Abstract
Correct cellular patterning is central to tissue morphogenesis, but the role of epithelial junctions in this process is not well-understood. The Drosophila pupal eye provides a sensitive and accessible model for testing the role of junction-associated proteins in cells that undergo dynamic and coordinated movements during development. Mutations in polychaetoid (pyd), the Drosophila homologue of Zonula Occludens-1, are characterized by two phenotypes visible in the adult fly: increased sensory bristle number and the formation of a rough eye produced by poorly arranged ommatidia. We found that Pyd was localized to the adherens junction in cells of the developing pupal retina. Reducing Pyd function in the pupal eye resulted in mis-patterning of the interommatidial cells and a failure to consistently switch cone cell contacts from an anterior-posterior to an equatorial-polar orientation. Levels of Roughest, DE-Cadherin and several other adherens junction-associated proteins were increased at the membrane when Pyd protein was reduced. Further, both over-expression and mutations in several junction-associated proteins greatly enhanced the patterning defects caused by reduction of Pyd. Our results suggest that Pyd modulates adherens junction strength and Roughest-mediated preferential cell adhesion.
Keywords: adhesion, Pyd, Polychaetoid, eye, epithelia, patterning
Introduction
Epithelial morphogenesis and tissue patterning are critical aspects of animal development. In particular, regulation of cell adhesion and cell-cell junctions plays a key role in maintaining tissue integrity and function (Aijaz et al., 2006; Gumbiner, 2005; Lecuit and Wieschaus, 2002; Muller, 2000; Schock and Perrimon, 2002; Tepass et al., 2001). Nevertheless, the mechanisms by which junctions and junction-associated molecules operate during epithelial morphogenesis remains poorly understood. We use the Drosophila pupal retina to analyze the role of Polychaetoid (Pyd), the Drosophila orthologue of Zonula Occludens-1 (ZO-1), during reorganization and patterning of a developing epithelium.
ZO-1 is a member of the MaGUK (membrane-associated guanylate kinase) family of proteins. MaGUK proteins act as protein scaffolds by coordinating protein interactions through PDZ (PSD-95, Discs-large, ZO-1) domains, an SH3 domain, and an inactive guanylate kinase (GuK) domain (Gonzalez-Mariscal et al., 2000). MaGUK family members have been implicated in a wide variety of morphogenetic and developmental processes including the regulation of synaptic plasticity, renal glomerular filtration, vulval induction in C. elegans and control of cell overgrowth (Ehrlich et al., 2007; Funke et al., 2005; Gonzalez-Mariscal et al., 2000; Kaech et al., 1998; Schnabel et al., 1990; Tejedor et al., 1997; Woods et al., 1996). ZO-1 is found at tight junctions in epithelial cells but associates with cadherin-based adherens junctions (AJs) both in non-epithelial cells and before mature junctions are fully formed (Imamura et al., 1999; Itoh et al., 1997; Itoh et al., 1993; Rajasekaran et al., 1996). The role of ZO-1 at the AJ is not well-understood, in part because it is likely to be functionally redundant with two other related proteins, ZO-2 and ZO-3 (Haskins et al., 1998; Ikenouchi et al., 2007; Itoh et al., 1999a; Itoh et al., 1999b; Umeda et al., 2006; Umeda et al., 2004). The Drosophila ZO-1 orthologue Polychaetoid (Pyd) plays a role in eye development, specification of sensory organ precursors, dorsal closure and tracheal development (Chen et al., 1996; Jung et al., 2006; Takahashi et al., 1998; Takahisa et al., 1996; Wei and Ellis, 2001). Pyd localizes strongly to sites of cell junctions in both embryos and imaginal discs (Wei and Ellis, 2001) and has been proposed to act in the dynamic remodeling of the cytoskeleton (Takahashi et al., 1998).
Cells within the developing pupal eye undergo a coordinated series of movements that lead to precise patterning of the epithelium (Cagan, 1993; Cagan and Ready, 1989; Ready et al., 1976). These movements are directed by the cell adhesion molecule Roughest: reducing roughest activity leads to ectopic cells that fail to move properly within the epithelium, while artificially high levels of Roughest direct cells to expand their contacts with neighboring cells (Bao and Cagan, 2005; Reiter et al., 1996; Wolff and Ready, 1991) (D. Larson and R. Cagan, in preparation). Roughest has, in turn, been linked to the AJ protein Drosophila E-Cadherin (DE-Cadherin) (Bao and Cagan, 2005; Grzeschik and Knust, 2005; Tepass and Harris, 2007). Similar to its vertebrate orthologue E-Cadherin, DE-Cadherin binds homophilically across adjacent cell membranes to form the core of the AJ and associates with a complex of proteins that includes β-Catenin, α-Catenin, and p120-Catenin (Gumbiner, 2005). Vertebrate E-Cadherin has also been proposed to link to the actin cytoskeleton through direct or indirect association with α-Catenin and ZO-1 (Itoh et al., 1997; Rimm et al., 1995; Weis and Nelson, 2006). Although many of the molecular effectors have been identified, we only poorly understand how the dynamic regulation of the AJ contributes to morphogenesis and patterning in situ.
Here, we demonstrate that Pyd is required for patterning the pupal retina. Decreasing Pyd activity leads to defects in the patterning of two classes of support cells within the pupal eye, ‘interommatidial precursor cells’ (IPCs) and glial-like ‘cone cells’. Both phenotypes have been previously associated with changes in cell-cell adhesion. We present evidence indicating that Pyd is found specifically at the AJ, regulates a core group of junctional proteins, and is functionally linked to the adhesion molecule Roughest. Our data support a role for Pyd in the modulation of adhesion and in pattern formation during pupal retinal development.
Materials and Methods
Fly lines
All crosses and staging took place at 25°C unless otherwise noted. Wild-type Canton-S (CS), GMR-Gal4, shg-lacZ and Df(3R)-XT103 lines were from the Bloomington Drosophila Stock Center. The following fly lines were used in this study: pyd1 (Chen et al., 1996; Phillis et al., 1993); pydtam1 (Takahisa et al., 1996); pydC5 (Chen et al., 1996; Jung et al., 2006; Takahisa et al., 1996); UAS-roughest (Reiter et al., 1996); hs-pyd-RB (hs-tam1-F424) (Takahisa et al., 1996); UAS-de-cadherin-GFP (UAS-DEFL)(Oda and Tsukita, 1999); UAS-α-catenin-GFP (Oda and Tsukita, 1999); shgR69 (Godt and Tepass, 1998); shg1H (Tepass et al., 1996); UAS-tkv-RNAi (Cordero et al., 2007); tkv8 and mad12 lines were provided by Laurel Rafferty; rstCT, a C-terminal truncation mutant of the IrreC-Rst protein, was provided by Karl Fischbach (Wolff and Ready, 1991); scalloped-Gal4 was provided by Sarah Bray; the GMR-flp; n-cadM12, FRT42D, shgR69/CyO (y+) line and the GMR-flp; n-cadM19, FRT42D/CyO (y+) line by the Carthew lab (Hayashi and Carthew, 2004); FRT40A, n-cadM19 (Iwai et al., 1997). The rst3 line was as described (Tanenbaum et al., 2000). The pydtex1 and pydtex2 were generated by Δ2–3 mediated imprecise excision of pydtam1.
Heat shock over-expression and clonal analysis
Control CS or hs-pyd-RB pupae were placed at 37°C for 15 minutes at 17 hours APF and then dissected at 41–42 hours APF. Discrete shg1H or n-cadM19 clones were generated by FRT-mediated recombination (Golic and Lindquist, 1989; Xu and Rubin, 1993) larvae were heat-shocked for 25 min at 37°C either 72 hours after egg laying (AEL) in the case of n-cadM19 clones or as 3rd instar larvae for shg1H clones. Flp-out clones of UAS transgenes were generated by heat-shocking larvae for 18 min at 37°C at 48–72hours AEL to induce FRT-mediated excision of the following stop cassette: act>stop, y+>gal4, UAS-lacZ (FlyBase stock #4410; (Ito et al., 1997). Clonal analysis was done in flies of the following genotypes: i.) FRTG13, shg1H/CyO; ii.) FRT40A, n-cadM19/SM6-TM6b; iii.) hsflp; UAS-pyd-RNAi; iv.) hsflp; UAS-pyd-RFP; v.) hs-flp; UAS-pydNT-RFP; vi.) hs-flp; UAS-pydCT-RFP; vii.) hs-flp; UAS-roughest/SM6-TM6b; (viii.) hs-flp; UAS-de-cadherin-GFP; (ix.) hs-flp; UAS-α-catenin-GFP; (x.) hs-flp; shg-lacZ; UAS-pyd-RNAi/S-T.
In situ hybridization and antibody production
Digoxin-labeled full-length pyd-RB RNA probes were synthesized from a tam cDNA plasmid provided by Ryu Ueda (Takahisa et al., 1996). 25 hours APF pupal retinas were dissected in RNAse-free conditions and processed as previously described (Bao and Cagan, 2005).
A fragment of Pyd was PCR-amplified using primers to a region from 2605bp to 3156bp of pyd-RB using the tam cDNA plasmid. The PCR fragment was cloned into a GST expression vector (pGex2T). The GST-Pyd2605–3156 peptide was generated and used to raise rabbit polyclonal antibodies by Proteintech Group, Inc.
Immmunohistochemistry and SEM
Dissection and immunohistochemistry of pupal eye discs were performed as previously described (Bao and Cagan, 2005). Retinas were imaged using a Zeiss Axiophot epifluorescence microscope with a Quantix CCD camera (Photometrics, Ltd) and Image Pro Plus software or a Leica TCS confocal microscope. Images were processed with Adobe Photoshop.
The following antibodies were used: rabbit anti-Pyd (1:1000 tissue, this work); mouse anti-Armadillo N2 7A1 (1:3) (Riggleman et al., 1990); mouse anti-Rst MAb24A5.1 (1:50) (Schneider et al., 1995); rat anti-DCAD2 (1:10 tissue; DSHB) (Oda et al., 1994); rat anti-DCAD1 (1:100 Western) (Oda et al., 1994); rat anti-DCAT1 (1:10 tissue; DSHB) (Oda et al., 1993); mouse anti-Discs Large 4F3E2 (1:50; DSHB) (Parnas et al., 2001); mouse anti-β-gal (1:100; J. Sanes); rabbit anti-pMad (1:5000; Tetsuya Tabata); rhodamine-phalloidin (1:50; Molecular Probes); rat anti-Par6 (1:250) (Rolls et al., 2003); rabbit anti-Echinoid (1:1000) (Rawlins et al., 2003); Alexa568, Alexa488 or Alexa 594-conjugated secondary antibodies (Molecular Probes); Cy5-conjugated secondary antibodies (Jackson Laboratories).
For SEM, flies were frozen at −80°C for 20 min and then processed for viewing as previously described (Cordero et al., 2007). Images were captured using a Hitachi S-2600H scanning electron microscope.
Construction of RNAi lines
RNAi constructs were cloned as inverted repeats as previously described (Bao and Cagan, 2006). UAS-pyd-RNAi1 targets a 519bp fragment beginning 13bp after the start codon of pyd-RB while UAS-pyd-RNAi3 targets a 519bp fragment beginning 2662bp after the start codon of pyd-RB. UAS-α-Cat-RNAi1 targets a 451bp fragment beginning 540bp after the start codon while UAS-α-cat-RNAi3 targets a 538bp fragment starting 2062bp after the start codon. UAS-DE-Cad-RNAi targets a 518bp fragment starting 1804bp after the start codon. These and all other constructs were either injected in our lab or by Rainbow Transgenics, Inc.
QRT-PCR
Efficacy of the pyd-RNAi transgenes was verified by QRT-PCR analysis of cDNA derived from L3 larvae expressing da>pyd-RNAi transgene. Analysis was performed using the MyiQ™ RT-PCR detection system with BioRad software and the Absolute SYBR Green ROX Mix (ABGene) with the primer sets GCAAGTCAACGATCGGATAA (5′/531) and CCTGCAGCATTAATGGGATT (3′/680); TTCTCCGATAGCATTTCCAA (5′/2152) and TGCAGTGGAGTCGAAAACTT (3′/2306); and TATCCCTGCAGCAACTGGAT (5′/1373) and AATTGCCTGCGAGAGCTGTA (3′/1514). Two primer sets recognizing rp49 were used as controls. Melting curve analysis showed a single peak for all samples. Products were confirmed by restriction digest analysis.
