Abstract
Background
For many years chemical preservatives have been used in food, to act as either antimicrobials or antioxidants or both. In general, consumers regard additive-free foods as safer since preservatives can cause health hazards like asthma and cancer and are suspected to be mutagenic and neurotoxic. The present study was carried out to evaluate the antimicrobial and antioxidant activity of methanolic extracts of seaweeds, with a view to developing safer food preservatives.
Methods
Ten edible seaweeds, which have wide pharmaceutical application, were collected from Central Marine Fisheries Research Institute, Tamil Nadu, India and evaluated for antioxidant and antimicrobial activity against food borne pathogens.
Results
The results indicate that Gelidiella acerosa has the highest antioxidant activity while Haligra sps exhibited antibacterial activity against Staphylococcus aureus (MTCC 96).
Conclusion
Quantitative analysis of the total phenolic content of the seaweeds indicated that Gelidella acerosa and Haligra sps have high phenolic contents, which correlated to their respective antioxidant and antimicrobial activity
Background
Refrigerated, ready-to-eat products, especially dairy foods, have become increasingly popular in recent years because of their convenience. Many pathogenic organisms spoil such foods, reducing their shelf life and often leading to food poisoning. It has been estimated that as many as 30% people in industrialized countries suffer from a food poisoning every year [1,2]. In addition to microbial contamination, all packed and refrigerated food also undergoes gradual changes during storage, due to auto oxidation which releases reactive oxygen species (ROS) including free radicals like superoxide anion (O2•-) and hydroxyl radicals (OH•) and non-free radical species like singlet oxygen (1O2) and hydrogen peroxide (H2O2) [3,4] into the food. These ROS induce peroxidation of lipids (polyunsaturated fatty acids) generating secondary oxidants like heptanol and hexanal [5], which contributes to oxidative rancidity, deteriorating the flavor of the food [6]. These not only cause a loss in food quality but are also believed to be associated with carcinogenesis, mutagenesis, arthritis, diabetes, inflammation, cancer and genotoxicity [7-9]. To overcome these problems a wide range of synthetic antimicrobial agents (sodium benzoate, calcium benzoate, sorbate) and synthetic antioxidants (butylhydroquinone, propyl gallate, butylated hydroxy toluene (BHT), butylated hydroxy anisole (BHA) [10], have been used as food preservatives. However, these preservatives can cause liver damage and are suspected to be mutagenic and neurotoxic. Hence, most consumers prefer additive-free foods [11,12] or a safer approach like the utilization of more effective antioxidants and antimicrobials of natural origin [8,13]. Recently, various phytochemicals like polyphenols, which are widely distributed in plants, have been reported to act as free radical scavengers and antimicrobial agents [14,15]. Marine plants, like seaweeds, also contain high amounts of polyphenols. For example, high concentrations of polyphenols such as catechin, epicatechin, epigalloctechin gallate and gallic acid are reported in the seaweed Halimada (Chlorophyceae) [16]. Since many types of seaweed have still to be investigated, we were prompted to take up this study. The Gulf of Mannar is a Marine Biosphere Reserve situated along the east coast of India and Sri Lanka, an area of about 10,500 sq. km which has a luxuriant growth of about 680 species of seaweed belonging to the Rhodophyta, Pheaophyta and Chlorophyta, in both the inter-tidal and deep water regions. Seaweed constitutes a commercially important marine renewable resource. Sargassum, Padina, Dictyota and Gracilaria sps. Are used by common people as fertilizers, food additives and animal feed [17]. The sulphated polysaccharides of Sargassum act as a potent free radical scavenger and anticancer agent [18,19]. Gelidella and Gracilaria sps are widely used for the production of agar and for the treatment of gastrointestinal disorders [20]. The methanolic extract of brown seaweeds such as Ecklonia cava [21] and Hizikia fusiformis [22] exhibit potent antioxidant activity. Although seaweeds possess wide application in food and in the pharmaceutical industry, the antioxidant and antimicrobial activity of many types of seaweed in the South Indian coastal area are still unexplored. The main objective of the present study is to evaluate the antioxidant and antimicrobial activities of seaweeds obtained from the Thondi, South Coastal Area of Tamil Nadu, India.
Methods
Chemicals used
Nicotiamide adenine dinucleotide (NADH), thiobarbituric acid (TBA), nitroblue tetrazolium (NBT), DPPH (1, 1-diphenyl,2-picrylhydrazyl), 2,4,6-tripyridyl-S-triazine (TPTZ) were purchased from Sigma (Sigma-Aldrich GmbH, Sternheim, Germany). All other chemicals and solvents used were of the highest purity grade commercially available.
Preparation of the seaweed extracts
Seaweeds were collected from Central Marine Fisheries Research Institute, Thondi, Tamil Nadu, and were identified by Dr. Nivedita Sahu, CSMCRI, India. The seaweeds used for study were Gelidiella acerosa (Rhodophyta), Gracilaria edulis (Rhodophyta), Turbinaria conoides (Phaeophyta), Padina gymnospora (Phaeophyta), Chondrococcus hornemanni (Rhodophyta), Hypnea pannosa (Rhodophyta), Dictyota dichotoma (Phaeophyta), Jania rubens (Rhodophyta), Sargassum wightii (Phaeophyta) and Haligra sps. The seaweeds were washed with water and alcohol, cut into small pieces and air-dried. One gm of dried sample was suspended in 10 ml of methanol for 72 h. The solution was filtered and evaporated to dryness under reduced pressure in a desiccator (Tarson Products Pvt Ltd, India). The dried powder was dissolved in distilled water containing less than 0.2% of methanol or Tween -20 (as solvents) and stored at -20°C until use [23].
Antioxidant Activity
Free radical scavenging assay
The free radical scavenging activity of seaweed extracts was measured by DPPH according to Blois method [24]. 1 ml of DPPH.(0.1 mM) solution in methanol was added to 3 ml of methanolic extract of seaweed (100 μg/ml) in water, shaken vigorously, allowed to stand at RT for 30 min and the absorbance was measured at 517 nm in a UV- visible spectrophotometer (U-2800 model, Hitachi, Japan). A low absorbance of the reaction mixture indicated a high free radical scavenging activity. BHT (20 – 100 μg/ml) was used as positive control. The percent DPPH. scavenging effect was calculated as follows
where Acont was the absorbance of the control reaction and Atest was the absorbance in the presence of the sample seaweeds.
