Abstract
As overall cesarean delivery rates have continued to rise, there has been growing interest in the rates of elective cesarean delivery (ECD), and its relative benefits and harms for the mother and neonate. This article explores the effects of elective cesarean delivery at term on neonatal morbidity and mortality. Available data are subject to a number of limitations, and do not provide conclusive evidence regarding the safety of planned elective cesarean versus planned vaginal delivery. Nevertheless, some data suggest an association between ECD and increased neonatal respiratory morbidity and lacerations, and possibly decreased central and peripheral nervous system injury. Potentially increased risks of neonatal mortality with ECD at term may be counterbalanced by risks of fetal demise in ongoing pregnancies. Patients and physicians considering ECD should carefully review competing risks and benefits; further research is needed to inform these discussions.
Keywords: cesarean delivery, elective, neonatal, morbidity, mortality, respiratory morbidity, injury
The cesarean delivery (CD) rate in the United States reached 30.2% in 2005, an all-time high 1. A number of factors are contributing to this rise: an increase in the rates of first-time, or primary CD, coupled with a decrease in rates of vaginal birth after cesarean, are felt to be major components. In addition to the growing numbers of elective repeat cesarean deliveries, there has been increasing attention given to elective cesarean delivery without medical or obstetric indications, which may be performed on maternal request. Clinicians and patients considering elective cesarean delivery (ECD) should undertake a thorough discussion of the risks and benefits of planned ECD versus planned vaginal delivery, related to both maternal and infant outcomes.
In this article, we will explore the effects of elective cesarean delivery on neonatal morbidity and mortality. Available data are subject to a number of limitations. There are no randomized trial data comparing outcomes among births from planned elective cesarean versus planned vaginal delivery in otherwise uncomplicated pregnancies; it’s possible that such a trial can never be accomplished. Furthermore, it has been difficult to identify and report rates of elective cesarean deliveries in many observational studies because this procedure option may not be included in hospital coding systems, or among payers’ reimbursable insurance claims. Thus, we focus primarily on available data on neonatal outcomes associated with cesarean delivery without labor, most commonly in the context of elective repeat cesarean and cesarean for breech, recognizing that data from these patient groups may not be fully generalizable to other types of elective CD, e.g. cesarean delivery on maternal request.
Perinatal and infant mortality
For more than 15 years, United States vital statistics data have indicated a 1.5-fold increased risk of neonatal mortality after cesarean delivery (both planned and unplanned) compared to vaginal delivery, though this has been assumed to be due to the greater proportion of high-risk pregnancies that are delivered operatively 2. Data more specific to elective cesarean delivery in uncomplicated pregnancies are conflicting. In a meta-analysis of 9 studies including more than 33,000 women, Mozurkewich and colleagues reported a significant increase in intrapartum and neonatal deaths among term, non-malformed infants who underwent a trial of labor, compared to those who underwent elective repeat cesarean delivery (OR 2.05, 95% CI 1.17 – 3.57) 3. A recent U.S. population-based study of neonatal and infant mortality by mode of delivery among women with “no indicated risk,” however, showed that neonatal mortality was increased more than two-fold after birth by cesarean, even after excluding infants with congenital anomalies and presumed intrapartum hypoxic events (Apgar score < 4) and adjusting for demographic and medical covariates 2. In these studies and others, the reported rates of neonatal death after elective repeat or “no indicated risk” cesareans are low, ranging from 0.01 – 0.17% 2–5.
When considering the risk of neonatal death after elective cesarean delivery, one should also give consideration to the competing risk of fetal demise in an ongoing pregnancy. Multiple investigators have reported an increase in unexplained intrauterine fetal demise rates that begins near term and continues with advancing gestation 6–8. Smith 9 calculated the cumulative probability of antepartum stillbirth as 0.08% at 38 weeks gestation, rising to 0.34% at 41 weeks’ gestation.
In an attempt to reconcile the competing risks of neonatal death after elective cesarean at term and antepartum stillbirth in ongoing term pregnancies, we 10 conducted a decision analysis, modeling the probability of perinatal death among a hypothetical cohort of 2,000,000 women with uncomplicated pregnancies at 39 weeks, half of whom underwent elective cesarean delivery, and half who were managed expectantly. After taking multiple chance probabilities into account, the model estimated that while neonatal deaths were increased among women delivered by elective cesarean, overall perinatal mortality was increased among women managed expectantly, because of the ongoing risk of fetal death in pregnancies that continue beyond 39 weeks. Of note, it was estimated that 1,441 elective cesarean deliveries would need to be performed to prevent one perinatal death (Table 1). In a separate analysis, Hankins and colleagues reached a similar conclusion 7.
Table 1.
