Summary
ESCRT-II plays a pivotal role in receptor downregulation and multivesicular body biogenesis, and is conserved from yeast to humans. The crystal structures of two human ESCRT-II complex structures have been determined at 2.6 and 2.9 Å resolution, respectively. The complex has three lobes and contains one copy each of VPS22 and VPS36, and two copies of VPS25. The structure reveals a dynamic helical domain to which both the VPS22 and VPS36 subunits contribute, which connects the GLUE domain to the rest of the ESCRT-II core. Hydrodynamic analysis shows that intact ESCRT-II has a compact, closed conformation. ESCRT-II binds to the ESCRT-I VPS28 C-terminal domain subunit through a helix immediately C-terminal to the VPS36-GLUE domain. ESCRT-II is targeted to endosomal membranes by the lipid binding activities of both the Vps36 GLUE domain and the first helix of Vps22. These data provide a unifying structural and functional framework for the ESCRT-II complex.
Introduction
The ESCRT (Endosomal Sorting Complex Required for Transport) machinery directs the budding of intralumenal vesicles from the limiting membrane of endosomes to form multivesicular bodies (MVBs) in yeast, insect, and animal cells (Babst, 2005; Hurley, 2008; Saksena et al., 2007; Williams and Urbe, 2007); the budding of HIV-1 and other enveloped viruses from the plasma membrane of animal cells (Bieniasz, 2006); and the membrane abscission step in cytokinesis (Carlton and Martin-Serrano, 2007; Morita et al., 2007). ESCRT-I and ESCRT-II are soluble and constitutively assembled complexes, which are targeted to the endosomal membrane by interactions with membrane-bound phosphoinositides and ubiquitinated membrane proteins (Babst, 2005; Hurley, 2008; Saksena et al., 2007; Williams and Urbe, 2007). ESCRT-III assembles into an insoluble array on the endosomal membrane and is thought to play a key role in membrane deformation (Babst, 2005; Hurley, 2008; Saksena et al., 2007; Williams and Urbe, 2007). ESCRT-III monomers are thought to be targeted to endosomes in part by interactions with ESCRT-II (Bowers et al., 2006; Langelier et al., 2006; Martin-Serrano et al., 2003; Teo et al., 2004; von Schwedler et al., 2003; Yorikawa et al., 2005), among other factors. Thus ESCRT-II connects the upstream cargo binding components of the system with the downstream membrane remodeling machinery.
In yeast, ESCRT-II is required for the lysosomal degradation of the mating factor receptor Ste2 (Babst et al., 2002), among other cargo. In Drosophila, the ESCRT-II subunit VPS25 acts as a tumor suppressor by promoting the degradation of the Notch and DPP receptors (Herz et al., 2006; Thompson et al., 2005; Vaccari and Bilder, 2005). In human cells, ESCRT-II is required for the degradation of internalized EGF receptors (Langelier et al., 2006; Malerod et al., 2007), the chemokine receptor CXCR4 (Malerod et al., 2007), and ferroportin (De Domenico et al., 2007). ESCRT-II has at least one clear-cut non-endosomal role, in establishing the bicoid mRNA gradient (Irion and St Johnston, 2007). In another potential non-endosomal function, the subunits of human ESCRT-II associate with the ELL transcriptional elongation complex (Kamura et al., 2001), although the implications of this are largely unexplored. Furthermore, ESCRT-II is responsible for connecting MVBs to the Rab7 effector RILP (Progida et al., 2006; Wang and Hong, 2006), which in turn binds to the dynein-dynactin motor complex and may coordinate MVB biogenesis to dynein-mediated motility (Progida et al., 2007).
ESCRT-II is targeted to endosomal membranes, binds ubiquitinated cargo, and assembles with other ESCRT complexes through interdependent interactions. The N-terminal region of Vps36 contains a phosphoinositide-binding GLUE domain (Slagsvold et al., 2005; Teo et al., 2006) important for membrane targeting. The yeast Vps36 GLUE domain contains a large sequence insert comprising two Npl4-type zinc finger domains, NZF1 and NZF2. The structure of the former has been determined in complex with the C-terminal domain of yeast Vps28 (Gill et al., 2007), a subunit of ESCRT-I. However, human VPS36 lacks this domain, and the nature of its interaction with ESCRT-I is unknown. The complex of the yeast VPS36 NZF2 with its ligand, ubiquitin, has been modeled on the basis of an NMR structure of the Npl4 zinc finger bound to ubiquitin (Alam et al., 2004). Human VPS36 also binds to ubiquitin, but it does so via a non-conserved site on the GLUE domain (Alam et al., 2006; Hirano et al., 2006; Slagsvold et al., 2005).
The core of the yeast ESCRT-II complex is shaped like the letter “Y” and contains two subunits of the Vps25 subunit, and one copy each of the Vps22 and Vps36 subunits (Hierro et al., 2004; Teo et al., 2004). The N-termini of the Vps22 and Vps36 subunits project from the ordered core of the complex. The N-terminus of Vps22 contains a long helical region of unknown function. A ~100 residue predicted helical region of unknown structure and function connects the Vps36 GLUE domain to the core. Structural information has been rapidly accumulating for most of the ESCRT components, allowing, for example, the reconstruction of a hydrodynamic model of intact ESCRT-I from the crystal structures of the constituent fragments (Kostelansky et al., 2007). The main obstacle to a similar model for the intact ESCRT-II has been the lack of information on the ~100 residue helical connector in Vps36 and the N-terminal helical region of Vps22. Here we report the structures of two human ESCRT-II constructs that include these regions. These two regions fold into a compact helical bundle, except for the N-terminal ~25 amino acids of Vps22, which we find has a role in membrane targeting. On the basis of this and previous structures (Alam et al., 2006; Hirano et al., 2006) and hydrodynamic measurements, we develop a unified model for the structure of intact ESCRT-II. We extend the observation on the N-terminus of Vps22 to develop a model of dual membrane targeting by this region and the GLUE domain.
RESULTS
Crystallographic analysis of human ESCRT-II
The 2.6 Å crystal structure of the human ESCRT-II complex lacking the GLUE domain was solved by multiple isomorphous replacement (Form I; Table 1; Fig. 1A–D). The complex contains two molecules of VPS25, one molecule of VPS22, and one copy of VPS36. It has a trilobal shape similar to that of yeast ESCRT-II (Hierro et al., 2004; Teo et al., 2004) (Supplementary Fig. 1). Two of the lobes consist of VPS25 subunits, and the third consists of VPS36 and VPS22 (Fig. 1B,C). The VPS22 and VPS36 subunits form a roughly parallel side-to-side arrangement. There is an extensive interface between VPS22 and VPS36, with a buried surface area of 2225 Å2. The first four helices of VPS22 and the first three helices VPS36 in this structure from a novel helical domain (HD, Fig. 2A). This conserved helical domain was absent in the structure of yeast ESCRT-II because the sequences corresponding to the first three helices of Vps36 were absent from the crystallized portion of yeast ESCRT-II (Fig. 1A) (Hierro et al., 2004; Teo et al., 2004). The HD is one of the most mobile regions of the human ESCRT-II crystal structure. Indeed, the HD helices of VPS22 and VPS36 subunits have sausage-like electron density. The presence of a few protruding bulky side chains allowed for sequence assignment. The linker connecting helices H1 and H2 (residues 202–211) in VPS36 subunit was not visible in the structure. The overall B-value of the ESCRT-II core complex is 78.7 Å2. However, the average B-value of the HD is 124.7, compared to an average B value of 61.2 Å2 for the remainder of the structure (Fig. 1E, left hand panel). This suggested to us that the HD was either loosely packed in the crystal lattice, or that is had intrinsically high mobility.