Hydroxyl Radical Scavenging Activity
The ability of seaweeds to scavenge OH. was assessed using the classic deoxyribose degradation assay as described by Halliwell et al [25]. Briefly, 2.0 ml of the assay mixture containing EDTA (1 mM), FeCl3(10 mM), H2O2 (10 mM), deoxyribose (10 mM) and seaweed extract (100 μg/ml) was dissolved in distilled water with ascorbic acid (1 mM) in 50 mM phosphate The mixture was incubated at 37°C for 1 h and 1.0 ml of the incubated mixture was mixed with 1 ml of 10% TCA and 1 ml of 0.4% TBA (in glacial acetic acid, pH adjusted by NaOH) to develop the pink chromagen measured at 532 nm. BHT (20 to 100 μg/ml) was used as positive control. The hydroxyl radical scavenging activity of the extract is reported as % inhibition of deoxyribose degradation and was calculated as above.
Scavenging of Hydrogen Peroxide (H2O2)
The ability of the seaweeds to scavenge H2O2 was determined according to the method of Ruch et al., [26] with the slight modification of Gulcin et al., [27]. Briefly, 40 mM H2O2 was prepared in phosphate buffer (pH 7.4) and the H2O2concentration was determined spectrophotometrically by measuring the absorption with the extinction coefficient for H2O2 of 81 M-1cm-1. Extracts (100 μg/ml) in distilled water and ascorbic acid (20 – 100 μg/ml, positive control) were added to 0.6 ml of 40 mM H2O2 solution and the absorbance of H2O2 was determined at 230 nm after 10 min incubation against a blank solution containing phosphate buffer without H2O2. The percentage of scavenging of H2O2 was calculated as above
Nitric Oxide Radical (NO.) Scavenging Activity
NO. generated from sodium nitroprusside in aqueous solution at physiological pH interacts with oxygen to produce nitrite ions, which were measured by the Griess reaction [28]. Briefly, 3 ml of the reaction mixture containing 10 mM sodium nitroprusside and the seaweed extract (100 μg/ml) in phosphate buffer were incubated at 25°C for 150 min. After incubation, 0.5 ml of the reaction mixture was mixed with 1 ml of sulfanilic acid reagent (0.33% in 20% glacial acetic acid) and allowed to stand for 5 min for complete diazotization. Then 1 ml of naphthyl ethylene diamine dihydrochloride (0.1%) was added and the solution mixed and allowed to stand for 30 min at 25°C. A pink colored chromophore is formed in diffused light. The absorbance of these solutions was measured at 540 nm against the corresponding blank solutions. BHT (50 – 250 μg/ml) was used as positive control. The NO. scavenging activity of the seaweeds extract is reported as % inhibition and was calculated as above.
Total Reducing Power
Total reducing capacity of seaweeds was determined according to the method of Oyaizu [29]. The seaweed extract (100 μg/ml) in distilled water and 1% potassium ferricyanide were mixed with phosphate buffer (0.2 M, pH 6.6) and the mixture was incubated at 50°C for 20 min. 2.5 ml of 10% TCA was added to the reaction mixture which was centrifuged at 1000×g for 10 min. The upper layer of solution (2.5 ml) was mixed with distilled water (2.5 ml), FeCl3 (0.5 ml, 0.1%) and the absorbance was measure at 700 nm. Ascorbic acid (20 – 100 μg/ml) was used as positive control. The higher the absorbance of the reaction mixture the greater is the reducing power.
FRAP (Ferric reducing ability plasma) assay
The FRAP assay was performed according to the method of Benzie et al [30]. It depends on the ability of the sample to reduce the ferric tripyridyltriazine (Fe (III)-TPTZ) complex to ferrous tripyridyltriazine (Fe (II)-TPTZ) at low pH. Fe (II)-TPTZ has an intensive blue color which can be read at 593 nm. 1.5 ml of freshly prepared FRAP reagent (25 ml of 300 mM/L of acetate buffer pH 3.6, 2.5 ml of 10 mM/L 2,4,6 tripyridyl S triazine (TPTZ) in 40 mM/L of HCl, 20 mM/L of ferric chloride solution) were mixed with 50 μl of seaweed extract (100 μg/ml) in 150 μl of distilled water. The absorbance was monitored for 4 min (every 10 sec) at 593 nm. ΔA is proportional to the combined ferric reducing/antioxidant power (FRAP value) of the antioxidants in the sample. The results are expressed as mMol of FRAP/L and were estimated using aqueous FeSO4 .7H2O (200 – 1000 mM) as standard for calibration. The relative activity of the sample was compared with standard ascorbic acid (2–10 μg/ml).
Lipid peroxidation Assay
The lipid peroxidation level is measured as the thiobarbituric acid reactive substance (TBARS), based on the method of Yagi et al [31], with the limitation that the TBARS assay involves interference. Erythrocytes were hemolysed with an equal volume of ice-cold milliQ to yield 50% hemolysate, which was diluted to 1:20 with phosphate buffer containing 0.05 M dithioethreitol. The final volume of the reaction mixture was 1 ml, which consisted of 500 μl of the hemolysate, 300 μl of the buffer containing the plant extract (100 μg/ml) and 100 μl of 10 mM H2O2 to start the peroxidation. Samples were incubated at 37°C for 1 h, after which lipid peroxidation was measured using the reaction with thiobarbituric acid (TBA) to form thiobarbituric acid reactive substance (TBARS) a pink color chromogen read at 532 nm. BHT (20 – 100 μg/ml) was used as positive control. Lipid peroxidation is expressed as nM of malodialdehyde (nM)/gm of Hb, extrapolated from a standard curve prepared using known amounts of MDA.
Determination of Total phenolics
The total soluble phenolic compounds in the seaweeds were determined with the Folin-Ciocalteu reagent according to the method of Singleton et al [32] using gallic acid as standard. 100 μl of the sample (1 gm of dry sample in 10 ml of acetone) in duplicate was incubated with 1 ml of diluted Folin-Ciocalteu reagent (1:2 with water) at RT for 5 min. 1 ml of 7% Na2CO3 was added to the reaction mixture which was incubated at RT for 90 min. and the absorbance was read at 750 nm. The total phenolic content is expressed as gallic acid equivalent (GAE) in milligrams per gram of dry sample.
