Abstract
Adult stem cells have been identified in most mammalian tissues of the adult body and are known to support the continuous repair and regeneration of tissues. A generalized decline in tissue regenerative responses associated with age is believed to result from a depletion and/or a loss of function of adult stem cells, which itself may be a driving cause of many age-related disease pathologies. Here we review the striking similarities between tissue phenotypes seen in many degenerative conditions associated with old age and those reported in age-related nuclear envelope disorders caused by mutations in the LMNA gene. The concept is beginning to emerge that nuclear filament proteins, A-type lamins, may act as signalling receptors in the nucleus required for receiving and/or transducing upstream cytosolic signals in a number of pathways central to adult stem cell maintenance as well as adaptive responses to stress. We propose that during ageing and in diseases caused by lamin A mutations, dysfunction of the A-type lamin stress-resistant signalling network in adult stem cells, their progenitors and/or stem cell niches leads to a loss of protection against growth-related stress. This in turn triggers an inappropriate activation or a complete failure of self-renewal pathways with the consequent initiation of stress-induced senescence. As such, A-type lamins should be regarded as intrinsic modulators of ageing within adult stem cells and their niches that are essential for survival to old age.
Keywords: adult stem cells, ageing, A-type lamins, laminopathies, senescence, signalling pathways, stem cell niche, tissue regeneration
Introduction
Adult stem cell maintenance involves a fine balance between genetic and molecular cell mechanisms, external factors in the local and systemic niches in the body and multiple signalling pathways which interface in the regulation of adult stem cell homeostasis. Age-dependent decreases in tissue regenerative responses have been linked to a decreased number of adult stem cells, their dysfunction in self-renewal and lineage potential, and/or the inhibitory activity of the local and systemic factors in the aged stem cell niches (Conboy & Rando, 2005). Hence, gaining insights into the complex regulation of adult stem cell maintenance has been regarded as the key to understanding the processes of generalized tissue degeneration associated with old age. Nuclear filament proteins, A-type lamins, have been linked to a number of progeroid syndromes and degenerative adult-onset diseases, and are proposed to be regulators of adult mesenchymal stem cell (MSC) homeostasis (Hutchison & Worman, 2004; Gotzmann & Foisner, 2006). In this review, we will introduce some of the most important mechanisms critical in determining the effects of ageing on tissue regeneration and adult stem cell maintenance. Following from this, we will discuss some of the latest findings implicating the involvement of A-type lamins in the homeostasis of adult tissues, particularly in a number of signalling pathways central to adult stem cell maintenance and tissue regeneration as well as adaptive responses to stress. In so doing, we will put forward a hypothesis for A-type lamins as intrinsic modulators of ageing in adult stem cells and their niches and thus of organismal lifespan. Therefore, human diseases and animal models with lamin A mutations may offer important clues to understanding the maintenance of adult stem cells and provide links to the physiology of adult stem cell ageing.
Adult stem cells and their niches
The ability of stem cells to self-renew is crucial in the maintenance and repair of adult tissues throughout life as much as it is in development. During development, embryonic stem cells give rise to germ cells and to a wide range of other more specialized somatic stem cells that maintain tissues within the adult organism. Adult stem cells can replace cells within tissues that have either high turnover such as blood, vascular endothelium and epithelia of skin, intestine, and respiratory tract, or those that have low turnover but a high regenerative potential upon injury or disease such as skeletal muscle, liver, pancreas and bone (Rando, 2006). In adult organisms, stem cells have been predominantly found in the bone marrow but they have also been identified from an ever increasing array of tissues including skeletal muscle, adipose tissue, hair follicle, peripheral circulation, intestinal epithelium, myocardium, lung, breast, kidney and brain (Wagers & Weissman, 2004). Bone marrow stem cells include pluripotent haematopoietic stem cells (HSCs) and MSCs (Serakinci & Keith, 2006). HSCs can give rise to all different blood cell lineages such as the myeloid and lymphoid cell lineages whilst MSCs give rise to stromal cells which in turn give rise to multiple mesoderm-type cell lineages such as osteogenic, adipogenic, chondrogenic and myogenic lineages. In addition, MSCs have been known to give rise to non-mesoderm-type cell lineages including fibroblastic cells, neuronal-like cells, cardiomyocytes and vascular cells such as smooth muscle-like and endothelial-like precursor cells (Barry & Murphy, 2004).
The process of self-renewal allows a stem cell to replicate and thus generate either two (symmetric division in embryonic stem cells) or one (asymmetric division in germinal and adult stem cells) daughter stem cell whilst preserving their broad developmental potential (Molofsky et al. 2004). This potential refers to the competence of daughter stem cells to express each potential fate whilst at the same time inhibiting their lineage commitment and differentiation. The offspring of stem cells are termed progenitor cells and can be classified as either early or late according to the degree of their lineage commitment. Stem cells have varying degrees of developmental potential from the pluripotency of embryonic stem cells, which give rise to all cell types of the embryo proper to the multipotentiality (MSCs) and unipotentiality (endothelial progenitor cells) of adult stem cells, which can differentiate into either a subset of cell types or only the cell type specific to the tissue from which they are derived, respectively. However, recent evidence suggests that many ‘unipotential’ adult stem cells have the ability to differentiate or trans-differentiate into cell types other than their tissue of origin (Qian et al. 2007).
Stem cells normally reside in the stem cell microenvironment or niche, which can modulate how stem cells participate in tissue regeneration, maintenance and repair (Scadden, 2006). Stem cell niches are diverse and comprise a variety of cells, extracellular matrices and paracrine hormonal and endocrine signals, which all interact with each other. This important interplay between stem cells and their niches mediates appropriate responses to the metabolic needs of tissues and the organism as a whole, especially under conditions of physiological challenge such as increased levels of oxidative stress following inflammation or injury. As such, the stem cell niche can have a dual role in stem cell regulation. Whilst on the one hand it may act as a protective barrier against damaging stimuli that lead to stem cell depletion or excessive proliferation, on the other hand, it can negatively modify the external stimuli and alter the function of stem cells leading to disease pathologies. Interestingly, a hallmark of stem cell populations in young animals is their quiescent state. Yet, in older animals, an increased number of stem cells is found in the cell cycle (Morrison et al. 1996). The increased stem cell cycle kinetics observed in older animals is thought to be a consequence of chronic inflammation or infection that occurs with age. Therefore, maintaining a balance between stem cell activity and periods of quiescence is a feature of the functional niche (Moore & Lemischka, 2006).
Ageing of ‘disposable soma’ and loss of adult stem cell potential
The finite lifespan of adult somatic cells and their loss in the adult human body is balanced through the production of new daughter cells from adult (somatic) stem cells. In simple multicellular organisms such as Caenorhabditis elegans and Drosophila melanogaster, which are almost entirely made up of post-mitotic cells, the maximum lifespan of an organism as a whole is determined by the lifespan of their ‘disposable soma’. Interestingly, however, the midgut in D. melanogaster has recently been found to be actively replenished by adult intestinal stem cells (Micchelli & Perrimon, 2006; Ohlstein & Spradling, 2006). It has been speculated that the acquisition of adult stem cells during evolution in more complex organisms has resulted in a major extension of organismal lifespan and thus the role of adult stem cells primarily lies in the rejuvenation of ageing somatic tissues (Kamminga & de Haan, 2006). As such, ageing of an organism could be considered as a gradual loss of adult stem cell potential. How would this occur? One theory that successfully bridges both evolutionary and genetic perspectives on the ageing process, the ‘disposable soma’ theory, provides an unanticipated explanation. In nature, there is a trade-off between somatic maintenance and repair on the one hand and reproduction costs on the other so that those organisms that invest more in the former have longer lifespans than those that invest in the latter (Kirkwood, 1977). Bearing this in mind, it seems very probable that the more complex organisms have evolved to live longer lives by investing more in maintaining and repairing their ‘disposable soma’ through adult stem cell regeneration. Following on from this argument, given that stem cell hyper-activation has been linked to cancers (Pelicci, 2004), it is envisaged that short-lived organisms would show an increased incidence of germ-cell-derived tumours whilst long-lived organisms would show an increased incidence of tumours in a wide range of somatic tissues.
The ageing of organisms is characterized by declining tissue repair and regeneration in response to injury which is thought to be a driving cause of many age-related tissue pathologies (Rando, 2006). The cause of this decline seems to be tissue-specific and is believed to be a consequence of both intrinsic stem cell ageing and external alterations of both the local factors within the stem cell niche and the systemic factors within the systemic organ environment. The connection between the replicative potential of stem cells and tissue ageing is not fully understood. Whilst a substantial number of adult stem cells seem to be sustained in later life (Collins et al. 2007), their replicative activity and thus regenerative potential, at least in mouse models, has been shown to decline sharply with age (Schlessinger & Van Zant, 2001; Shefer et al. 2006). Moreover, recent studies performed in HSCs and muscle satellite cells of aged mice show that in tissues of high turnover and/or regenerative potential, the loss of the replicative ability of stem cells may not be the main cause of tissue degeneration. Apparently, it was not the loss of replicative ability that directly contributed towards a decrease in regenerative potential in these aged adult stem cell types but the latter was a consequence of external alterations within the aged local stem cell niches and systemic organ environments in which these cell types resided (Conboy et al. 2005). Namely, these authors show that parabiotic pairings of young and old mice significantly improved both muscle and liver regeneration in the aged mice by restoring the regenerative capacity of the pre-existing aged satellite cells and liver progenitors, respectively, through exposure to the systemic factors of young mice. In addition to such inhibitory effects of aged systemic niches, it was also found that the replicative ability and myogenic capacity of satellite cells isolated from an injured young muscle was inhibited when co-cultured with the aged myofibre, demonstrating the negative influence of local aged muscle niches on satellite cell regenerative capacity (Carlson & Conboy, 2007). Remarkably, the similarity in the inhibitory properties of systemic and local organ niches indicates that either the inhibitory factors produced by the local aged niches have circulatory/endocrine activity or that the age-specific systemic inhibitory factors become deposited in the old tissues.
Cellular senescence: a window into stem cell ageing
A number of inefficient intrinsic cellular processes can limit lifespan, including errors in DNA repair, failures in protection against oxidative damage and protein cross-linking, the accumulation of defective mitochondria, the extent of protein or cell turnover, and failures in tumour protection mechanisms (von Zglinicki, 2002; Wright & Shay, 2002). One model that explains the functional decline of various organ systems with increasing age is that the cells essential for tissue function and regeneration reach a finite lifespan termed ‘cellular senescence’ and as such accumulate in the organism. Although cellular senescence has been mainly studied as an in vitro cellular phenomenon concerning finite replicative lifespan (Hayflick & Moorhead, 1961), it is proposed and more recently demonstrated in vivo that senescent cells can account for over 15% of the cell populations in the mitotic tissues of ageing primates (Herbig et al. 2006; Jeyapalan et al. 2007). Premature senescence is also a hallmark of the so-called premature ageing syndromes or segmental progerias which show an early or exacerbated manifestation of one or a few aspects of ageing such as Hutchinson Gilford Progeria Syndrome (HGPS) and Werner Syndrome (WS) (Bridger & Kill, 2004; Kipling et al. 2004). It was proposed that premature ageing in HGPS individuals may be a consequence of premature stem cell exhaustion (Halaschek-Wiener & Brooks-Wilson, 2007). The premature ageing syndromes in humans and mice could therefore provide insights into the mechanisms of accelerated ageing in stem cell populations. Recently, it has been demonstrated that the Klotho mouse models of accelerated ageing show a decrease in stem cell number and an increase in progenitor stem cell senescence in various tissues and organs (Liu et al. 2007). The Klotho gene encodes a single-pass transmembrane protein but can also function as a humoral factor when its extracellular domain becomes cleaved and secreted, in which case it binds to the cell surface receptors and inhibits both the insulin/IGF-1 (insulin growth factor-1) and Wnt (Wingless/INT) signalling pathways.
Senescent cells accumulate at many tissue sites associated with age-related pathologies such as atherosclerotic lesions, skin ulcers, arthritic joints and hyper-proliferative regions in the prostate and liver (Faragher & Kipling, 1998). How may senescent cells affect stem cells and their niches? Stem cells are prime targets of carcinogenesis and the ability of stem cells to undergo senescence is considered an important tumour-suppressive mechanism which prevents the accumulation of oncogenic mutations in the self-renewing compartments (Beausejour & Campisi, 2006). However, the accumulation of senescent cells in tissues over time would not only deplete the pool of mitotically competent stem and progenitor cells but would also change the surrounding stem cell niche and thus compromise tissue repair and renewal (Campisi, 2001, 2005). For example, interstitial fibroblasts synthesize stromal support for all renewable epithelial tissues. Senescent fibroblasts secrete epithelial growth factors, inflammatory cytokines and matrix metalloproteinases that alter the tissue structure and cause local inflammation (Campisi, 2005). This is thought to be an important causative factor in the altered tissue remodelling seen in atherosclerosis and hyperplastic epithelial lesions. As a result, senescent cells may be an example of ‘antagonistic pleiotropy’ involved in inhibiting stem cell hyper-proliferation as the protective tumour-suppressive mechanism in the young organisms whilst fostering a microenvironment that promotes age-associated diseases and/or neoplastic transformation in the old organisms (Krtolica et al. 2001).
Age-associated tissue degeneration
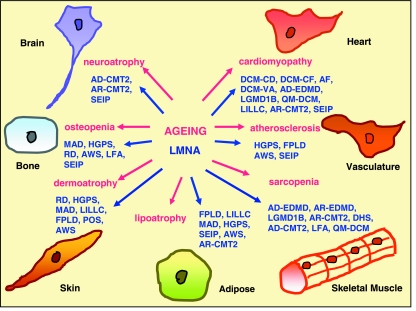
Ageing is associated with a number of degenerative disorders and inflammatory conditions including sarcopenia, osteoarthritis and osteopenia, lipo-atrophy with insulin resistance and diabetes, and cardiovascular conditions such as hypertension, atherosclerosis and cardio-myopathy (see Fig. 1). These disorders are believed to reflect an altered proliferative homeostasis between cell loss and cell replacement through adult stem cells in aged tissues and include a number of processes ranging from tissue fibrosis, sclerosis and hyperplasia to atrophy (Fehrer & Lepperdinger, 2005; Martin, 2007). Most commonly, the atrophy of specialized differentiated cells within aged tissues is associated with either a compensatory hyperplasia of the same cell types or an interstitial fibrosis in tissues such as heart and muscle. Whilst fibrotic tissue is critical in rejoining broken tissue during muscle or heart regeneration, its engagement post-injury interferes with tissue regenerative potential and prevents proper innervation (Mourkioti & Rosenthal, 2005).