Table 1.
Statistics of data collection, MIR phasing, and crystallographic refinement
| Crystal | Native Form I | TMLA derivative | K2PtCl6 derivative | KAuCl4 derivative | Native Form II |
|---|---|---|---|---|---|
| Constructs | VPS36 (170–386) | VPS36 (149–386) | |||
| VPS22 (26–258) | VPS22 (1–258) | ||||
| VPS25 (1–176) | VPS25 (1–102) | ||||
| Space group, unit cell | P21, a = 70.2, b = 89.2, c = 91.4, β = 101.5 | P3212, a, b = 81.5, c = 226.2 | |||
| Heavy atom soaking condition | 10 mM, 1day | 1 mM, 2hr | 2 mM, 1days | ||
| X-ray source | SER-CAT 22ID | CuKα | CuKα | CuKα | SER-CAT 22-ID |
| Wavelength (Å) | 1.0000 | 1.5418 | 1.5418 | 1.5418 | 1.0000 |
| Resolution (Å) (last shell) | 2.6 (2.69 – 2.60) | 3.6 (3.73 – 3.60) | 3.1 (3.21 – 3.10) | 3.2 (3.31 – 3.20) | 2.9 (3.0 – 2.9) |
| No. of unique reflections | 30680 | 11712 | 19725 | 17604 | 19408 |
| I/σ (last shell) | 33.0 (3.1) | 16.1 (5.0) | 28.8 (4.5) | 23.8 (4.2) | 41.3 (3.3) |
| Rsym a(%) | 4.9 (30.2) | 10.6 (33.1) | 7.1 (41.3) | 8.4 (43.3) | 4.7 (42.9) |
| Data completeness (%) | 91.4 (61.1) | 94.7 (96.5) | 99.9 (100.0) | 99.8 (99.5) | 99.6 (99.5) |
| Phasing and refinement | |||||
| Mean FOM (50 – 3.6 Å) | 0.54 (SOLVE) | MR | |||
| Overall FOM (50 –3.6 Å) | 0.75 (RESOLVE) | ||||
| R factor b (%) | 23.8 | 23.2 | |||
| Free R factor c (%) | 29.4 | 31.7 | |||
| R.m.s. bond length (Å) | 0.013 | 0.010 | |||
| R.m.s. bond angle (°) | 1.463 | 1.275 | |||
| Average B value (Å2) d | 78.7 | 89.8 | |||
| HD (V22 34-76, V36 172-225) 124.7 | HD 101.6 | ||||
| core (V22 77–252, V36 226–385) 61.2 | core 79.1 | ||||
| VPS25 84.7 | VPS25 100.8 |
The values in parentheses relate to highest resolution shells.
Rsym = Σh Σi |Ii(h) -<I>|/ Σh Σi Ii (h), where I is the observed intensity and <I> is the average intensity of multiple observations of symmetry-related reflections.
R = Σ ‖Fo|-k|Fc‖/ Σ |Fo|, where Fo and Fc are observed and calculated structure factor amplitudes, respectively.
Rfree is calculated for a randomly chosen 5% of reflections; the R factor is calculated for the remaining 95% of reflections used for structure refinement.
Average B value of all atoms in an asymmetric unit.
Figure 1. Structure of human ESCRT-II complex.

(A) Schematic of ordered regions visualized in human (this study) and yeast ESCRT-II (Hierro et al., 2004; Teo et al., 2004) complexes, with a comparison to the domain structures of intact human and yeast ESCRT-II complexes. (B) Overall structure of the complex. The WH2 domain of one of the VPS25 subunits was poorly visible in electron density map and it was not included in the structure refinement. The missing VPS25-WH2 was modeled in the figure using the structure of other subunit. (C) A top view of the complex showing a relatively flat “profile” of the complex. (D) Electron density from solvent-flattened MIR map contoured at 1.0 σ in the vicinity of VPS22–VPS36 portion of the core. The final refined structure was shown in a tube model. (E) Crystal structures in forms I and II are shown colored by B-factor to show regions of high (red) and low (blue) mobility.
Figure 2. The helical domain of ESCRT-II.

(A) N-terminal helical domain (HD) of VPS22 and VPS36. (B) Comparison of human and yeast ESCRT-II in the HD region. The newly observed N-terminal helical domain formed by human VPS22 and VPS36 is shown at left, as compared to the isolated Vps22 fragment of this domain seen in the yeast structure at right.
VPS22, VPS25 and VPS36 each contain two repeats of a winged helix (WH) domain with an H1/β1/H2/H3/β2/β3 topology. The N-terminal WH domain (WH1) packs against the C-terminal WH domain (WH2) in head to tail manner. All of the WH domains in the ESCRT-II complex superimpose on each other with an r.m.s.d of less than 2 Å, although there is no recognizable sequence similarity between the subunits.
The N-terminal domain of one VPS25 subunit contacts the C-terminal domain of VPS22, and the other VPS25 subunit contacts both VPS36 and VPS22. The VPS25 subunit buries 762 Å2 (63 Å2 for VPS22) of surface area upon binding to VPS36 and the other VPS25 subunit buries 1149 Å2 of surface area upon binding to VPS22 and VPS36. The WH2 domain of the VPS36-proximal VPS25 subunit, which packs in the crystal lattice along the c-axis, appears to be completely disordered and could not be visualized in electron density map. This suggested to us that VPS25 has intrinsic flexibility between its two WH domains.
The N-terminal 25 residues of VPS22 contain 8 basic charged residues and are predicted to form an α-helix (Supplementary Fig. 2). We refer to this predicted helix as VPS22-H0. The linker between GLUE domain and the C-terminal core of VPS36 (residues 140 – 169) also contains a predicted α- helix, which we refer to as VPS36- H0 (Fig. 2D). In an effort to reveal the structure of these N-terminal regions of VPS22 and VPS36, and to obtain more detailed information on the HD, we determined the 2.9 Å crystal structure (Form II, Table 1) of a second construct (construct a, Fig. 3C). The flexible WH2 domain of VPS25 was deleted in the second construct in an attempt to generate more ordered crystals. The structures of forms I and II are almost identical with an r.m.s.d. of 1.25 Å2. Form II manifested better electron density in the HD. It was possible to visualize the linker between H1 and H2 of VPS36. The improved density also allowed us to confirm the assignment of amino acids in the HD. The VPS22- H0, VPS36-H0, and adjoining residues were disordered. A solvent channel takes the space which is presumably occupied by the disordered N-terminal region in the crystal. The average B-value of HD is 101.6 Å2, which is higher than the value of 79.1 Å2 for the remainder of the structure, although lower than the HD in form I (Fig. 1E). The finding that the HD region has such high B-factors in two different crystal forms leads us to conclude that this domain is inherently dynamic.
Figure 3. Solution structure and ESCRT-I interactions of ESCRT-II.
