Abstract
Homeostatic regulation of cardiac function is dependent on the balance of inputs from the sympathetic and parasympathetic nervous systems. We investigated whether the p75 neurotrophin receptor plays a developmental role in cardiac innervation by analyzing sympathetic and parasympathetic fibers in the atria of p75 knockout and wildtype mice at several stages of postnatal development, and examining the effect on control of heart rate. We found that parasympathetic innervation of the atria in p75-/-mice was similar to wildtype at all time points, but that the density of sympathetic innervation was dynamically regulated. Compared to wildtype mice, the p75-/- mice had less innervation at postnatal day 4, an increase at day 28, and decreased innervation in adult mice. These changes reflect defects in initial fiber ingrowth and the timing of the normal developmental decrease in sympathetic innervation density in the atria. Thus, p75 regulates both the growth and stability of cardiac sympathetic fibers. The distribution of sympathetic fibers was also altered, so that many regions lacked innervation. Basal heart rate was depressed in adult p75-/- mice, and these mice exhibited a diminished heart rate response to restraint stress. This resulted from the lack of sympathetic innervation rather than increased parasympathetic transmission or a direct effect of p75 in cardiac cells. Norepinephrine was elevated in p75-/- atria, but stimulating norepinephrine release with tyramine produced less tachycardia in p75-/- mice than wild type mice. This suggests that altered density and distribution of sympathetic fibers in p75-/- atria impairs the control of heart rate.
Keywords: axon outgrowth, axon maintenance, sympathetic, parasympathetic, development, autonomic control
Introduction
The proper development and modulation of the autonomic nervous system is critical for maintaining internal homeostasis in the midst of a changing environment. The heart is an important target of the sympathetic and parasympathetic nervous systems, and balance between the stimulatory sympathetic inputs and inhibitory parasympathetic inputs are crucial for control of cardiac function. Post-ganglionic sympathetic neurons require the neurotrophin nerve growth factor (NGF) for survival (Francis and Landis, 1999; Levi-Montalcini and Angeletti, 1968) while parasympathetic neurons do not (Airaksinen and Saarma, 2002; Hiltunen et. al., 2000). In the heart NGF signaling is required for innervation by sympathetic axons in addition to its effects on cell survival (Glebova and Ginty, 2004). Thus, disruption of neurotrophin signaling would be expected to impact the development of the cardiac sympathetic innervation, while having little effect on the cardiac parasympathetic innervation.
Neurotrophins, including NGF, act through two distinct types of receptors: the Tropomyosin-related tyrosine kinase (Trk) receptors, and the lower affinity p75 receptor (reviewed by Glebova and Ginty, 2005; Zampieri and Chao, 2006). NGF and Neurotrophin-3 promote sympathetic neuron survival both in vitro (Birren et. al., 1993; DiCicco-Bloom et. al., 1993) and in vivo (Levi-Montalcini and Booker, 1960; Francis et. al., 1999) and this survival response is mediated via the TrkA receptor (Belliveau et. al., 1997; Fagan et. al., 1996; Tessarollo et. al., 1997). Both neurotrophins act through TrkA to promote the outgrowth of sympathetic neurons during development, with NGF playing a key role in final target innervation (Kuruvilla et. al., 2004; Glebova and Ginty, 2004). The role of the p75 receptor in development of the sympathetic innervation is less clear. In mice lacking p75 many sympathetic targets have normal innervation, while the innervation of other targets is disrupted (Jahed and Kawaja, 2005; Kuruvilla et. al., 2004; Lee et. al., 1992). Both of these disparate observations have been made concerning the sympathetic innervation of the heart. The lack of p75 during embryogenesis results in significantly fewer sympathetic axons projecting into the heart at E16.5 (Kuruvilla et. al., 2004) compared to wild type mice. In contrast, the density of sympathetic innervation in the heart is apparently normal 11-14 days after birth in mice lacking p75 (Jahed and Kawaja, 2005).
We examined the sympathetic and parasympathetic innervation of the right atria in mice lacking p75 at several postnatal time points to determine if there was a developmental delay in the establishment of the cardiac innervation. Furthermore, we asked if this led to functional consequences for the heart by monitoring heart rate. Sympathetic neurons innervating the sinoatrial node (SA node) in the right atria stimulate heart rate through release of norepinephrine (NE) and activation of β adrenergic receptors. Parasympathetic neurons inhibit heart rate at the SA node through release of acetylcholine (ACh) and activation of m2 muscarinic ACh receptors. We found that parasympathetic innervation of p75-/- atria was similar to wildtype at all time points that were examined, but that the density of sympathetic innervation was decreased at postnatal day 4. We observed a transient sympathetic hyperinnervation of p75-/- atria compared to the wildtype at postnatal day 28, and decreased sympathetic innervation in adult p75-/- mice compared to age-matched wildtype mice. The loss of sympathetic fibers in adults corresponded with a lower basal heart rate and an impaired stress response. These functional changes resulted from decreased sympathetic transmission, not increased parasympathetic transmission or altered cardiac responsiveness. These data indicate that p75 is involved in both the development and maintenance of the cardiac sympathetic innervation, and the lack of p75 alters autonomic control of heart rate.
Materials and Methods
Materials
Drugs and chemicals were obtained from Sigma (St. Louis, MO) unless otherwise noted. Antibodies were as follows: rabbit anti-tyrosine hydroxylase (TH), sheep anti-TH, and goat anti-vesicular acetylcholine transporter (VAChT) were from Chemicon (Temecula, California); donkey anti-rabbit Cy5, donkey anti-sheep Cy5, and donkey anti-goat Cy3 were from Jackson ImmunoResearch (West Grove, PA). Dobutamine hydrochloride was from Hospira, Inc. (Lake Forest, IL).
Animals
Wild type C57BL/6J and p75 KO mice (B6.129S4-Ngfrtm1Jae/J) were obtained from Jackson Laboratories, Bar Harbor, Maine. All animal experiments were carried out in accordance with animal protocols approved by the Brandeis University Institutional Animal Care and Use Committee or by the Portland VA Medical Center Institutional Animal Care and Use Committee. Adult mice were at least 7 months old and ranged from 7 to 15 months of age.
Immunohistochemistry of Mouse Atria
Sympathetic fibers were identified by staining mouse atrial whole mounts for tyrosine hydroxylase (TH), the rate limiting enzyme in norepinephrine synthesis. Parasympathetic neurons were identified in the same tissue by staining for the vesicular acetylcholine transporter (VAChT).