Fig. 1.
Schematic diagram drawing parallels between mesenchymal tissues affected in a number of age-associated degenerative disorders (labelled in pink) and human diseases associated with mutations in the LMNA gene (labelled in blue). The full names for LMNA-associated diseases are given in the body of the article.
In aged skin, there is a reduction of both epidermal and dermal cell compartments leading to age-associated dermo-atrophy. As a result of age-associated alterations in these compartments, a characteristic wrinkling of skin occurs, reflecting changes in the collagen and elastin fibre matrix as well as a loss of hydration (Boukamp, 2005). Environmental stressors such as exposure to cigarette smoke and UV radiation also lead to alterations in skin connective tissue and accumulation of elastotic material in the dermis (Uitto & Bernstein, 1998). There is a decline in skin dermal fibroblast proliferation, motility and adhesion properties whilst their contractile behaviour increases with age. Cutaneous wound healing is impaired in terms of inflammatory profile, proliferation and remodelling leading to the chronic wound healing states associated with conditions such as diabetic ulcers, pressure sores and venous stasis ulcers (Ashcroft et al. 1995). A decreased number of hair follicle epidermal stem cells is thought to be involved in this wound closure defect (Ito et al. 2005).
Visceral obesity with a loss of subcutaneous adipose tissue and insulin resistance (i.e. metabolic syndrome) is another characteristic of old age (Karagiannides et al. 2001). Ageing is characterized by tissue damage through cytotoxic lipids and altered fatty acid handling by adipose cells (Guo et al. 2007). Adipose progenitor stem cells, the pre-adipocytes, are thought to play a crucial role in altered fat tissue function and age-associated lipo-atrophy (Kirkland & Dobson, 1997). The pre-adipocytes in aged rats fail to undergo adipogenesis in response to a fatty acid diet and show an increased tendency towards cell death. Yet, in aged tissues such as muscle and liver there is an abnormal accumulation of lipid droplets while adipose connective tissue is found to replace muscle fibres, heart tissue and bone matrix (Kirkland et al. 2002). Redistribution of lipid to extra-adipose sites with ageing could result from a loss of lipid storage capacity in adipose cells, altered fatty acid handling by non-adipose cells and/or de-differentiation of mesenchymal precursors into a partial adipocyte phenotype.
Senile osteoporosis and osteopenia are characterized by a progressive and irreversible decline in bone mass. Age-associated low bone density results from the lower activity of bone-producing osteoblasts as compared to bone-reabsorbing osteoclasts, the cell types involved in remodelling and maintenance of bone tissue (Hartmann, 2006). These cell types regulate bone homeostasis by secreting factors that regulate the activities of one another, which are believed to be altered in aged bone. Interestingly, a loss of bone density associated with osteoporosis and osteopenia is accompanied by an increase in bone marrow adipose tissue (Mueller & Glowacki, 2001). This is thought to result from the alterations in differentiation pathways of bone marrow MSCs (Duque et al. 2004). In addition, an inappropriate ossification of other cell types such as interstitial fibroblasts in ligaments, tendons and muscles in the aged individuals leads to a stiffness in the affected areas and limits movement in the affected joints (Mourkioti & Rosenthal, 2005).
Muscle ageing is associated with a loss of muscle mass, strength and velocity of contraction known as sarcopenia. A progressive muscle denervation, loss of muscle fibres, decreased synthesis of myofibrillar components and the accumulation of connective tissue are some of the known cellular changes that accompany sarcopenia (Shi & Garry, 2006). Skeletal muscle consists of a single differentiated cell type, the contractile myofibre, which upon injury activates satellite cells, the resident stem cells located beneath the basal lamina surrounding each myofibre. With age, there is a gradual decline in the regenerative response of satellite cells to damage, often accompanied by a fibrous scar and/or adipose connective tissue (Renault et al. 2002; Lees et al. 2006). The alterations in local stem cell niches in aged muscles are found to affect directly the regenerative ability of muscle satellite cells (Carlson & Conboy, 2007).
Age-associated dysfunction in vasculature is a main cause of cardiovascular diseases such as atherosclerosis and hypertension (Shantsila et al. 2007). The vascular wall and endothelium undergo constant processes of injury and repair to both mechanical and chemical injury. Microvascular wall alterations of widening and atrophy occur with age together with poor angiogenesis. It is thought that the vascular progenitor stem cells from the bone marrow, peripheral blood or vascular adventitia can all give rise to endothelial cells and smooth muscle cells which play an important role in vascular integrity and endothelial repair (Hill et al. 2003; Qian et al. 2007). During ageing and in vascular pathological conditions such as coronary artery disease, these processes are thought to become impaired due to a decreased number of circulating endothelial progenitor cells (Boos et al. 2006; Tao et al. 2006).
Age-related deficits in heart function often lead to ischaemic cardio-myopathy and heart failure in old age (Torella et al. 2006). In the adult mammalian heart, cellular cardiomyocyte hypertrophy and proliferation of cardiac fibroblasts primarily characterizes the regenerative response to injury (Ballard & Edelberg, 2007). Moreover, cardiac stem cells (CSCs) have been identified in the heart, which are able to differentiate into cardiomyocytes and vascular cells and replace damaged myocardium and vascular tissues (Beltrami et al. 2003). CSCs are believed to be subject to age-dependent changes that impair their function and contribute to deregulated repair mechanisms following injury in the aged heart leading to cardiomyopathy (Torella et al. 2004). In addition, an abnormal ossification of heart valves due to osteogenic differentiation and mineralization of interstitial fibroblasts is a common cause of their age-dependent degeneration (Moioli et al. 2007).
The aging brain is characterized by a significant decrease in weight and volume, particularly after the age of 50. This atrophy is thought to result from the loss of neurons and a decline in myelination of axons by the myelinating glial cells, oligodendrocytes, in response to injury (Franklin et al. 2002). In contrast, gliosis, which exhibits both astrocytic and microglial markers, is a prominent feature of the aged brain and neuropathic conditions such as stroke, which involves production of a dense fibrous tissue in the areas of damage (Conde & Streit, 2006). Recent evidence supports the view that neurogenesis in the adult mammalian brain occurs in discrete regions via multipotent adult neuronal stem cells (NSCs), which give rise to all three main neuronal cell types, the neurons, astrocytes and oligodendrocytes (Johansson et al. 1999; Rietze et al. 2001). Although the identity of these NSCs is still a matter of debate, the astroglial origin for NSCs in the adult brain has received much support (Doetsch et al. 1999; Sanai et al. 2004). There is a decreased rate of neurogenesis with increasing age in the adult brains of mammals (Kuhn et al. 1996; Amrein et al. 2004), which may contribute to memory deficits and learning impediments known to occur in advanced age (Shors et al. 2001; Winocur et al. 2006).
A-type lamins as guardians of the ageing soma
According to ‘disposable soma’ theory the strongest candidates for longevity genes are those regulating somatic maintenance and repair, including the cellular responses to stress. Recently, the lamin A gene has been linked to longevity and was proposed to be a guardian of somatic cells during their lifetime (Hutchison & Worman, 2004). Mutations in the lamin A gene cause a spectrum of 20 age-related human disorders termed laminopathies, affecting the maintenance of one or more tissues of mesenchymal origin including skeletal muscle, tendons, adipose, skin and skin appendages, bone, peripheral neurons, myocardium and vasculature. These disorders represent ‘a functional continuum of related conditions rather than separate diseases’ (Bonne & Levy, 2003) with the same underlying cause, which is modulated by genetic and/or environmental factors (Stewart et al. 2007). Remarkably, many tissues affected by mutations in the lamin A gene are also affected in many degenerative conditions common to old age including sarcopenia, osteoporosis, atherosclerosis, cardio-myopathy, dermo-atrophy, neuropathy and lipo-atrophy (see Fig. 1). The compromised tissue functions in laminopathy diseases are proposed to be a consequence of decreased cellular proliferation (Pekovic et al. 2007), a failure to maintain a differentiated state and/or a loss of tissue repair during regeneration (Mounkes & Stewart, 2004; Bakay et al. 2006). Mouse models of lamin A knock-out, mutated lamin A knock-in or transgenic mice manifesting either muscular dystrophy (Sullivan et al. 1999; Arimura et al. 2005), premature ageing (Mounkes et al. 2003; Yang et al. 2005; Varga et al. 2006) or dilated cardiomyopathy (Mounkes et al. 2005), respectively, have shortened lifespans and die prematurely. Moreover, mouse models null for the pre-lamin A processing enzyme Zmpste24 also have shortened lifespans and show progeria-like pathologies of bone and muscle (Bergo et al. 2002; Pendas et al. 2002) whilst a down-regulation of Ce-lamin in C. elegans leads to a 16% decrease in lifespan (Haithcock et al. 2005).
Laminopathies can be loosely divided into four categories: those affecting primarily one specific tissue such as either striated muscle, adipose and bone tissues or peripheral neurons, and those affecting several tissues in a systemic manner as it occurs in the premature ageing syndromes and their related progeroid-like disorders (Broers et al. 2006; Worman & Bonne, 2007). However, it is important to stress that even disorders affecting predominantly one or two tissues often have a variable degree of involvement in other tissues (see Fig. 1). Disorders of striated muscle include: the autosomal dominant and recessive forms of Emery–Dreyfuss muscular dystrophy (AD- & AR-EDMD, Bonne et al. 1999; Raffaele Di Barletta et al. 2000), Limb-Girdle Muscular Dystrophy with atrioventricular conduction disturbances type 1B (LGMD1B, Muchir et al. 2000) and Dilated Cardiomyopathy with conduction system disease (DCM-CD; Fatkin et al. 1999). Other atypical striated muscle conditions include: Dilated Cardiomyopathy with early-onset cardiac fibrosis (DCM-CF, van Tintelen et al. 2007), Dilated Cardiomyopathy with apical left ventricular aneurysm (DCM-VA, Forissier et al. 2003), Early-onset Atrial Fibrillation (AF, Sebillon et al. 2003), Amyotrophic Quadricipital Myopathy with dilated cardiomyopathy (QM-DCM, Charniot et al. 2003) and Dropped Head Syndrome (DHS, D’Amico et al. 2005). Disorders of peripheral neurons include: the autosomal recessive and dominant forms of Charcot-Marie-Tooth disorder type 2 (AR- and AD-CMT2, De Sandre-Giovannoli et al. 2002; Chaouch et al. 2003; Goizet et al. 2004; Benedetti et al. 2005). Disorders affecting adipose tissue include: Dunnigan-type Familial Partial Lipodystrophy (FPLD, Cao & Hegele 2000; Shackleton et al. 2000) or FPLD with variable skeletal and heart involvement (Garg et al. 2002; van der Kooi et al. 2002; Vantyghem et al. 2004), Polycystic ovaries with type A insulin resistance syndrome (POS, Young et al. 2005) and Mandibuloacral Dysplasia (MAD, Novelli et al. 2002). The two premature ageing syndromes include: HGPS (De Sandre-Giovanolli et al. 2003; Cao & Hegele 2003; Eriksson et al. 2003) and Atypical Werner Syndrome (AWS, Chen et al. 2003a,b). The progeroid-like disorders include: Restrictive Dermopathy (RD, Navarro et al. 2004), congenital Seip–Berardinelli Syndrome (Seip, Csoka et al. 2004), Lethal Foetal Akinesis (LFA, Muchir et al. 2003) and Systemic laminopathy with generalized lipoatrophy, insulin-resistant diabetes, leukomelanodermic papules, liver steatosis and hypertrophic cardiomyopathy (LILLC, Caux et al. 2003). Moreover, mutations in the pre-lamin A processing enzyme Zmpste24 have been found in patients with RD and MAD (Agarwal et al. 2003; Navarro et al. 2005). Whilst most laminopathies are caused by missense mutations spread throughout the protein, most cases of HGPS are caused by a de novo silent mutation which activates a cryptic splice site within exon 11 of the LMNA gene and leads to the production of an alternatively spliced truncated lamin A variant lacking 150 nucleotides from its 3′ end (De Sandre-Giovanoli et al. 2003; Eriksson et al. 2003). In addition, there are other rare single base mutations within exon 11 or other exons of the LMNA gene that have been reported to cause atypical HGPS (Rankin & Ellard, 2006).
The nuclear lamina as a stress-resistant filamentous network
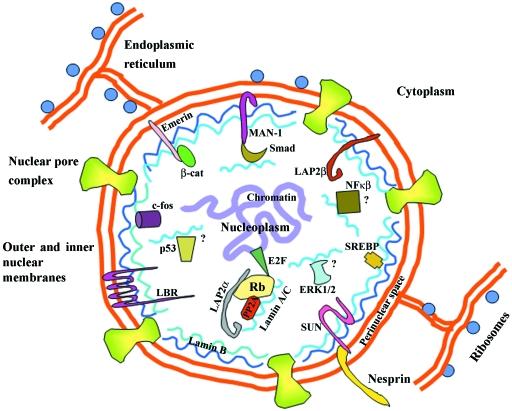
The vertebrate nuclear lamins are type V intermediate filament proteins of A-type and B-type that together with a diverse array of inner nuclear membrane proteins form a stress-resistant elastic network termed the nuclear lamina, which lies beneath the inner nuclear membrane and also veils the nucleus (Hutchison & Worman, 2004; Mounkes & Stewart, 2004; Smith et al. 2005; Broers et al. 2006). The nuclear envelope (NE) consists of the outer and inner nuclear membranes (ONM & INM) (the former being continuous with the rough endoplasmic reticulum, ER), the nuclear pore complexes (NPCs) and the nuclear lamina (NL), which together bridge the cytoskeleton and the various cytosolic organelles in the cytoplasm with the chromatin, the nuclear bodies and the nucleoskeleton inside the nucleus (Salpingidou et al. 2007) (see Fig. 2). Although it has been historically assumed that the nuclear lamina is an inert scaffolding structure which essentially functions to provide structural support to the nucleus and protect the peripheral chromatin, it is now known that the nuclear lamina is directly or indirectly involved in a number of fundamental molecular processes ranging from DNA replication and RNA transcription (Spann et al. 1997, 2002) to genome silencing and DNA repair (Liu et al. 2005, 2006; Reddy et al. 2008).