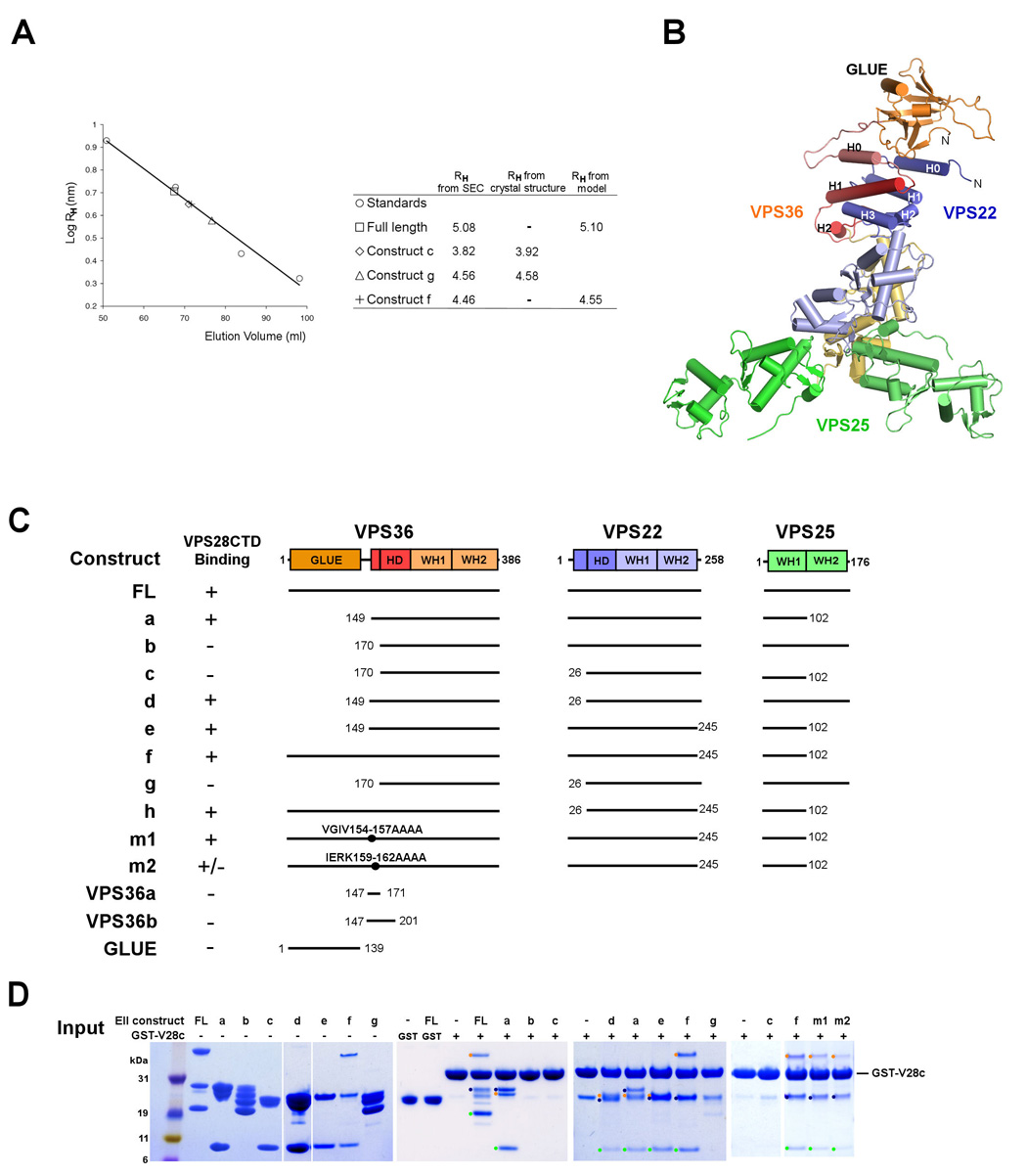
(A) Analysis of recombinant ESCRT-II complex on a Superdex 200 (16/60) column monitored by absorption at 280 nm. Comparison of the intact ESCRT-I Stokes radius RH derived from size exclusion data. The calculated RH values from crystal structures of the ESCRT-II constructs correspond precisely to the value expected from fitting to gel filtration standards. The standards are shown in open circles (BioRad, Hercules, CA) consist of bovine thyroglobulin (670 kDa, RH = 8.5 nm), bovine γ-globulin (158 kDa, RH = 5.3 nm), chicken ovalbumin (44 kDa, RH = 2.7 nm), and horse myoglobin (17 kDa, RH = 2.1 nm). (B) Solution conformation of intact ESCRT-II derived by fitting structural coordinates to hydrodynamic data from the four constructs shown in (A) using Hydropro (Garcia de la Torre et al., 2000). (C) Constructs used in this study. (D) GST-pull down experiment showing a direct interaction between VPS28-CTD and the various ESCRT-II constructs. The ESCRT-II constructs used for the assay are shown in lanes 2 – 8 for reference. The absence of binding of full length ESCRT-II to GST bound beads is shown in Lanes 9 and 10 as a control. Bands corresponding to subunits of complexes that are positive for VPS28-CTD binding are highlighted with dots colored as in panel (B).
Solution conformation of the complete ESCRT-II complex
To investigate the conformation of the full length ESCRT-II complex in solution, we measured the Stokes radii RH of various ESCRT-II constructs. The Stokes radii of the constructs were obtained using size exclusion chromatography (SEC) calibrated with standards of known RH (Fig. 3A). To gain insight on the overall shape of full length ESCRT-II, we positioned the GLUE domain structure (Alam et al., 2006; Hirano et al., 2006) based on the RH values from SEC experiments (Fig. 3A,B). The position of the GLUE domain was adjusted until the calculated RH agreed with the experimental values to within 0.1 nm. Conformations involving an extended linker between the crystallized core and the GLUE domain can be ruled out because these predict RH values substantially higher than are actually observed (Supplementary Fig. 3). On the other hand, conformations in which the GLUE domain is packed against the WH domains lead to predicted RH values that are lower than the experimental value. Based on this exercise, we concluded that the GLUE domain is probably packed against the HD domain of the crystallized ESCRT-II core, and that human ESCRT-II is in a compact, closed conformation in solution (Fig. 3B).
Interactions between human ESCRT-I and ESCRT–II
The yeast ESCRT-I interacts with ESCRT-II via the C-terminal domain (CTD) of Vps28 (Kostelansky et al., 2006; Teo et al., 2006) and the NZF1 domain of Vps36 (Gill et al., 2007; Teo et al., 2006). However, human ESCRT-II contains no NZF domains. Evidence for binding between human ESCRT-I and II comes from yeast two-hybrid analyses of pairwise interactions between subunits of ESCRT-I and II (Langelier et al., 2006). However, isolated ESCRT subunits expressed for two-hybrid studies contain extensive unpartnered hydrophobic regions. Under physiological conditions these would be buried within the larger complex, leading to a potentially elevated frequency of false positives. Given the presence of significant conserved sequences on the surface of the Vps28 CTD (Gill et al., 2007; Pineda-Molina et al., 2006) and 36 % sequence identity between the yeast and human CTD sequences, we speculated that this domain might have a conserved role in binding to ESCRT-II. Thus we sought to revisit the interaction between human ESCRT-I and II using the purified, stable, independently folded VPS28 CTD and various forms of the purified, fully assembled ESCRT-II complex in direct binding assays (Fig. 3C, D).