Mice were sacrificed by CO2 asphyxiation and hearts were dissected in dishes of phosphate buffered saline (PBS) under low magnification. Intact atria were separated from the ventricles and cleaned of other tissue, and the right atrium was dissected away from the left. Following removal of the left atrium, the interatrial septum was removed and the underside of the right atrium which attaches to the ventricle was cut away, leaving a flattened surface that was used for whole-mount immunohistochemistry. Atria were then fixed by submersion in 4% paraformaldehyde at 4°C. Postnatal day 4 (P4), P28, and adult atria were fixed for 4 hours; P1 atria were fixed overnight. Following fixation, atria were rinsed 3 × 10 min. in 80% ethanol and stored in PBS at 4°C. Differences in fixation time (and blocking time, see below) were used to minimize background staining. However, there were no significant differences in staining quality between any of the conditions.
Atria from younger mice (P4 and P28-35) were blocked overnight at 4°C in 5% horse serum (HS)/0.4% Triton X-100 in PBS, while adult atria were blocked in the same solution at room temperature for 30 minutes. Atria were incubated overnight at 4°C with primary antibodies diluted in 1% (P4 and P28-35) or 5% horse serum (adults). All double-labeling was done with rabbit anti-TH (1:500) and goat anti-VAChT (1:1000 or 1:4000). In some experiments in which only TH staining was examined a sheep anti-TH antibody (1:500) was used. Staining with this antibody showed results similar to the rabbit TH antibody and the data was combined. Sections were washed 3 × 20 minutes in PBS, and then incubated for 2 hours at room temperature with secondary antisera: donkey anti-sheep Cy5, or donkey anti-goat Cy5 and donkey anti-rabbit Cy3 diluted 1:400 or 1:600. Atria were rinsed 3×1 hr in PBS at room temperature and placed on flat glass slides with N-propyl-gallate, sealed with nail polish, and stored at -20°C in the dark.
Imaging and Analysis of Atrial Whole Mounts
Immunohistochemical staining was visualized on a Leica TCS confocal microscope with the 10× objective. Images of individual optical sections were collected at 10 micron intervals. The z-series were compressed to form a composite image that was then used for fiber measurements. There was some variability in the thickness of the sections, which were generally greater than 100 microns thick. Fibers were traced by hand using the cursor (Fig. 1a, b) using ImageJ 1.30v by Wayne Rasband (NIH), with the NeuronJ plugin by Eric Meijering of the Swiss Federal Institute of Technology (Meijering et al. 2004). Images were shrunk to 500×500 pixels and converted into 8 bit black and white before analysis. For grid experiments, atria were divided into 25 squares and each square was analyzed for fiber density. The 25 square grid was overlaid onto the compressed z-series from each animal and total fiber length within each square was traced and measured. Tissues were oriented in an anatomically consistent manner throughout the analysis. Each square was 300 by 300 microns. The whole block of 25 squares was thus 1500 by 1500 microns. The squares were binned according to total fiber density per square for all of the animal analyzed and the data presented as the fraction of squares that fell within each bin.
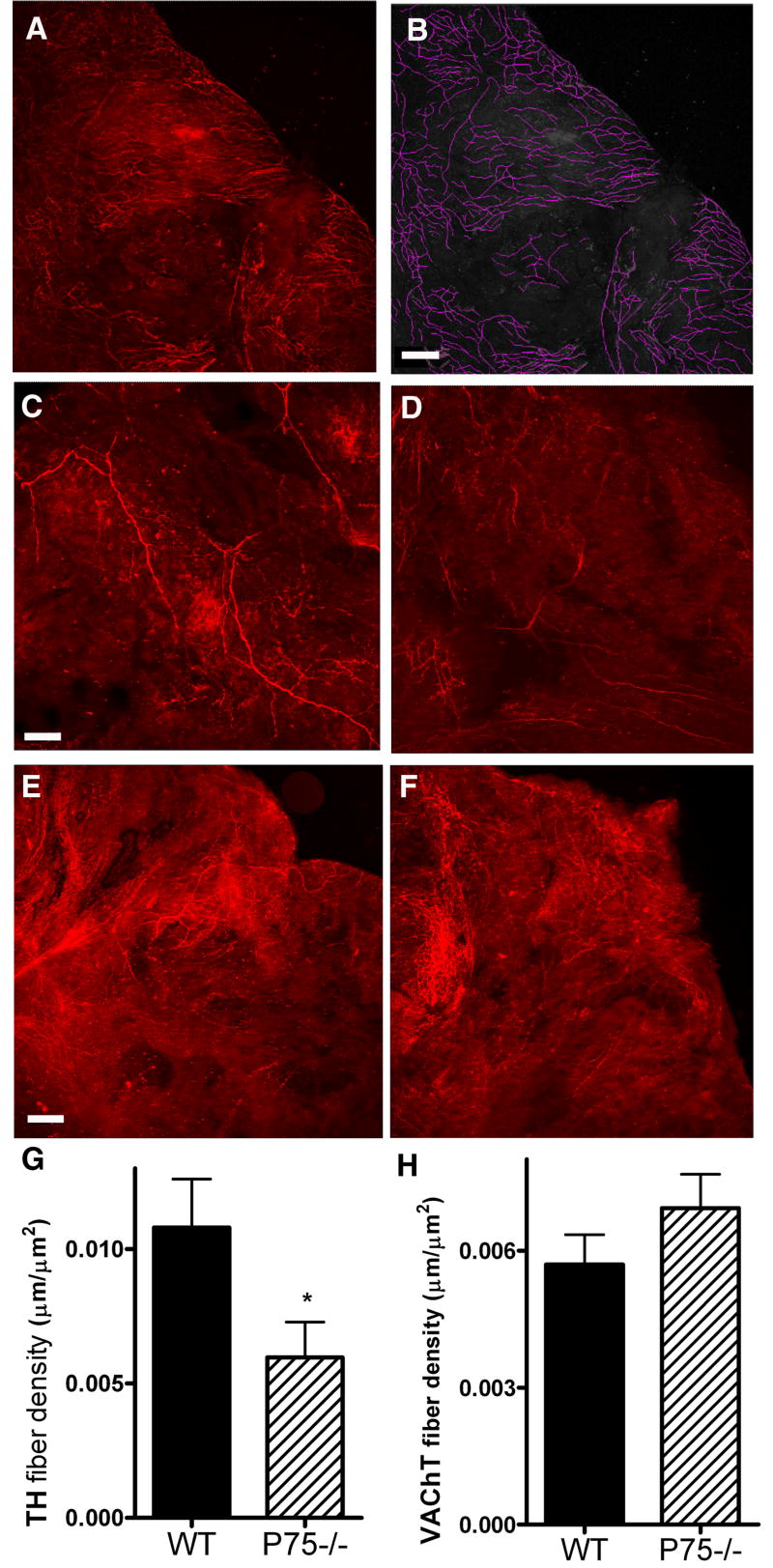
Figure 1.
