Abstract
Tissue engineering scaffolds are designed to influence the physical, chemical and biological environment surrounding a cell population. In this review we focus on our own work and introduce a range of strategies and materials used for tissue engineering, including the sources of cells suitable for tissue engineering: embryonic stem cells, bone marrow-derived mesenchymal stem cells and cord-derived mesenchymal stem cells. Furthermore, we emphasize the developments in custom scaffold design and manufacture, highlighting laser sintering, supercritical carbon dioxide processing, growth factor incorporation and zoning, plasma modification of scaffold surfaces, and novel multi-use temperature-sensitive injectable materials.
Keywords: controlled growth factor delivery, embryonic stem cells, injectable scaffolds, mesenchymal stem cells, regenerative medicine, surface engineering, tissue engineering, zonated scaffolds
Introduction
Tissue engineering, as viewed today, is ‘an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function or a whole organ’ (Langer & Vacanti, 1993). This utilizes scaffold matrices to fill the tissue void, to provide structural support and to deliver growth factors and/or cells that have the ability to form tissues within the body upon transplantation.
Tissue engineering/regenerative medicine strategies require interaction and integration with tissue and cells through incorporation of appropriate physical and cellular signals. Therefore, inclusion of modifying factors such as biologically active proteins and DNA are critical to success. Currently, simpler procedures are more successful and include using primary chondrocytes for the replacement of damaged cartilage (Brittberg et al. 1994; Richardson et al. 1999) as well as skin cell sheets for damaged skin (Hernon et al. 2006). However, some larger and more complex tissue reconstructions, notably the bladder, have been successfully performed (Atala et al. 2006), offering hope for more complex tissue engineered procedures in the future.
Although basic functional tissue engineered strategies have been key there is still considerable scope for future developments of cell sources, individually tailored cell supports, immune modulation, vascularization, and the predictive abilities of computer and mathematical modelling for more complex materials.
In this review we introduce some of the components and strategies that are currently in development with a bias toward some of the work within our group.
Overview of tissue engineering strategies
Two main approaches are utilized in this area to produce engineered tissue. First, scaffolding can be used as a cell support device upon which cells are seeded in vitro; cells are then encouraged to lay down matrix to produce the foundations of a tissue for transplantation. The second approach involves using the scaffold as a growth factor/drug delivery device. This strategy involves the scaffold being combined with growth factors, so upon implantation cells from the body are recruited to the scaffold site and form tissue upon and throughout the matrices. These two approaches are not mutually exclusive and can be easily combined.
The manor in which a cell type and scaffolding are combined should be carefully matched for purpose as it has been demonstrated that composition, topography and architecture of scaffolds are able to interact and influence cell behaviour. Scaffold architecture has been shown to modify the response of cells and subsequent tissue formation, as demonstrated by the generation of mineralization fronts in specific regions of scaffolds (Ripamonti, 2004). Nano to microscale topography has been demonstrated to affect cell behaviour by modification of cytoskeleton arrangements (Meredith et al. 2007). Furthermore, different cell types react to different materials; for example, different scaffold materials produced different levels of glycos-amino glycans in tissue engineered cartilage (Freed et al. 1993).
The source of cells is also an important choice for scaffolds, as is the culture regime used (Francioli et al. 2007). A range of cell types can now be combined with scaffolds to produce tissue engineered constructs; the merits of the choice of stem cells is discussed below.
Sources of cells for tissue engineering strategies
The production of an engineered tissue in vitro requires the use of cells to populate matrices and produce matrix resembling that of the native tissue. The main successes in this field have come from the use of primary cells, taken from the patient, and used in conjunction with scaffolds to produce tissue for re-implantation. However, this strategy has limitations, because of the invasive nature of cell collection and the potential for cells to be in a diseased state. Therefore, attention has become focused upon the use of stem cells, including embryonic stem (ES) cells, bone marrow mesenchymal stem cells (BM-MSCs) and umbilical cord-derived mesenchymal stem cells (UC-MSCs).
