Abstract
Mycobacterium tuberculosis CDC 1551, a highly immunogenic outbreak strain, previously reported to have unique surface distribution of capsular polysaccharide, was used to generate novel monoclonal antibodies (mAbs) to surface mycobacterial targets. Two Immunoglobulin G1 (IgG1) mAbs, 16a1 and 16a6 were generated. The mAbs originated from the same B cell, bound strongly to whole cell M. tuberculosis CDC1551 and to its cell wall, membrane and cytosol fractions recognizing a 90 kDa protein. Immunoprecipitation using mAb 16a1 isolated a protein with amino acid peptide sequences matching MPT51 from the cytosol. This immunogenic protein of unknown function, was previously reported only in culture filtrates of M. tuberculosis. Our findings suggest for the first time that this protein is found within the M. tuberculosis cell.
Keywords: Mycobacterium tuberculosis, Monoclonal antibodies, MPT51
Introduction
The role of antibodies in protection against tuberculosis (TB) was historically discounted. However, recent studies demonstrated that certain antibodies have beneficial effects on the course of mycobacterial infection (1-5;5-8). Monoclonal antibodies (mAbs) directed to arabinomannan (AM) and lipoarabinomannan (LAM) were described in several of these studies (1;3;5).
The surface of M. tuberculosis is rich in lipids and carbohydrates with an outermost capsular layer composed primarily of the polysaccharides AM and glucan (9). The capsular material was previously shown to be easily removed or shed from the mycobacterial surface (10;11). Using mAbs to AM, we previously demonstrated that all M. tuberculosis strains tested shed AM into the culture media (12). However, while most of these strains also retained capsular AM on the cell surface, some shed their entire capsular AM (12). Shedding of capsular material may have implications on the interactions of M. tuberculosis with antibodies and host cells. In this study we used M. tuberculosis CDC 1551, an outbreak strain from which the capsular AM is largely shed and possibly entirely removed from the surface, to generate novel mAbs to immunogenic non-capsular surface epitopes. These mAbs were important for understanding their target antigen localization.
Materials and Methods
Mycobacterial strains
M. tuberculosis CDC 1551 originated from an Albert Einstein College of Medicine collection. Irradiated M. tuberculosis CDC 1551 was supplied by J. T. Belisle (Colorado State University). Nine drug-susceptible M. tuberculosis strains from sputa of TB patients originated from the Microbiology Laboratory, Montefiore Medical Center, Bronx, NY. M. tuberculosis Erdman (TMC 107) originated from Trudeau Institute Mycobacterium Culture Collection Saranac Lake, N.Y. M. tuberculosis Erdman mutant strain with double deletion ΔRD1ΔpanCD was previously described (13).
Culture of mycobacterial strains
Virulent M. tuberculosis strains were grown in biosafety level 3 (BSL3). For the generation of mAbs, M. tuberculosis CDC 1551 was grown in 7H9 medium (Difco, Detroit, Mich.) containing 1% glycerol (Sigma, St. Louis, Mo.) and OADC (Becton-Dickinson, MD) without Tween 80 for 14 days at 37°C, heat killed at 80°C for 2 hours, washed twice in PBS, sonicated (Branson Ultrasonics, Danbury, Conn.) for 5 seconds and frozen at -80°C. M. tuberculosis clinical strains and Erdman were grown and lyophilized as previously described (12). M. tuberculosis ΔRD1ΔpanCD was grown in 7H9 medium containing 0.05% Tween 80 and pantothenate (24 μg/ml) (13).
Antibodies
MAbs 9d8 (IgG3) to AM, 24c5 (IgG1) to glucan, 5c11 (IgM) to LAM and AM, and 4f11 to a polysaccharide epitope of mycolyl-arabinogalactan-peptidoglycan (mAGP) complex were previously described (14;15).
Antigens
M. tuberculosis CDC 1551 whole cell lysate, cytosol, cell wall, membrane and culture filtrate protein as well as M. tuberculosis H37Rv LAM, mycolyl arabinogalactan-peptidoglycan complex (mAGP), total lipid fractions, and mycolic acids were supplied by J. T. Belisle. MPT51 was generated as previously described (16).
Generation of mAbs to M. tuberculosis CDC 1551
6-8 week old female BALB/c mice (Charles River Laboratories, Wilmington, Mass.) were each immunized intraperitoneally with 1 × 107 heat killed M. tuberculosis CDC 1551 bacilli suspended in 200 μl of PBS and mixed 1:1 with Incomplete Freund's Adjuvant. They were boosted, with 1 × 107 bacilli on days 14, 28, and 42. The mouse with the highest serum antibody titers to M. tuberculosis CDC 1551 was boosted again 4 days prior to fusion and its spleen cells were fused with NSO cells as previously described (15). Hybridomas producing antibodies were screened and cloned as previously described (14).
Whole cell ELISA for M. tuberculosis
ELISA was adapted from a previously described protocol (14) using M. tuberculosis CDC1551 (heat killed or irradiated) placed in microtiter plate wells and blocked with 5% milk (dry, nonfat). Hybridoma supernatants, were added, serially diluted if necessary and incubated for 1 h at 37°C. Alkaline Phospatase (AP) goat anti-mouse isotype-specigic Ig (1 μg/ml) (Southern Biotechnology Associates, INC) was added and color was developed using p-nitrophenylphosphate (PNPP) (Southern Biotechnology Associates, INC). Negative control wells had no M. tuberculosis. Whole cell ELISA with M. tuberculosis clinical strains and Erdman was performed as previously described (12).
