Abstract
Background
Abdominal adiposity is a growing clinical and public health problem. It is not known whether it is similarly associated with cardiovascular disease (CVD) and diabetes in different regions around the world, and thus whether measuring waist circumference (WC) in addition to body mass index (BMI) is useful in primary care practice.
Methods and Results
Randomly chosen primary care physicians (PCPs) in 63 countries recruited consecutive patients aged 18 to 80 years, on two pre-specified half-days. WC and BMI were measured and the presence of CVD and diabetes recorded. Of the patients consulting the PCPs, 97% agreed to participate in this study. Overall, 24% of 69,409 men and 27% of 98,750 women were obese (BMI ≥ 30 kg/m2). A further 40% and 30% of men and women, respectively, were overweight (BMI 25 to 30 kg/m2). In men and women, respectively, increased WC (>102/88cm, men/women) was recorded in 29% and 48%, CVD in 16% and 13%, and diabetes in 13% and 11%. There was a statistically significant graded increase in the frequency of CVD and diabetes with both BMI and WC, with a stronger relationship for WC than for BMI across regions, for both genders. This relationship between WC, CVD and particularly diabetes was seen even in lean patients (BMI <25 kg/m2).
Conclusions
Among men and women consulting PCPs, BMI and particularly WC were both strongly linked to CVD and especially to diabetes. Strategies to address this global problem are required to prevent an epidemic of these major causes of morbidity and mortality.
Keywords: cardiovascular disease, diabetes mellitus, epidemiology, obesity, risk factors
Keywords: Abdominal Fat, Adiposity, Adolescent, Adult, Aged, 80 and over, Body Mass Index, Cardiovascular Diseases, epidemiology, etiology, Diabetes Mellitus, Female, Humans, International Cooperation, Male, Middle Aged, Obesity, complications, Physicians, Family, Primary Health Care, Random Allocation, World Health
The beneficial impact of favorable trends in population control of classic risk factors such as smoking, hypercholesterolemia, and hypertension, on cardiovascular morbidity and mortality may be reversed by the current epidemic of obesity.1–6 Obesity — in particular abdominal adiposity — is associated with increased risk of cardiovascular disease (CVD) and diabetes.7–22 The prevalence of diabetes is increasing,23 and already in the USA, the lifetime risk of developing diabetes is high: 33% for men and 38% for women.24 Given that patients with diabetes have at least a twofold higher risk of cardiovascular mortality than non-diabetic patients,25 obesity has become a major clinical and public health problem that threatens to overwhelm already extended healthcare services in many countries.
Recent publications have shown that the “overweight” and particularly the “obese” have progressively higher morbidity and mortality.26,27 The multinational INTERHEART case-control study confirmed the importance of obesity, particularly abdominal adiposity, as a potent risk factor for myocardial infarction.15,16 Despite this interest, worldwide data on the distribution of even simple measures, such as waist circumference (WC), using a standardized protocol, are not available. This is crucial if geographic or ethnic thresholds are to be established for interventional programs. It is also important to extend the INTERHEART observations to determine the impact of adiposity on CVD and diabetes in different regions of the world and in a more widely based population consulting primary care physicians (PCPs).
The International Day for Evaluation of Abdominal obesity (IDEA)28 was a large international, non interventional, cross-sectional study evaluating abdominal adiposity (WC) measured by a standardized protocol in 168,000 PCPs on two pre-specified half-days in 63 countries. This report describes the geographic distribution of WC, overweight and obesity, and evaluates whether WC in addition to body mass index (BMI) is a useful clinical marker of physician-reported CVD and diabetes.
Methods
Between May 9 and July 6 2005, all consecutive patients aged 18 to 80 years consulting the randomly selected PCPs for any reason, were invited to participate in this study, on two half-days pre-specified for each of the 63 countries.28 Women who were known to be pregnant were excluded.
Physicians recorded the patient’s age, gender, smoking status, presence of known CVD (defined on the form as coronary heart disease, stroke, or revascularization), and known diabetes (which could be type 1 or type 2). Disease status used a combination of the physician’s knowledge of the patient’s history, medical notes and patient recall. The PCPs were trained to measure WC in the standing position, midway between the lowest rib and the iliac crest.1 Weight and height were measured and BMI calculated.
Ethics committee approval was obtained for each participating site and each patient provided written informed consent.
A sample size of >1100 patients per country enabled the frequency of abdominal obesity (with any definition) to be estimated to within 3% with 95% confidence. PCPs were randomly selected in each country by geographic or administrative strata from an exhaustive list of active PCPs, as reported previously,28 to ensure that participants in this study were representative of PCP patients.
Statistical Analysis
Data from 63 countries were grouped into 11 regions. Patient characteristics are described by percentages, means, and standard deviations (SD). Regional frequencies of obesity (BMI ≥ 30 kg/m2), overweight (BMI 25–30 kg/m2), CVD, and diabetes were standardized according to the age distribution of the overall study population. Box and whisker plots describe regional distributions of WC (median, quartiles). Age- and regionadjusted frequencies of CVD and diabetes were calculated according to gender-specific WC quintiles using logistic regression in SAS PROC GENMOD, with age as a continuous variable. Age- and region- adjusted Odds Ratios (ORs) were calculated according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP)29 and the International Diabetes Federation (IDF)30 defined thresholds for waist circumference, comparing groups: 102/88 cm and 94(90 for Asian)/80 cm with age as a continuous variable. ORs were also calculated for 1 SD increases in WC or BMI. Linearity was tested by adding squared terms, and ridge regression was used when BMI and WC were jointly analyzed. Age-, region- and smoking-adjusted frequencies of CVD and diabetes were calculated according to gender-specific WC tertiles and BMI categories using logistic regression in SAS PROC GENMOD, with age as a continuous variable. Trend tests were used to show whether the frequencies of CVD and diabetes increased with WC tertiles within each BMI category, and similarly with BMI categories within each WC tertile. Age- and region-adjusted ORs for CVD and diabetes were also determined for a 1 SD increase in WC within each BMI category.
All statistical analyses used SAS® statistical software (version 8.2; SAS Institute Inc., Cary, NC, USA).
Results
Study Participation
A total of 182,970 patients attending 6,393 PCP offices in 63 countries were screened: 177,345 (97%) agreed to participate in the study. Patient (84–100%) and physician (4–77%, average 30%) participation is given by country in Online Data Supplement 1.28 Data were analyzed from the 95% of patients (69,409 men, 98,750 women) who met the inclusion criteria and for whom data were available for age, gender, anthropometric parameters, CVD, and diabetes (Table 1). The mean(SD) age was 48.7(16.1) and 48.3(16.1) years for men and women.