Embryonic stem cells
ES cells could allow the production of type-matched tissues for each patient, either through stem cell banking or by the use of therapeutic cloning. ES cells have the ability to be maintained for long (theoretically indefinite) culture periods, therefore potentially providing large amounts of cells for tissues that could not be derived directly from a tissue source. Proof of the true pluripotent nature of ES cells is teratoma formation. This property demonstrates the ability of stem cells to tissue-engineer multiple tissue types but also highlights the importance of using a terminally differentiated cell stock without latent stem cell-like properties. The use of stem cells will therefore require a method to ensure differentiation, either by demonstration of selection of only non-stem cells or by removal of all stem cells (Hewitt et al. 2007) and by in vivo demonstration of an absence of teratoma formation.
One of the critical steps of stem cell usage for regenerative medicine is therefore the ability to control the differentiation of the cells to the desired tissue lineages. Differentiation of ES cells has been achieved using protocols modified from BM-MSC protocols whereby ES cells can be directed to express features of bone, notably the accumulation of mineral (Fig. 1) (Buttery et al. 2001; Sottile et al. 2003; Bielby et al. 2004). However, there are indistinct steps in the use and differentiation of ES cells, notably embryoid body formation, which aids in the formation of ectodermal, endodermal and mesodermal liniages before terminal differentiation is initiated. Efforts have been made to understand this process but also to control the procedure using chemical aggregation. This process utilizes the affinity of Biotin to Avidin to provide a standardized and enriched system for differentiation (Fig. 2) (De Bank et al. 2007). This process has also been standardized by using cell suspensions, from which the cells are forced together using centrifugation (Burridge et al. 2007).
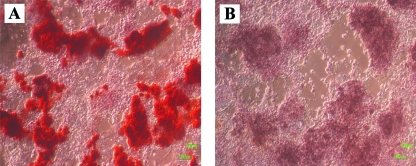
Fig. 1.
Differentiation of mouse embryonic stem cells to the osteogenic lineage shown by (A) alizarin red-stained mineral accumulation compared with (B) control (field of view 1100 × 950 µm).
Fig. 2.
Aggregation differentiation of embryonic stem cells is enhanced by using biotin–avidin linkers attached to the cell surface: (A) 500 000 cells mL−1 untreated at 10 h and (B) treated with avidin–biotin. The treatment has been shown to increase the rate of osteogenic differentiation and make the procedure more reproducible (field of view 1100 × 800 µm).
Many stem cell lines are cultured on feeder cells to provide a conducive environment for growth, but there are implications for the transmission of xenogenic materials so systems for growing stem cells in feeder-free systems are being established (Denning et al. 2006).
Bone marrow-derived mesenchymal stem cells
A stem cell type for bone and cartilage repair is the adult BM-MSC; these cells have been shown to be able to differentiate from a generic marrow cell population to an osteogenic lineage and have been used to augment repair of bone (Bruder et al. 1998; Yang et al. 2001; Howard et al. 2002). The MSC cell population can be isolated as a fraction of the adherent bone marrow colony forming units – fibroblastic (CFU-F; Friedenstein et al. 1966) and can be differentiated to the osteogenic and other lineages (Ashton et al. 1980; Pittenger et al. 1999).
As marrow is a complex mixture of cells a more defined starting cell population subset can be isolated from the mixture on the basis of epitope expression such as Endoglin (Haynesworth et al. 1992; Majumdar et al. 2000) and STRO-1 (Simmons & Torok-Storb, 1991; Howard et al. 2002; Stewart et al. 2003) antibody selection procedures. These cells can be removed from marrow and used to enhance materials such as the filler used for stabilizing artificial hip joints or for joining critical sized defects in bone that would not otherwise heal (Tilley et al. 2006).
Cord derived mesenchymal stem cells
Since the discovery that umbilical cord blood contains MSCs that can undergo multi-lineage differentiation, much research has been focused on determining their applications. The analysis of their gene expression profile reveals similarities to BM-MSCs (Jeong et al. 2005), with an ability to differentiate into adipocytes, osteoblasts (Lee et al. 2004), hepatocytes (Kang et al. 2006) and neuronal-like cells (Hou et al. 2003).
If this type of stem cell does function as a BM-MSC it would greatly improve the availability of matched tissues for treatments. With 669 531 births in 2006 in the UK alone (Office for National Statistics) this source of stem cells would provide a large pool of material, which could be purified using non-invasive techniques and could be recipient matched. In addition, as BM-MSCs differentiating potential may decrease with age (Oreffo et al. 1998; D’Ippolito et al. 1999) an alternative such as UC-MSCs would be of huge benefit.