Construction of pyd-RFP lines
Full-length pyd-PB, an N-terminal fragment of pyd-PB (comprising the 3 PDZ domains and associated regions from aa 1–489) and a C-terminal fragment of pyd-PB (the SH3 domain onward: aa 494–1371) were amplified from the tam cDNA plasmid and inserted into a C-terminal mRFP-pUAST vector (generated by amplifying mRFP (DsRed) from pRSETB-mRFP1 (Robert Campbell, UCSD) using primers that introduced AvrII and NheI sites; the PCR product was ligated into pUAST destroying the XbaI site). pydexon6- was cloned in two pieces from the tam cDNA plasmid and includes one amino acid change from pyd-PC, substituting a lysine for arginine residue at aa790.
In vivo imaging
In vivo imaging was performed as previously described (Cordero et al., 2007; Vidal et al., 2006). Pupae were raised at 25°C. Retinas were imaged every 15 min using a Zeiss Axiophot microscope equipped with a Xenon bulb. Images were processed and assembled as a composite picture using Adobe Photoshop and Quicktime. The following genotypes were imaged: (1.) GMR-Gal4, UAS-α-catenin-GFP/+ and (2.) GMR-Gal4, UAS-α-catenin-GFP/UAS-pyd-RNAi3.
Western blot analysis
6–10 retinas were dissected in PBS + protease inhibitors (Roche) and processed using standard SDS-PAGE, Western transfer and immunodetection.
Counting IPCs per hexagon
IPCs were quantified by drawing a hexagon with vertices at the center of six ommatidia surrounding a central ommatidium. IPCs were counted within the area bounded by this hexagon.
Results
Pyd protein localizes to the AJ in the Drosophila pupal retina
The Drosophila compound eye is composed of approximately 750 evenly spaced unit eyes known as ‘ommatidia’. The ommatidial core is established during late larval and early pupal stages and consists of eight underlying photoreceptors, four glial-like cone cells, and two primary pigment cells (1°s) that enwrap the cone cells (Fig 1A–D). Surrounding each ommatidium are additional support cells collectively known as secondary and tertiary pigment cells (2°/3°s), which organize to form a precise hexagonal pattern at the apical surface (Fig 1D). 2°/3°s emerge from ‘interommatidial precursor cells’ (IPCs). Patterning of IPCs involves their dynamic sorting by a process of cell intercalation (Fig 1A, B); changes in cell shape and cell positioning progressively refine the hexagonal array while excess IPCs are eliminated by programmed cell death (Brachmann and Cagan, 2003; Monserrate and Brachmann, 2007; Rusconi et al., 2000). From the pool of IPCs, six secondary pigment cells (2°s) elongate to form the sides of the hexagonal lattice while three of the IPCs become tertiary pigment cells (3°s) and lie at each vertex of the hexagon; the tertiary position is typically the last to be resolved (Fig 1D) (Cagan, 1993; Cagan and Ready, 1989; Ready et al., 1976).
Fig 1.
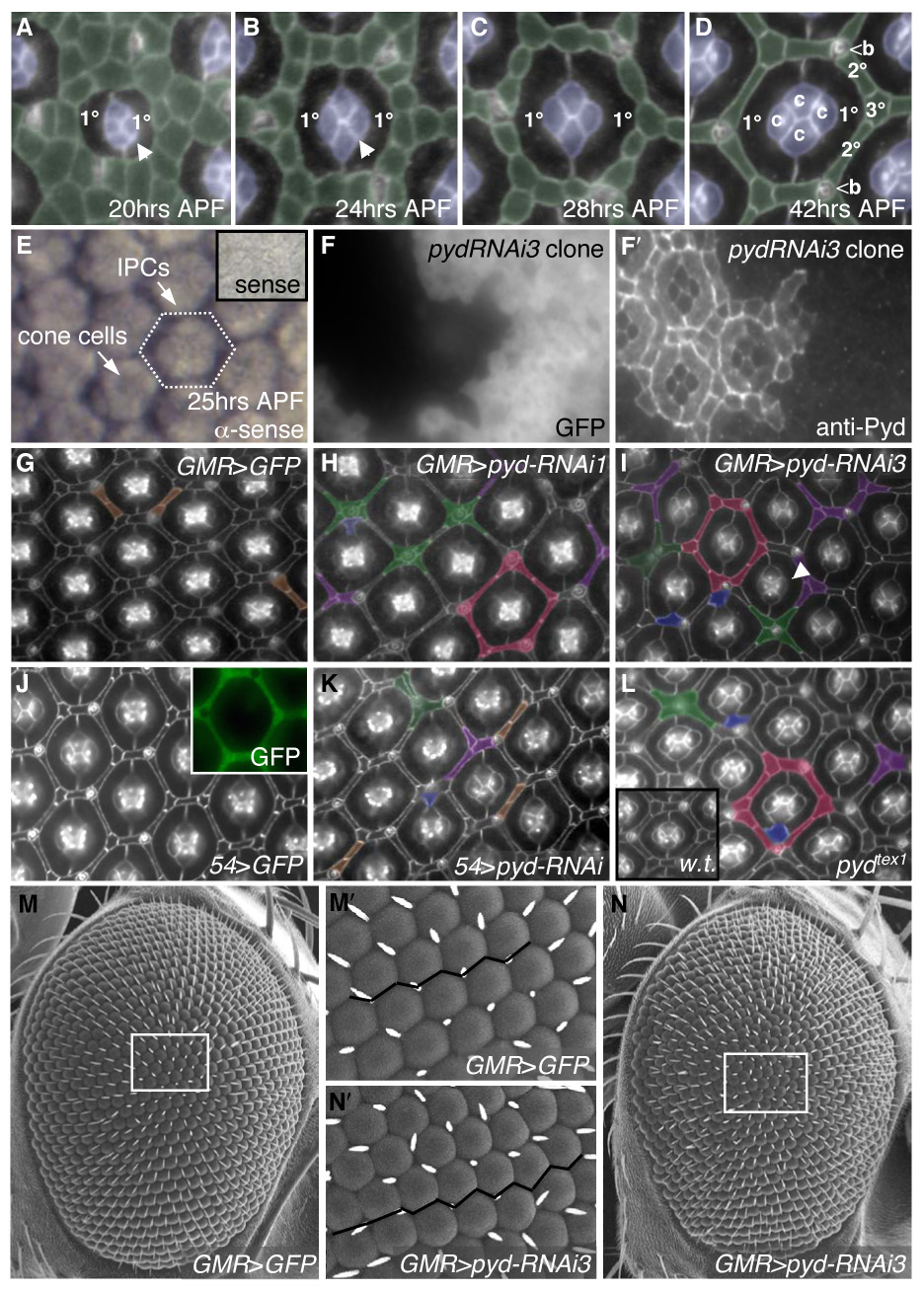
pyd regulates patterning of IPCs. Apical cell profiles were detected with anti-Arm antibodies at 41–42 hours APF (after puparium formation) unless otherwise indicated. Anterior is to the right in this and all subsequent images. A–D. Normal development of the pupal eye is marked by sorting of the IPCs (pseudo-colored in green) into a hexagonal lattice. Between 18–24 hours APF, cell intercalation narrows the rows of IPCs to single file (B). The hexagon is composed of six 2°s forming the sides of the hexagon and three 3°s at the vertices, alternating with bristles (b) (D). E–E′. In situ hybridization using full-length pyd-RB anti-sense probe in 25 hr APF pupal retinas: pyd was expressed at highest levels in IPCs and at lower levels in cone cells and 1°s. Inset shows pyd-RB sense probe. F–F′. Expression of pyd-RNAi3 in clonal patches in the eye marked by GFP (F) demonstrated cell-autonomous loss of Pyd antibody staining at the apical membrane (F′). G–L. Examples of patterning defects are pseudo-colored as follows: extra cells in the absence of other defects (brown), clustering of cells around bristles (green), pentagonal ommatidia (red), IPC-cone contact cells (blue) and a failure to specify a single 3° cell (purple). G. Control GMR>GFP retinas. H–I. GMR>pyd-RNAi1 (H) or GMR>pyd-RNAi3 (I) resulted in far stronger IPC patterning defects. Note also the failure to switch cone-cell contacts (I; arrowhead). J. 54>GFP retinas have no patterning defects (J). Expression of 54-Gal4, visualized with GFP, is found primarily in IPCs (inset). K. 54>pydRNAi exhibited errors in IPC organization. L. pydtex1 mutant eyes had defects that were similar to RNAi expression; inset shows wild-type retina. M–N. SEM of control (M) and pyd-RNAi3 (N) expressing eyes. The white tracing outlines an area that is shown at higher magnification in M′ and N′, respectively. The black line emphasizes the slightly uneven ommatidial rows in pyd-RNAi expressing eyes (N′) vs. control (M′).
During our search for new effectors of patterning we identified pyd as required for proper 2°/3° assembly. In situ hybridization detected high levels of pyd mRNA in cones and IPCs and lower levels in 1°s at 25 hours after puparium formation (APF; Fig 1E). We generated an antibody directed to a region C-terminal to the GuK domain (amino acids 869–1052 of Pyd-PB) that is shared by all potential isoforms except Pyd-PE (Supp Fig S1A). Antibody staining demonstrated localization of Pyd protein to apical regions of the IPCs, 1°s, and cone cells, where it co-localized with markers of the AJs, including DE-Cadherin, β-Catenin and α-Catenin (Fig 2A–L′ and data not shown) as well as with the cell adhesion molecule Roughest (Fig 2M–M″). Lower levels of Pyd antibody staining were observed along the basolateral membrane, overlapping partially with the septate junction marker Discs Large (Fig 2C, G and K), and in the axons leaving the eye (data not shown). Although Pyd was present around the entire cell circumference, it was strongly enriched in regions where three or more cells contacted (“tricellular junctions”; Fig 2N). This localization mirrored a similar accumulation described for Tricellulin, a tight junction-related protein that directly interacts with ZO-1 in mammals (Ikenouchi et al., 2005; Riazuddin et al., 2006).
Fig 2.
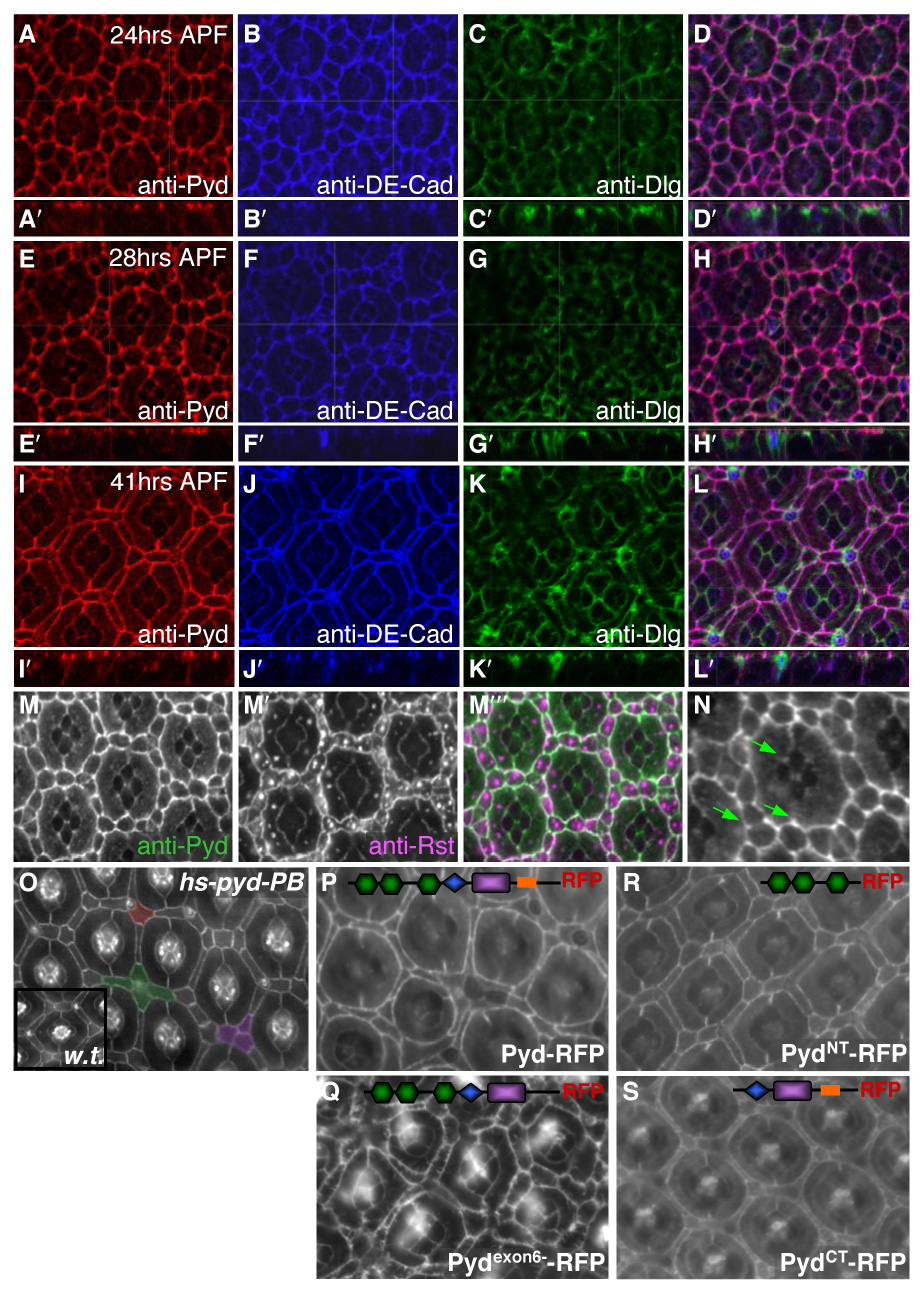
Pyd localized to AJs and its over-expression led to pupal eye patterning defects. A–L. Apical confocal sections of wild type pupal eye were taken at 24 hours APF (A–D), 28 hours APF (E–H) and 41 hours APF (I–L). Anti-Pyd immunofluorescence (A, E, I) co-localized extensively with anti-DE-Cad (B, F, J) at the AJ but only overlapped slightly with the septate junction marker Dlg (C, G, K). D, H, L. Overlay of anti-Pyd, anti-DE-Cad and anti-Dlg. Inset panels A′–L′ are lateral projections. M–M″. Anti-Pyd (M) co-localized with anti-Rst (M′) at the IPC/1° interface at 28 hours APF (overlay in M″). N. Anti-Pyd was concentrated where three or more cells came together (‘tricellular junctions’; green arrows). O. 41 hours APF pupal CS (inset) or hs-pyd-RB (O) eyes with a 30 min 37°C heat-shock at 17 hours APF. Notice that some phenotypes were shared with pyd-RNAi, such as the clustering of cells around bristles (pseudo-colored green) and the failure to specify a single 3° cell (pseudo-colored purple). Occasionally, a 2° extended to cover both the 2° and 3° niches (pseudo-colored red). P–S. GMR>UAS-pyd-RFP expression in 42 hr APF eyes; the relevant domains of each construct are schematized (see Supp Fig 3A). Pyd-RFP (P) localized to the AJ and directed patterning defects similar to those observed with pyd-RNAi expression. Pydexon6--RFP localized to the AJ and had severe cone cell and IPC defects (Q). PydNT-RFP and PydCT-RFP localized to the AJ but both rarely exhibited patterning defects (R and S, respectively).
