Abstract
Objective
To evaluate the hypothesis that cytokine levels are associated with miscarriage risk using serum samples collected prior to report of miscarriage.
Design
A nested case-control study.
Setting
Biospecimens from the multi-site Collaborative Perinatal Project, University of Florida laboratory assessment of IL-1ra, IL-1β, IL-4, IL-6, IFN-γ, TNF-α, TPO and G-CSF.
Patients
were obtained from cases of miscarriage (N=439) matched to controls (N=373) by gestational age (GA) at sample collection.
Intervention
None.
Main outcome measures
,Miscarriage.
Results
Increased risk of miscarriage was associated with elevated TPO (AOR: 1.16, 95% confidence interval (CI):1.00 – 1.36) and decreased G-CSF (AOR: 0.78, 95% CI 0.64 – 0.95). When analysis was restricted to samples collected more than 35 days prior to miscarriage, the effect of G-CSF was not observed (AOR: 0.96, 95% CI 0.72 – 1.28), whereas increased risk related to higher TPO remained.
Conclusions
Circulating levels of TPO may be associated with increased risk of miscarriage.
Keywords: cytokines, epidemiology, hematopoiesis, miscarriage, placentation
Introduction
Human reproduction is a complex and highly regulated process. In humans this process is prone to failures. Fecundability has been estimated to be less than 30% (1). Estimates of the proportion of recognized pregnancies that end in miscarriage range from 15 to 31% (1,2). Although some causes of miscarriage have been identified, the etiology is poorly understood (1,2).
Immune-related cytokines are among the molecules recognized to play key roles in pregnancy (3–5). These cytokines are primarily produced by cells of the immune system, but are also expressed at the maternal-fetal interface by decidua and trophoblast cells. Among their major regulatory functions, cytokines participate in differentiation of naïve T-helper cells into T-helper type (Th)-1 cells, or Th-2 cells. Murine and human studies have suggested a shift toward Th2 in successful pregnancy, though human studies have been less conclusive (5–15). Local production of cytokines by uterine and placental cells is considered to influence embryo implantation, decidualization, and placentation (3,16).
In addition, growth factors like vascular endothelial growth factor (VEGF), thrombopoietin (TPO), granulocyte colony stimulating factor (G-CSF) and granulocyte macrophage colony stimulating factor (GM-CSF) have been observed in various stages of pregnancy (4,17). VEGF and TPO are involved in angiogenesis and thrombopoiesis. These factors have been investigated in the context of preeclampsia, and both are critical for successful pregnancy (4,17).
Previous studies suggesting altered cytokine production in miscarriage have included small study populations, evaluated few factors, and/or utilized samples collected at diagnosis of miscarriage, subsequent to fetal demise (5,11,18–23). Given the ambiguous findings regarding the association among Th1 and Th2 cytokines and miscarriage, the complex biological interplay between these factors, and the lack of information regarding the relation between TPO and miscarriage, we evaluated the hypothesis that cytokine levels are altered in miscarriage using a large number of serum samples collected prior to report of miscarriage for simultaneous assessment of multiple cytokines. In addition to a nested case-control approach, we utilized serum samples from women who experienced pregnancies that ended in miscarriage and normal pregnancies for a case-crossover analysis. These within-woman comparisons address factors that might differ between women who experience miscarriage and those who do not.
Materials and Methods
Study Design and Population
Subjects were selected from the Collaborative Perinatal Project (CPP) cohort. The CPP was a multi-site prospective study conducted from 1959 to 1974 that enrolled participants at presentation for prenatal care, and is described in detail elsewhere (24). Serum samples were collected at entry to the CPP and at subsequent bimonthly visits and stored at −20°C. Gestation was estimated using self-reported date of last menstrual period. Miscarriage was defined as involuntary loss of a clinically recognized intrauterine pregnancy at less than 140 days of gestation. Subjects with serum samples collected less than 10 days prior to miscarriage (n = 355), or with unavailable serum samples (n = 36) were excluded. After exclusions, 439 cases of miscarriage were selected for this study.
Control selection followed a modified nested case-control design. Control serum samples were matched to cases by gestational age (GA) at sample collection to address underlying biologic variability of cytokine levels across gestation. Among women with eligible case samples, some also experienced normal pregnancies in the CPP; serum samples from these ‘crossover’ women were preferentially sampled, allowing for the addition of a case-crossover analysis to address time-invariant confounding (25). The case-control analysis was limited to independent pregnancies—excluding normal pregnancies from 86 women—and therefore included serum samples for 439 cases and 373 controls; the case-crossover analysis included 186 serum samples from 86 case pregnancies and 100 serum samples from the control pregnancies of the same 86 women.
Exposure Assessment
Serum cytokine levels were measured using the multiplex Fluorokine MAP Human Cytokine detection system (R&D Systems, Inc. Minneapolis, MN) as previously described (26). Briefly, the assays use 96-well plates with 50 µl of sera in duplicates in a sandwich ELISA-based approach. The solid phase consists of fluorescent beads covalently linked with cytokine-specific monoclonal antibodies allowing capture of each cytokine and corresponding biotinylated antibody. After addition of streptavidin-phycoerythrin, intensity is measured using the Luminex 100 IS system (Luminex Corp, Austin, TX).
Preliminarily, we evaluated use of CPP serum samples by comparing cytokine levels with serum samples freshly collected at first trimester from healthy term pregnancies, as well as to levels of IL-6 measured by standard ELISA. The levels of most measured cytokines were consistent across these groups. The feasibility assessment is discussed in more detail in the Appendix. Multiplex assays evaluated concentrations of IL-1 receptor antagonist (ra), IL-1β, IL-4, IL-6, γ-interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), G-CSF and TPO.