We are currently working, in collaboration with Dr Paul Genever (University of York), on comparing the osteogenic potential of a range of cell types that have previously been shown to have the ability to differentiate into osteoblasts and produce mineralized bone nodules. However, until now, no work has been done on comparing these cells directly. This research will further our knowledge of the osteogenic process and also indicate which stem cell types are best suited to different procedures.
Scaffold design and manufacture
As the field of tissue engineering progresses, the need for novel scaffold structures and reproducible fabrication techniques has become of paramount importance. The use of biodegradable polymers, such as poly lactic acid (PLA), has become widespread, but the manner in which these polymers are processed and the additives used at the time of manufacture allows the final properties of the scaffold to be tailored.
Some of the scaffold types discussed include: high-pressure CO2 foamed scaffolds, injectable scaffolds, novel custom scaffolds and how these can be further modified using growth factors, zonation of materials and plasma polymerization deposition.
Poly-hydroxyl acids such as PLA and poly lactic-co-glycolic acid (PLGA) have been extensively used for tissue engineering procedures, as these materials bulk-degrade by hydrolysis, providing a controllable drug release and degradation profile to match tissue in-growth. With careful use of molecular weights, cross links and side chains, materials can be produced with tailor-made properties making them ideal for use in tissue engineering matrices. Furthermore, poly-hydroxyl acid materials also have a long history of in vivo usage as degradable sutures, drug delivery devices and biodegradable surgical components.
Injectable materials for tissue engineering/regenerative medicine
A scaffold developed for orthopaedic use is ‘Injectabone’, a novel biodegradable, particulate, scaffold system which can be injected into a site of bone trauma (Hamilton et al. 2006). The scaffold forms via the use of two types of PLGA microparticles. Type 1 is a temperature-sensitive PLGA/polyethylene glycol (PEG) composite that acts as an adhesive for the type II PLGA particles. The dynamics of this scaffold type allows injection at room temperature and solidification at body temperature allowing for a non-invasive delivery system for treatment of non-union bone defects.
Microparticles are small enough to be delivered by syringe and can be used as an injectable scaffold by incorporating temperature and mositure sensitive or adherent systems. These versatile subunits can be produced using droplet formation of solvents (Suciati et al. 2006) or by spraying (Hao et al. 2004; Whitaker et al. 2005). Setting of a microparticle slurry was initially performed using the attraction between biotin in one set of beads and avidin in another. Furthermore, live cells could be incorporated into this system such that scaffolds could be injected containing evenly distributed cells. The range of applications can be increased with the incorporation of various drugs and surface modifications.
Growth factor incorporation into scaffolds
In addition to scaffolds being used as a support for cell growth they can simultaneously be used as a vehicle for drug delivery. In theory, the scaffolds can be used to deliver growth factors/drugs to the sites of repair, thus expediting the recovery process. Owing to the kinetics and complexity of biological growth factor release, the process has required extensive investigation. One of the major issues is maintaining the conformation and function of proteins during the process of scaffold manufacture. However, once this issue has been solved many more complications lie ahead, including the control of growth factor release to match the kinetics of physiological processes, as well as the independent release of many factors at different stages.
Recently, vascular endothelial growth factor (VEGF), a peptide growth factor, has been incorporated into PLA scaffolds to provide a controlled release of angiogenic signals from a scaffold (Kanczler et al. 2007). Release of bioactive VEGF was confirmed using the in vitro human umbilical vascular endothelial cell (HUVEC) assay and in vivo chick allantoic membrane (CAM) angiogenesis assay. It was demonstrated that the VEGF retained its angiogenic properties and encouraged vascularization of the PLA scaffold.
Growth factors can also be attached to the surface of scaffolds following manufacture through the use of functional groups to chemically attach the proteins and/or drugs. Chen et al. (2006) used this method to attach basic firoblastic growth factors (bFGF) to the surface of alginate beads via an –NH functional group. This scaffold provided a microenvironment permissive for the growth and differentiation of human neuronal stem cells prior to their use in tissue engineering procedures.