Antimicrobial Assay
Bacterial strains
The microorganisms (food borne pathogens) used for assessing the antimicrobial activity of seaweeds were Staphylococcusaureus (MTCC 96), Bacillus cereus (MTCC 1272), Vibrio vulnificans (MTCC 1145), Salmonella typhi (MTCC 733), Listeria monocytogens (MTCC 657), Enterococcus faecalis (MTCC 2729) and E.coli 0517; H7 (ATCC 43895),
Preparation of seaweed extract
Seaweed extract was filtered through a series of sterile, ethanol resistant cellulose acetate filters (0.2 μm, Advantec, Toyo Rashi Kaisha Ltd, Japan). The sterilized extract was stored in pre sterilized glass vials at -80°C until use.
Preparation of food samples
Ten grams of skimmed milk powder was mixed with 90 ml of distilled water and autoclaved at 121°C for 15 min prior to use to eliminate the contamination from organisms that may already be present in the food. The pH of the food samples ranged between 6 and 7.
Bacterial media
Brain Heart Infusion Agar (BHA), Brain Heart Infusion Broth (BHIB), Muller-Hinton Agar (MHA) and Muller-Hinton Broth (MHB), Nutrient Agar and Nutrient Broth were supplied by Hi media laboratories, India. Sodium benzoate was obtained from SRL chemicals Ltd, India. All media were prepared in deionised water and autoclaved at 121°C for 15 min prior to use.
Antibacterial Screening
The antibacterial activity of the seaweed extract was screened by the Disc diffusion method. All tests were performed in triplicate with different concentration of seaweed extracts. Sodium benzoate was used as the standard antimicrobial agent.
Agar – Disc Diffusion Method
Agar cultures of the test microorganisms were prepared as described by Mackeen et al [33]. Broth cultures were incubated overnight at 37°C. The concentration of the cultures was standardized by matching to the McFarland 0.5 turbidity standard using sterile saline to produce approximately 1.5 × 108 colony forming units (cfu) per ml. A suspension of the tested microorganisms was swabbed on the Muller Hinton agar medium. For screening, sterile 6 mm diameter filter paper disc were impregnated with different concentrations of plant extract (200 μg – 1 mg/ml). Discs were placed on MHA agar plates. Methanol or Tween-20 was used as negative control and sodium benzoate (200 μg) was used as positive control. Plates were incubated at 37°C for 24 h. Results were recorded by measuring the zones of growth inhibition surrounding the disc. Clear inhibition zones around the discs indicated the presence of antimicrobial activity. All data on antimicrobial activity are the average of triplicate analyses.
Determination of minimum bactericidal concentration (MBC)
The test substance was dispersed into an appropriate medium (MHB, reconstituted skimmed milk powder) and serial, two fold dilutions were made in the same medium to produce dilutions ranging from 1:10 to 1:640. 10 μl of standardized ON culture was added to each dilution. Following overnight incubation at 37°C, 50 μl from each dilution was spread on BHIA plates which were incubated overnight at 37°C. The procedure was performed in reconstituted skimmed milk powder. The minimum bactericidal concentration (MBC) was defined as the lowest concentration of plant extract that completely prevented microbial growth and was determined by visible inspection of the BHIA plates. MBC assays were carried out in duplicate.
Statistical Analysis
All experiments were conducted in triplicate (n = 3) and one-way ANOVA (using SPSS 11.5 statistical software) was used to compare the mean values of each treatment. Significant differences between the means of parameters were determined by using the Duncan test (P < 0.05).
Results
Yield of seaweed Extracts
The % yield of the methanolic extract of G. acerosa, G. edulis, T. conoides, P. gymnospora, C. hornemanni, H. pannosa, D. dichotoma, J. rubens, S. wightii and Haligra sps were 4.5%, 1.5%, 5%, 3.5%, 1%, 4%, 11%, 2%, 5% and 5% respectively
DPPH Radical Scavenging activity
The free radical scavenging activity of alcoholic extract of seaweed was assessed by the DPPH assay. Figure 1 illustrates a significant (P < 0.05) decrease in the concentration of DPPH radical due to scavenging ability of the seaweeds and the standard BHT. The results show that G. acerosa had the highest DPPH• scavenging activity (72.5 ± 2.78%) among the seaweeds, significantly (P < 0.05) higher than that of the standard BHT (70 ± 2%). This indicates that G. acerosa as a good source of natural antioxidants.
Figure 1.
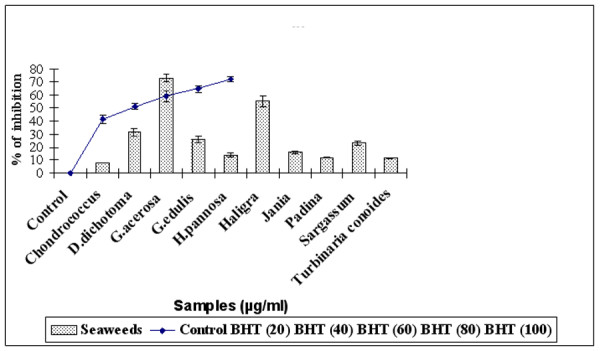
Comparison of free radical scavenging activity of methanolic extract of seaweeds (100 μg/ml) with standard BHT (20–100 μg/ml). Results are mean ± SD (n = 3).
Lipid peroxidation
The seaweeds not only exhibited excellent radical scavenging activity but also were potent in suppressing TBARS formation by H2O2 induced lipid peroxidation in RBC. Figure 2 show that G. acerosa (100 μg/ml) exhibited significant (P < 0.05) inhibition of 65.4 ± 0.9% of H2O2 induced lipid peroxidation when compared with other seaweeds. However it exhibited similar inhibition when compared with same dosage of BHT (65% ± 0.9%).
Figure 2.
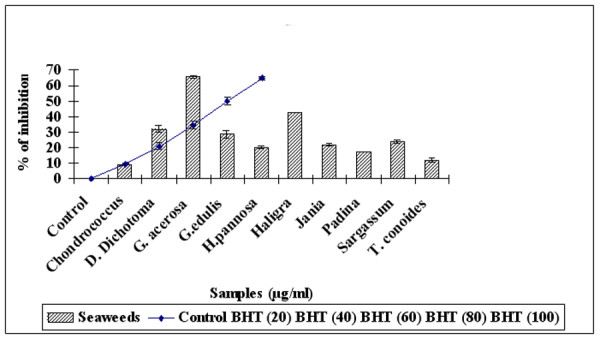
Lipid peroxidation of methanolic fraction of seaweeds (100 μg/ml) in comparison with standard BHT (20 – 100 μg/ml). Results are mean ± SD (n = 3).