Immunelectron microscopy
M. tuberculosis CDC 1551 grown for 9 days in 7H9 media without Tween 80, was fixed with 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M sodium cacodylate buffer, dehydrated through a graded series of ethanol, embedded in LR White resin and cut into ultra thin sections. Grids were blocked and then incubated with mAbs (10 μg /ml and 100 μg/ml) in BSA-gel PB for 2 hr at room temperature. No mAb was added to negative control grids. Grids were then incubated with goat anti-mouse Ig conjugated to 5 nm gold spheres (Aurion) in BSA-gel PB, fixed in PB containing 2% glutaraldehyde, washed with PB and distilled water followed by the addition of 4% phosphotungstenic (Fisher) and incubation with Lead Citrate. Grids were viewed with a 1200EX transmission electron microscope (JOEL, Peabody, Mass.).
Chemical analysis of mAbs epitope
Proteinase K and Sodium meta-periodate were used in ELISA as previously described (14) to investigate whether mAbs recognized epitopes containing protein or carbohydrate, respectively.
Binding of MAbs to cellular fractions of M. tuberculosis by ELISA
Cytosol, membrane, cell wall and whole cell lysate preparations of M. tuberculosis CDC1551 dissolved in PBS at 10 μg/ml, were placed in polystyrene microtiter ELISA plate wells (50 μl/well) and incubated for 1 hr at 37°C. The plates were blocked with 5% milk for 1 hr at room temperature. MAbs at 10 μg/ml in 1% BSA were added and serially diluted. The rest followed as previously described (12). Binding of mAbs to surface carbohydrate and lipid fractions was determined by ELISA, as previously described (14). LAM (10 μg/ml) and mAGP complex (1 mg/ml) in carbonate buffer (pH 9.6) were each incubated in microtiter ELISA plates at 37°C for 1 hr. TLF and mycolic acids suspended in 100% ethanol, were added to microtiter plates, serially diluted starting at 1 mg/ml, and air dried overnight prior to proceding with the ELISA protocol.
Western blot analysis
Western blot analysis was performed as previously described (17;18). Cytosol, membrane, cell wall, and samples of purified protein preparation suspended in PBS were separated by 10 and 12% SDS-PAGE gel (maximum voltage and current of 250 mA) for 3 hours. Gels were trans-blotted onto nitrocellulose membranes (Protran), blocked with BLOTTO (Tris buffered saline with 0.2% Tween, 2% dry non fat milk, and azide), incubated with 10 μg/ml of mAb diluted in BLOTTO overnight at 4°C, and then probed with goat anti-mouse-AP labeled IgG1 (Southern Biotechnology Associates, INC) at 1 μg/ml in Tris buffered saline with 0.2% Tween (TBST). Blots were washed with TBST between steps and color development reagents (Bio-Rad Laboratories) were applied. Negative controls consisted of mAb 2H1 to Cryptococcus neoformans or omission of primary mAb (19).
Antigen identification
For antigen identification, immunoprecipitation of cytosol was performed with purified mAb 16a1 using Seize® Protein G Immunoprecipitation Kit (Pierce, Rockford, IL). The immunoprecipitation product was separated on a 12% SDS-PAGE gel and silver stained. A separate gel was trans-blotted onto a nitrocellulose memebrane and incubated with mAb 16a1. The band identified by the immunoblot and silver stain was cut, processed and digested into peptides as previously described (20). Matrix-assisted laser desorption/ionization (MALDI) time-of-flight (TOF) mass spectrosocopy was performed using Voyager-DE™ Biosystems STR Biospectrometry™ workstation (Applied Biosystems, Foster city, CA). Data base search using MS-FIT was performed at the Mass Spectrosocopy Facility of the University of California, San Francisco (21). Tandem Mass Spectrometry was performed on an ABI 4700 TOF/TOF (Applied Biosystems) and data search was done using Mascot search engine (Matrix Science) (22).
MAb fine specificity studies
To examine the antibody specificity, mAb 16a6 was purified (immunopure L or G Immunoglobulin purification kit, Pierce, Rockford, IL), biotynilated (EZ-Link Sulfo-NHS-LC-Biotinylation kit, Pierce, Rockford, IL) and used in competition ELISA with mAb 16a1 for binding to M. tuberculosis CDC 1551 whole cell. Competition ELISA was performed twice; once adding constant concentration of biotinylted mAb 16a6 and varying concentrations of mAb 16a1, and once adding constant concentration of mAb 16a1 and varying concentrations of biotinylated mAb 16a6. After incubation and washing, alkaline phosphatase conjugated streptavidine was added followed by color development with PNPP in substrate buffer.