Fig. 2.
Schematic diagram depicting an overview of the major structural components of the nuclear envelope and their interactions and organization in the nucleus. These include the outer and inner nuclear membranes (ONM & INM) [the former of which is continuous with the endoplasmic reticulum (ER) lumen lined with ribosomes], the nuclear pore complexes (NPCs) and the nuclear lamina (NL) composed of A-type and B-type lamin oligomers, and INM & ONM proteins. Some selected INM proteins include: lamin B receptor (LBR), lamina-associated protein 2β (LAP2β), MAN1, emerin and Sun1. Sun1 acts as a tether for Nesprin 2 in the ONM through interactions which span the perinuclear space and link cytoskeleton to the nuclear membrane. Chromatin domains are also anchored to the nuclear lamina. A-type lamins and their binding partners (emerin, MAN1, LAP2α, Sun1) form functional complexes at the nuclear periphery and/or in the nucleoplasm, which have a role in gene regulation and signalling pathways. Not depicted are complexes involving B-type lamin binding partners such as LAP2β and LBR. Some selected nucleoplasmic partners include LAP2α, PP2A, Rb, E2F, b-catenin (b-cat), c-fos, Smads and SREBP1. Question marks indicate suggested but not yet proven A-type lamin interactions with p53, NF-κβ and ERK1/2.
Lamin monomers consist of a central alpha-helical rod domain flanked by a short amino-terminal head domain and a larger carboxy-terminal globular tail and become assembled in the nucleus through a process of dimerization, polymerization and higher-order assembly (Stuurman et al. 1998). It is suspected that lamins form a variety of oligomers and polymers with distinct binding properties both at the nuclear envelope and in the nucleoplasm (Broers et al. 1999; Zastrow et al. 2004). In vertebrates, A-type lamins are produced by an alternative splicing of the LMNA gene and include several somatic (A, C, A delta10) and germ cell-specific (C2) isoforms whilst B-type lamins are produced from two distinct genes, LMNB1 (B1) and LMNB2 (somatic B2 and germ cells-specific B3) (Hutchison, 2002). In D. melanogaster, only one A-type (lamin C) and one B-type lamin have been identified (Dm0), whilst in C. elegans only one B-type lamin (with A-type lamin features) has been found (Ce-lamin). Interestingly, lamin C is first expressed in the gut tissue of Drosophila(Riemer et al. 1995), which is so far the only tissue found in this organism able to replenish itself by adult intestinal stem cells (Ohlstein & Spradling, 2006; Micchelli & Perrimon, 2006). In C. elegans, the B-type lamin, Ce-lamin, is crucial in germ cell development (Margalit et al. 2005). As the complexity of lamin proteins increased during evolution (Cohen et al. 2001), it is tempting to speculate that B-type lamins evolved to protect germ cells in all multicellular organisms whilst A-type lamin isoforms co-evolved with the appearance of adult stem cells in a wide range of tissues in long-lived species.
A-type and B-type lamins differ in their post-translational modifications of the C-terminal CAAX motif (C, cysteine; A, any aliphatic amino acid; X, any amino acid) which is an isoprenylation signal found in many proteins including Ras, Ras-related proteins and G protein subunits, and is required for their attachment to the inner nuclear membrane (Rusinol & Sinensky, 2006). Mature lamin A is synthesized as a precursor prelamin A containing the CAAX motif, which similarly to B-type lamins, undergoes a sequence of reversible modifications including cysteine isoprenylation, –AAX proteolytic cleavage and cysteine carboxy-methylation (Rusinol & Sinensky, 2006). Whilst B-type lamins retain the modified C-terminal cysteine and as such are tightly coupled to the inner nuclear membrane, the maturation of lamin A requires a unique 15 amino acid cleavage at a conserved hexapeptide cleavage site which removes the downstream C-terminal modified cysteine. In contrast, an A-type lamin isoform, lamin C, lacks the C-terminal CAAX motif, and is thought to become attached to the inner nuclear membrane via interaction with prelamin A (Hutchison et al. 2001). In a majority of individuals with HGPS, the splicing mutations in the LMNA gene lead to the 50 amino acid truncated form of prelamin A, termed progerin, that has lost the second endoprotease cleavage site and thus permanently acquired the C-terminal modified cysteine which interferes with mitotic disassembly (Cao et al. 2007; Dechat et al. 2007).
One of the hallmarks of laminopathy cells are dramatic deformations of the nuclear morphology ranging from dysmorphic nuclear shapes to envelope raffling, blebbing, loss peripheral heterochromatin and/or INM proteins from one pole of the nucleus, and nuclear fragility upon stress (Hutchison, 2002; Goldman et al. 2004). Mutations in A-type lamins not only lead to nuclear envelope fragility but also to nucleoplasmic lamin disorganization (Broers et al. 2005; Wiesel et al. 2008), and can affect both the structural organization of lamin dimers and filaments as well as protein–protein and protein–DNA interactions (Dhe-Paganon et al. 2002; Krimm et al. 2002).
A-type lamins have a diverse spectrum of interacting partners ranging from the nuclear membrane proteins such as B-type lamins (Schirmer et al. 2001), emerin (Clements et al. 2000; Lee et al. 2001; Vaughan et al. 2001; Holaska & Wilson, 2007) and MAN1 (Mansharamani & Wilson, 2005), cytoskeletal linker proteins such as nesprins (Mislow et al. 2002; Zhang et al. 2005; Libotte et al. 2005) and SUN (Sad/UNC-84) domain proteins (Haque et al. 2006; Padmakumar et al. 2005) to gene regulatory proteins such as Rb (Ozaki et al. 1994; Mancini et al. 1994), LAP2α (lamina-associated polypeptide 2α) (Dechat et al. 2000), Smads (van Berlo et al. 2005), SREBP1 (sterol-regulatory element-binding protein) (Lloyd et al. 2002), c-fos (Ivorra et al. 2006) and MyoD (Bakay et al. 2006) (see Fig. 2). Mutations in A-type lamin binding partners including emerin, nesprins 1/2, LAP2α, MAN1 and B-type lamins have been implicated in X-linked EDMD (Bione et al. 1994), AD-EDMD (Zhang et al. 2007), DCM (Taylor et al. 2005), disorders of increased bone density (Osteopoikilosis, Busche–Ollendorf Syndrome and Melorheostosis) (Hellemans et al. 2004), AD-Leukodystrophy (Padiath et al. 2006) and Acquired Partial Lipodystrophy (Hegele et al. 2006). In the absence of A-type lamins, emerin (Sullivan et al. 1999; Vaughan et al. 2001) and nesprins (Muchir et al. 2000; Libotte et al. 2005) mislocalize to the ER whilst LAP2α forms aggregates in the nucleus (Muchir et al. 2000; Pekovic et al. 2007) and Rb is targeted to the proteasome and/or subnuclear splicing domains (Johnson et al. 2004; Pekovic et al. 2007). In addition, an absence of A-type lamins leads to the premature nuclear entry of transcription factors such as Smads (van Berlo et al. 2005) and c-fos (Ivorra et al. 2006).
A-type lamins as defenders against growth-related postnatal stress
B-type lamins are dynamically expressed throughout all stages of embryonic development and in all cells of the adult organism (Broers et al. 1997) and are essential for growth, development and survival (Harborth et al. 2001; Vergnes et al. 2004). In contrast, A-type lamin expression is developmentally regulated. Some of the transcriptional regulators that control the promoter activity of the LMNA gene include AP1 (activating protein 1), Sp1/3 (Muralikrishna & Parnaik, 2001), hepatocyte nuclear factor-3β, retinoic X receptor β (Arora et al. 2004) and p53 (Rahman-Roblick et al. 2007). Although A-type lamins are initially present in fertilized oocytes, they are absent during early embryonic development and become expressed in a tissue-specific manner during the middle stages of embryonic development as well as during postnatal development (Stewart & Burke 1987; Broers et al. 1997). Indeed, lamin A/C has recently been reported as a marker of both mouse and human embryonic differentiation (Constantinescu et al. 2006). For example, during mouse embryonic development, lamins A/C appear first during myogenesis of the eye and trunk muscles followed by their appearance during epidermis stratification whereas the expression in skeletal muscle, heart, liver, lung and brain is postnatal (Stewart & Burke, 1987; Rober et al. 1989). Consistent with these observations, in adult organisms, A-type lamins continue to be expressed in most differentiated adult cells except for undifferentiated lymphoid cells of the spleen, thymus, blood and bone marrow (Stewart & Burke, 1987). In addition, neural and neuro-endocrine cells of adult mice are also absent for A-type lamins (Broers et al. 1997). However, caution should be exercised with some of these conclusions, as some of these earlier studies relied solely on immunofluorescence microscopy and the absence of A-type lamins could be a result of epitope masking (Tunnah et al. 2005).
Recently, Takamori and co-workers gained a valuable insight into the expression of A-type lamins during neurogenesis in the adult rat brain. Remarkably, whilst lamins A/C were expressed in the primary neuronal progenitor stem cells as well as in the mature neurons of the adult rat brain, they were not or only marginally present in the early and late progenitor cells, respectively (Takamori et al. 2007). Therefore, in the adult mammalian tissues, lamin A/C expression seems to be reduced or absent in undifferentiated or proliferative cells, but is observed in differentiated or non-proliferative cells such as quiescent adult stem cells. Moreover, the primary embryonic fibroblasts from LMNA knockout (ko) mice models show no differences in proliferation patterns from wild-type cells across their lifespan (Sullivan et al. 1999). However, fibroblasts from early postnatal LMNA ko mice show severe proliferation defects and rapidly die (Mounkes & Stewart, 2004). Taken together, it becomes apparent that A-type lamins are required for postnatal growth and the maintenance of quiescence and differentiation. Given the argument that the rate of ageing of more complex tissues depends on the maintenance of their post-mitotic cells through adult stem cell regeneration, this leads us to suggest that the longer lifespans in more complex organisms may be linked to the protection of adult stem cell function by A-type lamins.
The nuclear lamina and signalling networks in adult stem cell homeostasis
The control of stem cell homeostasis within tissues such as the balance between proliferation, differentiation, apoptosis and senescence is strictly linked to the regulation of tissue repair and regeneration. It has been proposed that disease-causing mutations in A-type lamins perturb the balance between proliferation and differentiation in adult stem cells, leading to less efficient tissue regeneration (Gotzmann & Foisner, 2006; Vlcek & Foisner, 2007). There are at least three signalling pathways central to adult stem cell regeneration which have been linked to the functions of A-type lamins and their binding partners, including the Rb/E2F and Rb/MyoD pathways, the Wnt/β-catenin pathway and the TGF-β/Smad pathway (see Figs 3 and 5A). Therefore, A-type lamins can be viewed as ‘signalling receptors’ of the nucleus which can receive and transduce signals from the extracellular matrix and cytosol through covalent or non-covalent changes. This has been extensively studied in the satellite muscle cells involved in postnatal muscle growth and regeneration as well as the pre-adipocytes and dermal fibroblasts with A-type lamin, emerin, MAN1 or LAP2α mutations.
Fig. 3.
Schematic diagram illustrating how A-type lamins in association with other INM proteins modulate different signalling pathways that participate in either adult stem cell maintenance (TGF-β pathway) or growth-related stress responses (Ras/MAPK pathway). In the TGF-β pathway, TGF-β ligands in the extracellular space bind to dimerized TGF-β serine/threonine kinase receptors (type I and type II R) found on the plasma membrane. Ligand-receptor binding results in the phosphorylation of the receptor Smads 2/3, which disassociate from the receptor and form a heteromeric complex with the co-mediator Smad 4 in the cytoplasm, which can then translocate across the nuclear membrane. A-type lamins alone or together with the INM protein MAN1 bind to the Smad complex in the nucleus and inhibit their activation of transcription factors (TF) and target genes. In the Ras/MAPK pathway, growth factors in the extracellular matrix bind to heterotrimeric G protein-coupled receptors (GPCR) or receptor tyrosine kinases (not shown) on the plasma membrane. This leads to the activation of the G protein Ras (in a complex with Grb2 and SOS proteins) through GDP/GTP exchange, which is then free to bind to serine/threonine protein kinase Raf and/or G protein Rac (not shown). In the cytosol, downstream kinase cascade from Raf includes phosphorylation of protein kinase MEK 1/2, which leads to activation and enhanced phosphorylation of ERK 1/2 dimers and their subsequent translocation across the nuclear membrane. A-type lamins alone or together with some unknown INM proteins may bind to phosphorylated ERK 1/2 and inhibit their activation of transcription factors (TF) and gene expression. Grb, growth factor receptor-bound protein; SOS, son-of-sevenless; GDP/GTP, guanosine di/triphosphate; MEK, mitogen- or extracellular signal-related kinase.
Fig. 5.
Schematic representation of the involvement of A-type lamins in the signalling pathways central to adult stem cell regeneration (A) and adaptive responses to stress (B), which control the balance between proliferation, differentiation, senescence and apoptosis. (A) A-type lamins and their binding partners regulate at least three regenerative pathways including Rb/E2F and Rb/MyoD, Wnt/β-catenin and TGF-β/Smad. A number of extracellular stimuli including growth factors, cytokines and mitogens trigger the activation of either the Wnt-Frizzled receptor (and co-receptor LRP5/6), which protects their cytosolic target β-catenin against proteosomal degradation, or activation of the TGF-β receptor kinases 1/2 that phosphorylate receptor-associated Smads 2/3 in the cytosol. This allows β-catenin or Smads 2/3 (in complex with Smad 4) to translocate to the nucleus from the cytosol and control expression of genes. A-type lamin-binding partner emerin binds to β-catenin in the nucleus and promotes its nuclear export, which represses activation of TCF/LEF-responsive genes. A-type lamins alone or in concert with their binding partner MAN1 bind to Smads 2/3 in the nucleus and repress Smad-responsive genes by inducing their de-phosphorylation through nuclear phosphatase PP2A. A-type lamins and their binding partner LAP2α bind to transcription factor Rb and regulate the expression of E2F-dependent genes during S-phase or the activation of MyoD-dependent genes during muscle differentiation. (B) In response to mechanical stress, DNA damage, increased ROS levels or inflammatory cytokines in the extracellular space, cells activate the stress-associated signalling pathways through a number of cytosolic receptors such as TNF-α, PDGF-β and G protein coupled receptors, which activate cytosolic I-kβ kinase complex and its target NF-kβ, ATM kinase and its target p53 or one of the kinase branches of the Ras/MAPK cascade (ERK1/2, JNK/SAPK or p38), respectively. A-type lamins alone or in concert with other unknown binding partners regulate the nuclear entry, phosphorylation, DNA binding and/or transcriptional activity of these nuclear targets and thus their control of gene expression. Note that it is not known which cytosolic receptors lead to the activation of Rb in the cytosol but Rb is a downstream nuclear effector of a number of pathways including TGF-β, Ras/MAPK and p53.