Samples were randomly ordered by case status and batches organized by GA at sample collection. Case samples and matched controls were analyzed in the same batch. Because specimens had been collected previously and identifying information removed, the Office of Human Subjects Research at the NIH and IRB at the University of Florida determined this study as exempt from further IRB review.
Risk Factor Assessment
Maternal age, race, and smoking status were considered as possible confounding factors based on having a suspected association with miscarriage, and a possible relation with cytokine levels (2,27–29). Information on risk factors from the CPP examinations and interviews were recorded by study staff at entry into the study and at subsequent visits to prenatal care providers. Smoking was self-reported as cigarettes per day. Information on parity, gravidity, and previous pregnancy outcomes was abstracted from medical records. Information regarding factors measured at multiple time points was taken from the visit concurrent with sample collection, usually corresponding to the initial visit.
Statistical Analysis
Demographics, risk factors, and outcomes were evaluated among the 812 women comprising the study sample. For continuous variables, mean and standard error were calculated; for discrete variables, proportions were calculated. The study sample was grouped by case status into 439 case samples and 373 control samples, and bivariate relations between potential confounders and miscarriage were evaluated. For continuous variables Student t-tests were utilized and chi-square tests were used to compare categorical variables. Values for the biomarkers were divided by their standard deviation within the controls to yield standardized measures.
Conditional logistic regression models were utilized to estimate risk of miscarriage matched on GA in weeks at time of sample provision. These models were used to obtain crude and adjusted estimates of the odds ratio (OR). Specification of the final multivariable model was determined from bivariate analyses and/or biologic relevance (30), with confounding determined by a change in estimate of at least ten percent (31). Estimated adjusted odds ratios (AOR) correspond to the effect of one standard deviation change in biomarker on odds of miscarriage. Conditional logistic regression models were also used for analysis of the self-matched case-crossover data, with each individual woman serving as the matching factor.
To evaluate the importance of the time between sample collection and miscarriage on estimates, observations were classified in overlapping categories—no restriction (i.e., not beyond the exclusion criteria), at least 14 days, at least 21 days, at least 28 days, and the most restrictive subset, at least 35 days from sample collection to miscarriage.
Results
Characteristics of the 812 women in the study are shown in Table I. Mean maternal age at the time of sample provision was significantly higher among miscarriage cases than among controls (27.0 vs. 25.5 years, respectively). Additionally, a significantly higher proportion of cases had a history of three or more miscarriages (6.6% vs. 1.6%).
Table 1.
Descriptive characteristics of the study population by case status (n = 812)
| Cases Mean (SE) | Controls Mean (SE) | P-value | |
|---|---|---|---|
| Maternal age (years) | 27.0 (0.3) | 25.5 (0.3) | < 0.001 |
| Maternal BMI | 22.6 (0.2) | 22.5 (0.2) | 0.80 |
| n (%) | n (%) | ||
| Family income | |||
| (median income in 1961 = $5,700) | 0.63 | ||
| <$1,000 | 3 (0.9) | 7 (1.9) | |
| $1,000 – $2,999 | 72 (21.8) | 67 (18.7) | |
| $3,000 – $4,999 | 120 (36.4) | 122 (34.0) | |
| $5,000 – $6,999 | 66 (20.0) | 84 (23.4) | |
| $7,000 – $8,999 | 38 (11.5) | 42 (11.7) | |
| $9,000 + | 31 (9.4) | 37 (10.3) | |
| Cigarettes smoked per day at delivery | 0.16 | ||
| 0 | 202 (51.6) | 215 (58.0) | |
| 1 – 10 | 93 (23.4) | 84 (22.6) | |
| 11 – 20 | 72 (18.1) | 50 (13.5) | |
| 21 + | 30 (7.6) | 22 (5.9) | |
| Maternal race | 0.26 | ||
| White | 270 (61.5) | 247 (66.4) | |
| Black | 145 (33.0) | 103 (27.7) | |
| other | 24 (5.5) | 22 (5.9) | |
| Prior pregnancy losses | < 0.001 | ||
| 0 | 202 (50.9) | 215 (58.0) | |
| 1 | 93 (23.4) | 84 (22.6) | |
| 2 | 72 (18.1) | 50 (13.5) | |
| 3 + | 30 (7.6) | 22 (5.9) |
Conditional logistic regression models were used to estimate crude and adjusted ORs (Table II). In the multivariable model, which included terms for all cytokines as well as maternal age, the AOR estimate for TPO was 1.16 (95% CI 1.00 – 1.36). The AOR estimate for IL-1β was 0.76 (95% CI 0.59 – 0.97) and for G-CSF was 0.78 (95% CI 0.64 – 0.95).
Table 2.
