Abstract
Members of the soil-dwelling prokaryotic genus Streptomyces produce many secondary metabolites, including antibiotics and anti-tumour agents. Their formation is coupled with the onset of development, which is triggered by the nutrient status of the habitat. We propose the first complete signalling cascade from nutrient sensing to development and antibiotic biosynthesis. We show that a high concentration of N-acetylglucosamine—perhaps mimicking the accumulation of N-acetylglucosamine after autolytic degradation of the vegetative mycelium—is a major checkpoint for the onset of secondary metabolism. The response is transmitted to antibiotic pathway-specific activators through the pleiotropic transcriptional repressor DasR, the regulon of which also includes all N-acetylglucosamine-related catabolic genes. The results allowed us to devise a new strategy for activating pathways for secondary metabolite biosynthesis. Such ‘cryptic' pathways are abundant in actinomycete genomes, thereby offering new prospects in the fight against multiple drug-resistant pathogens and cancers.
Keywords: development, nutrient sensing, regulation, secondary metabolism, signalling
Introduction
As producers of around two-thirds of all known antibiotics, the soil-dwelling Gram-positive filamentous streptomycetes are a paradigm of secondary metabolite-producing microorganisms (Hopwood, 1999). Streptomyces coelicolor A3(2) is the most-studied streptomycete; its complete genome sequence (Bentley et al, 2002) and that of S. avermitilis (Ikeda et al, 2003) each revealed more than 20 ‘cryptic' (silent) biosynthetic gene clusters for secondary metabolites (Challis & Hopwood, 2003). Although there is a clear temporal correlation between the onset of antibiotic production and development, a detailed understanding of the mechanisms underlying this global control is lacking (Bibb, 2005).
Similar to filamentous fungi, streptomycetes form a mycelium of branching hyphae. On nutrient depletion, aerial hyphae are erected on top of the substrate mycelium and these eventually develop chains of unigenomic spores (Chater & Losick, 1997). Antibiotic production is switched on at a time that corresponds to the early stages of development. What are the control mechanisms that tie chemical differentiation—antibiotic production—to the onset of morphological differentiation? Premature commitment to sporulation means that the opportunity for maximum biomass accumulation is lost, whereas failure to initiate development in time means that the required nutrients become depleted (Gonzalez-Pastor et al, 2003; Dworkin & Losick, 2005). The lytic dismantling of the vegetative mycelium in Streptomyces colonies, which is necessary to provide nutrients for the build-up of the spore-forming aerial mycelium, is a striking example of programmed cell death (Fernandez & Sanchez, 2002; Manteca et al, 2006). In eukaryotes, the programmed removal of cells during development or for eradicating defective cells is well studied (Baumann et al, 2002; van Loo et al, 2002), but it has only recently been recognized as important in bacteria (Rice & Bayles, 2003). The suggestion that antibiotic production is controlled by an apoptosis-like mechanism is reinforced by the failure of most non-developing mutants to produce antibiotics (Bibb, 2005). The remarkable coincidence between the onset of development and of secondary metabolism suggests the presence of a master switch superimposed on the pathway-specific control mechanisms. Here, we describe the first, to our knowledge, complete signalling cascade from the perception of the nutritional status of the environment to the onset of antibiotic production, which revolves around the global regulatory protein DasR.
Results And Discussion
N-acetylglucosamine as a signal for antibiotic production
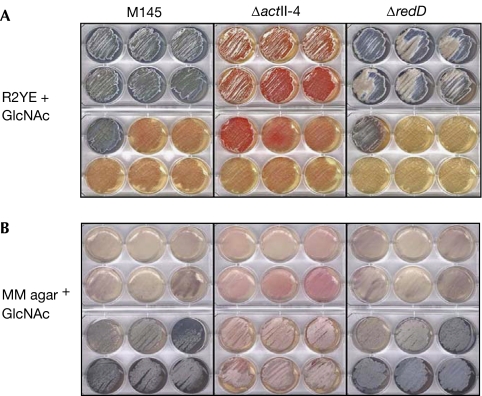
The carbon and nitrogen source N-acetylglucosamine (GlcNAc), which is part of the bacterial peptidoglycan and the monomer of the abundant natural polymer chitin, causes developmental arrest of S. coelicolor on rich media (Rigali et al, 2006). This contrasts with the comparable carbon and nitrogen source glutamate, which as a carbon source is preferred to glucose, but has no apparent effect on development (van Wezel et al, 2006). As antibiotic production coincides with morphogenesis, we investigated the effect of GlcNAc on the production of the red-pigmented antibiotic undecylprodigiosin (Red) and the blue-pigmented actinorhodin (Act) of S. coelicolor grown on rich (R2YE) and minimal medium (MM) agar plates. The act and red biosynthetic clusters are transcriptionally activated by RedD/RedZ and ActII-ORF4, respectively (Bibb, 2005). Interestingly, as shown in Fig 1, GlcNAc blocks development and antibiotic production under rich growth conditions (R2YE agar+10 mM GlcNAc or higher), whereas it triggers Act and Red production and sporulation under poor nutritional conditions (MM agar; 5 mM GlcNAc or higher).