Results of decision analyses comparing outcomes among 1 million elective cesarean deliveries and 1 million planned vaginal deliveries: Estimated neonatal morbidity and mortality by management strategy
| Elective Cesarean Delivery at 39w | Expectant Management | # of Cesarean Deliveries Needed to Prevent One Case* | |
|---|---|---|---|
| Perinatal Deaths | 804 | 1496 | 1441 |
| Stillbirths | 0 | 1118 | |
| Neonatal Deaths | 804 | 378 | |
| Respiratory Morbidity (TTN and RDS) | 11,000 | 2524 | |
| Intracranial Hemorrhage | 490 | 1007 | 1934 |
| Brachial Plexus Injury | 410 | 787 | 2653 |
| PPH | 3700 | 1488 | |
| Suspected Sepsis | 20,000 | 33,211 | 76 |
| Confirmed Sepsis | 0 | 2635 | 380 |
| Laceration | 8000 | 2464 |
Numbers shown are number of cases per million deliveries. TTN, transient tachypnea of the newborn; RDS, respiratory distress syndrome; PPH, persistent pulmonary hypertension
Results shown only for those outcomes estimated to occur more frequently with expectant management.
Adapted from Signore C, Hemachandra A, Klebanoff M. Neonatal mortality and morbidity after elective cesarean delivery versus routine expectant management: a decision analysis. Semin Perinatol. 2006;30:288–295.
Though this type of modeling is subject to limitations 10–13, it is clear that elective delivery—by cesarean or induction of labor, for that matter—of a healthy fetus at 39 weeks by accurate dating essentially eliminates the risk of future in utero fetal demise. Three important caveats should be stated, however. First, the results of these analyses should not be taken as an impetus for elective delivery before 39 weeks. Neonatal deaths increase with each week of decreasing gestational age, such that by 37 weeks, the association between elective cesarean delivery and reduced perinatal mortality appears to be lost 10, and perinatal deaths may be expected to increase with surgical intervention (Figure 1). Thus, clinicians should adhere to American College of Obstetricians and Gynecologists’ (ACOG) practice guidelines for confirming gestational age (or lung maturity) before elective delivery 14.
Figure 1. Estimated perinatal deaths associated with elective cesarean delivery versus expectant management, by gestational age.
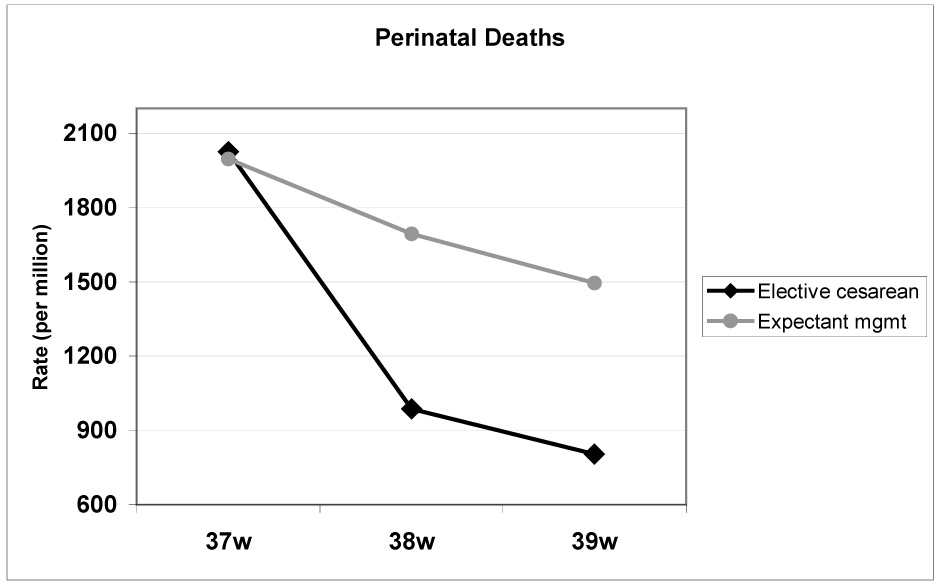
Perinatal mortality increases for both modes of delivery as gestational age decreases below 39 weeks. At 37 weeks’ gestation, more perinatal deaths would be expected with elective cesarean delivery than with expectant management.
Adapted from Signore C, Hemachandra A, Klebanoff M. Neonatal mortality and morbidity after elective cesarean delivery versus routine expectant management: a decision analysis. Semin Perinatol. 2006;30:288–295, with permission.
Second, these analyses assume that a liveborn infant whose impending stillbirth was prevented by ECD at 39 weeks would have the same risk of neonatal mortality as an infant who would not have died in utero if managed expectantly. This assumption may not hold in reality, as a fetal condition that may predispose to stillbirth at 40 weeks may similarly predispose to neonatal death after a “rescue” delivery at 39 weeks.