Crude and adjusted odds ratio estimates from conditional logistic regression models of risk of miscarriage.
| Unadjusted models | Adjusted model a | ||||
|---|---|---|---|---|---|
| OR | [95% CI] | OR | [95% CI] | ||
| Th1-type cytokines b | IL-1β | 0.90 | [0.77, 1.05] | 0.76 | [0.59, 0.97] |
| IFN-γ | 1.01 | [0.87, 1.18] | 1.09 | [0.91, 1.30] | |
| TNF-α | 0.92 | [0.79, 1.08] | 0.93 | [0.77, 1.13] | |
| Th2-type cytokines b | IL-1ra | 1.01 | [0.89, 1.14] | 1.06 | [0.90, 1.24] |
| IL-4 | 1.00 | [0.86, 1.17] | 1.05 | [0.88, 1.25] | |
| IL-6 | 0.99 | [0.86, 1.15] | 1.19 | [0.96, 1.48] | |
| Growth factors b | G-CSF | 0.84 | [0.72, 0.99] | 0.78 | [0.64, 0.95] |
| TPO | 1.09 | [0.95, 1.25] | 1.16 | [1.00, 1.36] | |
Abbreviations: IL, interleukin; RA, receptor antagonist; G-CSF, granulocyte colony stimulating factor; IFN, interferon; TNF, tumor necrosis factor; TPO, thrombopoietin
Adjusted model included terms for all cytokines, maternal age, and gestational age at sample collection
Biomarkers were standardized by dividing assay-determined concentration by the standard deviation among controls
Table III displays estimates from multivariable models derived from increasingly restrictive non-mutually exclusive subsets of the study population based on time between sample provision and pregnancy outcome—no restriction, more than 14 days, more than 21 days, more than 28 days, and more than 35 days. Regardless of interval, confidence intervals included the null value of 1 for IFN-γ, IL-1ra, IL-4, and TNF-α. The AOR estimates for IL-1β progressively moved further from the null as the time restriction went from no restriction (OR: 0.76, 95% CI 0.59 – 0.97) to greater than 35 days (OR: 0.51, 95% CI 0.28 – 0.90). OR estimates for G-CSF approached 1 with increasing time between sample collection and miscarriage, with an AOR of 0.96 (95% CI 0.72, 1.28) when the analysis was restricted to serum samples taken more then 35 days prior to miscarriage. Estimates for IL-6 and TPO were largely stable, though the AOR for IL-6 was largest in the analysis restricted to samples collected more than 35 days prior to miscarriage.
Table 3.
Adjusted odds ratio estimates a for cytokines within subsets of the study population defined by the interval between sample collection and pregnancy outcome, from conditional logistic regression models of miscarriage risk.
| Interval ≥ 10 days | Interval > 14 days | Interval > 21 days | Interval > 28 days | Interval > 35 days | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (439 cases, 373 controls) | (350 cases, 373 controls) | (259 cases, 373 controls) | (197 cases, 371 controls) | (135 cases, 371 controls) | ||||||
| AOR | [95% CI] | AOR | [95% CI] | AOR | [95% CI] | AOR | [95% CI] | AOR | [95% CI] | |
| IL-1β b | 0.76 | [0.59, 0.97] | 0.72 | [0.57, 0.92] | 0.65 | [0.46, 0.91] | 0.58 | [0.38, 0.90] | 0.51 | [0.28, 0.90] |
| IFN-γ b | 1.09 | [0.91, 1.31] | 1.03 | [0.85, 1.24] | 0.94 | [0.76, 1.16] | 0.97 | [0.77, 1.22] | 0.98 | [0.74, 1.28] |
| TNF-α b | 0.94 | [0.78, 1.13] | 0.93 | [0.77, 1.14] | 0.96 | [0.78, 1.18] | 0.95 | [0.75, 1.21] | 0.90 | [0.67, 1.22] |
| IL-1RA b | 1.06 | [0.91, 1.25] | 1.06 | [0.90, 1.25] | 1.05 | [0.87, 1.27] | 1.02 | [0.81, 1.29] | 0.98 | [0.73, 1.31] |
| IL-4 b | 1.05 | [0.89, 1.25] | 1.08 | [0.90, 1.29] | 1.13 | [0.93, 1.38] | 1.07 | [0.89, 1.29] | 1.05 | [0.86, 1.29] |
| IL-6 b | 1.20 | [0.97, 1.48] | 1.22 | [0.96, 1.55] | 1.34 | [1.02, 1.75] | 1.45 | [1.04, 2.00] | 1.62 | [1.08, 2.42] |
| G-CSF b | 0.78 | [0.65, 0.95] | 0.84 | [0.68, 1.02] | 0.85 | [0.67, 1.06] | 0.91 | [0.71, 1.17] | 0.96 | [0.72, 1.28] |
| TPO b | 1.17 | [1.00, 1.36] | 1.19 | [1.01, 1.40] | 1.19 | [1.00, 1.41] | 1.19 | [0.98, 1.43] | 1.19 | [0.96, 1.48] |
Abbreviations: IL, interleukin; RA, receptor antagonist; TPO, thrombopoietin; G-CSF, granulocyte colony stimulating factor; IFN, interferon; TNF, tumor necrosis factor
Adjusted model included terms for all cytokines, maternal age, and gestational age at sample collection
Biomarkers were standardized by dividing assay-determined concentration by the standard deviation among controls
Case-crossover analysis
Table IV displays the results of the case-crossover analysis (n = 186). In this analysis, TPO was associated with increased risk of miscarriage (AOR 2.78, 95% CI 1.48 – 5.22). The AOR for IL-1β was high (3.37) but the 95% CI was wide and included 1 (0.76 – 14.84). None of the other biomarkers was significantly associated with miscarriage risk.
Table 4.
