Abstract
Cluster N is a cluster of forebrain regions found in night-migratory songbirds that shows high activation of activity-dependent gene expression during night-time vision. We have suggested that Cluster N may function as a specialized night-vision area in night-migratory birds and that it may be involved in processing light-mediated magnetic compass information. Here, we investigated these ideas. We found a significant lateralized dominance of Cluster N activation in the right hemisphere of European robins (Erithacus rubecula). Activation predominantly originated from the contralateral (left) eye. Garden warblers (Sylvia borin) tested under different magnetic field conditions and under monochromatic red light did not show significant differences in Cluster N activation. In the fairly sedentary Sardinian warbler (Sylvia melanocephala), which belongs to the same phyolgenetic clade, Cluster N showed prominent activation levels, similar to that observed in garden warblers and European robins. Thus, it seems that Cluster N activation occurs at night in all species within predominantly migratory groups of birds, probably because such birds have the capability of switching between migratory and sedentary life styles. The activation studies suggest that although Cluster N is lateralized, as is the dependence on magnetic compass orientation, either Cluster N is not involved in magnetic processing or the magnetic modulations of the primary visual signal, forming the basis for the currently supported light-dependent magnetic compass mechanism, are relatively small such that activity-dependent gene expression changes are not sensitive enough to pick them up.
Keywords: European robin, garden warbler, light-mediated magnetoreception, magnetic compass, night vision
Introduction
Migratory passerines have orientation skills that enable them to travel thousands of kilometres every spring and autumn between their breeding and wintering grounds. Passerines migrating at night can use a geomagnetic compass and/or celestial cues to orientate (e.g. Wiltschko, 1967; species overviews, Wiltschko & Wiltschko, 1996; Cochran et al., 2004). Despite intensive experimental efforts, the underlying physiological and molecular mechanism(s) of magnetoreception and detection of celestial constellations at night are still not fully understood. Currently, two hypotheses on how birds may sense the magnetic field are both supported by experimental evidence (review: Mouritsen & Ritz, 2005). One is based on superparamagnetic iron particles in the upper beak region (Fleissner et al., 2003; Mora et al., 2004). Whether in the beak or elsewhere, it has been proposed that magnetic field distortions affect ion channels, leading in turn to a change in membrane potential (Kirschvink et al., 2001). This magnetite hypothesis is mainly discussed in connection with a magnetic map or signpost sense (e.g. Munro et al., 1997a; Mora et al., 2004). The other hypothesis is based on a magnetic compass sense, where the bird extracts global directional information via a light-dependent radical-pair-based mechanism (Ritz et al., 2000, 2004). This mechanism requires light input and suggests that light-sensitive molecules in the retina form radical pairs upon photoexcitation. The direction of the magnetic field relative to the axis of the molecules will modulate the number of radical pairs existing in one of two states and thus indirectly affect the light sensitivity of specific chemical reactions (Ritz et al., 2000). Cryptochromes are the most probable candidates as radical-pair-forming photoreceptor molecules in the eyes of birds (Ritz et al., 2000). In support of this idea, cryptochromes are expressed in the retina of night-migratory birds (Möller et al., 2004; Mouritsen et al., 2004a), magnetic orientation of night-migratory birds is dependent on the availability of light with wavelengths (e.g. Wiltschko et al., 1993; Munro et al., 1997b; Wiltschko & Wiltschko, 1999; Rappl et al., 2000; Muheim et al., 2002) similar to the absorption spectrum of plant cryptochromes (Lin et al., 1995), and high-frequency oscillating magnetic fields that disturb the effects of magnetic fields on radical-pair reactions disrupt magnetic orientation under certain conditions (Ritz et al., 2004; Thalau et al., 2005).
If light-dependent processes in the eyes are involved in magneto-perception, birds should possess a brain area that is specialized in processing visual information at night. In a previous study, we identified such a candidate forebrain area and named it Cluster N; it is specifically activated during the night in dim light in night-migratory songbirds (Mouritsen et al., 2005). Blocking visual input inhibited activation in Cluster N. We could not find a well-defined Cluster N in nonmigratory birds. Thus, in night-migratory songbirds, Cluster N seems to be a brain region specialized for enhanced night-time vision, and it could possibly be used to sense geomagnetic field modulations of light-dependent magnetic compass signals and/or to perceive directional information from the stars.
To further elucidate the function of Cluster N and its possible role in processing magnetic information, we measured expression of neuronal activity-dependent immediate–early gene (IEG) expression in Cluster N at night under dim light following various visual, light and magnetic field manipulations. Clear differences in the neuronal activation pattern of Cluster N under any of these conditions would indicate involvement of Cluster N in processing light-mediated magnetic compass information, whereas no differences in Cluster N expression under these conditions have to be interpreted with caution (for details see Discussion). We also investigated Cluster N in a closely related species that shows different degrees of migratory behaviour.
Materials and methods
Animals
We examined two distantly related species of wild-caught night-migratory songbirds, European robins (Erithacus rubecula) and garden warblers (Sylvia borin), and a closely related nonmigratory songbird, the Sardinian warbler (Sylvia melanocephala). Twenty-six European robins were caught around Oldenburg during spring of 2003 and 2004. A total of 43 garden warblers were caught on Helgoland, around Oldenburg, Germany, or in Rybachy, Russia, during autumn 2001–02 and spring 2003. Five Sardinian warblers were caught in Navarra, Spain, in August 2004. All birds were housed indoors under a simulated local photoperiod. They were kept at least 3 days in captivity in Oldenburg before testing. Behavioural tests on European robins were performed during the spring migratory season from April 15 to May 25, 2003 and April 14–29, 2004. Tests on 35 garden warblers were performed during the autumn migratory season from August 22 to October 18, 2002 and 2003. Three garden warblers were tested during the nonmigratory season between July 17 and August 3, 2003. The test under monochromatic red light on five garden warblers was carried out during the nonmigratory season between December 13 and 22, 2004. The Sardinian warblers were tested during the autumn migratory season between September 11 and October 8, 2004. Some of these animals were also included in the previous experiments (Mouritsen et al., 2005). All animal procedures were approved by the Animal Care and Use Committees of the Bezirksregierung Weser-Ems (Oldenburg, Germany).