The full length ESCRT-II construct binds to VPS28-CTD, but not to the GST control (Fig. 3D). Constructs in which the GLUE domain, the VPS25 WH2 domain, and VPS22-H0 (Construct a, d, e, and f) were deleted, respectively, also bind to the VPS28-CTD. However the constructs lacking residues 149 – 169, which encompass VPS36-H0, did not interact with VPS28-CTD, suggesting that the VPS36-H0 and/or adjacent residues are required for binding to the VPS28-CTD. The role of this region of VPS36 is consistent with the two-hybrid analysis (Langelier et al., 2006). In order to pinpoint this site, two blocks of conserved residues were mutated to polyalanine. A quadruple mutant within VPS36-H0 (construct m2), IERK(159–162)AAAA, showed substantially reduced binding as compared to wild-type and to another, mutant (construct m1, Fig. 3C). GST-tagged constructs of short regions of VPS36 (residues 147 – 171 and residues 147 – 201) were made to test if these isolated regions were sufficient for binding. These constructs did not pull down VPS28-CTD (Fig. 3C,D), nor did the isolated GLUE domain (Fig. 3C, data not shown). Thus VPS36-H0 is necessary but not sufficient for interaction with the VPS28-CTD. We sought to characterize the conformation of the VPS28-CTD-ESCRT-II complex in solution. The mixture of these two normally soluble proteins resulted in their precipitation. The simplest interpretation is that the binding of the VPS28-CTD and ESCRT-II leads to a conformational change in one or both partners.
Determinants for membrane targeting of human ESCRT-II
To investigate the lipid specificity and membrane binding sites in human ESCRT-II, we tested binding of various ESCRT-II constructs to lipid vesicles of various compositions (Fig. 4). For expediency, we used a pseudo-intact ESCRT-II (construct f, Fig. 3C) lacking the VPS25-WH2 as the baseline for comparison. This construct bound to vesicles as well as, or better than, the intact complex (Supplementary Fig. 4), and we found that it was more stable and could be purified with several-fold higher yields than the intact complex. The following deletion constructs were made in the context of this pseudo-intact complex: a deletion of GLUE domain (construct e), a deletion of the basic N-terminal 24 residues (Δ1–24) of VPS22 (construct h), and a double deletion of both the GLUE and VPS22- H0 (construct c). The purified pseudo-intact ESCRT-II showed strong binding to liposomes made of synthetic lipids composed of phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphoinositides (PIPs) (Fig. 4D–K), and bound weakly to PC:PE and PC:PE:phosphatidylinositol (PI) liposomes (Fig. 4B, C, K). Deletion of the VPS22-H0 (construct h) significantly decreased the membrane binding to all PIPs tested. Deletion of VPS22- H0 had no apparent effect on binding to PC:PE liposomes, however (Fig. 4B, K). The GLUE domain deletion lost binding to PC:PE and PC:PE:PI (Fig. 4B, C, K). Deletion of both GLUE and Δ1–24 of VPS22 (construct c) essentially abolished binding to all compositions tested (Fig. 4B–K). From these data, we draw the following conclusions. Human ESCRT-II binds strongly but relatively promiscuously to PIPs, and binds weakly to uncharged lipids. Both the GLUE domain and VPS22- H0 are required for full membrane binding.
Figure 4. Liposome binding of ESCRT-II complex.

Purified constructs of the ESCRT-II complex were mixed with liposomes. (A) The ESCRT-II constructs used for liposome binding assay are shown for reference. All constructs used for the binding assay lack the VPS25-WH2 for expedience. However the binding of this construct and full-length ESCRT-II are essentially identical (Supplementary Fig. 3). (B) Binding to PC:PE liposomes. Molecular weight markers are shown in lane 1. Unbound samples in supernatants were shown in lanes 2 – 5 for reference. (C) – (J) Liposome binding results with different lipid compositions. Variable amounts of PI and PIPs were mixed to the PE : PC mixture to examine the specificity. The mole fractions of PIPs were chosen to maintain a constant charge density on the membrane. (K) The relative amounts of proteins in the pellets were shown in bars. The construct double deletion construct (c), which shows negligible binding to all liposomes tested, is not shown in bar graphs.
A functional role for Vps22-H0
The Vps22- H0 is conserved from yeast to humans, with 30% sequence identity between these two species in this region (Supplementary Fig. 2). To determine if the Vps22- H0 was important for the cargo sorting function of ESCRT-II in vivo, the localization of the ESCRT substrate Cps1 was assayed in yeast expressing wild-type and mutant alleles of vps22. Yeast Vps22 contains 9 basic charged residues in the N-terminal 50 residues. To test the contribution of the N-terminal region of Vps22 on cargo sorting function in vivo, we constructed an N-terminal 30 residue deletion mutant, Vps22Δ1–30 (Supplementary Fig. 2). As expected (Babst et al., 2002), vps22Δmanifests GFP-Cps1 mislocalized to a prominent class E compartment, and absent from the vacuolar lumen (Fig. 5B). The wild-type phenotype is completely rescued by a plasmid bearing wild-type VPS22 (Fig. 5E). Expression of VPS22Δ1–30 results in some vacuolar GFP-Cps1 localization (Fig. 5F), but most of the cargo is retained on the limiting membrane, consistent with a weak class E phenotype. This indicates that the Vps22-H0 is important for the cargo sorting function of ESCRT-II.
Figure 5. Cargo sorting and localization of Vps22 and Vps36 mutants.
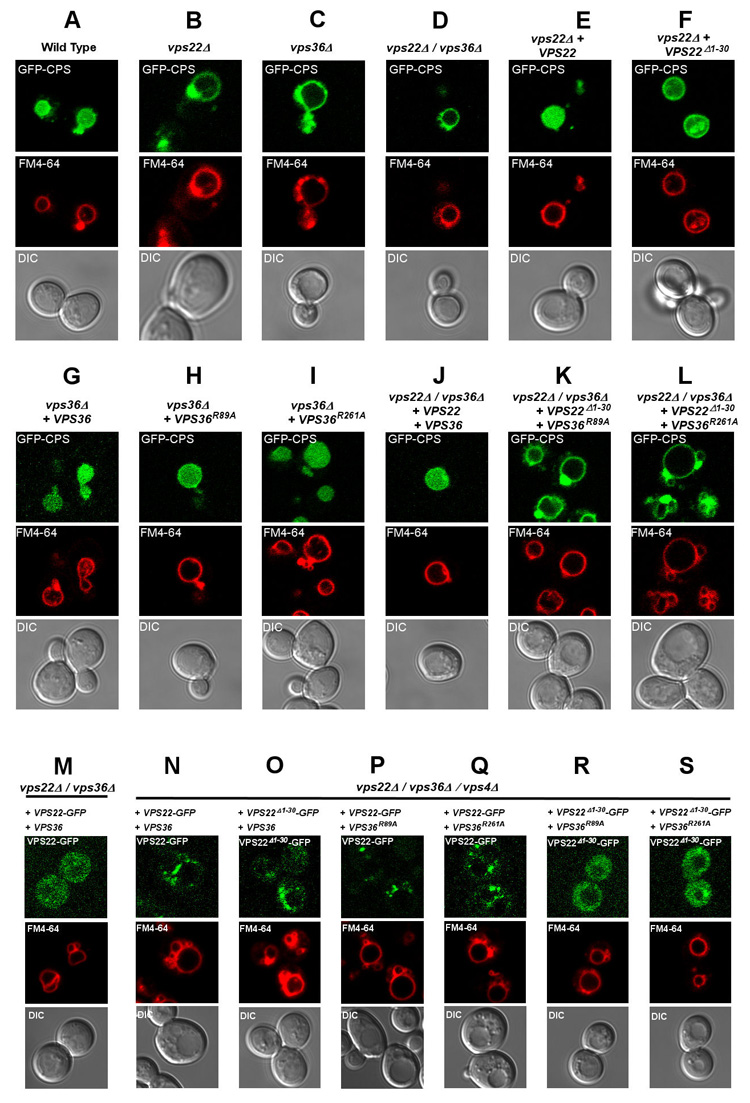
(A–L) The uppermost panel of each column shows the sorting of the GFP-Cps1 construct (green) in various strains, as indicated at the top of each column. (M–S) The localization of the designated ESCRT-II constructs in the indicated strains, as monitored by Vps22-GFP. The middle panels show the limiting membrane of the vacuole as labeled by FM4-22 64 (red), and the lower panels show the DIC image. Results presented here are characteristic of observations of > 100 cells for each strain shown.