TABLE 1.
Characteristics of Patients in IDEA by Region
| Region | Countries | No. of Patients | Mean Age (SD), Years | Men (%) | Mean Waist Circumference (SD), cm | Mean BMI (SD), kg/m2 | ||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||
| NW Europe | Austria, Belgium, Denmark, Finland, France, Germany, Ireland, The Netherlands, Norway, Sweden, Switzerland | 29,582 | 51.7(16.4) | 43.3 | 97.8 (13.5) | 88.3(14.8) | 27.2(4.6) | 26.4(5.6) |
| South Europe | Greece, Italy, Portugal, Spain, Turkey | 31,289 | 53.0(15.8) | 43.0 | 99.4(12.9) | 91.3(14.7) | 28.2(4.5) | 27.9(5.6) |
| E Europe | Bulgaria, Czech Republic, Estonia, Hungary, Latvia, Lithuania, Poland, Russia, Slovakia, Slovenia, Ukraine | 30,375 | 50.2(15.9) | 36.8 | 96.9(13.7) | 89.7(15.7) | 27.5(4.8) | 27.6(6.0) |
| N Africa | Egypt, Morocco, Tunisia | 5,028 | 43.1(14.7) | 37.2 | 93.6(15.3) | 93.1(16.2) | 26.6(5.4) | 28.3(6.4) |
| S Africa | Republic of South Africa | 2,492 | 42.0(13.8) | 43.2 | 93.6(15.5) | 89.8(16.4) | 26.9(5.6) | 28.9(7.2) |
| Middle East | Israel, Kuwait, Lebanon, Qatar, Saudi Arabia, United Arab Emirates | 5,457 | 41.9(13.9) | 56.0 | 98.2(14.2) | 93.4(16.5) | 28.2(5.4) | 28.7(6.9) |
| E Asia | China, Hong Kong, Korea, Taiwan | 11,402 | 48.4(15.9) | 38.6 | 86.4(10.7) | 80.2(10.7) | 24.4(4.0) | 23.9(4.1) |
| S Asia | India, Indonesia, Malaysia, Pakistan, The Philippines, Singapore, Thailand, Vietnam | 19,381 | 43.3(14.7) | 50.0 | 89.3(13.4) | 84.1(13.9) | 24.7(4.8) | 25.0(5.6) |
| Australia | Australia | 1,846 | 49.3(16.7) | 44.5 | 99.1(14.9) | 89.0(15.9) | 28.0(5.2) | 27.5(6.3) |
| Canada | Canada | 3,062 | 51.9(15.7) | 43.8 | 101.4(15.2) | 92.2(16.2) | 29.2(5.7) | 28.9(6.9) |
| Latin America | Argentina, Brazil, Chile, Colombia, Dominican Republic, Ecuador, Guatemala, Jamaica, Mexico, Peru, Trinidad & Tobago, Venezuela | 28,245 | 44.1(15.5) | 34.4 | 96.4(13.4) | 89.7(13.8) | 27.8(4.9) | 27.6(5.7) |
| Total | 168,159 | 48.5(16.1) | 41.3 | 95.8(14.0) | 88.7(14.9) | 27.1(4.9) | 27.0(5.9) | |
BMI indicates body mass index; SD, standard deviation.
Distribution of Overweight and Obesity
In all regions except East and South Asia, more than 60% of men and 50% of women were either overweight or obese (BMI ≥ 25 kg/m2). The overall frequency of overweight (BMI 25–30 kg/m2) was 40% in men and 30% in women, and this was remarkably similar across regions (Figure 1). In contrast, the frequency of obesity (BMI ≥ 30 kg/m2) differed between regions and was consistently low in both men and women in South and East Asia. Obesity ranged from just over 7% in men and women in East Asia to 36% in both genders in Canada, and 38% to 40% in women in the Middle East and North and South Africa.
Figure 1.
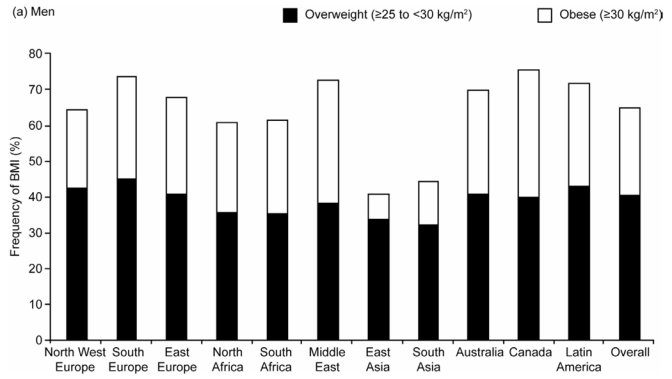
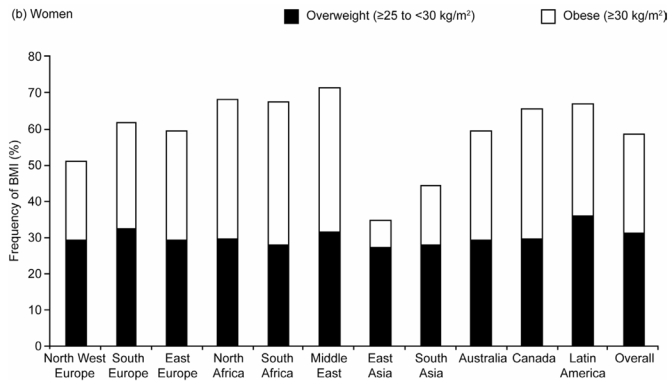
Age-standardized Frequency of Overweight (Body Mass Index [BMI] 25–30kg/m2) and Obesity (BMI ≥ 30kg/m2) by Region in (a) Men and (b) Women
Distribution of Waist Circumference
There was a wide distribution of WCs within each region, with median WCs higher in men than in women (Figure 2). Overall, the median WC (quartiles) was 95(86–104) cm for men and 88(78–98) cm for women. According to the NCEP criteria [WC>102/88 cm men/women],29 29% of men and 48% of women had abdominal adiposity; with the IDF Caucasian criteria [WC ≥ 94/80 cm men/women]30 these frequencies increased to more than half of the population: men, 56% and women, 71%.
Figure 2.