Rb pathways
Regulation of the cell cycle via the Rb/E2F pathway involves the cell cycle-regulated activity of cyclins, cyclin-dependent kinases (CDK), CDK inhibitors p16ink4 and p21cip1 and their transcription factor targets, the retinoblastoma protein Rb and E2F (Galderisi et al. 2006). A-type lamins and their binding partner LAP2α bind to and tether Rb/E2F transcription factor complexes in the nucleus during G1 phase of the cell cycle, which is essential for its role in repressing activation of E2F-dependent S-phase genes (Markiewicz et al. 2002; Dorner et al. 2006). Hyper-phosphorylation of Rb during the G1/S phase transition by cyclin/CDKs, which are activated by mitogenic signalling, releases active E2F and leads to S-phase progression (Mittnacht et al. 1997). This regulation of Rb by the nucleoplasmic A-type lamin/LAP2α complexes is exerted through either protecting Rb from proteosomal degradation (Johnson et al. 2004) and targeting to the subnuclear splicing speckles (Pekovic et al. 2007), or through controlling its rate of phosphorylation via protein phosphatases such as PP2A (van Berlo et al. 2005). Loss of Rb regulation by either RNAi knock-down of LAP2α or A-type lamins in human and mouse cells leads to accelerated S-phase entry, which is followed by either inadequate growth arrest following withdrawal of mitogens (Dorner et al. 2006) or S-phase delay and G2 arrest (Pekovic et al. 2007). Similarly, in the absence of Rb, skeletal muscle cells progress slowly through S phase but then arrest in S and G2 phases of the cell cycle, most likely due to incomplete replication and/or DNA damage (Novitch et al. 1996). A deregulated Rb/E2F pathway in laminopathy cells could therefore lead to premature stem cell exhaustion through increased proliferation and/or the accumulation of DNA damage.
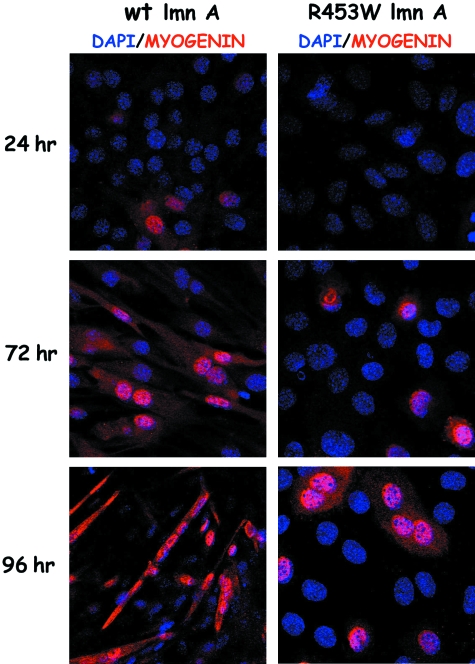
Rb can cooperate with a number of tissue-specific transcription factors in order to regulate tissue-specific differentiation. The Rb/MyoD pathway has a well-established role in myogenesis of satellite myoblasts whereby the interaction of Rb with the myogenic transcription factor MyoD leads to the activation of MyoD-dependent target genes and initiation of muscle differentiation (Novitch et al. 1996). A number of recent studies have shed new light on the role of A-type lamins and their binding partner emerin in satellite cell differentiation and regeneration upon injury. Mouse satellite cells null for A-type lamins or emerin have impaired differentiation potential as a result of delayed ability to exit the cell cycle and show a decreased expression of proteins critical in muscle differentiation including Rb, MyoD, desmin and M-cadherin (Frock et al. 2006). Similarly, two further studies have implicated a deregulated Rb/MyoD pathway as a cause of decreased differentiation potential in A-type lamin or emerin null regenerating muscle using mRNA expression profiling (Bakay et al. 2006; Melcon et al. 2006). Interestingly, other myogenic factors such as Myf5 and Pax7 are up-regulated in lamin A/C null cells, indicating that they still retain myogenic properties (Frock et al. 2006). Over-expression of Myf5 in the absence of MyoD is thought to be a marker for self-renewing satellite cells or ‘reserve cells’ in the adult muscle (Yablonka-Reuveni et al. 1999). In this regard, lamin A/C and emerin null myoblasts resemble the behaviour of MyoD null myoblasts which undergo an enhanced self-renewal at the expense of producing progeny that undergoes differentiation (Megeney et al. 1996). Two previous studies on mouse myoblasts transfected with mutant lamin A demonstrated the inhibition of muscle differentiation and decreased expression of late myogenic factors such as myogenin and cathepsin B (Favreau et al. 2004; Markiewicz et al. 2005, see Fig. 4). These myoblasts exhibited a failure to exit the cell cycle as seen by persistently high levels of hyper-phosphorylated Rb and proliferation marker PCNA.
Fig. 4.
C2C12 mouse myoblasts stably transfected with GFP-R453W lamin A linked to muscular dystrophy fail to undergo muscle differentiation and up-regulation of muscle-specific gene products. C2C12 mouse myoblasts stably transfected with GFP-wt lamin A or GFP-R453W lamin A were grown for 3 days after which they were induced to differentiate for 4 days by replacing their growth media (DMEM/10% FCS) with differentiation media (DMEM/2% horse serum). Coverslips with cells were taken after 24, 72 and 96 h and processed for confocal microscopy using the antibody against late muscle differentiation marker myogenin and DNA-intercalating dye DAPI. Images were collected on a Zeiss confocal microscope and projected as blue/red colour merged micrographs in which myogenin is in red and DAPI is in blue. DMEM, Dulbecco's modified Eagle's medium.
In preadipocytes, Rb stimulates adipocyte differentiation through modulating adipogenic factors such as CAAT/enhancer binding protein-α (C/EBP-α) and peroxisome proliferator-activated receptor-γ (PPAR-γ) (Classon et al. 2000). Prelamin A-binding protein SREBP1 also activates PPAR-γ when its activated form gets translocated to the nucleus (Lloyd et al. 2002; Capanni et al. 2005). In fibroblasts of FPLD, MAD and AWS patients which accumulate prelamin A, SREBP1 is sequestered to the nuclear envelope which prevents the activation of PPAR-γ and thus inhibits adipogenesis. Similarly, the over-expression of both wild-type and the lipodystrophy-causing mutant lamin A inhibits lipid accumulation and the expression of adipogenic markers (Boguslavsky et al. 2006). Moreover, the over-expression of A-type lamin-binding partner LAP2α in pre-adipocytes initiates early events in the adipogenic pathway but inhibits lipid accumulation and terminal adipogenesis (Dorner et al. 2006). In contrast, embryonic fibroblasts lacking A-type lamins undergo adipogenesis more readily than controls and these lamin A null mice do not develop lipodystrophy (Sullivan et al. 1999). These studies suggest that down-regulation of A-type lamins in physiological conditions may be necessary to induce normal adipogenesis. Given that in cells from lipodystrophy patients A-type lamin levels are normal, a loss of adipogenic potential may be attributed to their increased stability in cells. The ability of A-type lamins to dynamically reorganize themselves (Markiewicz et al. 2005; Mariappan & Parnaik, 2005) and/or to modulate their stability (E. Markiewicz, personal communication) may be important regulators of mesenchymal differentiation.
Wnt/β-catenin pathway
The canonical Wnt/β-catenin pathway is one of the most important regulators of mesenchymal tissue proliferation and differentiation. It acts via the Wnt-Frizzled receptor and co-receptor LRP5/6 (low-density lipoprotein receptor-related protein) through inactivating a GSK3-β/Axin/APC destruction complex which regulates β-catenin ubiquitination and proteosomal degradation (GSKβ-glycogen synthase kinaseβ; APC-adenomatous polyposis coli) (Hartmann, 2006). This leads to a stabilized cytoplasmic pool of β-catenin which can then translocate to the nucleus resulting in the co-activation of transcription factors TCF/LEF (T cell factor/lymphoid enhancer factor). Emerin binds to β-catenin via its C-terminal APC-like domain which restricts the nuclear accumulation of β-catenin in a CRM1-export-dependent manner (CRM1-exportin chromosome region maintenance1) and thus represses the transcriptional activation of TCF/LEF-responsive genes (Markiewicz et al. 2006). Indeed, a number of genes associated with Wnt signalling were found to be up-regulated in X-EDMD patient fibroblasts (Tsukahara et al. 2002). The downstream nuclear targets of β-catenin include a number of proliferative genes such as cyclin D1 and c-myc which are both up-regulated in emerin-null human fibroblasts leading to auto-stimulatory growth phenotype (Markiewicz et al. 2006). Abnormal β-catenin activity has been implicated in a number of mesenchymal fibro-proliferative disorders (Bowley et al. 2007). As cardiac and skeletal muscles accumulate fatty fibrotic tissue in patients with emerin and LMNA mutations, the aberrant Wnt signalling is suggested to lead to this phenotype in X-EDMD patients through accelerated cell turnover (Markiewicz et al. 2006). Remarkably, increased Wnt/β-catenin signalling has been found to induce the conversion of aged muscle satellite cells into fibroblastic cell lineage during muscle regeneration leading to increased fibrosis associated with poor regenerative response in the aged muscle (Brack et al. 2007). However, in recent studies of both human and mouse EDMD models of muscle regeneration, abnormal Wnt signalling has not yet been implicated.
TGF-β/Smad pathway
The canonical tumour growth factor-β (TGF-β)/Smad pathway is another important regulator of mesenchymal tissue homeostasis (Verrecchia et al. 2006). It acts through the TGF-β receptor kinases 1/2 that phosphorylate the receptor-associated Smads 2 and 3 which then interact with the co-mediator Smad 4 and as such can be translocated to the nucleus where they control the expression of various genes such as collagen (see Fig. 3). TGF-β can also induce an activation of nuclear phosphatase PP2A whereby it requires Rb protein for the execution of its growth-suppressing role (Herrera et al. 1996; Derynck & Zhang, 2003). A-type lamins have been found to bind to both the TGF-β-induced Smads 2/3 and Rb and to repress TGF-β/Smad-dependent gene expression in mouse embryonic fibroblasts (MEFs) by promoting their dephosphorylation via PP2A (van Berlo et al. 2005). Interestingly, in senile osteoporosis, decreases in the expression of TGF-β signalling in somatic cells and MSCs of aged mice (Moerman et al. 2004; Han et al. 2005) induce a lack of osteoblasts at the expense of adipocytes in the bone matrix (Chan & Duque, 2002). This resembles the situation in MSCs of lmna-deficient mice, which show a preference towards adipocyte as opposed to osteoblast differentiation (Mounkes et al. 2003). Moreover, an A-type lamin-binding partner MAN1, which interacts with R-Smads and antagonizes TGF-β signalling (Lin et al. 2005; Pan et al. 2005) when mutated, leads to the over-stimulation of this pathway and increased bone density. Over-expression of TGF-β signalling is also implicated in tissue fibrosis in muscle and heart due to increased extracellular matrix production (Khan & Sheppard, 2006). It is thus suggested that the increased proliferation rate and collagen production as a result of deregulated TGF-β/Smad signalling in lmna-null mice has implications for the tissue fibrosis seen in their heart and muscle (van Berlo et al. 2005).
A-type lamins in regulation of stress-activated signalling pathways
In addition to pathways associated with tissue regeneration, A-type lamins have been linked to the signalling pathways associated with cellular responses to stress including MAPK signalling pathways, the p53/p21 and Rb/p16 pathways and the NF-κβ pathway, which are either chronically up-regulated or fail to activate upon stress in the cells and tissues with lamin A/C mutations (see Figs 3 and 5B).