The function of growth factor incorporation can be further enhanced by zoning, offering an interesting way of controlling tissue integration and development, which potentially allows the regionalized release of proteins to act on specific cell populations or initiate physiological processes, i.e. angiogenesis, at particular sites throughout scaffolds. This system has been demonstrated by Suciati et al. 2006, in which PLA/PEG microparticles were loaded with proteins such as horseradish peroxidase, trypsin or BMP-2. These particles were then sintered to form distinct layers. These scaffolds could maintain release over a period of up to 30 days, with the BMP-2 loaded particles able to initiate zonal osteogenic differentiation of responsive C2C12 cells in vitro.
An alternative to growth factor incorporation is to integrate DNA plasmids encoding a gene and mammalian promoter into the polymer; transfection with the DNA programmes the cells to produce their own growth factors. Once optimized, changing the inserted gene to alter the growth factor produced would allow a range of factors to be produced; however, uptake rates and toxicity are still major issues to this promising technique (Heyde et al. 2007).
Supercritical carbon dioxide processing of polymers
Processing of polymers into reticulated tissue engineering scaffolds often requires organic solvents and a method to provide pores, such as inclusion of salt granules, which are later removed by leaching, or by addition of blowing or foaming agents. Organic solvents, used in scaffold fabrication, such as dichloromethane, also often interact with many sensitive structural motifs found in peptide drugs, and can leave toxic residues behind (the upper FDA limit for DCM residues is only 600 parts per million).
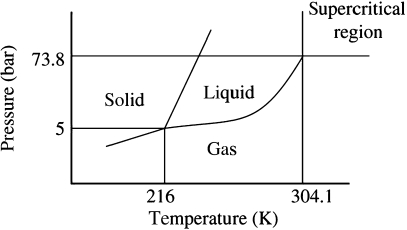
Supercritical CO2 forms a phase between liquid and gas (Fig. 3) that is able to penetrate many polymers and plasticize them. Evaporation results in solidification of the polymer and can be controlled to fuse separate bubble nucleation points, providing a reticulated and interconnected scaffold with a high strength to weight ratio (Fig. 4). Supercritical CO2 is also able to incorporate peptide drugs with minimal damage (Kanczler et al. 2007); if exposed briefly it is sufficiently inert to incorporate living cells by plasticizing a scaffold around cells (Ginty et al. 2006). The use of CO2 is not without limitations, as careful control of the supercritical foaming process is key to the correct formation of interconnected chamber structures and the use of this process requires quality control of the scaffolds produced. However, the structures produced are architecturally very strong and the ability easily to incorporate otherwise sensitive peptide drugs is a major advantage.
Fig. 3.
Phase diagram for the point at which CO2 becomes supercritical and can be used to melt polymers at ambient temperatures to produce porous scaffolds.
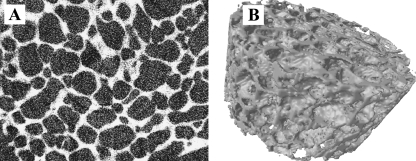
Fig. 4.
Supercritical CO2 produced poly lactic acid foamed porous block (5 mm) viewed using (A) X-ray micro-computed tomography in section and (B) reconstructed.
Custom scaffold production
There is also a need for development of custom matrices either tailored for purpose, or for the individual patient. Scaffolds have been produced for individuals via custom three-dimensional (3D) printing using a laser stereo lithography technique. This allows the scaffold to be built from computed 3D information derived from patient scans or from computer simulations (Antonov et al. 2004). The process is similar to rapid prototyping procedures whereby layers of particles are selectively sintered using a directed laser; these fused particles are further layered and sintered until several to several hundred layers have been bonded together, producing a custom 3D scaffold. Scaffolds may also be printed to include the cells using systems such as the fusing gel and cell bead system (Jakab et al. 2004).
Certain tissues, such as muscle, may require different material properties as this tissue needs flexibility as a fundamental part of its mode of action. To address this, modification of a flexible polymer, poly(1,8-octanediol-co-citric acid) (POC), to make it more suitable for culture of muscle cells has been developed (Yang et al. 2005; Hidalgo-Bastida et al. 2007).
