Abstract
Nitric oxide (NO) has been long assumed to play a key role in mammalian olfaction. This was based largely on circumstantial evidence, i.e. prominent staining for nitric oxide synthase (NOS) and cyclic GMP or soluble guanylyl cyclase, an effector enzyme activated by NO, in local interneurons of the olfactory bulb. Here we employ innovative custom-fabricated NO micro-sensors to obtain the first direct, time-resolved measurements of NO signaling in the olfactory bulb. In 400 μm thick mouse olfactory bulb slices, we detected a steady average basal level of 87 nM NO in the extracellular space of mitral or granule cell layers. This NO ‘tone’ was sensitive to NOS substrate manipulation (200 μM L-arginine, 2 mM L-NAME) and Mg2+ modulation of NMDA receptor conductance. Electrical stimulation of olfactory nerve fibers evoked transient (peak at 10 s) increments in NO levels 90 – 100 nM above baseline. In the anesthetized mouse, NO micro-sensors inserted into the granule cell layer detected NO transients averaging 55 nM in amplitude and peaking at 3.4 sec after onset of a 5 sec odorant stimulation. These findings suggest dual roles for NO signaling in the olfactory bulb – tonic inhibitory control of principal neurons, and regulation of circuit dynamics during odor information processing.
Keywords: arginine, electrode, granule cell, microsensor, NOS, odorant
INTRODUCTION
Nitric oxide (NO) is likely to be intimately involved in odor information processing, based on the high expression levels of one isoform of its synthetic enzyme, neuronal nitric oxide synthase (nNOS), in specific cellular populations in the main olfactory bulb (MOB) (Kishimoto et al., 1993; Spessert et al., 1994; Dellacorte et al., 1995; Hopkins et al., 1996; Alonso et al., 1998; Crespo et al., 2003; Gutierrez-Mecinas et al., 2005). Among the principal neurons, nNOS is expressed in a subset of tufted cells participating in an intrabulbar association system (Kosaka and Kosaka, 2007). The intrinsic neurons of the MOB containing nNOS include subpopulations of periglomerular cells, granule cells, short-axon cells and stellate cells. The nNOS content of periglomerular and granule cells has been determined using three different cytochemical methods (Kishimoto et al., 1993). The periglomerular (PG) cells containing nNOS are non-dopaminergic and receive direct synaptic contacts from olfactory nerve terminals (type 1 PG) (Crespo et al., 2003). A separate subset of PG cells stains positive for the β1 subunit of soluble guanylyl cyclase, an effector enzyme that makes cyclic GMP (cGMP) when activated by NO. The β1–positive PGs do not receive synaptic contacts from olfactory nerve terminals (type 2 PG) and they are mostly calbindin 28k-positive (Gutierrez-Mecinas et al., 2005). This cellular compartmentalization suggests that NO acts as a diffusible intraglomerular signal between type 1 and type 2 PG cells. Similarly, NOS expression (indicated by NADPH-diaphorase staining) and cGMP synthesis are partitioned into mutually exclusive subsets of granule cells (Hopkins et al., 1996).
The precise role of NO in regulating developmental events (Willse et al., 2005) or modifying sensory processing (Gelperin et al., 2000; Wilson et al., 2007) in neural circuits in the olfactory bulb has not yet been determined. Expression of nNOS is required for development of the glomerular organization of the bulb (Chen et al., 2004; Moreno-Lopez and Gonzalez-Forero, 2006). An indication of a role for NO in synaptic interactions comes from in vivo microdialysis measurements. Vaginocervical stimulation during pro-oesterus/oestrus elicits citrulline production, a marker of NO synthesis, in the MOB (Guevara-Guzman et al., 2000). This effect may depend on noradrenergic activation of the NO/cGMP/protein kinase G pathway (Gonzalez-Flores et al., 2007). Indirect evidence that NO affects olfactory processing in the main olfactory bulb is based on the ability of nNOS blockers applied to the main olfactory bulb to block olfactory memory formation (Kendrick et al., 1997). Chronic systemic application of nNOS blockers improves olfactory memory by removing the inhibitory effect of NO on neurogenesis in the subventricular zone, thereby increasing the number of new periglomerular neurons in the glomerular layer of the OB (Romero-Grimaldi et al., 2006). The accessory olfactory bulb (AOB) also stains densely for nNOS and has an NO-dependent memory formation mechanism activated by olfactory signals during mating (Okere et al., 1996; Okere and Kaba, 2000). Nitric oxide is implicated in a variety of mechanisms for synaptic plasticity and learning, involving both olfactory cues and processing in other sensory systems (Susswein et al., 2004; Tsutsuki et al., 2007; Wang et al., 2007).
Methods for electrochemical detection of NO have recently become available (Wink et al., 1995; Christodoulou et al., 1996; Allen et al., 2000; Brunet et al., 2003; Zhang, 2004; Nunemaker et al., 2007), enabling measurements of NO on the second and sub-second time scales in local regions of neural circuitry in vivo (Buerk et al., 1996; Buerk et al., 2003a; Buerk et al., 2003b), in brain slices in vitro (Leonard et al., 2001; Ledo et al., 2002; Ferreira et al., 2005) and in neuronal cell cultures (Oni et al., 2004; Xu et al., 2004; Pereira-Rodriques et al., 2005). Here, we show for the first time that NO-selective microelectrodes can be used to measure NO production in the living mouse olfactory bulb. We can detect both tonic and stimulus-evoked NO production in bulb slices. We also show that odorant stimulation can evoke NO production in the intact olfactory bulb of anesthetized mice.
METHODS
NO microsensor fabrication and calibration
NO micro-sensors were Nafion-coated recessed gold-plated microelectrodes (Buerk et al., 1996; Buerk and Riva, 1998; Fukamura et al., 2001; Thom et al., 2002; Buerk et al., 2003a) with tip diameters ranging between 5 and 10 μm. The recessed design in this and other types of NO microelectrodes has been briefly reviewed (Buerk, 2001). Microelectrodes were fabricated from Wood’s metal-filled glass micropipettes, plated with gold inside the recess at the tip, thoroughly cleaned, and then the tip was dip coated with Nafion polymer to reduce interference from ascorbate, catecholamines, nitrates, tyrosine, and other chemical species. The remaining recess, typically 10 to 20 μm deep, protects the membrane and also acts as a diffusion barrier to larger molecules that might be oxidized. For example, Bohlen (1998) reported that the current sensitivity to ascorbate and norepinephrine for recessed NO microelectrodes with similar tip and recess dimensions was over three orders of magnitude smaller than for NO (Bohlen, 1998). Current generated by the electrochemical oxidation of NO gas at a potential of +850 mV relative to a grounded Ag/AgCl reference was measured with a sensitive electrometer (Model 610C; Keithley Instruments, Cleveland, Ohio). Prior to brain tissue measurements, two-point calibrations of each electrode were made in deoxygenated saline at 37°C using compressed gases (100% nitrogen and 1800 ppm NO in nitrogen) corresponding to NO concentrations of 0 and 2.76 μM, with typical sensitivities ranging between 20–100 nM/pA. The recessed NO micro-sensor has excellent spatial and temporal resolution, with 90% response time <100 ms and linear response characteristics in the range of physiological NO concentrations (typically <1 μM). The raw data from the NO micro-sensor were sampled and digitized at rates of 10, 20 or 100 Hz.