Sympathetic, but not parasympathetic innervation of the right atria is altered in P4 p75 knockout mice. Right atria were stained for TH to identify sympathetic fibers or VAChT to identify parasympathetic fibers. Confocal images were captured at 10 μm intervals and fibers were manually traced using from a compressed z-stack using ImageJ software with the NeuronJ plug-in. Fiber density was calculated for each atria. Panels A and B show an example of fiber tracing on an image taken from an adult atrium stained with VAChT. C, D: Representative TH staining from P4 WT (C) and p75-/- (D) atria. E, F: Representative VAChT staining from P4 WT (E) and p75-/- (F) atria. Images C-F show representative images from the same region of the right atria, fiber tracing was carried out over the entire atrium. G, H: Average TH (G) and VAChT (H) fiber densities for wildtype (WT, solid bars) or p75-/- (hatched bars) atria. Data shown are the mean±sem, *p < 0.05, n=10-11 for TH, n=6 for VAChT. Scale bar = 125 μm.
Heart rate measurements
Mice were sedated with 2% isoflurane and placed in a supine position on a heating pad with a nosecone in place for administering isoflurane at 2%. ECG electrodes were placed subcutaneously on all four limbs and the chest, and a rectal temperature probe was used to monitor body temperature. The animal's head was covered with a black cloth to prevent light stimulation of sympathetic reflexes. Body temperature and heart rate were allowed to stabilize for 15 minutes prior to drug treatments or collection of basal heart rate data. Heart rate and temperature data were collected with a PowerLab data acquisition system and analyzed using Chart Software v.5.4.
Drug treatments
Atropine sulfate
To test the role of parasympathetic transmission, the muscarinic antagonist atropine sulfate (2 mg/kg) was injected IP and heart rate monitored for 15 minutes. The right jugular vein was exposed and cannulated with PE50 tubing with a PE10 tip for infusion of the following drugs, which were dissolved in saline: Hexamethonium chloride (5 mg/kg body weight in 100 μl) was infused over a 30 sec period to abolish autonomic reflexes. Ten minutes later the mouse was infused with a bolus dose of dobutamine hydrochloride (20 μg/kg in 30μl). After washout of the dobutamine, tyramine hydrochloride (200 μg/kg in 100 μl) was infused over 30 seconds to trigger release of NE from sympathetic nerve terminals. These concentrations of dobutamine and tyramine were chosen because they stimulated maximal increases in heart rate in our preliminary experiments.
Restraint Experiment
Baseline heart rate measurements were obtained in sedated animals, and anesthetics can alter sympathetic control of the cardiovascular system (Just et. al., 2000). Therefore, heart rate was monitored in conscious adult mice upon waking and following 10 minutes of restraint stress to activate the sympathetic nervous system. Mice were sedated with 2% isoflurane and placed in a supine position on a heating pad with a nosecone in place for administering isoflurane at 2%. ECG electrodes were placed subcutaneously on all four limbs and the chest and fixed in place with adhesive to prevent removal of the electrodes upon waking. The animal was immobilized in a restraint chamber while under anesthesia and then allowed to wake up. The heart rate immediately upon waking was identified as the basal heart rate for this experiment. For 10 minutes the conscious animal was restrained and heart rate monitored. The “stress” heart rate was the average heart rate during minutes 6-10 of the stress period.
Norepinephrine assay
NE levels in heart tissue were measured by HPLC with electrochemical detection as described in detail previously (Li et al., 2004). Frozen heart tissue samples were pulverized with a mortar and pestle, chilled on dry ice and stored at -80°C until assayed. The frozen powdered tissue was weighed and homogenized with a microhomogenizer in 250 μL of perchloric acid (PCA, 0.1 M) containing 1.0 μM of the internal standard, DHBA (dihydroxybenzylamine), to correct for sample recoveries. The catechols in the sample were extracted with alumina, desorbed with 100 μL of 0.1 M PCA, and 50 μl aliquots were fractionated by reversed-phase HPLC (C18; 5 μm particle size, Rainin) using a mobile phase containing 75 mM sodium phosphate (pH 3.0), 360 mg/L of sodium octane sulfonate, 100 μL/L triethylamine and 3.0% acetonitrile. Detection limits for NE were about 0.05 pmol with recoveries greater than 60%.
Statistics
Differences between genotypes were analyzed by unpaired two-tailed student's t-test using Prism v4.2.
Results
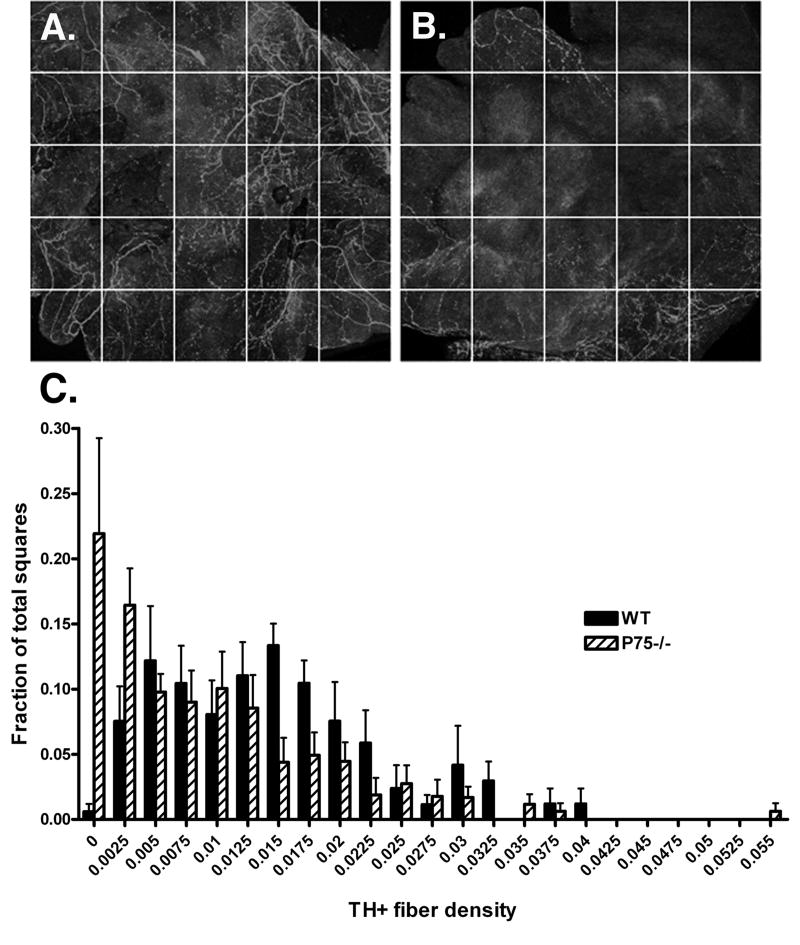
The autonomic innervation of the right atria was examined at several time points during postnatal development in order to determine if the absence of p75 altered the cardiac innervation. Whole mount preparations of right atrial were stained for tyrosine hydroxylase (TH) to stain sympathetic fibers and vesicular acetylcholine transporter (VAChT) to label parasympathetic fibers. Fiber tracing of confocal images was used to measure innervation density (Fig. 1A,B). The overall density of TH-positive sympathetic fibers in the right atrium was decreased in p75-/- mice compared to wild type mice at P4 (Fig. 1). There was no corresponding difference in the VAChT-positive parasympathetic innervation (Fig. 1), resulting in a decrease in the ratio of sympathetic to parasympathetic fibers in the P4 right atrium (TH/VAChT- WT, 1.94; p75-/-, 0.85). In addition to lower overall sympathetic innervation density, the pattern of innervation was altered in the P75-/- atria. In the absence of p75 the atria had many areas that were completely devoid of sympathetic fibers (Fig. 2), compared to the more even distribution of sympathetic fibers across WT atria.