Plasma modification of scaffold surfaces
The ability to change the adherence properties of cells to a scaffold allows the manipulation of the important cell intrusion phase and subsequent tissue development. By guiding cells to specific locations and preventing cell build-up or layer formation a better integration could be achieved. By modification of the surface chemistry of scaffolds with charged gas plasma polymerization deposition, it is possible to manipulate the regions to which cells will adhere and grow.
Deposition of plasma polymerized allyl amine (ppAm) allows stronger cell adherence to coated surfaces; conversely, deposition of plasma polymerized hexane (ppHex) strongly repels cell adhesion (Barry et al. 2006). As the plasma easily penetrates 3D structures such as tissue engineering scaffolds, changing the properties of scaffolds for cell attachment is possible by generation of gradients of surface chemistries by overlaying low to high cell adhesion zones. By using the deposition of ppHex surface over a ppAm core, cells can be moved from the non-adherent surface layer to the more adherent inner core, thus allowing some redress of the typical cell attachment primarily to the surface of scaffolds (Fig. 5).
Fig. 5.
Different distributions of cells (white) shown using X-ray micro-computed tomography cross-sections of CO2 foamed poly lactic acid scaffolds (black; 10 × 4.7 mm) with (A) cells located mainly on the surface on untreated scaffolds but (B) cells throughout the scaffold treated using plasma polymerized deposition of a cell adherent core (ppAm) and non-adherent surface (ppHex).
Summary
Although tissue engineering is in its relative infancy, huge advances are being made through the collaborations between stem cell biologists and material chemists. However, issues surrounding cell source and scaffold structure dominate investigations into engineered tissues. Identification of novel stem cell populations, such as UC-MSCs and scaffolds, with unique properties will expedite this process. It is hoped that by combining stem cell technologies with materials able to deliver combinations of growth factors we may be able to treat conditions requiring reconstructive surgery or organ replacement. The growth of knowledge in both stem cell biology, through the investigation of the differentiation process, and materials science, by development of novel polymers and scaffold structures, is bringing the time of routine engineered tissues closer.
Acknowledgments
Many thanks are due to the various funding bodies, BBSRC, EPSRC, EU (Osteocord), MRC and many researchers without whom this work would not have been possible. Thanks also go to David Gothard and Lloyd Hamilton for help in preparing the manuscript.
References
- Antonov EN, Bagratashvili VN, Whitaker MJ, et al. Three-dimensional bioactive and biodegradable scaffolds fabricated by surface-selective laser sintering. Adv Mater Deerfield. 2004;17:327–330. doi: 10.1002/adma.200400838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton BA, Allen TD, Howlett CR, Eaglesom CC, Hattori A, Owen M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res. 1980;151:294–307. [PubMed] [Google Scholar]
- Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- Barry JJA, Howard D, Shakesheff KM, Howdle SM, Alexander MR. Using a core-sheath distribution of chemistry through tissue engineering scaffolds to control cell ingress. Advanced Materials. 2006;18:1–6. [Google Scholar]
- Bielby RC, Boccaccini AR, Polak JM, Buttery LD. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. 2004;10:1518–1525. doi: 10.1089/ten.2004.10.1518. [DOI] [PubMed] [Google Scholar]
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998;16:155–162. doi: 10.1002/jor.1100160202. [DOI] [PubMed] [Google Scholar]
- Burridge PW, Anderson D, Priddle H, et al. Improved human embryonic stem cell embryoid body homogeneity and cardiomyocyte differentiation from a novel V-96 plate aggregation system highlights interline variability. Stem Cells. 2007;25:929–938. doi: 10.1634/stemcells.2006-0598. [DOI] [PubMed] [Google Scholar]