Behavioural setup
We used the behavioural setup described in Mouritsen et al. (2004a,b, 2005). It consists of a cylindrical, transparent Plexiglas cage (height 40 cm, diameter 40 cm) with a circular perch (diameter 20 cm) placed 8.5 cm above the ground in the centre of the cage. The cage was placed inside a 2 × 2 × 2 m Helmholtz coil system (Mouritsen, 1998), controlled by high-precision constant-current power supplies (Kepco BOP 50-2M). This allowed for controlled manipulation of the magnetic field in any direction. Four small light bulbs placed on the floor provided uniform dim illumination of ~1 mW/m2 intensity (equivalent to 0.04 lux, simulating a moonlit night). Two infrared-sensitive cameras, providing top and side views of the cage, were connected to a split-screen surveillance monitor to allow for real-time observation and recording to video (25 frames/s) and PC (5 frames/s) during day and night. Migratory birds tested in this setup performed stereotyped migratory restlessness behaviour or sat still and awake on the perch. Experiments took place in a wooden house providing access to an undisturbed natural magnetic field (67°± 1° inclination, 47 700 ± 600 nT total intensity).
Testing procedure
On the day of testing, a thin stripe of infrared-reflective tape (Retroreflective tape, 3M) was glued to the top of the bird’s head. The infrared reflective tape allowed us to track and analyse the bird’s orientation on digital videos using a custom-written Matlab program. This program computes the position of the bird’s head by screening each single frame for the white strip stemming from the reflective tape and relating its position to the centre of the cage. The birds were placed into the cylindrical cage between 12.30 and 13.30 h; food was removed 90 min before onset of darkness (onset of darkness during the experimental period was between 20.00 and 20.30 h). For the daytime control group, the birds were placed into the cage during daytime, without food, and observation started after ~1 h of habituation to the cage. This habituation period was necessary to let any handling-induced IEG expression decrease to baseline. After a bird performed a minimum of 45 min of the desired constant behaviour, i.e. either showing migratory restlessness (or regular movements during the day) or sitting still but awake, the animal was killed by decapitation and the brain was rapidly dissected. The two hemispheres were separated along the mid-sagittal plane, embedded in TissueTek O.C.T. (Sakura Finetek), and quick-frozen to −80 °C in a dry ice and ethanol bath.
Lateralization experiment
In birds, fibres of the optic nerve are 99% crossed and interhemispheric commissures are comparatively small (Weidner et al., 1985). Thus, primary visual input from the left eye is predominantly processed by the right hemisphere and vice versa. Testing birds with monocular light-tight eye caps therefore allowed us to analyse the extent of visual input perceived in the brain via each eye separately. It has been suggested that monocular eye covers have strong effects on magnetic orientation in European robins (Wiltschko et al., 2002). Therefore, we fitted European robins with light-tight eye caps (n = 8 left eye covered, n = 7 right eye covered), built of small black plastic–velour cylinders (diameter 10 mm) enlarged with Leucoplast (Beiersdorf AG, Hamburg, Germany) and light-tight black tape. The eye caps were glued to the bird’s head so that they covered the eyes completely without any direct contact with the eyes themselves. Controls for lateralization had either no eyes covered (n = 6) or both eyes covered (n = 5). The birds wearing unilateral eye caps were given 1 day to adapt to wearing the eye cap (the eye cover was fitted at noon the day before the experiment) before they were tested in the experiment. For birds wearing bilateral eye caps, these were fixed onto the bird’s head 3–5 h before onset of darkness. In order to avoid gene expression due to motor behaviour (G. Feenders, H. Mauritsen and E. D. Jarvis, personal communication) that might mask small differences in expression level due to potential magnetic sensing, we only included birds that had been sitting still but awake for a minimum of 45 min. Birds of all four groups were tested in a changing magnetic field. This allowed for direct comparison with the garden warblers (see below).
Magnetic field experiment
It is known that redstarts (Phoenicurus phoenicurus) cannot orientate in a zero magnetic field (ZMF; Mouritsen, 1998). Therefore, we placed garden warblers in the cylindrical cage and exposed them to one of three magnetic field conditions: (i) natural magnetic field (NMF) during night-time (n = 5); (ii) changing magnetic field (CMF) during night time with magnetic north switching 120° (clockwise then back) every 5 min (n = 13); and (iii) ZMF during night-time to eliminate all directional information from the geomagnetic field (n = 10; Mouritsen et al., 2004b). At ~1 h after onset of darkness, the birds were exposed to one of the three magnetic field conditions for a minimum of 45 min. As a control group we tested garden warblers during the day in a NMF (NMFday; n = 10).
Monochromatic red light experiment
Exposure to dim monochromatic red light with peak wavelength between 617 and 635 nm is known to disturb or disrupt magnetic orientation, at least temporarily, and the disruption seems strongest in the upper part of the range (Wiltschko et al., 1993; Rappl et al., 2000; Muheim et al., 2002; Wiltschko et al., 2004a,b). Therefore, we replaced the white light bulbs used to generate the dim light with an array of diodes emitting light in the red range between 600 and 700 nm with a clear peak at 650 nm. We then tested four garden warblers for 45 min under this red light (intensity 1.02 mW/m2) in a NMF and compared their Cluster N activation with simultaneously hybridized brain sections from animals obtained in the previous study (Mouritsen et al., 2005): five garden warblers tested in a NMF under dim white light and 10 garden warblers tested in a NMF during the day. We added one individual under the standard dim white light condition just to help normalize any potential differences between experiments.
Sardinian warblers
Three Sardinian warblers were tested under a CMF during night-time in dim white light, and two Sardinian warblers were tested in a NMF during day-time in full room light. We chose CMF in order to allow for direct comparison of the results with previous data collected from garden warblers.