Polychaetoid is required for patterning the Drosophila pupal retina
The allele pydC5 (reported as a null allele; Jung et al., 2006) and the hypomorphic alleles pyd1 and pydtam1 failed to show significant reduction in Pyd staining (data not shown) and exhibited little or no phenotypic defects. In particular, no significant bristle or eye phenotypes were observed in homozygotes of several independent strains of pydC5; these strains were out-crossed by others (Jung et al., 2006) and us to remove background mutations. Therefore, we utilized imprecise excision of the pydtam1 P-element to recover two semi-viable fly lines (pydtex1 and pydtex2); both exhibited ectopic thoracic bristles and a mildly rough eye (Supp Fig S1F–G and data not shown). pydtex1 contains a deletion of at least the first three exons of Pyd-PB but leaves at least exon 7 intact (data not shown). The pydtex2 allele contains a smaller deletion, though this allele (i) generated more severe phenotypic defects and (ii) exhibited less Pyd protein in homozygous mutant tissue compared to pydtex1 (data not shown). We also generated transgenic lines containing targeted RNA-interference (see Methods): UAS-pyd-RNAi1 targets an N-terminal region common to all predicted isoforms of pyd while UAS-pyd-RNAi3 targets four of five predicted transcripts (Fig S1A). QRT-PCR confirmed reduced pyd transcript levels (see Methods). Discrete clonal patches of pyd-RNAi-expressing cells had almost no detectable Pyd protein (Fig 1F–F′). Significantly, targeted expression of either UAS-pyd-RNAi produced both a mildly rough eye and ectopic thoracic bristles (Fig 1M–N′; Supp Fig S1).
The phenotype of pydtex1 in the pupal eye was similar—though somewhat milder— to that of GMR>pyd-RNAi (Fig1L). Consistent with this observation, residual antibody staining was present especially in the basolateral membrane, indicating that at least some protein was produced (data not shown). Due to pydtex1’s residual protein and the potential for a neomorphic gene product, we utilized UAS-pyd-RNAi3 for all subsequent loss-of-function analyses except where noted.
Expression of UAS-pyd-RNAi in the pupal retina with the eye specific promoter GMR-Gal4 (GMR>pyd-RNAi) led to consistent IPC patterning errors. For example, three cells were commonly observed to share the 3° cell niche equally (Fig 1H and 1I); normally a single cell is found in this position (Fig 1D). There was a mild increase in the total number of IPCs surrounding each ommatidium, especially around the bristles (GMR>pyd-RNAi1: 13.4 ± 1.4 S.D., n=37 hexagons vs. GMR>pydRNAi3: 12.8 ± 1.2, n=83 vs. control GMR>GFP: 12.3 ± 0.8 n=50). IPCs were poorly placed, resulting in breakdown of the hexagonal pattern to pentagonal or squared ommatidial arrays (Fig 1H and 1I). Similar defects were observed with pyd-RNAi targeted predominantly to IPCs (54>pyd-RNAi; Fig 1J–K); expressing pyd-RNAi in clonal patches in the eye resulted in patterning defects almost exclusively within the clone (data not shown), also indicating that Pyd is required locally for patterning of the pupal IPCs.
The integrity of the 1°/1° interface was occasionally (1.6%, n=1975 ommatidia vs. control GMR>GFP: 0%, n=1514) disrupted by an IPC that made direct contact with the cone cells, hereafter referred to as IPC cone-contact cells (Fig 1H and 1I). The number of cone cells in each ommatidium was only occasionally altered, but we frequently observed incorrect contacts within a cone cell quartet. During normal development, the anterior and posterior (A/P) cone cell membranes initially contact one another but, between 22–28 hours APF, the equatorial and polar (E/P) cone cells reach across to contact and exclude the A/P cones. In pyd-RNAi retinas, the cone cells occasionally (8.1%, n=1975 ommatidia vs. GMR>GFP: 0.1%, n=1514) failed to execute this shift in contacts (Fig 1I).
Over-expression of Pyd-RB is associated with patterning errors
Ectopic Pyd expression (Takahisa et al., 1996) during early pupal development led to mild IPC patterning errors similar to those observed with pyd-RNAi expression (Fig 2O). The contribution of the various protein interaction domains of Pyd to its function is not well understood, although exon 6 has been implicated in targeting to the AJ (Wei and Ellis, 2001). We generated four different C-terminal-tagged RFP constructs (Supp Fig S3A). Expression of UAS-pyd-RFP or UAS-pydexon6--RFP (lacking exon six; see Methods) with an eye-specific driver (GMR>pyd-RFP) led to a broad palate of defects in IPC patterning (Fig 2P); the effects of GMR-pydexon6--RFP expression were, in general, more severe (Fig 2Q). Both Pyd-RFP and Pydexon6--RFP localized to the AJ, although Pydexon6--RFP extended radially from the membrane as well (Fig 2P–Q, Supp Fig S3B–E′ and data not shown). Expression of GMR-pydNT-RFP or GMR-pydCT-RFP, comprising the N-terminal and C-terminal halves of the protein, respectively, resulted in only very rare patterning defects, although both protein fragments expressed well and localized to the AJ (Fig 2R–S). These results suggest that only expression of the full-length Pyd can disrupt IPC organization.
Reducing pyd activity leads to a loss of directed movement
Utilizing live imaging techniques (Cordero et al., 2007; Vidal et al., 2006), we observed defects in genotypically GMR>pyd-RNAi pupal eyes as early as 20 hours APF. Starting at this stage, control cells made directed apical movements past their neighbors, rapidly sorting into single rows so that each IPC bridged between two adjacent 1°s (Fig 3A–A′; see Movie 1 in supplementary material). GMR>pyd-RNAi cells also exhibited local movements, indicating that the processes contributing to dynamic cytoskeletal changes were likely intact. However, these movements were undirected and unproductive: GMR>pyd-RNAi cells frequently failed to undergo intercalation to create single cell rows (Fig 3B–B′; see Movie 2 in supplementary material). During later stages, wild-type cells moved dynamically into and out of the 3° position (Fig 3C–C′; see Movie 3 in supplementary material); even at these later time points, pyd-RNAi-expressing cells failed to resolve the defects in IPC patterning and to pattern the 3° position (Fig 3D–D′; see Movie 4 in supplementary material). In summary, we found that cells expressing pyd-RNAi were capable of cell movement but failed to make the directed translocations necessary to pattern correctly.
Fig 3.
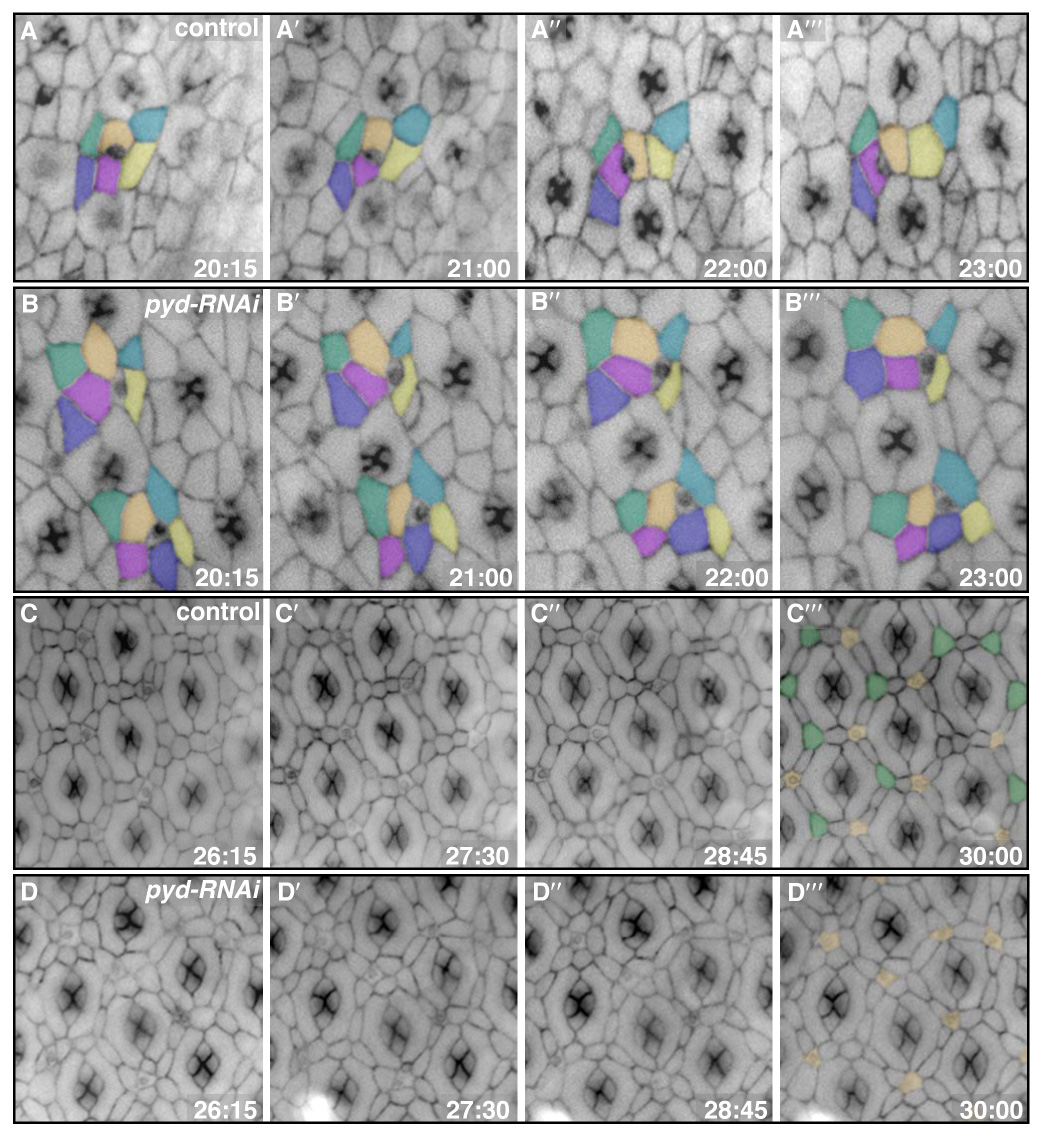
pyd-RNAi expressing cells failed to execute productive movements or sort appropriately. Membranes were labeled with α-Catenin-GFP. Times represent hours APF. A–B‴. Panels from four time points during cell intercalation for both control (A–A‴) and pyd-RNAi (B–B‴) retinas. Cells were pseudo-colored to emphasize particular cell movements. Typically, pyd-RNAi-expressing cells failed to undergo intercalation, instead remaining in double rows. C–D‴. Panels from four time points during 3° cell patterning for both control (C–C‴) and pyd-RNAi (D–D‴) retinas. Control cells moved into and out of the 3° position early; by 30 hours, however, one cell had taken over the niche in most cases (green in C‴; bristles were pseudo-colored in orange in C‴ and D‴). By contrast, the pyd-RNAi-expressing cells maintained initial contacts and, in most cases, failed to establish a single 3° (D‴).