Unadjusted and adjusted case-crossover analysis: odds ratio estimates from conditional logistic regression models of risk of miscarriage among women who experienced pregnancies that ended in miscarriage and normal pregnancies (n = 186)
| Unadjusted models | Adjusted model a | ||||
|---|---|---|---|---|---|
| OR | [95% CI] | OR | [95% CI] | ||
| Th1-type cytokines b | IL-1β | 1.50 | [0.79, 2.83] | 3.37 | [0.76, 14.84] |
| IFN-γ | 1.35 | [0.94, 1.94] | 1.40 | [0.83, 2.37] | |
| TNF-α | 1.16 | [0.73, 1.86] | 0.94 | [0.38, 2.34] | |
| Th2-type cytokines b | IL-1RA | 1.14 | [0.81, 1.61] | 0.52 | [0.25, 1.08] |
| IL-4 | 0.90 | [0.51, 1.58] | 0.97 | [0.42, 2.24] | |
| IL-6 | 1.60 | [0.55, 4.61] | 0.94 | [0.11, 8.29] | |
| Growth factors b | G-CSF | 0.79 | [0.52, 1.19] | 0.65 | [0.33, 1.26] |
| TPO | 1.62 | [1.13, 2.34] | 2.78 | [1.48, 5.22] | |
Abbreviations: IL, interleukin; RA, receptor antagonist; G-CSF, granulocyte colony stimulating factor; IFN, interferon; TNF, tumor necrosis factor; TPO, thrombopoietin
Adjusted model included terms for all cytokines, maternal age, and gestational age at sample collection
Biomarkers were standardized by dividing assay-determined concentration by the standard deviation among controls
Discussion
To our knowledge, this is the largest study to assess the circulating levels of cytokines and growth factors in relation to risk of miscarriage using prospectively collected samples. We observed an increased risk associated with higher levels of TPO and with lower levels of IL-1β and G-CSF. The association with miscarriage persisted for IL-1β and TPO even when assessment was restricted to those who provided serum 35 days or more prior to experiencing miscarriage, suggesting a causal role in miscarriage. In contrast, the protective effect of G-CSF observed in the analysis unrestricted by the interval between sample collection and miscarriage was not observed with more restrictive definitions of this interval. In a case-crossover analysis, which matched serum samples from case pregnancies to serum samples from normal pregnancies of the same women, elevated TPO, but not lower levels of IL-1β or of G-CSF, was significantly associated with increased risk of miscarriage.
The observation of increased risk of miscarriage associated with higher circulating levels of TPO was independent of the time between sample collection and miscarriage, and was also observed in within-woman comparisons of TPO levels in miscarriage and normal pregnancy. Whether altered circulating levels of TPO reflect local production at the feto-placental interface has yet to be established, though a previous study reported elevated serum TPO during normal pregnancy compared to non-pregnant levels (32), Although the biological significance of increased TPO level in women experiencing miscarriage requires further investigation, TPO is the primary growth-factor for the megakaryocyte lineage and may function as a non-redundant hematopoietic cytokine throughout pregnancy (33). Thrombocytosis, commonly observed in preterm infants, has been associated with elevated circulating levels of TPO resulting from low platelet expression of TPO receptor that persists until one month after birth, reducing TPO clearance. High levels of free TPO in blood is believed to promote platelet production from megakaryocytes or their progenitors in bone marrow, resulting in thrombocytosis in preterm infants (34). Additionally, TPO acting through hypoxia inducible factor-1 has been shown to promote the expression of vascular endothelial growth factor (VEGF), a potent mitogen and the primary factor involved in angiogenesis (33). Since angiogenesis is critical in placentation and highly regulated by VEGF and oxygen pressure (35), elevated TPO in miscarriage cases relative to controls may indicate that TPO exerts its effect by mimicking hypoxia that eventually leads to fetal death.
We also observed a protective effect of G-CSF in the main analysis. However, in models restricted to subsets of the population defined by the time between sample collection and miscarriage, the protective effect disappeared as the interval increased. Thus, the association of G-CSF with miscarriage risk may be acute, and would only be observed in samples collected close to miscarriage; alternately, altered G-CSF levels may be a consequence rather than cause of the miscarriage. It is unclear why G-CSF production might be reduced in response to miscarriage. The AORs for G-CSF remained protective in all but those samples from 35 days or more prior to miscarriage (data not shown). Additionally, among case samples there was some overlap between those with short intervals to miscarriage and those where miscarriage occurred early in pregnancy, potentially confusing these two issues. G-CSF may regulate production of IFN-γ (36), critical in early pregnancy for implantation and trophoblast invasion. However, the precise physiologic role of G-CSF in pregnancy has not been established.
We observed low circulating levels of several cytokines— IL-1β, IL-4, IL-6, IFN-γ and TNF-α—and lack of consistent associations with miscarriage risk. Other traditional Th1 and Th2 cytokines, such as IL-2 and IL-10, were not detected in our preliminary study and thus not included in this investigation. Previous reports have suggested a “Th2 bias” in successful pregnancy (11,18,20,22,37–39), but results from human studies have not uniformly been in agreement; some have yielded null findings (5,23,40–42) and others observed a protective effect of certain Th1 cytokines (41,42). A possible explanation for inconsistency in human studies may be related to timing of sample collection relative to the miscarriage. Since our study involved analysis of serum samples collected at least 10 days prior to diagnosis of the miscarriage, we are better able to consider the conditions leading to demise of pregnancy than analyses carried out using samples obtained during or immediately preceding the abortive process (40,41). Our study conclusion is confined to circulating levels of these cytokines, which were not elevated at the time of sample collection prior to miscarriage. We cannot comment on local production. However, elevated serum cytokine levels are observed in certain pathologies (20).