Figure 1.
The dual GlcNAc signal depends on media conditions. On nutrient-rich R2YE plates (A), GlcNAc blocks morphogenesis and antibiotic production, whereas on nutrient-depleted MM agar plates (B), GlcNAc has the opposite effect. GlcNAc concentrations (left to right, top to bottom) are 0, 0.001, 0.01, 0.1, 1, 5, 10, 20, 50, 100, 150 and 200 mM. The effect of GlcNAc on each antibiotic was assessed using the actII-ORF4 and redD null mutants for monitoring the production of undecylprodigiosin and actinorhodin, respectively. GlcNAc, N-acetylglucosamine; MM, minimal medium.
To assess whether the effect of GlcNAc on antibiotic production is widespread among streptomycetes, several Streptomyces species were plated onto MM with mannitol (25 mM) as the sole carbon source and with or without GlcNAc (50 mM), using Bacillus subtilis as the indicator strain (Fig 2). GlcNAc had a stimulating effect on antibiotic production by the Streptomyces species S. clavuligerus, S. collinus, S. griseus, S. hygroscopicus and S. venezuelae, whereas there was no effect on visible antibiotic production by S. acrimycini, S. avermitilis, S. cinnamonensis, S. limosus and S. rimosus. An inhibitory effect was observed only for S. roseosporus. This suggests that the antibiotic-triggering effect of GlcNAc is common in streptomycetes, although not universal, at least under the conditions we studied.
Figure 2.
Conservation of the GlcNAc-dependent antibiotic-inducing pathway among streptomycetes. Streptomycetes were grown on minimal medium agar plates with 25 mM mannitol alone (left panel) or with the addition of 50 mM GlcNAc (middle panel). Bacillus subtilis was used as an indicator strain. For strains (right panel), see Table 1. GlcNAc triggered antibiotic activity in S. hygroscopicus, S. collinus, S. venezuelae, S. clavuligerus and S. griseus, whereas it inhibited antibiotic production in S. roseosporus. GlcNAc, N-acetylglucosamine.
DasR as a master switch for antibiotic production
In S. coelicolor, GlcNAc is internalized and phosphorylated by the sugar phosphotransferase system (PTS; Nothaft et al, 2003). The GlcNAc regulon is controlled by the GntR regulator DasR (Rigali et al, 2002, 2004), the DNA-binding activity of which is inhibited by glucosamine-6-phosphate (Rigali et al, 2006). Interestingly, the S. coelicolor dasR mutant BAP29 showed enhanced and accelerated production of pigmented antibiotics, suggesting a role for DasR in the control of antibiotic production (supplementary Fig S1 online). The relative increase in antibiotic production in the dasR mutant was quantified by spectrophotometry, and showed that production of Act and Red was consistently enhanced in BAP29 by factors of 3.2 (±0.2) and 3.9 (±0.3), respectively.