Pyd affects patterning by modulating AJ protein levels
In normal development, heterophilic interactions between two adhesion molecules, Roughest in IPCs and Hibris in 1°s, lead to a characteristic ‘scalloping’ that serves to maximize the IPC/1° contact interface (Bao and Cagan, 2005). Reducing pyd activity exaggerated this scalloping (Fig 4F–F‴), suggesting that reducing pyd altered Roughest-mediated adhesion between IPCs and 1°s. Interestingly, clonal patches of cells expressing pyd-RNAi exhibited a strong and consistent increase in Roughest levels at 24 and 28 hours APF (Fig 4B). Previous work has demonstrated that directed cell movement in the pupal eye requires DE-Cadherin and Roughest, though the relationship between properly regulated AJs and cell-cell adhesion is unclear (Bao and Cagan, 2005; Cordero et al., 2007; Grzeschik and Knust, 2005; Reiter et al., 1996). Clonal pyd-RNAi patches exhibited a similar increase in the AJ-associated proteins DE-Cadherin, α-Catenin, and β-Catenin (Fig 4A, C and data not shown). The levels of DE-Cadherin and Rst were similarly increased in tissue homozygous for pydtex1 (data not shown) and pydtex2 (Fig 4D,E).
Fig 4.
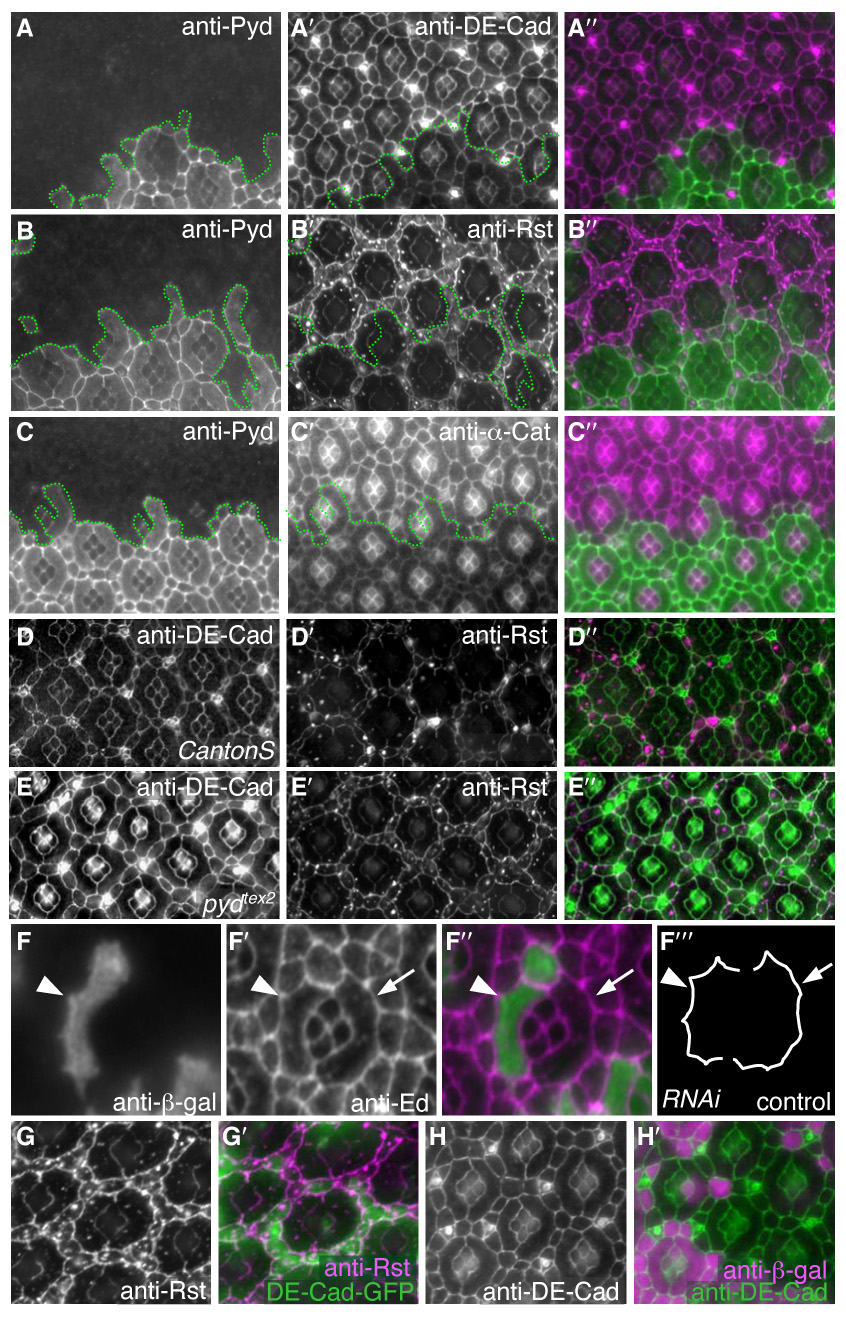
Cells with reduced Pyd exhibited increased levels of core AJ proteins at the membrane. All images were taken at 28 hours APF. A–C″. pyd-RNAi expressing cells were marked by lack of anti-Pyd immunofluorescence (A, B, C). DE-Cad (A′), Rst (B′) and α-Cat (C′) levels were increased specifically at the apical membrane in cells expressing pyd-RNAi. Overlay in A″, B″ and C″, respectively. D–E″. Homozygous pydtex2 retinas also had increased levels of DE-Cad (E) and Rst (E′) – compare to wild type tissue (D and D′). Overlay in D″ and E″. F–F‴. Single 1° cell and IPC clones expressing pyd-RNAi were marked by β-gal expression (F). Anti-Echinoid (F′) outlines the cells (overlay in F″). Tracing of control and pyd-RNAi expressing cells (F‴) demonstrating that scalloping of the IPC/1° boundary (e.g., arrowhead) was increased when cells expressed pyd-RNAi. G–G′. DE-Cad-GFP over-expression in a patch of cells (green in G′) did not alter the localization or level of anti-Rst immunofluorescence (G and magenta in G″). H–H′. Rst over-expression in a patch of cells (marked by anti-β-gal; magenta in H′) did not alter the localization or level of anti-DE-Cad (green in H″).
This pyd-RNAi-mediated increase in DE-Cadherin and Roughest did not reflect a general increase in AJ-localized proteins, as Echinoid did not increase; also, the septate junction marker Discs Large (Woods et al., 1997), the subapical complex marker Par6 (Petronczki and Knoblich, 2001), and the underlying actin network as assessed by phalloidin staining were unaffected (data not shown). Altering Roughest levels directly did not alter DE-Cadherin levels or vice versa (Fig 4G–H’), indicating Pyd affected each protein independently. Total protein levels were unaffected as assessed by Western blot analysis (Supp Fig S2A) and shotgun/de-cadherin transcriptional reporter activity was unaffected by pyd-RNAi expression in clones (Supp Fig S2B). The pyd-RNAi phenotype was not dominantly modified by mutations in any of several components of the endocytosis or endosome recycling pathways including Rab4, Rab5, Rab11, Sec6, or Hook (data not shown), suggesting that Pyd does not alter levels of junctional proteins through a general inhibition of endocytosis.
Expression of ectopic Pyd-RFP—but not PydNT-RFP or PydCT-RFP— in clonal patches of cells also resulted in an increase in the levels of Roughest (Supp Fig S3F–F″), suggesting that either (i) over-expressing at least one Pyd isoform can act in a dominant negative fashion or (ii) levels of Pyd expression require tight control. Together, our data suggest that Pyd regulates IPC patterning at least in part by modulating the localization or stability of key AJ proteins.
Pyd interacts with AJ proteins to coordinate patterning
Genetic modifier tests functionally linked Pyd and components of the AJ. The pyd-RNAi phenotype was dominantly enhanced in the presence of one copy of a de-cadherin/shotgun (shgR69) or roughest (rstCT or rst3) mutation; each strongly enhanced the buildup of IPCs around the ommatidium (Fig 5A–F and data not shown, quantified in 5A′–F′). A similar genetic enhancement was observed when either Roughest or DE-Cadherin was over-expressed in IPCs in the presence of 54>pyd-RNAi (Supp Fig S2C–D″). In addition, the mild shg-RNAi and rst heterozygous or homozygous phenotypes were dominantly enhanced by pydtex1 and pydtex2 (Fig. S4 and data not shown). The ability of both increased and decreased levels of AJ proteins to enhance the pyd-RNAi phenotype suggests that Pyd is involved in finely tuning regulation of junctional proteins.
Fig 5.
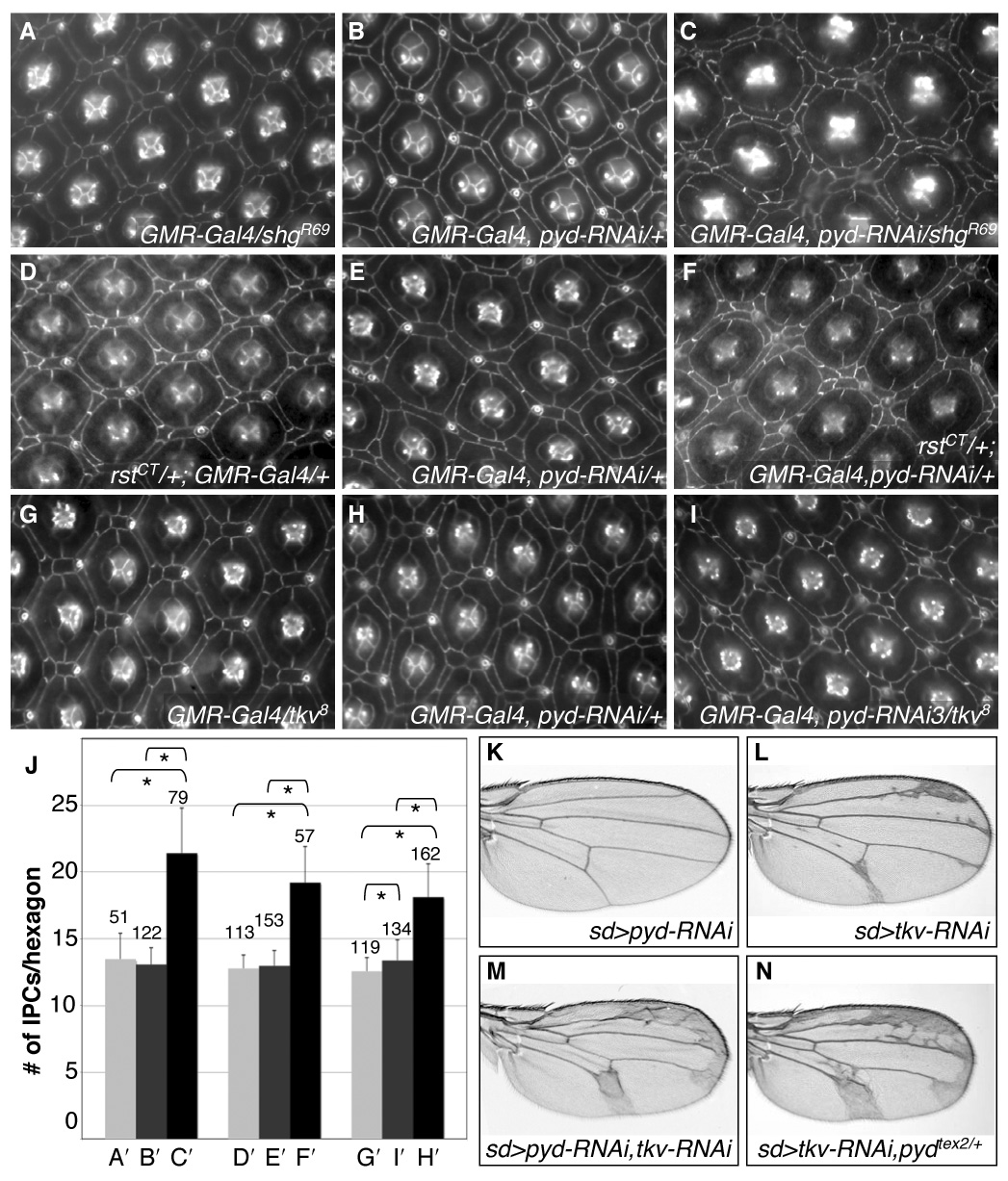
pyd is functionally linked to AJ proteins involved in adhesion. A–I. Control GMR-Gal4/shgR69 (A), rstCT/+; GMR-Gal4/+(D) and GMR-Gal4/tkv8 retinas exhibited few IPC patterning errors. GMR>pyd-RNAi retinas (B, E, H) had mild IPC patterning errors. GMR>pyd-RNAi defects were strongly enhanced by removal of one functional copy of de-cad/shg (C; GMR-Gal4, pyd-RNAi/shgR69), rst (F; rstCT/+; GMR-Gal4, pyd-RNAi/+) or the Dpp pathway Type I receptor tkv (I; GMR-Gal4, pyd-RNAi/tkv8). J. Number of IPCs per hexagonal area (see Methods) for each genotype, respectively. Error bars represent standard deviation, N numbers are given above each bar. Brackets with stars represent statistically significant differences at p<0.001 by the Mann-Whitney U test. K–N. Reducing Pyd with the wing driver scalloped-Gal4 (sd>pyd-RNAi) led to no visible effect on the wing veins (K). Reducing Tkv protein levels using scalloped-Gal4 (sd>tkv-RNAi) resulted in mild expansion of the wing vein material (L). When Pyd and Tkv were jointly reduced (sd>pyd-RNAi, tkv-RNAi) the wing vein phenotype was strongly enhanced (M). Similarly the tkv-RNAi phenotype was greatly enhanced in pydtex heterozygotes (N). All wings are from females.