MAPK pathways
Genome-wide profiling of hearts in a mouse model of AD-EDMD revealed increases in the expression of several genes associated with MAPK signalling such as c-jun, Elk1, pERK1/2 and pJNK as well as the genes involved in fibrosis and inflammation (Muchir et al. 2007). Activation of MAPK (mitogen-activated protein kinase) signalling either through ERK or JNK in hearts has been associated with the development of cardiomyopathy in mouse models (Nicol et al. 2001; Petrich et al. 2003). MAPK signalling is initiated from a number of receptor and non-receptor tyrosine kinases or heterotrimeric receptors coupled to small G proteins such as Ras (Iwasa et al. 2003). There are a number of effector pathways downstream of Ras, the best known being the mitogenic signalling pathway, the MAPK cascade (see Fig. 3). MAPK pathways consist of three distinct signalling branches including ERKs (extracellular signal-related kinases), JNK/SAPKs (Jun N-terminal kinases/stress-activated protein kinases) and p38 which, when activated by phosphorylation, translocate to the nucleus and transactivate transcription factors involved in gene regulation. Whilst the ERK pathway is activated by mitotic stimuli, the latter two pathways are activated by cellular stresses such as UV light, ROS (reactive oxygen species) and inflammatory cytokines. Ras pathway over-activation leads to premature senescence in cells with intact cell cycle controls (Serrano et al. 1997) by activating the stress-kinases JNK/SAP and p38 (Hutter et al. 2002; Wang et al. 2002; Iwasa et al. 2003). Thus, the activation of JNK in lamin A/C mutant hearts could lead to cardiomyopathy by inducing premature senescence of cardiomyocytes or resident cardiac stem cells. Interestingly, downstream targets of Ras-mediated signalling involve the regulation of cyclin D1, pRb phosphorylation and its partner MyoD (Mittnacht et al. 1997). Suppression of Ras signalling using MAPK inhibitors leads to the trans-activation of Rb/MyoD pathway and restores myogenic differentiation in Rb-null muscle cells (Lee et al. 1999). It would be worth investigating whether increased Ras/MAPK activity also prevents proper cardiomyocyte differentiation in cardiac cells with lamin A/C or emerin mutations through modulating the Rb/MyoD pathway as has recently been demonstrated for myogenic differentiation of C2C12 myoblasts expressing EDMD-linked lamin A mutant (Favreau et al. 2008).
p53/p21 and Rb/p16 pathways
The p53/p21 and Rb/p16 pathways are essential gatekeepers of senescent arrest in both mouse and human models (Shay et al. 1991). Activation of these pathways in somatic stem cell compartments limits the regenerative capacity of their respective tissues. Mouse models with high expression or activity of p53 have a shorter lifespan and show signs of premature ageing (Tyner et al. 2002; Maier et al. 2004) and over-expression of CDK inhibitor p16 in stem cells leads to premature exhaustion of their activity (Janzen et al. 2006). The p53/p21 pathway is induced in response to a variety of signals following platelet-derived growth factor-β (PDGF-β) receptor activation of ataxia telangiectasia mutated (ATM) kinases which sense DNA damage (Chen et al. 2003a). Hyper-activation of the p53 pathway has recently been reported in Zmpste24-null and lmna-null mouse models of accelerated ageing (Varela et al. 2005), which is triggered by increased DNA damage and/or a reduced level of anti-oxidant enzymes (Liu et al. 2005; Varela et al. 2005). Moreover, oxidative stress has been implicated in the pathophysiology of lamin A-linked Amyotrophic Quadricipital Syndrome with cardiac involvement and in human FPLD fibroblasts with lamin A/C mutations which undergo p16-dependent senescent arrest (Caron et al. 2007; Charniot et al. 2007). Interestingly, lmna-null and pre-lamin A processing mutant mouse cells which have low levels of Rb cannot arrest in response to DNA damage or increased activity of CDK inhibitor p16ink4(Johnson et al. 2004; Nitta et al. 2006). These data imply that A-type lamin/Rb complexes may be important in both DNA damage-induced and p16-dependent senescent arrest. How would this regulation be exerted? Rb has a well-known role in activating growth arrest following DNA damage and preventing the accumulation of DNA damage in the face of unscheduled DNA synthesis, which would otherwise give rise to double-stranded breaks (DSBs) and genomic instability (Bosco et al. 2004). Impaired DNA repair, genomic instability and premature senescence have also been demonstrated in Zmpste24-null MEFs, bone marrow cells and human HGPS fibroblasts (Liu et al. 2005).
NF-κβ pathway
The nuclear factor-κβ (NF-κβ) pathway is involved in the regulation of diverse cellular processes including survival, apoptosis, cell growth, differentiation, immunity and cellular responses to stress (Jones et al. 2003). It can be activated by the canonical cytokine-mediated pathways such as tumour necrosis factor-α (TNF-α) or by signal transduction cascades involving the ERK1/2 branch of MAPK signalling. Many of these cascades activate NF-κβ by activating the inhibitor I-κβ kinase complex, which then allows its translocation to the nucleus and activation of target genes. NF-κβ functions as a key regulator of cardiac gene expression in many physiological states such as adaptive cardiac hypertrophy as well patho-physiological states such as dilated cardiomyopathy and atherosclerosis (Jones et al. 2003). Following cytokine stimulation or mechanical stress, both lamin A/C-null and emerin-null mouse fibroblasts show impaired mechano-sensitive transduction pathways involving NF-κβ at a step downstream of its nuclear entry and DNA binding (Lammerding et al. 2004, 2005). Moreover, in response to mechanical loads, lamin A/C-null cells are not able to resist forces of compression and are prone to apoptosis (Broers et al. 2004; Lammerding et al. 2004), which is not the case for emerin null cells (Lammerding et al. 2005). However, it is not yet known how precisely lamin A/C and emerin are involved in the regulation of NF-κβ transactivation and whether each protein acts independently or in combination with one another or other INM proteins. It has been suggested that the lack of dephosphorylation of NF-κβ subunit relA (reticuloendotheliosis viral oncogene A) by A-type lamin-binding phosphatase PP2A may explain its altered activity in lamin A/C- or emerin-null cells (van Berlo et al. 2005).
A-type lamins at the crossroads between tissue regeneration and stem cell ageing: a summary
Adult stem cell maintenance involves a fine balance between intrinsic mechanisms regulating genome integrity and repair, external factors in the local and systemic niches in the body, and multiple signalling pathways which incorporate these mechanisms as their integral components and regulate the decision as to whether stem cells engage in self-renewal, lineage specification, growth arrest or stress response. Age-dependent tissue degeneration is associated with the alterations in these intrinsic and extrinsic factors in adult stem cells and their niches, which leads to decreased regeneration of tissues upon injury (Conboy & Rando, 2005). As a result, tissue-degeneration in old age is accompanied by a compensatory hyperplasia of the same tissue-specific cell types or interstitial fibrosis (Martin, 2007). One model that explains the decline in adult stem cell regeneration with age in the context of both intrinsic and extrinsic mechanisms concerns the accumulation of senescent cells with age. Senescent cells arise as a result of a stress response to a number of inefficient intrinsic cellular mechanisms that alter the tissue microenvironment, which becomes inhibitory to stem cell activity (Campisi, 2005).
Recently, A-type lamins have been linked to the maintenance and regeneration of a number of mesenchymal tissues and have been proposed to be regulators of mesenchymal stem cell regeneration (Gotzmann & Foisner, 2006). Both lamin A and progerin have been implicated in physiological ageing (Duque & Rivas, 2006; McClintock et al. 2006; Scaffidi & Misteli, 2006; Afilalo et al. 2007; Cao et al. 2007; Ukekawa et al. 2007; Candelario et al. 2008; Huang et al. 2008). Tissues affected by lamin A/C mutations often show degenerative changes accompanied by increased fibrosis and/or lipid accumulation (Hutchison & Worman, 2004). A number of degenerative mesenchymal diseases associated with increasing age show significant clinical overlap with those diseases caused by lamin A/C mutations. How could A-type lamins regulate adult stem cell function and their onset/rate of ageing? A-type lamins interact with a diverse spectrum of interacting partners, which together form a stress-resistant signalling network in the nucleus (Lammerding et al. 2005) that can receive external signals from the extracellular matrix and cytosol and transduce them into intrinsic control of gene expression. The signalling pathways regulating adult stem cell homeostasis as well as cellular responses to stress have been linked to the function of A-type lamins as ‘signalling receptors’ of the nucleus. Regulation of these pathways by A-type lamins in combination with their distinct binding partners may provide an additional level of complexity which may determine the extent of cross-talks between different pathways. For instance, it would be important to investigate whether TGF-β signalling (van Berlo et al. 2005) cooperates with Wnt signalling (Markiewicz et al. 2006) or the MAPK stress signalling (Muchir et al. 2007) in lamin A/C- and emerin-null cells to induce rapid growth and increased fibrosis in hearts and skeletal muscles of patients with EDMD. Wnt signalling in the ageing context has recently been implicated in the abnormal fibrogenic conversion of the aged muscle satellite cells during muscle regeneration (Brack et al. 2007). Moreover, increased TGF-β signalling via the MAPK kinase can lead to premature senescence in human fibroblasts (Kim et al. 2004), which is known to induce β-catenin activation (Sato, 2006). Therefore, the increased fibrosis in the heart and muscle of patients with lamin A/C and emerin mutations may result from the induction of premature senescence in resident stem cells as a result of the abnormal cross-talk between these pathways.
Furthermore, in cases where the affected tissues show severe degeneration, damaged myofibres may send the signals that trigger increased satellite cell renewal and thus lead to a vicious circle of premature stem cell exhaustion. The accumulation of DNA damage in lamin A/C mutant tissues as a result of either decreased NF-κβ-dependent adaptive mechanical responses (Lammerding et al. 2004), inappropriate DNA damage repair (Liu et al. 2005; Manju et al. 2006), inability of cells to prevent unscheduled DNA replication via Rb (Johnson et al. 2004; Nitta et al. 2006) or increased oxidative stress via MAPK stress signalling (Caron et al. 2007; Charniot et al. 2007; Muchir et al. 2007) could all mimic an injury environment in the stem cell niches and lead to constant cycles of stem cell regeneration in those tissues and their depletion. This scenario would lead to stress-induced premature stem cell senescence by up-regulation of p53/p21 signalling and/or the Rb/p16 pathway depending on whether the latter is still intact. In conclusion, a loss of appropriate regulation of a number of regenerative pathways intrinsic to adult stem cell maintenance may underpin the premature demise of tissues with lamin A/C mutations. This may lead to a loss of protection against growth-related stress as well as defects in a number of cellular processes in adult stem cells and/or their progenitors including growth, differentiation, apoptosis and repair, which may in turn switch on the chronic stress signalling pathways that serve to maintain organismal survival but at the same time promote the ageing phenotype. Therefore, A-type lamins should be considered as intrinsic modulators of ageing within the adult stem cells and their niches.
Update
Since this article was submitted for publication, two new complementary studies pertinent to this topic have been published. The first study shows that the expression of progerin in immortalized human MSCs negatively affects their molecular identity and differentiation potential by interfering with the Notch signalling pathway, which is essential in stem cell regulation and maintenance (Scaffidi & Misteli, 2008). The second study demonstrates that the Zmpste24-null mouse model of accelerated ageing with prelamin A accumulation shows a defective proliferative potential of bulge cells in the hair follicle niche due to a decreased Wnt signalling pathway, which leads to dysfunction of epidermal stem cell renewal (Espada et al. 2008). These findings strongly support the hypothesis that lamin A regulates stem cell maintenance via a range of regenerative signalling pathways and provide the insight that the regulation of adult stem cell ageing may occur at a number of levels that intersect with lamin A (wt lamin A, progerin and prelamin A) including adult stem cells, their progenitors and/or stem cell niches.
Acknowledgments
We thank Dr Ewa Markiewicz for the kind provision of C2C12 mouse myoblast cell lines stably transfected with GFP-lamin A wt or GFP-R453W lamin A. We would like to give special thanks to Veronika Boczonadi for kindly providing us with the diagrams in Figs 2 and 3. We are also very grateful to Christopher Vaughan for his kind assistance with bibliography and for proof-reading of the manuscript. Our work is supported by the AICR, the MDC and the EU F-6 to C.J.H. This article is dedicated to the memory of Charlotte.