Third, calculation of a “number needed to treat” as above, assumes a causal relationship between exposure—in this case, mode of delivery—and outcome. This assumption may also be contested; existing observational data do not warrant the conclusion that the association between ECD and perinatal death (or other outcomes considered here) is causal. Similarly, one should keep in mind that it is delivery of an infant that prevents future stillbirth, not elective cesarean delivery per se.
Respiratory morbidity
Though characterized by varying definitions and methodologies, a consistent body of evidence indicates that infants delivered by elective cesarean experience higher rates of respiratory morbidity than infants delivered vaginally 15,16. In term infants, respiratory difficulty is most often manifested as transient tachypnea of the newborn (TTN), though more serious disorders, such as respiratory distress syndrome (RDS) and persistent pulmonary hypertension (PPH) do occur 17,18. In a cohort study of more than 33,000 births between 37 and 42 weeks, infants delivered by prelabor cesarean (N=2341) were nearly 7 times more likely to develop respiratory morbidity (RDS or TTN) than infants delivered vaginally (3.6% versus 0.5%, OR 6.8, 95% CI 5.2 – 8.9) 19. In this and other studies 20–22, the risk of respiratory morbidity in term infants is decreased with advancing gestational age. Hansen, et al. 20 recently showed that infants delivered by elective cesarean at 37 weeks had a 10% incidence of respiratory morbidity (defined as TTN, RDS, or PPH), compared to 2.8% among infants delivered vaginally (OR 3.7, 95% CI 2.2 – 6.1). By 40 weeks, the rate of respiratory morbidity with elective cesarean decreased to 1.5%, and was no longer significantly different from the rate seen with vaginal deliveries. Of note, even among term infants, respiratory difficulty associated with elective cesarean can be serious. In the Hansen study, 1.9% of infants delivered by ECD at 37 weeks experienced serious respiratory morbidity, defined as that requiring treatment for ≥3 days with continuous oxygen, nasal continuous positive airway pressure, or any period of mechanical ventilation 20.
Proposed mechanisms for the association between cesarean delivery and respiratory morbidity include iatrogenic prematurity with surfactant deficiency 18,23, and an attenuation of the fetal catecholamine surge during labor 24,25. Some 19,26, but not all 27,28 authors report a decrease in respiratory morbidity if cesarean delivery is performed after the onset of labor. This has prompted some authors to recommend deferral of elective cesarean delivery until after the onset of spontaneous labor 29.
As mentioned earlier, ACOG practice guidelines specify that without biochemical assessment of fetal lung maturity, elective delivery should not be undertaken prior to 39 weeks gestation by strict criteria 14. This recommendation was affirmed by an expert panel conducting the recent NICHD State of the Science Conference on Cesarean Delivery on Maternal Request 30. Of interest, a recent British randomized trial showed that a single course of antenatal corticosteroids prior to elective cesarean delivery at 37 weeks or later significantly decreased special care nursery admissions for respiratory distress (2.4% versus 5.1%, P=0.021, RR 0.46, 95% CI 0.23 – 0.93) 31. The treatment effect was particularly pronounced at 37 – 38 weeks, but even with treatment, respiratory distress was substantially more common at these gestational ages than at ≥39 weeks. Again, delaying elective cesarean delivery until 39 weeks or later decreases rates of newborn respiratory distress, but steroids may be helpful in preventing respiratory complications in the fetus at 37 or 38 weeks who has a non-immediate indication for prelabor cesarean delivery.
Neonatal asphyxia or encephalopathy and permanent neurologic injury
Early advocates for elective cesarean delivery proposed that these “atraumatic” deliveries would decrease the risk for intrapartum neurologic injury and cerebral palsy 32. Neonatal encephalopathy is a clinical syndrome of abnormal neurologic function in late preterm and term infants characterized by altered level of consciousness, abnormal muscular tone and reflexes, respiratory difficulty, and/or seizure activity 33. An infant with neonatal encephalopathy may or may not develop permanent neurologic impairment, such as cerebral palsy. Despite consistently rising cesarean delivery rates over the last three decades, cerebral palsy rates in term (≥ 2500 gram) infants have not decreased 34, supporting the now widely-held premise that a small minority of cases (approximately 10%) of encephalopathy and cerebral palsy in these infants are related to intrapartum hypoxic events and are thus amenable to prevention by altering route of delivery 35,36. On the other hand, in at least some cases, ECD at 39 weeks would be expected to preempt an unpredictable catastrophic obstetric event (e.g. acute placental abruption at 40 weeks) that might result in permanent neurological injury.