- Buttery LD, Bourne S, Xynos JD, et al. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 2001;7:89–99. doi: 10.1089/107632700300003323. [DOI] [PubMed] [Google Scholar]
- Chen YW, Chiou SH, Wong TT, et al. Using gelatin scaffold with coated basic fibroblast growth factor as a transfer system for transplantation of human neural stem cells. Transplant Proc. 2006;38:1616–1617. doi: 10.1016/j.transproceed.2006.02.084. [DOI] [PubMed] [Google Scholar]
- D’Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115–1122. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- De Bank PA, Hou Q, Warner RM, et al. Accelerated formation of multicellular 3-D structures by cell-to-cell cross-linking. Biotechnol Bioeng. 2007;97:1617–1625. doi: 10.1002/bit.21343. [DOI] [PubMed] [Google Scholar]
- Denning C, Allegrucci C, Priddle H, et al. Common culture conditions for maintenance and cardiomyocyte differentiation of the human embryonic stem cell lines, BG01 and HUES-7. Int J Dev Biol. 2006;50:27–37. doi: 10.1387/ijdb.052107cd. [DOI] [PubMed] [Google Scholar]
- Francioli SE, Martin I, Sie CP, et al. Growth factors for clinical-scale expansion of human articular chondrocytes, relevance for automated bioreactor systems. Tissue Eng. 2007;13:1227–1234. doi: 10.1089/ten.2006.0342. [DOI] [PubMed] [Google Scholar]
- Freed LE, Marquis JC, Nohria A, Emmanual J, Mikos AG, Langer R. Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J Biomed Mater Res. 1993;27:11–23. doi: 10.1002/jbm.820270104. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Piatetzky II S, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- Ginty PJ, Howard D, Rose FR, et al. Mammalian cell survival and processing in supercritical CO2. Proc Natl Acad Sci USA. 2006;103:7426–7431. doi: 10.1073/pnas.0508895103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton L, France RM, Shakesheff KM. Development of an injectable scaffold for application in regenerative medicine to deliver stem cells and growth factors. J Pharm Pharmacol. 2006;58:A52–A53. [Google Scholar]
- Hao J, Whitaker MJ, Wong B, Serhatkulu G, Shakesheff KM, Howdle SM. Plasticization and spraying of poly (DL-lactic acid) using supercritical carbon dioxide, control of particle size. J Pharm Sci. 2004;93:1083–1090. doi: 10.1002/jps.20002. [DOI] [PubMed] [Google Scholar]
- Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69–80. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- Hernon CA, Dawson RA, Freedlander E, et al. Clinical experience using cultured epithelial autografts leads to an alternative methodology for transferring skin cells from the laboratory to the patient. Regen Med. 2006;1:809–821. doi: 10.2217/17460751.1.6.809. [DOI] [PubMed] [Google Scholar]
- Hewitt Z, Priddle H, Thomson AJ, Wojtacha D, McWhir J. Ablation of undifferentiated human embryonic stem cells, exploiting innate immunity against the Gal alpha1–3Galbeta1–4GlcNAc-R (alpha-Gal) epitope. Stem Cells. 2007;25:10–18. doi: 10.1634/stemcells.2005-0481. [DOI] [PubMed] [Google Scholar]
- Heyde M, Partridge KA, Howdle SM, Oreffo RO, Garnett MC, Shakesheff KM. Development of a slow non-viral DNA release system from P(DL)LA scaffolds fabricated using a supercritical CO2 technique. Biotechnol Bioeng. 2007;98:679–693. doi: 10.1002/bit.21446. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Bastida LA, Barry JJ, Everitt NM, et al. Cell adhesion and mechanical properties of a flexible scaffold for cardiac tissue engineering. Acta Biomater. 2007;3:457–462. doi: 10.1016/j.actbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Hou L, Cao H, Wang D, et al. Induction of umbilical cord blood mesenchymal stem cells into neuron-like cells in vitro. Int J Hematol. 2003;78:256–261. doi: 10.1007/BF02983804. [DOI] [PubMed] [Google Scholar]
- Howard D, Partridge K, Yang X, et al. Immunoselection and adenoviral genetic modulation of human osteoprogenitors, in vivo bone formation on PLA scaffold. Biochem Biophys Res Commun. 2002;299:208–215. doi: 10.1016/s0006-291x(02)02561-5. [DOI] [PubMed] [Google Scholar]