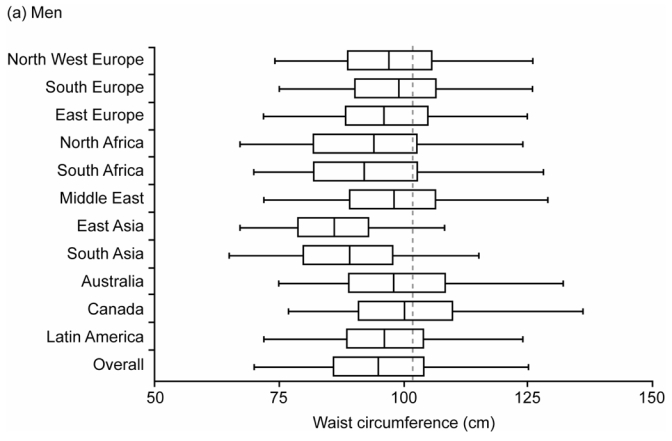
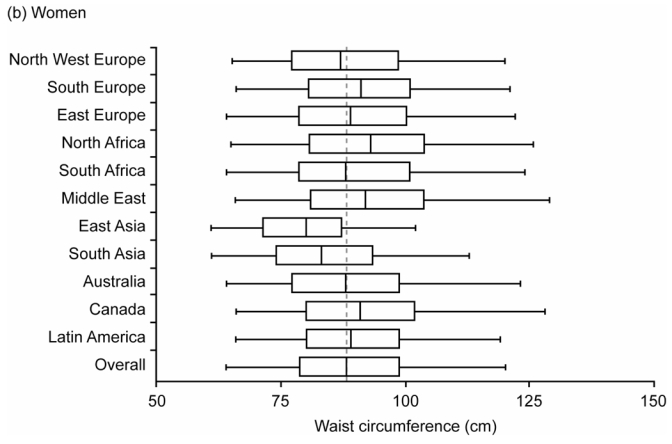
Box and Whisker Plot Showing the Distribution of Waist Circumference (WC) by Region for (a) Men and (b) Women. Data shown are Medians, Quartiles, and 2.5 and 97.5 Percentiles. Vertical lines show WC=102cm (men) and WC=88cm (women)
Frequency of CVD and Diabetes According to Region
The overall frequency of CVD was 16% in men and 13% in women, and were higher in men than in women in all regions except East Asia, with wide variability across regions (Figure 3 There was a very high frequency of CVD in Eastern Europe in both men (27%) and women (24%), in contrast to other regions where frequencies ranged from 8% (Canadian women) to 16% (North West European men).
Figure 3.
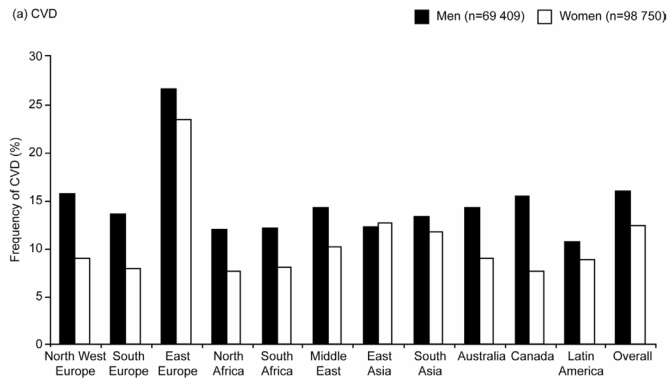
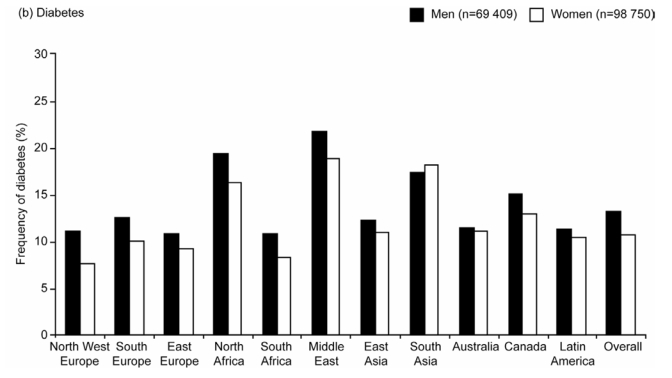
Age-standardized Frequencies of (a) Cardiovascular Disease (CVD) and (b) Diabetes by Region in Men and Women
The frequency of diabetes showed more regional variability than CVD (Figure 3). Overall, 13% of men and 11% of women had known diabetes. Both diabetes and CVD were more common in men than women in all regions except South Asia. The highest age-standardized frequency of diabetes was in the Middle East (22% in men and 19% in women), followed by North Africa (19% and 16%) and South Asia (17% and 18%).
Relationship between WC, CVD and Diabetes
The age- and region-adjusted frequency of CVD increased with WC in both men and women (Figure 4). Men in the highest WC quintile (≥107 cm) had 2.2 times more CVD than those in the lowest quintile (<84 cm); for women, comparing the most to the least abdominally obese (≥101 vs <76 cm), the ratio was 2.6.
Figure 4.
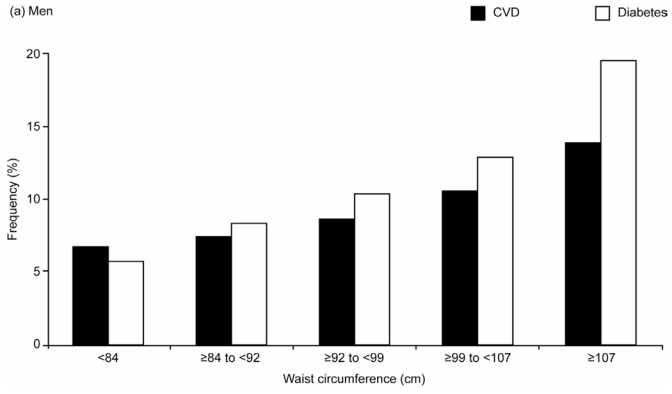
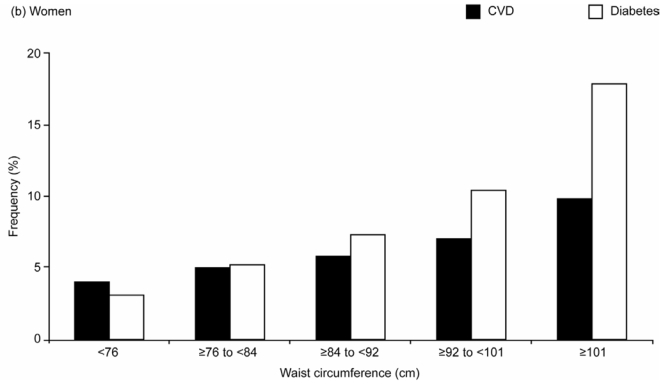
Age- and Region-adjusted Frequencies of Cardiovascular Disease (CVD) and Diabetes Across Gender-specific Quintiles of Waist Circumference in the Overall Study Population of (a) Men (n=69,409), (b) Women (n=98,750)
The frequency of diabetes showed an even stronger graded increase across the WC quintiles: in men it increased more than threefold (from 5.7% in the lowest to 19.4% in the highest WC quintile group) and in women it increased by almost sixfold (3.1% to 17.8%) (Figure 4).