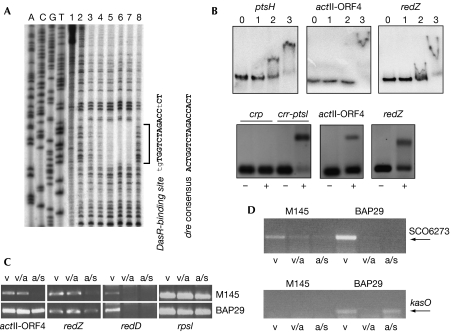
To identify the precise DasR-responsive element (dre), we performed DNase I footprinting on the promoter region of the crr-ptsI operon, encoding the PTS enzyme IIA (IIACrr) and enzyme I (EI; Fig 3A). The protected sequence (TGTGGTCTAGACCTCT) corresponded to positions −130 to −115 relative to the start of crr, and had a 13- out of 16-bp match to the in silico-predicted consensus DasR-binding site (Colson et al, 2007). This information was used to determine the precise dre sites of five known target genes. By using this training set, we built a position weight matrix to scan the genome of S. coelicolor with the PREDetector program for putative new dre sites (supplementary Table S1 online; Rigali et al, 2004; Hiard et al, 2007). Interestingly, PREDetector identified putative dre boxes upstream from actII-ORF4 and redZ, encoding transcriptional activators of the act and red gene clusters, respectively (supplementary Table S2 online). The dre site upstream from actII-ORF4 is located between the −35 and −10 sequences of the promoter, whereas that of redZ is situated about 50 bp upstream from the −35 sequence of the redZ promoter.
Figure 3.
DasR controls antibiotic production in Streptomyces coelicolor. (A) Identification of the DasR-binding site by DNase I footprint analysis of the crr-ptsI upstream region. The crr-ptsI probe was incubated with DNase I (0.4 μg/ml) and increasing amounts of purified DasR (0, 10, 20, 40, 60 or 80 pmol of DasR in lanes 2, 3, 4, 5, 6 and 7, respectively). Controls: lane 1, no DasR or DNase I; lane 8, DNase I and 350 pmol of bovine serum albumin. ACGT, DNA sequence lanes. (B) Electrophoretic mobility gel shift assays showing direct interaction of DasR with dre sites predicted upstream from actII-ORF4 and redZ. Top: binding of DasR to the entire promoter regions of ptsH, actII-ORF4 and redZ; lane 0, probe; lanes 1, 2 and 3, probe and 1, 5 and 20 nM DasR, respectively; bottom: double-stranded oligonucleotide probes encompassing dre sites for crr-ptsI (positive control), actII-ORF4 and redZ were incubated with (+) or without (−) purified His-tagged DasR. The crp promoter region was used as a negative control. (C) Transcription of the pathway-specific activator genes for Act (actII-ORF4) and Red (redD) analysed by semiquantitative RT–PCR. Samples were collected from S. coelicolor M145 and the dasR mutant BAP29 grown on minimal medium mannitol plates after 30 h (vegetative growth (v)), 42 h (initiation of aerial growth (a)) and 72 h (aerial growth and spores (s)). rpsI (for ribosomal protein S9) was used as an RNA integrity control. (D) Transcriptional analysis of the kas ‘cryptic' type I polyketide cluster of S. coelicolor (SCO6273–6288) by semiquantitative RT–PCR. Transcriptional repression of kasO is relieved by deletion of dasR. RNA samples are as in (C). RT–PCR, reverse transcription–PCR.
Electrophoretic mobility gel shift assays (EMSAs) with purified His6-tagged DasR on 32P-radiolabelled DNA probes (in the presence of a 100-fold molar excess of unlabelled ϕX174 DNA) encompassing the promoter regions of actII-ORF4, redZ or ptsH (positive control, encoding the PTS HPr protein) showed direct binding of DasR to all three DNA fragments, with a single large conformational shift for actII-ORF4, redZ and two binding sites for ptsH, which correspond to the two known dre sites (Fig 3B). Use of double-stranded oligonucleotide probes containing only the dre elements with short flanking sequences showed that DasR bound to the dre elements for the actII-ORF4, redZ and crr-ptsI promoter regions, but not to the cis-acting element of Crp (negative control; Fig 3B).
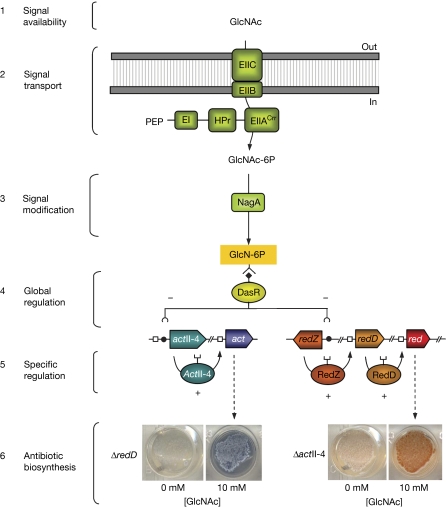
Semiquantitative reverse transcription–PCR (RT–PCR) analyses further showed upregulation of actII-ORF4 and redZ in the dasR mutant (Fig 3C). Enhanced expression of redZ strongly induces transcription of the red pathway-specific activator gene redD (Guthrie et al, 1998), and redD was also upregulated in the mutant. Hence, the activator genes of the act and red clusters are downregulated by DasR, explaining the enhanced production of the respective antibiotics in the dasR mutant. A signalling cascade is thus described from nutrient sensing to antibiotic production (Fig 4).
