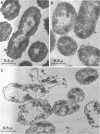
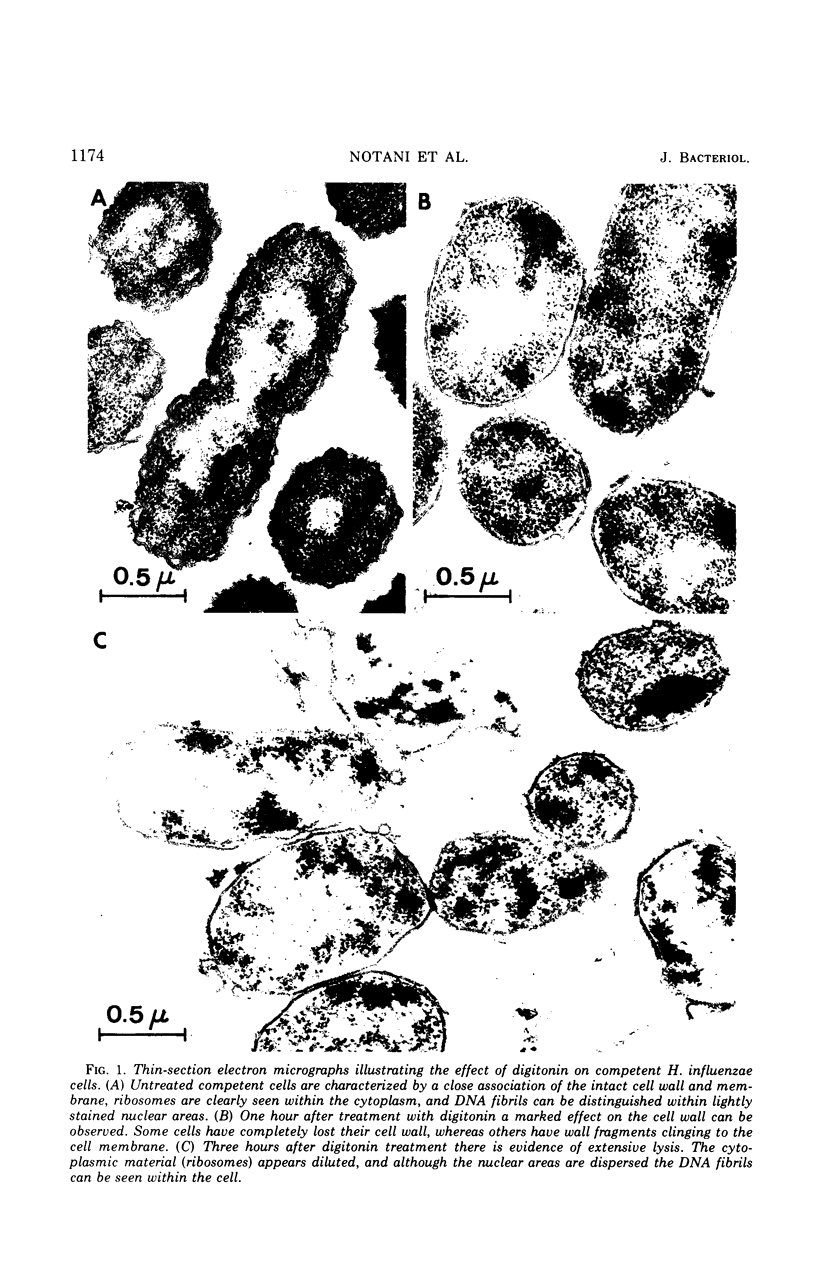
Abstract
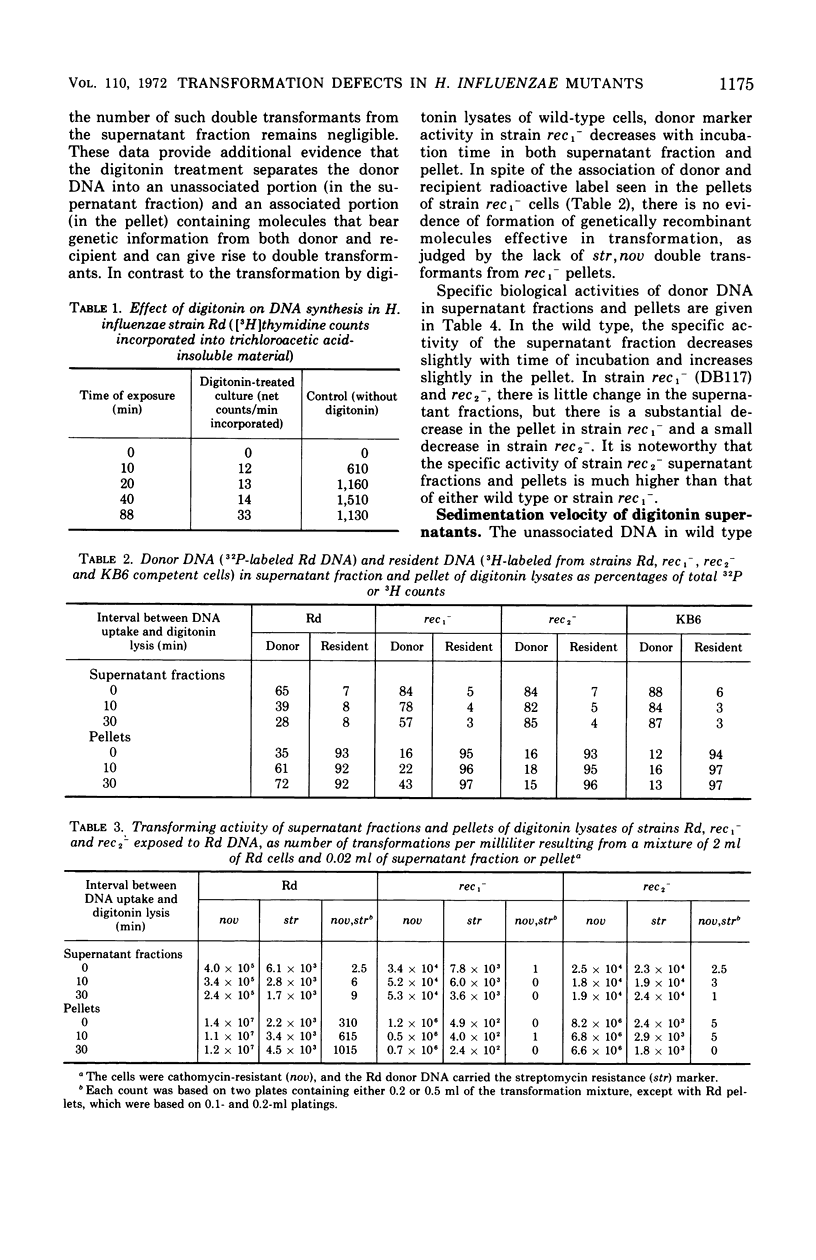
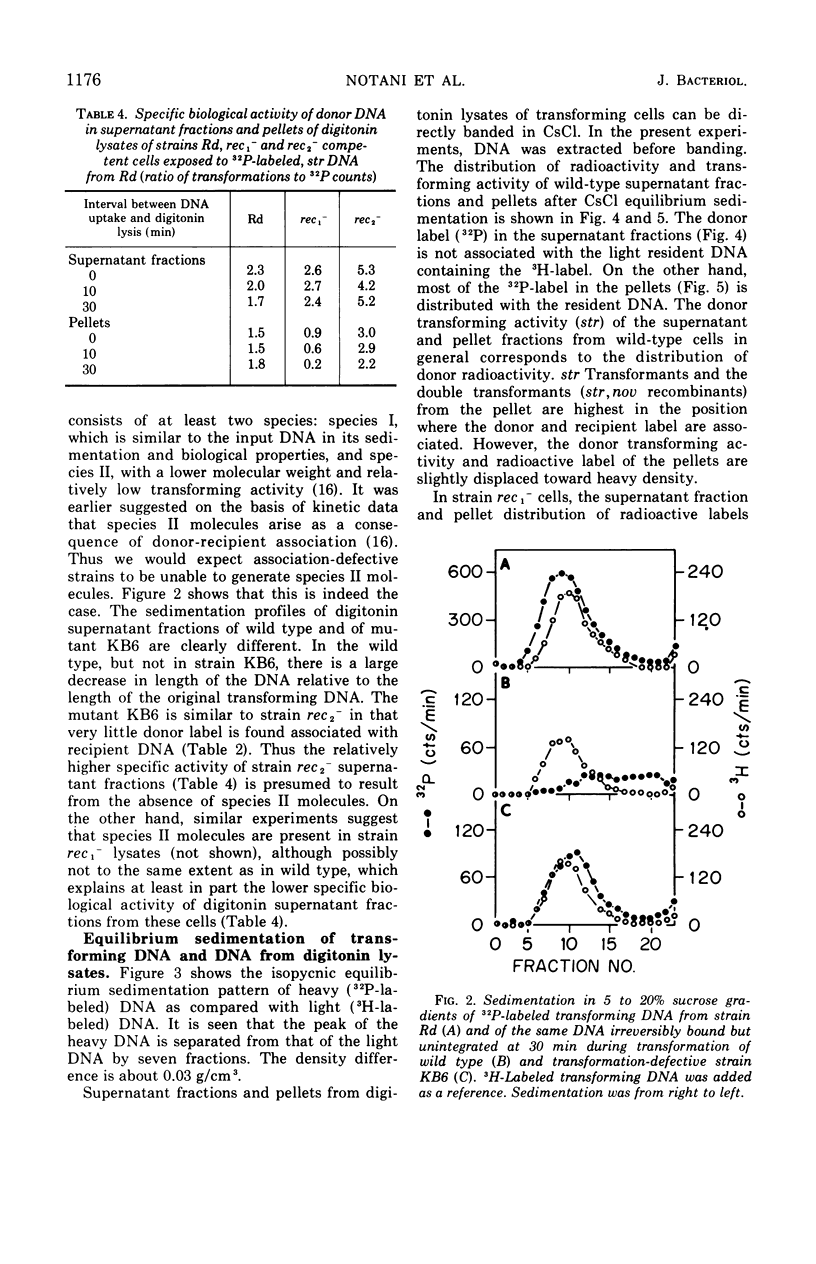
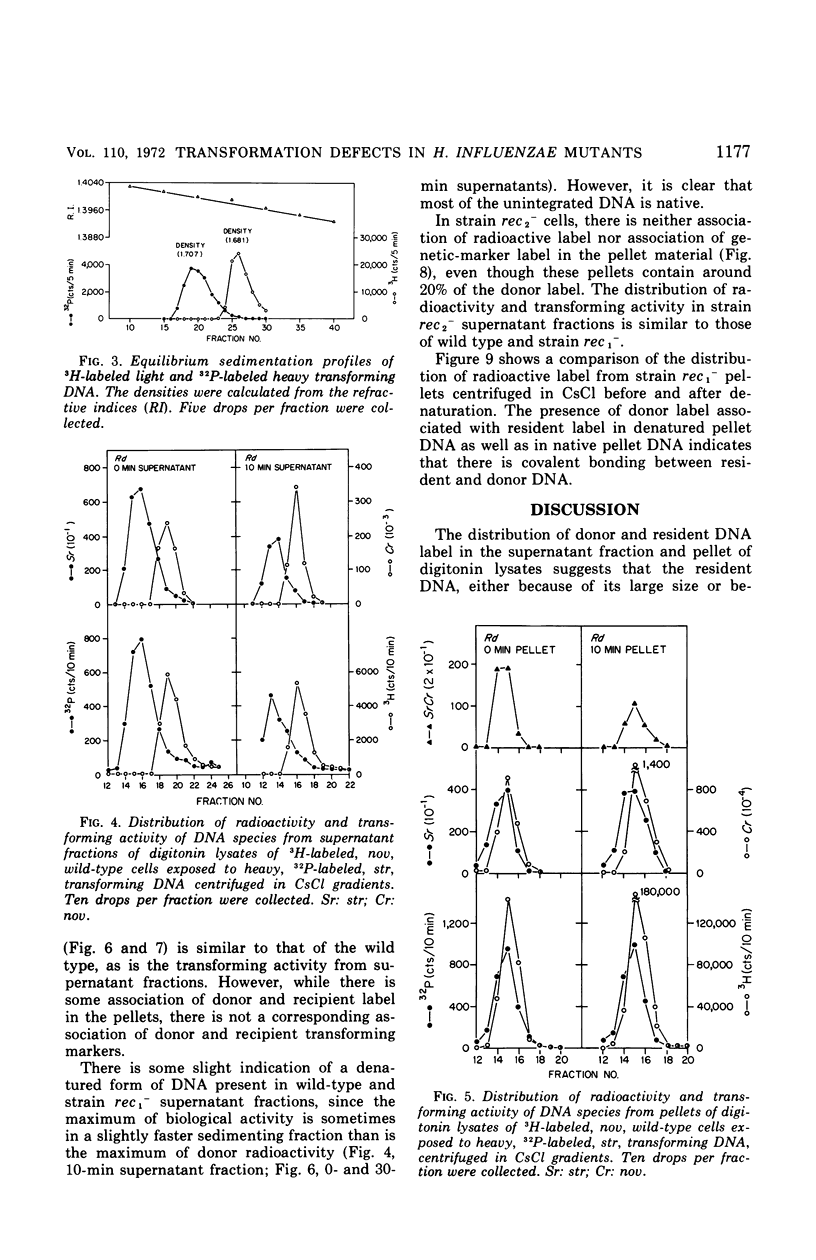
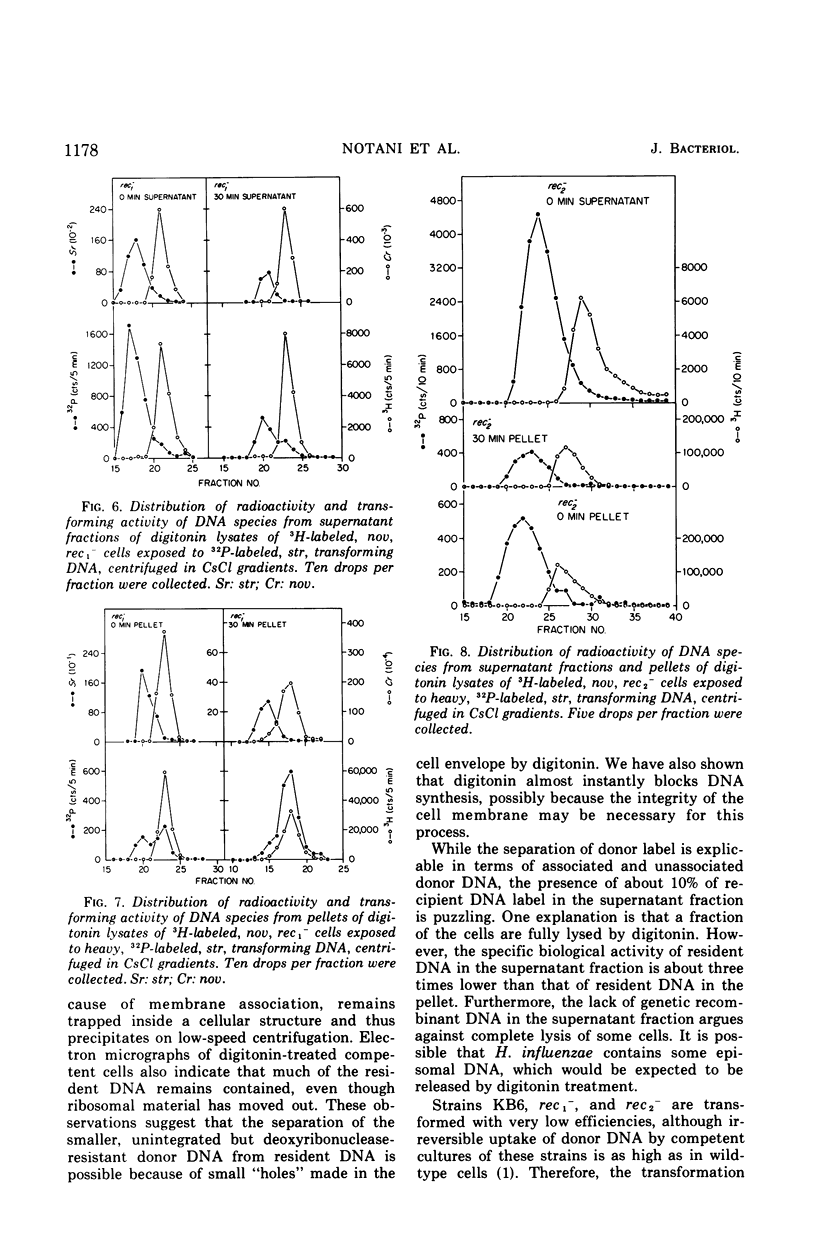
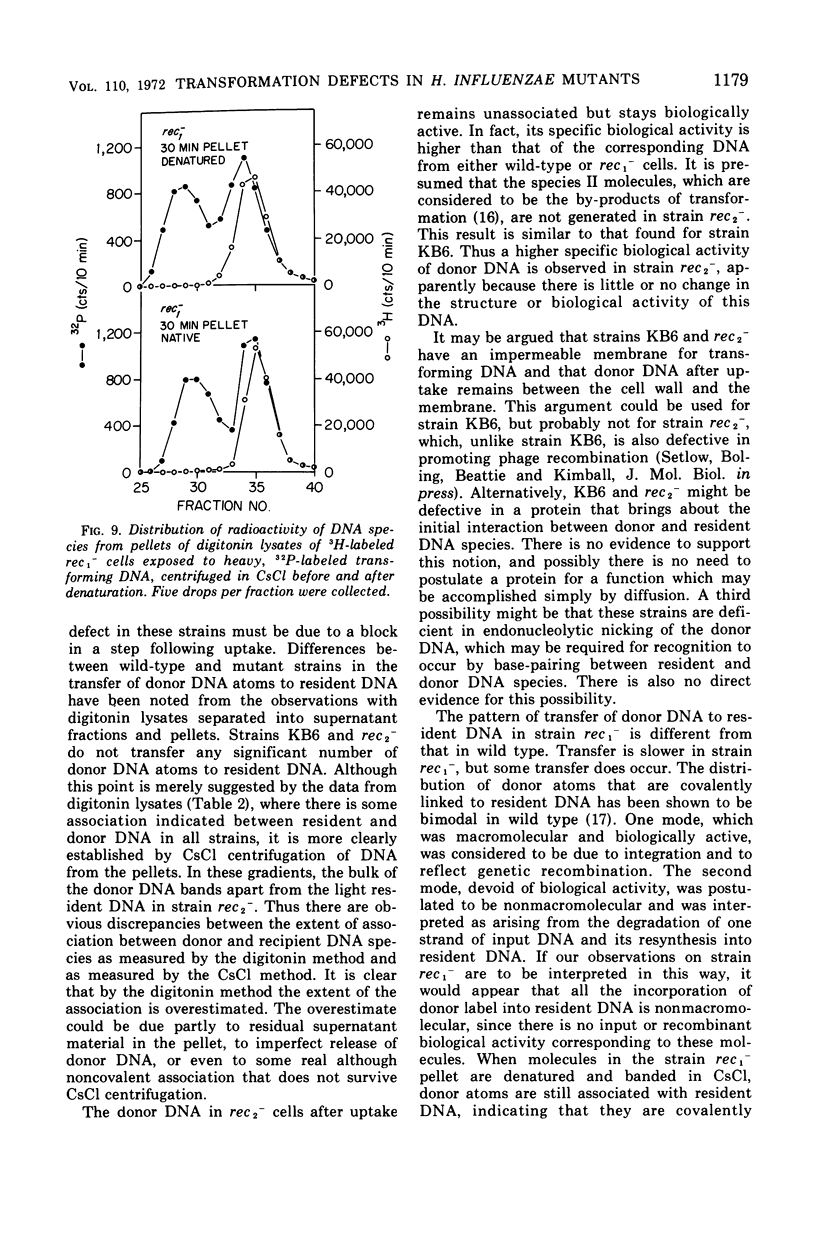
To determine the molecular basis of transformation defects in Haemophilus influenzae, the fate of genetically marked, 32P-labeled, heavy deoxyribonucleic acid (DNA) was examined in three mutant strains (rec1−, rec2−, and KB6) and in wild type having 3H-labeled DNA and a second genetic marker. Transforming cells upon lysis with digitonin followed by low-speed centrifugation are separable into the supernatant fraction, containing mainly the unintegrated donor DNA, and the pellet, containing most of