Hydroxyl Radical scavenging activity
The scavenging effect of OH• was investigated using the Fenton reaction and the results areshown as an inhibition rate in Figure 3. G. acerosa exhibited the highest inhibition of about 70 ± 4.27% of the seaweeds but thisis lower than the standard BHT (100 μg/ml) whose % of inhibition is 89.15 ± 0.007% with a significance of P < 0.01.
Figure 3.
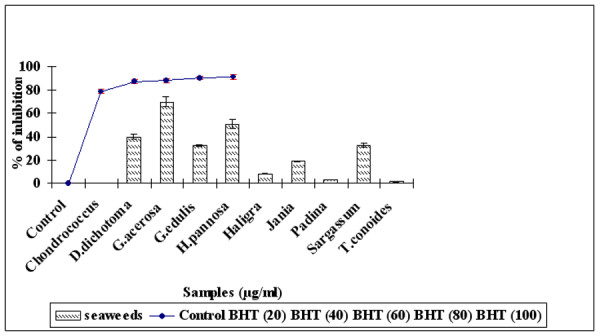
Hydroxyl radical scavenging activity of Seaweeds (100 μg/ml) against positive control BHT (20–100 μg/ml). Results are expressed as mean ± SD (n = 3).
Nitric Oxide Radical scavenging Activity
Suppression of NO• release may be attributed to a direct NO• scavenging effect as all the seaweed extracts decreased the amount of nitrite generated from the decomposition of sodium nitroprusside in vitro as shown in Figure 4. The results show that Haligra sps and G. acerosa (100 μg/ml) had scavenging activity of 39.8 ± 3.52 and 33.3 ± 1.7% respectively, similar to the standard BHT (33 ± 2.07%) with no significant differences.
Figure 4.
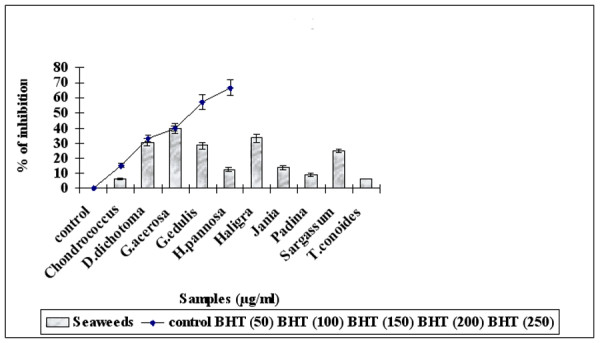
Scavenging effect of Seaweeds extract (100 μg/ml) and Standard BHT (50–250 μg/ml) on Nitric Oxide radical.
Scavenging of Hydrogen Peroxide
The ability of seaweeds to scavenge H2O2 was determined according to the method of Ruch and Gulcin et al [26,27]. Figure 5 indicates that G. acerosa (100 μg/ml) exhibits a maximum H2O2scavenging activity of 61.9 ± 1.27% which is significantly (P < 0.05) higher than the standard L-ascorbic acid whose scavenging effect is 51.7 ± 1.7%.
Figure 5.
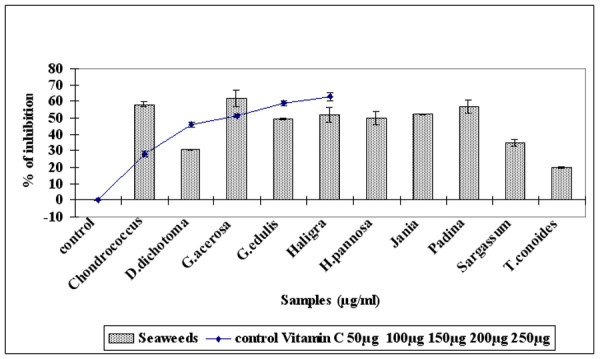
Comparison of Percent Scavenging of Hydrogen peroxide radical by different seaweed extract (100 μg/ml) with same dosage of L-Ascorbic acid a positive control. Results are expressed as mean ± SD (n = 3).
Reducing power
Figure 6 shows the reducing capacity of methanolic seaweed extract compared to standard BHT (50–250 μg/ml). G. acerosa (100 μg/ml) showed significantly (P < 0.01) higher reducing ability (absorbance 1.326 ± 0.042) when compared with the control (absorbance 0.402 ± 0.1) and the same dosage of the standard BHT (1.18 ± 0.043). Since the reducing capacity of a compound serves as a significant indicator of its potential antioxidant ability [34]G. acerosa can be considered as a potent source of natural antioxidants.
Figure 6.
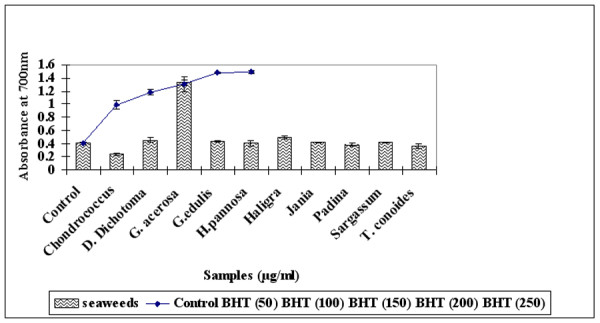
Reducing Ability of different seaweed extract and Standard BHT (50–250 μg/ml).
Antioxidative power as measured by the FRAP assay
The antioxidant power was measured by the FRAP method at λ = 593 nm which is based on comparison of the total amount of antioxidant with the reducing capacity of the samples. Figure 7 illustrates the total antioxidative power of the seaweed extracts (100 μg/ml) in comparison with vitamin C (2–10 μg/ml). FeSO4 was used as standard for calibration. The. FRAP value is expressed as mMol equivalent of Fe (II)/L. The results show that G. acerosa (100 μg/ml) exhibits significant (P < 0.05) ferric reducing capacity with an absorbance of 0.103 ± 0.005 which is equivalent to the absorbance of 800 mM/L of Fe (II) in FeSO4 in comparison with positive control vitamin C (10 μg/ml) with an absorbance of 0.112 ± 0.0005. When compared with the standard antioxidant L-ascorbic acid the antioxidative power was significantly less.