Figure 4.
Model of GlcNAc-dependent signalling in streptomycetes. GlcNAc enters the cytoplasm and is phosphorylated by the PTS, which is composed of intracellular general PTS proteins EI, HPr and EIIA, and the GlcNAc-specific EIIB and EIIC components. N-acetylglucosamine-6-phosphate (GlcNAc-6P) is deacetylated by NagA. The resulting glucosamine-6-phosphate (GlcN-6P) is an allosteric effecter of DasR that inhibits its DNA binding, resulting in the loss of transcriptional repression of actII-ORF4 and redZ, and thereby activating actinorhodin (Act) and undecylprodigiosin (Red) production, respectively. The stimulatory effect of GlcNAc on antibiotic production is visualized by using the S. coelicolor mutants M511 (Act non-producer) and M510 (Red non-producer), respectively. E, enzyme; GlcNAc, N-acetylglucosamine; NagA, N-acetylglucosamine deacetylase; PEP, phosphoenolpyruvate; PTS, phosphotransferase system.
A major question concerns the origin of the GlcNAc-derived signal. Previously, we reported a marked difference in the effects of either GlcNAc or its polymeric form, chitin, on differentiation, as GlcNAc blocks development and antibiotic production under nutrient-rich conditions, whereas chitin has no effect (Colson et al, 2008). This suggests that the cell can distinguish between chitin-derived GlcNAc (signalling abundance) and GlcNAc derived from cell-wall lysis (signalling starvation). According to our bioinformatic predictions, chitinase genes and the gene for D-Ala-D-Ala aminopeptidase (DppA, SCO6489), responsible for catabolism of the cell-wall precursor D-Ala-D-Ala under nutrient deficiency (Cheggour et al, 2000), are part of the DasR regulon. To explain this, we measured the overall chitinase and DppA activities. Under conditions in which antibiotics are overproduced in the dasR mutant (that is, on MM), there was a fivefold higher activity of DppA, whereas at the same time BAP29 lost substrate induction of the chitinolytic system by chitin (S.R., F.T., S.B., S.M., A.W.T., D.A.H. & G.P.v.W., unpublished data). This suggests coordination between antibiotic production and cell-wall lysis in a DasR-dependent manner (see below).
Awakening cryptic clusters
Understanding the control of cryptic biosynthetic clusters is a major challenge in modern antibiotic research (Van Lanen & Shen, 2006; Wilkinson & Micklefield, 2007). We analysed the role of DasR in the control of a biosynthetic gene cluster for a hypothetical antibiotic made by a type I modular polyketide synthase (SCO6273–6288), the induction of which depends on kasO (SCO6280; Takano et al, 2005). kasO transcripts were identified in RNA from early and late samples of BAP29, but not in wild-type samples (Fig 3D). Most probably as a result of the induction of kasO, transcription of SCO6273, the last open reading frame of the cryptic cluster, was markedly increased during vegetative growth (Fig 3D). No dre consensus sequence was found upstream from kasO, and the manner in which the kas gene cluster is controlled by DasR is now under investigation in our laboratories.
Speculation
The signalling function of GlcNAc should depend on environmental conditions: development is undesirable under nutrient-rich (‘feast') conditions, but should be triggered under ‘famine' conditions. Therefore, the decision depends on the availability of the two principal sources of GlcNAc in nature: chitin and the bacterium's own cell wall. Both chitinases and DppA are part of the DasR regulon. Both antibiotic production and hydrolysis of cell-wall precursors by the D-Ala-D-Ala aminopeptidase seem to be strongly enhanced in the dasR mutant, while this strain has lost its ability to induce the chitinolytic system. Thus, in a situation in which DasR is inactive and antibiotic production is switched on, chitin degradation is repressed while the cell activates catalytic enzymes that break down peptidoglycan building blocks. This supports our hypothesis that the accumulation of GlcNAc during cell-wall hydrolysis (famine) triggers development and antibiotic production, whereas utilization of chitin-derived GlcNAc (feast) should block development. This fine-tuning allows the organism to recruit an omnipresent compound as a signalling molecule, and to decide between growth and commitment to the irreversible final lifecycle step of sporulation.