Another limit to study inference regards our case definition; karyotype information on miscarriages is not available, and thus cases likely include losses of some chromosomally abnormal fetuses. Prior research suggests that fetal losses due to chromosomal defects tend to occur early in pregnancy (43). Most miscarriages in our study occurred during the second trimester of pregnancy, and effect estimates for TPO did not vary by gestational age at miscarriage. Nevertheless, we cannot be certain that the observed relation is not affected by karyotypic abnormalities.
Our study is among the largest studies to evaluate miscarriage risk and cytokines. We used multivariable regression models to evaluate circulating levels of cytokines and risk of miscarriage simultaneously for all measured cytokines, adjusting for maternal age (2,44). Given the pleiotropic and redundant nature of cytokines, this multivariable approach is well suited to evaluate the relations among cytokines and miscarriage. We used information regarding timing, taking advantage of the prospective nature of the CPP, to account for GA at entry to study, which might be systematically different between cases and controls. Moreover, we considered the interval between sample collection and report of the miscarriage in order to discriminate between changes in cytokine levels causally related to miscarriage and those coincidentally related to miscarriage, occurring as a response to missed miscarriage. Ultrasound studies have observed the interval between occurrence of fetal loss and its detection to be up to several weeks (45,46). Thus, interpretation of studies that have not collected samples from women prior to experiencing miscarriage is uncertain (11,19–21,37,38,46). We conducted analyses by time between sample collection and diagnosis to help address this concern. Finally, we conducted a small self-matched analysis using serum samples from women who participated in the CPP multiple times with at least one pregnancy that ended in miscarriage and at least one pregnancy that did not. Since all time-invariant factors are inherently equal when comparing specimens from the same women taken at multiple time points, the case-crossover analysis is an effective tool to address residual confounding.
In summary, we observed that higher circulating levels of TPO are significantly related to increased risk of miscarriage in models adjusting for cytokines and maternal age. Our results did not indicate a general increase in levels of Th2 cytokines in normal pregnancy. These results may help to explain the complex molecular etiology of miscarriage; however, future study is needed to better understand the molecular basis of miscarriage, and the utility of TPO as a biomarker for early detection and perhaps as a target for treatment.
Figure 1. Circulating levels of thrombopoietin in miscarriage cases and controls of the study sample by week of gestation at serum collection.
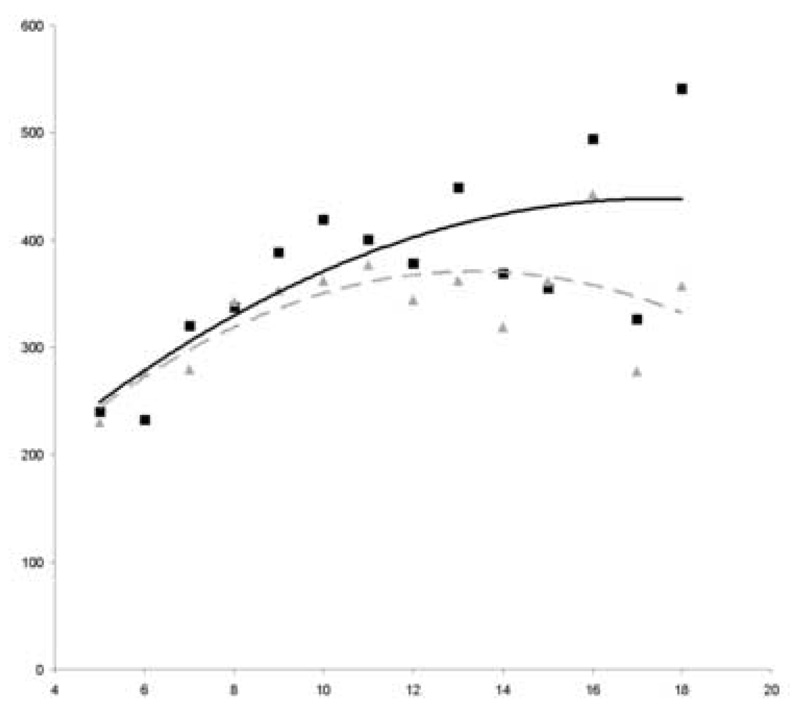
A higher level of thrombopoietin in women whose pregnancy resulted in miscarriage compared to those that did not is evident starting at approximately 8 weeks of gestation.
Y-axis label: Picograms/mL
X-axis label: Gestational age (weeks)
■ Cases
—— Trendline (cubic) in cases
▲ Controls
---- Trendline (cubic) in controls
Table A1.
Medians and interquartile range for serum cytokine concentrations, as determined by multiplex assays, in serum samples—frozen samples from the Collaborative Perinatal Project and fresh samples from the University of Florida.
| Factor | CPP samples | UFL samples– 3rd trimester | ||
|---|---|---|---|---|
| Median | IQRa | Median | IQRa | |
| IL-1a | 0 | 0 – 0 | 0 | 0 – 0 |
| IL-1β | 0.5 | 0.3 – 0.6 | 0 | 0 – 0 |
| IL-1RA | 345.5 | 263.2 – 457.5 | 100.7 | 38.8 – 167.7 |
| IL-2 | 0 | 0 – 0 | 0 | 0 – 0 |
| IL-4 | 0 | 0 – 0 | 0 | 0 – 0 |
| IL-5 | 0 | 0 – 0 | 0 | 0 – 0 |
| IL-6 | 0 | 0 – 0 | 0 | 0 – 0 |
| IL-10 | 0 | 0 – 0 | 0 | 0 – 0 |
| IL-17 | 0 | 0 – 0 | 0 | 0 – 0 |
| FGFb | 0 | 0 – 0 | 11.1 | 10.5 – 13.5 |
| G-CSF | 6.7 | 3.9 – 10.9 | 4.1 | 3.1 – 5.8 |
| GM-CSF | 0 | 0 – 0 | 0 | 0 – 0 |
| IFN-γ | 0.0 | 0.0 – 1.0 | 0 | 0 – 0 |
| TNF-α | 1.1 | 1.1 – 1.1 | 0.9 | 0.6 – 1.7 |
| TPO | 83.6 | 50.1 – 106.5 | 99.7 | 76.6 – 123.9 |
| VEGF | 1.4 | 1.1 – 2.1 | 1.4 | 1.4 – 1.8 |
| IL-6, ELISA | 2.1 | 1.5 – 3.1 | 2.5 | 2.1 – 4.3 |
IQR, interquartile range, shows the 25th – 75th percentiles
Abbreviations: CPP, Collaborative Perinatal Project; UFL, University of Florida
Table A2.