Data addressing the impact of route of delivery on immediate and long-term neurological outcome are sparse and conflicting. Badawi and colleagues 37 conducted a case-control study of 164 term infants with moderate to severe newborn encephalopathy and 400 randomly selected control infants and found a lower risk of encephalopathy in infants delivered by elective cesarean (defined as cesarean planned at least 24 hours prior to surgery) than in those delivered via spontaneous vaginal delivery (OR 0.17, 95% CI 0.05 – 0.56). On the other hand, Towner, et al. 38 found higher rates of convulsions and central nervous system (CNS) depression among infants weighing 2500 – 4000 grams delivered by cesarean without labor than in infants delivered spontaneously, though the difference was only significant for CNS depression (OR 2.2). A limitation of this study is that the “cesarean without labor” group likely included pregnancies with precarious maternal or fetal status, which would confound the relationship between mode of delivery and neonatal neurological outcome 16. Another consideration is that the vaginal delivery comparison groups in both studies consisted of actual spontaneous births, not all births from planned vaginal delivery, some of which would have been vacuum, forceps, and cesarean deliveries during labor. In both the Badawi and Towner papers, rates of neonatal neurologic abnormalities were significantly higher in those delivered by operative vaginal delivery (OR 2.3 – 6.9) and cesarean during labor (OR 2.2 – 10.8) as compared to spontaneous vaginal delivery 37,38. If elective cesarean prevents cerebral palsy, given that the U.S. rate of cerebral palsy is 2 to 3 per thousand, and that 10% of cases arise intrapartum, approximately 3000 to 5000 elective cesarean deliveries would need to be performed to prevent 1 case of cerebral palsy related to labor events 10,32,39.
Intracranial Hemorrhage
Mode of delivery may be expected to influence rates of intracranial bleeding in neonates, i.e. subdural or cerebral, intraventricular, and subarachnoid hemorrhage. Towner and colleagues 38 conducted a large, population based retrospective review of more than 583,000 births of 2500 – 4000 gram infants to nulliparous women in California, and found a combined rate of intracranial hemorrhage of 0.4% among operative vaginal deliveries, 0.1% in cesarean with labor, 0.05% in spontaneous vaginal deliveries, and 0.05% in cesarean deliveries without labor. While risk was significantly increased for operative vaginal delivery and cesarean during labor, there was no significant difference in the risk of intracranial hemorrhage between women who underwent prelabor cesarean and those who had a spontaneous vaginal delivery. These data suggest that intracranial hemorrhage may be related to underlying abnormalities of labor, as operative vaginal deliveries and labored cesarean deliveries are often undertaken because of dysfunctional labor.
Suspected and confirmed neonatal sepsis
Suspected neonatal infection is a major reason for admissions to neonatal intensive care units and invasive procedures in infants. Infection and inflammation have been linked to higher rates of cerebral palsy 40,41. There are scant data comparing rates of suspected and confirmed neonatal sepsis in infants delivered by elective cesarean and by planned vaginal delivery. Hook and colleagues 42 compared infectious outcomes among 497 women undergoing elective repeat cesarean delivery and 492 who attempted vaginal birth after cesarean (VBAC). Rates of both suspected and confirmed neonatal sepsis were significantly lower in the elective repeat cesarean group (2% versus 5%, P<0.05 for suspected sepsis, and 0% versus 1% P<0.05 for proven sepsis). The rate of suspected sepsis was 12% in neonates born after a failed trial of labor, compared to 2% after a successful VBAC (P<0.0001). Based on these data, a decision analytic model estimated that evaluations for suspected neonatal sepsis would be decreased with a policy of elective cesarean delivery at term, but that 76 elective cesarean deliveries would need to be performed to prevent 1 confirmed case of neonatal sepsis 10.
Brachial plexus injury
Shoulder dystocia leading to brachial plexus injury remains a feared complication of attempted vaginal delivery. Shoulder dystocia remains notoriously difficult to predict, despite identification of risk factors such as maternal diabetes, maternal obesity, and fetal macrosomia. A number of studies have examined the potential benefit of prophylactic cesarean delivery for preventing brachial plexus injury to the suspected macrosomic fetus 43–46, but there is little data regarding the impact of elective cesarean delivery on the rate of brachial plexus injury in non-macrosomic infants. One large population-based study in California examined rates of brachial plexus injuries by mode of delivery in infants weighing 2500 – 4000g and found that, compared to spontaneous vaginal deliveries, brachial plexus injuries were significantly less common in cesarean deliveries (0.03% vs. 0.08%, OR 0.4, 95% CI 0.3 – 0.5) and significantly more common in operative vaginal deliveries (0.5% vs. 0.08%, OR 6.0, 95% CI 3.3 – 10.7) 38. Brachial plexus injury occurred in 0.04% of cesarean deliveries without labor; this rate was not significantly different from that of spontaneous vaginal deliveries (0.08%, OR 0.5, 95% CI 0.3 – 1.0) 38.