MAb sequencing
RNA was prepared from hybridoma cell lines 16a1 and 16a6 as previously described (23). RNA was used for the synthesis of cDNA from mRNA, using oligo(dT) primer and superscript II reverse transcriptase (GIBCO). The cDNA encoding the immunoglobulin variable domains was then amplified by PCR as previously described (23), using the following universal 5′ (sense) variable-region and specific 3′ (antisense) constant region primers: 3′msCgamma, AGA CCG ATG GGG CTG TTG TTT TGG C; 3′msCkappa, TGG ATA CAG TTG GTG CAG CAT CAG C; 5′vh Uni, TGA GGT GCA GCT GGA GGA GTC; 5′Vk Uni GAC ATT CTG ATG ACC CAG TCT. PCR products (Qiagen) were ligated into pCR2.1 (Invitrogen) according to manufacturer instructions. Constructs containing inserts were detected by restriction digestion with EcoR1 (Roche) and were visualized by 2% agarose gel electrophoresis. Alternatively a one-step PCR Kit (Qiagen) was used according to the manufacturer instruction, under the following conditions: 50°C for 30 min, 95°C for 15 min, followed by 94°C for 1 min, followed by 54°C for 1 min, and 72°C for 1 min, followed by a final 10-min extension at 72°C. Amplified variable domains cDNA samples were sequenced bidirectionally by automated gel sequencing using the appropriate 5′ (sense) and 3′ (antisense) primers for each variable domain.
Agglutination assay
To evaluate for mAb-mediated agglutination, ΔRD1ΔpanCD double mutant of M. tuberculosis Erdman was thawed from frozen stocks and 100 μl aliquots were added to 10 ml liquid medium and incubated at 37°C. The aggregation status of mycobacteria was determined visually using Olympus BX61 microscope with Cooke SensiCamQE High Performance camera. For this, 10 μl of culture were placed on glass microscope slide and covered with a coverslip. The slides were sealed with nail polish. To obtain a solution containing only single bacilli, vortexing, precipitation of clumps by gravity or centrifugation for 1 min using VMSC-13 minicentrifuge at 6.5 RPM to precipitate clumped bacilli were employed as necessary. The bacilli status was determined after screening multiple fields. Sets of 3 to 5 pictures were taken from each preparation for re-evaluation. Aliquots of 100 μl media containing single bacilli were then added to media containing purified mAb at concentrations of 10 or 100 μg/ml. The mycobacterial inocula CFU were 4.88×105 and 3.47×105, respectively. At designated times, aliquots were removed to determine the bacilli status by microscopy. Plating on 7H10 agar and absorbance measurements were performed to confirm viability.
Results
Isolation of hybridomas producing mAbs to M. tuberculosis CDC 1551
36 antibody producing hybridoma cell lines were identified by whole cell ELISA. After two cloning procedures in soft agar, 6 stable clones were isolated. We continued our work with two hybridoma cell lines, 16a1 and 16a6, both producing IgG1 with kappa light chains, because they bound both heat-killed and irradiated M. tuberculosis (see below) suggesting a heat-stable target epitope.
Binding of mAbs to whole cell M. tuberculosis ELISA
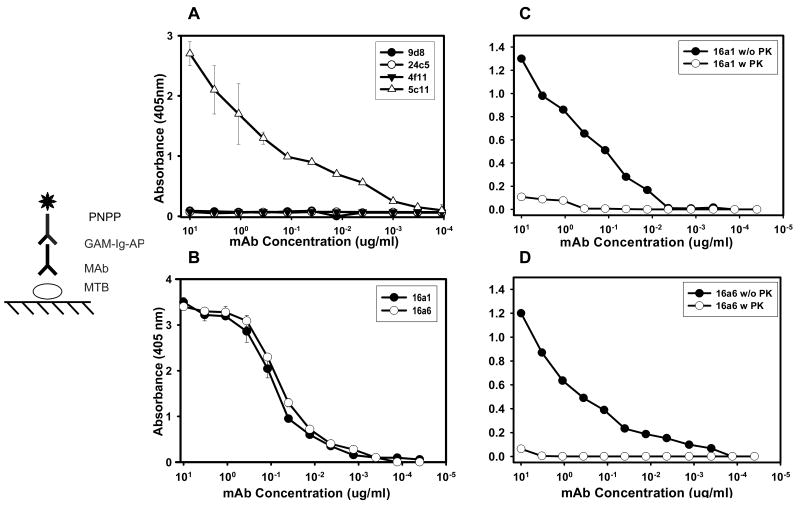
To confirm the surface properties of M. tuberculosis CDC 1551, mAbs 5c11 (IgM), 9d8 (IgG3) 24c5 (IgG1) and 4f11 (IgM) were reacted with heat killed whole-cell M. tuberculosis CDC 1551 by ELISA. Strong reactivity of mAb 5c11 and no reactivity of mAbs 9d8, 24c5 and 4f11 were demonstrated (fig. 1A).
FIG. 1.
Binding of MAbs to M. tuberculosis CDC 1551 by whole-cell ELISA. Binding at different dilutions of (A) MAbs 5c11, 24c5, 9d8 (B) MAbs 16a1, and 16a6. Similar binding of mAbs 16a1 and 16a6 were obtained using irradiated killed M. tuberculosis CDC 1551. (C) and (D) represent binding of mAbs 16a1 and 16a6 to M. tuberculosis in the presence (open symbols) and absence (filled symbols) of proteinase K, by whole-cell ELISA.
The diagrams represent the ELISA configurations. Values represent the average of three measurements. Error bars indicate standard deviation from the mean. Experiment was repeated three times with similar results.; MTB, M. tuberculosis; AP, alkaline phosphatase
MAbs 16a1 and 16a6 bound similarly heat-killed (Fig 1B), and irradiated M. tuberculosis CDC 1551 as well as M. tuberculosis Erdman and all clinical strains (TB1 through TB 9) (Table 1). No reactivity was detected in negative control wells.