NO measurements in slices of mouse olfactory bulb
Olfactory bulb slices from mice were prepared as described previously (Lowe, 2002, 2003). Briefly, male P21 C57BL6 mice (Charles River Laboratories, Wilmington, Massachusetts) were sacrificed by decapitation under deep halothane anesthesia and the bulbs extracted into ice cold sucrose slicing solution (mM): 240 sucrose, 2.5 KCl, 10 Na-HEPES, 10 glucose, 1 CaCl2, 4 MgCl2, 0.2 ascorbic acid, pH 7.2. Horizontal olfactory bulb slices (350 μm thickness) were cut and incubated 1–3 h (32°C–23°C) in an interface chamber with high Mg2+, L-arginine-supplemented artificial cerebrospinal fluid (ACSF) (mM): 124 NaCl, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 10 glucose, 1 CaCl2, 3 MgCl2, 200 μM L-arginine (95% O2, 5% CO2). For NO measurements, slices were submerged in an open chamber, immobilized with a platinum harp and perfused (2 ml/min) with L-arginine-supplemented standard ACSF (mM): 124 NaCl, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 25 glucose, 2 CaCl2, 1.3 MgCl2, 200 μM L-arginine (bubbled with 95% O2, 5% CO2, 23°C). Bath solutions were changed by using a teflon valve to switch between different perfusion reservoirs.
To make measurements of NO release in slices, NO micro-sensors were positioned in mitral and granule cell layers of the slice under visual control, using a Nikon E600 FN upright microscope equipped with a Leica HCX APO L 63X/0.90 water-immersion objective, infrared differential interference contrast (IR-DIC) optics, and an infrared video camera (C2400–79H, Hamamatsu Photonics K. K.). A concentric bipolar platinum/iridium electrode (125 μm outer pole, 25 μm inner pole) (Frederick Haer & Co, Bowdoinham, Maine) was placed in the overlying olfactory nerve layer and 100 – 200 μs, 0.2 – 0.4 mA pulses were delivered as single stimuli or as trains at 40Hz by a constant current generator (A320, World Precision Instruments, Sarasota, Florida). This type of stimulation has been shown previously to evoke neuronal synchrony and oscillatory network activity in bulb slices (Friedman and Strowbridge, 2003). NO oxidation currents were amplified by a sensitive electrometer, and the output recorded by a computer controlled data acquisition system at a sampling rate of 10 Hz. Changes in current are shown, subtracting the current for basal NO in some cases, or the residual current for zero NO in the bath in other cases.
NO measurements in anesthetized mouse olfactory bulb Male
C57BL6 mice (20–25 g) were anesthetized with 2% isoflurane. When unresponsive to paw pinch, the head of the mouse was mounted in a precision stereotaxic device, the head shaved and the scalp incised and retracted. Mouse body temperature was monitored and maintained at 37°C with a rectal thermistor and heating pad. A craniotomy (2 mm diameter) was made using a dental drill to allow access of an NO micro-sensor to the dorsal surface of the left or right olfactory bulb. Measurements of NO release in the olfactory bulb of the anesthetized mouse used the same micro-sensors and instrumentation as in recordings from the mouse olfactory bulb slices. We made NO measurements at different depths in the olfactory bulbs of 3 mice. In some experiments, the depths of the dorsal and ventral mitral cell layers were first determined with a tungsten single unit extracellular recording electrode. In these experiments an NO micro-sensor was then advanced along the same path traversed by the single unit electrode. In all experiments the depth of the granule cell layer was identified stereotaxically, relative to bregma and the depth below the dura. Both NO micro-sensor and single unit electrode signals were sampled at 100 Hz, along with a timing signal when solenoids were switched on and off. Stimuli included seven different odorant mixtures (Table 1), delivered to the nares by a custom-built olfactometer that used a bank of solenoid valves to switch the saturated headspace of odorant sample vials into the isoflurane supply line. Odorants were obtained from Sigma-Aldrich (St. Louis, Missouri) and each component of the odorant mixture was present at 1% (vol/vol). All components of a given mixture were in either water or odorless mineral oil as a solvent.
Table 1.
Odorant mixtures used to evoke NO signals in olfactory bulbs of anesthetized mice.
| Mixture #1 | Mixture #2 | Mixture #3 | Mixture #4 |
|---|---|---|---|
| L-carvone | 1-hexanol | isoamyl acetate | allyl nonate |
| D-carvone | t-amyl alcohol | hexyl propionate | allyl hexanoate |
| 3-hexanone | ETOH | ethyl salicylate | 2-methylbutyl isovalerate |
| 3-heptanone | +/− linalool | ethyl valerate | benzyl isovalerate |
| 3-nonanone | 1-pentanol | n-amyl acetate | 2-phenylpropyl isovalerate |
| 2-decanone | decyl alcohol | amyl butyrate | allyl propionate |
| 1-propanol | allyl butyrate | ||
| carvactol | |||
| isopropanol | |||
| benzyl alcohol | |||
| Mixture #5 | Mixture #6 | Mixture #7 | |
| Citral | piperonyl acetate | L- heptanol | |
| Octanal | benzyl acetate | trans-anethole | |
| Cineole | citral diethyl acetal | hexanal | |
| mint extract | n-butyl acetate | isoamyl acetate | |
| Carvactol | decyl alcohol | benzaldehyde | |
| orange extract | hexyl acetate | 5-nonanone | |
| cherry extract | eugenol | geraniol | |
| banana extract | 2-octanone | ||
| ethanolamine | |||
| ethyl acetate |
All experimental procedures complied with U.S. Public Health Service guidelines for the humane use and care of laboratory animals. The number of animals used was minimized and pain was alleviated with anesthetics.
RESULTS
Nitric oxide gradients in mouse olfactory bulb slices in vitro
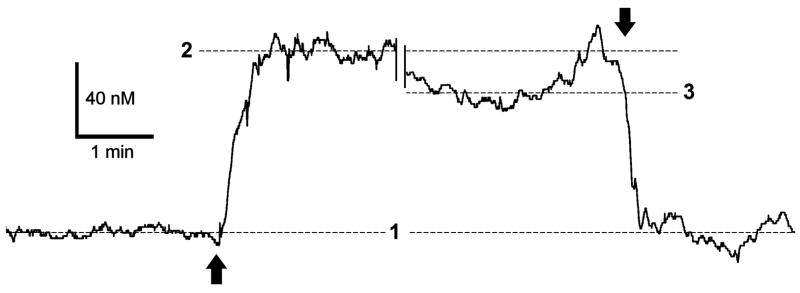
Olfactory bulb circuits generate robust spontaneous spike activity in the resting, unstimulated state (Walsh, 1956; Chaput and Holley, 1979; Doving, 1987; Wellis et al., 1989; Motokizawa and Ogawa, 1997; Kay and Laurent, 1999; Rinberg et al., 2006). Spontaneous spiking is also strongly manifested in olfactory bulb neurons in vitro (Isaacson, 1999; Carlson et al., 2000) being driven by intrinsic pacemaker action in specific cell types (Hayar et al., 2004; Puopolo et al., 2005). We reasoned that this spontaneous activity might drive tonic production of nitric oxide in MOB slices that would be detectable with our NO micro-sensor technology. To determine resting levels of NO in slices, we took sensor readings with the probe tip immersed in fresh ACSF perfusion medium flowing ~300 μm above the slice surface, and then after the tip penetrated the slice. Figure 1 shows typical data recorded before, during and after penetration into an area near the mitral cell body layer of a slice. The electrode current was stable in bath ACSF, but we observed an abrupt upward deflection as the tip entered the slice, and the current quickly settled to a new steady level. After 42 min, the probe tip was retracted from the slice, and electrode current fell back to the mean level recorded before in clean ACSF. These observations indicate that there is endogenous NO production in mouse MOB slices. From our micro-sensor calibration, we estimated that resting levels of NO in slices were maintained at a steady level of 86.5 ± 6.2 nM (mean ± SE, range 60 to 135 nM, for N = 9 measurements obtained with 5 different micro-sensors).
Figure 1. Nitric oxide gradients in the mouse olfactory bulb slice.