Fetal lacerations
That infants delivered by cesarean would be at risk for laceration from sharp instruments is intuitive, and has been borne out in published reports. Fetal laceration occurs in 0.1 – 3.1% of cesarean deliveries 47,47–52. The risk of fetal laceration is greater during emergent (5.3%) and unscheduled labored cesarean deliveries (1.8%) than in elective cesareans without labor (1.0%) 47,52. Other risk factors for fetal laceration at cesarean are abnormal presentation 49,50 and rupture of membranes 52. Though moderate to severe injuries requiring plastic surgical repair have been reported, lacerations that occur during elective cesarean are usually mild and rarely require treatment beyond application of sterile strips 47,52.
Other outcomes
There are limited data examining other outcomes that may be influenced by elective cesarean delivery. A number of authors have expressed concern that cesarean delivery, by separating mother and infant after birth, may negatively impact bonding and early initiation of breastfeeding 53. In the Term Breech Trial, 77% of women randomized to planned vaginal delivery and 73% randomized to planned cesarean initiated breastfeeding “within a few hours” of delivery (P=0.05) 54. The median duration of breastfeeding was 8 months in both groups 55.
Another area of interest has been the “hygiene hypothesis,” i.e. an alteration in microbial colonization of neonates who are not exposed to vaginal flora during delivery, which may affect postnatal maturation of T cells and predispose to illnesses later in childhood 56. A number of investigators have reported associations between cesarean delivery and childhood asthma 57–59, but these findings have not been replicated in other studies 60–62.
Summary
Increasing cesarean delivery rates in the U.S. and worldwide are of intense interest and public health importance. Elective repeat cesarean delivery rates have been increasing steadily since the late 1990’s, and there may be a growing trend in cesarean delivery on maternal request. There are insufficient data on which to base conclusions regarding rates of neonatal morbidity and mortality between planned elective cesarean and planned vaginal delivery. Nevertheless, existing data suggests that elective cesarean delivery is associated with greater risk of neonatal respiratory morbidity and fetal laceration, and potentially decreased risk of brachial plexus injury, neonatal sepsis, intracranial hemorrhage, intrapartum asphyxia, and neonatal encephalopathy. Though neonatal deaths may be increased among infants delivered via elective cesarean, overall perinatal mortality may be reduced due to prevention of antepartum stillbirths. To minimize potential neonatal risks of elective cesarean, these deliveries should not be undertaken prior to 39 weeks gestation. Patients considering elective cesarean delivery should be made aware of available data on potential risks and benefits to the fetus and neonate. Further research is needed to inform these discussions.
Acknowledgments
This work was supported in part by the by the Intramural Research Program of the National Institute of Child Health and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2005. Natl Vital Stat Rep. 2006;55:1–18. [PubMed] [Google Scholar]
- 2.Macdorman MF, Declercq E, Menacker F, Malloy MH. Infant and neonatal mortality for primary cesarean and vaginal births to women with "no indicated risk," United States, 1998–2001 birth cohorts. Birth. 2006;33:175–182. doi: 10.1111/j.1523-536X.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- 3.Mozurkewich EL, Hutton EK. Elective repeat cesarean delivery versus trial of labor: a meta-analysis of the literature from 1989 to 1999. Am J Obstet Gynecol. 2000;183:1187–1197. doi: 10.1067/mob.2000.108890. [DOI] [PubMed] [Google Scholar]
- 4.Richardson BS, Czikk MJ, daSilva O, Natale R. The impact of labor at term on measures of neonatal outcome. Am J Obstet Gynecol. 2005;192:219–226. doi: 10.1016/j.ajog.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 5.Smith GC, Pell JP, Cameron AD, Dobbie R. Risk of perinatal death associated with labor after previous cesarean delivery in uncomplicated term pregnancies. JAMA. 2002;287:2684–2690. doi: 10.1001/jama.287.20.2684. [DOI] [PubMed] [Google Scholar]