Figure 2.
Sympathetic fiber density across the atria at P4. Confocal images of individual TH-stained wildtype (A) or p75-/- (B) atria were obtained. A grid containing 25 squares (300 × 300 μm/square) was overlaid onto the compressed z-series images and each square was analyzed for total fiber density (C). Squares were binned according to fiber density and the fraction of squares with a given density was calculated over multiple animals. Wildtype, solid bars; p75-/-, hatched bars. Data shown are the mean±sem of the fraction of squares with a given density, n=7-8. Significantly more squares lacked sympathetic fibers in the p75-/- atria (p<0.02).
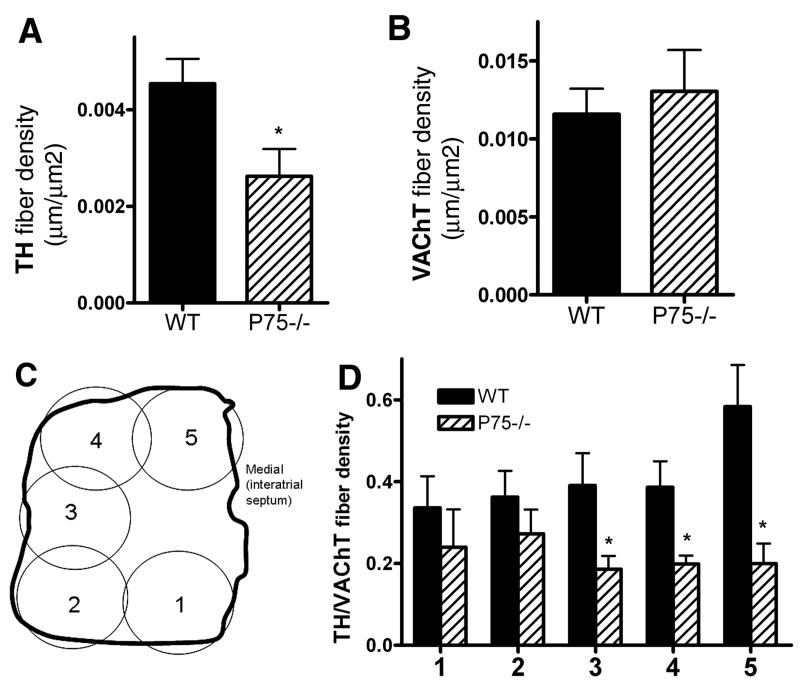
While sympathetic innervation is decreased in neonatal p75-deficient animals, by the time the mice reach 28-35 days of age the density of sympathetic fibers was greater in the right atrium of p75-/- mice than in the wild-type controls (Fig. 3). However, sympathetic innervation density in the right atria was decreased in adult mice lacking p75 compared to age-matched wild-type controls (Fig. 4). Parasympathetic innervation was not different between wild-type and p75-/- animals in adult mice and the density of parasympathetic fibers increased to a similar extent between P4 and adult in both genotypes. Parasympathetic innervation was not examined at the P28 time point. The lower density of sympathetic fibers in the adult was not uniformly distributed throughout the right atria, rather, the greatest loss in fiber density in the p75-deficient mice was observed in the highly innervated region that is near the SA node (Fig. 4).
Figure 3.
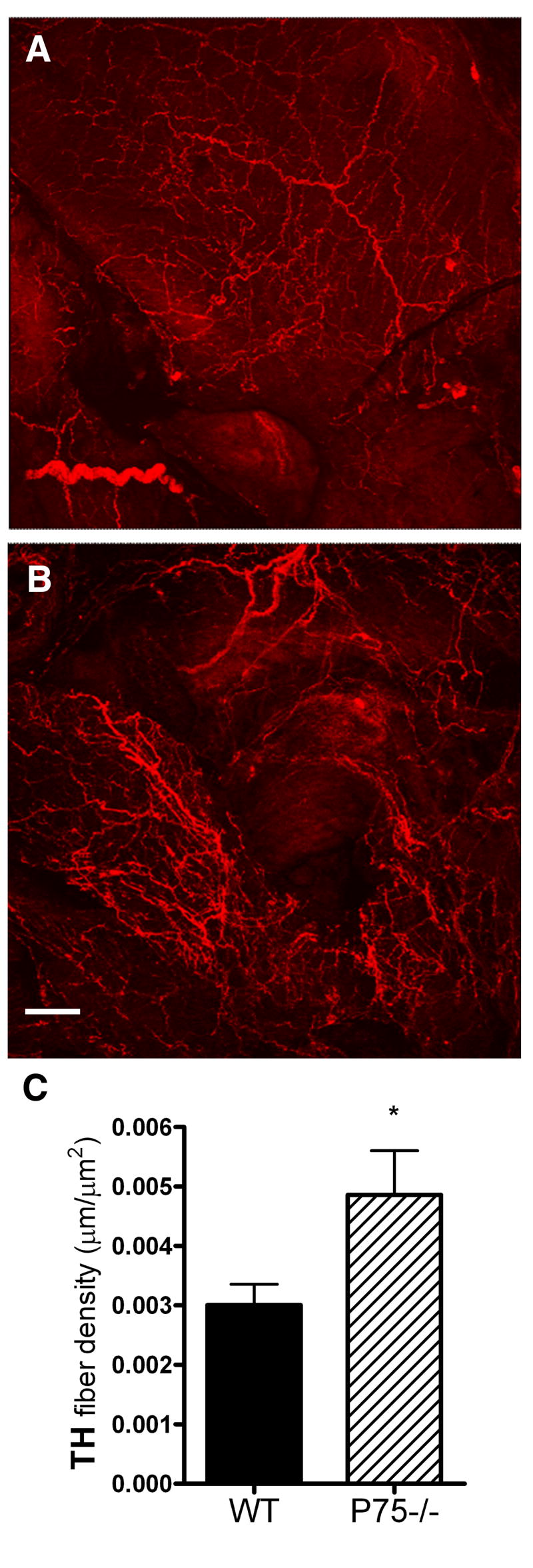
Increased sympathetic fibers in the right atria of P28-35 p75-/- mice. Representative TH staining of (A) wildtype or (B) p75-/- right atria. Fibers were traced using NeuronJ software and the fiber density was normalized to the area of the atria for each experiment. (C) Data shown are the mean±sem from 10 wildtype (WT, solid bars) and 10 p75-/- (hatched bars) atria. *p < 0.05. Scale bar = 100 μm.