A calibrated NO micro-sensor was positioned in the slice perfusion chamber with the tip initially located ~300 μm above the slice surface. The sensor was subsequently driven along its axis with a micromanipulator until the tip penetrated the slice near the mitral cell body layer to a depth of ~ 50 μm. Electrode current was sampled at 10 Hz during this procedure. After a 42 minute gap in the record during which several electrical stimulation trials were done, the NO sensor tip was retracted from the slice. The up and down arrows indicate the times of sensor penetration and retraction, respectively. Dashed lines indicate mean NO readings before (1) and a few minutes after (2) slice penetration, and a few minutes before retraction (3).
Substrate modulation of NO synthesis in mouse olfactory bulb slices in vitro
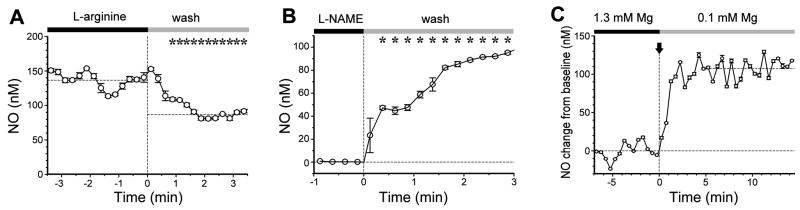
To show that the elevated electrode currents recorded when our micro-sensors are inserted into MOB slices are due to tonic NO production, we performed manipulations known to affect the synthesis of NO by NOS. The amino acid L-arginine is the sole physiological substrate for the synthesis of NO, so the availability of arginine is rate-limiting for the NOS enzyme. In cerebellar slice preparations, supplementing the bath medium with arginine increases NO production (Garthwaite et al., 1989). Conversely, we expect that removal of extracellular arginine may decrease NO production in brain slices (Wiesinger, 2001). Fig. 2A shows that the electrode current from a micro-sensor located in the granule cell layer of a mouse MOB slice was decreased significantly within 1 min after washout of 200 μM L-arginine from the bath ACSF. The opposite effect (increase in current) was observed when 200 μM L-arginine was added to a slice that had been incubated in arginine-free ACSF (data not shown). This effect is not due to electrode detection of L-arginine, because tests of the NO micro-sensor in other physiological studies have shown that it is not sensitive to L-arginine (Vukosavljevic et al., 2006)
Figure 2. Tonic nitric oxide production in the mouse olfactory bulb slice is altered by modulating NOS and neuronal activity.
A. Removal of the nNOS substrate L-arginine (at time 0) resulted in a significant decrease in NO micro-sensor signal, which settled to a new steady current. Starred values are significantly less than mean pre-wash out values (p < 0.05, Mann Whitney rank sum test). Horizontal dashed lines show mean steady state values before and after perfusion switch.
B. Removal of L-NAME, a competitive inhibitor of nNOS, resulted in a significant increase in NO micro-sensor signal (zero current corresponding to zero NO level for this case, as L-NAME completely inhibited NO synthesis). Starred values are significantly higher than mean pre-wash out value (p < 0.05, Mann Whitney rank sum test). Data in A and B were acquired at 10 Hz and binned in 15 s intervals (mean values with standard deviations indicated). Horizontal dashed line shows mean steady state value before perfusion switch. Electrode calibration was 20 nM/pA.
C. An NO micro-sensor was inserted into the granule cell layer of a slice (depth ~ 50 μm) and electrode current was recorded as the slice perfusion solution was switched from 1.3 mM Mg2+ to 100 μM Mg2+ (arrow, time 0) which increases excitability of neurons. Current was sampled at 20 Hz and binned in 30 s intervals bins (mean values with standard deviations indicated). Horizontal dashed lines show mean steady state values before and after perfusion switch. The time delay between valve switch and completion of solution change is 1.5 min. Electrode calibration was 24 nM/pA.
NO production can also be modulated by synthetic arginine analogs that act as competitive inhibitors at the arginine binding site of NOS (Moore and Handy, 1997). Fig. 2B shows that removal of the analog L-NAME (NG-Nitro-L-arginine methyl ester) from the extracellular bath after a 30 min period of perfusion with this compound (2 mM) resulted in a significant increase in electrode current. The data points in the period 0.5 –3 min of recording after L-NAME washout in Fig. 2B are significantly different from data in the minute prior to L-NAME washout. The opposite effect (decrease in current) was observed when a slice in control ACSF was switched to ACSF + 2 mM L-NAME (data not shown). These results confirm that the micro-sensor reports tonic NO production in the MOB slice, controlled by availability of the NOS substrate L-arginine.
Extracellular Mg2+ modulates NO production in the mouse olfactory bulb slice in vitro
The excitability of many brain circuits depends critically on extracellular Mg2+, which acts as a voltage-dependent open-channel blocker of the NMDA receptor. In low Mg2+ ACSF, spontaneous activity in slices is greatly potentiated as glutamate is able to activate NMDA receptors at more negative membrane potentials, facilitating the depolarization and spiking of neurons. This is predicted to up-regulate NO synthesis by increasing Ca2+ influx into neurons through voltage-gated Ca2+ channels, or through the NMDA receptors themselves. Indeed, calcium entry through NMDA receptors coupled to the nNOS enzyme in the postsynaptic density by an adaptor protein (PSD-95) can directly enhance NO synthesis (Christopherson et al., 1999; Sattler et al., 1999; Ishii et al., 2006). We tested this prediction in the MOB slice by using our micro-sensor to monitor tonic NO production as a function of extracellular Mg2+. We observed a large and immediate increase in the electrode current when bath Mg2+ was reduced from 1.3 mM to 100 μM (Fig. 2C). This degree of Mg2+ reduction is estimated to relieve voltage-dependent block and increase the conductance of NMDA receptor channels by ~5.6-fold at −60 mV in 200 μM glutamate (Wollmuth et al., 1998). This result suggests that NMDA receptors play an important role in determining NO synthesis in the olfactory bulb. It implies that tonic NO production is not just due to constitutive activity of nNOS, but is actually controlled by glutamatergic transmission and spontaneous neuronal activity.
Electrical stimulation evokes NO production in the mouse olfactory bulb slice in vitro
Since we can modulate tonic NO levels by altering spontaneous neuronal activity in the slice, we also expect to evoke increased NO synthesis by directly stimulating afferent neural pathways of the olfactory bulb. In the MOB slice, sensory input can be mimicked by electrical stimulation of the olfactory nerve layer above the glomeruli. This evokes glutamate release from olfactory nerve terminals within nearby glomeruli, exciting dendrites of principal neurons - mitral and tufted cells. These in turn excite local inhibitory interneurons – periglomerular and granule cells – propagating oscillatory activity through modular circuits associated with the excited glomeruli (Lagier et al., 2004). Oscillatory network activity is linked to NO signaling in other systems (Gelperin, 1994; Gelperin et al., 2000; Freund and Katona, 2007; Makara et al., 2007; Wilson et al., 2007). We therefore sought to detect NO production in mouse MOB slices stimulated with brief gamma frequency trains of current pulses that have been shown to evoke robust oscillations in the rat MOB slice (Friedman and Strowbridge, 2003). Figure 3 shows averaged data for responses recorded by an NO micro-sensor at a single site in the granule cell layer, in response to brief pulse trains (20 shocks of 200 μs, 1 mA at 100 Hz) delivered by a concentric bipolar stimulus electrode in the olfactory nerve layer. The stimulus electrode was placed adjacent to glomeruli that were oriented radial with respect to the micro-sensor in the granule cell layer. Stimulation evoked a significant increase in micro-sensor current that peaked within 10 s and was significantly elevated for 30 s. From the electrode calibration, we estimate that the increment in NO concentration for the 15 sec period immediately after stimulation was 90 – 100 nM. When spike activity was blocked by 1 μM TTX and 200 μM cadmium, trains of 20 glomerular shocks failed to elicit detectable NO production (data not shown).