Note added in proof
Recently, McArthur and Bibb used an elegant technique to scan the actII-ORF4 promoter region for new regulatory elements and to monitor their effects in vivo (McArthur & Bibb, 2008). This highlighted a regulatory element located between the −35 and −10 consensus sequences of the actII-ORF4 promoter that should be a target for a transcriptional repressor. This regulatory element is in fact the dre identified in this work, providing further in vivo substantiation for the direct repressing activity of DasR on actinorhodin production.
Methods
Bacterial strains and culture conditions. The species of Streptomyces used in this work are shown in Table 1. All media and routine Streptomyces techniques have been described previously (Kieser et al, 2000). Phenotypic characterization of mutants was carried out on MM agar plates (Kieser et al, 2000) with carbon sources as indicated. Quantification of Act and Red was performed as described previously (Martinez-Costa et al, 1996).
Table 1.
Streptomyces species used in this work
| Streptomyces species | Genotype | Reference or source |
|---|---|---|
| S. coelicolor M145 | WT | JIC |
| S. coelicolor BAP29 | ΔdasR | Rigali et al (2006) |
| S. coelicolor M510 | ΔactII-ORF4 | Floriano & Bibb (1996) |
| S. coelicolor M511 | ΔredD | Floriano & Bibb (1996) |
| S. coelicolor M512 | ΔactII-ORF4 ΔredD | Floriano & Bibb (1996) |
| S. acrimycini DSM 40540 | WT | DSMZ |
| S. avermitilis NRRL 8165 | WT | ATCC |
| S. cinnamonensis DSM 40467 | WT | DSMZ |
| S. clavuligerus NRRL 358 | WT | DSMZ |
| S. collinus DSM 40733 | WT | DSMZ |
| S. griseus NRRL B2682 | WT | DSMZ |
| S. hygroscopicus ATCC27438 | WT | ATCC |
| S. limosus ATCC 19778 | WT | ATCC |
| S. lividans 1326 | WT | JIC |
| S. rimosus ATCC 10970 | WT | ATCC |
| S. roseosporus ATCC 31568 | WT | ATCC |
| S. venezuelae ATCC15439 | WT | ATCC |
| The parts of the species names shown in bold refer to the abbreviations used in Fig 2. ATCC, American Type Culture Collection; DSMZ, Deutsche Sammlung für Mikroorganismen und Zellkulturen; JIC, John Innes Centre Strain collection; WT, wild type. | ||
DNase I footprinting. A 211 bp PCR-amplified DNA fragment corresponding to the −202–+8 region relative to the start of S. coelicolor crr (SCO1390) was chosen for DNase I footprinting. A 50 fmol portion of 32P-end-labelled probe was incubated with the relevant proteins (DasR-His6 and/or BSA) and DNase I (0.4 μg/ml) as described previously (Sambrook et al, 1989).
Electrophoretic mobility gel shift assay. For EMSAs on the entire promoter regions, we used 1–20 nM of His6-tagged DasR and 1 nM of 32P-end-labelled PCR-amplified DNA probes, whereas EMSAs on double-stranded oligonucleotides were performed using 50 nM of non-radiolabelled probes and 200 nM of His-tagged DasR. All the reactions were carried out in binding buffer (20 mM Tris–HCl pH 7.5, 10 mM MgCl2, 100 mM NaCl and 10% glycerol) containing 100 μg/ml bovine serum albumin and a 100- or 10-fold molar excess of HpaII-digested ϕX174 DNA for EMSAs on PCR fragments and oligonucleotides, respectively. Promoter fragments were the −134–+97 fragment of actII-ORF4, the −280–+78 fragment of redZ and the −249–+103 fragment of ptsH (nucleotide position relative to the translational start sites), and double-stranded oligonucleotide probes encompassing the predicted dre elements were taken from the promoter regions of actII-ORF4 (SCO5085), redZ (SCO5881) and crr (SCO1390); the cis-acting element of crp (SCO3571; not controlled by DasR) was used as a negative control. For oligonucleotides, see the supplementary Table S3 online.