the resident DNA along with integrated donor DNA. Electron micrographs of digitonin-treated cells also indicate that the resident DNA is trapped inside a cellular structure but that cytoplasmic elements such as ribosomes are extensively released. DNA synthesis in digitonin-treated cells is immediately blocked, as is any further integration of donor DNA into the resident genome. Isopycnic and sedimentation analysis of supernatant fluids and pellets revealed that in strains rec2− and KB6 there is little or no association between donor and resident DNA, and thus there is negligible transfer of donor DNA genetic information. In these strains, the donor DNA is not broken into pieces of lower molecular weight as it is in strain rec1− and in the wild type, both of which show association between donor and recipient DNA. In strain rec1−, although some donor DNA atoms become covalently linked to resident DNA, the incorporated material does not have the donor DNA transforming activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BODMER W. F., GANESAN A. T. BIOCHEMICAL AND GENETIC STUDIES OF INTEGRATION AND RECOMBINATION IN BACILLUS SUBTILIS TRANSFORMATION. Genetics. 1964 Oct;50:717–738. doi: 10.1093/genetics/50.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie K. L., Setlow J. K. Transformation-defective strains of Haemophilus influenzae. Nat New Biol. 1971 Jun 9;231(23):177–179. doi: 10.1038/newbio231177a0. [DOI] [PubMed] [Google Scholar]
- Broker T. R., Lehman I. R. Branched DNA molecules: intermediates in T4 recombination. J Mol Biol. 1971 Aug 28;60(1):131–149. doi: 10.1016/0022-2836(71)90453-0. