Abstract
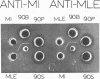
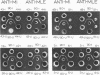
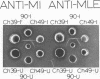
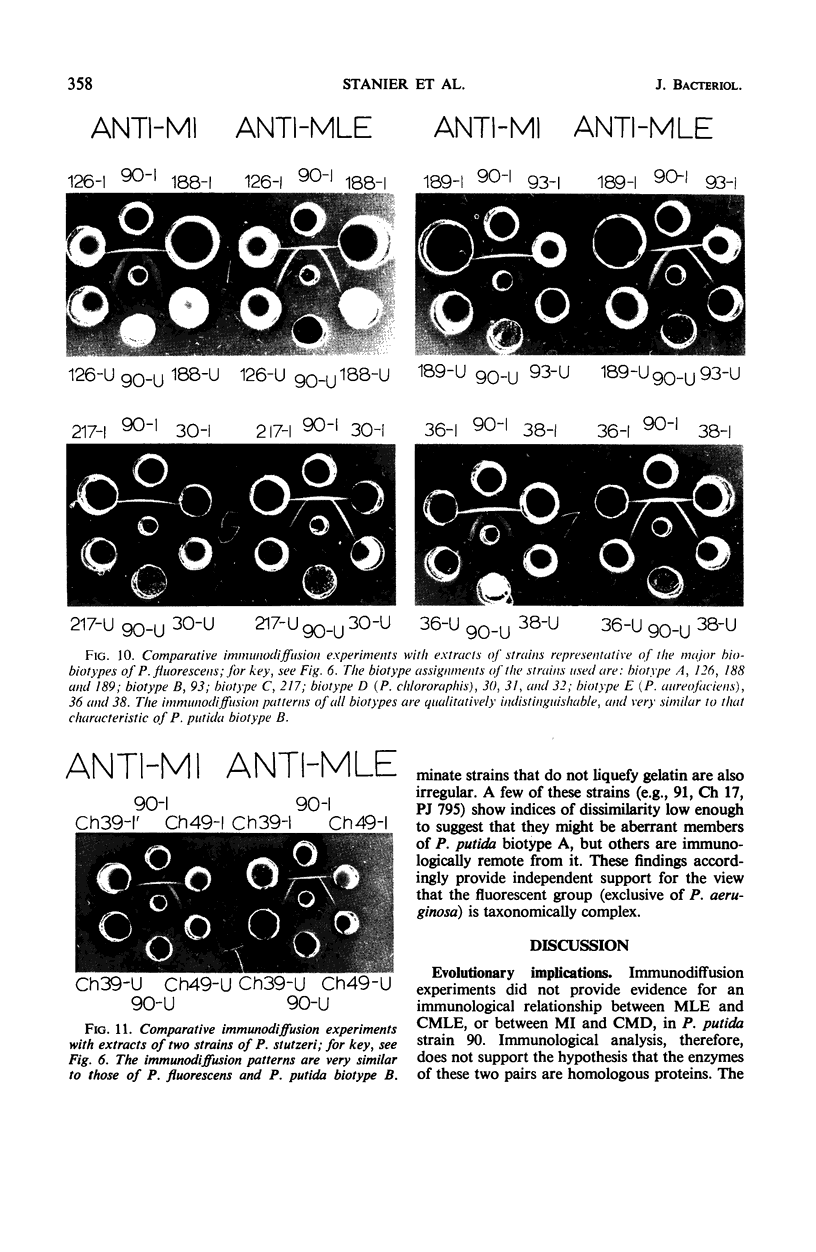
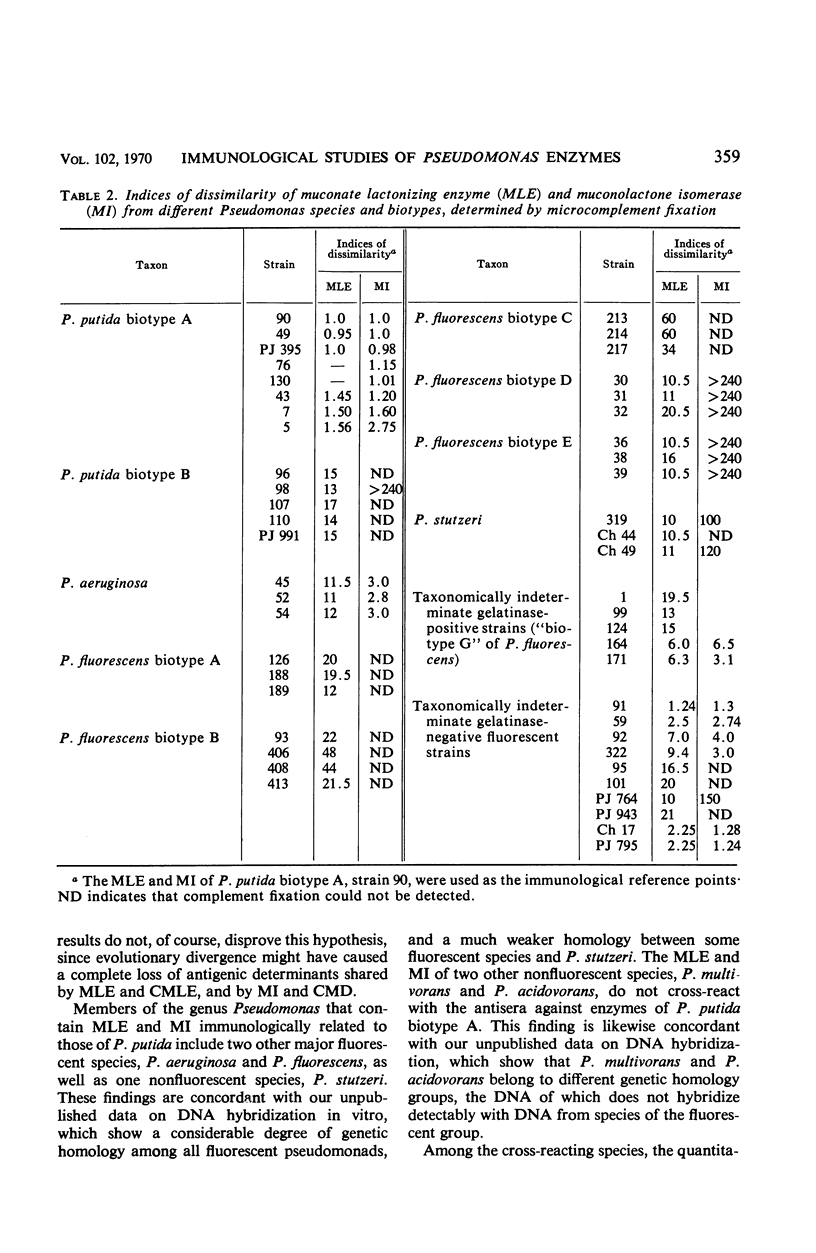
Crystalline preparations of muconate lactonizing enzyme and muconolactone isomerase, two inducible enzymes that catalyze successive steps in the catechol branch of the β-ketoadipate pathway, were used to prepare antisera. Both enzymes were isolated from a strain of Pseudomonas putida biotype A. The antisera did not cross-react with enzymes of the same bacterial strain that catalyze the chemically analogous steps in the protocatechuate branch of the β-ketoadipate pathway, carboxymuconate lactonizing enzyme and carboxymuconolactone decarboxylase. The antisera gave heterologous cross-reactions of varying intensities with the muconate lactonizing enzymes and muconolactone isomerases of P. putida biotype B, P. aeruginosa, P. stutzeri, and all biotypes of P. fluorescens, but did not cross-react with the isofunctional enzymes of P. acidovorans, of P. multivorans, and of two bacterial species that belong to other genera. The evolutionary and taxonomic implications of the findings are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheim N., Prager E. M., Wilson A. C. Immunological prediction of sequence differences among proteins. Chemical comparison of chicken, quail, and phesant lysozymes. J Biol Chem. 1969 Apr 25;244(8):2085–2094. [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Cocks G. T., Wilson A. C. Immunological detection of single amino acid substitutions in alkaline phosphatase. Science. 1969 Apr 11;164(3876):188–189. doi: 10.1126/science.164.3876.188. [DOI] [PubMed] [Google Scholar]