Figure 3. Electrical stimulation evokes nitric oxide production in the mouse olfactory bulb slice.
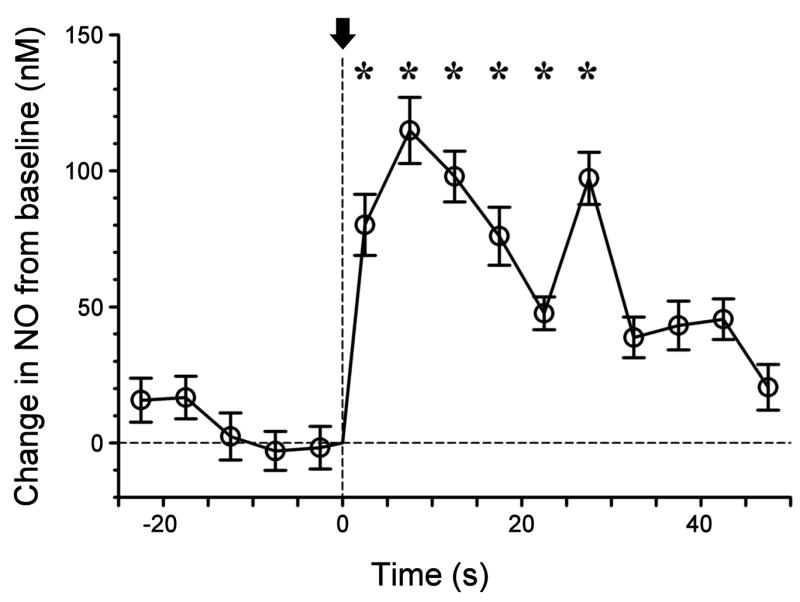
An NO micro-sensor was inserted into the granule cell layer of a slice (depth ~ 50 μm) and the electrode current was recorded as the slice was stimulated by current pulses from concentric bipolar electrode placed in olfactory nerve layer radial to the sensor site (a 200 ms train of 20 current pulses at 100 Hz, initiated at time 0, indicated by arrow). The plot shows data sampled at 20 Hz binned in 5 s intervals (mean values with standard deviations indicated). Starred values are significantly greater than mean pre-shock values (p < 0.05, Mann Whitney rank sum test). Electrode calibration was 24 nM/pA.
Odorant-evoked nitric oxide transients in the mouse olfactory bulb in vivo
The ability to evoke NO production by simulated sensory stimulation in the in vitro bulb slice suggests that increased NO synthesis may also occur when neurons in the intact olfactory system respond to odorant stimuli. To detect stimulus-evoked NO production in vivo, we inserted NO micro-sensors into the granule cell layer of the MOB of anesthetized mice, and delivered odorants to the animals through an olfactometer. Different odorants evoke different spatial patterns of activity in the MOB (Mori et al., 2006; Johnson and Leon, 2007). To increase the probability of activating neurons near sensor recording sites, we used odorant stimuli consisting of mixtures containing from 6 to 10 different compounds in liquid phase at 0.01% (vol/vol) (Table 1). During each trial, solenoid valves delivering stimuli were actuated for a period of 5 s. At each recording site, each of 6 or 7 mixtures was tested in two or more trials. In some trials, transient increases in NO micro-sensor current were observed during the stimulation period. As expected, these responses were not evoked by all mixtures at all recording sites. Figure 4 shows responses averaged over trials and odorant mixtures at two recording depths in the same mouse. The time course of the response was dependent on the depth. At 500 μm we recorded a brief monophasic response lasting ~5 s. At 900 μm a slower response was recorded (> 10 s duration) that was followed by oscillatory fluctuations. From the calibration of the micro-sensor, we estimated that the responses corresponded to increases in basal NO concentration of 30 – 60 nM. Similar results were obtained from two additional mice. The set of odorant-evoked increases in NO level over the set of 52 odorant applications at various depths in the olfactory bulbs of 3 mice is shown in Table 2. The average increment in NO level for the 52 trials of odorant application was 54.6 nM, with peak response occurring 3.4 sec after onset of the 5 sec odorant application. In Fig. 4B weak oscillatory changes in NO levels are evident after odorant offset.
Figure 4. Odorant-evoked nitric oxide transients in the anesthetized mouse olfactory bulb.
A. Signal recorded from a calibrated NO micro-sensor inserted into the granule cell layer at a depth of 500 μm relative to the surface of the dura. The plot is the averaged response for multiple trials applying 6 of 7 odorant mixtures (Table 1).
B. Signal recorded from the granule cell layer at a depth of 900 μm (averaged response for all 7 odorant mixtures in Table 1). Signal was sampled at 100 Hz and then averaged in intervals of 500 ms. Bars indicate standard error of the mean. Dashed lines indicate the period of actuation of solenoid valves controlling odorant stimulation.
Table 2.
Nitric oxide levels in the mouse olfactory bulb are increased by odorant application to the anesthetized mouse.
Odorant mixtures used for stimulation are shown in Table 1. Odorant stimuli were 5 sec in duration. Each odorant in the mixture was present at 0.01 % (vol/vol). Odorants were introduced into the flow of isoflurane used to maintain anesthesia.
| Depth (μm) | Trials | Mean peak ± SE (nM) | Time to peak (s) | |
|---|---|---|---|---|
| Mouse 1 | 750 | 5 | 40.5 ± 12.2 | 5.75 |
| 950 | 7 | 63.4 ± 20.5 | 3.65 | |
| Mouse 2 | 500 | 7 | 64.5 ± 28.6 | 2.45 |
| 800 | 7 | 20.1 ± 9.8 | 1.45 | |
| 1500 | 7 | 17.7 ± 12.4 | 3.65 | |
| Mouse 3 | 500 | 6 | 81.7 ± 11.9 | 2.25 |
| 900 | 7 | 84.3 ± 14.3 | 2.25 | |
| 1500 | 6 | 64.2 ± 19.0 | 5.75 |
DISCUSSION
An extensive literature documents the intense expression of the NOS enzyme in olfactory centers of diverse species of vertebrates (Bredt et al., 1991; Kishimoto et al., 1993; Spessert et al., 1994; Dellacorte et al., 1995; Hopkins et al., 1996; Alonso et al., 1998; Crespo et al., 2003; Gutierrez-Mecinas et al., 2005; Herrmann et al., 2007) and invertebrates (Gelperin, 1994; Elphrick et al., 1995; Muller and Hildebrandt, 1995; Nighorn et al., 1998; Fujie et al., 2002; Seki et al., 2005; Settembrini et al., 2007; Watanabe et al., 2007). However, odorant-evoked NO production was only recently demonstrated in the moth antennal lobe using a fluorescent indicator (Collmann et al., 2004). Measurements of citrulline production also indicated indirectly that some non-olfactory stimuli can increase NO synthesis in the vertebrate olfactory bulb (Hopkins et al., 1996; Alonso et al., 1998; Guevara-Guzman et al., 2000). The methods used in previous studies do not provide quantitative estimates of NO signals. By using innovative micro-sensor technology, we have obtained the first absolute measurements of physiological levels of NO in a mammalian olfactory system. We show that there is both a tonic background level of NO synthesis in the mitral or granule cell layers of the mouse olfactory bulb, as well as phasic NO increments evoked by electrical and odorant stimulation. Tonic synthesis maintained a steady basal level of 60 – 135 nM NO in the extracellular space of 400 μm thick in vitro slices. Both electrical (in vitro) and odorant (in vivo) stimuli evoked NO increments above baseline of 20 – 100 nM.