- 6.Froen JF, Arnestad M, Frey K, Vege A, Saugstad OD, Stray-Pedersen B. Risk factors for sudden intrauterine unexplained death: epidemiologic characteristics of singleton cases in Oslo, Norway, 1986–1995. Am J Obstet Gynecol. 2001;184:694–702. doi: 10.1067/mob.2001.110697. [DOI] [PubMed] [Google Scholar]
- 7.Hankins GD, Clark SM, Munn MB. Cesarean section on request at 39 weeks: impact on shoulder dystocia, fetal trauma, neonatal encephalopathy, and intrauterine fetal demise. Semin Perinatol. 2006;30:276–287. doi: 10.1053/j.semperi.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Yudkin PL, Wood L, Redman CW. Risk of unexplained stillbirth at different gestational ages. Lancet. 1987;1:1192–1194. doi: 10.1016/s0140-6736(87)92154-4. [DOI] [PubMed] [Google Scholar]
- 9.Smith GC. Life-table analysis of the risk of perinatal death at term and post term in singleton pregnancies. Am J Obstet Gynecol. 2001;184:489–496. doi: 10.1067/mob.2001.109735. [DOI] [PubMed] [Google Scholar]
- 10.Signore C, Hemachandra A, Klebanoff M. Neonatal mortality and morbidity after elective cesarean delivery versus routine expectant management: a decision analysis. Semin Perinatol. 2006;30:288–295. doi: 10.1053/j.semperi.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Goel V. Decision analysis: applications and limitations. The Health Services Research Group. CMAJ. 1992;147:413–417. [PMC free article] [PubMed] [Google Scholar]
- 12.Kassirer JP, Moskowitz AJ, Lau J, Pauker SG. Decision analysis: a progress report. Ann Intern Med. 1987;106:275–291. doi: 10.7326/0003-4819-106-2-275. [DOI] [PubMed] [Google Scholar]
- 13.Rouse DJ, Owen J. Decision analysis. Clin Obstet Gynecol. 1998;41:282–295. doi: 10.1097/00003081-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 14.American College of Obstetrics and Gynecology. Washington DC: American College of Obstetrics and Gynecology; Induction of labor. ACOG Technical Bulletin [No. 10] 1999
- 15.Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB. Elective caesarean section and respiratory morbidity in the term and near-term neonate. Acta Obstet Gynecol Scand. 2007;86:389–394. doi: 10.1080/00016340601159256. [DOI] [PubMed] [Google Scholar]
- 16.Viswanathan M, Visco AG, Hartmann K, Wechter ME, Gartlehner G, Wu JM, Palmieri R, Funk MJ, Lux LJ, Swinson T, Lohr KN. Rockville, MD: Agency for Healthcare Research and Quality; Cesarean Delivery on Maternal Request. Evidence Report/Technology Assessment No. 133. AHRQ Publication No. 06-E009. 2006 [PMC free article] [PubMed]
- 17.Keszler M, Carbone MT, Cox C, Schumacher RE. Severe respiratory failure after elective repeat cesarean delivery: a potentially preventable condition leading to extracorporeal membrane oxygenation. Pediatrics. 1992;89:670–672. [PubMed] [Google Scholar]
- 18.Parilla BV, Dooley SL, Jansen RD, Socol ML. Iatrogenic respiratory distress syndrome following elective repeat cesarean delivery. Obstet Gynecol. 1993;81:392–395. [PubMed] [Google Scholar]
- 19.Morrison JJ, Rennie JM, Milton PJ. Neonatal respiratory morbidity and mode of delivery at term: influence of timing of elective caesarean section. Br J Obstet Gynaecol. 1995;102:101–106. doi: 10.1111/j.1471-0528.1995.tb09060.x. [DOI] [PubMed] [Google Scholar]
- 20.Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB. Risk of respiratory morbidity in term infants delivered by elective caesarean section: cohort study. BMJ. 2008;336:85–87. doi: 10.1136/bmj.39405.539282.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visco AG, Viswanathan M, Lohr KN, et al. Cesarean delivery on maternal request: maternal and neonatal outcomes. Obstet Gynecol. 2006;108:1517–1529. doi: 10.1097/01.AOG.0000241092.79282.87. [DOI] [PubMed] [Google Scholar]
- 22.Zanardo V, Simbi AK, Franzoi M, Solda G, Salvadori A, Trevisanuto D. Neonatal respiratory morbidity risk and mode of delivery at term: influence of timing of elective caesarean delivery. Acta Paediatr. 2004;93:643–647. doi: 10.1111/j.1651-2227.2004.tb02990.x. [DOI] [PubMed] [Google Scholar]
- 23.Wax JR, Herson V, Carignan E, Mather J, Ingardia CJ. Contribution of elective delivery to severe respiratory distress at term. Am J Perinatol. 2002;19:81–86. doi: 10.1055/s-2002-23558. [DOI] [PubMed] [Google Scholar]
