Berlyn M. B., Ahmed S. I., Giles N. H. Organization of polyaromatic biosynthetic enzymes in a variety of photosynthetic organisms. J Bacteriol. 1970 Nov;104(2):768–774. doi: 10.1128/jb.104.2.768-774.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Yanofsky C. ON THE SEPARATION OF THE TRYPTOPHAN SYNTHETASE OF ESCHERICHIA COLI INTO TWO PROTEIN COMPONENTS. Proc Natl Acad Sci U S A. 1958 Dec 15;44(12):1161–1170. doi: 10.1073/pnas.44.12.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E., Yanofsky C. Association of the alpha and beta-2 subunits of the tryptophan synthetase of Escherichia coli. J Biol Chem. 1966 Feb 25;241(4):980–990. [PubMed] [Google Scholar]
- DEMOSS J. A. Studies on the mechanism of the tryptophan synthetase reaction. Biochim Biophys Acta. 1962 Aug 13;62:279–293. doi: 10.1016/0006-3002(62)90041-0. [DOI] [PubMed] [Google Scholar]
- Delmer D. P., Mills S. Tryptophan synthase from Nicotiana tabacum. Biochim Biophys Acta. 1968 Oct 8;167(2):431–443. doi: 10.1016/0005-2744(68)90223-4. [DOI] [PubMed] [Google Scholar]
- Demoss J. A., Bonner D. M. STUDIES ON NORMAL AND GENETICALLY ALTERED TRYPTOPHAN SYNTHETASE FROM NEUROSPORA CRASSA. Proc Natl Acad Sci U S A. 1959 Sep;45(9):1405–1412. doi: 10.1073/pnas.45.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATANAKA M., WHITE E. A., HORIBATA K., CRAWFORD I. P. A study of the catalytic properties of Escherichia coli tryptophan synthetase, a two-component enzyme. Arch Biochem Biophys. 1962 Jun;97:596–606. doi: 10.1016/0003-9861(62)90129-7. [DOI] [PubMed] [Google Scholar]
- Hütter R., DeMoss J. A. Enzyme analysis of the tryptophan pathway in Aspergillus nidulans. Genetics. 1967 Feb;55(2):241–247. doi: 10.1093/genetics/55.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütter R., DeMoss J. A. Organization of the tryptophan pathway: a phylogenetic study of the fungi. J Bacteriol. 1967 Dec;94(6):1896–1907. doi: 10.1128/jb.94.6.1896-1907.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Manney T. R. Physiological advantage of the mechanism of the tryptophan synthetase reaction. J Bacteriol. 1970 May;102(2):483–488. doi: 10.1128/jb.102.2.483-488.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ A. K., BONNER D. M. TRYPTOPHAN SYNTHETASE IN BACILLUS SUBTILIS: EFFECTS OF HIGH POTASSIUM ION CONCENTRATION ON A TWO COMPONENT ENZYME. Biochim Biophys Acta. 1964 Aug 26;89:337–347. doi: 10.1016/0926-6569(64)90223-8. [DOI] [PubMed] [Google Scholar]
- SMITH O. H., YANOFSKY C. 1-(o-Carboxyphenylamino)-1-deoxyribulose 5-phosphate, a new intermediate in the biosynthesis of tryptophan. J Biol Chem. 1960 Jul;235:2051–2057. [PubMed] [Google Scholar]
- Sakaguchi K. The similarity of tryptophan synthetases of Anabaena variabilis and Chlorella ellipsoidea with that of bacteria. Biochim Biophys Acta. 1970 Dec 16;220(3):580–593. doi: 10.1016/0005-2744(70)90288-3. [DOI] [PubMed] [Google Scholar]
- Wegman J., DeMoss J. A. The enzymatic conversion of anthranilate to indolylglycerol phosphate in Neurospora crassa. J Biol Chem. 1965 Oct;240(10):3781–3788. [PubMed] [Google Scholar]