Heavy expression of NOS and the NO target enzyme, soluble guanylyl cyclase, in selective subpopulations of the GABAergic PG and granule cells in the mammalian olfactory bulb indicates that NO signaling is intimately involved in the inhibitory control of olfactory principal neurons – mitral and tufted cells. Our measurements suggest that NO may regulate both tonic and stimulus-evoked dynamics of these principal neurons for olfactory signal processing. It is becoming clear that basal NO production can play a variety of roles in regulating functions of neuronal circuits. For example, in supraoptic nucleus, NO tone generated by magnocellular neurons exerts inhibitory control over oxytocin and vasopressin systems (Stern and Zhang, 2005). During late pregnancy, nNOS mRNA is reduced and endogenous NO production markedly down-regulated, facilitating oxytocin release during parturition (Srisawat et al., 2000). In cerebellum, basal NO production by nNOS reduces the input resistance of granule cells and enhances their tonic GABAergic inhibition by golgi cells (Wall, 2003).
In addition to soluble guanylyl cyclase, NO can also act through other effectors to alter neuronal excitability, for example S-nitrosylation (Ahern et al., 2002) and mono-ADP-ribosyltransferase (Edwards and Rickard, 2007). Potential S-nitrosylation targets in the bulb include NMDA receptors, olfactory cyclic nucleotide-gated channels (Broillet, 2000; Murphy and Isaacson, 2003) large conductance calcium-activated potassium channels (Isaacson and Murphy, 2001) and ryanodine receptor calcium release (RyR) channels (Carlson et al., 1997). Another pathway through which olfactory input could change NO levels in the olfactory bulb is by modulating capillary blood flow in glomeruli (Chaigneau et al., 2003; Gurden et al., 2006; Chaigneau et al., 2007), which may stimulate endothelial release of NO (Garthwaite et al., 2006). NO could also influence olfactory bulb network activity by modulating gap junctional communication (Hatton, 1998) between cells in glomeruli (Chen and Shepherd, 2005; Kosaka et al., 2005; Kosaka and Kosaka, 2005; Hayar et al., 2005).
What functions might NO signaling serve in the olfactory bulb? One idea is that NO plays an integral role in odor learning and memory. Odor learning is accompanied by changes in olfactory bulb synaptic interactions which may depend critically on both resting NO levels and odorant-stimulated phasic changes in NO levels. Regulation of NO levels within a certain range is critical for interactions between NO and processes mediating synaptic plasticity in brain slice preparations (Hopper and Garthwaite, 2006). Both tonic levels and phasic changes of NO are required for hippocampal long-term potentiation (LTP). Olfactory circuits generate rhythmic oscillatory activity (Gelperin, 2006) that can be NO-dependent (Gelperin, 1994; Fujie et al., 2005) and modulated by odor learning (Kay, 2005; Martin et al., 2006), and odor learning can be dependent on NO synthesis (Kendrick et al., 1997; Sakura et al., 2004; Susswein et al., 2004; Edwards and Rickard, 2007).
It has been 15 years since it was suggested that NO plays an integral role in vertebrate olfactory processing, both at the sensory periphery and in central structures (Breer and Shepherd, 1993). Technology has finally advanced to the stage that we can test this directly. Our demonstration that odorant stimulation activates NO signaling in the olfactory bulb supports this idea, and it raises many new questions to be addressed in future studies.
Acknowledgments
This research was supported by U.S.Public Health Service grants DC04208-04 (GL) and NIH HL068164 (DB), and Army Research Office & Whitehall Foundation grants (AG).
List of Abbreviations
- ACSF
artificial cerebrospinal fluid
- cGMP
guanosine 3′, 5′-cyclic monophosphate
- L-NAME
NG-Nitro-L-arginine methyl ester
- MOB
main olfactory bulb, NO, nitric oxide
- NOS
nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- PG
periglomerular
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahern GP, Klyachko VA, Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci. 2002;25:510–517. doi: 10.1016/s0166-2236(02)02254-3. [DOI] [PubMed] [Google Scholar]
- Allen BW, Piantadosi CA, Coury LAJ. Electrode materials for nitric oxide detection. Nitric Oxide: Biology and Chemistry. 2000;4:75–84. doi: 10.1006/niox.2000.0273. [DOI] [PubMed] [Google Scholar]
- Alonso JR, Porteros A, Crespo C, Arevalo R, Brinon JG, Weruaga E, Aijon J. Chemical anatomy of the macaque monkey olfactory bulb: NADPH-diaphorase/nitric oxide synthase activity. J Comp Neurol. 1998;402:419–434. [PubMed] [Google Scholar]
- Bohlen HG. Mechanism of increased vessel wall nitric oxide concentrations during intestinal absorption. Am J Physiol Heart Circ Physiol. 1998;275:H542–H550. doi: 10.1152/ajpheart.1998.275.2.H542. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM, Snyder SH. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991;7:615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- Breer H, Shepherd GM. Implications of the NO/cGMP system for olfaction. Trends Neurosci. 1993;16:5–9. doi: 10.1016/0166-2236(93)90040-s. [DOI] [PubMed] [Google Scholar]
- Broillet MC. A single intracellular cysteine residue is responsible for the activation of the olfactory cyclic nucleotide-gated channel by NO. J Biol Chem. 2000;275:15135–15141. doi: 10.1074/jbc.275.20.15135. [DOI] [PubMed] [Google Scholar]
- Brunet A, Pailleret A, Devyneck MA, Devynck J, Bedioui F. Electrochemical sensing of nitric oxide for biological systems: methodological approach and new insights in examining interfering compounds. Talanta. 2003;61:53–59. doi: 10.1016/S0039-9140(03)00359-X. [DOI] [PubMed] [Google Scholar]
- Buerk D, Ances B, Greenberg J, Detre J. Temporal dynamics of brain tissue nitric oxide during functional forepaw stimulation in rats. Neuroimage. 2003a;18:1–9. doi: 10.1006/nimg.2002.1314. [DOI] [PubMed] [Google Scholar]
- Buerk DG. Can we model NO biotransport? A survey of mathematical models for a simple diatomic molecule with surprisingly complex biological activities. Annu Rev Biomed Eng. 2001;3:109–143. doi: 10.1146/annurev.bioeng.3.1.109. [DOI] [PubMed] [Google Scholar]
- Buerk DG, Atochin DN, Riva CE. Investigating the role of nitric oxide in regulating blood flow and oxygen delivery from in vivo electrochemical measurements in eye and brain. Adv Exp Med Biol. 2003b;530:359–370. doi: 10.1007/978-1-4615-0075-9_33. [DOI] [PubMed] [Google Scholar]
- Buerk DG, Riva CE. Vasomotor and spontaneous low frequency oscillations in blood flow and nitric oxide in cat optic nerve head. Microvasc Res. 1998;55:103–112. doi: 10.1006/mvre.1997.2053. [DOI] [PubMed] [Google Scholar]
- Buerk DG, Riva CE, Cranstoun SD. Nitric oxide has a vasodilatory role in cat optic nerve head during flicker stimuli. Microvasc Res. 1996;52:13–26. doi: 10.1006/mvre.1996.0040. [DOI] [PubMed] [Google Scholar]
- Carlson GC, Shipley MT, Keller A. Long-lasting depolarizations in mitral cells of the rat olfactory bulb. J Neurosci. 2000;20:2011–2021. doi: 10.1523/JNEUROSCI.20-05-02011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, Slawecki ML, Lancaster E, Keller A. Distribution and activation of intracellular Ca2+ stores in cultured olfactory bulb neurons. J Neurophysiol. 1997;78:2176–2185. doi: 10.1152/jn.1997.78.4.2176. [DOI] [PubMed] [Google Scholar]