- 24.Falconer AD, Lake DM. Circumstances influencing umbilical-cord plasma catecholamines at delivery. Br J Obstet Gynaecol. 1982;89:44–49. doi: 10.1111/j.1471-0528.1982.tb04633.x. [DOI] [PubMed] [Google Scholar]
- 25.Faxelius G, Hagnevik K, Lagercrantz H, Lundell B, Irestedt L. Catecholamine surge and lung function after delivery. Arch Dis Child. 1983;58:262–266. doi: 10.1136/adc.58.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerten KA, Coonrod DV, Bay RC, Chambliss LR. Cesarean delivery and respiratory distress syndrome: does labor make a difference? Am J Obstet Gynecol. 2005;193:1061–1064. doi: 10.1016/j.ajog.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 27.Levine EM, Ghai V, Barton JJ, Strom CM. Mode of delivery and risk of respiratory diseases in newborns. Obstet Gynecol. 2001;97:439–442. doi: 10.1016/s0029-7844(00)01150-9. [DOI] [PubMed] [Google Scholar]
- 28.Sutton L, Sayer GP, Bajuk B, Richardson V, Berry G, Henderson-Smart DJ. Do very sick neonates born at term have antenatal risks? 2. Infants ventilated primarily for lung disease. Acta Obstet Gynecol Scand. 2001;80:917–925. doi: 10.1034/j.1600-0412.2001.801008.x. [DOI] [PubMed] [Google Scholar]
- 29.Cohen M, Carson BS. Respiratory morbidity benefit of awaiting onset of labor after elective cesarean section. Obstet Gynecol. 1985;65:818–824. [PubMed] [Google Scholar]
- 30.NIH State-of-the-Science Conference Statement on cesarean delivery on maternal request. NIH Consens State Sci Statements. 2006;23:1–29. [PubMed]
- 31.Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662. doi: 10.1136/bmj.38547.416493.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wax JR, Cartin A, Pinette MG, Blackstone J. Patient choice cesarean: an evidence-based review. Obstet Gynecol Surv. 2004;59:601–616. doi: 10.1097/01.ogx.0000133942.76239.57. [DOI] [PubMed] [Google Scholar]
- 33.American College of Obstetricians and Gynecologists' Task Force on Neonatal Encephalopathy and Cerebral Palsy, American College of Obstetricians and Gynecologists and American Academy of Pediatrics. Neonatal encephalopathy and cerebral palsy: Defining the pathogenesis and pathophysiology. Washington, DC: American College of Obstetricians and Gynecologists; 2003. [Google Scholar]
- 34.Paneth N, Hong T, Korzeniewski S. The descriptive epidemiology of cerebral palsy. Clin Perinatol. 2006;33:251–267. doi: 10.1016/j.clp.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Clark SL, Hankins GD. Temporal and demographic trends in cerebral palsy--fact and fiction. Am J Obstet Gynecol. 2003;188:628–633. doi: 10.1067/mob.2003.204. [DOI] [PubMed] [Google Scholar]
- 36.Scheller JM, Nelson KB. Does cesarean delivery prevent cerebral palsy or other neurologic problems of childhood? Obstet Gynecol. 1994;83:624–630. doi: 10.1097/00006250-199404000-00025. [DOI] [PubMed] [Google Scholar]
- 37.Badawi N, Kurinczuk JJ, Keogh JM, et al. Intrapartum risk factors for newborn encephalopathy: the Western Australian case-control study. BMJ. 1998;317:1554–1558. doi: 10.1136/bmj.317.7172.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Towner D, Castro MA, Eby-Wilkens E, Gilbert WM. Effect of mode of delivery in nulliparous women on neonatal intracranial injury. N Engl J Med. 1999;341:1709–1714. doi: 10.1056/NEJM199912023412301. [DOI] [PubMed] [Google Scholar]
- 39.Blair E, Stanley FJ. Intrapartum asphyxia: a rare cause of cerebral palsy. J Pediatr. 1988;112:515–519. doi: 10.1016/s0022-3476(88)80161-6. [DOI] [PubMed] [Google Scholar]
- 40.Nelson KB, Willoughby RE. Infection, inflammation and the risk of cerebral palsy. Curr Opin Neurol. 2000;13:133–139. doi: 10.1097/00019052-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284:1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 42.Hook B, Kiwi R, Amini SB, Fanaroff A, Hack M. Neonatal morbidity after elective repeat cesarean section and trial of labor. Pediatrics. 1997;100:348–353. doi: 10.1542/peds.100.3.348. [DOI] [PubMed] [Google Scholar]
- 43.Boulet SL, Salihu HM, Alexander GR. Mode of delivery and birth outcomes of macrosomic infants. J Obstet Gynaecol. 2004;24:622–629. doi: 10.1080/01443610400007828. [DOI] [PubMed] [Google Scholar]