References
- Afilalo J, Sebag IA, Chalifour LE, et al. Age-Related Changes in Lamin A/C Expression in Cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;293(3):1451–1456. doi: 10.1152/ajpheart.01194.2006. [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Fryns JP, Auchus RJ, Garg A. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet. 2003;12:1995–2001. doi: 10.1093/hmg/ddg213. [DOI] [PubMed] [Google Scholar]
- Amrein I, Slomianka L, Poletaeva II, Bologova NV, Lipp HP. Marked species and age-dependent differences in cell proliferation and neurogenesis in the hippocampus of wild-living rodents. Hippocampus. 2004;14:1000–1010. doi: 10.1002/hipo.20018. [DOI] [PubMed] [Google Scholar]
- Arimura T, Helbling-Leclerc A, Massart C, et al. Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum Mol Genet. 2005;14:155–169. doi: 10.1093/hmg/ddi017. [DOI] [PubMed] [Google Scholar]
- Arora P, Muralikrishna B, Parnaik VK. Cell-type-specific interactions at regulatory motifs in the first intron of the lamin A gene. FEBS Lett. 2004;568:122–128. doi: 10.1016/j.febslet.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Horan MA, Ferguson MW. The effects of ageing on cutaneous wound healing in mammals. J Anat. 1995;187:1–26. [PMC free article] [PubMed] [Google Scholar]
- Bakay M, Wang Z, Melcon G, et al. Nuclear envelope dystrophies show a transcriptional fingerprint suggesting disruption of Rb-MyoD pathways in muscle regeneration. Brain. 2006;129:996–1013. doi: 10.1093/brain/awl023. [DOI] [PubMed] [Google Scholar]
- Ballard VL, Edelberg JM. Stem cells and the regeneration of the aging cardiovascular system. Circ Res. 2007;100:1116–1127. doi: 10.1161/01.RES.0000261964.19115.e3. [DOI] [PubMed] [Google Scholar]
- Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Beausejour CM, Campisi J. Ageing: balancing regeneration and cancer. Nature. 2006;443:404–405. doi: 10.1038/nature05221. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- Benedetti S, Bertini E, Iannaccone S, et al. Dominant LMNA mutations can cause combined muscular dystrophy and peripheral neuropathy. J Neurol Neurosurg Psychiatry. 2005;76:1019–1021. doi: 10.1136/jnnp.2004.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergo MO, Gavino B, Ross J, et al. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc Natl Acad Sci USA. 2002;99:13049–13054. doi: 10.1073/pnas.192460799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berlo JH, Voncken JW, Kubben N, et al. A-type lamins are essential for TGF-beta1 induced PP2A to dephosphorylate transcription factors. Hum Mol Genet. 2005;14:2839–2849. doi: 10.1093/hmg/ddi316. [DOI] [PubMed] [Google Scholar]
- Bione S, Maestrini E, Rivella S, et al. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet. 1994;8:323–327. doi: 10.1038/ng1294-323. [DOI] [PubMed] [Google Scholar]
- Boguslavsky RL, Stewart CL, Worman HJ. Nuclear lamin A inhibits adipocyte differentiation: implications for Dunnigan-type familial partial lipodystrophy. Hum Mol Genet. 2006;15:653–663. doi: 10.1093/hmg/ddi480. [DOI] [PubMed] [Google Scholar]
- Bonne G, Levy N. LMNA mutations in atypical Werner's syndrome. Lancet. 2003;362:1585–1586. doi: 10.1016/S0140-6736(03)14761-7. author reply 1586. [DOI] [PubMed] [Google Scholar]
- Bonne G, Di Barletta MR, Varnous S, et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet. 1999;21:285–288. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- Boos CJ, Goon PK, Lip GY. Endothelial progenitor cells in the vascular pathophysiology of hypertension: arterial stiffness, ageing and more. J Hum Hypertens. 2006;20:475–477. doi: 10.1038/sj.jhh.1001991. [DOI] [PubMed] [Google Scholar]
- Bosco EE, Mayhew CN, Hennigan RF, et al. RB signaling prevents replication-dependent DNA double-strand breaks following genotoxic insult. Nucleic Acids Res. 2004;32:25–34. doi: 10.1093/nar/gkg919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukamp P. Skin aging: a role for telomerase and telomere dynamics? Curr Mol Med. 2005;5:171–177. doi: 10.2174/1566524053586644. [DOI] [PubMed] [Google Scholar]
- Bowley E, O’Gorman DB, Gan BS. Beta-catenin signaling in fibroproliferative disease. J Surg Res. 2007;138:141–150. doi: 10.1016/j.jss.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Bridger JM, Kill IR. Aging of Hutchinson-Gilford progeria syndrome fibroblasts is characterised by hyperproliferation and increased apoptosis. Exp Gerontol. 2004;39:717–724. doi: 10.1016/j.exger.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Broers JL, Machiels BM, Kuijpers HJ, et al. A- and B-type lamins are differentially expressed in normal human tissues. Histochem Cell Biol. 1997;107:505–517. doi: 10.1007/s004180050138. [DOI] [PubMed] [Google Scholar]
- Broers JL, Machiels BM, van Eys GJ, et al. Dynamics of the nuclear lamina as monitored by GFP-tagged A-type lamins. J Cell Sci. 1999;112:3463–3475. doi: 10.1242/jcs.112.20.3463. [DOI] [PubMed] [Google Scholar]
- Broers JL, Peeters EA, Kuijpers HJ, et al. Decreased mechanical stiffness in LMNA–/– cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum Mol Genet. 2004;13:2567–2580. doi: 10.1093/hmg/ddh295. [DOI] [PubMed] [Google Scholar]
- Broers JL, Kuijpers HJ, Ostlund C, Worman HJ, Endert J, Ramaekers FC. Both lamin A and lamin C mutations cause lamina instability as well as loss of internal nuclear lamin organization. Exp Cell Res. 2005;304:582–592. doi: 10.1016/j.yexcr.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Broers JL, Ramaekers FC, Bonne G, Yaou RB, Hutchison CJ. Nuclear lamins: laminopathies and their role in premature ageing. Physiol Rev. 2006;86:967–1008. doi: 10.1152/physrev.00047.2005. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–S31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Candelario J, Sudhakar S, Navarro S, Reddy S, Comai L. Perturbation of wild-type lamin A metabolism results in a progeroid phenotype. Aging Cell. 2008;7(3):355–367. doi: 10.1111/j.1474-9726.2008.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Hegele RA. Nuclear lamin A/C R482Q mutation in canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet. 2000;9:109–112. doi: 10.1093/hmg/9.1.109. [DOI] [PubMed] [Google Scholar]
- Cao H, Hegele RA. LMNA is mutated in Hutchinson-Gilford progeria (MIM 176670) but not in Wiedemann-Rautenstrauch progeroid syndrome (MIM 264090) J Hum Genet. 2003;48:271–274. doi: 10.1007/s10038-003-0025-3. [DOI] [PubMed] [Google Scholar]
- Cao K, Capell BC, Erdos MR, Djabali K, Collins FS. A lamin A protein isoform overexpressed in Hutchinson-Gilford progeria syndrome interferes with mitosis in progeria and normal cells. Proc Natl Acad Sci USA. 2007;104:4949–4954. doi: 10.1073/pnas.0611640104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capanni C, Mattioli E, Columbaro M, et al. Altered pre-lamin A processing is a common mechanism leading to lipodystrophy. Hum Mol Genet. 2005;14:1489–1502. doi: 10.1093/hmg/ddi158. [DOI] [PubMed] [Google Scholar]
- Carlson ME, Conboy IM. Loss of stem cell regenerative capacity within aged niches. Aging Cell. 2007;6:371–382. doi: 10.1111/j.1474-9726.2007.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron M, Auclair M, Donadille B, et al. Human lipodystrophies linked to mutations in A-type lamins and to HIV protease inhibitor therapy are both associated with prelamin A accumulation, oxidative stress and premature cellular senescence. Cell Death Differ. 2007;14:1759–1767. doi: 10.1038/sj.cdd.4402197. [DOI] [PubMed] [Google Scholar]
- Caux F, Dubosclard E, Lascols O, et al. A new clinical condition linked to a novel mutation in lamins A and C with generalized lipoatrophy, insulin-resistant diabetes, disseminated leukomelanodermic papules, liver steatosis, and cardiomyopathy. J Clin Endocrinol Metab. 2003;88:1006–1013. doi: 10.1210/jc.2002-021506. [DOI] [PubMed] [Google Scholar]
- Chan GK, Duque G. Age-related bone loss: old bone, new facts. Gerontology. 2002;48:62–71. doi: 10.1159/000048929. [DOI] [PubMed] [Google Scholar]
- Chaouch M, Allal Y, De Sandre-Giovannoli A, et al. The phenotypic manifestations of autosomal recessive axonal Charcot-Marie-Tooth due to a mutation in Lamin A/C gene. Neuromuscul Disord. 2003;13:60–67. doi: 10.1016/s0960-8966(02)00196-7. [DOI] [PubMed] [Google Scholar]
- Charniot JC, Pascal C, Bouchier C, et al. Functional consequences of an LMNA mutation associated with a new cardiac and non-cardiac phenotype. Hum Mutat. 2003;21:473–481. doi: 10.1002/humu.10170. [DOI] [PubMed] [Google Scholar]
- Charniot JC, Bonnefont-Rousselot D, Marchand C, et al. Oxidative stress implication in a new phenotype of amyotrophic quadricipital syndrome with cardiac involvement due to lamin A/C mutation. Free Radic Res. 2007;41:424–431. doi: 10.1080/10715760601110046. [DOI] [PubMed] [Google Scholar]
- Chen K, Albano A, Ho A, Keaney JF., Jr Activation of p53 by oxidative stress involves platelet-derived growth factor-beta receptor-mediated ataxia telangiectasia mutated (ATM) kinase activation. J Biol Chem. 2003a;278:39527–39533. doi: 10.1074/jbc.M304423200. [DOI] [PubMed] [Google Scholar]
- Chen L, Lee L, Kudlow BA, et al. LMNA mutations in atypical Werner's syndrome. Lancet. 2003b;362:440–445. doi: 10.1016/S0140-6736(03)14069-X. [DOI] [PubMed] [Google Scholar]
- Classon M, Kennedy BK, Mulloy R, Harlow E. Opposing roles of pRB and p107 in adipocyte differentiation. Proc Natl Acad Sci USA. 2000;97:10826–10831. doi: 10.1073/pnas.190343597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements L, Manilal S, Love DR, Morris GE. Direct interaction between emerin and lamin A. Biochem Biophys Res Commun. 2000;267:709–714. doi: 10.1006/bbrc.1999.2023. [DOI] [PubMed] [Google Scholar]
- Cohen M, Lee KK, Wilson KL, Gruenbaum Y. Transcriptional repression, apoptosis, human disease and the functional evolution of the nuclear lamina. Trends Biochem Sci. 2001;26:41–47. doi: 10.1016/s0968-0004(00)01727-8. [DOI] [PubMed] [Google Scholar]
- Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005;4:407–410. doi: 10.4161/cc.4.3.1518. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Conde JR, Streit WJ. Microglia in the aging brain. J Neuropathol Exp Neurol. 2006;65:199–203. doi: 10.1097/01.jnen.0000202887.22082.63. [DOI] [PubMed] [Google Scholar]
- Constantinescu D, Gray HL, Sammak PJ, Schatten GP, Csoka AB. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells. 2006;24:177–185. doi: 10.1634/stemcells.2004-0159. [DOI] [PubMed] [Google Scholar]
- Csoka AB, Cao H, Sammak PJ, et al. Novel lamin A/C gene (LMNA) mutations in atypical progeroid syndromes. J Med Genet. 2004;41:304–308. doi: 10.1136/jmg.2003.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico A, Haliloglu G, Richard P, et al. Two patients with ‘Dropped head syndrome’ due to mutations in LMNA or SEPN1 genes. Neuromuscul Disord. 2005;15:521–524. doi: 10.1016/j.nmd.2005.03.006. [DOI] [PubMed] [Google Scholar]
- De Sandre-Giovannoli A, Chaouch M, Kozlov S, et al. Homozygous defects in LMNA, encoding lamin A/C nuclear-envelope proteins, cause autosomal recessive axonal neuropathy in human (Charcot-Marie-Tooth disorder type 2) and mouse. Am J Hum Genet. 2002;70:726–736. doi: 10.1086/339274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sandre-Giovannoli A, Bernard R, Cau P, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125. [DOI] [PubMed] [Google Scholar]
- Dechat T, Korbei B, Vaughan OA, et al. Lamina-associated polypeptide 2alpha binds intranuclear A-type lamins. J Cell Sci. 2000;113:3473–3484. doi: 10.1242/jcs.113.19.3473. [DOI] [PubMed] [Google Scholar]
- Dechat T, Shimi T, Adam SA, et al. Alterations in mitosis and cell cycle progression caused by a mutant lamin A known to accelerate human aging. Proc Natl Acad Sci USA. 2007;104:4955–4960. doi: 10.1073/pnas.0700854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Dhe-Paganon S, Werner ED, Chi YI, Shoelson SE. Structure of the globular tail of nuclear lamin. J Biol Chem. 2002;277:17381–17384. doi: 10.1074/jbc.C200038200. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Dorner D, Vlcek S, Foeger N, et al. Lamina-associated polypeptide 2alpha regulates cell cycle progression and differentiation via the retinoblastoma-E2F pathway. J Cell Biol. 2006;173:83–93. doi: 10.1083/jcb.200511149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque G, Rivas D. Age-related changes in lamin A/C expression in the osteoarticular system: laminopathies as a potential new aging mechanism. Mech Ageing Dev. 2006;127:378–383. doi: 10.1016/j.mad.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Duque G, Macoritto M, Kremer R. 1,25(OH)2D3 inhibits bone marrow adipogenesis in senescence accelerated mice (SAM-P/6) by decreasing the expression of peroxisome proliferator-activated receptor gamma 2 (PPARgamma2) Exp Gerontol. 2004;39:333–338. doi: 10.1016/j.exger.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Brown WT, Gordon LB, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espada J, Varela I, Flores I, et al. Nuclear envelope defects cause stem cell dysfunction in premature-aging mice. J Cell Biol. 2008;181:27–35. doi: 10.1083/jcb.200801096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faragher RG, Kipling D. How might replicative senescence contribute to human ageing. Bioessays. 1998;20(12):985–991. doi: 10.1002/(SICI)1521-1878(199812)20:12<985::AID-BIES4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Fatkin D, MacRae C, Sasaki T, et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999;341:1715–1724. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- Favreau C, Higuet D, Courvalin JC, Buendia B. Expression of a mutant lamin A that causes Emery-Dreifuss muscular dystrophy inhibits in vitro differentiation of C2C12 myoblasts. Mol Cell Biol. 2004;24:1481–1492. doi: 10.1128/MCB.24.4.1481-1492.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favreau C, Delbarre E, Courvalin JC, Buendia B. Differentiation of C2C12 myoblasts expressing lamin A mutated at a site responsible for Emery-Dreifuss muscular dystrophy is improved by inhibition of the MEK-ERK pathway and stimulation of the PI3-kinase pathway. Exp Cell Res. 2008;314:1392–1405. doi: 10.1016/j.yexcr.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Fehrer C, Lepperdinger G. Mesenchymal stem cell aging. Exp Gerontol. 2005;40:926–930. doi: 10.1016/j.exger.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Forissier JF, Bonne G, Bouchier C, et al. Apical left ventricular aneurysm without atrio-ventricular block due to a lamin A/C gene mutation. Eur J Heart Fail. 2003;5:821–825. doi: 10.1016/s1388-9842(03)00149-1. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Zhao C, Sim FJ. Ageing and CNS remyelination. Neuroreport. 2002;13:923–928. doi: 10.1097/00001756-200205240-00001. [DOI] [PubMed] [Google Scholar]
- Frock RL, Kudlow BA, Evans AM, et al. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev. 2006;20:486–500. doi: 10.1101/gad.1364906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galderisi U, Cipollaro M, Giordano A. The retinoblastoma gene is involved in multiple aspects of stem cell biology. Oncogene. 2006;25:5250–5256. doi: 10.1038/sj.onc.1209736. [DOI] [PubMed] [Google Scholar]