- Chaigneau E, Oheim M, Audinat E, Charpak S. Two-photon imaging of capillary blood flow in olfactory bulb glomeruli. Proc Natl Acad Sci U S A. 2003;100:13081–13086. doi: 10.1073/pnas.2133652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaigneau E, Tiret P, Lecoq J, Ducros M, Knopfel T, Charpak S. The relationship between blood flow and neuronal activity in the rodent olfactory bulb. J Neurosci. 2007;27:6452–6460. doi: 10.1523/JNEUROSCI.3141-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput M, Holley A. Spontaneous activity of olfactory bulb neurons in awake rabbits, with some obervations on the effects of pentobarbital anaesthesia. J Physiol Paris. 1979;75:939–948. [PubMed] [Google Scholar]
- Chen JJ, Tu YJ, Moon C, Matarazzo V, Palmer AM, Ronnett GV. The localization of neuronal nitric oxide synthase may influence its role in neuronal precursor proliferation and synaptic maintenance. Develop Biol. 2004;269:165–182. doi: 10.1016/j.ydbio.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Chen WR, Shepherd GM. The olfactory glomerulus: a cortical module with specific functions. J Neurocytol. 2005;34:353–360. doi: 10.1007/s11068-005-8362-0. [DOI] [PubMed] [Google Scholar]
- Christodoulou D, Kudo S, Cook JA, Krishna MC, Miles A, Grisham MB, Murugesan R, Ford PC, Wink DA. Electrochemical methods for detection of nitric oxide. Methods in Enzymology. 1996;268:69–83. doi: 10.1016/s0076-6879(96)68010-0. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- Collmann C, Carlsson MA, Hansson BS, Nighorn A. Odorant-evoked nitric oxide signals in the antennal lobe of Manduca sexta. J Neurosci. 2004;24:6070–6077. doi: 10.1523/JNEUROSCI.0710-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo C, Gracia-Llanes FJ, Blasco-Ibanez JM, Gutierrez-Mecinas M, Marques-Mari AI, Martinez-Guijarro FJ. Nitric oxide synthase containing periglomerular cells are GABAergic in the rat olfactory bulb. Neurosci Lett. 2003;3449:151–154. doi: 10.1016/s0304-3940(03)00819-x. [DOI] [PubMed] [Google Scholar]
- Dellacorte C, Kalinoski DL, Huque T, Wysocki LM, Resrepo D. NADPH diaphorase staining suggests localization of nitric oxide synthase within mature vertebrate olfactory neurons. Neurosci. 1995;66:215–225. doi: 10.1016/0306-4522(94)00530-i. [DOI] [PubMed] [Google Scholar]
- Doving KB. Response properties of neurones in the rat olfactory bulb to various parameters of odour stimulation. Acta Physiol Scand. 1987;130:285–298. doi: 10.1111/j.1748-1716.1987.tb08139.x. [DOI] [PubMed] [Google Scholar]
- Edwards TM, Rickard NS. New perspectives on the mechanisms through which nitric oxide may affect learning and memory processes. Neurosci Biobehav Rev. 2007;31:413–425. doi: 10.1016/j.neubiorev.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Elphrick MR, Rayne RC, Riveros-Moreno V, Moncada S, O’Shea M. Nitric oxide synthesis in locust olfactory interneurons. J Exp Biol. 1995;198:821–829. doi: 10.1242/jeb.198.3.821. [DOI] [PubMed] [Google Scholar]
- Ferreira NR, Ledo A, Frade JG, Gerhardt GA, Laranjinha J, Barbosa RM. Electrochemical measurement of endogenously produced nitric oxide in brain slices using Nafion/o-phenylenediamine modified carbon fiber electrodes. Analytica Chimica Acta. 2005;535:1–7. [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Friedman D, Strowbridge BW. Both electrical and chemical synapses mediate fast network oscillations in the olfactory bulb. J Neurophysiol. 2003;89:2601–2610. doi: 10.1152/jn.00887.2002. [DOI] [PubMed] [Google Scholar]
- Fujie S, Aonuma H, Ito I, Gelperin A, Ito E. The nitric oxide/cyclic GMP pathway in the olfactory processing system of the terrestrial slug Limax marginatus. Zool Sci. 2002;19:15–26. doi: 10.2108/zsj.19.15. [DOI] [PubMed] [Google Scholar]
- Fujie S, Yamamoto T, Murakami J, Hatakeyama D, Shiga H, Suzuki N, Ito E. Nitric oxide synthase and soluble guanylyl cyclase underlying the modulation of electrical oscillations in a central olfactory organ. J Neurobiol. 2005;62:14–30. doi: 10.1002/neu.20046. [DOI] [PubMed] [Google Scholar]
- Fukamura D, Gohongi T, Kadambi A, Ang J, Yun C-O, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in VEGF-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite G, Bartus K, Malcolm D, Goodwin D, Kollb-Sielecka M, Dooldeniya C, Garthwaite J. Signaling from blood vessels to CNS axons through nitric oxide. J Neurosci. 2006;26:7730–7740. doi: 10.1523/JNEUROSCI.1528-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J, Garthwaite G, Palmer RM, Moncada S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol. 1989;172:413–416. doi: 10.1016/0922-4106(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Gelperin A. Nitric oxide mediates network oscillations of olfactory interneurons in a terrestrial mollusc. Nature. 1994;369:61–63. doi: 10.1038/369061a0. [DOI] [PubMed] [Google Scholar]
- Gelperin A. Olfactory computations and network oscillations. J Neurosci. 2006;26:1663–1668. doi: 10.1523/JNEUROSCI.3737-05b.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelperin A, Flores J, Raccuia-Behling F, Cooke IRC. Nitric oxide and carbon monoxide modulate oscillations of olfactory interneurons in a terrestrial mollusc. J Neurophysiol. 2000;83:116–127. doi: 10.1152/jn.2000.83.1.116. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Flores O, Beyer C, Lima-Hernandez FJ, Gomora-Arrati P, Gomez-Camarillo MA, Hoffman K, Etgen AM. Facilitation of estrous behavior by vaginal cervical stimulation in female rats involves alpha1-adrenergic receptor activation of the nitric oxide pathway. Behav Brain Res. 2007;176:237–243. doi: 10.1016/j.bbr.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara-Guzman R, Barrera-Mera B, de la Riva C, Kendrick KM. Release of classical transmitters and nitric oxide in the rat olfactory bulb, evoked by vaginocervical stimulation and potassium, varies with the oestrus cycle. Eur J Neurosci. 2000;12:80–88. doi: 10.1046/j.1460-9568.2000.00882.x. [DOI] [PubMed] [Google Scholar]
- Gurden H, Uchida N, Mainen ZF. Sensory-evoked intrinsic optical signals in the olfactory bulb are coupled to glutamate release and uptake. Neuron. 2006;52:335–345. doi: 10.1016/j.neuron.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Mecinas M, Crespo C, Blasco-Ibanez JM, Garcia-Llanes FJ, Marques-Mari AI, Martinez-Guijarrro FJ. Soluble guanylyl cyclase appears in a specific subset of periglomerular cells in the olfactory bulb. Eur J Neurosci. 2005;21:1443–1448. doi: 10.1111/j.1460-9568.2005.03960.x. [DOI] [PubMed] [Google Scholar]