For the NCEP/IDF WC categories, in comparison to the lowest WC group <94cm(90 for Asian)/80 cm in men/women, the age- and region-adjusted CVD ORs (95% CI) for CVD were for men: 1.28(1.21–1.36) for WC:94–102 cm(90–102 cm for Asians), 1.90(1.80–2.02) for WC>102 cm; for women: 1.31(1.22–1.41) for WC:80–88 cm, 1.97(1.85–2.09) for WC>88 cm; the corresponding ORs for diabetes were higher, 1.60(1.51–1.71), 2.65(2.49–2.81) and 1.78(1.64–1.94), 3.94(3.66–4.24).
Relationship between WC, BMI and CVD
In men, for all regions except North Africa and the Middle East, the OR for CVD associated with a 1 SD increase in WC was greater than that for a 1 SD increase in BMI (Table 2); for women this was the case in all but four of the eleven regions. Overall, the standardized ORs for CVD were higher for WC than for BMI in both men and women. This was also the case after adjustment of WC for BMI, and of BMI for WC: the ORs (WC vs BMI) were 1.24(1.19–1.28) vs 1.13(1.09–1.17) for men and 1.21(1.17–1.25) vs 1.20(1.16–1.24) for women, showing that both WC and BMI were independently associated with CVD.
TABLE 2.
Age-adjusted, Standardized ORs for CVD and Diabetes in Men and Women Associated with a 1 SD Increase in WC or BMI, by Region
| Age-adjusted ORs for CVD Associated with a 1 SD Increase in WC or BMI
|
Age-adjusted ORs for Diabetes Associated with a 1 SD Increase in WC or BMI
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Men
|
Women
|
Men
|
Women
|
|||||
| Region | WC | BMI | WC | BMI | WC | BMI | WC | BMI |
| NW Europe | 1.29(1.23–1.36)* | 1.27(1.21–1.33)* | 1.37(1.30–1.44)* | 1.31(1.24–1.37)* | 1.76(1.67–1.86)* | 1.72(1.63–1.81)* | 2.17(2.05–2.29)* | 1.96(1.86–2.06)* |
| S Europe | 1.31(1.25–1.38)* | 1.22(1.17–1.28)* | 1.30(1.23–1.37)* | 1.22(1.16–1.28)* | 1.45(1.38–1.53)* | 1.39(1.33–1.46)* | 1.78(1.69–1.87)* | 1.68(1.60–1.76)* |
| E Europe | 1.42(1.35–1.49)* | 1.41(1.34–1.48)* | 1.48(1.42–1.54)* | 1.53(1.47–1.59)* | 1.83(1.72–1.95)* | 1.73(1.63–1.83)* | 1.98(1.88–2.09)* | 1.82(1.73–1.91)* |
| N Africa | 1.48(1.25–1.74)* | 1.51(1.30–1.77)* | 1.50(1.28–1.75)* | 1.52(1.32–1.74)* | 1.57(1.38–1.79)* | 1.57(1.38–1.78)* | 1.63(1.46–1.82)* | 1.44(1.30–1.60)* |
| S Africa | 1.93(1.49–2.50)* | 1.63(1.29–2.06)* | 1.51(1.19–1.92)† | 1.43(1.11–1.84)‡ | 1.71(1.38–2.11)* | 1.52(1.25–1.85)* | 1.89(1.52–2.34)* | 1.70(1.38–2.09)* |
| Middle East | 1.44(1.25–1.66)* | 1.49(1.30–1.70)* | 1.51(1.28–1.79)* | 1.52(1.29–1.78)* | 1.35(1.22–1.50)* | 1.40(1.27–1.54)* | 1.86(1.64–2.11)* | 1.89(1.68–2.12)* |
| E Asia | 1.33(1.21–1.47)* | 1.32(1.20–1.46)* | 1.32(1.22–1.44)* | 1.21(1.12–1.30)* | 1.38(1.25–1.51)* | 1.25(1.14–1.37)* | 1.45(1.33–1.57)* | 1.32(1.23–1.43)* |
| S Asia | 1.27(1.18–1.36)* | 1.26(1.17–1.35)* | 1.30(1.21–1.39)* | 1.26(1.18–1.35)* | 1.53(1.44–1.62)* | 1.43(1.35–1.51)* | 1.57(1.47–1.67)* | 1.41(1.33–1.50)* |
| Australia | 1.76(1.39–2.22)* | 1.53(1.24–1.89)* | 1.19(0.95–1.50) | 1.11(0.87–1.40) | 1.49(1.20–1.85)† | 1.50(1.22–1.83)† | 1.45(1.20–1.75)† | 1.54(1.28–1.86)* |
| Canada | 1.26(1.08–1.47)‡ | 1.19(1.03–1.39)§ | 1.50(1.25–1.81)* | 1.49(1.25–1.77)* | 1.92(1.65–2.23)* | 1.81(1.57–2.09)* | 2.40(2.06–2.80)* | 2.02(1.76–2.32)* |
| Latin America | 1.28(1.19–1.38)* | 1.23(1.15–1.32)* | 1.36(1.28–1.44)* | 1.37(1.29–1.44)* | 1.36(1.27–1.46)* | 1.34(1.26–1.43)* | 1.66(1.57–1.75)* | 1.51(1.44–1.59)* |
| Overall | 1.36(1.33–1.39)* | 1.32(1.29–1.35)* | 1.40(1.37–1.43)* | 1.38(1.35–1.41)* | 1.59(1.56–1.63)* | 1.52(1.49–1.56)* | 1.83(1.79––1.87)* | 1.67(1.64–1.71)* |
P<0.0001;
P<0.001;
P<0.01.
P<0.05
BMI indicates body mass index; CI, confidence interval; CVD, cardiovascular disease; OR, odds ratio; SD, standard deviation; WC, waist circumference.
Data are age-adjusted ORs (95% CI) for CVD and for diabetes for an increase of 1 SD (region-specific) in WC or BMI (see Table 1 for SDs by region and overall). Overall data are also adjusted for region.