Reliability of multiplex assays for measurement of cytokine concentrations in serum samples—frozen samples from the Collaborative Perinatal Project and fresh samples from the University of Florida.
| Factor | CPP samples | UFL samples | ||
|---|---|---|---|---|
| ICC | Detected | ICC | Detected | |
| IL-1a | - | 0 | - | 0 |
| IL-1β | 0.894 | 100 | - | 0 |
| IL-1RA | 0.996 | 100 | 0.824 | 90 |
| IL-2 | - | 0 | - | 0 |
| IL-4 | - | 0 | - | 5 |
| IL-5 | - | 0 | - | 0 |
| IL-6 | - | 0 | 10 | |
| IL-10 | - | 0 | - | 0 |
| IL-17 | - | 0 | - | 0 |
| FGFb | - | 0 | 0.989 | 100 |
| G-CSF | 0.994 | 90 | 0.743 | 95 |
| GM-CSF | - | 0 | - | 0 |
| IFN-γ | - | 0 | - | 5 |
| TNF-α | 0.995 | 10 | 0.941 | 100 |
| TPO | 0.998 | 100 | 0.856 | 100 |
| VEGF | 0.912 | 20 | 0.954 | 95 |
| IL-6, ELISA | 0.918 | 100 | 0.984 | 100 |
Abbreviations: ICC, intraclass correlation statistic; CPP, Collaborative Perinatal Project; UFL, University of Florida
Acknowledgments
Financial support: This research was funded by an intramural grant from the Epidemiology Branch of the Division of Epidemiology, Statistics and Prevention Research at the National Institute of Child Health and Human Development.
Appendix
In consideration of the possible effects of the age and storage conditions of the serum samples from the CPP, a feasibility study was conducted to evaluate their use for the current study. For this study, CPP samples from women in the third trimester of normal pregnancies (“CPP samples”) were randomly selected for inclusion in the feasibility study. Additionally, samples were collected from patients at the University of Florida affiliated Shands Hospital seen at routine prenatal visits; serum from 20 women at term (“UFL samples”) were obtained after obtaining approval from the University of Florida Institutional Review Board. All samples were assayed in duplicate for cytokine levels using the R & D Systems, Inc. (Minneapolis, MN) Fluorokine MAP Multiplex Human Cytokine Panel A and the Luminex 100IS platform (Luminex Corp, Austin, TX). Samples were also tested for IL-6 using standard ELIM assays (R & D Systems, Inc., Minneapolis, MN). Samples were compared regarding the proportion above the assay sensitivity limits, absolute values of each analyte, and assay reliability (test-retest agreement).
Levels of IL-1a, IL-2, IL-4, IL-5, IL-10, IL-17 and GM-CSF were consistently not detected in both frozen CPP or UFL samples. For two cytokines, basic fibroblast growth factor (bFGF) and VEGF, levels were detectable and measured with good reliability in UFL samples but not detected in the frozen CPP samples. Conversely, IL-1β was detected in all CPP samples, albeit at very low levels, but not detected in the fresh UFL samples. Similar proportions of CPP and UFL samples measured detectable levels of IL-1RA and G-CSF. The cytokine TPO was detected in all samples and at similar levels in CPP and UFL samples. Levels of TNF-α, IFN-γ, and IL-6 were detected in few samples in both of the groups. Standard ELISA assays for IL-6 measured detectable levels in all samples at levels above the manufacturer’s reported assay sensitivity limit; median concentrations were similar between groups. Additionally, the cytokine levels we observed in the frozen CPP samples were similar to those published by other investigations using freshly collected serum (141).
Test-retest reliability, as measured by intraclass-correlation coefficients (ICC), was above 0.95 for most consistently detected analytes. Among the CPP samples, an exception was IL-1β, which had ICCs of 0.89. No ICC statistics were calculated for those cytokines not detected by the assay—IL-1a, IL-2, IL-4, IL-5, IL-6, IL-10, IL-17, IFN-γ, bFGF and GM-CSF.