- Jakab K, Neagu A, Mironov V, Markwald RR, Forgacs G. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc Natl Acad Sci USA. 2004;101:2864–2869. doi: 10.1073/pnas.0400164101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JA, Hong SH, Gang EJ, et al. Differential gene expression profiling of human umbilical cord blood-derived mesenchymal stem cells by DNA microarray. Stem Cells. 2005;23:584–593. doi: 10.1634/stemcells.2004-0304. [DOI] [PubMed] [Google Scholar]
- Kanczler JM, Barry J, Ginty P, Howdle SM, Shakesheff KM, Oreffo RO. Supercritical carbon dioxide generated vascular endothelial growth factor encapsulated poly(DL-lactic acid) scaffolds induce angiogenesis in vitro. Biochem Biophys Res Commun. 2007;352:135–141. doi: 10.1016/j.bbrc.2006.10.187. [DOI] [PubMed] [Google Scholar]
- Kang XQ, Zang WJ, Bao LJ, Li DL, Xu XL, Yu XJ. Differentiating characterization of human umbilical cord blood-derived mesenchymal stem cells in vitro. Cell Biol Int. 2006;30:569–575. doi: 10.1016/j.cellbi.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Banks V, Peluso DP, Morris EA. Isolation, characterization, and chondrogenic potential of human bone marrow-derived multipotential stromal cells. J Cell Physiol. 2000;185:98–106. doi: 10.1002/1097-4652(200010)185:1<98::AID-JCP9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Meredith DO, Eschbach L, Riehle MO, Curtis AS, Richards RG. Microtopography of metal surfaces influence fibroblast growth by modifying cell shape, cytoskeleton, and adhesion. J Orthop Res. 2007;25:1523–1533. doi: 10.1002/jor.20430. [DOI] [PubMed] [Google Scholar]
- Oreffo RO, Bord S, Triffitt JT. Skeletal progenitor cells and ageing human populations. Clin Sci (Lond) 1998;94:549–555. doi: 10.1042/cs0940549. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Richardson JB, Caterson B, Evans EH, Ashton BA, Roberts S. Repair of human articular cartilage after implantation of autologous chondrocytes. J Bone Joint Surg Br. 1999;81:1064–1068. doi: 10.1302/0301-620x.81b6.9343. [DOI] [PubMed] [Google Scholar]
- Ripamonti U. Soluble, insoluble and geometric signals sculpt the architecture of mineralized tissues. J Cell Mol Med. 2004;8:169–180. doi: 10.1111/j.1582-4934.2004.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- Sottile V, Thomson A, McWhir J. In vitro osteogenic differentiation of human ES cells. Cloning Stem Cells. 2003;5:149–155. doi: 10.1089/153623003322234759. [DOI] [PubMed] [Google Scholar]
- Stewart K, Monk P, Walsh S, Jefferiss CM, Letchford J, Beresford JN. STRO-1, HOP-26 (CD63), CD49a and SB-10 (CD166) as markers of primitive human marrow stromal cells and their more differentiated progeny, a comparative investigation in vitro. Cell Tissue Res. 2003;313:281–290. doi: 10.1007/s00441-003-0762-9. [DOI] [PubMed] [Google Scholar]
- Suciati T, Howard D, Barry J, Everitt NM, Shakesheff KM, Rose FR. Zonal release of proteins within tissue engineering scaffolds. J Mater Sci Mater Med. 2006;17:1049–1056. doi: 10.1007/s10856-006-0443-9. [DOI] [PubMed] [Google Scholar]
- Tilley S, Bolland BJ, Partridge K, New AM, Latham JM, Dunlop DG, Oreffo RO. Taking tissue-engineering principles into theater: augmentation of impacted allograft with human bone marrow stromal cells. Regen Med. 2006;1:685–692. doi: 10.2217/17460751.1.5.685. [DOI] [PubMed] [Google Scholar]
- Whitaker MJ, Hao J, Davies OR, et al. The production of protein-loaded microparticles by supercritical fluid enhanced mixing and spraying. J Control Release. 2005;101:85–92. doi: 10.1016/j.jconrel.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Yang J, Motlagh D, Webb AR, Ameer GA. Novel biphasic elastomeric scaffold for small-diameter blood vessel tissue engineering. Tissue Eng. 2005;11:1876–1886. doi: 10.1089/ten.2005.11.1876. [DOI] [PubMed] [Google Scholar]
- Yang XB, Roach HI, Clarke NM, et al. Human osteoprogenitor growth and differentiation on synthetic biodegradable structures after surface modification. Bone. 2001;29:523–531. doi: 10.1016/s8756-3282(01)00617-2. [DOI] [PubMed] [Google Scholar]