Gene expression analysis
We used behavioural molecular mapping to visualize neuronal activation in Cluster N and the rest of the brain. Behavioural molecular mapping relies on the expression of IEGs to identify functional areas in the brain showing an increase in neural activation during specific behaviour and processing tasks (Jarvis & Nottebohm, 1997). We used in situ hybridization to visualize expression patterns of the IEG called ZENK (bird acronym; gene is known in other species as zif-268, egr-1, NGF-IA & krox-2; for methodological details see Wada et al., 2004 and Mouritsen et al., 2005) in 12-μm frozen sections. For the magnetic field condition experiments, frozen sections were cut throughout the whole brain of one individual per group: the left hemisphere in the sagittal plane and the right hemisphere in the frontal plane. As no magnetic condition-dependent differences were observed between the two hemispheres, we continued to cut the left hemisphere only in the remaining birds. We chose the sagittal plane as it provided the best overview of the Cluster N expression pattern. The sections were fixed in 4% paraformaldehyde and processed by in situ hybridization with antisense S35-UTP riboprobes made from a zebra finch ZENK cDNA. Expression of ZENK mRNA is driven by neuronal activation and occurs throughout the brain at high levels except in primary thalamic recipient neurons of the forebrain (field L2, entopallium and nucleus basorostralis), the globus pallidus, and parts of the thalamus (Mello & Clayton, 1994; Jarvis, 2004). Accumulated mRNA can be detected in neurons ~10–60 min after activation occurred, with a peak after ~30–45 min (Jarvis & Nottebohm, 1997). The hybridized sections were first exposed to X-ray film (Biomax; Kodak) and then dipped into autoradiographic emulsion (NTB2; Kodak) diluted 1 : 1 with water, incubated for 4–6 weeks at 4 °C, developed using Kodak developer (D19) and fixer, Nissl-stained with Cresyl Violet acetate (Sigma) and coverslipped with permount glue (Fisher). For quantification, the X-ray films of brain images were digitally scanned using a dissecting microscope and either a Diagnostic Instruments SPOT III or a Leica DFC320 camera. Using the Adobe Photoshop (Adobe Systems Inc.) software histogram tools, brain regions of interest were encircled and the mean pixel density was measured on a 256-level grey scale.
Quantification of ZENK expression in Cluster N was carried out as previously described (Mouritsen et al., 2005); the pixel density of the anterior ventral part of HA, IHA and MD was subtracted from the pixel density of the Cluster N area [comprising the posterior dorsal parts of the hyperpallium apicale (HA), intercalated hyperpallium apicale (IHA), dorsal mesopallium (MD), dorsal nucleus of the hyperpallium (DNH) and the DNH shell] see Fig. 3A and B. To test for asymmetries in Cluster N expression between the right and left unilateral eye-cover conditions, we determined the differences in Cluster ZENK activation (pixel density) between hemispheres of the same bird on the same microscope slide. We also examined nonsubtracted expression levels between groups of birds, but the variations among birds was too high to reliably use this measure for all comparisons.
Fig. 3.
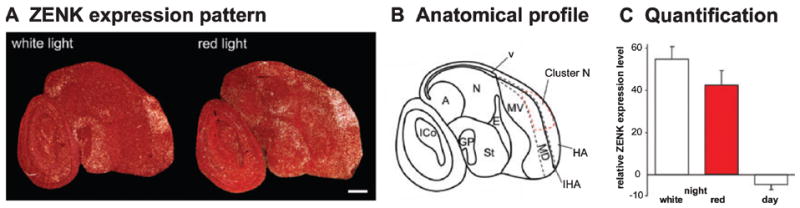
ZENK expression in garden warbler brains under dim white vs. dim red light. (A) Darkfield image of Cluster N ZENK expression under dim white light at sagittal section, night (left) and after 45 min exposure to dim-monochromatic red light (right). Rostral is right, dorsal is up. (B) Anatomical profile of the right image shown in A. (C) Quantification of ZENK expression levels in Cluster N, comparing exposure to standard white light with red light. IHA, intercalated hyperpallium apicale; for other abbreviations see Fig. l legend. Error bars are SEM. Scale bar, 1 mm.
Results
Lateralization
To further investigate the role of vision in Cluster N activation, we performed monocular and binocular eye-cover experiments with European robins. Prior to covering the eyes, the European robins showed correct magnetic orientation directed towards north-east consistent with the birds’ natural spring migratory direction (Fig. 1A; α = 38°, r = 0.64, P < 0.001). No celestial cues were available to the birds so they had to rely on magnetic field information only. Birds with monocular eye covers showed less neuronal activation of Cluster N in the brain hemisphere contralateral to the covered eye than to the ipsilateral hemisphere (Fig. 1C and D; one-way ANOVA, followed by Holm–Sidak multicomparison test; P < 0.001). However, ZENK activation in the contralateral Cluster N (with respect to the covered eye) was still significantly higher than the levels seen in the corresponding hemisphere of birds that had both their eyes covered (Fig. 1B and D; t-test comparing the contralateral expression (with respect to the covered eye) in birds with one eye covered vs. the expression in the equivalent hemisphere in birds with both eyes covered, P = 0.005). This significant activation of Cluster N in the hemisphere that receives its main input from the occluded contralateral eye is presumably due to ipsilateral post-thalamic recrossing of pathways from the open eye (Schmidt & Bischof, 2001).
Fig. 1.