The frequency of CVD, adjusted for age, region, and smoking status, increased with WC in each BMI category and with BMI in each WC tertile (all p<0.01) (Figures 5a and b). Importantly, CVD was significantly associated with WC even in “lean” individuals (BMI <25 kg/m2), with standardized ORs 1.10(1.05–1.15) in men and 1.15(1.11–1.19) in women, corresponding to a 9.3 cm increase in WC in both men and women (Table 3).
Figure 5.
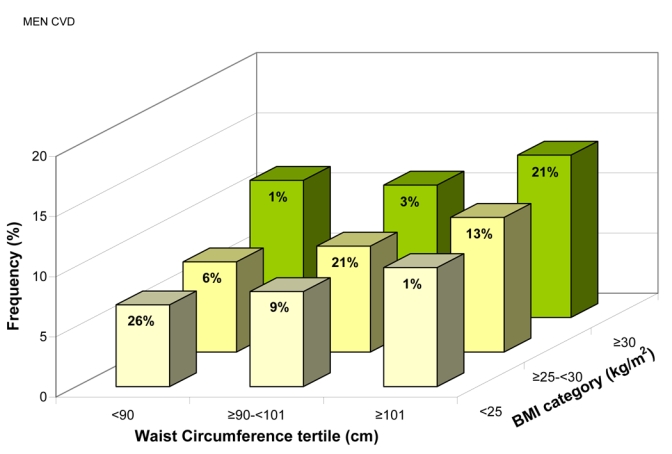
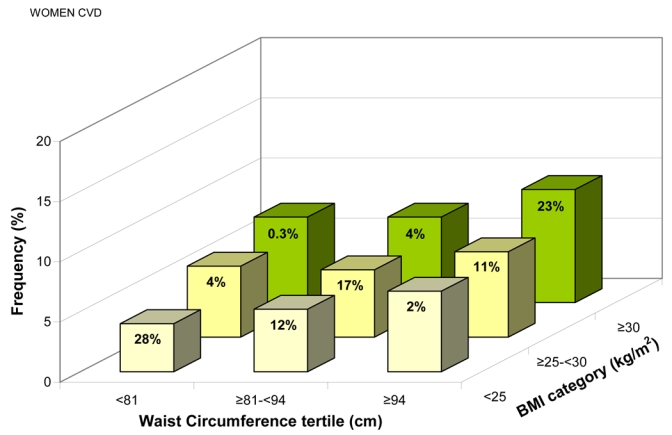
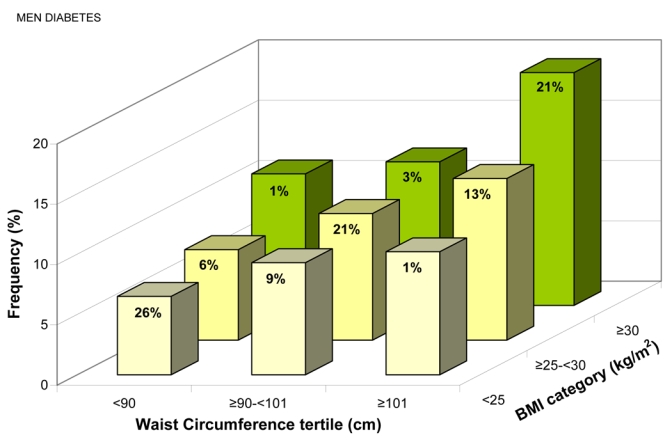
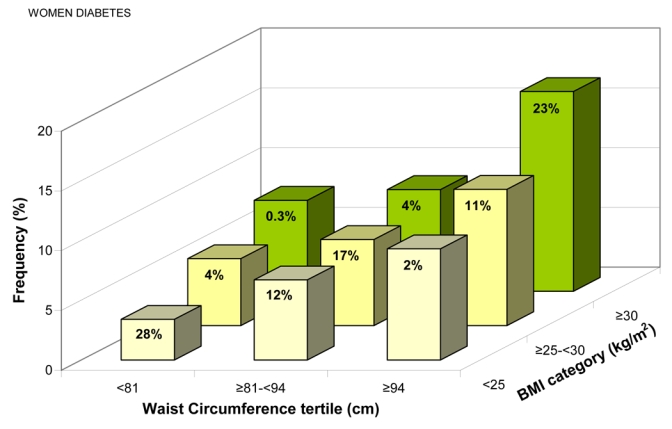
Frequency of Known Cardiovascular Disease for (a) Men, (b) Women, and Known Diabetes for (c) Men, (d) Women, Adjusted for Age, Region, and Smoking Status, by Gender-specific Waist Circumference (WC) Tertiles and Body Mass Index (BMI) Categories. The percentage of patients in each of the nine groups is shown.
TABLE 3.
Age- and Region-adjusted, Standardized ORs for CVD and Diabetes in Men and Women Associated with a 1 SD Increase in WC by BMI categories
| Men
|
Women
|
||||||
|---|---|---|---|---|---|---|---|
| BMI Category | 1 SD WC (cm) | OR CVD | OR Diabetes | 1 SD WC (cm) | OR CVD | OR Diabetes | |
| BMI < 25 kg/m2 | 9.3 | 1.10(1.05–1.15)* | 1.27(1.21–1.33)* | 9.3 | 1.15(1.11–1.19)* | 1.43 (1.37–1.48)* | |
| BMI 25–30 kg/m2 | 8.5 | 1.19(1.15–1.23)* | 1.23(1.18–1.27)* | 9.2 | 1.12(1.08–1.16)* | 1.32(1.27–1.37)* | |
| BMI ≥ 30 kg/m2 | 11.4 | 1.23 (1.18–1.28)* | 1.34(1.29–1.39)* | 11.9 | 1.24(1.20–1.29)* | 1.43 (1.38–1.47)* | |
| Overall | 14.0 | 1.36(1.33–1.39)* | 1.59(1.56–1.63)* | 14.9 | 1.40(1.37–1.43)* | 1.83 (1.79–1.87)* | |
P<0.0001
BMI indicates body mass index; CI, confidence interval; CVD, cardiovascular disease; OR, odds ratio; SD, standard deviation; WC, waist circumference. Data are age- and region-adjusted ORs (95% CI) for CVD and for diabetes for an increase of 1 SD (gender-specific) in WC.