Figure 7.
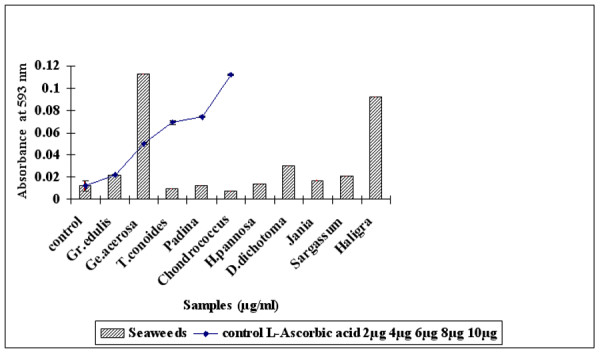
Total antioxidant power of methanolic extract of seaweeds(100 μg/ml) in comparison with L-Ascorbic acid (2–10 μg/ml) measured by FRAP assay.
Antimicrobial Activity
The antibacterial activities of ten seaweeds were assessed against seven food borne pathogens using a disc diffusion assay. Of the ten seaweeds screened Haligra sps (50 mg/ml) showed antibacterial activity against S. aureus (MTCC 96), a gram-positive bacteria, which causes food poisoning in humans. ZOI of Haligra sps was 16 ± 0.9 mm which is significantly (P < 0.05) higher than the standard antimicrobial agent sodium benzoate (200 mg/ml) whose ZOI was 14 ± 0.5 mm. The entire assay was carried out in triplicate.
The MBC assay performed in MHA and skimmed milk powder determined the lowest concentration of extract that produced a bactericidal effect. Table 1 shows the MBC result of Haligra sps. The highest MBC of Haligra sps extract against S. aureus was observed at a concentration of 7.5 mg/ml (1:20 dilution) in MHA and 5 mg/ml (1:40) in skimmed milk. Sodium benzoate, a standard antimicrobial agent, exhibited MBC at a concentration of 30 mg/ml (1:10) for both MHA and skimmed milk.
Table 1.
Determination of MBC of Haligra sps extract against Staphylococcus aureus (MTCC 96)
| Sample | MHA | Milk |
| Haligra | 1:20 | 1:40 |
| Sodium Benzoate | 1:10 | 1:10 |
Total Phenolic Compounds
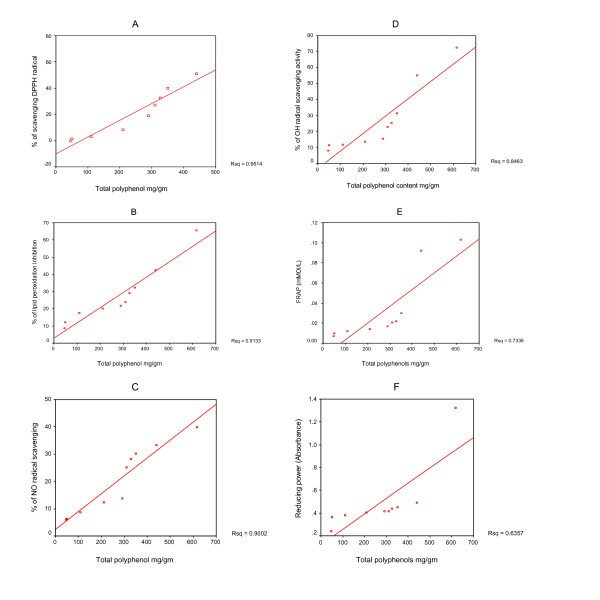
The total phenolic content of the seaweed extracts was measured spectrophotometrically by the Folin-Ciocalteau method and the results are expressed as gallic acid equivalents (GAE). Table 2 shows that the total phenolic content of the G. acerosa (0.616 ± 0.0063 g/g) and Haligra sps (0.440 ± 0.0043 g/g) extracts was significantly higher than the other seaweeds. The correlation between the total polyphenol contents and the results of the determination of the antioxidant capacity were used to compare the sensitivity of the relevant tests as shown in Fig. 8(A, B, C, D, E, F). From the result it is clear that the correlation between the total polyphenolic content and antioxidant capacity, as determined by antioxidant assays using the DPPH• scavenging assay, is the highest (R2 = 0.9514) and with reducing power is the lowest (R2 = 0.6357). Therefore the best method for determination the antioxidant capacity of seaweed is the DPPH method. We have already demonstrated that G. acerosa exhibits the greatest antioxidative activity and Haligra sps is the most effective antimicrobial agent when compared with the other seaweeds.
Table 2.
Total Phenolic Content of Methanolic Extracts from Seaweedsa
| S.No: | Samples | Total phenolic content/gm (mg Gallic acid Equivalent/g)b |
| 1 | Gellidela acerosa | 616.3 ± 6.3 |
| 2 | Gracilaria edulis | 327 ± 13.5 |
| 3 | Turbinaria conoides | 50 ± 0.5 |
| 4 | Padina | 109.5 ± 1.9 |
| 5 | Chondrococcus | 47.5 ± 4.5 |
| 6 | Hypnea pannosa | 210.5 ± 2.1 |
| 7 | Dictyota dichotoma | 350 ± 3.5 |
| 8 | Jania | 290 ± 2.5 |
| 9 | Sargassum | 309.5 ± 3.9 |
| 10 | Haligra | 440.3 ± 4.3 |
a The experiments were measured according to the Folin-Cioclateu method.
b Each experiment was performed in triplicate and the results are mean ± SD.
Figure 8.
Correlation between the contents of total phenols in seaweeds and their antioxidant capacity as determined by Antioxidant Assay (AA) using DPPH method (A), Lipid peroxidation (B), Nitric oxide method (C), Hydroxyl radical method (D), FRAP method (E) and Reducing power (F).
Discussion
Free radicals are highly reactive molecules with an unpaired electron and are produced by radiation or as by-products of metabolic processes. They initiate chain reactions which lead to disintegration of cell membranes and cell compounds, including lipids, proteins and nucleic acids. Besides damage to living cells, free radicals are the major cause of food deterioration through lipid oxidation, which ultimately affects the organoleptic properties and edibility of foods. Hence, intervention of an antioxidant may have a therapeutic effect and also maintain the freshness of food products. Antioxidant compounds scavenge free radicals such as peroxide, hydroperoxide or lipid peroxyl and thus reduce the level of oxidative stress and slow/prevent the development of complications associated with oxidative stress-related diseases [35] Many synthetic antioxidants have shown toxic and mutagenic effects, which have shifted attention towards naturally occurring antioxidants. A great number of naturally occurring substances like seaweeds have been recognized to have antioxidant abilities [36].