We next sought to determine if Vps22-H0 cooperated with the Vps36-GLUE domain in function. The yeast Vps36 GLUE domain PI(3)P binding pocket double mutant, VPS36R89A/R261A, has a strong class E phenotype (Teo et al., 2006). We found that the single GLUE domain mutants VPS36R89A and VPS36R261A expressed in a vps36Δ background had near normal and normal function, respectively (Fig. 5H, I). However, when either of these mutants are co-expressed with VPS22Δ1–30 in a vps22Δ vps36Δ background, a strong class E phenotype is observed (Fig. 5K, L). The loss of function is much stronger than for any of the individual mutants, consistent with the concept that the Vps22-H0 and the Vps36-GLUE domain cooperate in the sorting function of ESCRT-II.
In order to determine if the defects in sorting where due to changes in membrane targeting, the localization of wild-type and mutant Vps22-GFP constructs was evaluated (Fig. 5 M–S). ESCRT components are diffusely localized in wild-type yeast because of the rapid recycling of endosomal ESCRTs to the cytosol as part of their normal function. However, in vps4Δstrains the dissociation of ESCRT components from endosomal compartments is inhibited, allowing their endosomal localization to be monitored. Consistent with previous results (Babst et al., 2002), Vps22-GFP was diffusely distributed in a vps22 Δ background (Fig. 5M), but found in large punctate structures in a vps4 Δ vps22 Δ vps36 Δ strain (Fig. 5N). The single GLUE domain mutants VPS36R89A and VPS36R261A manifested, respectively, complete and predominant punctate localization of Vps22-GFP in the vps4 Δ vps22 Δ vps36 Δ strain (Fig. 5P, Q). Therefore these mutations did not substantially impair ESCRT-II targeting. The VPS22 Δ1–30-GFP allele manifested mostly punctate localization, with a small increase in diffuse localization compared to wild-type VPS22 (Fig. 5O). The VPS22 Δ1–30-GFP allele in combination with either VPS36R89A or VPS36R261A, however, showed a completely diffuse localization pattern (Fig. 5R,S). Thus these defects in the Vps22-H0 and GLUE domain abolish endosomal localization of ESCRT-II only in combination.
Discussion
A major goal of structural analysis is to obtain a holistic picture of molecular assemblies. This is typically accomplished by integrating high resolution x-ray and NMR structures of smaller fragments with lower resolution data on the intact complex using electron microscopy or solution-state techniques. In the case of ESCRT-II, atomic structures have been available for the WH domain core (Hierro et al., 2004; Teo et al., 2004), the NZF1 domain (Gill et al., 2007), and the GLUE domain of the yeast complex (Teo et al., 2006), and the GLUE domain of the human complex (Alam et al., 2006; Hirano et al., 2006). One essential component was lacking to model the complete ESCRT-II structure: the structure of the predicted helical regions in the Vps22 N-terminus and in Vps36 between the GLUE and WH domains. We have now found that the most N-terminal predicted helix of the core of each subunit (H0) is flexibly attached to the core assembly. We have been able to assign functions to each of these two regions. The VPS22-H0 participates in membrane binding, while the VPS36-H0 interacts with ESCRT-I. The most significant finding from the crystallographic analysis is that ESCRT-II contains a helical domain composed of portions of both the VPS22 and VPS36 subunits. As described below, this domain appears to serve as a structural platform for the GLUE domain.
While ESCRT-II is too small to yield a cryo-EM reconstruction, we have been able to use hydrodynamic analysis (Garcia de la Torre et al., 2000; Kostelansky et al., 2007) together with the ESCRT-II core structure and the previously determined structure of the GLUE domain (Alam et al., 2006; Hirano et al., 2006) to model the conformation of the complete human complex in solution. This analysis shows unambiguously that human ESCRT-II has a compact conformation in solution. This is in sharp contrast to the ESCRT-I complex, where the functional domains extend away from the core on freely flexing linkers (Kostelansky et al., 2007). The constraints imposed by known crystal structures and by experimental hydrodynamic data indicate that the GLUE domain must directly contact the helical domain. Thus one function of the helical domain appears to be to scaffold the GLUE domain in the closed conformation of ESCRT-II.
Current models of ESCRT function invoke either the sequential or cooperative assembly of multiple ESCRT complexes on the endosomal membrane (Hurley, 2008; Nickerson et al., 2007; Saksena et al., 2007; Williams and Urbe, 2007). The yeast ESCRT-I and II complexes assemble together through a required interaction between the ESCRT-I Vps28-CTD and the ESCRT-II Vps36 NZF1 domain (Gill et al., 2007), although the existence of additional interactions has not been ruled out. Indeed, we find that the yeast ESCRT-I/II supercomplex is compact in solution (Kostelansky and J.H.H., unpublished), consistent with the presence of more than one interaction site. Human ESCRT-II lacks an NZF domain, and the available evidence that it interacts directly with human ESCRT-I has been limited to two-hybrid studies of isolated subunits (Langelier et al., 2006). Here we find that human ESCRT-II interacts robustly with the human VPS28-CTD. A motif within the ESCRT-II VPS36-H0 is necessary but not sufficient for this interaction. This motif is conserved in yeast Vps36, consistent with the possibility that the yeast ESCRT-I/II interaction involves more than one point of contact. The observation that the motif is necessary but not sufficient for the interaction suggests to us that ESCRT-I recognition by ESCRT-II involves an extended epitope.
Based on the highly dynamic nature of the ESCRT-II HD region and the observation of a dramatic solubility change upon mixing ESCRT-II and the ESCRT-I VPS28-CTD, we speculate that formation of the ESCRT I/II supercomplex entails a large conformational change. A similar solubility change was observed when yeast ESCRT-II or its Vps25 subunit was mixed with the yeast ESCRT-III subunit Vps20 (Teo et al., 2004). It will be interesting to assess whether there are widespread conformational changes when ESCRTs assemble with one another and with membranes. Such changes could have important implications for the membrane remodeling interactions that are catalyzed by the ESCRT system.