Relationship between WC, BMI and Diabetes
The standardized ORs for diabetes (Table 2) were higher for WC than for BMI in almost all regions, with overall ORs of 1.59(1.56–1.63) vs 1.52(1.49–1.56) in men and 1.83(1.79–1.87) vs 1.67(1.64–1.71) in women. The exceptions were the Middle East and Australia, where the ORs for diabetes were consistently higher for BMI than for WC in both genders. In North African men with diabetes, the ORs for WC and BMI were equivalent. After adjustment (WC for BMI and BMI for WC), WC had a higher OR for diabetes than BMI in the overall patient population (men: 1.35[1.30–1.40] vs 1.23[1.19–1.27]; women: 1.55[1.50–1.60] vs 1.23[1.19–1.27]), thus both WC and BMI were independently associated with diabetes. Furthermore, both WC and BMI were more strongly associated with diabetes than with CVD, particularly in women (Table 2).
Diabetes frequency, adjusted for age, region and smoking status increased with WC tertiles within each BMI category, and with BMI categories within each WC tertile (all p < 0.01) (Figures 5c and d). Diabetes was significantly associated with WC even in “lean” individuals (BMI <25 kg/m2): for a 9.3 cm increase in WC, the standardized ORs were 1.27(1.21–1.33) in men, 1.43(1.37–1.48) in women (Table 3).
Discussion
IDEA is the largest study to assess the frequency of adiposity in primary care patients: nearly 170,000 ambulant patients were studied from 63 countries across 5 continents, providing a snapshot of patients worldwide. The study results show that excess body weight is pandemic (apart from in East and South Asia), with one-half to two-thirds of the population being overweight or obese by current definitions. Abdominal obesity, measured by the WC, showed a graded relationship with both CVD and diabetes at all levels of BMI, importantly even in so called “lean” subjects. These relationships were consistent across regions for both men and women, despite the up to threefold difference in background frequency of CVD and diabetes between regions. Our findings support the need for worldwide population-based initiatives to target adiposity. If improvements are not forthcoming, recent favorable trends in cardiovascular morbidity and mortality are likely to be reversed.
Comparisons with Other Studies
In this study, the ranking of regional frequencies of both CVD and diabetes from patients in primary care are consistent with other published data. In Eastern Europe, CVD mortality rates are very high31 for both men and women, and the frequency of diabetes in the Middle East and North Africa is known to be high and increasing.32 Patterns of adiposity contribute to these geographic variations in disease, but other genetic, environmental and behavioral characteristics are also clearly important.
The findings of IDEA, associating WC with diabetes and with all stages of CVD, extend those from the INTERHEART study which involved only myocardial infarction.15,16 It is likely that early intervention to target adiposity would have the greatest impact on lifetime manifestations of diabetes and atherosclerosis. The greater impact of WC compared to BMI on diabetes is consistent with prospective studies in American men (the Health Professionals’ Follow-up Study22) and women (the Nurses Health Study19). These studies also showed that WC was a better predictor of diabetes than the waist:hip ratio. These epidemiologic data are supported by animal and human reports of differences in subcutaneous and visceral fat phenotype, with visceral fat associated with inflammation, unfavorable adipokine profiles, and disturbances of insulin and glucose homeostasis.33,34
The mechanisms by which abdominal adiposity may lead to clinical disease are not clarified by the findings of the IDEA study. Two new, large-scale multinational studies have been initiated (INSPIRE ME and INSPIRE ME IAA) that will involve detailed profiling of risk factors, inflammatory biomarkers, as well as computed tomography (CT) to quantify visceral fat. Previous small-scale studies using CT have shown very strong associations between intra-abdominal fat and the development of CVD and diabetes in Japanese-Americans who are not obese, but at high risk of developing diabetes.21,35
Strengths and Limitations of this Study
The IDEA Study was very large and covered most regions of the world. Particular care was taken with the random selection of PCPs to ensure that the physicians, and consequently their patients, were representative within each geographic area of each country. The study population included all patients consulting their PCPs, with only two exclusion criteria: age <18 or >80 years, and pregnancy. Patient participation was high, but in some countries the PCP participation was low - when contacted the PCPs either did not answer the call or refused to participate for different reasons (“Not interested”, “Not available on target days”, “No time to participate”). This may affect the reported frequencies and associations, but it is not possible to determine the extent and direction of any bias this might cause.
This study was conducted in primary care, as this was a feasible way of recruiting large numbers of individuals in each country - the response rate was 97%, even though patients were not remunerated. Results cannot be extrapolated to the general population of the countries, only to the population consulting PCPs.
An important strength of IDEA was the uniform documentation, translated into the various languages, and the physicians’ training to measure WC using a standardized approach.1 In this study, we chose to use WC as a measure of abdominal adiposity, because a close correlation between WC and the amount of intra-abdominal fat has been observed using CT.36 In addition, WC requires only a single measurement, making it easier, less time consuming and less subject to error than combining measurements of waist and hip for the waist:hip ratio. Intra-and inter-physician variability in WC measurement was not formally assessed. Despite possible measurement error, clear relationships were shown between WC and both CVD and diabetes. Furthermore, the relationship was stronger in most cases for WC than BMI, even though the latter should have less measurement error.
Physician-reported diagnosis is probably more reliable than patient-reported diagnosis.19 In our study no diagnostic procedure was carried out either for diabetes or CVD (defined as CHD, stroke or revascularization). The relationships were clear, despite the potentially different definitions used in clinical practice between regions for both conditions. The IDEA questionnaire did not distinguish between type 1 and type 2 diabetes. As the majority of patients with diabetes (85% to 95%) have type 2 diabetes,32 the observed relationships between diabetes and adiposity would be primarily driven by type 2 diabetes.
It was not possible to determine whether the relationships between WC and CVD or diabetes differed across ethnic groups within geographic regions, as race was not recorded in IDEA due to legal restrictions in some countries. Race within regions may have an impact on the risk of CVD and diabetes associated with WC, as suggested by INTERHEART,16 but race is difficult to document accurately.
IDEA showed that the frequencies of adiposity are high in all regions of the world, even in Asian populations that are usually considered to be lean. The regional frequencies of “overweight” were surprisingly similar, but there were regional differences in the frequency of “obesity”. The lower levels of marked adiposity in regions such as South East Asia are not necessarily reassuring, as the impact of adiposity — particularly abdominal adiposity — is rising, and may be more acute in certain populations. IDEA emphasizes the need for a global approach to adiposity from early in life. Adiposity in teenagers is rapidly increasing and is associated with adverse changes in arterial wall structure and function, the early stages of atherosclerosis.37 The Diabetes Prevention Program has shown the beneficial effects of weight reduction38 and objective measures of improvements in the arterial wall can be rapidly identified after lifestyle modifications, including diet and physical exercise, in the young.39
Conclusions
IDEA has already increased global awareness of abdominal adiposity among PCPs, but the importance of this risk factor remains inadequately recognized. Routine measurement of WC — a convenient and inexpensive measure in primary care — provides a clinical marker for risk of CVD and diabetes in all regions of the world, even in patients with “normal” weight. The rise in adiposity worldwide is likely to contribute to major increases in morbidity and mortality from diabetes and CVD unless adequately addressed by public health programs.