Figure 4.
Reduced TH, but not VAChT, fiber density in the right atria of adult p75-/- mice. Adult (12+ months) right atria were stained for TH (A) or VAChT (B), and fiber densities were calculated using ImageJ software and the NeuronJ plug-in. Data shown are the mean±sem, *p < 0.05, n=5-6. Regional fiber distribution was analyzed by examining five regions of the adult atria (C, 1-5) and calculating the ratio of TH to VAChT fiber density in each area (D). Data shown are the mean±sem, *p < 0.05, n=5-6. Wildtype, solid bars; p75-/-, hatched bars.
Heart rate was monitored in 7 month-old adult mice lacking p75 to determine if the lower density of sympathetic nerve fibers in the right atria resulted in decreased heart rate. Baseline heart rate was significantly lower in mice lacking p75 compared to wild type mice (p75-/- 426±12 bpm; WT 475±16 bpm; mean±sem, n=7-8, p<0.03). Atropine injection increased heart rate to a similar extent in both genotypes (Fig. 5), suggesting that the level of cardiac parasympathetic tone was unchanged in p75-/- mice. Since the extent of sympathetic innervation in the right atria of p75-/- mice was variable over developmental time and was increased compared to wild-type in young animals, we asked if the difference in heart rate in adult p75-/- mice was absent at the younger age. Heart rate was not significantly different between age-matched wild type and p75-/- mice at postnatal days 30-34 (WT 594±4 bpm; P75-/- 584±6 bpm; mean±sem, n=11). Thus, the increased innervation density seen at this age compared to the older animals is associated with increased heart rate that reaches, but does not exceed, the basal heart rate in age-matched controls.
Figure 5.
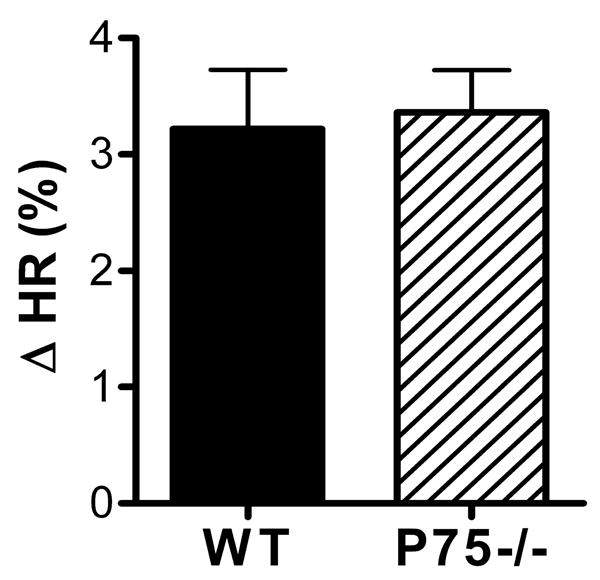
Heart rate response to atropine. Atropine sulfate (2 mg/kg) was injected to block parasympathetic transmission in the heart, resulting in increased heart rate (HR). Wildtype, solid bar, p75-/-, hatched bar. Data are expressed as percent change within each animal, and are mean±sem, n=5-6. Average basal HR: WT 546±6, P75-/- 517±19; average HR after atropine: WT 564±6, P75-/- 539±16.
Basal heart rates were higher in conscious mice of both genotypes, but restraint stress triggered a significant rise in heart rate only in WT mice, not in p75-/- mice (Fig. 6). Restraint stress causes widespread activation of the sympathetic nervous system, including the release of epinephrine from the adrenal medulla (Popper et. al., 1977). This raised the possibility that the lack of a heart rate response in p75-/- mice was due to impaired cardiac responsiveness rather than decreased sympathetic innervation in the right atria. To determine if the SA node pacemaker or cardiac responsiveness was altered in p75-/- mice, we determined the intrinsic heart rate by blocking ganglionic transmission and then monitored the heart rate increase following infusion of the beta agonist dobutamine (20 μg/kg). Intrinsic heart rate following hexamethonium blockade of ganglionic transmission was not significantly altered in p75-/- mice (WT 520±14 bpm, n=11; p75-/- 510±11 bpm n=14, mean±sem). In contrast, direct activation of beta receptors with dobutamine stimulated a larger increase in heart rate in p75-/- mice than in age-matched WT mice (Fig. 7).
Figure 6.
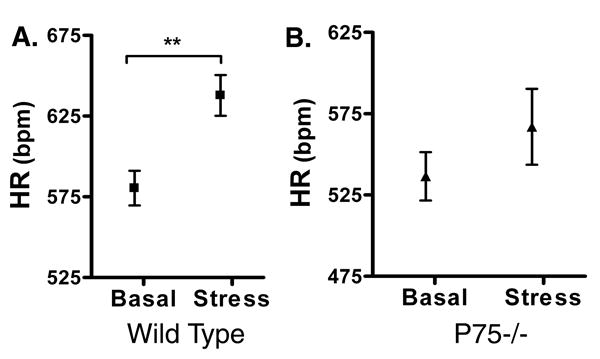
Restraint stress increases heart rate in wildtype, but not p75-/- mice. Heart rates were monitored in conscious WT (A) and p75-/- mice (B) before (basal) and after restraint stress (Stress). Data shown are mean±sem, n= 4 WT, n=6 P75-/-; ** p<0.01.
Figure 7.