The mechanism of membrane targeting by ESCRT-II has attracted considerable attention. ESCRT-II is functionally activated by ESCRT-I. However, ESCRT-I is not required for the recruitment of ESCRT-II, since the loss of ESCRT-I can be rescued by the overexpression of ESCRT-II subunits (Babst et al., 2002). Yeast ESCRT-II binds with moderate to high affinity to PIP-containing liposomes in the absence of ESCRT-I (Kostelansky et al., 2007; Teo et al., 2004; Teo et al., 2006). The principal site for PIP binding was first localized to the GLUE domain in studies of human ESCRT-II by Stenmark and colleagues (Slagsvold et al., 2005). This study showed that the human ESCRT-II GLUE domain bound in vitro to immobilized PI(3,4,5)P3 in a dot-blot format, and to a lesser extent to PI(3,4)P2 and PI(3,5)P2 (Slagsvold et al., 2005). PI(3,4,5)P3 is typically present at the plasma membrane of cells stimulated by hormones such as insulin and PDGF, but is not characteristic of endosomal membranes. The GLUE domain of yeast ESCRT-II, in contrast, bound preferentially to PI(3)P when presented in liposomes, and the isolated yeast GLUE domain targets to endosomes when expressed in cultured human cells (Teo et al., 2006). Here we deduce, based on comparison of the intact and GLUE-domain deleted ESCRT-II complexes, that the human ESCRT-II GLUE domain binds to a variety of phosphoinositides. Deletion of the GLUE domain sharply reduces binding to the endosomal lipids PI(3)P and PI(3,5)P2, but more modestly reduces binding to PI(3,4,5)P3. Deleting Vps22-H0 has a modest effect on binding to PI(3)P and most other PIPs compared to the GLUE domain deletion. However, the Vps22-H0 deletion reduces binding to PI(3,5)P2 and PI(3,4,5)P3 by about the same extent as the GLUE domain deletion. We infer there is a strong electrostatic interaction between Vps22-H0 and the polyanionic PI(3,5)P2 and PI(3,4,5)P3. The ability of human ESCRT-II to bind to PI(3)P and PI(3,5)P2 is consistent with the endosomal function of ESCRT-II. The binding to PI(3,4,5)P3 and other non-endosomal PIPs is consistent with the previous report of a GLUE-PI(3,4,5) P3 interaction (Slagsvold et al., 2005), but hard to rationalize in terms of the endosomal role of ESCRT-II. The GLUE domain of Drosophila ESCRT-II, which more closely resembles human as opposed to yeast ESCRT-II, binds to bicoid mRNA (Irion and St Johnston, 2007). Thus the propensity of human ESCRT-II to bind highly acidic lipids may reflect this or other non-endosomal functions (Slagsvold et al., 2006) that involve binding to various highly acidic ligands.
The observation that both the Vps36 GLUE domain and the Vps22 H0 contribute to lipid binding in vitro suggested to us that a combinatorial mechanism evolved to drive high affinity membrane targeting of ESCRT-II (Fig. 6). Multivalent membrane targeting is widely used in the ESCRT system and other trafficking pathways for signal integration and coincidence detection. Given that ESCRT-I is involved in the functional activation of ESCRT-II, it is intriguing that ESCRT-I binds to a region of ESCRT-II very close to the locus of membrane binding, and that this interaction appears to trigger a structural change. In conclusion, we have derived here a structural and conceptual framework for a more precise mechanistic understanding of the interplay between ESCRT-I and membrane binding by ESCRT-II. More importantly, the organization of ESCRT-II deduced here, together with similar models for the overall structures of the Vps27-Hse1 (Prag et al., 2007) and ESCRT-I (Kostelansky et al., 2007) complexes, will underpin higher order structural studies of the ESCRT system.
Figure 6. Combinatorial membrane targeting of ESCRT-II.

(A) Overall schematic representation of full length ESCRT-II structure showing the binding site for VPS28-CTD and the VPS22-H0 examined in this study, and the previously described binding sites for PI(3)P (Teo et al., 2006), ubiquitin (Alam et al., 2006; Hirano et al., 2006), and VPS20 (Langelier et al., 2006). (B) Model for combinatorial targeting by specific and nonspecific interactions with membrane (represented by the solid horizontal bar) lipids.
EXPERIMENTAL PROCEDURES
Protein expression and purification
Full length genes for human VPS22, VPS25, and VPS36 were synthesized by PCR-based gene synthesis. Oligonucleotides were designed by the program DNAWorks (Hoover and Lubkowski, 2002). The nucleotide sequences of the genes were optimized for expression in E. coli, and restriction enzyme recognition sequences in the multiple cloning sites of pST39 vector were removed from the genes for efficient cloning of multiple genes into the vector. VPS22 was tagged with an N-terminal hexahistidine and a TEV protease cleavage site. The plasmid was transformed into E. coli strain BL21(DE3) Star and expressed overnight at 30 °C. Cells were resuspended in buffer (2X PBS plus 20 mM imidazole) and lysed by sonication. The ESCRT-II complex was isolated using Ni2+ affinity chromatography. The eluate was concentrated and the histidine tag was removed with TEV protease. The ESCRT-II complex was further purified by a Superdex S200 size exclusion chromatography. The fractions containing the complex were concentrated in buffer 10 mM Tris-HCl (pH 8.0), 100 mM NaCl.
Crystallization
Form I crystals of construct g (Fig. 3C) were grown by vapor-diffusion methods at 25 °C over a reservoir of 100 mM Na-Acetate (pH 4.5), 5% PEG 4000, 15% glycerol for one week. Crystals were cryoprotected in reservoir solution supplemented with 20% (v/v) glycerol and flash frozen under N2 gas at 95 K. Heavy atom derivative crystals were prepared by co-crystallization or by soaking crystals in heavy atom solutions. Form II crystals of construct a (Fig. 3C) were obtained in 100 mM Tris-HCl (pH 8.5), 40% PEG300 using a microseeding technique.
Crystallographic analysis
Native data for the form I crystal VPS36 GLUE domain and VPS22 H0 truncation (construct g, Fig. 3) were collected to 2.6 Å resolution from a single frozen crystal with an MAR CCD detector at beamline 22-ID, APS. The data sets were anisotropic with weak diffraction along c extending only to 2.9 Å resolution. All data were processed and scaled using HKL2000 (HKL Research). Heavy atom derivative data sets were collected with an R-AXIS IV image-plate system attached to a Rigaku rotating-anode generator providing Cu Kα radiation. MIR phasing was carried out using the programs SOLVE (Terwilliger and Berendzen, 1999) at 3.6 Å resolution (Table 1) and the phases were further improved by RESOLVE (Terwilliger, 2000). The initial model was built manually into the density-modified map using the programs O (Jones et al., 1991) and Coot (Emsley and Cowtan, 2004). Tracing of the backbones was facilitated by comparing the homologous structure of yeast ESCRT-II complex. Then the density map was improved using model phase combination and the resolution was extended to 2.6 Å resolution. The refinement was carried out using CNS (Brunger et al., 1998) and Refmac (CCP4, 1994). The final model for form I consisted of residues 172–385 from VPS36, residues 34–252 from VPS22, and residues 4–176 and 5–101 from VPS25. There are 97.2% of the residues in the most favored and additional allowed regions of the Ramachandran plot. Seven residues have conformations in disallowed regions, and all of these are located in regions of high mobility at the extreme termini of chains, in the helical domain, or in the flexible VPS25 WH2 domain. The structure of the form II crystal (construct a, Fig. 3) was determined by molecular replacement with the program MOLREP using the form I structure as a starting model. Residues 149–169 from VPS36 and residues 1–25 from VPS22 are present in construct a, but could not be visualized in electron density, and are presumed to be disordered. All structural figures were prepared using the program PyMOL (W. Delano, http://pymol.sourceforge.net/)
Hydrodynamic modeling
Residues in the N- and C- termini the subunits (residues 1–3 in VPS25, 27–33 and 253–258 in VPS22) that were present in the crystallized construct of form I but missing in the electron density were modeled as random coils, and the random coil conformation was adjusted iteratively with interactive graphics until the calculated and experimental RH values were in agreement within < 0.1 nm (Fig. 3A). Essentially the same procedure was used to place VPS22-H0 and VPS36-H0, which were modeled as helices on the basis of secondary structure prediction, using the crystal structure of form II and the corresponding RH value (Fig. 3A). Full length ESCRT-II was modeled by positioning the GLUE domain interactively so as to obtain agreement with between the calculated and experimental RH values (Fig. 3A). The structure of the human ESCRT-II GLUE domain was obtained from PDB entry 2HTH (Alam et al., 2006).