- Kemp M. B., Hegeman G. D. Genetic control of the beta-ketoadipate pathway in Pseudomonas aeruginosa. J Bacteriol. 1968 Nov;96(5):1488–1499. doi: 10.1128/jb.96.5.1488-1499.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. L., Jukes T. H. Non-Darwinian evolution. Science. 1969 May 16;164(3881):788–798. doi: 10.1126/science.164.3881.788. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- Mandel M. Deoxyribonucleic acid base composition in the genus Pseudomonas. J Gen Microbiol. 1966 May;43(2):273–292. doi: 10.1099/00221287-43-2-273. [DOI] [PubMed] [Google Scholar]
- Murphy T. M., Mills S. E. Immunochemical and enzymatic comparisons of the tryptophan synthase alpha subunits from five species of Enterobacteriaceae. J Bacteriol. 1969 Mar;97(3):1310–1320. doi: 10.1128/jb.97.3.1310-1320.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan C., Margoliash E. Comparative aspects of primary structures of proteins. Annu Rev Biochem. 1968;37:727–790. doi: 10.1146/annurev.bi.37.070168.003455. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. 3. Enzymes of the catechol pathway. J Biol Chem. 1966 Aug 25;241(16):3795–3799. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. II. Enzymes of the protocatechuate pathway. J Biol Chem. 1966 Aug 25;241(16):3787–3794. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- Robert-Gero M., Poiret M., Stanier R. Y. The function of the beta-ketoadipate pathway in Pseudomonas acidovorans. J Gen Microbiol. 1969 Aug;57(2):207–214. doi: 10.1099/00221287-57-2-207. [DOI] [PubMed] [Google Scholar]
- Sarich V. M., Wilson A. C. Quantitative immunochemistry and the evolution of primate albumins: micro-complement fixation. Science. 1966 Dec 23;154(3756):1563–1566. doi: 10.1126/science.154.3756.1563. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- WILSON A. C., KAPLAN N. O., LEVINE L., PESCE A., REICHLIN M., ALLISON W. S. EVOLUTION OF LACTIC DEHYDROGENASES. Fed Proc. 1964 Nov-Dec;23:1258–1266. [PubMed] [Google Scholar]