Acknowledgments
The IDEA Steering Committee wishes to express their gratitude to the physicians who participated in the study, to Nadia Rolland who managed the study, to Alain-Jean Richard who supervised the data analysis, and to Sandrine Brette who provided statistical support. Editorial assistance was provided through the Global Publication Group at sanofi-aventis.
Funding
IDEA was funded by a grant from sanofi-aventis.
Footnotes
Contributors
The IDEA Steering Committee supervised the study conduct and design, together with analysis and interpretation of data. Beverley Balkau and Steven Haffner had primary responsibility for writing the paper, and all other authors critically reviewed the manuscript for important intellectual content. Beverley Balkau also validated the statistical analysis.
IDEA Steering Committee
Members of the IDEA Steering Committee were: Beverley Balkau* (France); Jean-Pierre Després* (Canada); Steven Haffner* (USA); Phil Barter (Australia); Jean-Pierre Bassand (France); John E. Deanfield (UK); Keith A. A. Fox (UK); Christine Massien (France); Alain-Jean Richard (France); Sidney Smith (USA); Chee-Eng Tan (Singapore); Luc Van Gaal (Belgium); and Hans-Ulrich Wittchen (Germany). *Members of the Executive Steering Committee.
IDEA National Coordinators and Investigators
Argentina: J. Krauss; Australia: M. Nelson; Austria: E. Rebhandl; Belgium: G. De Backer; Brazil: A. Avezum; Bulgaria: S. Zaharieva; Canada: A. Sharma; Chile: S. Kunstmann; China: Y. Wu; Colombia: A. Ruiz; Czech Republic: V. Hainer; Denmark: O. L. Svendsen; Dominican Republic, Jamaica, and Trinidad & Tobago: A. Gonzalez Medina; Ecuador: M. Pasquel; Egypt: M. Ibrahim; Estonia: M. Vigimaa; Finland: M. Savolainen; France: P. Amouyel; Germany: H.-U. Wittchen; Greece: S. Raptis, D. Kremastinos; Guatemala: M. A. Rodas; Hong Kong: C.-P. Lau; Hungary: L. Halmy; India: A. Misra; Indonesia: S. Soegondo; Ireland: V. Maher; Israel: A. Porath; Italy: M. Carrruba; Korea: H-J. Yoo; Latvia: A. Kalveli, G. Bahs; Lithuania: V. Kasiulevicius; Malaysia: M. Z. Morad, R. Zambahari; Mexico: A. L. Esqueda; Morocco: C. Abdelkhirane; The Netherlands: F. L. J. Visseren; Norway: T. Pedersen; Pakistan: A. Jabbar; Peru: R. Gamboa; The Philippines: M. L. Abrahan; Poland: K. Narkiewicz; Portugal: V. Gil; Russia: R. G. Oganov; Saudi Arabia: M. Halawa; Singapore: E. S. Tai; Slovakia: A. Dukát; Slovenia: I. Švab; Republic of South Africa: M-T. van der Merwe; Spain: B. Moreno, F. Casanueva; Sweden: Å. Sjöholm; Switzerland: R. Darioli, A. Gallino, G. Noll; Taiwan: C. J. Chang; Thailand: P. Sritara; Tunisia: A. Belhani; Turkey: V. Sansoy; Ukraine: A. Parkhomenko; United Arab Emirates: Kuwait, and Qatar: A. Binbrek; Venezuela: A. Perez Monteverde.
Conflict of Interest Statement
B. Balkau, J.-P. Després, J.-P. Bassand, K. A. A. Fox, P. Barter, L. Van Gaal, H.-U. Wittchen, and S. M. Haffner are consultants to sanofi-aventis and have received honoraria for acting on the IDEA Steering Committee. C. E. Tan has received honoraria for acting on the IDEA Steering Committee. H-U. Wittchen has received an unrestricted educational grant from Sanofi-Synthelabo, Germany, to conduct an epidemiologic study on hypertension and diabetes in primary care. C. Massien is an employee of sanofi-aventis. J. E. Deanfield and S. C. Smith have no conflicts of interest.
Contributor Information
Beverley Balkau, Recherche en épidémiologie et biostatistique INSERM : U780, INSERM : IFR69, Université Paris Sud - Paris XI, 16, Avenue Paul Vaillant-Couturier 94807 VILLEJUIF CEDEX,FR.
John E. Deanfield, Great Ormond Street Hospital for Children Hospital for Chidren London, GB
Jean-Pierre Després, Laval Hospital Research Center Hospital Research Center Sainte-Foy, CA.
Jean-Pierre Bassan, Hôpital Jean-Minjoz Université Besançon, FR.
Keith A.A. Fox, Royal Infirmary Royal Infirmary of Edinburgh, GB
Sidney C. Smith, University of North Carolina North of Carolina, Chapel Hill, US
Philip Barter, The heart Research Institute The Heart Research Institute, AU.
Chee E. Tan, Centre for Molecular Epidemiology National University of Singapore, SG
Luc Van Gaal, Antwerp University Hospital Antwerp University Hospital, BE.
Hans-Ulrich Wittchen, Institut für Klinische Psychologie und Psychotherapie TU Dresden, DE.
Christine Massien, Sanofi-Aventis Sanofi-Aventis, FR.