Table 1.
Analysis of murine mAbs 16a 1 and 16a6
| MAb | Isotype | Binding to M. tuberculosis CDC 1551 (Heat-killed) | Binding to M. tuberculosis CDC 1551 (irradiated) | Binding to M. tuberculosis strains Erdman & TB1-TB9 | Peptide sequences of MPT51 identified following immunoprecipiation with mAb | VH | JH | VK | JK |
|---|---|---|---|---|---|---|---|---|---|
| 16a1 | IgG1 | + | + | + | APYENLMVPSPSMGR
GISVVAPAPAGGAYSMYTNWEQDGSK GLAPGGHAAVGAAQGGYGAMALAAFHPDR WHDPWVHASLLAQNNTR VWVWSPTNPGASDPAAMIGQAAEAMGNSR MFYNQYRS |
7183 | 4 | 4
(subgroup 50) |
2 |
|
|
|
||||||||
| 16a6 | IgG1 | + | + | + | 7183 | 4 | 4
(subgroup 50) |
2 | |
Chemical analysis of epitopes
Binding of mAbs 16a1 and 16a6 to proteinase K treated M. tuberculosis CDC 1551 was significantly reduced relative to non-treated mycobacterial cells (Fig. 1C and 1D). Binding of mAbs was not affected by sodium meta-periodate treatment (which oxidizes carbohydrate sugar rings and can affect the binding of antibodies to polysaccharide epitopes). Neither mAb bound to LAM, mAGP, total lipid fraction or mycolic acids.
Localization of mAb binding
MAbs 16a1 and 16a6 bound strongly to cytosol, membrane, cell wall and cell lysate tested by ELISA. Western blot analysis with M. tuberculosis cell fractions confirmed the ELISA results and demonstrated that both mAbs bound to a 90 kDa protein antigen (Fig. 2A) in all cell fractions tested.
FIG. 2.
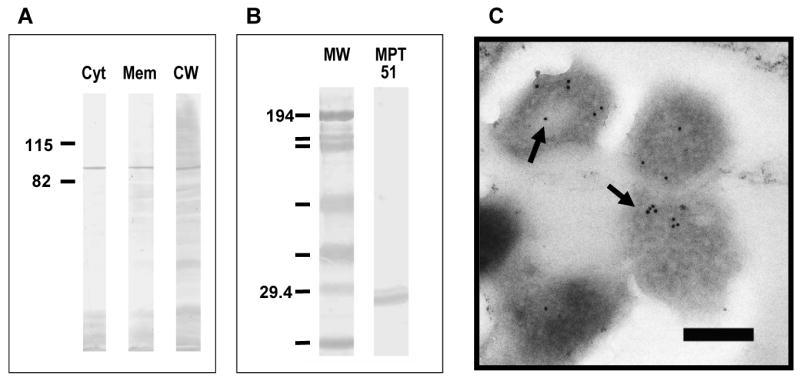
(A) Western blot analysis using MAb 16a6 with M. tuberculosis CDC1551 cellular fractions and recombinant protein. Assay using mAb 16a1 was identical. (B) Western Blot analysis with MPT 51. Results were identical for mAb 16a1 and 16a6. (C) Immunoelectron microscopy demonstrating the binding of MAb 16a1 to M. tuberculosis. Gold particles denote secondary antibody binding to primary MAb. Similar binding patterns were obtained with mAb 16a6. Control experiments with irrelevant isotype-matched MAbs revealed no binding of gold particles to mycobacteria (data not shown). Bars = 200 nm
Immunelectron microscopy
Gold labeling localized primarily within the mycobacterium when mAbs 16a1 and 16a6 were used (Fig. 2C). No gold labeling was detected in control samples, including those utilizing mAbs 9d8 and 24c5.
Antigen identification
The immunoprecipitation product obtained using mAb 16a1 migrated to the 90 kDa range on SDS-PAGE gel. Mass spectroscopy of the protein recovered, obtained unique monoisotropic ions used for peptide mass fingerprinting of the Mycobacterium NCBInr database. The top 2 hits, with MOWSE scores of 2.049e+06 and 4.603e+04, identified 5 peptides that matched with peptides of fbpC1 also known as MPT 51. Tandem Mass Spectrometry data search using Mascot (22) identified 2 peptide sequences that belong to MPT51, with a search score of 70 and 39. Altogether 6 peptides of MPT51 were identified covering 124 of the 299 (41%) amino acids of the protein (Table 1). The binding of mAbs 16a1 and 16a6 to MPT 51 was confirmed by running purified recombinant MPT51 on SDS-PAGE gel and performing a western blot as described above (Fig. 2B).
MAb fine specificity
Due to the similarities in binding characteristics of mAbs 16a1 and 16a6, competition ELISA was done to assess the mAb fine specificity. Inhibition of the binding of biotinylated mAb 16a6 to M. tuberculosis CDC 1551 was observed in the presence mAb 16a1 both when mAb 16a1 concentration was kept constant and mAbs 16a6 concentration was variable and vise versa (not shown).