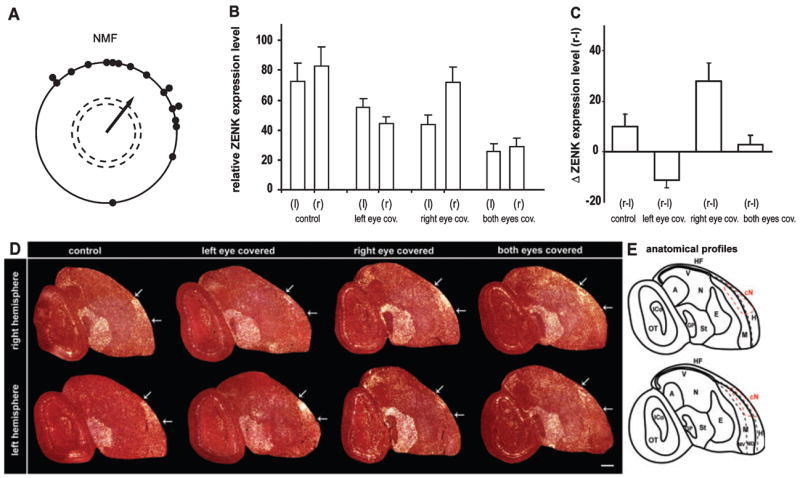
ZENK expression in European robin brains under different eye-cover conditions. (A) Orientation of European robins during spring migration in an NMF in our behavioural apparatus. Each dot represents the mean orientation of one individual bird. The arrow indicates the mean orientation of the group. The length of the arrow represents the length (r-value) of the group mean vector. The dashed circles indicate the radius of the group mean vector needed for significance (P < 0.05 and P < 0.01) according to the Rayleigh Test of uniformity. (B) ZENK expression levels in Cluster N of the right (r) and left (l) hemisphere for four groups of birds with various eye open and covered conditions. (C) Direct within-bird subtracted comparisons of the interhemispheric differences in ZENK expression levels for the four experimental groups. One-way ANOVA comparison between all groups revealed highly significant differences for all group comparisons (P < 0.001), except for the comparison between the two control groups: birds with both eyes open (control) and birds with both eyes covered. (D) Darkfield image of ZENK expression in right and left hemisphere saggital sections showing Cluster N expression under different eye-cover conditions. White arrows mark the caudal and rostral boundaries of Cluster N for each brain section. Rostral is right, dorsal is up. (E) Anatomical profile of the right image (condition: both eyes covered) shown in (D). Abbreviations: A, arcopallium; Cn, Cluster N; E, entopallium; GP, globus pallidus; HF, hippocampal formation; H, hyperpallium; ICo, inferior colliculus; M, mesopallium including ventral (MV) and dorsal (MD) part; N, nidopallium; OT, optic tectum; St, striatum; v, ventricle; W, visual Wulst. Error bars are SEM. Scale bar, 1 mm.
The extent of ipsilateral Cluster N activation was similar for the two unilateral eye-cover conditions (Fig. 1B; t-test, P = 0.94; comparing Cluster N activation of the left hemisphere for birds with the right eye covered and the right hemisphere of birds with the left eye covered). In contrast, the contralateral activation of Cluster N between the hemispheres of birds with one eye covered was significantly higher when the right eye was covered than when the left eye was covered (Fig. 1B and C; t-test, P = 0.021, comparing left minus right hemisphere for left eye covered birds with right minus left hemisphere for right eye covered birds). This asymmetry was also present in birds with both eyes open, where ZENK activation in the right Cluster N of European robins was significantly higher than in the left Cluster N (see Fig. 1C; paired t-test, P = 0.007, within-bird comparisons). When both eyes were covered, in addition to the ZENK decrease in both hemispheres, the hemispheric asymmetry disappeared (Fig. 1C; paired t-test, P = 0.989, within-bird comparisons). These results indicate that, in European robins, Cluster N is a lateralized night-vision brain area that is right-dominant.
Magnetic conditions
To investigate a possible role of Cluster N in magnetic field processing, we placed garden warblers in the cylindrical cage and tested them under different magnetic field conditions. We chose garden warblers as this is the species in which Cluster N was initially characterized (Mouritsen et al., 2005). Prior to the ZENK mapping experiments, as previously described (Mouritsen et al., 2004b, 2005), our garden warblers showed a preference for orientation, which was towards south-west in autumn under NMF, whereas they did not show significant directional orientation under ZMF (Fig. 2A and B). Thus, our birds did use the magnetic field for orientation. However, the expression level of ZENK in Cluster N did not differ significantly between birds that were exposed to NMF, ZMF or CMF (Fig. 2C, one-way ANOVA followed by Holm–Sidak multicomparison test: NMF vs. ZMF, P = 0.085; NMF vs. CMF, P = 0.145; CMF vs. ZMF, P = 0.660). All three groups were significantly different from the day-time NMF group (Fig. 2C, one-way ANOVA, followed by Holm–Sidak multicomparison test, P < 0.001).
Fig. 2.
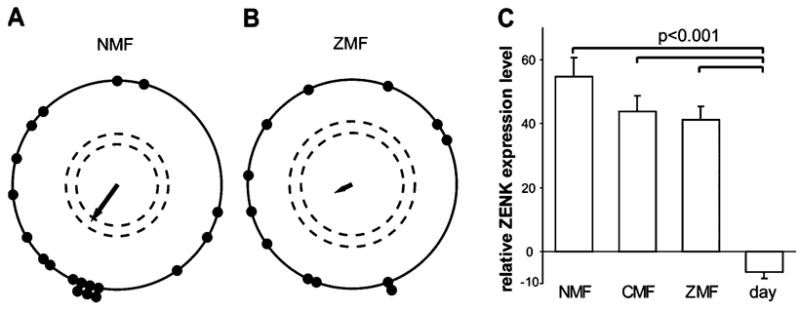
Relative ZENK expression levels in Cluster N (difference in expression between Cluster N and the brain subdivisions rostral to Cluster N; see Materials and methods) in garden warbler brains under different magnetic field conditions. (A and B) Orientation of garden warblers during autumn migration in (A) an NMF and (B) a ZMF (symbols are as in Fig. 1). Panels A and B reproduced (with permission from Elsevier) from Mouritsen et al. (2004b), as the results reported here originate from the same individual birds as used in that study. (C) Relative ZENK expression levels in Cluster N in birds exposed to NMF, CMF or ZMF compared with birds collected during day-time (day, NMF). Error bars are SEM.
Monochromatic red light
To determine whether the wavelength of dim light is important for Cluster N neuronal activation, we tested garden warblers exposed to red light with a peak wavelength of 650 nm. Red light exposure with a peak wavelength from 617 to 635 nm has previously been shown to temporarily disrupt or lead to inappropriately orientated magnetic orientation behaviour in several bird species including garden warblers (Wiltschko et al., 1993; Rappl et al., 2000; Muheim et al., 2002; Wiltschko et al., 2004b; but see Wiltschko et al., 2004a for a weakness of this result). ZENK expression in Cluster N in garden warblers exposed to dim (intensity 1.02 mW/m2) red light did not differ significantly from the expression seen under dim white light; under red light, ZENK expression was still significantly higher than the expression level during the day (Fig. 3, one-way ANOVA followed by Holm–Sidak multicomparison test, red light vs. white light, P = 0.342; red light vs. day, P < 0.001). As we conducted these experiments during the winter, when garden warblers are not migrating, the results provided further evidence that Cluster N is activated to a similar degree throughout the year (Mouritsen et al., 2005). The present data complete the annual cycle to show that Cluster N activation is affected neither by season nor by migratory behaviour itself.