Steven M. Haffner, University of Texas Health Science Center University of San Antonio, US
References
- 1.Seidell JC, Kahn HS, Williamson DF, Lissner L, Valdez R. Report from a Centers for Disease Control and Prevention Workshop on use of adult anthropometry for public health and primary health care. Am J Clin Nutr. 2001;73:123–126. doi: 10.1093/ajcn/73.1.123. [DOI] [PubMed] [Google Scholar]
- 2.Caterson ID, Hubbard V, Bray GA, Grunstein R, Hansen BC, Hong Y, Labarthe D, Seidell JC, Smith SC, Jr the American Heart Association. Prevention Conference VII: Obesity, a worldwide epidemic related to heart disease and stroke: Group III: worldwide comorbidities of obesity. Circulation. 2004;110:e476–e483. doi: 10.1161/01.CIR.0000140114.83145.59. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F the American Heart Association/National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Mokdad AH, Giles WH. Trends in waist circumference among U.S. adults. Obes Res. 2003;11:1223–1231. doi: 10.1038/oby.2003.168. [DOI] [PubMed] [Google Scholar]
- 6.York DA, Rossner S, Caterson I, Chen CM, James WP, Kumanyika S, Martorell R, Vorster HH the American Heart Association. Prevention Conference VII: Obesity, a worldwide epidemic related to heart disease and stroke: Group I: worldwide demographics of obesity. Circulation. 2004;110:e463–e470. doi: 10.1161/01.CIR.0000140125.26161.49. [DOI] [PubMed] [Google Scholar]
- 7.Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. BMJ. 1984;289:1257–1261. doi: 10.1136/bmj.289.6454.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsson B, Svardsudd K, Welin L, Wilhelmsen L, Bjorntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. BMJ. 1984;288:1401–1404. doi: 10.1136/bmj.288.6428.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welin L, Svardsudd K, Wilhelmsen L, Larsson B, Tibblin G. Analysis of risk factors for stroke in a cohort of men born in 1913. N Engl J Med. 1987;317:521–526. doi: 10.1056/NEJM198708273170901. [DOI] [PubMed] [Google Scholar]
- 10.Després JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990;10:497–511. doi: 10.1161/01.atv.10.4.497. [DOI] [PubMed] [Google Scholar]
- 11.Kannel WB, Cupples LA, Ramaswami R, Stokes J, III, Kreger BE, Higgins M. Regional obesity and risk of cardiovascular disease: the Framingham Study. J Clin Epidemiol. 1991;44:183–190. doi: 10.1016/0895-4356(91)90265-b. [DOI] [PubMed] [Google Scholar]
- 12.Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Willett WC, Manson JE. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 13.Rexrode KM, Buring JE, Manson JE. Abdominal and total adiposity and risk of coronary heart disease in men. Int J Obes Relat Metab Disord. 2001;25:1047–1056. doi: 10.1038/sj.ijo.0801615. [DOI] [PubMed] [Google Scholar]
- 14.Dagenais GR, Yi Q, Mann JF, Bosch J, Pogue J, Yusuf S. Prognostic impact of body weight and abdominal obesity in women and men with cardiovascular disease. Am Heart J. 2005;149:54–60. doi: 10.1016/j.ahj.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P, Jr, Razak F, Sharma AM, Anand SS the INTERHEART Study Investigators. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a casecontrol study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 16.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L the INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 17.Ohlson LO, Larsson B, Svardsudd K, Welin L, Eriksson H, Wilhelmsen L, Bjorntorp P, Tibblin G. The influence of body fat distribution on the incidence of diabetes mellitus: 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34:1055–1058. doi: 10.2337/diab.34.10.1055. [DOI] [PubMed] [Google Scholar]
- 18.Kissebah AH, Peiris AN. Biology of regional body fat distribution: relationship to non-insulin-dependent diabetes mellitus. Diabetes Metab Rev. 1989;5:83–109. doi: 10.1002/dmr.5610050202. [DOI] [PubMed] [Google Scholar]
- 19.Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, Speizer FE, Manson JE. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol. 1997;145:614–619. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- 20.Wei M, Gaskill SP, Haffner SM, Stern MP. Waist circumference as the best predictor of noninsulin dependent diabetes mellitus (NIDDM) compared to body mass index, waist/hip ratio and other anthropometric measurements in Mexican Americans: a 7-year prospective study. Obes Res. 1997;5:16–23. doi: 10.1002/j.1550-8528.1997.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 21.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care. 2000;23:465–471. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81:555–563. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 23.Burke JP, Williams K, Gaskill SP, Hazuda HP, Haffner SM, Stern MP. Rapid rise in the incidence of type 2 diabetes from 1987 to 1996: results from the San Antonio Heart Study. Arch Intern Med. 1999;159:1450–1456. doi: 10.1001/archinte.159.13.1450. [DOI] [PubMed] [Google Scholar]
- 24.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 25.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 26.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 27.Jee SH, Sull JW, Park J, Lee SY, Ohrr H, Guallar E, Samet JM. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 28.Wittchen H-U, Balkau B, Massien C, Richard A-J, Haffner SM, Després J-P on behalf of the IDEA Steering Committee. International Day for the Evaluation of Abdominal obesity: rationale and design of a primary care study on the prevalence of abdominal obesity and associated factors in 63 countries. Eur Heart J Suppl. 2006;8(suppl B):B26–B33. [Google Scholar]
- 29.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 30.Alberti KG, Zimmet P, Shaw J for the IDF Epidemiology Task Force Consensus Group. The metabolic syndrome — a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 31.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 32.King H, Aubert RA, Herman WH. Global burden of diabetes, 1995–2005. Prevalence, numerical estimates, projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 33.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 34.Boucher J, Castan-Laurell I, Daviaud D, Guigne C, Buleon M, Carpene C, Saulnier-Blache JS, Valet P. Adipokine expression profile in adipocytes of different mouse models of obesity. Horm Metab Res. 2005;37:761–767. doi: 10.1055/s-2005-921098. [DOI] [PubMed] [Google Scholar]
- 35.Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, Shofer JB, Wahl PW. Visceral adiposity and incident coronary heart disease in Japanese–American men. The 10-year follow-up results of the Seattle Japanese–American Community Diabetes Study. Diabetes Care. 1999;22:1808–1812. doi: 10.2337/diacare.22.11.1808. [DOI] [PubMed] [Google Scholar]
- 36.Pouliot MC, Després JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 37.Whincup PH, Gilg JA, Donald AE, Katterhorn M, Oliver C, Cook DG, Deanfield JE. Arterial distensibility in adolescents: the influence of adiposity, the metabolic syndrome, and classic risk factors. Circulation. 2005;112:1789–1797. doi: 10.1161/CIRCULATIONAHA.104.532663. [DOI] [PubMed] [Google Scholar]
- 38.Wing RR, Hamman RF, Bray GA, Delahanty L, Edelstein SL, Hill JO, Horton ES, Hoskin MA, Kriska A, Lachin J, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner JG, Venditti B, Wylie-Rosett J for the Diabetes Prevention Program Research Group. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12:1426–1434. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watts K, Beye P, Siafarikas A, Davis EA, Jones TW, O’Driscoll G, Green DJ. Exercise training normalizes vascular dysfunction and improves central adiposity in obese adolescents. J Am Coll Cardiol. 2004;43:1823–1827. doi: 10.1016/j.jacc.2004.01.032. [DOI] [PubMed] [Google Scholar]