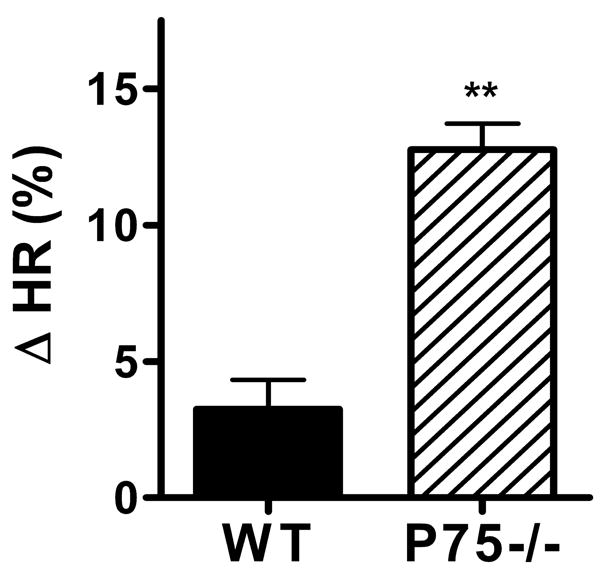
Heart rate response to dobutamine. Ganglionic transmission was blocked with hexamethonium (average HR after hexamethonium: WT 538±18 bpm, p75-/- 514±19 bpm), and then the beta adrenergic agonist dobutamine (20 μg/kg) was infused. Dobutamine stimulated a larger heart rate increase in p75-/- mice (hatched bar) than wild type mice (solid bar). Data are mean±sem, n=4 WT, n=6 P75-/-, **p< 0.01.
The augmented heart rate response to an injected beta agonist, coupled with the lack of response to endogenous NE release following stress, suggested that noradrenergic transmission was impaired in the right atria. This might result entirely from the decreased innervation density or altered pattern of innervation, or might also be due to impaired NE synthesis or release. Therefore, we measured NE content in right and left atria from wild type and p75-/- mice. Surprisingly, NE levels were higher in the atria of p75-/- mice compared to wild type mice (Fig. 8). This suggested that the lack of increased heart rate following stress-induced activation of the sympathetic nervous system was due to impaired NE release in p75-/- mice. Therefore, we used a maximally active dose of tyramine (200 μg/kg) to stimulate NE release from sympathetic nerve terminals. Ganglionic transmission was blocked with hexamethonium (5 mg/kg) to prevent reflex bradycardia following the NE-induced increase in arterial pressure. Tyramine infusion induced tachycardia as expected in both WT and p75-/- mice, but it generated a smaller heart rate increase in p75-/- mice than WT mice (Fig. 9). Given the higher levels of NE in the atria of p75-/- mice, this suggests that impaired NE release alone does not account for the low heart rate in p75-/- mice, and that the lack of sympathetic fibers or aberrant distribution of sympathetic fibers relative to the SA node has significant functional consequences.
Figure 8.
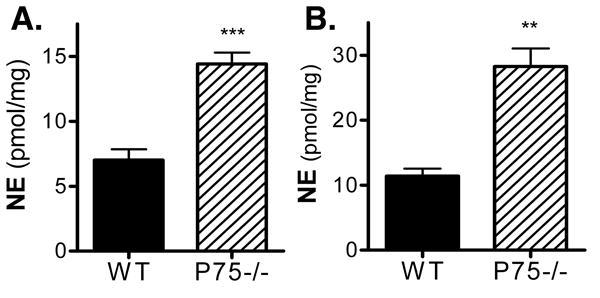
Higher levels of norepinephrine in the atria of p75-/- mice. NE content (pmol/mg protein) was measured in the left (A) and right (B) atria of WT (solid bars) and P75-/-mice (hatched bars). Data are mean±sem, n=5 WT, n=6 P75-/-, ** p<0.0005, *** p<0.0002.
Figure 9.
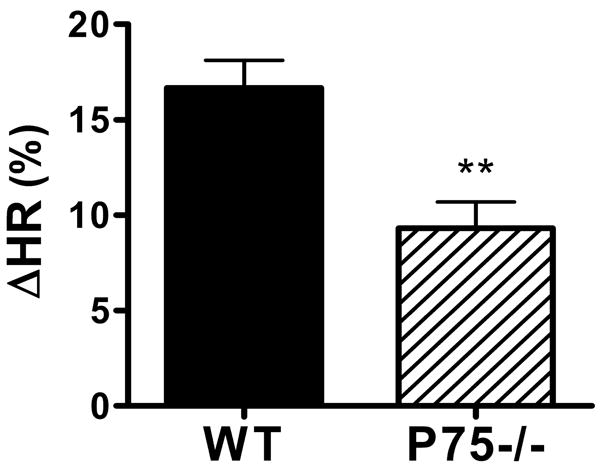
Heart rate response to tyramine. Ganglionic transmission was blocked with hexamethonium, bringing heart rates to a similar level in both genotypes (WT 499±19, P75-/- 504±9). Tyramine (200 μg/kg) was infused to maximally stimulate release of NE from sympathetic nerve terminals, triggering increased heart rate in wild type (solid bars) and P75-/- (hatched bars) mice. Data are mean±sem, n=5 WT, n=6 P75-/-, ** p <0.005
Discussion
We examined the cardiac innervation of mice lacking the p75 neurotrophin receptor and found that the timing and pattern of innervation in the right atria was altered significantly compared to age-matched control mice. Sympathetic fibers were scarce in p75-/- mice shortly after birth, but innervation density increased in comparison to wild-type controls during postnatal development, resulting in a relative hyperinnervation by P28. This innervation pattern was not maintained in adult mice and did not follow the normal developmental profile of sympathetic innervation that we observed in wild-type animals, defining a role for the p75 receptor in the development and stability of cardiac sympathetic innervation. Alterations in cardiac sympathetic innervation had consequences for cardiac function as p75-/- animals had lower baseline heart rates and an attenuated stress response. p75-deficient atria contained higher NE levels, but changes in release properties and/or fiber loss and redistribution within the atria resulted in decreased availability of NE within the right atria in the knockout animals.
Developmental changes in cardiac innervation patterns suggest that p75 may regulate the stability of sympathetic fibers. Such an effect could help to explain published studies showing variable effects of p75 loss on target innervation in different tissues and at different developmental times. p75-/- mice have normal sympathetic innervation of the iris and salivary gland but deficits in the pineal gland, meningeal arteries and sweat pads (Kawaja, 1998; Lee et. al., 1992; Lee et. al., 1994). Cardiac innervation is decreased in embryonic E16 mice, but appears normal in P10-14 animals (Glebova and Ginty, 2004; Jahed and Kawaja, 2005). We have shown a decrease in fiber density in P4 p75-deficient mice compared to wild-type mice with an increase by P28-35, suggesting a developmental delay in fiber in-growth and disruption of normal mechanisms that regulate fiber growth within the heart. While the wildtype animals show a decrease in innervation over time, the knockouts showed a delay in this normal developmental decrease, which is likely to result in the relatively higher innervation relative to the wildtype at P28. The knockout mice do eventually show a developmental decrease resulting in a final adult innervation density that is less than the wildtype.