Pyd is functionally linked to the Dpp pathway
Recently, the Dpp signal transduction pathway—analogous to the mammalian BMP2/4 pathway— has been linked to patterning of the eye at least in part through its interactions with both DE-Cadherin and Roughest (Cordero et al., 2007). Null alleles of the Dpp pathway Type I receptor thickveins (tkv8) or the downstream target mad (mad12) dominantly enhanced the pyd-RNAi phenotype (Fig 5G–I and data not shown). Similarly, pyd-RNAi expression, the pyd-overlapping deficiency Df(3R)XT103, pydtex1 and pydtex2 enhanced a tkv-RNAi-dependent wing vein and retinal phenotype (Fig 5K–N, Fig S4D–F and data not shown) consistent with previous results genetically linking de-cadherin/shotgun and tkv-RNAi in the wing (Cordero et al., 2007). Expression of pyd-RNAi did not detectably affect the level of Tkv staining; surprisingly, it also did not alter phosphorylation of Mad, a standard readout of downstream Dpp activity (data not shown). Our results suggest that Pyd functions with Dpp to modulate cell sorting and adhesion but in a manner that is independent of significant changes in Dpp pathway signaling.
Pyd alters adhesion in the cone cells
We also utilized the cone cell switching phenotype (described above; Fig 6A) to explore dynamic changes in cell-cell contacts. A moderate number of GMR>pyd-RNAi cone cell quartets failed to make the transition from A/P to E/P contacts (Fig 6D). Over-expression of DE-Cadherin or α-Catenin in the pupal eye caused a very low frequency of similar errors (Fig 6B–C) (Hayashi and Carthew, 2004). Reducing Pyd activity can direct an increase in the AJ proteins DE-Cadherin and α-Catenin (see Fig 4A′ and 4C′), and we hypothesized that this increase might be responsible for the GMR>pyd-RNAi cone cell phenotype. Consistent with this model, co-expression of GMR>pyd-RNAi and either de-cadherin or α-catenin strongly increased the penetrance of cone cell switching defects (Fig 6E–G).
Fig 6.
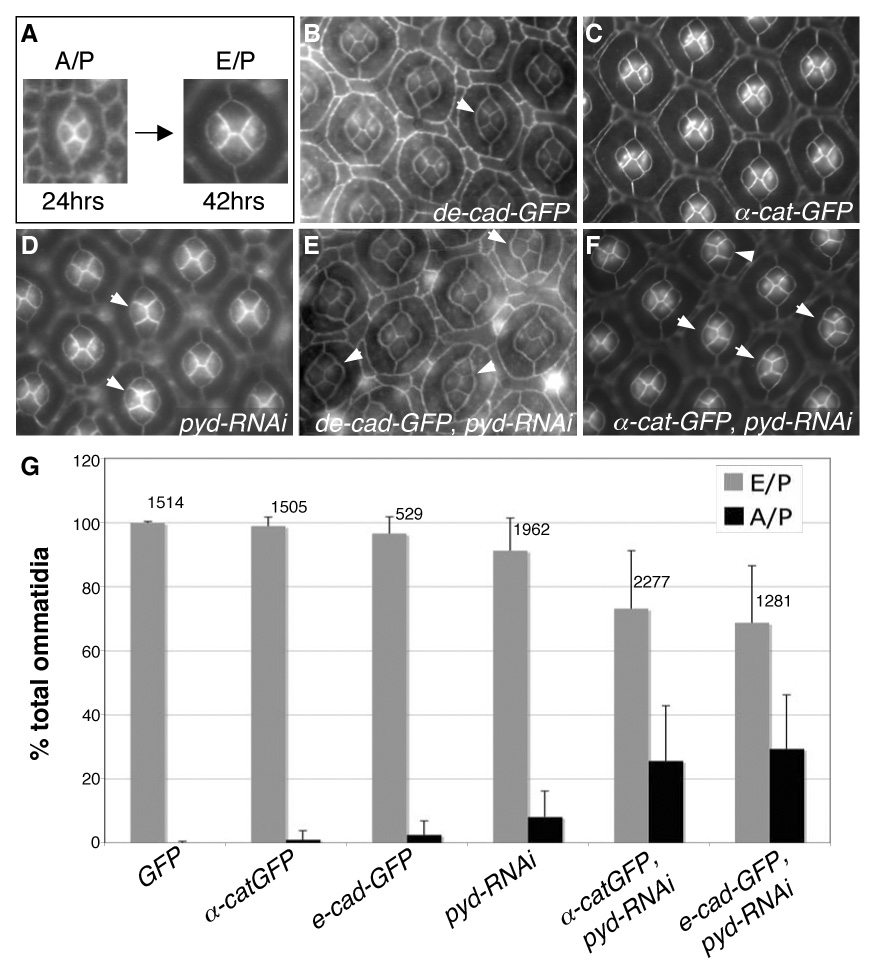
pyd regulates the A/P to E/P cone cell contact switch. A. Between 24–28 hours APF, cone cells switch their apical contacts from an anterior/posterior (A/P) to an equatorial/polar (E/P) dominant configuration. B–G. GMR>pyd-RNAi expression interfered with this switching, and a subset of cone cell groups maintained the immature A/P arrangement through 42 hours APF (D, arrows). Expression of either UAS-de-cad-GFP (B, arrow) or UAS-α-cat-GFP (C) during pupal development resulted in a low level failure to switch cone cell-contacts. Expression of UAS-de-cad-GFP or UAS-α-cat-GFP strongly enhanced the pyd-RNAi cone cell switching phenotype (arrowheads in E and F, respectively). Data from the relevant genotypes is quantified in G; error bars represent standard deviation for each eye field, N numbers are given above each set of bars.
Pyd localization to the AJ is dependent on DE-Cadherin and α-Catenin
Apical Pyd protein became diffusely cytoplasmic in de-cadherin/shotgun (shg1H) null 1°s and IPCs (Fig 7A–A″ and data not shown). The effects of removing de-cadherin/shotgun were locally non-cell autonomous: loss of DE-Cadherin from one cell affected Pyd staining in adjacent cells, emphasizing the dependence of Pyd on DE-Cadherin/DE-Cadherin interactions (Fig 7A′). Cells expressing high ectopic levels of DE-Cadherin, however, did not exhibit changes in the levels or localization of Pyd (Fig 7E–E′).
Fig 7.
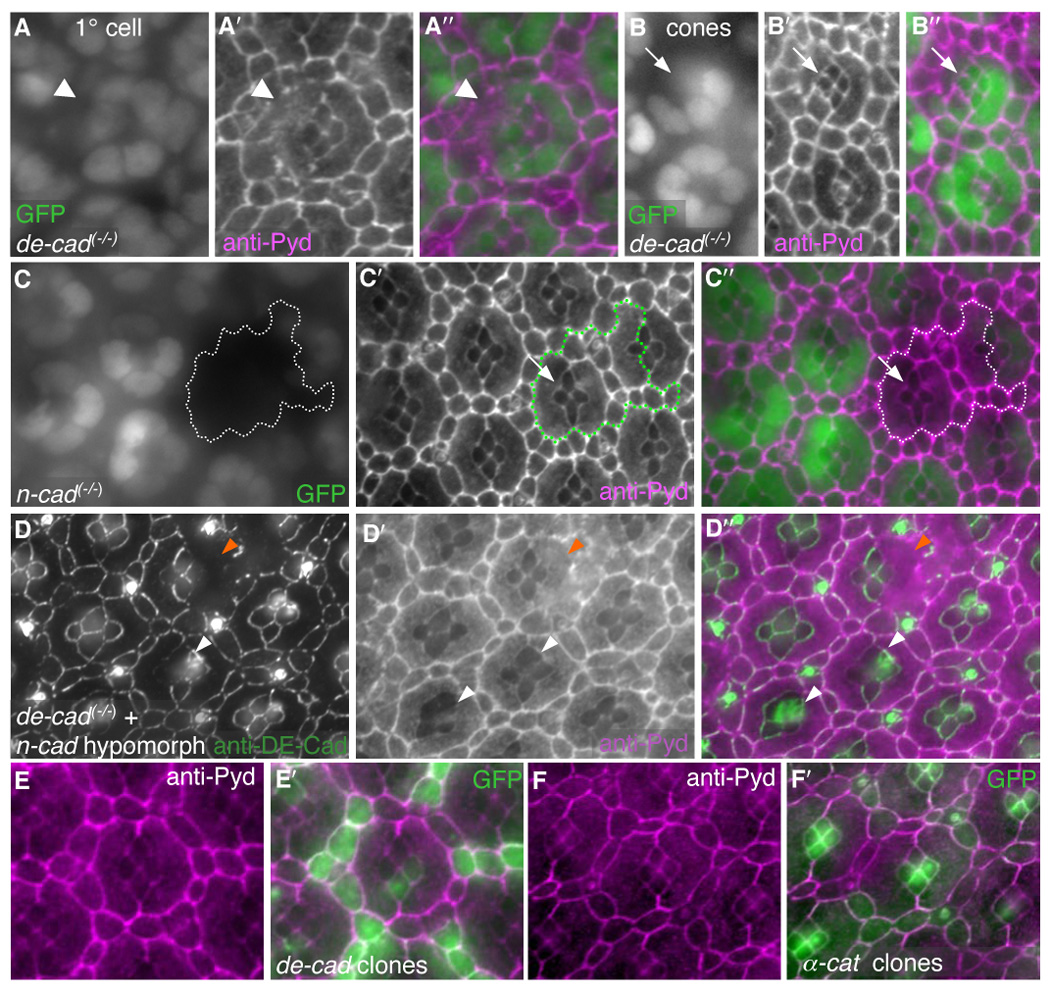
DE-Cad is necessary for Pyd to localize to the AJ. All images taken at 28 hours APF. A–B′. Single-cell null de-cad/shotgun (shg1H) 1° clones (A, arrowhead) and cone cell clones (B, arrow) are marked by loss of nuclear GFP expression. In isolated 1° cell clones (A), Pyd de-localized from the membranes of the adjoining IPC and cone cells that contacted the de-cad deficient cell membrane (A′, arrowhead). Pyd was maintained at the cone/cone interface in de-cad null cone cell clones (B′, arrow). C–C″. Null n-cadherin (n-cadM19) clones, marked by lack of nuclear GFP (C), showed no change in Pyd localization (C′) at the cone/cone interface (arrow, overlay in C″). D–D″. Null de-cad (shgR69) single-cell clones (marked by loss of DE-Cad staining in D) in the background of a strong reduction in n-cadherin (n-cadM12/n-cadM19) exhibited de-localized Pyd staining at the cone/cone interface (D′, white arrowheads). Null de-cad clones in 1°s were also observed (orange arrrowheads). D″ shows overlay. E–E″. DE-Cad-over-expression in single cells (labeled with GFP, green in E′) did not alter the localization or level of anti-Pyd immunofluorescence (E and magenta in E′). F–F′. UAS-α-catenin expression in single cells (labeled with GFP, F′) did not alter the localization or level of anti-Pyd immunofluorescence (F and magenta in F′).