- 44.Herbst MA. Treatment of suspected fetal macrosomia: a cost-effectiveness analysis. Am J Obstet Gynecol. 2005;193:1035–1039. doi: 10.1016/j.ajog.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 45.Mollberg M, Hagberg H, Bager B, Lilja H, Ladfors L. High birthweight and shoulder dystocia: the strongest risk factors for obstetrical brachial plexus palsy in a Swedish population-based study. Acta Obstet Gynecol Scand. 2005;84:654–659. doi: 10.1111/j.0001-6349.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- 46.Rouse DJ, Owen J, Goldenberg RL, Cliver SP. Determinants of the optimal time in gestation to initiate antenatal fetal testing: a decision-analytic approach. Am J Obstet Gynecol. 1995;173:1357–1363. doi: 10.1016/0002-9378(95)90615-0. [DOI] [PubMed] [Google Scholar]
- 47.Haas DM, Ayres AW. Laceration injury at cesarean section. J Matern Fetal Neonatal Med. 2002;11:196–198. doi: 10.1080/jmf.11.3.196.198. [DOI] [PubMed] [Google Scholar]
- 48.Okaro JM, Anya SE. Accidental incision of the fetus at caesarian section. Niger J Med. 2004;13:56–58. [PubMed] [Google Scholar]
- 49.Puza S, Roth N, Macones GA, Mennuti MT, Morgan MA. Does cesarean section decrease the incidence of major birth trauma? J Perinatol. 1998;18:9–12. [PubMed] [Google Scholar]
- 50.Smith JF, Hernandez C, Wax JR. Fetal laceration injury at cesarean delivery. Obstet Gynecol. 1997;90:344–346. doi: 10.1016/s0029-7844(97)00284-6. [DOI] [PubMed] [Google Scholar]
- 51.Wiener JJ, Westwood JF. lacerations at caesarean section. J Obstet Gynaecol. 2002;22:23–24. doi: 10.1080/01443610120101655. [DOI] [PubMed] [Google Scholar]
- 52.Dessole S, Cosmi E, Balata A, et al. Accidental fetal lacerations during cesarean delivery: experience in an Italian level III university hospital. Am J Obstet Gynecol. 2004;191:1673–1677. doi: 10.1016/j.ajog.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 53.Rowe-Murray HJ, Fisher JR. Baby friendly hospital practices: cesarean section is a persistent barrier to early initiation of breastfeeding. Birth. 2002;29:124–131. doi: 10.1046/j.1523-536x.2002.00172.x. [DOI] [PubMed] [Google Scholar]
- 54.Hannah ME, Hannah WJ, Hodnett ED, et al. Outcomes at 3 months after planned cesarean vs planned vaginal delivery for breech presentation at term: the international randomized Term Breech Trial. JAMA. 2002;287:1822–1831. doi: 10.1001/jama.287.14.1822. [DOI] [PubMed] [Google Scholar]
- 55.Hannah ME, Whyte H, Hannah WJ, et al. Maternal outcomes at 2 years after planned cesarean section versus planned vaginal birth for breech presentation at term: the international randomized Term Breech Trial. Am J Obstet Gynecol. 2004;191:917–927. doi: 10.1016/j.ajog.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Prescott SL, Macaubas C, Smallacombe T, Holt BJ, Sly PD, Holt PG. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200. doi: 10.1016/S0140-6736(98)05104-6. [DOI] [PubMed] [Google Scholar]
- 57.Bager P, Melbye M, Rostgaard K, Benn CS, Westergaard T. Mode of delivery and risk of allergic rhinitis and asthma. J Allergy Clin Immunol. 2003;111:51–56. doi: 10.1067/mai.2003.34. [DOI] [PubMed] [Google Scholar]
- 58.Renz-Polster H, David MR, Buist AS, et al. Caesarean section delivery and the risk of allergic disorders in childhood. Clin Exp Allergy. 2005;35:1466–1472. doi: 10.1111/j.1365-2222.2005.02356.x. [DOI] [PubMed] [Google Scholar]
- 59.Salam MT, Margolis HG, McConnell R, McGregor JA, Avol EL, Gilliland FD. Mode of delivery is associated with asthma and allergy occurrences in children. Ann Epidemiol. 2006;16:341–346. doi: 10.1016/j.annepidem.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 60.Juhn YJ, Weaver A, Katusic S, Yunginger J. Mode of delivery at birth and development of asthma: a population-based cohort study. J Allergy Clin Immunol. 2005;116:510–516. doi: 10.1016/j.jaci.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 61.Maitra A, Sherriff A, Strachan D, Henderson J. Mode of delivery is not associated with asthma or atopy in childhood. Clin Exp Allergy. 2004;34:1349–1355. doi: 10.1111/j.1365-2222.2004.02048.x. [DOI] [PubMed] [Google Scholar]
- 62.Werner A, Ramlau-Hansen CH, Jeppesen SK, Thulstrup AM, Olsen J. Caesarean delivery and risk of developing asthma in the offspring. Acta Paediatr. 2007;96:595–596. doi: 10.1111/j.1651-2227.2006.00150.x. [DOI] [PubMed] [Google Scholar]