MAb variable domain sequencing
Due to the similarity in the specificity, localization and isotype of mAbs 16a1 and 16a6, we sequenced their VH and VK domains. A 100% sequence homology for the heavy and light chain variable domains was observed for the 2 mAbs. Based on 317 nucleotides, the sequence of the Variable Heavy chain (VH) was demonstrated to use the germ line family VH 7183, and JH family JH4. The sequence of the Variable light (VL) chain (based on 325 nucleotides) used the germ line family VK 4 subgroup 50 and JK family JK 2 (Table 1). The consensus sequences were deposited in GenBank (accession numbers EF 409992 and EF 409993).
Determination of agglutination by mAbs
M. tuberculosis has a natural tendency to clump (24). Thus establishing the role of antibody in the agglutination of mycobacteria can be difficult. To overcome this problem, we devised a method for identifying single bacilli visually prior to the addition of antibodies and observing their aggregation status thereafter. When mAbs 16a1 and 16a6 were used at a concentration of 10 μg/ml, no clumping was observed 3 days after the addition of mAbs. At a mAb concentration of 100 μg/ml, widespread clumping was observed (Figure 3B), while control samples containing mAb 18B7 or no mAb had predominance of single bacilli (Figure 3A). Microscopic observation 20 days later, demonstrated predominance of single bacilli (with only few clumps) in samples to which mAbs 16a1 and 16a6 were added, regardless of antibody purification method (by protein A or L). Viability of bacilli was confirmed by growth of bacilli on 7H10 agar plates and increasing absorbance values.
Fig. 3.
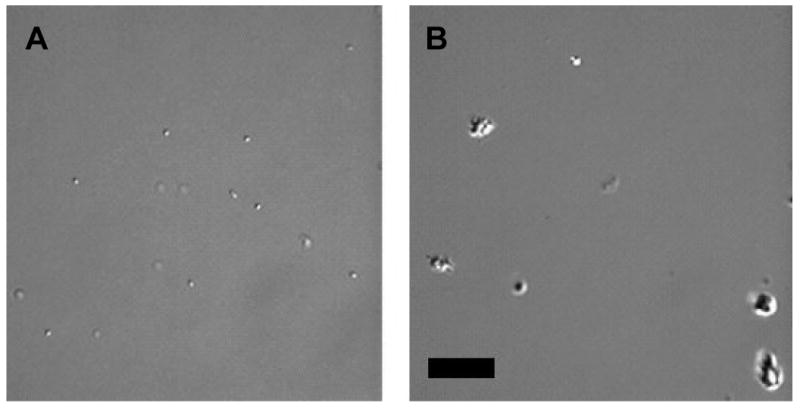
Figures of M. tuberculosis ΔRD1ΔpanCD under DIC microscope. (A) Control sample 3 days after inoculation with M. tuberculosis ΔRD1ΔpanCD without addition of mAb, demonstrating single bacilli. (B) Antibody (mAb 16a1) - treated sample demonstrating clumps; similar results were obtained with mAb 16a6 (Bar 20 μm).
Discussion
The beneficial effect of mAbs to AM on the outcome of M. tuberculosis infection (1;3;5) combined with the reports that AM conjugate vaccine candidates elicited antibody responses (12), lowered CFU early after mycobacterial infection (12) and prolonged survival (25), suggest that AM is a useful antigen to be considered in vaccine development against TB. However, the shedding of capsular AM from the mycobacterial surface which in few M. tuberculosis strains appears to be complete (12;26), raises concerns regarding the ability of antibodies directed towards certain capsular epitopes to protect effectively against those strains. Hence, we directed our efforts toward identifying additional surface antigens that could potentially be useful for eliciting protective antibody responses. We hypothesized that using a M. tuberculosis strain that sheds the capsular polysaccharide completely will allow an enhanced exposure of other surface antigens to the immune system. We used M. tuberculosis CDC 1551, which was shown in our previous studies (12) to lack the capsular AM on its surface after washing, to generate mAbs. This strain of M. tuberculosis was previously found to induce a more vigorous host response in vivo and in vitro as compared to other strains (27).
Our results of whole-cell ELISA and immunoelectron microscopy showed that mAb 9d8 and mAb 24c5 (directed to the capsular polysaccharides AM and glucan respectively) did not bind the surface of M. tuberculosis CDC 1551, confirming that their respective epitopes were absent from the cell surface after separation from the growth media and washing. The lack of binding of mAb 4f11 which recognizes a polysaccharide epitope of mycolyl-arabinomannan-peptidoglycan (mAGP) (14), could suggest either the absence of this epitope from the surface of M. tuberculosis CDC 1551, or a different distribution of this epitope with respect to the cell surface of this strain.
The two new IgG1 mAbs generated, mAbs 16a1 and 16a6, bound to M. tuberculosis CDC 1551, by whole-cell ELISA, immunoelectron microscopy and western blot. Each mAb recognized protein epitopes not affected by irradiation or heat killing of M. tuberculosis CDC 1551, suggesting a heat-stable target. The binding of these mAbs to cytosol, membrane and cell wall fractions suggests that their epitopes were found on the cell surface as well as within the mycobacterial cell. The reactivity of both mAbs with all clinical strains tested suggests a target epitope that is ubiquitous among M. tuberculosis isolates.