By contrast, staining of Pyd between genotypically shg cone cells remained unaltered (Fig 7B–B″). N-Cadherin is found specifically at the interface between cone cells and has been shown to act redundantly with E-Cadherin in controlling cone cell morphology (Fig 7G) (Hayashi and Carthew, 2004). Clones of a null allele of n-cadherin (n-cadM19) did not disturb Pyd localization to cone cell/cone cell junctions (Fig 7C–C″). However, within null shg clones produced in the background of a strong viable hypomorph of n-cadherin (n-cadM19/ n-cadM12) we frequently observed de-localization of Pyd from the cone cell-cone cell junctions (Fig 7D–D″). Together these results indicate that DE-Cadherin is necessary to tether Pyd to the AJs in IPCs and suggest that DE-Cadherin and N-Cadherin may act redundantly to localize Pyd at the cone cell/cone cell contacts.
Previous experiments have indicated that mammalian ZO-1 is linked to E-Cadherin at least in part through α-Catenin (Itoh et al., 1997; Itoh et al., 1993). We generated fly lines containing inducible α-catenin-RNAi (α-cat-RNAi) transgenes that reduced α-Catenin protein levels in situ (see Methods). Results were confirmed with two independent RNA-interference constructs targeting a portion of either the N-or C-terminus, respectively. Reduction of α-Catenin activity caused de-localization of Pyd from the AJ of all lattice cells (Fig 8I–J) as well as severe disruptions in patterning of IPCs, manifesting as a ‘rough’ adult eye (Fig 8C–D). Interestingly, localization of all AJ components tested were similarly dependent on the presence of α-Catenin, including DE-Cadherin, β-Catenin, Roughest and Echinoid (Fig 8G–H, Fig 8K–L, and data not shown). The septate junction protein Discs Large was unaffected (Fig 8M–N). However, expression of ectopic α-Catenin in clones in the eye did not change the level or localization of Pyd (Fig 7F–F′). In summary, disrupting AJs— through removal of DE-Cadherin or α-Catenin— led Pyd protein to dissociate from the apical membrane of IPCs and become predominantly cytoplasmic.
Fig 8.
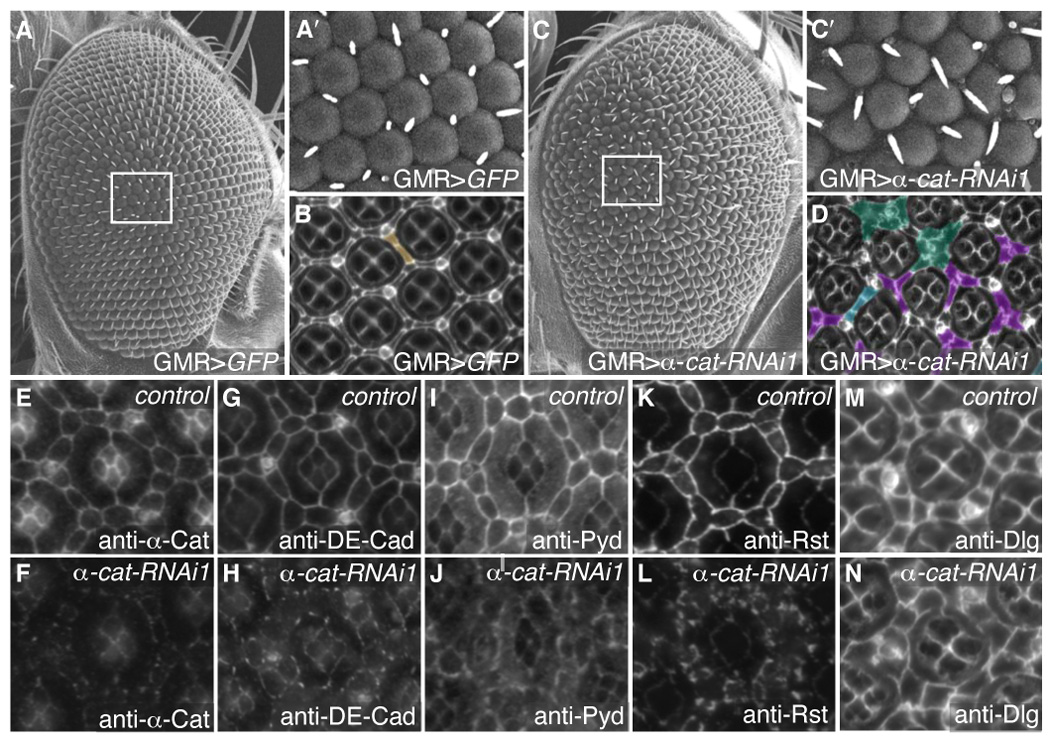
α-Catenin is necessary for AJ protein localization. A–A′. SEM of control GMR>GFP adult eyes demonstrating even ommatidial rows (A) with boxed area shown enlarged in A′. B. 41 hours APF pupal eye stained with anti-Discs large to mark cell membranes. Extra cell was pseudo-colored in brown. C–C′. Reduction of α-Catenin (GMR>α-cat-RNAi) disrupted the orderly array of ommatidial rows in the adult eye (C). C′ shows an enlargement of the boxed area C. D. 41 hours APF pupal eye stained with anti-Discs large and pseuso-colored to show examples of patterning errors: extra cells (blue), clustering of IPCs around bristles (green), and the failure of only one cell to occupy the 3° niche (purple). E–N. 28 hours APF pupal eyes. E–F. GMR>α-cat-RNAi retinas showed greatly reduced levels of α-Catenin (F) compared to GMR>GFP expressing eyes (E): the image in F represents >8-fold longer exposure than E. G–N. Control GMR>GFP retinas visualizing DE-Cad (G), Pyd (I), Rst (K) and Dlg (M). Expression of GMR>α-cat-RNAi in the pupal retina resulted in de-localization of DE-Cad (H), Pyd (J) and Rst (L), but no change in anti-Dlg staining (N).
Discussion
Pyd and DE-Cadherin act together to regulate patterning
Cell-cell junctions play a key role in maintaining organ system integrity and are required for embryonic viability (Aijaz et al., 2006; Gumbiner, 2005). In dynamically restructured tissues such as the pupal eye, junctions and junction-associated proteins do not simply play a permissive role in restricting cell movement. Instead, the levels, localization and turnover of junctional proteins appear to be tightly regulated. This dynamic interplay between the establishment and turnover of junctions is central to the timing and precision of the subtle movements that direct cells into their proper niches.
Our data demonstrate that Pyd is an AJ-associated protein that is required for patterning of the pupal lattice cells. Live imaging of the developing eye indicates that Pyd is necessary for the directed movements of IPCs that allow cell sorting into defined niches. Membrane contacts are dynamically exchanged in the pupal eye: each shift in the position of a cell requires the removal of previous contacts and the establishment of new ones. Pyd regulates patterning at least in part through modulating levels of the AJ-associated proteins DE-Cadherin, β-Catenin, and α-Catenin. Other studies have suggested that cell adhesion is necessary both to facilitate and restrict cell movement within the eye epithelium; the interplay between these two processes requires tight regulation of the levels of both cell adhesion molecules and junctional proteins (Bao and Cagan, 2005; Cordero et al., 2007; Grzeschik and Knust, 2005; Hayashi and Carthew, 2004; Reiter et al., 1996). Our data indicate that removal of Pyd from the AJ compromises this tightly-regulated system and biases the cells toward poorly-directed movements, perhaps because of dysregulation of the timing or function of the mechanisms that control the stability of AJ proteins. This failure in precise regulation of adhesion was also highlighted in the inability of cone cells to exchange their membrane contacts: the apical interfaces of pyd-RNAi expressing cone cells were locked in place. Ectopic DE-Cadherin further increased the percentage of ommatidia affected, again emphasizing the link between pyd activity and the AJ.
Pyd localization to the AJ is controlled by DE-Cadherin and α-Catenin
The localization of Pyd to the AJ in the pupal eye was dependent on both DE-Cadherin and α-Catenin. However, we found that ectopic expression of either junctional protein was not sufficient to alter the localization of Pyd. Taken together, our data indicate that DE-Cadherin and α-Catenin are necessary to build or maintain the AJ and to localize Pyd but that, in excess, they are not sufficient to attract ectopic Pyd. This suggests that either Pyd protein levels are not easily altered or that Pyd may be binding to proteins other than the core AJ constituents. Recent work demonstrated that E-Cadherin was necessary for the initial steps of AJ formation while α-Catenin was essential for both the establishment and maintenance of the junction; only when α-Catenin was reduced was ZO-1 lost from established junctions (Capaldo and Macara, 2007). Our results suggest that in dynamically restructured tissues such as the eye, both E-Cadherin and α-Catenin are necessary for the localization of AJ-associated proteins.
Pyd affects Roughest, a key regulator of patterning in the pupal eye
The immunoglobulin superfamily member Roughest is necessary for appropriate sorting of IPCs during pupal eye development (Reiter et al., 1996). We found that reducing Pyd increased Roughest protein levels specifically at the AJ. Roughest is the Drosophila orthologue of Neph1, a cell adhesion molecule necessary for the structure and function of the glomerular slit diaphragm in the mammalian kidney (Cagan, 2003; Donoviel et al., 2001). The slit diaphragm is the main size-selective barrier in the filtration apparatus of the kidney and retains many characteristics of both the tight and AJ complexes from which it was derived (Kerjaschki, 2001; Lee et al., 2006; Reiser et al., 2000). The Hibris orthologue Nephrin also forms part of the physical structure of the slit diaphragm and both cell adhesion molecules have been reported to bind to each other as well as to ZO-1 (Barletta et al., 2003; Holzman et al., 1999; Huber et al., 2003; Kestila et al., 1998; Lehtonen et al., 2004; Putaala et al., 2001). Perhaps ZO-1, as with Pyd, has a role in regulating the localization or levels of cell adhesion molecules such as Neph1 and Nephrin.
Connections between DE-Cadherin and Roughest: Pyd and the Dpp pathway
The Dpp pathway has emerged as a major contributor to patterning of the Drosophila pupal eye. Its role requires functional connections to both DE-Cadherin and Roughest. For example, mutations in shotgun— the locus that encodes DE-Cadherin—suppressed the roughest eye phenotype but enhanced Dpp pathway-dependent phenotypes in the eye and wing (Cordero et al., 2007). Together, these data suggest a model in which 1.) Roughest acts to promote the stability of membrane contacts to drive directed cell movements and 2.) the Dpp pathway and Pyd act to destabilize the adherens junction complex and local cell contacts to allow for proper IPC sorting. Consistent with this view, we observed that reducing pyd enhanced the effects of reduced Dpp pathway activity in the eye and wing. Thus, Pyd appears to act in concert with the Dpp pathway to regulate select core components of the AJ during development.
We have shown that Pyd is required specifically for patterning the interommatidial cells of the Drosophila pupal eye. Pyd appears to regulate both cell shape and cell positioning by controlling the levels of AJ proteins such as DE-Cadherin and adhesion proteins such as Roughest. Thus, Pyd provides a link between adhesion and junction formation; a further understanding of its role in the pupal eye will shed light on how these processes are coordinated to generate precise cellular movements during epithelial patterning.
Supplementary Material
Movie 1 Control cells undergo cell intercalation to generate single-file rows of IPCs. Time-lapse movie of GMR>α-catenin-GFP from 20.25hr–23 hours APF. In this and all subsequent movies, membranes were visualized with α-Catenin-GFP and cells were pseudo-colored to emphasize particular cell movements. Multiple images were taken every 15 minutes and assembled as a composite image in Adobe Photoshop (see Materials and Methods)‥ Wild-type cells moved past one another to contact two or more 1°s during early pupal retinal patterning.
Movie 2 pyd-RNAi expressing cells failed to sort appropriately during cell intercalation. Time-lapse movie of GMR>pyd-RNAi, α-catenin-GFP retina from 20.25–23 hours APF. Typically, pyd-RNAi-expressing cells failed to undergo intercalation, instead remaining in double rows.
Movie 3 Time-lapse movie of GMR>α-catenin-GFP from 26.25hr–30 hours APF. Control IPCs moved into and out of the 3° niche until one cell assumed the 3° position in most cases. IPCs that successfully patterned the 3° niche by 30 hours APF were pseudo-colored in green in the final frame. Bristles were pseudo-colored in orange.