Reverse transcription–PCR. RNA was isolated from the mycelium of S. coelicolor M145 and BAP29 collected after 30 h (vegetative growth), 42 h (initiation of aerial growth) or 72 h (aerial growth and spores) from MM mannitol plates with cellophane discs (grown at 28°C). RT–PCR analyses were conducted using the Superscript III one-step RT–PCR Kit (Invitrogen, Carlsbad, CA, USA). RT–PCRs without reverse transcription were used as a control for the absence of residual DNA. For semiquantitative analysis, samples were taken at four-cycle intervals between cycles 24 and 35 to compare non-saturated PCR product formation (van Wezel et al, 2005). Data were verified in three independent experiments. For oligonucleotides, see supplementary Table S3 online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank K.-P. Yan and M. Merzbacher for excellent technical assistance, and E. Vijgenboom, S. Bialek, B. Joris, J.-M. Frère and S. Colson for discussions and for sharing unpublished information. This work was supported by grants SFB473 and GK805 of the Deutsche Forschungsgemeinschaft to A.W.T. and F.T., and by a grant from the Netherlands Organization for Scientific Research (NWO) to G.P.v.W. We dedicate the work to the memory of Shanna Johansen (1979–2007).
Footnotes
The authors declare that they have no conflict of interest.
References
- Baumann S, Krueger A, Kirchhoff S, Krammer PH (2002) Regulation of T cell apoptosis during the immune response. Curr Mol Med 2: 257–272 [DOI] [PubMed] [Google Scholar]
- Bentley SD et al. (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417: 141–147 [DOI] [PubMed] [Google Scholar]
- Bibb MJ (2005) Regulation of secondary metabolism in streptomycetes. Curr Opin Microbiol 8: 208–215 [DOI] [PubMed] [Google Scholar]
- Challis GL, Hopwood DA (2003) Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proc Natl Acad Sci USA 100(Suppl 2): 14555–14561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater KF, Losick R (1997) Mycelial life style of Streptomyces coelicolor A3(2) and its relatives. In Bacteria as Multicellular Organisms, Shapiro JA, Dworkin M (eds), pp 149–182. New York, NY, USA: Oxford University Press [Google Scholar]
- Cheggour A, Fanuel L, Duez C, Joris B, Bouillenne F, Devreese B, Van Driessche G, Van Beeumen J, Frere JM, Goffin C (2000) The dppA gene of Bacillus subtilis encodes a new D-aminopeptidase. Mol Microbiol 38: 504–513 [DOI] [PubMed] [Google Scholar]
- Colson S, Stephan J, Hertrich T, Saito A, van Wezel GP, Titgemeyer F, Rigali S (2007) Conserved cis-acting elements upstream of genes composing the chitinolytic system of streptomycetes are DasR-responsive elements. J Mol Microbiol Biotechnol 12: 60–66 [DOI] [PubMed] [Google Scholar]
- Colson S, van Wezel GP, Craig M, Noens EE, Nothaft H, Mommaas AM, Titgemeyer F, Joris B, Rigali S (2008) The chitobiose-binding protein, DasA, acts as a link between chitin utilization and morphogenesis in Streptomyces coelicolor. Microbiology 154: 373–382 [DOI] [PubMed] [Google Scholar]
- Dworkin J, Losick R (2005) Developmental commitment in a bacterium. Cell 121: 401–409 [DOI] [PubMed] [Google Scholar]
- Fernandez M, Sanchez J (2002) Nuclease activities and cell death processes associated with the development of surface cultures of Streptomyces antibioticus ETH 7451. Microbiology 148: 405–412 [DOI] [PubMed] [Google Scholar]
- Floriano B, Bibb M (1996) afsR is a pleiotropic but conditionally required regulatory gene for antibiotic production in Streptomyces coelicolor A3(2). Mol Microbiol 21: 385–396 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Pastor JE, Hobbs EC, Losick R (2003) Cannibalism by sporulating bacteria. Science 301: 510–513 [DOI] [PubMed] [Google Scholar]
- Guthrie EP, Flaxman CS, White J, Hodgson DA, Bibb MJ, Chater KF (1998) A response-regulator-like activator of antibiotic synthesis from Streptomyces coelicolor A3(2) with an amino-terminal domain that lacks a phosphorylation pocket. Microbiology 144: 727–738 [DOI] [PubMed] [Google Scholar]