Disruptions in the growth dynamics of sympathetic fibers in the knockout mice suggest a role for p75 in the growth and patterning of sympathetic fibers within the heart. This role for p75 is supported by the observation of disorganized distribution of sympathetic fibers in the atria of p75-/- hearts. Disorganization of sympathetic innervation has also been observed in the sweat pads of p75-deficient mice where some sweat pads show functional loss of sympathetic innervation, while nearby pads have normal responses (Lee et. al., 1994).
The depressed baseline heart rate in adult mice lacking p75 is likely due to the loss of sympathetic transmission. Heart rate remained lower in p75 knockouts compared to age-matched controls even after administration of the muscarinic antagonist atropine to inhibit parasympathetic transmission. Although p75-/- mice maintain a lower heart rate following atropine injection the heart rate in each genotype was raised approximately 3.4%, which is consistent with other studies in the mouse (Desai et. al., 1997; Janssen et. al., 2000), suggesting that parasympathetic tone in p75-/- mice is normal. This finding is also consistent with the normal parasympathetic innervation pattern seen in the knockout animals. Pharmacological studies indicate that basal sympathetic tone has a larger impact on heart rate than parasympathetic tone in the mouse (Just et. al., 2000). Therefore, it is not surprising that decreased sympathetic innervation density in the right atria, which contains the SA node, would result in a lower basal heart rate.
The lack of significant tachycardia following a 10 minute restraint stress is also consistent with a loss of sympathetic transmission in the heart. However, restraint stress stimulates release of epinephrine into the circulation in addition to stimulating NE release (Popper et. al., 1977). One might expect that this circulating epinephrine would be sufficient to elevate heart rate even if sympathetic transmission in the heart was impaired. Sino-atria node cells and the cardiac conduction system are excitable cells and have many neuron-like characteristics. The lack of a heart rate response following stress raised the possibility that p75 was involved in SA node function, and that cardiac responsiveness was impaired in these mice. Intrinsic heart rate in the absence of neural transmission was similar in p75-/- mice and WT controls, suggesting that the SA node is functional. We used the short-acting beta agonist dobutamine to determine if cardiac responsiveness was impaired in p75-/- mice, and found that dobutamine stimulated a larger increase in heart rate in p75-/- than in wild type mice. This type of increased response to beta agonists is characteristic of cardiac sympathetic denervation and results from upregulation of cardiac beta receptors (Vatner et. al., 1985). An increased heart rate response to dobutamine is typical of the denervated heart after transplantation (Akosah et. al., 1999), while a decreased response to dobutamine is characteristic of heart failure (reviewed by Bristow et. al., 1990; Vatner et. al., 1996). Thus, the augmented heart rate response to dobutamine observed in p75-/- mice is consistent with the lack of sympathetic innervation and impaired noradrenergic transmission in the heart.
Given the decreased density of sympathetic nerve fibers in the adult right atria, the lack of heart rate change following restraint stress, and an increased response to dobutamine, we expected that NE content would be low in the right atria of p75-/- compared to wild type mice. Surprisingly, NE content in the right atria of p75 deficient mice was elevated 3-fold compared to age-matched controls. Thus, fewer sympathetic fibers were producing significantly more NE. The elevated NE content, coupled with functional changes that were consistent with denervation, suggested that NE release was impaired in the cardiac innervation of p75-/- mice.
If NE levels are increased, but release is impaired in p75-/- mice, we would expect that treatments that by-pass normal release would result in enhanced tachycardia. Thus, to determine if NE release was deficient in these mice, tyramine was used to stimulate non-exocytotic release of NE from sympathetic terminals. Since tyramine-induced release of NE from vascular sympathetic neurons stimulates a spike in blood pressure, ganglionic transmission was blocked to prevent reflex bradycardia. However, tyramine-induced release of NE triggered a smaller increase in heart rate in p75-/- mice than in WT mice. This unexpected result suggests that impaired release alone does not account for the differences in sympathetic control of heart rate in p75-/- mice. Although NE content was elevated in p75-deficient atria, it is possible that only a fraction of the NE present in p75-/- sympathetic fibers is in a pool accessible to release by tyramine (Langeloh and Trendelenburg, 1987). Thus, the decreased density and altered distribution of sympathetic fibers in the right atria may both contribute to the lower heart rate response. This interpretation is consistent with studies in humans showing that infusion of tyramine onto the SA node does not stimulate tachycardia when the heart is fully denervated, but re-innervation of the SA node following transplantation is identified by the onset of a tyramine-induced increase in heart rate and NE release (Odaka et. al., 2001; Wilson et. al., 1993; Wilson et. al., 2000).
In conclusion, we have examined the development and function of the cardiac innervation in mice lacking the p75 neurotrophin receptor. The parasympathetic innervation of the right atria appeared normal, but there was a developmental lag in axon outgrowth in the right atria, and a deficit in the maintenance of sympathetic axon arbors in adult mice. This resulted in fewer sympathetic fibers that were distributed unevenly so that much of the right atria had no sympathetic innervation while other regions had dense innervation. These changes resulted in a functional decrease in sympathetic transmission resulting in lower basal heart rate and impaired stress response despite elevated cardiac NE content. Thus, the p75 receptor regulates the development and stability of sympathetic innervation of the heart and the physical distribution of sympathetic fibers appears to have critical function consequences.