Movie 4 GMR>pyd-RNAi-expressing cells do not make productive movements during 3° specification. Time-lapse movie of GMR>pyd-RNAi, α-catenin-GFP retina from 26.25–30 hours APF. pyd-RNAi-expressing cells maintained initial contacts and commonly failed to establish a single cell in the 3° position. The few IPCs that successfully patterned the 3° niche by 30 hours APF were pseudo-colored in green in the final frame. Bristles were pseudo-colored in orange.
Supp Fig 1 Ubiquituous expression of pyd-RNAi phenocopied mutations in pyd. A. The five predicted isoforms of Pyd emphasizing important protein-protein interaction domains; exon 6 is also schematized. pyd-RNAi1 (red bar) targets an N-terminal region shared by all isoforms while Pyd-RNAi3 (red bar) targets 4 of the 5 potential isoforms. The region used to generate a polyclonal anti-Pyd antibody is marked with a blue triangle. B–G. Adult thoracic bristles, dorsal view. The placement and number of bristles are stereotyped in wild-type flies (B). A characteristic pyd mutant phenotype is the appearance of extra bristles, visible on the thorax of flies homozygous for the hypomorphic pyd1 allele (C, arrowheads). Expression of UAS-pyd-RNAi3 with the ubiquitous driver da-Gal4 also resulted in the formation of extra thoracic bristles (D, arrowheads). Flies homozygous for the pyd hypomorphic mutation pydtam1 exhibited a very small increase in bristle number (E, arrowheads). Excision of the pydtam1-associated P-element yielded two fly lines pydtex1 (F, arrowheads) and pydtex2 (G, arrowheads) with extra thoracic bristles.
Supp Fig 2 Expression of pyd-RNAi or pyd-RFP did not alter the overall protein levels of DE-Cad or Rst. Total DE-Cad or Rst, levels (A) were not altered in eyes expressing pyd-RNAi (lane 2) or pyd-RFP (lane 3) as compared with control eyes expressing β-gal (lane 1). Anti-Lamin was used as a loading control. Likewise, the transcription of de-cad was not altered in clonal patches of pyd-RNAi-expressing cells (marked by GFP expression in B), as assessed by a shg-lacZ reporter construct at 27 hrs APF (C). D. Overlay of GFP (green) and anti-β-gal (magenta). C–C″. Expression of 54>de-cad-GFP did not result in patterning errors (C, GFP). The mild IPC errors of 54>pyd-RNAi retinas (C′, anti-Arm) were consistently exacerbated by concurrent expression of de-cad-GFP (C″, GFP). D–D″. Expression of 54>rst resulted in mild defects in IPC organization (D, anti-Arm) that were similar to 54>pyd-RNAi (D′, anti-Arm). The IPC errors of 54>pyd-RNAi retinas were strongly enhanced by concurrent expression of rst (D″, anti-Arm).
Supp Fig 3 Pyd-RFP localizes to the adherens junction and ectopic expression leads to defects in pupal eye patterning. A. Pyd-RFP constructs are depicted with important domains emphasized. Pyd-RFP is full-length Pyd-PB linked to a C-terminal mRFP. PydNT-RFP contains the first 3 PDZ domains of Pyd-PB, while PydCT-RFP contains the SH3 domain, GuK domain, exon 6 and the C-terminal proline-rich domain of Pyd-PB. Pydexon6--RFP lacks exon 6 and has a single amino acid substitution (see Methods). B–E′. Pyd-RFP localizes to the adherens junction (B) as marked by anti-DE-Cad (C). There is very little if any overlap of Pyd-RFP with the SJ marker Dlg (D, as well as overlay in E and lateral projection in E′). F–F″. Increased levels of anti-Rst immunofluorescence (F′) are present in clonal patches of cells expressing UAS-pyd-RFP (F). F″. Overlay of RFP (magenta) and anti-Rst (green). G–H″. Expression of either GMR>pydNT-RFP or GMR>pydCT-RFP transgenes (G and H, respectively) does not result in an increase in anti-Rst immunofluorescence (G′ and H′, respectively). Overlay in G″ and H″ of RFP (magenta) and anti-Rst (green).
Supp Fig 4 pyd is functionally linked to AJ proteins involved in adhesion. Control GMR-Gal4/+; shg-RNAi/+ (A), rst3/+ (C) and Control GMR-Gal4/+; tkv-RNAi/+ retinas exhibited few IPC patterning errors. These defects were strongly enhanced by removal of one functional copy of pydtex1 or pydtex2 (B, D and F, anti-Arm). G. Number of IPCs per hexagonal area (see Methods) for each genotype, respectively. Error bars represent standard deviation and N numbers are given above each bar. Brackets with stars indicate a statistically significant interaction between tkv-RNAi and pydtex2 (at p<0.001 by the Mann-Whitney U test). Brackets only indicate a marginally statistically significant interaction between rst3 and pydtex2 (at p<0.05). pydtex1 strongly enhanced the mispatterning phenotype of shg-RNAi without modifying the number of IPCs.
Acknowledgments
We would like to thank our colleagues, the Developmental Studies Hybridoma Bank, and the Bloomington Stock Center for generously providing reagents. We thank the members of the Cagan Lab for unflagging support and constructive criticism of the design and analysis of the experiments and the writing of the paper. Thank you to Dr. Craig Micchelli for many helpful comments on experimental design and direction. In addition, we thank Dave Larson for designing the UAS-α-catenin-RNAi constructs and Alan Fanning for constructive discussions and helpful advice. We apologize to our colleagues whose work was not cited due to space restriction. This work was supported by NIH grant NIH R01 EY1149.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol. 2006;248:261–298. doi: 10.1016/S0074-7696(06)48005-0. [DOI] [PubMed] [Google Scholar]
- Bao S, Cagan R. Preferential adhesion mediated by Hibris and Roughest regulates morphogenesis and patterning in the Drosophila eye. Dev Cell. 2005;8:925–935. doi: 10.1016/j.devcel.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Bao S, Cagan R. Fast cloning inverted repeats for RNA interference. Rna. 2006;12:2020–2024. doi: 10.1261/rna.258406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barletta GM, Kovari IA, Verma RK, Kerjaschki D, Holzman LB. Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J Biol Chem. 2003;278:19266–19271. doi: 10.1074/jbc.M301279200. [DOI] [PubMed] [Google Scholar]
- Brachmann CB, Cagan RL. Patterning the fly eye: the role of apoptosis. Trends Genet. 2003;19:91–96. doi: 10.1016/S0168-9525(02)00041-0. [DOI] [PubMed] [Google Scholar]
- Cagan R. Cell fate specification in the developing Drosophila retina. Dev Suppl. 1993:19–28. [PubMed] [Google Scholar]
- Cagan R. The signals that drive kidney development: a view from the fly eye. Curr Opin Nephrol Hypertens. 2003;12:11–17. doi: 10.1097/00041552-200301000-00003. [DOI] [PubMed] [Google Scholar]
- Cagan RL, Ready DF. The emergence of order in the Drosophila pupal retina. Dev Biol. 1989;136:346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- Capaldo CT, Macara IG. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2007;18:189–200. doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Freedman JA, Bettler DR, Jr, Manning SD, Giep SN, Steiner J, Ellis HM. Polychaetoid is required to restrict segregation of sensory organ precursors from proneural clusters in Drosophila. Mech Dev. 1996;57:215–227. doi: 10.1016/0925-4773(96)00548-5. [DOI] [PubMed] [Google Scholar]
- Cordero JB, Larson DE, Craig CR, Hays R, Cagan R. Dynamic Decapentaplegic signaling regulates patterning and adhesion in the Drosophila pupal retina. Development. 2007;134:1861–1871. doi: 10.1242/dev.002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA, Starbuck M, Brandt M, Gupta A, Ramirez-Solis R, Zambrowicz BP, Powell DR. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol. 2001;21:4829–4836. doi: 10.1128/MCB.21.14.4829-4836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci U S A. 2007;104:4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke L, Dakoji S, Bredt DS. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem. 2005;74:219–245. doi: 10.1146/annurev.biochem.74.082803.133339. [DOI] [PubMed] [Google Scholar]
- Godt D, Tepass U. Drosophila oocyte localization is mediated by differential cadherin-based adhesion. Nature. 1998;395:387–391. doi: 10.1038/26493. [DOI] [PubMed] [Google Scholar]
- Golic KG, Lindquist S. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell. 1989;59:499–509. doi: 10.1016/0092-8674(89)90033-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Betanzos A, Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol. 2000;11:315–324. doi: 10.1006/scdb.2000.0178. [DOI] [PubMed] [Google Scholar]
- Grzeschik NA, Knust E. IrreC/rst-mediated cell sorting during Drosophila pupal eye development depends on proper localisation of DE-cadherin. Development. 2005;132:2035–2045. doi: 10.1242/dev.01800. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Carthew RW. Surface mechanics mediate pattern formation in the developing retina. Nature. 2004;431:647–652. doi: 10.1038/nature02952. [DOI] [PubMed] [Google Scholar]
- Holzman LB, St John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 1999;56:1481–1491. doi: 10.1046/j.1523-1755.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- Huber TB, Schmidts M, Gerke P, Schermer B, Zahn A, Hartleben B, Sellin L, Walz G, Benzing T. The carboxyl terminus of Neph family members binds to the PDZ domain protein zonula occludens-1. J Biol Chem. 2003;278:13417–13421. doi: 10.1074/jbc.C200678200. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi J, Umeda K, Tsukita S, Furuse M, Tsukita S. Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J Cell Biol. 2007;176:779–786. doi: 10.1083/jcb.200612080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y, Itoh M, Maeno Y, Tsukita S, Nagafuchi A. Functional domains of alpha-catenin required for the strong state of cadherin-based cell adhesion. J Cell Biol. 1999;144:1311–1322. doi: 10.1083/jcb.144.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development. 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999a;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Morita K, Tsukita S. Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin. J Biol Chem. 1999b;274:5981–5986. doi: 10.1074/jbc.274.9.5981. [DOI] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S, Tsukita S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Y, Usui T, Hirano S, Steward R, Takeichi M, Uemura T. Axon patterning requires DN-cadherin, a novel neuronal adhesion receptor, in the Drosophila embryonic CNS. Neuron. 1997;19:77–89. doi: 10.1016/s0896-6273(00)80349-9. [DOI] [PubMed] [Google Scholar]
- Jung AC, Ribeiro C, Michaut L, Certa U, Affolter M. Polychaetoid/ZO-1 is required for cell specification and rearrangement during Drosophila tracheal morphogenesis. Curr Biol. 2006;16:1224–1231. doi: 10.1016/j.cub.2006.04.048. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Whitfield CW, Kim SK. The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell. 1998;94:761–771. doi: 10.1016/s0092-8674(00)81735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest. 2001;108:1583–1587. doi: 10.1172/JCI14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Wieschaus E. Junctions as organizing centers in epithelial cells? A fly perspective. Traffic. 2002;3:92–97. doi: 10.1034/j.1600-0854.2002.030202.x. [DOI] [PubMed] [Google Scholar]
- Lee DB, Huang E, Ward HJ. Tight junction biology and kidney dysfunction. Am J Physiol Renal Physiol. 2006;290:F20–F34. doi: 10.1152/ajprenal.00052.2005. [DOI] [PubMed] [Google Scholar]
- Lehtonen S, Lehtonen E, Kudlicka K, Holthofer H, Farquhar MG. Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby canine kidney cells expressing nephrin. Am J Pathol. 2004;165:923–936. doi: 10.1016/S0002-9440(10)63354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monserrate JP, Brachmann CB. Identification of the death zone: a spatially restricted region for programmed cell death that sculpts the fly eye. Cell Death Differ. 2007;14:209–217. doi: 10.1038/sj.cdd.4401947. [DOI] [PubMed] [Google Scholar]
- Muller HA. Genetic control of epithelial cell polarity: lessons from Drosophila. Dev Dyn. 2000;218:52–67. doi: 10.1002/(SICI)1097-0177(200005)218:1<52::AID-DVDY5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Oda H, Tsukita S. Dynamic features of adherens junctions during Drosophila embryonic epithelial morphogenesis revealed by a Dalpha-catenin-GFP fusion protein. Dev Genes Evol. 1999;209:218–225. doi: 10.1007/s004270050246. [DOI] [PubMed] [Google Scholar]
- Oda H, Uemura T, Harada Y, Iwai Y, Takeichi M. A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell-cell adhesion. Dev Biol. 1994;165:716–726. doi: 10.1006/dbio.1994.1287. [DOI] [PubMed] [Google Scholar]