- Hiard S, Maree R, Colson S, Hoskisson PA, Titgemeyer F, van Wezel GP, Joris B, Wehenkel L, Sebastien R (2007) PREDetector: a new tool to identify regulatory elements in bacterial genomes. Biochem Biophys Res Commun 357: 861–864 [DOI] [PubMed] [Google Scholar]
- Hopwood DA (1999) Forty years of genetics with Streptomyces: from in vivo through in vitro to in silico. Microbiology 145: 2183–2202 [DOI] [PubMed] [Google Scholar]
- Ikeda H, Ishikawa J, Hanamoto A, Shinose M, Kikuchi H, Shiba T, Sakaki Y, Hattori M, Omura S (2003) Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat Biotechnol 21: 526–531 [DOI] [PubMed] [Google Scholar]
- Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical Streptomyces Genetics. Norwich, UK: John Innes Foundation [Google Scholar]
- Manteca A, Mader U, Connolly BA, Sanchez J (2006) A proteomic analysis of Streptomyces coelicolor programmed cell death. Proteomics 6: 6008–6022 [DOI] [PubMed] [Google Scholar]
- Martinez-Costa OH, Arias P, Romero NM, Parro V, Mellado RP, Malpartida F (1996) A relA/spoT homologous gene from Streptomyces coelicolor A3(2) controls antibiotic biosynthetic genes. J Biol Chem 271: 10627–10634 [DOI] [PubMed] [Google Scholar]
- McArthur M, Bibb MJ (2008) Manipulating and understanding antibiotic production in Streptomyces coelicolor A3(2) with decoy oligonucleotides. Proc Natl Acad Sci USA 105: 1020–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothaft H, Dresel D, Willimek A, Mahr K, Niederweis M, Titgemeyer F (2003) The phosphotransferase system of Streptomyces coelicolor is biased for N-acetylglucosamine metabolism. J Bacteriol 185: 7019–7023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice KC, Bayles KW (2003) Death's toolbox: examining the molecular components of bacterial programmed cell death. Mol Microbiol 50: 729–738 [DOI] [PubMed] [Google Scholar]
- Rigali S, Derouaux A, Giannotta F, Dusart J (2002) Subdivision of the helix–turn–helix GntR family of bacterial regulators in the FadR, HutC, MocR, and YtrA subfamilies. J Biol Chem 277: 12507–12515 [DOI] [PubMed] [Google Scholar]
- Rigali S, Schlicht M, Hoskisson P, Nothaft H, Merzbacher M, Joris B, Titgemeyer F (2004) Extending the classification of bacterial transcription factors beyond the helix–turn–helix motif as an alternative approach to discover new cis/trans relationships. Nucleic Acids Res 32: 3418–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigali S et al. (2006) The sugar phosphotransferase system of Streptomyces coelicolor is regulated by the GntR-family regulator DasR and links N-acetylglucosamine metabolism to the control of development. Mol Microbiol 61: 1237–1251 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press [Google Scholar]
- Takano E, Kinoshita H, Mersinias V, Bucca G, Hotchkiss G, Nihira T, Smith CP, Bibb M, Wohlleben W, Chater K (2005) A bacterial hormone (the SCB1) directly controls the expression of a pathway-specific regulatory gene in the cryptic type I polyketide biosynthetic gene cluster of Streptomyces coelicolor. Mol Microbiol 56: 465–479 [DOI] [PubMed] [Google Scholar]
- Van Lanen SG, Shen B (2006) Microbial genomics for the improvement of natural product discovery. Curr Opin Microbiol 9: 252–260 [DOI] [PubMed] [Google Scholar]
- van Loo G, Saelens X, van Gurp M, MacFarlane M, Martin SJ, Vandenabeele P (2002) The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ 9: 1031–1042 [DOI] [PubMed] [Google Scholar]
- van Wezel GP, Mahr K, König M, Traag BA, Pimentel-Schmitt EF, Willimek A, Titgemeyer F (2005) GlcP constitutes the major glucose uptake system of Streptomyces coelicolor A3(2). Mol Microbiol 55: 624–636 [DOI] [PubMed] [Google Scholar]
- van Wezel GP, Krabben P, Traag BA, Keijser BJ, Kerste R, Vijgenboom E, Heijnen JJ, Kraal B (2006) Unlocking Streptomyces spp. for use as sustainable industrial production platforms by morphological engineering. Appl Environ Microbiol 72: 5283–5288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B, Micklefield J (2007) Mining and engineering natural-product biosynthetic pathways. Nat Chem Biol 3: 379–386 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information