Pull-down assays
For GST-pull-downs, 50 µl of gluthathione sepharose resin was prewashed with binding buffer (1X PBS). GST-tagged human VPC28 C-terminal domain (residues 123–220) was bound to the resin. Various purified constructs of human ESCRT-II complex were mixed with the GST-VPS28 CTD-bound resin for 30 min at room temperature. GST-bound resin was used as a negative control. Then the beads were washed three times with 1X PBS and the bound proteins were analyzed by SDS-PAGE.
Liposome binding experiments
The synthetic lipids used in this study were all purchased from Avanti Polar Lipids except for PI(3)P from Echelon. All liposomes contain 0.5 % of a dye, lissamine rhodamine B for quantitation of the liposomes. The liposomes were prepared at a total lipid concentration of 1 mg/ml by evaporating the solvent from the desired lipid mixture using a nitrogen stream. The dried lipids were resuspended in 150 ml of 0.3 M sucrose, and the solution was incubated at room temperature under nitrogen for 1 hr with periodic vortexing; 1ml of water was added, and the sample was sedimented in an ultracentrifuge at 128,000 × g for 30 min at 4°C. The supernatant was removed, and the pellet was frozen and thawed three times in liquid nitrogen. The pellet was dissolved in 1 ml of buffer A (20 mM HEPES at pH 7.4 and 150 mM NaCl) and extruded 10 times through a 0.1 µm filter. For binding experiments, 100 µg of the liposomes were mixed with 80 µg of protein and were brought up to a total volume of 200 µl with buffer A, incubated at room temperature for 30 min, and sedimented at 128,000 × g at 4°C for 30 min. The pellet was washed once with 200 µl of the buffer A and again sedimented for 30 min. Samples of the supernatant (10 µl) and pellet were analyzed by SDS-PAGE. The intensities of bands in the SDS-PAGE gels were measured using the LabWorks 5.6 program (UVP).
Plasmid construction and yeast strains
The vps22Δ vps36Δ strain was prepared by replacing the VPS36 gene with a nourseothrisin resistance gene from a vps22 Δ strain by homologous recombination. The vps4 Δ vps22 Δ vps36 Δ strain was made by homologous recombination between the VPS4 and URA3 genes in the vps22 Δ vps36 Δ strain. The complete expression cassette of Vps22 and the open reading frame of Vps36 were amplified from yeast genomic DNA and cloned into YCplac111 and pRS413MET25 vectors respectively. The N-teminal 30 amino acid deletion of Vps22 and the R89A and R261A mutations of Vps36 were introduced by Quickchange mutagenesis (Stratagene). DNA coding for green fluorescent protein (GFP) was fused to the 3’ end of the vps22 cassette using PCR, and the PCR product was cloned to the YCplac111 vector. Plasmids encoding VPS22 and VPS36 genes, and the pGO45 vector, were transformed to wild-type and mutant strains. The following yeast strains were used: BY4741 (MATa his3Δ 1 leu2Δ0 met15Δ0 ura3Δ0), BY4741 vps22Δ::KanR, BY4741 vps36Δ::KanR, BY4741 vps22Δ::KanR vps36Δ::NATR, BY4741 vps22Δ::KanR vps36Δ::NATR vps4Δ::URA3.
Microscopy
Yeast strains expressing the appropriate alleles were harvested at an A660 of 0.4–0.6, labeled with FM4-64 for vacuolar membrane staining (Vida and Emr, 1995). Uptake of FM4-64 by live cells was performed at 30 °C for 1 hr, after which cells were resuspended in selection media and incubated for 30 min at 30 °C. Visualization of cells was performed on a LSM510 fluorescence microscope (Carl Zeiss MicroImaging, Inc.) equipped with fluorescein isothiocyanate (FITC) and rhodamine filters, captured with a digital camera.
Supplementary Material
ACKNOWLEDGEMENTS
We thank to Will Prinz and Beverly Wendland for technical advice on yeast experiments, W.P. and Greg Odorizzi for providing plasmids and yeast strains, Boris Baibakov for technical assistance on yeast microscopy imaging, the SER-CAT staff for user support at the Advanced Photon Source (APS), and B.W. for comments on the manuscript. Use of the APS was supported by the U. S. DOE, Basic Energy Sciences, Office of Science, under Contract No.W-31–109-Eng-38. This research was supported by NIH intramural support, NIDDK and IATAP.
Footnotes
COORDINATES
Crystallographic coordinates have been deposited in the Protein Data Bank with accession codes 3CUQ for crystal form I and 2ZME for form II.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alam SL, Langelier C, Whitby FG, Koirala S, Robinson H, Hill CP, Sundquist WI. Structural basis for ubiquitin recognition by the human ESCRT-II EAP45 GLUE domain. Nat Struct Mol Biol. 2006;13:1029–1030. doi: 10.1038/nsmb1160. [DOI] [PubMed] [Google Scholar]
- Alam SL, Sun J, Payne M, Welch BD, Black BK, Davis DR, Meyer HH, Emr SD, Sundquist WI. Ubiquitin interactions of NZF zinc fingers. Embo J. 2004;23:1411–1421. doi: 10.1038/sj.emboj.7600114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M. A Protein's Final ESCRT. Traffic. 2005;6:2–9. doi: 10.1111/j.1600-0854.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell. 2002;3:283–289. doi: 10.1016/s1534-5807(02)00219-8. [DOI] [PubMed] [Google Scholar]
- Bieniasz PD. Late budding domains and host proteins in enveloped virus release. Virology. 2006;344:55–63. doi: 10.1016/j.virol.2005.09.044. [DOI] [PubMed] [Google Scholar]
- Bowers K, Piper SC, Edeling MA, Gray SR, Owen DJ, Lehner PJ, Luzio JP. Degradation of endocytosed epidermal growth factor and virally ubiquitinated major histocompatibility complex class I is independent of mammalian ESCRTII. J. Biol. Chem. 2006;281:5094–5105. doi: 10.1074/jbc.M508632200. [DOI] [PubMed] [Google Scholar]
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. Sect. D-Biol. Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316:1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- CCP4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr A. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, Ganz T, Musci G, Kaplan J. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18:2569–2578. doi: 10.1091/mbc.E07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. Sect. D-Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Garcia de la Torre J, Huertas ML, Carrasco B. Calculation of hydrodynamic properties of globular proteins from their atomic-level structure. Biophys. J. 2000;78:719–730. doi: 10.1016/S0006-3495(00)76630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DJ, Teo H, Sun J, Perisic O, Veprintsev DB, Emr SD, Williams RL. Structural insight into the ESCRT-I/-II link and its role in MVB trafficking. Embo J. 2007;26:600–612. doi: 10.1038/sj.emboj.7601501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz HM, Chen Z, Scherr H, Lackey M, Bolduc C, Bergmann A. vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis. Development. 2006;133:1871–1880. doi: 10.1242/dev.02356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierro A, Sun J, Rusnak AS, Kim J, Prag G, Emr SD, Hurley JH. Structure of the ESCRT-II endosomal trafficking complex. Nature. 2004;431:221–225. doi: 10.1038/nature02914. [DOI] [PubMed] [Google Scholar]