1,1-Diphenyl-2-picrylhydrazyl (DPPH) is a stable nitrogen centered free radical which can be effectively scavenged by antioxidants [37]. Hence it has been widely used for rapid evaluation of the antioxidant activity of plant and microbial extracts relative to other methods [38]. DPPH is also considered as a good kinetic model for peroxyl radicals [39]. The ability of seaweeds to scavenge DPPH radicals was determined by the decrease in its absorbance at 517 nm. The present investigation has shown that the extracts of all the seaweeds exhibited DPPH scavenging activity, the most effective being G.acerosa which exhibited significantly higher DPPH scavenging activity (72.5% inhibition) followed by Haligra species (55% inhibition) when compared with the highest concentration of the standard BHT (72% inhibition) (Fig 1). The result is indicative of the hydrogen donating ability of G. acerosa, since the effects of antioxidants on DPPH radical scavenging is thought to be due to their hydrogen donating ability [40]
In addition, the ability to scavenge the DPPH radicals is related to the inhibition of lipid peroxidation [41]. Hence the ability of the seaweeds to prevent lipid peroxidation was assessed in RBC by inducing lipid peroxidation with H2O2. During lipid peroxidation, low molecular weight end products, probably malonaldehyde, are formed by oxidation of polyunsaturated fatty acids and react with two molecules of thiobarbituric acid to give a pinkish red chromagen. As shown in Fig 2, at a concentration of 100 μg/ml, G. acerosa inhibited lipid peroxidation to 65.6% similar to the reference compound BHT which showed 64.9% inhibition.
OH• has a short half-life and is the most reactive and damaging ROS. It causes oxidative damage to DNA, lipids and proteins [42]. The extract was examined for its ability to scavenge OH• radicals generated by the Fenton reaction. When the seaweed extract and standard BHT (100 μg/ml) were added to the reaction mixture they removed hydroxyl radicals and prevented the degradation of 2-deoxyribose-2-ribose. G. acerosa exhibited the greatest scavenging effect of OH• among the seaweeds but less than the standard BHT. OH• is known to be capable of abstracting hydrogen atoms from membranes and they bring about peroxidic reactions of lipids [43]. It is thus anticipated that G. acerosa would show antioxidant effects against lipid peroxidation on biomembranes and would scavenge OH• radicals at the stages of initiation and termination.
NO radicals play an important role in inducing inflammatory response and their toxicity multiplies only when they react with O2•- radicals to form peroxynitrite, which damages biomolecules like proteins, lipids and nucleic acids [44]. Nitric oxide is generated when sodium nitroprusside reacts with oxygen to form nitrite. Seaweeds inhibit nitrite formation by competing with oxygen to react with nitric oxide directly. The methanolic extract of G.acerosa and Haligra sp at 100 μg/ml exhibited 39.8% and 33.3% inhibition which was comparable to the standard BHT, which exhibited 39.6% inhibition at 150 μg/ml. The present results suggest that G.acerosa and Haligra sp might be potent and novel therapeutic agents for scavenging of NO and the regulation of pathological conditions caused by excessive generation of NO and its oxidation product, peroxynitrite
Hydrogen peroxide is a weak oxidizing agent and can inactivate a few enzymes directly, usually by oxidation of essential thiol (-SH) groups. H2O2can cross cell membranes rapidly and once inside the cell it can probably react with Fe2+ and possibly Cu2+ to form hydroxyl radicals and this may be the origin of many of its toxic effects [45]. It is therefore advantageous for cells to control the amount of hydrogen peroxide that is allowed to accumulate. Figure 5 illustrates that the strongest anti- H2O2 activity was observed for G.acerosa at 100 μg/ml when compared with the standard BHT while T conoides appeared to be a very weak scavenger of hydrogen peroxide. The result suggest that G acerosa can be a better antioxidant for removing H2O2 and thus protecting food systems
The reducing ability of a compound greatly depends on the presence of reductones, which have exhibit antioxidative potential by breaking the free radical chain by donating a hydrogen atom [46]. For the measurement of reductive ability, we investigated the Fe3+ to Fe2+ transformation in the presence of an alcoholic extract using the method of Oyaizu et al [29]. Figure 6 illustrates that G. acerosa (100 μg/ml) showed higher absorbance when compared with the control and the same dosage as the standard BHT. The reducing capacity of G. acerosa is a significant indicator of its potential antioxidant activity. The results of the antioxidant assays indicate that G. acerosa is the best source of antioxidant compounds among the seaweeds investigated.
Apart from damage caused by free radicals, another common problem during food preservation is contamination by microbes. It not only affects the quality of food, but also causes serious health problems to those who consume the contaminated food. Of the ten seaweeds screened Haligra sps (50 mg/ml) showed antibacterial activity against S. aureus (MTCC 96) which was significantly (P < 0.05) higher than the standard antimicrobial agent sodium benzoate (200 mg/ml). The inhibition of growth of S.aureus by Haligra sp reveals that it can be used as an antibacterial agent against S.aureus which causes vomiting, diarrhoea, abdominal cramps and prostration and which also spoils raw meats, poultry, dairy products, salads, shrimp and ham [47].
The total phenolic content expressed as gallic acid equivalents was higher for the G. acerosa (0.616 ± 0.0063 g/g) and Haligra sps (0.440 ± 0.0043 g/g) extracts than for the other seaweeds. Plant phenolics in general are effective free radical scavengers and antioxidants. Phenolic compounds are commonly found in the edible brown, green and red seaweeds in which the antioxidative property has been correlated to their phenolic content [48-50]. Some authors claim that there is no correlation between the total phenolic content and the radical scavenging capacity [51], so it was very important to examine the correlation between the total phenolic contents and total antioxidant capacity of the studied seaweeds. The results of the present study reveal that there is a strong correlation between antioxidant activity and phenolic content. It is believed that the antioxidant properties of phenolics are a result of their ability to act as reducing agents, hydrogen donors, and free radical quenchers and phenolics can also act as metal chelators which prevent the catalytic function of metal in the process of initiating radicals [35]. It is possible that the antioxidant and antimicrobial activity of both the seaweed extract (G. acerosa and Haligra) can be the result of their high concentration of phenolic compounds.