- Hatton GI. Synaptic modulation of neuronal coupling. Cell Biol Int. 1998;22:765–780. doi: 10.1006/cbir.1998.0386. [DOI] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Ennis M, Shipley MT. External tufted cells: A major excitatory element that coordinates glomerular activity. J Neurosci. 2004;24:6676–6685. doi: 10.1523/JNEUROSCI.1367-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Shipley MT, Ennis M. Olfactory bulb external tufted cells are synchronized by multiple intraglomerular mechanisms. J Neurosci. 2005;25:8197–8208. doi: 10.1523/JNEUROSCI.2374-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann G, Hlushchuk R, Baum O, Scotti AL. Nitric oxide synthase protein levels, not the mRNA, are downregulated in olfactory bulb interneurons of reeler mice. J Chem Neuroanat. 2007;33:87–96. doi: 10.1016/j.jchemneu.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Hopkins DA, Steinbusch HW, Markerink-van Ittersum M, De Vente J. Nitric oxide synthase, cGMP, and NO-mediated cGMP production in the olfactory bulb of the rat. J Comp Neurol. 1996;375:641–658. doi: 10.1002/(SICI)1096-9861(19961125)375:4<641::AID-CNE6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Hopper RA, Garthwaite J. Tonic and phasic nitric oxide signals in hippocampal long-term potentiation. J Neurosci. 2006;26:11513–11521. doi: 10.1523/JNEUROSCI.2259-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS. Glutamate spillover mediates excitatory transmission in the rat olfactory bulb. Neuron. 1999;23:377–384. doi: 10.1016/s0896-6273(00)80787-4. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Murphy GJ. Glutamate-mediated extrasynaptic inhibition: direct coupling of NMDA receptors to Ca(2+)-activated K+ channels. Neuron. 2001;31:1027–1034. doi: 10.1016/s0896-6273(01)00428-7. [DOI] [PubMed] [Google Scholar]
- Ishii H, Shibuya K, Ohta Y, Mukai H, Uchino S, Takata N, Rose JA, Kawato S. Enhancement of nitric oxide production by association of nitric oxide synthase with N-methyl-D-aspartate receptors via postsynaptic density 95 in genetically engineered Chinese hamster ovary cells: real-time fluorescence imaging using nitric oxide sensitive dye. J Neurochem. 2006;96:1531–1539. doi: 10.1111/j.1471-4159.2006.03656.x. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Chemotopic odorant coding in a mammalian olfactory system. J Comp Neurol. 2007;503:1–34. doi: 10.1002/cne.21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM. Theta oscillations and sensorimotor performance. Proc Natl Acad Sci U S A. 2005;102:3863–3868. doi: 10.1073/pnas.0407920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Laurent G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nature Neurosci. 1999;2:1003–1009. doi: 10.1038/14801. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Guevara-Guzman R, Zorrilla J, Hinton MR, Broad KD, Mimmack M, Ohkura S. Formation of olfactory memories mediated by nitric oxide. Nature. 1997;388:670–674. doi: 10.1038/41765. [DOI] [PubMed] [Google Scholar]
- Kishimoto J, Keverne EB, Hardwick J, Emson PC. Localization of nitric oxide synthase in the mouse olfactory and vomeronasal system: a histochemical, immunological and in situ hybridization study. Eur J Neurosci. 1993;5:1684–1694. doi: 10.1111/j.1460-9568.1993.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Deans MR, Paul DL, Kosaka K. Neuronal gap junctions in the mouse main olfactory bulb: morphological analyses on transgenic mice. Neuroscience. 2005;134:757–769. doi: 10.1016/j.neuroscience.2005.04.057. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K. Intraglomerular dendritic link connected by gap junctions and chemical synapses in the mouse main olfactory bulb: electron microscopic serial section analyses. Neuroscience. 2005;131:611–625. doi: 10.1016/j.neuroscience.2004.11.050. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K. Heterogeneity of nitric oxide synthase-containing neurons in the mouse main olfactory bulb. Neurosci Res. 2007;57:165–178. doi: 10.1016/j.neures.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Lagier S, Carleton A, Lledo P-M. Interplay between local GABAergic interneurons and relay neurons generates gamma oscillations in the rat olfactory bulb. J Neurosci. 2004;24:4382–4392. doi: 10.1523/JNEUROSCI.5570-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledo A, Barbosa RM, Frade JG, Laranjinha J. Nitric oxide monitoring in hippocampal brain slices using electrochemical methods. Methods in Enzymology. 2002;359:111–125. doi: 10.1016/s0076-6879(02)59176-x. [DOI] [PubMed] [Google Scholar]
- Leonard CS, Michaelis EK, Mitchell KM. Activity-dependent nitric oxide concentration dynamics in the laterodorsal tegmental nucleus in vitro. J Neurophysiol. 2001;86:2159–2172. doi: 10.1152/jn.2001.86.5.2159. [DOI] [PubMed] [Google Scholar]
- Lowe G. Inhibition of backpropagating action potentials in mitral cell secondary dendrites. J Neurophysiol. 2002;88:64–85. doi: 10.1152/jn.2002.88.1.64. [DOI] [PubMed] [Google Scholar]
- Lowe G. Flash photolysis reveals a diversity of ionotropic glutamate receptors on the mitral cell somatodendritic membrane. J Neurophysiol. 2003;90:1737–1746. doi: 10.1152/jn.00180.2003. [DOI] [PubMed] [Google Scholar]
- Makara JK, Katona I, Nyiri G, Nemeth B, Ledent C, Watanabe M, de Vente J, Freund TF, Hajos N. Involvement of nitric oxide in depolarization-induced suppression of inhibition in hippocampal pyramidal cells during activation of cholinergic receptors. J Neurosci. 2007;27:10211–10222. doi: 10.1523/JNEUROSCI.2104-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Gervais R, Messaoudi B, Ravel N. Learning-induced oscillatory activities correlated to odour recognition: a network activity. Eur J Neurosci. 2006;23:1801–1810. doi: 10.1111/j.1460-9568.2006.04711.x. [DOI] [PubMed] [Google Scholar]
- Moore PK, Handy RL. Selective inhibitors of neuronal nitric oxide synthase--is no NOS really good NOS for the nervous system? Trends Pharmacol Sci. 1997;18:204–211. doi: 10.1016/s0165-6147(97)01064-x. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez B, Gonzalez-Forero D. Nitric oxide and synaptic dynamics in the adult brain: physiopathological aspects. Rev Neurosci. 2006;17:309–357. doi: 10.1515/revneuro.2006.17.3.309. [DOI] [PubMed] [Google Scholar]
- Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- Motokizawa F, Ogawa Y. Discharge properties of mitral/tufted cells in the olfactory bulb of cats. Brain Res. 1997;763:285–287. doi: 10.1016/s0006-8993(97)00511-8. [DOI] [PubMed] [Google Scholar]
- Muller U, Hildebrandt H. The nitric oxide/cGMP system in the antennal lobe of Apis mellifera is implicated in integrative processing of chemosensory stimuli. Eur J Neurosci. 1995;7:2240–2248. doi: 10.1111/j.1460-9568.1995.tb00645.x. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Isaacson JS. Presynaptic cyclic nucleotide-gated ion channels modulate neurotransmission in the mammalian olfactory bulb. Neuron. 2003;37:639–647. doi: 10.1016/s0896-6273(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Nighorn A, Gibson NJ, Rivers DM, Hildebrand JG, Morton DB. The nitric oxide-cGMP pathway may mediate communication between sensory afferents and projection neurons in the antennal lobe of Manduca sexta. J Neurosci. 1998;18:7244–7255. doi: 10.1523/JNEUROSCI.18-18-07244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, Buerk DG, Zhang M, Satin LS. Glucose-induced release of nitric oxide from mouse pancreatic islets as detected with nitric oxide-selective glass microelectrodes. Am J Physiol Endocrinol Metab. 2007;292:E907–912. doi: 10.1152/ajpendo.00518.2006. [DOI] [PubMed] [Google Scholar]