Sardinian warblers
We found high activation of Cluster N in fairly sedentary Sardinian warblers tested in CMF under dim white light at night (Fig. 4A). The ZENK expression pattern in Cluster N was very similar to that seen in garden warblers and European robins. Cluster N ZENK expression levels in Sardinian warblers during night-time was significantly higher than during day-time (Fig. 4C; t-test, P = 0.005, t = −7.677). However, in Sardinian warblers during day-time, the shell around the dorsal nucleus of the hyperpallium showed high ZENK expression especially in one of the two birds tested (not shown). This was never observed in the garden warblers. The reason for this variation in expression patterns remains unclear.
Fig. 4.
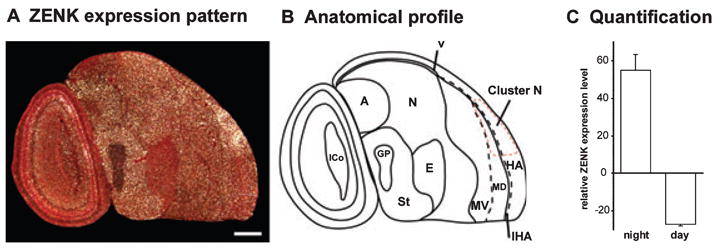
ZENK expression in Sardinian warblers. (A) Darkfield image of ZENK expression in a sagittal section showing Cluster N. Rostral is right, dorsal is up. (B) Anatomical profile of the image shown in (A). The red dashed line marks the boundary of Cluster N. (C) Quantification of ZENK expression levels in Cluster N, comparing day-time and night-time groups. For abbreviations see Fig. 1 legend. Error bars are SEM. Scale bar, 1 mm.
Discussion
Recently, we described Cluster N as a brain area that is specifically activated during night-time light conditions in night-migratory songbirds (Mouritsen et al., 2005). We suggested that this brain area could be involved in special night vision and possibly in information processing of magnetic field and/or star compass orientation. In the present study, our findings do not prove or disprove whether Cluster N is involved in processing of magnetic information, but they do strengthen the conclusion that Cluster N is a night-time vision brain area specialized in primarily migratory clades of birds.
The eye-cover experiments revealed a significant dominance of the right hemisphere in light-induced activation of Cluster N. This right hemisphere dominance in Cluster N activation is presumably due to contralateral rather than ipsilateral input because the activation of the ipsilateral Cluster N is the same whether the right or the left eye is covered. In other words, activation of the left Cluster N in birds with their right eye covered is not different from the activation of the right Cluster N in birds with their left eye covered. This observation adds to the mounting evidence which shows that there are asymmetries in the organization of both the tectofugal and thalamofugal visual pathways in birds (e.g. Bischof & Watanabe, 1997; for a review see Rogers, 1996; Güntürkün, 2002, 2006). Several studies have demonstrated a preferential use of the right eye and left hemisphere in food discrimination of chicks, pigeons and zebra finches (Alonso, 1998; Mench & Andrew, 1986; Güntürkün & Kesch, 1987), and the left eye and right hemisphere in social recognition (Vallortigara, 1998; George et al., 2006). Based upon these findings it has been proposed that, in birds, the left hemisphere is involved in sustaining attention on an object while the right hemisphere is used to analyse details and control rapid responses (George et al., 2006). If a similar property exists for Cluster N, then an intuitive interpretation would be that processing of the Earth’s magnetic field and/or starry sky may require attention to detail, a right hemisphere visual brain function.
Our findings suggesting dominance for Cluster N of the left-eye–right-hemisphere need to be reconciled with behavioural experiments that suggest a dominance of the right-eye–left-hemisphere in magnetic compass orientation behaviour (Wiltschko et al., 2002). However, this may not be a conflict. First, our data and those of Wiltschko et al. (2002) are not directly comparable. Wiltschko et al. (2002) used behavioural responses, which derive from a multitude of processes, whereas the present study uses ZENK activation patterns in the forebrain only. Second, it is possible that Cluster N is not involved in processing magnetic compass information. If true, then one or more other brain areas should be processing magnetic compass information in birds performing magnetic compass orientation and should be detectable as an active region by means of behavioural molecular mapping. Despite a careful analysis of the entire brain, we found no forebrain region other than Cluster N showing dominant and consistent ZENK expression during night-time magnetic compass orientation (Mouritsen et al., 2005). If, on the other hand, Cluster N indeed processes magnetic compass information then it would be one of the first times that less activation of a brain region (the right Cluster N) would be associated with behavioural dominance of the same hemisphere.
The data on garden warblers tested under various magnetic field conditions, including a ZMF, revealed consistently high IEG expression in Cluster N. The ZMF group did show the lowest ZENK expression levels, but differences between the magnetic field groups at night were nonsignificant. If Cluster N is integrating magnetic field information, one could assume this area to show a distinctly different activity pattern because a ZMF scenario does not provide any magnetic information at all. However, if we assume that the underlying receptive mechanism is light-based then light itself is the primary signal on which the magnetic field imposes small modulations. Consequently, whenever light is available, as it was in our magnetic field study, radical pairs should be formed as a result of photoexcitation. In a NMF, there are expected to be small modulations of the singlet–triplet proportions (probably a few per cent) in different parts of the retina (Ritz et al., 2000). In a ZMF, the radical pairs are expected to occur in a default proportion of singlet and triplet states throughout the eye and independently of the orientation of the radical-pair-forming molecules. In both NMF and ZMF, the light-induced signals will have to be sent to the brain for further processing (e.g. to compare the input from different parts of the retina) irrespective of the magnetic field condition. Therefore, if the putative magnetic modulations are generally small and/or modulated in opposite directions, behavioural molecular mapping may not be sensitive enough to detect magnetic modulations of neuronal signals in Cluster N that could occur under different magnetic field conditions. The IEG expression indicates the average expression level in all neurons within a certain brain area over a time span of ~45–60 min. Thus, the lack of differences in Cluster N ZENK activation in the manipulated magnetic field conditions does not rule out Cluster N as having a role in processing of magnetic compass information.