- Garg A, Speckman RA, Bowcock AM. Multisystem dystrophy syndrome due to novel missense mutations in the amino-terminal head and alpha-helical rod domains of the lamin A/C gene. Am J Med. 2002;112:549–555. doi: 10.1016/s0002-9343(02)01070-7. [DOI] [PubMed] [Google Scholar]
- Goizet C, Yaou RB, Demay L, et al. A new mutation of the lamin A/C gene leading to autosomal dominant axonal neuropathy, muscular dystrophy, cardiac disease, and leuconychia. J Med Genet. 2004;41:e29. doi: 10.1136/jmg.2003.013383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman RD, Shumaker DK, Erdos MR, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA. 2004;101:8963–8968. doi: 10.1073/pnas.0402943101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotzmann J, Foisner R. A-type lamin complexes and regenerative potential: a step towards understanding laminopathic diseases? Histochem Cell Biol. 2006;125:33–41. doi: 10.1007/s00418-005-0050-8. [DOI] [PubMed] [Google Scholar]
- Guo W, Pirtskhalava T, Tchkonia T, et al. Aging results in paradoxical susceptibility of fat cell progenitors to lipotoxicity. Am J Physiol Endocrinol Metab. 2007;292:E1041–E1051. doi: 10.1152/ajpendo.00557.2006. [DOI] [PubMed] [Google Scholar]
- Haithcock E, Dayani Y, Neufeld E, et al. Age-related changes of nuclear architecture in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2005;102:16690–16695. doi: 10.1073/pnas.0506955102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaschek-Wiener J, Brooks-Wilson A. Progeria of stem cells: stem cell exhaustion in Hutchinson-Gilford progeria syndrome. J Gerontol A Biol Sci Med Sci. 2007;62:3–8. doi: 10.1093/gerona/62.1.3. [DOI] [PubMed] [Google Scholar]
- Han KH, Choi HR, Won CH, et al. Alteration of the TGF-beta/SMAD pathway in intrinsically and UV-induced skin aging. Mech Ageing Dev. 2005;126:560–567. doi: 10.1016/j.mad.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Haque F, Lloyd DJ, Smallwood DT, et al. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol Cell Biol. 2006;26:3738–3751. doi: 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001;114:4557–4565. doi: 10.1242/jcs.114.24.4557. [DOI] [PubMed] [Google Scholar]
- Hartmann C. A Wnt canon orchestrating osteoblastogenesis. Trends Cell Biol. 2006;16:151–158. doi: 10.1016/j.tcb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Hegele RA, Cao H, Liu DM, et al. Sequencing of the reannotated LMNB2 gene reveals novel mutations in patients with acquired partial lipodystrophy. Am J Hum Genet. 2006;79:383–389. doi: 10.1086/505885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans J, Preobrazhenska O, Willaert A, et al. Loss-of-function mutations in LEMD3 result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis. Nat Genet. 2004;36:1213–1218. doi: 10.1038/ng1453. [DOI] [PubMed] [Google Scholar]
- Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- Herrera RE, Makela TP, Weinberg RA. TGF beta-induced growth inhibition in primary fibroblasts requires the retinoblastoma protein. Mol Biol Cell. 1996;7:1335–1342. doi: 10.1091/mbc.7.9.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- Holaska JM, Wilson KL. An emerin ‘proteome’: purification of distinct emerin-containing complexes from HeLa cells suggests molecular basis for diverse roles including gene regulation, mRNA splicing, signaling, mechanosensing, and nuclear architecture. Biochemistry. 2007;46:8897–908. doi: 10.1021/bi602636m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Risques RA, Martin GM, Rabinovitch PS, Oshima J. Accelerated telomere shortening and replicative senescence in human fibroblasts overexpressing mutant and wild-type lamin A. Exp Cell Res. 2008;314:82–91. doi: 10.1016/j.yexcr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison CJ. Lamins: building blocks or regulators of gene expression? Nat Rev Mol Cell Biol. 2002;3:848–858. doi: 10.1038/nrm950. [DOI] [PubMed] [Google Scholar]
- Hutchison CJ, Worman HJ. A-type lamins: guardians of the soma? Nat Cell Biol. 2004;6:1062–1067. doi: 10.1038/ncb1104-1062. [DOI] [PubMed] [Google Scholar]
- Hutchison CJ, Alvarez-Reyes M, Vaughan OA. Lamins in disease: why do ubiquitously expressed nuclear envelope proteins give rise to tissue-specific disease phenotypes? J Cell Sci. 2001;114:9–19. doi: 10.1242/jcs.114.1.9. [DOI] [PubMed] [Google Scholar]
- Hutter E, Unterluggauer H, Uberall F, Schramek H, Jansen-Durr P. Replicative senescence of human fibroblasts: the role of Ras-dependent signaling and oxidative stress. Exp Gerontol. 2002;37:1165–1174. doi: 10.1016/s0531-5565(02)00136-5. [DOI] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Ivorra C, Kubicek M, Gonzalez JM, et al. A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev. 2006;20:307–320. doi: 10.1101/gad.349506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa H, Han J, Ishikawa F. Mitogen-activated protein kinase p38 defines the common senescence-signalling pathway. Genes Cells. 2003;8:131–144. doi: 10.1046/j.1365-2443.2003.00620.x. [DOI] [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming HE, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev. 2007;128:36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, et al. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Johnson BR, Nitta RT, Frock RL, et al. A-type lamins regulate retinoblastoma protein function by promoting subnuclear localization and preventing proteasomal degradation. Proc Natl Acad Sci USA. 2004;101:9677–9682. doi: 10.1073/pnas.0403250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WK, Brown M, Ren X, He S, McGuinness M. NF-kappaB as an integrator of diverse signaling pathways: the heart of myocardial signaling? Cardiovasc Toxicol. 2003;3:229–254. doi: 10.1385/ct:3:3:229. [DOI] [PubMed] [Google Scholar]
- Kamminga LM, de Haan G. Cellular memory and hematopoietic stem cell aging. Stem Cells. 2006;24:1143–1149. doi: 10.1634/stemcells.2005-0345. [DOI] [PubMed] [Google Scholar]
- Karagiannides I, Tchkonia T, Dobson DE, et al. Altered expression of C/EBP family members results in decreased adipogenesis with aging. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1772–R1780. doi: 10.1152/ajpregu.2001.280.6.R1772. [DOI] [PubMed] [Google Scholar]
- Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118:10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ES, Kim MS, Moon A. TGF-beta-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int J Oncol. 2004;25:1375–1382. [PubMed] [Google Scholar]
- Kipling D, Davis T, Ostler EL, Faragher RG. What can progeroid syndromes tell us about human aging? Science. 2004;305:1426–1431. doi: 10.1126/science.1102587. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Dobson DE. Preadipocyte function and aging: links between age-related changes in cell dynamics and altered fat tissue function. J Am Geriatr Soc. 1997;45:959–967. doi: 10.1111/j.1532-5415.1997.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I. Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol. 2002;37:757–767. doi: 10.1016/s0531-5565(02)00014-1. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. Evolution of ageing. Nature. 1977;270:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- van der Kooi AJ, Bonne G, Eymard B, et al. Lamin A/C mutations with lipodystrophy, cardiac abnormalities, and muscular dystrophy. Neurology. 2002;59:620–623. doi: 10.1212/wnl.59.4.620. [DOI] [PubMed] [Google Scholar]
- Krimm I, Ostlund C, Gilquin B, et al. The Ig-like structure of the C-terminal domain of lamin A/C, mutated in muscular dystrophies, cardiomyopathy, and partial lipodystrophy. Structure. 2002;10:811–823. doi: 10.1016/s0969-2126(02)00777-3. [DOI] [PubMed] [Google Scholar]
- Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J, Schulze PC, Takahashi T, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding J, Hsiao J, Schulze PC, et al. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol. 2005;170:781–791. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Ladha MH, McMahon C, Ewen ME. The retinoblastoma protein is linked to the activation of Ras. Mol Cell Biol. 1999;19:7724–7732. doi: 10.1128/mcb.19.11.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KK, Haraguchi T, Lee RS, et al. Distinct functional domains in emerin bind lamin A and DNA-bridging protein BAF. J Cell Sci. 2001;114:4567–4573. doi: 10.1242/jcs.114.24.4567. [DOI] [PubMed] [Google Scholar]
- Lees SJ, Rathbone CR, Booth FW. Age-associated decrease in muscle precursor cell differentiation. Am J Physiol Cell Physiol. 2006;290:C609–C615. doi: 10.1152/ajpcell.00408.2005. [DOI] [PubMed] [Google Scholar]
- Libotte T, Zaim H, Abraham S, et al. Lamin A/C-dependent localization of Nesprin-2, a giant scaffolder at the nuclear envelope. Mol Biol Cell. 2005;16:3411–3424. doi: 10.1091/mbc.E04-11-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Morrison JM, Wu W, Worman HJ. MAN1, an integral protein of the inner nuclear membrane, binds Smad2 and Smad3 and antagonizes transforming growth factor-beta signaling. Hum Mol Genet. 2005;14:437–445. doi: 10.1093/hmg/ddi040. [DOI] [PubMed] [Google Scholar]
- Liu B, Wang J, Chan KM, et al. Genomic instability in laminopathy-based premature aging. Nat Med. 2005;11:780–785. doi: 10.1038/nm1266. [DOI] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rusinol A, Sinensky M, Wang Y, Zou Y. DNA damage responses in progeroid syndromes arise from defective maturation of prelamin A. J Cell Sci. 2006;119:4644–4649. doi: 10.1242/jcs.03263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DJ, Trembath RC, Shackleton S. A novel interaction between lamin A and SREBP1: implications for partial lipodystrophy and other laminopathies. Hum Mol Genet. 2002;11:769–777. doi: 10.1093/hmg/11.7.769. [DOI] [PubMed] [Google Scholar]
- Maier B, Gluba W, Berner B, et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini MA, Shan B, Nickerson JA, Penman S, Lee WH. The retinoblastoma gene product is a cell cycle-dependent, nuclear matrix-associated protein. Proc Natl Acad Sci USA. 1994;91:418–422. doi: 10.1073/pnas.91.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manju K, Muralikrishna B, Parnaik VK. Expression of disease-causing lamin A mutants impairs the formation of DNA repair foci. J Cell Sci. 2006;119:2704–2714. doi: 10.1242/jcs.03009. [DOI] [PubMed] [Google Scholar]
- Mansharamani M, Wilson KL. Direct binding of nuclear membrane protein MAN1 to emerin in vitro and two modes of binding to barrier-to-autointegration factor. J Biol Chem. 2005;280:13863–13870. doi: 10.1074/jbc.M413020200. [DOI] [PubMed] [Google Scholar]
- Margalit A, Liu J, Fridkin A, Wilson KL, Gruenbaum Y. A lamin-dependent pathway that regulates nuclear organization, cell cycle progression and germ cell development. Novartis Found Symp. 2005;264:231–240. discussion 240–245. [PubMed] [Google Scholar]
- Mariappan I, Parnaik VK. Sequestration of pRb by cyclin D3 causes intranuclear reorganization of lamin A/C during muscle cell differentiation. Mol Biol Cell. 2005;16:1948–1960. doi: 10.1091/mbc.E04-02-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz E, Dechat T, Foisner R, Quinlan RA, Hutchison CJ. Lamin A/C binding protein LAP2alpha is required for nuclear anchorage of retinoblastoma protein. Mol Biol Cell. 2002;13:4401–4413. doi: 10.1091/mbc.E02-07-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz E, Ledran M, Hutchison CJ. Remodelling of the nuclear lamina and nucleoskeleton is required for skeletal muscle differentiation in vitro. J Cell Sci. 2005;118:409–420. doi: 10.1242/jcs.01630. [DOI] [PubMed] [Google Scholar]
- Markiewicz E, Tilgner K, Barker N, et al. The inner nuclear membrane protein emerin regulates beta-catenin activity by restricting its accumulation in the nucleus. EMBO J. 2006;25:3275–3285. doi: 10.1038/sj.emboj.7601230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM. The genetics and epigenetics of altered proliferative homeostasis in ageing and cancer. Mech Ageing Dev. 2007;128:9–12. doi: 10.1016/j.mad.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock D, Gordon LB, Djabali K. Hutchinson-Gilford progeria mutant lamin A primarily targets human vascular cells as detected by an anti-Lamin A G608G antibody. Proc Natl Acad Sci USA. 2006;103:2154–2159. doi: 10.1073/pnas.0511133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996;10:1173–1183. doi: 10.1101/gad.10.10.1173. [DOI] [PubMed] [Google Scholar]
- Melcon G, Kozlov S, Cutler DA, et al. Loss of emerin at the nuclear envelope disrupts the Rb1/E2F and MyoD pathways during muscle regeneration. Hum Mol Genet. 2006;15:637–651. doi: 10.1093/hmg/ddi479. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Mislow JM, Holaska JM, Kim MS, et al. Nesprin-1alpha self-associates and binds directly to emerin and lamin A in vitro. FEBS Lett. 2002;525:135–140. doi: 10.1016/s0014-5793(02)03105-8. [DOI] [PubMed] [Google Scholar]
- Mittnacht S, Paterson H, Olson MF, Marshall CJ. Ras signalling is required for inactivation of the tumour suppressor pRb cell-cycle control protein. Curr Biol. 1997;7:219–221. doi: 10.1016/s0960-9822(97)70094-0. [DOI] [PubMed] [Google Scholar]
- Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moioli EK, Hong L, Mao JJ. Inhibition of osteogenic differentiation of human mesenchymal stem cells. Wound Repair Regen. 2007;15:413–421. doi: 10.1111/j.1524-475X.2007.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Morrison SJ. Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell Biol. 2004;16:700–707. doi: 10.1016/j.ceb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- Mounkes LC, Stewart CL. Aging and nuclear organization: lamins and progeria. Curr Opin Cell Biol. 2004;16:322–327. doi: 10.1016/j.ceb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Mounkes LC, Kozlov S, Hernandez L, Sullivan T, Stewart CL. A progeroid syndrome in mice is caused by defects in A-type lamins. Nature. 2003;423:298–301. doi: 10.1038/nature01631. [DOI] [PubMed] [Google Scholar]
- Mounkes LC, Kozlov SV, Rottman JN, Stewart CL. Expression of an LMNA-N195 K variant of A-type lamins results in cardiac conduction defects and death in mice. Hum Mol Genet. 2005;14:2167–2180. doi: 10.1093/hmg/ddi221. [DOI] [PubMed] [Google Scholar]
- Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol. 2005;26:535–542. doi: 10.1016/j.it.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Muchir A, Bonne G, van der Kooi AJ, et al. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B) Hum Mol Genet. 2000;9:1453–1459. doi: 10.1093/hmg/9.9.1453. [DOI] [PubMed] [Google Scholar]
- Muchir A, van Engelen BG, Lammens M, et al. Nuclear envelope alterations in fibroblasts from LGMD1B patients carrying nonsense Y259X heterozygous or homozygous mutation in lamin A/C gene. Exp Cell Res. 2003;291:352–362. doi: 10.1016/j.yexcr.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Muchir A, Pavlidis P, Decostre V, et al. Activation of MAPK pathways links LMNA mutations to cardiomyopathy in Emery-Dreifuss muscular dystrophy. J Clin Invest. 2007;117:1282–1293. doi: 10.1172/JCI29042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583–590. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- Muralikrishna B, Parnaik VK. SP3 and AP-1 mediate transcriptional activation of the lamin A proximal promoter. Eur J Biochem. 2001;268:3736–3743. doi: 10.1046/j.1432-1327.2001.02281.x. [DOI] [PubMed] [Google Scholar]