- Hirano S, Suzuki N, Slagsvold T, Kawasaki M, Trambaiolo D, Kato R, Stenmark H, Wakatsuki S. Structural basis of ubiquitin recognition by mammalian Eap45 GLUE domain. Nat Struct Mol Biol. 2006;13:1031–1032. doi: 10.1038/nsmb1163. [DOI] [PubMed] [Google Scholar]
- Hoover DM, Lubkowski J. DNA Works: an automated method for designing oligonucleotides for PCR-based gene synthesis. Nucleic Acids Res. 2002;30:e43. doi: 10.1093/nar/30.10.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH. ESCRT Complexes and the Biogenesis of Multivesicular Bodies. Curr. Opin. Cell Biol. 2008;20:4–11. doi: 10.1016/j.ceb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irion U, St Johnston D. bicoid RNA localization requires specific binding of an endosomal sorting complex. Nature. 2007;445:554–558. doi: 10.1038/nature05503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved Methods for Building Protein Models in Electron- Density Maps and the Location of Errors in These Models. Acta Crystallogr. Sect. A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kamura T, Burian D, Khalili H, Schmidt SL, Sato S, Liu WJ, Conrad MN, Conaway RC, Conaway JW, Shilatifard A. Cloning and characterization of ELL-associated proteins EAP45 and EAP20. a role for yeast EAP-like proteins in regulation of gene expression by glucose. J. Biol. Chem. 2001;276:16528–16533. doi: 10.1074/jbc.M010142200. [DOI] [PubMed] [Google Scholar]
- Kostelansky MS, Schluter C, Tam YYC, Lee S, Ghirlando R, Beach B, Conibear E, Hurley JH. Molecular architecture and functional model of the complete yeast ESCRT-I heterotetramer. Cell. 2007;129:485–498. doi: 10.1016/j.cell.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostelansky MS, Sun J, Lee S, Kim J, Ghirlando R, Hierro A, Emr SD, Hurley JH. Structural and functional organization of the ESCRT-I trafficking complex. Cell. 2006;125:113–126. doi: 10.1016/j.cell.2006.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langelier C, von Schwedler UK, Fisher RD, De Domenico I, White PL, Hill CP, Kaplan J, Ward D, Sundquist WI. Human ESCRT-II complex and its role in human immunodeficiency virus type 1 release. J. Virol. 2006;80:9465–9480. doi: 10.1128/JVI.01049-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malerod L, Stuffers S, Brech A, Stenmark H. Vps22/EAP30 in ESCRT-II mediates endosomal sorting of growth factor and chemokine receptors destined for lysosomal degradation. Traffic. 2007;8:1617–1629. doi: 10.1111/j.1600-0854.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- Martin-Serrano J, Yarovoy A, Perez-Caballero D, Bieniasz PD. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12414–12419. doi: 10.1073/pnas.2133846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita E, Sandrin V, Chung HY, Morham SG, Gygi S, Rodesch CK, Sundquist WI. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. Embo J. 2007;26:4215–4227. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson DP, Russell DW, Odorizzi G. A concentric circle model of multivesicular body cargo sorting. EMBO Rep. 2007;8:644–650. doi: 10.1038/sj.embor.7401004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda-Molina E, Belrhali H, Piefer AJ, Akula I, Bates P, Weissenhorn W. The crystal structure of the C-terminal domain of Vps28 reveals a conserved surface required for Vps20 recruitment. Traffic. 2006;7:1007–1016. doi: 10.1111/j.1600-0854.2006.00440.x. [DOI] [PubMed] [Google Scholar]
- Prag G, Watson H, Kim YC, Beach BM, Ghirlando R, Hummer G, Bonifacino JS, Hurley JH. The Vps27/Hse1 complex is a GAT domain-based scaffold for ubiquitin-dependent sorting. Dev. Cell. 2007;12:973–986. doi: 10.1016/j.devcel.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Progida C, Malerod L, Stuffers S, Brech A, Bucci C, Stenmark H. RILP is required for the proper morphology and function of late endosomes. J. Cell Sci. 2007;120 doi: 10.1242/jcs.017301. [DOI] [PubMed] [Google Scholar]
- Progida C, Spinosa MR, De Luca A, Bucci C. RILP interacts with the VPS22 component of the ESCRT-II complex. Biochem. Biophys. Res. Commun. 2006;347:1074–1079. doi: 10.1016/j.bbrc.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Saksena S, Sun J, Chu T, Emr SD. ESCRTing proteins in the endocytic pathway. Trends Biochem Sci. 2007;32:561–573. doi: 10.1016/j.tibs.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Slagsvold T, Aasland R, Hirano S, Bache KG, Raiborg C, Trambaiano D, Wakatsuki S, Stenmark H. Eap45 in mammalian ESCRT-II binds ubiquitin via a phosphoinositide-interacting GLUE domain. J Biol Chem. 2005;280:19600–19606. doi: 10.1074/jbc.M501510200. [DOI] [PubMed] [Google Scholar]
- Slagsvold T, Pattni K, Malerod L, Stenmark H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 2006;16:317–326. doi: 10.1016/j.tcb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Teo H, Perisic O, Gonzalez B, Williams RL. ESCRT-II, an endosome-associated complex required for protein sorting: Crystal structure and interactions with ESCRT-III and membranes. Dev. Cell. 2004;7:559–569. doi: 10.1016/j.devcel.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Teo HL, Gill DJ, Sun J, Perisic O, Veprintsev DB, Vallis Y, Emr SD, Williams RL. ESCRT-I core and ESCRT-II GLUE domain structures reveal role for GLUE in linking to ESCRT-I and membranes. Cell. 2006;125:99–111. doi: 10.1016/j.cell.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Terwilliger TC. Maximum-likelihood density modification. Acta Crystallogr. Sect. D-Biol. Crystallogr. 2000;56:965–972. doi: 10.1107/S0907444900005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger TC, Berendzen J. Automated MAD and MIR structure solution. Acta Crystallogr. Sect. D-Biol. Crystallogr. 1999;55:849–861. doi: 10.1107/S0907444999000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ, Mathieu J, Sung HH, Loeser E, Rorth P, Cohen SM. Tumor suppressor properties of the ESCRT-II complex component vps25 in Drosophila. Dev. Cell. 2005;9:711–720. doi: 10.1016/j.devcel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating Notch trafficking. Dev. Cell. 2005;9:687–698. doi: 10.1016/j.devcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A New Vital Stain for Visualizing Vacuolar Membrane Dynamics and Endocytosis in Yeast. J. Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schwedler UK, Stuchell M, Muller B, Ward DM, Chung HY, Morita E, Wang HE, Davis T, He GP, Cimbora DM, et al. The protein network of HIV budding. Cell. 2003;114:701–713. doi: 10.1016/s0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
- Wang TL, Hong WJ. RILP interacts with VPS22 and VPS36 of ESCRT-II and regulates their membrane recruitment. Biochem. Biophys. Res. Commun. 2006;350:413–423. doi: 10.1016/j.bbrc.2006.09.064. [DOI] [PubMed] [Google Scholar]
- Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat. Rev. Mol. Cell Biol. 2007;8:355–368. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- Yorikawa C, Shibata H, Waguri S, Hatta K, Horii M, Katoh K, Kobayashi T, Uchiyama Y, Maki M. Human CHMP6, a myristoylated ESCRT-III protein, interacts directly with an ESCRT-II component EAP20 and regulates endosomal cargo sorting. Biochem. J. 2005;387:17–26. doi: 10.1042/BJ20041227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