Birds exposed to monochromatic red light with a peak at 650 nm showed prominent ZENK expression in Cluster N at night. The activation in Cluster N under red light seemed to be slightly lower than under white light, but this difference was not significant. Initially, red light was shown to temporarily disrupt or lead to inappropriately aligned magnetic orientation under certain conditions (Wiltschko et al., 1993, 2001; Wiltschko & Wiltschko, 1999, 2001b; Muheim et al., 2002). However, recently Wiltschko et al. (2004a) subsequently showed that when European robins are pre-exposed to the red light for ~1 h, the birds seem to regain their orientation capabilities. Thus, possible explanations for the robust Cluster N activation we found in our birds tested under red light are: (i) the primary sensor is sensitive to red light but the brain needs time to interpret the unusual signals correctly; (ii) the 45 min of pre-exposure to red light was long enough to modify the sensitivity of the primary receptor to respond to red light; or (iii) a signal originating from an alternative receptor system (for discussion, see Wiltschko et al., 2004a) immediately leads to Cluster N activation. The latter interpretation is in line with recent findings supporting the suggestion that at least two different receptor types exist in the retina of birds (Wiltschko et al., 2004a,b). It could be that a second receptor type gets activated under our red-light conditions and sends a signal to the brain which is reflected as Cluster N activation.
Our finding that the neuronal activation of Cluster N is seen during both migratory and nonmigratory seasons suggest that the specialized night vision processing can occur at all times of the year. It is commonly observed in other seasonally relevant sensory systems that sensory input is processed year-round, even during seasons when the information is not of behavioural relevance. For example in canaries, which are seasonal breeders, ZENK is equally strongly activated in auditory forebrain areas year-round (Jarvis & Nottebohm, 1997) even though, for this and other species, song processing is more relevant during the breeding season and leads to important behavioural responses at that time (Nottebohm et al., 1987).
The experiment with Sardinian warblers revealed significant neuronal activation in Cluster N at night but not during the day, thus providing a similar pattern of Cluster N activation to that observed in garden warblers. Sardinian warblers are fairly sedentary in the Mediterranean and are frequently used as a nonmigratory Sylvia warbler in comparative studies (e.g. Healy et al., 1996; Mettke-Hofmann & Gwinner, 2003). Nevertheless, it has been observed that individuals of this species undertake directed movements in autumn and spring (Cramp, 1998), and some of our birds actually performed some migratory restlessness at night. Although there is no evidence in the literature about how Sardinian warblers may orientate, it is probable that this species is capable of orientating, e.g. according to the magnetic field. In fact, nonmigratory birds can also use a magnetic compass (homing pigeons, Columba livia: Keeton, 1971; Wiltschko & Wiltschko, 2001a; chick, Gallus domesticus: Freire et al., 2005), but it is not yet clear whether the mechanism is the same in (night) migrants and nonmigrants. Further evidence that at least a partial migratory behaviour is likely to exist in Sardinian warblers is provided by the studies of Pulido et al. (1996). They have shown that within natural populations of blackcaps (Sylvia atricapilla) and probably many other species there exist individuals showing more migratory behaviour, less migratory behaviour or no migratory behaviour at all, and that many populations can evolve into a migratory or nonmigratory state within a few generations. Therefore, it is probable that all species in a predominantly night-migratory clade of birds, such as the Sylvia warblers, possess the ability to orientate according to a magnetic compass because sedentary–migratory behaviour has probably evolved and re-evolved repeatedly over the last 10 000 years (review: Berthold, 1999; Piersma et al., 2005). Consequently, predominantly migratory clades are likely to possess the ability to orientate at night using the magnetic field and they would therefore be expected to have a functional Cluster N if it is indeed used for integrating light-mediated magnetic compass information.
In conclusion, our results suggest a marked dominance of the right hemisphere in processing the visual component integrated in Cluster N. The signal sensed and the information asymmetrically processed in Cluster N is light-mediated. If the vision-mediated information processed and integrated in Cluster N were of magnetic nature, this would be in line with theoretical (Ritz et al., 2000), molecular (Mouritsen et al., 2004a) and behavioural (e.g. Mouritsen et al., 2004b; Ritz et al., 2004) evidence suggesting that the Earth’s magnetic field modulates the light sensitivity of specialized receptor molecules differently in various parts of the retina. Tests under various magnetic field conditions and under red light could not be used to clarify whether Cluster N is an integrative area for magnetic compass orientation or whether it is ‘just’ a night vision area specialized in night-migratory species, probably including their close relatives. A specialized night vision area may be necessary to extract directional information from the starry sky, or it may trigger the bird’s alertness to initiate night-migratory behaviour. Electrophysiological recordings and studies with lesions of Cluster N will be crucial to elucidate the ultimate function of Cluster N.
Acknowledgments
This work was supported by a VolkwagenStiftung Nachwuchsgruppe grant to H.M. and the University of Oldenburg to H.M., and the NSF Waterman Award to E.D.J. We thank the Institut für Vogelforschung Wilhelmshaven und Helgoland and the Biological Station Rybachy for help catching and keeping the garden warblers, and A. Artázcoz and the Government of Navarra (Spain) for help and support in catching and exporting the Sardinian warblers. We thank the botanical garden in Oldenburg for allowing us to catch some of our Robins there. We thank Andreas Sommer (University of Oldenburg) for building the observation cages, and the staff of the electronics workshop at the University of Oldenburg for other assistance.