Footnotes
Capsule: The authors conducted a nested case-control study of the relation of circulating cytokine levels and miscarriage. Increased risk of miscarriage was associated with elevated levels of TPO and decreased G-CSF.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 2.Bulletti C, Flamigni C, Giacomucci E. Reproductive failure due to spontaneous abortion and recurrent miscarriage. Hum Reprod Update. 1996;2:118–136. doi: 10.1093/humupd/2.2.118. [DOI] [PubMed] [Google Scholar]
- 3.Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update. 2005;11:613–630. doi: 10.1093/humupd/dmi023. [DOI] [PubMed] [Google Scholar]
- 4.Smith SK. Angiogenesis and reproduction. BJOG. 2001;108:777–783. doi: 10.1111/j.1471-0528.2001.00211.x. [DOI] [PubMed] [Google Scholar]
- 5.Lim KJ, Odukoya OA, Ajjan RA, Li TC, Weetman AP, Cooke ID. The role of T-helper cytokines in human reproduction. Fertil Steril. 2000;73:136–142. doi: 10.1016/s0015-0282(99)00457-4. [DOI] [PubMed] [Google Scholar]
- 6.Wegmann TG. Foetal protection against abortion: is it immunosuppression or immunostimulation? Ann Immunol (Paris) 1984;135D:309–312. doi: 10.1016/s0769-2625(84)81196-4. [DOI] [PubMed] [Google Scholar]
- 7.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 9.Yui J, Garcia-Lloret M, Wegmann TG, Guilbert LJ. Cytotoxicity of tumour necrosis factor-alpha and gamma-interferon against primary human placental trophoblasts. Placenta. 1994;15:819–835. doi: 10.1016/s0143-4004(05)80184-5. [DOI] [PubMed] [Google Scholar]
- 10.Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. 1993;151:4562–4573. [PubMed] [Google Scholar]
- 11.Raghupathy R, Makhseed M, Azizieh F, Omu A, Gupta M, Farhat R. Cytokine production by maternal lymphocytes during normal human pregnancy and in unexplained recurrent spontaneous abortion. Hum Reprod. 2000;15:713–718. doi: 10.1093/humrep/15.3.713. [DOI] [PubMed] [Google Scholar]
- 12.Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med. 1998;4:1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- 13.Hill JA, Choi BC. Maternal immunological aspects of pregnancy success and failure. J Reprod Fertil Suppl. 2000;55:91–97. [PubMed] [Google Scholar]
- 14.Wilczynski JR. Th1/Th2 cytokines balance--yin and yang of reproductive immunology. Eur J Obstet Gynecol Reprod Biol. 2005;122:136–143. doi: 10.1016/j.ejogrb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Chaouat G, Ledee-Bataille N, Dubanchet S, Zourbas S, Sandra O, Martal J. TH1/TH2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining the TH1/TH2 paradigm. Int Arch Allergy Immunol. 2004;134:93–119. doi: 10.1159/000074300. [DOI] [PubMed] [Google Scholar]
- 16.Saito S. Cytokine network at the feto-maternal interface. J Reprod Immunol. 2000;47:87–103. doi: 10.1016/s0165-0378(00)00060-7. [DOI] [PubMed] [Google Scholar]
- 17.Levine RJ, Karumanchi SA. Circulating angiogenic factors in preeclampsia. Clin Obstet Gynecol. 2005;48:372–386. doi: 10.1097/01.grf.0000160313.82606.d7. [DOI] [PubMed] [Google Scholar]
- 18.Arslan E, Colakoglu M, Celik C, Gezginc K, Acar A, Capar M, et al. Serum TNF-alpha, IL-6, lupus anticoagulant and anticardiolipin antibody in women with and without a past history of recurrent miscarriage. Arch Gynecol Obstet. 2004;270:227–229. doi: 10.1007/s00404-003-0547-0. [DOI] [PubMed] [Google Scholar]
- 19.Koumantaki Y, Matalliotakis I, Sifakis S, Kyriakou D, Neonaki M, Goymenou A, et al. Detection of interleukin-6, interleukin-8, and interleukin-11 in plasma from women with spontaneous abortion. Eur J Obstet Gynecol Reprod Biol. 2001;98:66–71. doi: 10.1016/s0301-2115(01)00289-5. [DOI] [PubMed] [Google Scholar]
- 20.Makhseed M, Raghupathy R, Azizieh F, Farhat R, Hassan N, Bandar A A. Circulating cytokines and CD30 in normal human pregnancy and recurrent spontaneous abortions. Hum Reprod. 2000;15:2011–2017. doi: 10.1093/humrep/15.9.2011. [DOI] [PubMed] [Google Scholar]
- 21.Paradisi R, Maldini-Casadei M, Boni P, Busacchi P, Porcu E, Venturoli S. T-helper 2-cytokine levels in women with threatened abortion. Eur J Obstet Gynecol Reprod Biol. 2003;111:43–49. doi: 10.1016/s0301-2115(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 22.Yu XW, Yan CF, Jin H, Li X. Tumor necrosis factor receptor 1 expression and early spontaneous abortion. Int J Gynaecol Obstet. 2005;88:44–48. doi: 10.1016/j.ijgo.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Zenclussen AC, Fest S, Busse P, Joachim R, Klapp BF, Arck PC. Questioning the Th1/Th2 paradigm in reproduction: peripheral levels of IL-12 are down-regulated in miscarriage patients. Am J Reprod Immunol. 2002;48:245–251. doi: 10.1034/j.1600-0897.2002.01136.x. [DOI] [PubMed] [Google Scholar]
- 24.Hardy JB. The Collaborative Perinatal Project: lessons and legacy. Ann Epidemiol. 2003;13:303–311. doi: 10.1016/s1047-2797(02)00479-9. [DOI] [PubMed] [Google Scholar]