Although the cell lines producing the 2 mAbs differed in the amount of antibody produced (as manifested by significantly different mAb concentrations in equal amounts of culture supernatants and ascites), the similarities between the mAbs in binding characteristic and isotype, led us to hypothesized that they may be structurally very similar or perhaps identical. Inhibition ELISA further indicated that the 2 mAbs have similar specificities. The 100% homology of the variable light (VL) and heavy chains (VH) sequences, suggest that these mAbs originated from the same ancestral B cell that underwent clonal expansion. Thus, the experimental results obtained with mAbs 16a1 and 16a6 provide a valuable internal confirmation for our findings.
Immunoprecipitation of the cytosol fraction with mAb 16a1 identified a protein with peptides mass and sequences that matched MPT 51. MPT 51 is a 27-30 kDa protein, the exact function of which is unknown. It has about 40% sequence homology with members of the antigen 85 complex (A, B, and C) of M. tuberculosis. These are secreted proteins with molecular weight of 27 to 31 kDa (16;28;29) that catalyze mycolate transfer resulting in the formation of α,α′ –trehalose monomycolate (TMM) and α,α′ –trehalose dimycolate (TDM), the later also known as cord factor (30). Ag 85C has an additional role in the covalent attachment of mycolic acids to arabinogalactan (31). Several conserved residues of these proteins represent potential sites for interaction with human fibronectin (32;33), and they are also known as fibronectin-binding proteins (Fbp) A, B and C. The degree of sequence homology between MPT 51 and the members of the Ag 85 complex and the previous demonstration that it can bind fibronectin (34), led to speculations that this protein might constitute a forth member of this complex. However, MPT51 does not have structural elements that are required for mycolyltrandferase activity (16), suggesting that it may have function/s that is/are different than that/those of the members of Antigen 85 complex.
MPT 51 was identified as a B-cell antigen with specific antibodies detected in patients with TB, especially HIV-infected (35)(36). Detection of antibodies prior to the development of clinical TB (36), suggested that this antigen is expressed during active replication of M. tuberculosis. Protective cell-mediated immunity induced in mice with recombinant attenuated strains of Listeria monocytogenes carrying an expression plasmid for MPT51, suggest a role for MPT 51 as a T-cell antigen as well.
Previous studies demonstrated the presence of MPT51 in culture filtrate, but not in the cell wall or cytosol (37). Our results indicate for the first time the presence of this protein in subcellular fractions including cytosol and cell surface. The identification of 6 peptides with amino acid sequences that are unique to MPT51 and do not match with any of the Ag 85 complex members, provide a strong evidence for the existence of this protein in the cytosol. The migration of this protein in our SDS-page gels as a 90 kD protein suggests that it may be present in a trimeric form in these subcellular fractions.
The localization of proteins is of paramount importance to the definition of their function (38). Furthermore, the localization of MPT 51 to cell wall and cytosol, suggests that it may play more than one role, (30).
Antibodies can cause agglutination of the particles bearing their target antigen (39). Given the natural tendency of M. tuberculosis to clump it may be difficult to study antibody-mediated agglutination. Although sonication is often used to break clumps, our experience demonstrated that clumps could be present in significant amounts following the procedure. ΔRD1ΔpanCD is an attenuated double mutant strain of M. tuberculosis Erdman (13) which has been approved for use under Biosafety Level 2 (BSL2) conditions at the Albert Einstein College of Medicine and was suitable for DIC microscopy. Clumping of mycobacteria resulting from the addition of mAbs 16a1 and 16a6 at a concentration of 100 μg/ml, but not at 10 μg/ml, confirm the presence of these mAbs epitope on the surface of M. tuberculosis and provide useful information regarding the antibody concentration that can be used without causing agglutination. Agglutination is a potential mechanism of antibody function since clumping helps localize the microbe to the site of infection and agglutinated mycobacteria could potentially be handled differently by the immune system as compared to single bacteria. Additional studies are required to determine the role of antibody-mediated agglutination on the course of mycobacterial infections.
In conclusion, the use of a M. tuberculosis strain that sheds easily capsular epitopes resulted in the generation of MPT51-binding mAbs, which enabled the identification and extraction of the molecule from subcellular fraction. We also developed a method to evaluate for antibody-mediated agglutination and demonstrated a dose-dependent agglutination of mycobacteria by mAbs 16a1 and 16a6. The clumping provides further confirmation to the existence of their epitope on the mycobacterial surface. Further studies are needed to establish the function of MPT51, to define its exact role in the pathogenesis of Tb and the potential of MPT51-binding antibodies to modify the course of M. tuberculosis infection.