- Navarro CL, De Sandre-Giovannoli A, Bernard R, et al. Lamin A and ZMPSTE24 (FACE-1) defects cause nuclear disorganization and identify restrictive dermopathy as a lethal neonatal laminopathy. Hum Mol Genet. 2004;13:2493–2503. doi: 10.1093/hmg/ddh265. [DOI] [PubMed] [Google Scholar]
- Navarro CL, Cadinanos J, De Sandre-Giovannoli A, et al. Loss of ZMPSTE24 (FACE-1) causes autosomal recessive restrictive dermopathy and accumulation of Lamin A precursors. Hum Mol Genet. 2005;14:1503–1513. doi: 10.1093/hmg/ddi159. [DOI] [PubMed] [Google Scholar]
- Nicol RL, Frey N, Pearson G, et al. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 2001;20:2757–2767. doi: 10.1093/emboj/20.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta RT, Jameson SA, Kudlow BA, Conlan LA, Kennedy BK. Stabilization of the retinoblastoma protein by A-type nuclear lamins is required for INK4A-mediated cell cycle arrest. Mol Cell Biol. 2006;26:5360–5372. doi: 10.1128/MCB.02464-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli G, Muchir A, Sangiuolo F, et al. Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am J Hum Genet. 2002;71:426–431. doi: 10.1086/341908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitch BG, Mulligan GJ, Jacks T, Lassar AB. Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J Cell Biol. 1996;135:441–456. doi: 10.1083/jcb.135.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Saijo M, Murakami K, et al. Complex formation between lamin A and the retinoblastoma gene product: identification of the domain on lamin A required for its interaction. Oncogene. 1994;9:2649–2653. [PubMed] [Google Scholar]
- Padiath QS, Saigoh K, Schiffmann R, et al. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat Genet. 2006;38:1114–1123. doi: 10.1038/ng1872. [DOI] [PubMed] [Google Scholar]
- Padmakumar VC, Libotte T, Lu W, et al. The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J Cell Sci. 2005;118:3419–3430. doi: 10.1242/jcs.02471. [DOI] [PubMed] [Google Scholar]
- Pan D, Estevez-Salmeron LD, Stroschein SL, et al. The integral inner nuclear membrane protein MAN1 physically interacts with the R-Smad proteins to repress signaling by the transforming growth factor-{beta} superfamily of cytokines. J Biol Chem. 2005;280:15992–16001. doi: 10.1074/jbc.M411234200. [DOI] [PubMed] [Google Scholar]
- Pekovic V, Harborth J, Broers JL, et al. Nucleoplasmic LAP2alpha-lamin A complexes are required to maintain a proliferative state in human fibroblasts. J Cell Biol. 2007;176:163–172. doi: 10.1083/jcb.200606139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicci PG. Do tumor-suppressive mechanisms contribute to organism aging by inducing stem cell senescence? J Clin Invest. 2004;113:4–7. doi: 10.1172/JCI200420750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendas AM, Zhou Z, Cadinanos J, et al. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat Genet. 2002;31:94–99. doi: 10.1038/ng871. [DOI] [PubMed] [Google Scholar]
- Petrich BG, Molkentin JD, Wang Y. Temporal activation of c-Jun N-terminal kinase in adult transgenic heart via cre-loxP-mediated DNA recombination. FASEB J. 2003;17:749–751. doi: 10.1096/fj.02-0438fje. [DOI] [PubMed] [Google Scholar]
- Qian H, Yang Y, Li J, Huang J, Dou K, Yang G. The role of vascular stem cells in atherogenesis and post-angioplasty restenosis. Ageing Res Rev. 2007;6:109–127. doi: 10.1016/j.arr.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Raffaele Di Barletta M, Ricci E, Galluzzi G, et al. Different mutations in the LMNA gene cause autosomal dominant and autosomal recessive Emery-Dreifuss muscular dystrophy. Am J Hum Genet. 2000;66:1407–1412. doi: 10.1086/302869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman-Roblick R, Roblick UJ, Hellman U, et al. p53 targets identified by protein expression profiling. Proc Natl Acad Sci USA. 2007;104:5401–5406. doi: 10.1073/pnas.0700794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080–1086. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- Rankin J, Ellard S. The laminopathies: a clinical review. Clin Genet. 2006;70:261–274. doi: 10.1111/j.1399-0004.2006.00677.x. [DOI] [PubMed] [Google Scholar]
- Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452:243–247. doi: 10.1038/nature06727. [DOI] [PubMed] [Google Scholar]
- Renault V, Thornell LE, Butler-Browne G, Mouly V. Human skeletal muscle satellite cells: aging, oxidative stress and the mitotic clock. Exp Gerontol. 2002;37:1229–1236. doi: 10.1016/s0531-5565(02)00129-8. [DOI] [PubMed] [Google Scholar]
- Riemer D, Stuurman N, Berrios M, et al. Expression of Drosophila lamin C is developmentally regulated: analogies with vertebrate A-type lamins. J Cell Sci. 1995;108:3189–3198. doi: 10.1242/jcs.108.10.3189. [DOI] [PubMed] [Google Scholar]
- Rietze RL, Valcanis H, Brooker GF, et al. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412:736–739. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- Rober RA, Weber K, Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development. 1989;105:365–378. doi: 10.1242/dev.105.2.365. [DOI] [PubMed] [Google Scholar]
- Rusinol AE, Sinensky MS. Farnesylated lamins, progeroid syndromes and farnesyl transferase inhibitors. J Cell Sci. 2006;119:3265–3272. doi: 10.1242/jcs.03156. [DOI] [PubMed] [Google Scholar]
- Salpingidou G, Smertenko A, Hausmanowa-Petrucewicz I, Hussey PJ, Hutchison CJ. A novel role for the nuclear membrane protein emerin in association of the centrosome to the outer nuclear membrane. J Cell Biol. 2007;178:897–904. doi: 10.1083/jcb.200702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Sato M. Upregulation of the Wnt/beta-catenin pathway induced by transforming growth factor-beta in hypertrophic scars and keloids. Acta Derm Venereol. 2006;86:300–307. doi: 10.2340/00015555-0101. [DOI] [PubMed] [Google Scholar]
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol. 2008;10:452–459. doi: 10.1038/ncb1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer EC, Guan T, Gerace L. Involvement of the lamin rod domain in heterotypic lamin interactions important for nuclear organization. J Cell Biol. 2001;153:479–489. doi: 10.1083/jcb.153.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger D, Van Zant G. Does functional depletion of stem cells drive aging? Mech Ageing Dev. 2001;122:1537–1553. doi: 10.1016/s0047-6374(01)00299-8. [DOI] [PubMed] [Google Scholar]
- Sebillon P, Bouchier C, Bidot LD, et al. Expanding the phenotype of LMNA mutations in dilated cardiomyopathy and functional consequences of these mutations. J Med Genet. 2003;40:560–567. doi: 10.1136/jmg.40.8.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serakinci N, Keith WN. Therapeutic potential of adult stem cells. Eur J Cancer. 2006;42:1243–1246. doi: 10.1016/j.ejca.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Shackleton S, Lloyd DJ, Jackson SN, et al. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet. 2000;24:153–156. doi: 10.1038/72807. [DOI] [PubMed] [Google Scholar]
- Shantsila E, Watson T, Lip GY. Antioxidant protection: yet another function of endothelial progenitor cells? J Hum Hypertens. 2007;21:343–346. doi: 10.1038/sj.jhh.1002166. [DOI] [PubMed] [Google Scholar]
- Shay JW, Pereira-Smith OM, Wright WE. A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res. 1991;196:33–39. doi: 10.1016/0014-4827(91)90453-2. [DOI] [PubMed] [Google Scholar]
- Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev. 2006;20:1692–708. doi: 10.1101/gad.1419406. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Smith ED, Kudlow BA, Frock RL, Kennedy BK. A-type nuclear lamins, progerias and other degenerative disorders. Mech Ageing Dev. 2005;126:447–460. doi: 10.1016/j.mad.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Spann TP, Moir RD, Goldman AE, Stick R, Goldman RD. Disruption of nuclear lamin organization alters the distribution of replication factors and inhibits DNA synthesis. J Cell Biol. 1997;136:1201–1212. doi: 10.1083/jcb.136.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann TP, Goldman AE, Wang C, Huang S, Goldman RD. Alteration of nuclear lamin organization inhibits RNA polymerase II-dependent transcription. J Cell Biol. 2002;156:603–608. doi: 10.1083/jcb.200112047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C, Burke B. Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell. 1987;51:383–392. doi: 10.1016/0092-8674(87)90634-9. [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kozlov S, Fong LG, Young SG. Mouse models of the laminopathies. Exp Cell Res. 2007;313:2144–2156. doi: 10.1016/j.yexcr.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuurman N, Heins S, Aebi U. Nuclear lamins: their structure, assembly, and interactions. J Struct Biol. 1998;122:42–66. doi: 10.1006/jsbi.1998.3987. [DOI] [PubMed] [Google Scholar]
- Sullivan T, Escalante-Alcalde D, Bhatt H, et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–920. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori Y, Tamura Y, Kataoka Y, et al. Differential expression of nuclear lamin, the major component of nuclear lamina, during neurogenesis in two germinal regions of adult rat brain. Eur J Neurosci. 2007;25:1653–1662. doi: 10.1111/j.1460-9568.2007.05450.x. [DOI] [PubMed] [Google Scholar]
- Tao J, Wang Y, Yang Z, et al. Circulating endothelial progenitor cell deficiency contributes to impaired arterial elasticity in persons of advancing age. J Hum Hypertens. 2006;20:490–495. doi: 10.1038/sj.jhh.1001996. [DOI] [PubMed] [Google Scholar]
- Taylor MR, Slavov D, Gajewski A, et al. Thymopoietin (lamina-associated polypeptide 2) gene mutation associated with dilated cardiomyopathy. Hum Mutat. 2005;26:566–574. doi: 10.1002/humu.20250. [DOI] [PubMed] [Google Scholar]
- van Tintelen JP, Tio RA, Kerstjens-Frederikse WS, et al. Severe myocardial fibrosis caused by a deletion of the 5′ end of the lamin A/C gene. J Am Coll Cardiol. 2007;49:2430–2439. doi: 10.1016/j.jacc.2007.02.063. [DOI] [PubMed] [Google Scholar]
- Torella D, Rota M, Nurzynska D, et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- Torella D, Ellison GM, Mendez-Ferrer S, Ibanez B, Nadal-Ginard B. Resident human cardiac stem cells: role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl. 1):S8–S13. doi: 10.1038/ncpcardio0409. [DOI] [PubMed] [Google Scholar]
- Tsukahara T, Tsujino S, Arahata K. CDNA microarray analysis of gene expression in fibroblasts of patients with X-linked Emery-Dreifuss muscular dystrophy. Muscle Nerve. 2002;25:898–901. doi: 10.1002/mus.10085. [DOI] [PubMed] [Google Scholar]
- Tunnah D, Sewry CA, Vaux D, Schirmer EC, Morris GE. The apparent absence of lamin B1 and emerin in many tissue nuclei is due to epitope masking. J Mol Histol. 2005;36:337–344. doi: 10.1007/s10735-005-9004-7. [DOI] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- Uitto J, Bernstein EF. Molecular mechanisms of cutaneous aging: connective tissue alterations in the dermis. J Investig Dermatol Symp Proc. 1998;3:41–44. [PubMed] [Google Scholar]
- Ukekawa R, Miki K, Fujii M, Hirano H, Ayusawa D. Accumulation of multiple forms of lamin A with down-regulation of FACE-1 suppresses growth in senescent human cells. Genes Cells. 2007;12:397–406. doi: 10.1111/j.1365-2443.2007.01057.x. [DOI] [PubMed] [Google Scholar]
- Vantyghem MC, Pigny P, Maurage CA, et al. Patients with familial partial lipodystrophy of the Dunnigan type due to a LMNA R482W mutation show muscular and cardiac abnormalities. J Clin Endocrinol Metab. 2004;89:5337–5346. doi: 10.1210/jc.2003-031658. [DOI] [PubMed] [Google Scholar]
- Varela I, Cadinanos J, Pendas AM, et al. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature. 2005;437:564–568. doi: 10.1038/nature04019. [DOI] [PubMed] [Google Scholar]
- Varga R, Eriksson M, Erdos MR, et al. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA. 2006;103:3250–3255. doi: 10.1073/pnas.0600012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan A, Alvarez-Reyes M, Bridger JM, et al. Both emerin and lamin C depend on lamin A for localization at the nuclear envelope. J Cell Sci. 2001;114:2577–2590. doi: 10.1242/jcs.114.14.2577. [DOI] [PubMed] [Google Scholar]
- Vergnes L, Peterfy M, Bergo MO, Young SG, Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc Natl Acad Sci USA. 2004;101:10428–10433. doi: 10.1073/pnas.0401424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrecchia F, Mauviel A, Farge D. Transforming growth factor-beta signaling through the Smad proteins: role in systemic sclerosis. Autoimmun Rev. 2006;5:563–569. doi: 10.1016/j.autrev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Vlcek S, Foisner R. Lamins and lamin-associated proteins in aging and disease. Curr Opin Cell Biol. 2007;19:298–304. doi: 10.1016/j.ceb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- Wang W, Chen JX, Liao R, et al. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol Cell Biol. 2002;22:3389–3403. doi: 10.1128/MCB.22.10.3389-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel N, Mattout A, Melcer S, et al. Laminopathic mutations interfere with the assembly, localization, and dynamics of nuclear lamins. Proc Natl Acad Sci USA. 2008;105:180–185. doi: 10.1073/pnas.0708974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Worman HJ, Bonne G. ‘Laminopathies’: a wide spectrum of human diseases. Exp Cell Res. 2007;313:2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WE, Shay JW. Historical claims and current interpretations of replicative aging. Nat Biotechnol. 2002;20:682–688. doi: 10.1038/nbt0702-682. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rudnicki MA, Rivera AJ, et al. The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev Biol. 1999;210:440–455. doi: 10.1006/dbio.1999.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Bergo MO, Toth JI, et al. Blocking protein fornesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson-Gilford progeria syndrome mutation. Proc Natl Acad Sci USA. 2005;102:10291–10296. doi: 10.1073/pnas.0504641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Morbois-Trabut L, Couzinet B, et al. Type A insulin resistance syndrome revealing a novel lamin A mutation. Diabetes. 2005;54:1873–1878. doi: 10.2337/diabetes.54.6.1873. [DOI] [PubMed] [Google Scholar]
- Zastrow MS, Vlcek S, Wilson KL. Proteins that bind A-type lamins: integrating isolated clues. J Cell Sci. 2004;117:979–987. doi: 10.1242/jcs.01102. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ragnauth CD, Skepper JN, et al. Nesprin-2 is a multi-isomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle. J Cell Sci. 2005;118:673–687. doi: 10.1242/jcs.01642. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Bethmann C, Worth NF, et al. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet. 2007;16:2816–2833. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]