- Okere CO, Kaba H. Increased expression of neuronal nitric oxide synthase mRNA in the accessory olfactory bulb during the formation of olfactory recognition memory in mice. Eur J Neurosci. 2000;12:4552–4556. doi: 10.1046/j.0953-816x.2000.01325.x. [DOI] [PubMed] [Google Scholar]
- Okere CO, Kaba H, Higuchi T. Formation of an olfactory recognition memory in mice: reassessment of the role of nitric oxide. Neuroscience. 1996;71:349–354. doi: 10.1016/0306-4522(95)00467-x. [DOI] [PubMed] [Google Scholar]
- Oni J, Pailleret A, Isik S, Diab N, Radtke I, Blochl A, Jackson MB, Bedioui F, Schuhmann W. Functionalized electrode array for the detection of nitric oxide released by endothelial cells using different NO-sensing chemistries. Anal Bioanalyt Chem. 2004;378:1594–1600. doi: 10.1007/s00216-004-2512-6. [DOI] [PubMed] [Google Scholar]
- Pereira-Rodriques N, Zurgil N, Chang SC, Henderson JR, Bedioui F, McNeil CJ, Deutsch M. Combined system for the simultaneous optical and electrochemical monitoring of intra- and extracellular NO production by glioblastoma cells. Anal Chem. 2005;77:2733–2738. doi: 10.1021/ac0483163. [DOI] [PubMed] [Google Scholar]
- Puopolo M, Bean BP, Raviola E. Spontaneous activity of isolated dopaminergic periglomerular cells of the main olfactory bulb. J Neurophysiol. 2005;94:3618–3627. doi: 10.1152/jn.00225.2005. [DOI] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Sparse odor coding in the behaving mouse. J Neurosci. 2006;26:8857–8865. doi: 10.1523/JNEUROSCI.0884-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Grimaldi C, Gheusi G, Lledo PM, Estrada C. Chronic inhibition of nitric oxide synthesis enhances both subventricular zone neurogenesis and olfactory learning in adult mice. Eur J Neurosci. 2006;24:2461–2470. doi: 10.1111/j.1460-9568.2006.05127.x. [DOI] [PubMed] [Google Scholar]
- Sakura M, Kabetani M, Watanabe S, Kirino Y. Impairment of olfactory discrimination by blockade of nitric oxide activity in the terrestrial slug Limax valentianus. Neurosci Lett. 2004;370:257–261. doi: 10.1016/j.neulet.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- Seki Y, Aonuma H, Kanzaki R. Pheromone processing center in the protocerebrum of Bombyx mori revealed by nitric oxide-induced anti-cGMP immunocytochemistry. J Comp Neurol. 2005;481:340–351. doi: 10.1002/cne.20392. [DOI] [PubMed] [Google Scholar]
- Settembrini BP, Coronel MF, Nowicki S, Nighorn AJ, Villar MJ. Distribution and characterization of nitric oxide synthase in the nervous system of Triatoma infestans (Insecta: Heteroptera) Cell Tissue Res. 2007;328:421–430. doi: 10.1007/s00441-006-0359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spessert R, Wohlgemuth C, Reuss S, Layes E. NADPH-diaphorase activity of nitric oxide synthase in the olfactory bulb: co-factor specificity and characterization regarding the interrelation to NO formation. J Histochem Cytochem. 1994;42:569–575. doi: 10.1177/42.5.7512584. [DOI] [PubMed] [Google Scholar]
- Srisawat R, Ludwig M, Bull PM, Douglas AJ, Russell JA, Leng G. Nitric oxide and the oxytocin system in pregnancy. J Neurosci. 2000;20:6721–6727. doi: 10.1523/JNEUROSCI.20-17-06721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Zhang W. Cellular sources, targets and actions of constitutive nitric oxide in the magnocellular neurosecretory system of the rat. J Physiol. 2005;562:725–744. doi: 10.1113/jphysiol.2004.077735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susswein AJ, Katzoff A, Miller N, Hurwitz I. Nitric oxide and memory. Neuroscientist. 2004;10:153–162. doi: 10.1177/1073858403261226. [DOI] [PubMed] [Google Scholar]
- Thom SR, Bhopale V, Fisher D, Manevich Y, Huang PL, Buerk DG. Stimulation of nitric oxide synthase in cerebral cortex due to elevated partial pressures of oxygen: An oxidative stress response. J Neurobiol. 2002;51:85–100. doi: 10.1002/neu.10044. [DOI] [PubMed] [Google Scholar]
- Tsutsuki H, Kohda T, Hara M, Kozaki S, Ihara H. Nitric oxide inhibits depolarization-evoked glutamate release from rat cerebellar granule cells. Nitric Oxide. 2007;16:217–227. doi: 10.1016/j.niox.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Vukosavljevic N, Jaron D, Barbee KA, Buerk DG. Quantifying the L-arginine paradox in vivo. Microvasc Res. 2006;71:48–54. doi: 10.1016/j.mvr.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Wall MJ. Endogenous nitric oxide modulates GABAergic transmission to granule cells in adult rat cerebellum. Eur J Neurosci. 2003;18:869–878. doi: 10.1046/j.1460-9568.2003.02822.x. [DOI] [PubMed] [Google Scholar]
- Walsh RR. Single cell spike activity in the olfactory bulb. Am J Physiol. 1956;186:255–257. doi: 10.1152/ajplegacy.1956.186.2.255. [DOI] [PubMed] [Google Scholar]
- Wang S, Paton JF, Kasparov S. Differential sensitivity of excitatory and inhibitory synaptic transmission to modulation by nitric oxide in rat nucleus tractus solitarii. Exp Physiol. 2007;92:371–382. doi: 10.1113/expphysiol.2006.036103. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kikuchi M, Hatakeyama D, Shiga T, Yamamoto T, Aonuma H, Takahata M, Suzuki N, Ito E. Gaseous neuromodulator-related genes expressed in the brain of honeybee Apis mellifera. Dev Neurobiol. 2007;67:456–473. doi: 10.1002/dneu.20359. [DOI] [PubMed] [Google Scholar]
- Wellis DP, Scott JW, Harrison TA. Discrimination among odorants by single neurons of the rat olfactory bulb. J Neurophysiol. 1989;61:1161–1177. doi: 10.1152/jn.1989.61.6.1161. [DOI] [PubMed] [Google Scholar]
- Wiesinger H. Arginine metabolism and the synthesis of nitric oxide in the nervous system. Prog Neurobiol. 2001;64:365–391. doi: 10.1016/s0301-0082(00)00056-3. [DOI] [PubMed] [Google Scholar]
- Willse A, Belcher AM, Preti G, Wahl JH, Thresher M, Yang P, Yamazaki K, Beauchamp GK. Identification of major histocompatibility complex-regulated body odorants by statistical analysis of a comparative gas chromatography/mass spectrometry experiment. Anal Chem. 2005;77:2348–2361. doi: 10.1021/ac048711t. [DOI] [PubMed] [Google Scholar]
- Wilson CH, Christensen TA, Nighorn AJ. Inhibition of nitric oxide and soluble guanylyl cyclase signaling affects olfactory neuron activity in the moth, Manduca sexta. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2007;193:715–728. doi: 10.1007/s00359-007-0227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink DA, Christodoulou D, Ho M, Krishna MC, Cook JA, Haut H, Randolph JK, Sullivan M, Coia G, Murray RC, Meyer T. A discussion of electrochemical techniques for the detection of nitric oxide. Methods. 1995;7:71–77. [Google Scholar]
- Wollmuth LP, Kuner T, Sakmann B. Adjacent asparagines in the NR2-subunit of the NMDA receptor channel control the voltage-dependent block by extracellular Mg2+ J Physiol. 1998;506:13–32. doi: 10.1111/j.1469-7793.1998.013bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wink DA, Colton CA. Nitric oxide production and regulation of neuronal NOS in tyrosine hydroxylase containing neurons. Exp Neurol. 2004;188:341–350. doi: 10.1016/j.expneurol.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Zhang X. Real time and in vivo monitoring of nitric oxide by electrochemical sensors- from dream to reality. Front Biosci. 2004;9:3434–3446. doi: 10.2741/1492. [DOI] [PubMed] [Google Scholar]