Acknowledgments
This work was supported in part by a grant from the Center for AIDS Research Albert Einstein College of Medicine and by NIH grants AI 001691, AI 053192 to A.G-F. A. G-F., A.C. and W.R.J. Jr. are Center for AIDS Research investigators at the Albert Einstein College of Medicine. Special gratitude to Louis Weiss for critically reviewing the manuscript and helpful suggestions, John Belisle for providing research materials as part of contract N01 Al-75320 (Tuberculosis Materials and Vaccine Testing), Samir Hasan for technical support and advise, the Clinical Microbiology Laboratory at the Montefiore Medical Center, the Analytical Imaging facility, Fang Wang and Eddie Nieves from the laboratory of Macromolecular analysis and Proteonomics at the Albert Einstein College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glatman-Freedman A, Mednick AJ, Lendvai N, Casadevall A. Clearance and organ distribution of Mycobacterium tuberculosis lipoarabinomannan (LAM) in the presence and absence of LAM-binding IgM. Infect Immun. 2000;68:335–341. doi: 10.1128/iai.68.1.335-341.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glatman-Freedman A. Advances in antibody-mediated immunity against Mycobacterium tuberculosis: Implications for a novel vaccine strategy. FEMS Immunol Med Microbiol. 2003;39:9–16. doi: 10.1016/S0928-8244(03)00172-X. [DOI] [PubMed] [Google Scholar]
- 3.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins JB, Unanue E, Casadevall A, Bloom BR. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci USA. 1998;95:15688–15693. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers MA, Gavier-Widen D, Hewinson RG. Antibody bound to the surface antigen MPB83 of Mycobacterium bovis enhances survival against high dose and low dose challenge. FEMS Immunol Med Microbiol. 2004:93–100. doi: 10.1016/j.femsim.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Hamasur B, Haile M, Pawlowski A, Schroder U, Kallenius G, Svenson SB. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab')2 fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2004;138:30–38. doi: 10.1111/j.1365-2249.2004.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pethe K, Alonso S, Blet F, Delogu G, Brennan MJ, Locht C, Menozzi FD. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature. 2001;412:190–194. doi: 10.1038/35084083. [DOI] [PubMed] [Google Scholar]
- 7.Williams A, Reljic R, Naylor I, Clark SO, Falero-Diaz G, Singh M, Challacombe S, Marsh PD, Ivanyi J. Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs. Immunology. 2004;111:328–333. doi: 10.1111/j.1365-2567.2004.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glatman-Freedman A. The role of antibody-mediated immunity in defense against Mycobacterium tuberculosis: Advances towards a novel vaccine strategy. Tuberculosis. 2006;86:191–197. doi: 10.1016/j.tube.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Daffe M, Draper P. The envelope layers of mycobacteria with reference to their pathogenicity. Advances in Microbial Physiology. 1998;39:131–203. doi: 10.1016/s0065-2911(08)60016-8. [DOI] [PubMed] [Google Scholar]
- 10.Ortalo-Magne A, Dupont MA, Lemassu A, Andersen AB, Gounon P, Daffe M. Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiology. 1995;141:1609–1620. doi: 10.1099/13500872-141-7-1609. [DOI] [PubMed] [Google Scholar]
- 11.Ortalo-Magne A, Lemassu A, Laneelle MA, Bardou F, Silve G, Gounon P, Marchal G, Daffe M. Identification of the surface-exposed lipids on the cell envelope of Mycobacterium tuberculosis and other mycobacterial species. J Bacteriol. 1996;178:456–461. doi: 10.1128/jb.178.2.456-461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glatman-Freedman A, Casadevall A, Dai Z, Jacobs WR, Jr, Li A, Morris SL, Navoa JD, Piperdi S, Robbins JB, Schneerson R, Schwebach JR, Shapiro M. Antigenic evidence of prevalence and diversity of Mycobacterium tuberculosis arabinomannan. J Clin Microbiol. 2004;42:3225–3231. doi: 10.1128/JCM.42.7.3225-3231.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sambandamurthy VK, Derrick SC, Hsu T, Chen B, Larsen MH, Jalapathy KV, Chen M, Kim J, Porcelli SA, Chan J, et al. Mycobacterium tuberculosis DeltaRD1 DeltapanCD: A safe and limited replicating mutant strain that protects immunocompetent and immunocompromised mice against experimental tuberculosis. Vaccine. 2006;24:6309–6320. doi: 10.1016/j.vaccine.2006.05.097. [DOI] [PubMed] [Google Scholar]
- 14.Glatman-Freedman A, Martin JM, Riska PF, Bloom BR, Casadevall A. Monoclonal antibodies to surface antigens of Mycobacterium tuberculosis and their use in a modified enzyme-linked immunosorbent spot assay for detection of mycobacteria. J Clin Microbiol. 1996;34:2795–2802. doi: 10.1128/jcm.34.11.2795-2802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwebach JR, Glatman-Freedman A, Gunter-Cummins L, Dai Z, Robbins JR, Schneerson R, Casadevall A. Glucan is a component of the Mycobacterium tuberculosis surface that is expressed in vitro and in vivo. Infect Immune. 2002;70:2566–2575. doi: 10.1128/IAI.70.5.2566-2575.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson RA, Maughan WN, Kremer L, Besra GS, Futterer K. The structure of Mycobacterium tuberculosis MPT 51 (FbpC1) defines a new family of non-catalytic α/β hydrolases. J Mol Biol. 2004;335:519–530. doi: 10.1016/j.jmb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Towbin H, Staehelin T, Gordon J. Electophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets. Procedure and some applications. Pro Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee J, Scharff MD, Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun. 1992;60:4534–4541. doi: 10.1128/iai.60.11.4534-4541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez J, Gharahadaghi F, Mische SM. Routine identification of proteins from sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels on polyvinyl difluoride membranes using matrix assisted laser desorption/ionization time of flight-mass spectrometry (MALDI-TOF-MS) Electrophoresis. 1998;19:1036–1045. doi: 10.1002/elps.1150190619. [DOI] [PubMed] [Google Scholar]
- 21.Mass Spectrometry facility. MS-Fit. University of California San Francisco; USA: http://prospector.ucsf.edu/ucsfhtml4.0/msfit.htm. [Google Scholar]
- 22.Mascot. http://www.matrixscience.com.