Conclusion
The present study elucidated for the first time the antioxidant property of ten seaweeds. This study suggested that, among the ten seaweeds, the G. acerosa extract possesses high antioxidant activity which might be helpful in preventing or slowing the progress of various oxidative stress related disorders. Moreover,Haligra sps. possesses potent antimicrobial activity against S. aureus, a food borne pathogen. Therefore it can be concluded that G. acerosa and Haligra sps, in appropriate combination, can act as an effective food preservative. There are few reports on the antioxidant capacity of seaweeds and the mechanism of seaweeds as antioxidative agents is still not fully understood. Hence ffurther research is underway to analyze and isolate the active compounds responsible for the antioxidant and antimicrobial activity from both the seaweeds.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KPD and SKP conceived of the study, and participated in its design and coordination. PK carried out the assays. NS participated in the design of the study and performed the statistical analysis. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
Ms. P. Kesika wishes to thank Tamil Nadu State Council for Science and Technology (TNSCT), India for the financial assistance (TNSCT Students Projects Scheme). The authors also gratefully acknowledge the use of the Bioinformatics Infrastructure Facility, Alagappa University funded by the Department of Biotechnology, Ministry of Science and Technology, Government of India (No. BT/BI/04/055/2001).
Contributor Information
Kasi Pandima Devi, Email: devikasi@yahoo.com.
Natarajan Suganthy, Email: suganthy.n@gmail.com.
Periyanaina Kesika, Email: kesikabiotech@gmail.com.
Shanmugaiahthevar Karutha Pandian, Email: sk_pandian@redifmail.com.
References
- WHO Food safety and food borne illness. World health organization fact sheet 237, Geneva. revised January 2002.
- Tauxe RV. Emerging foodborne diseases: an evolving public health challenge. Emerg Infect Dis. 1997;3:425–434. doi: 10.3201/eid0304.970403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haliwell B, Gutteridge JMC. Free radicals in Biology and Medicine. 2. Clarendon: Oxford, U.K; 1989. p. 241. [Google Scholar]
- Yildirim A, Mavi A, Oktay M, Kara AA, Algur OF, Bilaloglu V. Comparison of antioxidant and antimicrobial activities of tilia (Tilia argenta Desf Ex Dc), Sage (Salvia triloba L.) and black tea (camellia sinesis) extracts. J Agric Food chem. 2000;48:5030–5034. doi: 10.1021/jf000590k. [DOI] [PubMed] [Google Scholar]
- St Angelo AJ, Vercellotti JR, Vinenett CH, Kuan JW, James C, Jr, Duputy HP. Chemical and instrumental analyses of warmed over flavor in beef. J Food sci. 1987;52:1163–1168. doi: 10.1111/j.1365-2621.1987.tb14034.x. [DOI] [Google Scholar]
- Ladikos D, Lougovois V. Lipid oxidation of muscle foods: a review. Food chem. 1990;35:295–314. doi: 10.1016/0308-8146(90)90019-Z. [DOI] [Google Scholar]
- Kourounakis AP, Galanakis D, Tsiakitzis K. Synthesis and pharmacological evaluation of novel derivatives of anti-inflammatory drugs with increased antioxidant and anti-inflammatory activities. Drug Dev Res. 1999;47:9–16. doi: 10.1002/(SICI)1098-2299(199905)47:1<9::AID-DDR2>3.0.CO;2-9. [DOI] [Google Scholar]
- Gulcin I, Buyukokuroglu MF, Oktay M, Kufrevioglu OI. On the in vitro antioxidant properties of melatonin. J Pineal Res. 2002;33:167–171. doi: 10.1034/j.1600-079X.2002.20920.x. [DOI] [PubMed] [Google Scholar]
- Buyukokuroglu ME, Gulcin I, Oktay M, Kufrevioglu OI. In vitro antioxidant properties of dantrolene sodium. Pharm Res. 2001;44:491–495. doi: 10.1006/phrs.2001.0890. [DOI] [PubMed] [Google Scholar]
- Sherwin FR. Antioxidants. In: Branen R, editor. Food Additives. New York: Marcel Dekker; 1990. pp. 139–193. [Google Scholar]
- Grice HC. Safety evaluation of butylated hydroxytoluene (BHT) in the liver, lung and gastrointestinal tract. Food Chem Toxicol. 1986;24:1127–1130. doi: 10.1016/0278-6915(86)90298-X. [DOI] [PubMed] [Google Scholar]
- Wichi HP. Enhanced tumour development by butylated hydroxyanisole (BHA) from the prospective of effect on forestomach and oesophageal squamous epithelium. Food Chem Toxicol. 1988;26:717–723. doi: 10.1016/0278-6915(88)90072-5. [DOI] [PubMed] [Google Scholar]
- Oktay M, Gulçin I, Kufrevioglu OI. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LEBENSM WISS TECHNOL. 2003;36:263–271. doi: 10.1016/S0023-6438(02)00226-8. [DOI] [Google Scholar]
- Shahidi F, Wanasundara PKJPD. Phenolic antioxidants: criterial review. Food sci Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- Sanchez-Moreno C, Larrauri JA, Saura-Calixto F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grapes juices and related polyphenolic constituents. Food Res Int. 1999;32:407–412. doi: 10.1016/S0963-9969(99)00097-6. [DOI] [Google Scholar]
- Yoshie Y, Wand W, Hsieh YP, Suzuki T. Compositional difference of phenolic compounds between two seaweeds, Halimeda spp. J Tokyo univ Fish. 2002;88:21–24. [Google Scholar]
- Qasim SZ. Bioactive substances, Glimpses of the Indian Ocean. Universities Press; 1998. pp. 75–79. [Google Scholar]
- Park PJ, Heo SJ, Park EJ, Kim SK, Byun HG, Jeon BT, Jeon YJ. Reactive oxygen scavenging effect of enzymatic extracts from Sargassum thunbergii. J Agric Food Chem. 2005;53:6666–6672. doi: 10.1021/jf050582+. [DOI] [PubMed] [Google Scholar]
- Dias PF, Siqueira JM, Vendruscolo LF, de Jesus Neiva T, Gagliardi AR, Maraschin M, Ribeiro-do-Valle RM. Antiangiogenic and antitumoral properties of a polysaccharide isolated from the seaweed Sargassum stenophyllum. Cancer chemoth Pharm. 2005;56:436–446. doi: 10.1007/s00280-004-0995-7. [DOI] [PubMed] [Google Scholar]