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Leick V. Rapid equilibrium isopycnic CsC1 gradients. Biochim Biophys Acta. 1969 Mar 18;179(1):136–144. doi: 10.1016/0005-2787(69)90129-4. [DOI] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Paper strip method for assaying gradient fractions containing radioactive macromolecules. Anal Biochem. 1971 Oct;43(2):427–432. doi: 10.1016/0003-2697(71)90272-7. [DOI] [PubMed] [Google Scholar]
- FOX M. S., ALLEN M. K. ON THE MECHANISM OF DEOXYRIBONUCLEATE INTEGRATION IN PNEUMOCOCCAL TRANSFORMATION. Proc Natl Acad Sci U S A. 1964 Aug;52:412–419. doi: 10.1073/pnas.52.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodgal S. H., Postel E. H. On the mechanism of integration following transformation with single-stranded DNA of Hemophilus influenzae. J Mol Biol. 1967 Sep 14;28(2):261–273. doi: 10.1016/s0022-2836(67)80008-1. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modak S. P., Setlow J. K. Synthesis of deoxyribonucleic acid after ultraviolet irradiation of sensitive and resistant Haemophilus influenzae. J Bacteriol. 1969 Jun;98(3):1195–1198. doi: 10.1128/jb.98.3.1195-1198.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammed A., Setlow J. K. Ultraviolet-induced decrease in integration of Haemophilus influenzae transforming deoxyribonucleic acid in sensitive and resistant cells. J Bacteriol. 1970 Feb;101(2):444–448. doi: 10.1128/jb.101.2.444-448.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N. K. Genetic and physical properties of unintegrated donor DNA molecules during Hemophilus transformation. J Mol Biol. 1971 Jul 14;59(1):223–226. doi: 10.1016/0022-2836(71)90426-8. [DOI] [PubMed] [Google Scholar]
- Notani N., Goodgal S. H. On the nature of recombinants formed during transformation in Hemophilus influenzae. J Gen Physiol. 1966 Jul;49(6):197–209. doi: 10.1085/jgp.49.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechowska M., Fox M. S. Fate of transforming deoxyribonucleate in Bacillus subtilis. J Bacteriol. 1971 Nov;108(2):680–689. doi: 10.1128/jb.108.2.680-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart W. L., Herriott R. M. Fate of recipient deoxyribonucleic acid during transformation in Haemophilus influenzae. J Bacteriol. 1968 Nov;96(5):1718–1724. doi: 10.1128/jb.96.5.1718-1724.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]