- 23.Navoa JD, Laal S, Pirofski L, McLean G, Robbins JB, Schneerson R, Dai Z, Casadevall A, Glatman-Freedman A. Specificity and Diversity of Antibodies to Mycobacrerium tuberculosis Arabinomannan. Clin Diagn Lab Immunol. 2003;10:88–94. doi: 10.1128/CDLI.10.1.88-94.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anton V, Rouge P, Daffe M. Identification of the Sugars involved in mycobacterial cell aggregation. FEMS Microbiol Letters. 1996;144:167–170. doi: 10.1111/j.1574-6968.1996.tb08525.x. [DOI] [PubMed] [Google Scholar]
- 25.Hamasur B, Haile M, Pawlowski A, Schroder U, Williams A, Hatch G, Hall G, Marsh P, Kallenius G, Svenson SB. Mycobacterium tuberculosis arabinomannan-protein conjugates protect against tuberculosis. Vaccine. 2003;21:4081–4093. doi: 10.1016/s0264-410x(03)00274-3. [DOI] [PubMed] [Google Scholar]
- 26.Schwebach JR, Casadevall A, Schneerson R, Dai Z, Wang X, Robbins JB, Glatman-Freedman A. Expression of a Mycobacterium tuberculosis Arabinomannan Antigen In Vitro and In Vivo. Infect Immun. 2001;69:5671–5678. doi: 10.1128/IAI.69.9.5671-5678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manca C, Tsenova L, Barry CE, 3, Bergtold A, Freeman S, Haslett PAJ, Musser JM, Freedman VH, Kaplan G. Mycobacterium tuberculosis CDC 1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J Immunol. 1999;162:6740–6746. [PubMed] [Google Scholar]
- 28.Nagai S, Wiker HG, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991;59:372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramalingam B, Baulard AR, Locht C, Narayanan PR, Raja A. Cloning, expression, and purification of the 27kDa (MPT51, Rv3803c) protein of Mycobacterium tuberculosis. Protein Expression and purification. 2004;36:53–60. doi: 10.1016/j.pep.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Belisle JT, Vissa VD, Sievert T, Takayama K, Brennan PJ, Besra GS. Role of major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 31.Jackson M, Raynaud C, Laneelle MA, Guilhot C, Laurent-Winter C, Ensergueix D, Gicquel B, Daffe M. Inactivation of the antigen 85C gene profoundly affects the mycolate content and alters the permeability of the Mycobacterium tuberculosis cell envelope. Mol Microbiol. 1999;31:1573–1587. doi: 10.1046/j.1365-2958.1999.01310.x. [DOI] [PubMed] [Google Scholar]
- 32.Naito M, Ohara N, Matsumoto S, Yamada T. The novel fibronectin-binding motif and key residues of mycobacteria. J Biol Chem. 1998;273:2905–2909. doi: 10.1074/jbc.273.5.2905. [DOI] [PubMed] [Google Scholar]
- 33.Ronning DR, Klabunde T, Besta GS, Vissa VD, Belisle JT, Sacchettini JC. Crystal structure of the secreted form of antigen 85C reveals potential targets for mycobacterial drugs and vaccines. Nat Struct Biol. 2000;7:141–146. doi: 10.1038/72413. [DOI] [PubMed] [Google Scholar]
- 34.Naito M, Ohara N, Matsumoto S, Yamada T. The novel fibronectin-binding motif and key residues of mycobacteria. J Biol Chem. 1998;273:2905–2909. doi: 10.1074/jbc.273.5.2905. [DOI] [PubMed] [Google Scholar]
- 35.Ramalingam B, Uma Devi KR, Raja A. Isotype-specific anti-38 and 27 kDa (mpt51) response in pulmonary tuberculosis with human immunodeficiency virus coinfection. Scand J Infect Dis. 2003;35:234–239. doi: 10.1080/00365540310000292. [DOI] [PubMed] [Google Scholar]
- 36.Singh KK, Dong Y, Belisle JT, Harder J, Arora VK, Laal S. Antigens of Mycobacterium tuberculosis recognized by antibodies during incipient, subclinical tuberculosis. Clin Diagn Lab Immunol. 2005;12:354–358. doi: 10.1128/CDLI.12.2.354-358.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenkrands I, Weldingh K, Jacobsen S, Hansen CV, Florio W, Gianetri I, Andersen P. Mapping and identification of Mycobacterium tuberculosis proteins by two-dimentional gel electrophoresis, microsequencing and immunodetection. Electrophoresis. 2000;21:935–948. doi: 10.1002/(SICI)1522-2683(20000301)21:5<935::AID-ELPS935>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 38.Belisle JT, Braunstein M, Rosenkrands I, Andersen P. The proteonome of Mycobacterium tuberculosis. In: Cole ST, Eisenach KD, McMurray DN, Jacobs WR Jr, editors. The proteonome of Mycobacterium tuberculosis. ASM press; Washington, D.C.: 2005. pp. 235–260. [Google Scholar]
- 39.Janeway CA, Travers P, Walport M, Shlomchik MJ, editors. Immunobiology. Garland Science; New York and London: 2005. Glossary; p. 753. [Google Scholar]