Abbreviations
- CMF
changing magnetic field
- IEG
immediate–early gene
- NMF
natural magnetic field
- ZENK
bird acronym
- gene is known in other species as zif-268
egr-1, NGF-IA & krox-2
- ZMF
zero magnetic field
References
- Alonso Y. Lateralization of visual guided behavior during feeding in zebra finches (Taeniopygia guttata) Behav Proc. 1998;43:257–263. doi: 10.1016/s0376-6357(98)00015-1. [DOI] [PubMed] [Google Scholar]
- Berthold P. A comprehensive theory for the evolution, control and adaptability of avian migration. Ostrich. 1999;70:1–11. [Google Scholar]
- Bischof HJ, Watanabe S. On the structure and function of the tectofugal visual pathway in laterally eyed birds. Eur J Morph. 1997;35:246–254. doi: 10.1076/ejom.35.4.246.13080. [DOI] [PubMed] [Google Scholar]
- Cochran WW, Mouritsen H, Wikelski M. Migratory songbirds recalibrate their magnetic compass daily from twilight cues. Science. 2004;304:405–408. doi: 10.1126/science.1095844. [DOI] [PubMed] [Google Scholar]
- Cramp S. The Complete Birds of the Western Palaearctic, CD-ROM. Oxford University Press; Oxford: 1998. [Google Scholar]
- Fleissner G, Holtkamp-Rötzler E, Hanzlik M, Fleissner G, Petersen N, Wiltschko W. Ultrastructural analysis of a putative magnetoreceptor in the beak of homing pigeons. J Comp Neurol. 2003;458:350–360. doi: 10.1002/cne.10579. [DOI] [PubMed] [Google Scholar]
- Freire R, Munro U, Rogers L, Wiltschko R, Wiltschko W. Chickens orient using a magnetic compass. Curr Biol. 2005;15:R620–R621. doi: 10.1016/j.cub.2005.08.017. [DOI] [PubMed] [Google Scholar]
- George I, Hara E, Hessler NA. Behavioral and neural lateralization of vision in courtship singing of the zebra finch. J Neurobiol. 2006;66:1164–1173. doi: 10.1002/neu.20273. [DOI] [PubMed] [Google Scholar]
- Güntürkün O. Hemispheric asymmetry in the visual system of birds. In: Hugdahl K, Davidson RJ, editors. Brain Asymmetries. 2. MIT Press; Cambridge, MA: 2002. [Google Scholar]
- Güntürkün O. Avian cerebral asymmetries: The view from the inside. Cortex. 2006;42:104–106. doi: 10.1016/s0010-9452(08)70331-9. [DOI] [PubMed] [Google Scholar]
- Güntürkün O, Kesch S. Visual lateralization during feeding in pigeons. Behav Neurosci. 1987;101:433–435. doi: 10.1037//0735-7044.101.3.433. [DOI] [PubMed] [Google Scholar]
- Healy S, Gwinner E, Krebs JR. Hippocampal volume in migratory and non-migratory warblers: Effects of age and experience. Behav Brain Res. 1996;81:61–68. doi: 10.1016/s0166-4328(96)00044-7. [DOI] [PubMed] [Google Scholar]
- Jarvis ED. Brains and birdsong. In: Marler P, Slabbekoorn H, editors. Nature’s Music: the Science of Bird Song. Elsevier-Academic Press; San Diego: 2004. pp. 226–271. [Google Scholar]
- Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc Natl Acad Sci USA. 1997;94:4097–4102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeton WT. Magnets interfere with pigeon homing. Proc Natl Acad Sci USA. 1971;68:102–106. doi: 10.1073/pnas.68.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschvink JL, Walker MM, Diebel CE. Magnetite-based magnetoreception. Curr Opin Neurobiol. 2001;11:462–467. doi: 10.1016/s0959-4388(00)00235-x. [DOI] [PubMed] [Google Scholar]
- Lin C, Ahmad M, Gordon D, Cashmore AR. Expression of an Arabidopsis cryptochrome gene in transgenic tobacco results in hypersensitivity to blue, UV-A, and green light. Proc Natl Acad Sci USA. 1995;92:8423–8427. doi: 10.1073/pnas.92.18.8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mench JA, Andrew RJ. Lateralization of a food search task in the domestic chick. Behav Neural Biol. 1986;46:107–114. doi: 10.1016/s0163-1047(86)90570-4. [DOI] [PubMed] [Google Scholar]
- Mettke-Hofmann C, Gwinner E. Long-term memory for a life on the move. Proc Natl Acad Sci USA. 2003;100:5863–5866. doi: 10.1073/pnas.1037505100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller A, Sagasser S, Wiltschko W, Schierwater B. Retinal cryptochrome in a migratory passerine bird: a possible transducer for the avian magnetic compass. Naturwissenschaften. 2004;91:585–588. doi: 10.1007/s00114-004-0578-9. [DOI] [PubMed] [Google Scholar]
- Mora CV, Davison M, Wild JM, Walker MM. Magnetoreception and its trigeminal mediation in the homing pigeon. Nature. 2004;432:508–511. doi: 10.1038/nature03077. [DOI] [PubMed] [Google Scholar]
- Mouritsen H. Redstarts, Phoenicurus phoenicurus, can orient in a true-zero magnetic field. Anim Behav. 1998;55:1311–1324. doi: 10.1006/anbe.1997.0696. [DOI] [PubMed] [Google Scholar]
- Mouritsen H, Feenders G, Liedvogel M, Kropp W. Migratory birds use head scans to detect the direction of the earth’s magnetic field. Curr Biol. 2004b;14:1946–1949. doi: 10.1016/j.cub.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Mouritsen H, Feenders G, Liedvogel M, Wada K, Jarvis ED. A night vision brain area in migratory songbirds. Proc Natl Acad Sci USA. 2005;102:8339–8344. doi: 10.1073/pnas.0409575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen H, Janssen-Bienhold U, Liedvogel M, Feenders G, Stalleicken J, Dirks P, Weiler R. Cryptochrome and activity markers co-localise in bird retina during magnetic orientation. Proc Natl Acad Sci USA. 2004a;101:14294–14299. doi: 10.1073/pnas.0405968101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen H, Ritz T. Magnetoreception and its use in bird navigation. Curr Opin Neurobiol. 2005;15:406–414. doi: 10.1016/j.conb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Muheim R, Backman J, Akesson S. Magnetic compass orientation in European robins is dependent on both wavelength and intensity of light. J Exp Biol. 2002;205:3845–3856. doi: 10.1242/jeb.205.24.3845. [DOI] [PubMed] [Google Scholar]