- Armisen R. World wide use and importance of Gracilaria. J Appl Phycol. 1995;7:231–243. doi: 10.1007/BF00003998. [DOI] [Google Scholar]
- Senevirathne M, Kim SH, Siriwardhana N, Ha JH, Lee K, Jown YJ. Antioxidant potential of Ecklonia cava on Reactive oxygen species scavenging, metal chelating, reducing power and lipid peroxidation inhibition. Food Sci Technol Int. 2006;12:27–38. doi: 10.1177/1082013206062422. [DOI] [Google Scholar]
- Siriwardhana N, Lee KW, Kim SH, Ha JW, Jeon YJ. Antioxidant activity of Hizikia fusiformis on reactive oxygen species scavenging and lipid peroxidation inhibition. Food Sci Technol Int. 2003;9:339–347. doi: 10.1177/1082013203039014. [DOI] [Google Scholar]
- Ratnasooriya WD, Premakumara GA, Tillerkaratne LMV. Postcoital contraceptive activity of crude extracts of Srilankan marine red algae. Contracep. 1994;50:291–299. doi: 10.1016/0010-7824(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Blois MS. Antioxidant determination by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Halliwell B, Gutteridge JMC, Arnoma OL. The deoxyribose method: A simple test tube assay for the determination of rate constant for reaction of hydroxyl radical. Anal Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogen. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- Gülçin I, Oktay M, Kireçci E, Küfreviolu O. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chem. 2003;83:371–382. doi: 10.1016/S0308-8146(03)00098-0. [DOI] [Google Scholar]
- Green LC, Wanger DA, Glogowski J. Analysis of nitrate, nitrite and (15 N) Nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jap J Nut. 1986;44:307–315. [Google Scholar]
- Benzie IFF, Strain JJ. Ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Yagi K, Rastogi R. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Annu Rev Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Mackeen MM, Ali AM, El-Sharkawy SH, Manap M, Salleh KM, Lajis NH, Kawazu K. Antimicrobial and cytotoxic properties of some Malaysian vegetables. Int J Pharmcog. 1997;35:237–243. [Google Scholar]
- Meir S, Kanner J, Akiri B. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agric Food chem. 1995;43:1813–1815. doi: 10.1021/jf00055a012. [DOI] [Google Scholar]
- Wu XJ, Hansen C. Antioxidant capacity, Phenolic content, Polysaccharide content of Lentinus edodes grown in Whey permeate-based submerged culture. J Food Sci. 2008;73:M1–M8. doi: 10.1111/j.1750-3841.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Formica JV, Regelson W. Review of the biology of the quercetin and related biflavonoids. Food chem Toxicol. 1995;33:1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Villano D, Fernandez-Pachona MS, Moyab ML, Troncosoa AM, Garcia-Parrilla MC. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta. 2007;71:230–235. doi: 10.1016/j.talanta.2006.03.050. [DOI] [PubMed] [Google Scholar]
- Hu C, Kitts DD. Studies on the antioxidant activity of Echinacea root extract. J Agric Food Chem. 2000;48:1466–1472. doi: 10.1021/jf990677+. [DOI] [PubMed] [Google Scholar]
- Rackova L, Oblozinsky M, Kostalova D, Kettmann V, Bezakova L. Free radical scavenging activity and lipoxygenase inhibition of Mahonia aquifolium extract and isoquinoline alkaloids. J Inflam. 2007;4:15. doi: 10.1186/1476-9255-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti F, Loizzo MR, Statti GA, Menichini F. Comparative radical scavenging and Antidiabetic activities of methanolic extract and fractions from Achillea ligustica ALL. Bio Pharm Bull. 2005;28:1791–1794. doi: 10.1248/bpb.28.1791. [DOI] [PubMed] [Google Scholar]
- Rekka E, Kourounakis PN. Effect of hydroxyethyl rutenosides and related compounds on lipid peroxidation and free radical scavenging activiy, some structural aspects. J Pharm Pharmacol. 1991;43:486–491. doi: 10.1111/j.2042-7158.1991.tb03519.x. [DOI] [PubMed] [Google Scholar]
- Spencer JPE, Jenner A, Aruoma OI. Intensive oxidative DNA damage promoted by L-DOPA and its metabolites implications for neurodegenerative disease. FEBS Lett. 1994;353:246–250. doi: 10.1016/0014-5793(94)01056-0. [DOI] [PubMed] [Google Scholar]
- Kitada M, Igarashi K, Hirose S, Kitagawa H. Inhibition by polyamines of lipid peroxide formation in rat liver microsomes. Biochem Biophys Res Commun. 1979;87:388–394. doi: 10.1016/0006-291X(79)91808-4. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Haliwell B. Reactive oxygen species in living systems: source, biochemistry and role in human disease. The Am J Med. 1991;91:14–22. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- Pin-Der-Duh X. Antioxidant activity of burdock (Arctium lappa Linne): it's scavenging effect on free radical and active oxygen. J Am Oil Chem Soc. 1998;75:455–461. doi: 10.1007/s11746-998-0248-8. [DOI] [Google Scholar]
- Prescott LM, Harley JP, Klein DA. Microbiology. 5. Mc Graw Hill:New York; 2002. p. 932. [Google Scholar]
- Jimenez-Escrig A, Jimenez-Jimenez I, Pulido R, Saura-Calixto F. Antioxidant activity of fresh and processed edible seaweeds. J Sci Food Agric. 2001;81:530–534. doi: 10.1002/jsfa.842. [DOI] [Google Scholar]
- Kuda T, Tsunekawa M, Hishi T, Araki Y. Antioxidant properties of dried 'kayamo-nori', a brown alga Scytosiphon lomentaria (Scytosiphonales, Phaeophyceae) Food Chem. 2006;89:617–622. [Google Scholar]
- Ganesan P, Kumar CS, Bhaskar N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour Technol. 2008;99:2717–2723. doi: 10.1016/j.biortech.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Yu L, Haley S, Perret J, Harris M, Wilson J, Aian M. Free radical scavenging properties of wheat extracts. J Agric Food Chem. 2002;50:1619–1624. doi: 10.1021/jf010964p. [DOI] [PubMed] [Google Scholar]