Acknowledgments
We would like to thank Eva Nokes for technical assistance. This work was sponsored by NIH HL68231 (BAH), NIH NS40168 and HD042716 (SJB), and P30 NS45713 supporting Core Facilities for Neuroscience at Brandeis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airaksinen MS, Saarma M. The GDNF Family: Signaling, Biological Functions and Therapeutic Value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Akosah KO, Denlinger B, Mohanty PK. Safety Profile and Hemodynamic Responses to beta-Adrenergic Stimulation by Dobutamine in Heart Transplant Patients. Chest. 1999;116:1587–1592. doi: 10.1378/chest.116.6.1587. [DOI] [PubMed] [Google Scholar]
- Belliveau DJ, Krivko I, Kohn J, Lachance C, Pozniak C, Rusakov D, Kaplan D, Miller FD. NGF and neurotrophin-3 both activate TrkA on sympathetic neurons but differentially regulate survival and neuritogenesis. J Cell Biol. 1997;136:375–388. doi: 10.1083/jcb.136.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birren SJ, Lo L, Anderson DJ. Sympathetic neuroblasts undergo a developmental switch in trophic dependence. Development. 1993;119:597–610. doi: 10.1242/dev.119.3.597. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Hershberger RE, Port JD, Gilbert EM, Sandoval A, Rasmussen R, Cates AE, Feldman AM. Beta-adrenergic pathways in nonfailing and failing human ventricular myocardium. Circulation. 1990;82:I12–I25. [PubMed] [Google Scholar]
- Desai KH, Sato R, Schauble E, Barsh GS, Kobilka BK, Bernstein D. Cardiovascular indexes in the mouse at rest and with exercise: new tools to study models of cardiac disease. Am J Physiol Heart Circ Physiol. 1997;272:H1053–H1061. doi: 10.1152/ajpheart.1997.272.2.H1053. [DOI] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Friedman WJ, Black IB. NT-3 stimulates sympathetic neuroblast proliferation by promoting precursor survival. Neuron. 1993;11:1101–1111. doi: 10.1016/0896-6273(93)90223-e. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Zhang H, Landis S, Smeyne RJ, Silos-Santiago I, Barbacid M. TrkA, but not TrkC, receptors are essential for survival of sympathetic neurons in vivo. J Neurosci. 1996;16:6208–6218. doi: 10.1523/JNEUROSCI.16-19-06208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis N, Farinas I, Brennan C, Rivas-Plata K, Backus C, Reichardt L, Landis S. NT-3, like NGF, is required for survival of sympathetic neurons, but not their precursors. Dev Biol. 1999;210:411–427. doi: 10.1006/dbio.1999.9269. [DOI] [PubMed] [Google Scholar]
- Francis NJ, Landis SC. Cellular and molecular determinants of sympathetic neuron development. Annu Rev Neurosci. 1999;22:541–566. doi: 10.1146/annurev.neuro.22.1.541. [DOI] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD. Heterogeneous Requirement of NGF for Sympathetic Target Innervation In Vivo. J Neurosci. 2004;24:743–751. doi: 10.1523/JNEUROSCI.4523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- Hiltunen JO, Laurikainen A, Airaksinen MS, Saarma M. GDNF family receptors in the embryonic and postnatal rat heart and reduced cholinergic innervation in mice hearts lacking ret or GFRalpha2. Dev Dyn. 2000;219:28–39. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1031>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Jahed A, Kawaja MD. The influences of p75 neurotrophin receptor and brain-derived neurotrophic factor in the sympathetic innervation of target tissues during murine postnatal development. Auton Neurosci. 2005;118:32–42. doi: 10.1016/j.autneu.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Janssen BJA, Leenders PJA, Smits JFM. Short-term and long-term blood pressure and heart rate variability in the mouse. Am J Physiol Regul Integr Comp Physiol. 2000;278:R215–R225. doi: 10.1152/ajpregu.2000.278.1.R215. [DOI] [PubMed] [Google Scholar]
- Just A, Faulhaber J, Ehmke H. Autonomic cardiovascular control in conscious mice. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2214–R2221. doi: 10.1152/ajpregu.2000.279.6.R2214. [DOI] [PubMed] [Google Scholar]
- Kawaja MD. Sympathetic and sensory innervation of the extracerebral vasculature: roles for p75NTR neuronal expression and nerve growth factor. J Neurosci Res. 1998;52:295–306. doi: 10.1002/(SICI)1097-4547(19980501)52:3<295::AID-JNR6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Langeloh A, Trendelenburg U. The mechanism of the 3H-noradrenaline releasing effect of various substrates of uptake1: role of monoamine oxidase and of vesicularly stored 3H-noradrenaline. Naunyn Schmiedebergs Arch Pharmacol. 1987;336:611–620. doi: 10.1007/BF00165751. [DOI] [PubMed] [Google Scholar]
- Lee KF, Bachman K, Landis S, Jaenisch R. Dependence on p75 for innervation of some sympathetic targets. Science. 1994;263:1447–1449. doi: 10.1126/science.8128229. [DOI] [PubMed] [Google Scholar]
- Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Angeletti PU. Nerve growth factor. Physiol Rev. 1968;48:534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Booker B. Destruction of the Sympathetic Ganglia in mammals by an Antiserum to a Nerve Growth Protein. Proc Natl Acad Sci U S A. 1960;46:384–391. doi: 10.1073/pnas.46.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odaka K, von Scheidt W, Ziegler SI, Ueberfuhr P, Nekolla SG, Reichart B, Bengel FM, Schwaiger M. Reappearance of Cardiac Presynaptic Sympathetic Nerve Terminals in the Transplanted Heart: Correlation Between PET Using 11C-Hydroxyephedrine and Invasively Measured Norepinephrine Release. J Nucl Med. 2001;42:1011–1016. [PubMed] [Google Scholar]
- Popper CW, Chiueh CC, Kopin IJ. Plasma catecholamine concentrations in unanesthetized rats during sleep, wakefulness, immobilization and after decapitation. J Pharmacol Exp Ther. 1977;202:144–148. [PubMed] [Google Scholar]
- Tessarollo L, Tsoulfas P, Donovan MJ, Palko ME, Blair-Flynn J, Hempstead BL, Parada LF. Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc Natl Acad Sci U S A. 1997;94:14776–14781. doi: 10.1073/pnas.94.26.14776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatner DE, Lavallee M, Amano J, Finizola A, Homcy CJ, Vatner SF. Mechanisms of supersensitivity to sympathomimetic amines in the chronically denervated heart of the conscious dog. Circ Res. 1985;57:55–64. doi: 10.1161/01.res.57.1.55. [DOI] [PubMed] [Google Scholar]
- Vatner DE, Sato N, Ishikawa Y, Kiuchi K, Shannon RP, Vatner SF. Beta-adrenoceptor desensitization during the development of canine pacing-induced heart failure. Clin Exp Pharmacol Physiol. 1996;23:688–692. doi: 10.1111/j.1440-1681.1996.tb01760.x. [DOI] [PubMed] [Google Scholar]
- Wilson RF, Laxson DD, Christensen BV, McGinn AL, Kubo SH. Regional differences in sympathetic reinnervation after human orthotopic cardiac transplantation. Circulation. 1993;88:165–171. doi: 10.1161/01.cir.88.1.165. [DOI] [PubMed] [Google Scholar]
- Wilson RF, Johnson TH, Haidet GC, Kubo SH, Mianuelli M. Sympathetic Reinnervation of the Sinus Node and Exercise Hemodynamics After Cardiac Transplantation. Circulation. 2000;101:2727–2733. doi: 10.1161/01.cir.101.23.2727. [DOI] [PubMed] [Google Scholar]
- Zampieri N, Chao MV. Mechanisms of neurotrophin receptor signalling. Biochem Soc Trans. 2006;34:607–611. doi: 10.1042/BST0340607. [DOI] [PubMed] [Google Scholar]