- 25.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 26.Li R, Luo X, Pan Q, Zineh I, Archer DF, Williams RS, et al. Doxycycline alters the expression of inflammatory and immune-related cytokines and chemokines in human endometrial cells: implication in irregular uterine bleeding. Hum Reprod. 2006 doi: 10.1093/humrep/del206. [DOI] [PubMed] [Google Scholar]
- 27.Coste J, Job-Spira N, Fernandez H. Risk factors for spontaneous abortion: a case-control study in France. Hum Reprod. 1991;6:1332–1337. doi: 10.1093/oxfordjournals.humrep.a137535. [DOI] [PubMed] [Google Scholar]
- 28.Kline J, Levin B, Kinney A, Stein Z, Susser M, Warburton D. Cigarette smoking and spontaneous abortion of known karyotype. Precise data but uncertain inferences. Am J Epidemiol. 1995;141:417–427. doi: 10.1093/oxfordjournals.aje.a117444. [DOI] [PubMed] [Google Scholar]
- 29.Kline J, Stein Z. The epidemiology of spontaneous abortion. In: Huisjes HJ, Lind T, editors. Early pregnancy failure. New York, NY: Churchill Livingstone; pp. 240–256. [Google Scholar]
- 30.Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155:176–184. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 31.Grayson DA. Confounding confounding. Am J Epidemiol. 1987;126:546–553. doi: 10.1093/oxfordjournals.aje.a114687. [DOI] [PubMed] [Google Scholar]
- 32.Frolich MA, Datta S, Corn SB. Thrombopoietin in normal pregnancy and preeclampsia. Am J Obstet Gynecol. 1998;179:100–104. doi: 10.1016/s0002-9378(98)70257-1. [DOI] [PubMed] [Google Scholar]
- 33.Kirito K, Kaushansky K. Thrombopoietin stimulates vascular endothelial cell growth factor (VEGF) production in hematopoietic stem cells. Cell Cycle. 2005;4:1729–1731. doi: 10.4161/cc.4.12.2197. [DOI] [PubMed] [Google Scholar]
- 34.Nakayama H, Ihara K, Hikino S, Yamamoto J, Nagatomo T, Takemoto M, et al. Thrombocytosis in preterm infants: a possible involvement of thrombopoietin receptor gene expression. J Mol Med. 2005;83:316–320. doi: 10.1007/s00109-004-0619-z. [DOI] [PubMed] [Google Scholar]
- 35.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 36.Calhoun DA, Chegini N, Polliotti BM, Gersting JA, Miller RK, Christensen RD. Granulocyte colony-stimulating factor in preterm and term pregnancy, parturition, and intra-amniotic infection. Obstet Gynecol. 2001;97:229–234. doi: 10.1016/s0029-7844(00)01120-0. [DOI] [PubMed] [Google Scholar]
- 37.Mallmann P, Mallmann R, Krebs D. Determination of tumor necrosis factor alpha (TNF alpha) and interleukin 2 (IL 2) in women with idiopathic recurrent miscarriage. Arch Gynecol Obstet. 1991;249:73–78. doi: 10.1007/BF02390365. [DOI] [PubMed] [Google Scholar]
- 38.Rezaei A, Dabbagh A. T-helper (1) cytokines increase during early pregnancy in women with a history of recurrent spontaneous abortion. Med Sci Monit. 2002;8:CR607–CR610. [PubMed] [Google Scholar]
- 39.Wilson R, Jenkins C, Miller H, McInnes IB, Moore J, McLean MA, et al. Abnormal cytokine levels in non-pregnant women with a history of recurrent miscarriage. Eur J Obstet Gynecol Reprod Biol. 2004;115:51–54. doi: 10.1016/j.ejogrb.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 40.Schust DJ, Hill JA. Correlation of serum cytokine and adhesion molecule determinations with pregnancy outcome. J Soc Gynecol Investig. 1996;3:259–261. doi: 10.1016/s1071-5576(96)00026-3. [DOI] [PubMed] [Google Scholar]
- 41.Yamada H, Morikawa M, Furuta I, Kato EH, Shimada S, Sata F, et al. Circulating cytokines during early pregnancy in women with recurrent spontaneous abortion: decreased TNF-alpha levels in abortion with normal chromosome karyotype. Hokkaido Igaku Zasshi. 2004;79:237–241. [PubMed] [Google Scholar]
- 42.Bates MD, Quenby S, Takakuwa K, Johnson PM, Vince GS. Aberrant cytokine production by peripheral blood mononuclear cells in recurrent pregnancy loss? Hum Reprod. 2002;17:2439–2444. doi: 10.1093/humrep/17.9.2439. [DOI] [PubMed] [Google Scholar]
- 43.Kline J, Stein Z, Susser M. Conception to birth: epidemiology of prenatal development. New York, NY: Oxford University Press; 1989. [Google Scholar]
- 44.Klebanoff MA, Levine RJ, DerSimonian R, Clemens JD, Wilkins DG. Maternal serum paraxanthine, a caffeine metabolite, and the risk of spontaneous abortion. N Engl J Med. 1999;341:1639–1644. doi: 10.1056/NEJM199911253412202. [DOI] [PubMed] [Google Scholar]
- 45.Mills JL, Simpson JL, Driscoll SG, Jovanovic-Peterson L, Van Allen M, Aarons JH, et al. Incidence of spontaneous abortion among normal women and insulin-dependent diabetic women whose pregnancies were identified within 21 days of conception. N Engl J Med. 1988;319:1617–1623. doi: 10.1056/NEJM198812223192501. [DOI] [PubMed] [Google Scholar]
- 46.McFadyen IR. Missed abortion, and later spontaneous abortion, in pregnancies clinically normal at 7–12 wk. Eur J Obstet Gynecol Reprod Biol. 1985;20:381–384. doi: 10.1016/0028-2243(85)90061-9. [DOI] [PubMed] [Google Scholar]