- Munro U, Munro JA, Philips JB, Wiltschko W. Effect of wavelength of light and pulse magnetisation on different magnetoreception systems in a migratory bird. Aust J Zool. 1997b;45:189–198. [Google Scholar]
- Munro U, Munro JA, Phillips JB, Wiltschko R, Wiltschko W. Evidence for a magnetite-based navigational ‘map’ in birds. Naturwissenschaften. 1997a;84:26–28. [Google Scholar]
- Nottebohm F, Nottebohm ME, Crane LA, Wingfield JC. Seasonal-changes in gonadal hormone levels of adult male canaries and their relation to song. Behav Neural Biol. 1987;47:197–211. doi: 10.1016/s0163-1047(87)90327-x. [DOI] [PubMed] [Google Scholar]
- Piersma T, Pérez-Tris J, Mouritsen H, Bauchinger U, Bairlein F. Is there a ‘migratory syndrome’ common to all migrant birds? Ann NY Acad Sci. 2005;1046:282–293. doi: 10.1196/annals.1343.026. [DOI] [PubMed] [Google Scholar]
- Pulido F, Berthold P, van Noordwijk AJ. frequency of migrants and migratory activity are genetically correlated in a bird population: Evolutionary implications. Proc Natl Acad Sci USA. 1996;93:14642–14647. doi: 10.1073/pnas.93.25.14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappl R, Wiltschko R, Weindler P, Berthold P, Wiltschko W. Orientation behavior of garden warblers (Sylvia borin) under monochromatic light of various wavelengths. Auk. 2000;117:256–260. [Google Scholar]
- Ritz T, Adem S, Schulten K. A model for photoreceptor-based magnetoreception in birds. Biophys J. 2000;78:707–718. doi: 10.1016/S0006-3495(00)76629-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T, Thalau P, Philips JB, Wiltschko R, Wiltschko W. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature. 2004;429:177–180. doi: 10.1038/nature02534. [DOI] [PubMed] [Google Scholar]
- Rogers L. Behavioral, structural and neurochemical asymmetries in the avian brain: a model system for studying visual development and processing. Neurosci Behav Rev. 1996;20:487–503. doi: 10.1016/0149-7634(95)00024-0. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Bischof HJ. Integration of information from both eyes by single neurons of nucleus rotundus, ectostriatum and lateral neostriatum in the zebra finch (Taeniopygia guttata castanotis Gould) Brain Res. 2001;923:20–31. doi: 10.1016/s0006-8993(01)03192-4. [DOI] [PubMed] [Google Scholar]
- Thalau P, Ritz T, Stapput K, Wiltschko R, Wiltschko W. Magnetic compass orientation of migratory birds in the presence of a 1.315 MHz oscillating field. Naturwissenschaften. 2005;92:86–90. doi: 10.1007/s00114-004-0595-8. [DOI] [PubMed] [Google Scholar]
- Vallortigara G. Right hemisphere advantage for social recognition in the chick. Neuropsychology. 1998;9:761–768. doi: 10.1016/0028-3932(92)90080-6. [DOI] [PubMed] [Google Scholar]
- Wada K, Sakaguchi H, Jarvis ED, Hagiwara M. Differential expression of glutamate receptors in avian neural pathways for learned vocalization. J Comp Neurol. 2004;476:44–64. doi: 10.1002/cne.20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner C, Reperant J, Miceli D, Haby M, Rio JP. An anatomical study of ipsilateral retinal projections in the quail using radioautographic, horseradish peroxidase, fluorescence and degeneration techniques. Brain Res. 1985;340:99–108. doi: 10.1016/0006-8993(85)90778-4. [DOI] [PubMed] [Google Scholar]
- Wiltschko W. Über den Einfluß statischer Magnetfelder auf die Zugorientierung der Rotkehlchen (Erithacus rubecula) Z Tierpsychol. 1967;25:537–558. [PubMed] [Google Scholar]
- Wiltschko W, Gesson M, Stapput K, Wiltschko R. Light-dependent magnetoreception in birds: interaction of at least two different receptors. Naturwissenschaften. 2004b;91:130–134. doi: 10.1007/s00114-003-0500-x. [DOI] [PubMed] [Google Scholar]
- Wiltschko R, Gesson M, Wiltschko W. Magnetic compass orientation of European robins under 565 nm green light. Naturwissenschaften. 2001;88:387–390. doi: 10.1007/s001140100248. [DOI] [PubMed] [Google Scholar]
- Wiltschko W, Möller A, Gesson M, Noll C, Wiltschko R. Light-dependent magnetoreception in birds: analysis of the behavior under red light after pre-exposure to red light. J Exp Biol. 2004a;207:1193–1202. doi: 10.1242/jeb.00873. [DOI] [PubMed] [Google Scholar]
- Wiltschko W, Munro U, Ford H, Wiltschko R. Red-light disrupts magnetic orientation of migratory birds. Nature. 1993;364:525–527. [Google Scholar]
- Wiltschko W, Traudt J, Güntürkün O, Wiltschko R. Lateralization of magnetic compass orientation in a migratory bird. Nature. 2002;419:467–470. doi: 10.1038/nature00958. [DOI] [PubMed] [Google Scholar]
- Wiltschko W, Wiltschko R. Magnetic orientation in birds. J Exp Biol. 1996;199:29–38. doi: 10.1242/jeb.199.1.29. [DOI] [PubMed] [Google Scholar]
- Wiltschko W, Wiltschko R. The effect of yellow and blue light on magnetic compass orientation in European robins, Erithacus rubecula. J Comp Physiol A. 1999;184:295–299. [Google Scholar]
- Wiltschko W, Wiltschko R. Clock-shift experiments with homing pigeons: a compromise between solar and magnetic information? Behav Ecol Sociobiol. 2001a;49:393–400. [Google Scholar]
- Wiltschko W, Wiltschko R. Light-dependent magnetoreception in birds: the behavior of European robins Erithacus rubecula, under monochromatic light of various wavelengths and intensities. J Exp Biol. 2001b;204:3295–3302. doi: 10.1242/jeb.204.19.3295. [DOI] [PubMed] [Google Scholar]