- Oda H, Uemura T, Shiomi K, Nagafuchi A, Tsukita S, Takeichi M. Identification of a Drosophila homologue of alpha-catenin and its association with the armadillo protein. J Cell Biol. 1993;121:1133–1140. doi: 10.1083/jcb.121.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas D, Haghighi AP, Fetter RD, Kim SW, Goodman CS. Regulation of postsynaptic structure and protein localization by the Rho-type guanine nucleotide exchange factor dPix. Neuron. 2001;32:415–424. doi: 10.1016/s0896-6273(01)00485-8. [DOI] [PubMed] [Google Scholar]
- Petronczki M, Knoblich JA. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat Cell Biol. 2001;3:43–49. doi: 10.1038/35050550. [DOI] [PubMed] [Google Scholar]
- Phillis RW, Bramlage AT, Wotus C, Whittaker A, Gramates LS, Seppala D, Farahanchi F, Caruccio P, Murphey RK. Isolation of mutations affecting neural circuitry required for grooming behavior in Drosophila melanogaster. Genetics. 1993;133:581–592. doi: 10.1093/genetics/133.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putaala H, Soininen R, Kilpelainen P, Wartiovaara J, Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001;10:1–8. doi: 10.1093/hmg/10.1.1. [DOI] [PubMed] [Google Scholar]
- Rajasekaran AK, Hojo M, Huima T, Rodriguez-Boulan E. Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol. 1996;132:451–463. doi: 10.1083/jcb.132.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Lovegrove B, Jarman AP. Echinoid facilitates Notch pathway signalling during Drosophila neurogenesis through functional interaction with Delta. Development. 2003;130:6475–6484. doi: 10.1242/dev.00882. [DOI] [PubMed] [Google Scholar]
- Ready DF, Hanson TE, Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976;53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11:1–8. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- Reiter C, Schimansky T, Nie Z, Fischbach KF. Reorganization of membrane contacts prior to apoptosis in the Drosophila retina: the role of the IrreC-rst protein. Development. 1996;122:1931–1940. doi: 10.1242/dev.122.6.1931. [DOI] [PubMed] [Google Scholar]
- Riazuddin S, Ahmed ZM, Fanning AS, Lagziel A, Kitajiri S, Ramzan K, Khan SN, Chattaraj P, Friedman PL, Anderson JM, Belyantseva IA, Forge A, Riazuddin S, Friedman TB. Tricellulin is a tight-junction protein necessary for hearing. Am J Hum Genet. 2006;79:1040–1051. doi: 10.1086/510022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggleman B, Schedl P, Wieschaus E. Spatial expression of the Drosophila segment polarity gene armadillo is posttranscriptionally regulated by wingless. Cell. 1990;63:549–560. doi: 10.1016/0092-8674(90)90451-j. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci U S A. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls MM, Albertson R, Shih HP, Lee CY, Doe CQ. Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia. J Cell Biol. 2003;163:1089–1098. doi: 10.1083/jcb.200306079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi JC, Hays R, Cagan RL. Programmed cell death and patterning in Drosophila. Cell Death Differ. 2000;7:1063–1070. doi: 10.1038/sj.cdd.4400767. [DOI] [PubMed] [Google Scholar]
- Schnabel E, Anderson JM, Farquhar MG. The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol. 1990;111:1255–1263. doi: 10.1083/jcb.111.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Reiter C, Eule E, Bader B, Lichte B, Nie Z, Schimansky T, Ramos RG, Fischbach KF. Restricted expression of the irreC-rst protein is required for normal axonal projections of columnar visual neurons. Neuron. 1995;15:259–271. doi: 10.1016/0896-6273(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Schock F, Perrimon N. Molecular mechanisms of epithelial morphogenesis. Annu Rev Cell Dev Biol. 2002;18:463–493. doi: 10.1146/annurev.cellbio.18.022602.131838. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Matsuo T, Katsube T, Ueda R, Yamamoto D. Direct binding between two PDZ domain proteins Canoe and ZO-1 and their roles in regulation of the jun N-terminal kinase pathway in Drosophila morphogenesis. Mech Dev. 1998;78:97–111. doi: 10.1016/s0925-4773(98)00151-8. [DOI] [PubMed] [Google Scholar]
- Takahisa M, Togashi S, Suzuki T, Kobayashi M, Murayama A, Kondo K, Miyake T, Ueda R. The Drosophila tamou gene, a component of the activating pathway of extramacrochaetae expression, encodes a protein homologous to mammalian cell-cell junction-associated protein ZO-1. Genes Dev. 1996;10:1783–1795. doi: 10.1101/gad.10.14.1783. [DOI] [PubMed] [Google Scholar]
- Tanenbaum SB, Gorski SM, Rusconi JC, Cagan RL. A screen for dominant modifiers of the irreC-rst cell death phenotype in the developing Drosophila retina. Genetics. 2000;156:205–217. doi: 10.1093/genetics/156.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejedor FJ, Bokhari A, Rogero O, Gorczyca M, Zhang J, Kim E, Sheng M, Budnik V. Essential role for dlg in synaptic clustering of Shaker K+ channels in vivo. J Neurosci. 1997;17:152–159. doi: 10.1523/JNEUROSCI.17-01-00152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U, Gruszynski-DeFeo E, Haag TA, Omatyar L, Torok T, Hartenstein V. shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev. 1996;10:672–685. doi: 10.1101/gad.10.6.672. [DOI] [PubMed] [Google Scholar]
- Tepass U, Harris KP. Adherens junctions in Drosophila retinal morphogenesis. Trends Cell Biol. 2007;17:26–35. doi: 10.1016/j.tcb.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Umeda K, Matsui T, Nakayama M, Furuse K, Sasaki H, Furuse M, Tsukita S. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J Biol Chem. 2004;279:44785–44794. doi: 10.1074/jbc.M406563200. [DOI] [PubMed] [Google Scholar]
- Vidal M, Larson DE, Cagan RL. Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev Cell. 2006;10:33–44. doi: 10.1016/j.devcel.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Wei X, Ellis HM. Localization of the Drosophila MAGUK protein Polychaetoid is controlled by alternative splicing. Mech Dev. 2001;100:217–231. doi: 10.1016/s0925-4773(00)00550-5. [DOI] [PubMed] [Google Scholar]
- Weis WI, Nelson WJ. Re-solving the cadherin-catenin-actin conundrum. J Biol Chem. 2006;281:35593–35597. doi: 10.1074/jbc.R600027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Cell death in normal and rough eye mutants of Drosophila. Development. 1991;113:825–839. doi: 10.1242/dev.113.3.825. [DOI] [PubMed] [Google Scholar]
- Woods DF, Hough C, Peel D, Callaini G, Bryant PJ. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J Cell Biol. 1996;134:1469–1482. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DF, Wu JW, Bryant PJ. Localization of proteins to the apico-lateral junctions of Drosophila epithelia. Dev Genet. 1997;20:111–118. doi: 10.1002/(SICI)1520-6408(1997)20:2<111::AID-DVG4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 1 Control cells undergo cell intercalation to generate single-file rows of IPCs. Time-lapse movie of GMR>α-catenin-GFP from 20.25hr–23 hours APF. In this and all subsequent movies, membranes were visualized with α-Catenin-GFP and cells were pseudo-colored to emphasize particular cell movements. Multiple images were taken every 15 minutes and assembled as a composite image in Adobe Photoshop (see Materials and Methods)‥ Wild-type cells moved past one another to contact two or more 1°s during early pupal retinal patterning.
Movie 2 pyd-RNAi expressing cells failed to sort appropriately during cell intercalation. Time-lapse movie of GMR>pyd-RNAi, α-catenin-GFP retina from 20.25–23 hours APF. Typically, pyd-RNAi-expressing cells failed to undergo intercalation, instead remaining in double rows.
Movie 3 Time-lapse movie of GMR>α-catenin-GFP from 26.25hr–30 hours APF. Control IPCs moved into and out of the 3° niche until one cell assumed the 3° position in most cases. IPCs that successfully patterned the 3° niche by 30 hours APF were pseudo-colored in green in the final frame. Bristles were pseudo-colored in orange.
Movie 4 GMR>pyd-RNAi-expressing cells do not make productive movements during 3° specification. Time-lapse movie of GMR>pyd-RNAi, α-catenin-GFP retina from 26.25–30 hours APF. pyd-RNAi-expressing cells maintained initial contacts and commonly failed to establish a single cell in the 3° position. The few IPCs that successfully patterned the 3° niche by 30 hours APF were pseudo-colored in green in the final frame. Bristles were pseudo-colored in orange.
Supp Fig 1 Ubiquituous expression of pyd-RNAi phenocopied mutations in pyd. A. The five predicted isoforms of Pyd emphasizing important protein-protein interaction domains; exon 6 is also schematized. pyd-RNAi1 (red bar) targets an N-terminal region shared by all isoforms while Pyd-RNAi3 (red bar) targets 4 of the 5 potential isoforms. The region used to generate a polyclonal anti-Pyd antibody is marked with a blue triangle. B–G. Adult thoracic bristles, dorsal view. The placement and number of bristles are stereotyped in wild-type flies (B). A characteristic pyd mutant phenotype is the appearance of extra bristles, visible on the thorax of flies homozygous for the hypomorphic pyd1 allele (C, arrowheads). Expression of UAS-pyd-RNAi3 with the ubiquitous driver da-Gal4 also resulted in the formation of extra thoracic bristles (D, arrowheads). Flies homozygous for the pyd hypomorphic mutation pydtam1 exhibited a very small increase in bristle number (E, arrowheads). Excision of the pydtam1-associated P-element yielded two fly lines pydtex1 (F, arrowheads) and pydtex2 (G, arrowheads) with extra thoracic bristles.
Supp Fig 2 Expression of pyd-RNAi or pyd-RFP did not alter the overall protein levels of DE-Cad or Rst. Total DE-Cad or Rst, levels (A) were not altered in eyes expressing pyd-RNAi (lane 2) or pyd-RFP (lane 3) as compared with control eyes expressing β-gal (lane 1). Anti-Lamin was used as a loading control. Likewise, the transcription of de-cad was not altered in clonal patches of pyd-RNAi-expressing cells (marked by GFP expression in B), as assessed by a shg-lacZ reporter construct at 27 hrs APF (C). D. Overlay of GFP (green) and anti-β-gal (magenta). C–C″. Expression of 54>de-cad-GFP did not result in patterning errors (C, GFP). The mild IPC errors of 54>pyd-RNAi retinas (C′, anti-Arm) were consistently exacerbated by concurrent expression of de-cad-GFP (C″, GFP). D–D″. Expression of 54>rst resulted in mild defects in IPC organization (D, anti-Arm) that were similar to 54>pyd-RNAi (D′, anti-Arm). The IPC errors of 54>pyd-RNAi retinas were strongly enhanced by concurrent expression of rst (D″, anti-Arm).
Supp Fig 3 Pyd-RFP localizes to the adherens junction and ectopic expression leads to defects in pupal eye patterning. A. Pyd-RFP constructs are depicted with important domains emphasized. Pyd-RFP is full-length Pyd-PB linked to a C-terminal mRFP. PydNT-RFP contains the first 3 PDZ domains of Pyd-PB, while PydCT-RFP contains the SH3 domain, GuK domain, exon 6 and the C-terminal proline-rich domain of Pyd-PB. Pydexon6--RFP lacks exon 6 and has a single amino acid substitution (see Methods). B–E′. Pyd-RFP localizes to the adherens junction (B) as marked by anti-DE-Cad (C). There is very little if any overlap of Pyd-RFP with the SJ marker Dlg (D, as well as overlay in E and lateral projection in E′). F–F″. Increased levels of anti-Rst immunofluorescence (F′) are present in clonal patches of cells expressing UAS-pyd-RFP (F). F″. Overlay of RFP (magenta) and anti-Rst (green). G–H″. Expression of either GMR>pydNT-RFP or GMR>pydCT-RFP transgenes (G and H, respectively) does not result in an increase in anti-Rst immunofluorescence (G′ and H′, respectively). Overlay in G″ and H″ of RFP (magenta) and anti-Rst (green).
Supp Fig 4 pyd is functionally linked to AJ proteins involved in adhesion. Control GMR-Gal4/+; shg-RNAi/+ (A), rst3/+ (C) and Control GMR-Gal4/+; tkv-RNAi/+ retinas exhibited few IPC patterning errors. These defects were strongly enhanced by removal of one functional copy of pydtex1 or pydtex2 (B, D and F, anti-Arm). G. Number of IPCs per hexagonal area (see Methods) for each genotype, respectively. Error bars represent standard deviation and N numbers are given above each bar. Brackets with stars indicate a statistically significant interaction between tkv-RNAi and pydtex2 (at p<0.001 by the Mann-Whitney U test). Brackets only indicate a marginally statistically significant interaction between rst3 and pydtex2 (at p<0.05). pydtex1 strongly enhanced the mispatterning phenotype of shg-RNAi without modifying the number of IPCs.


