Abstract
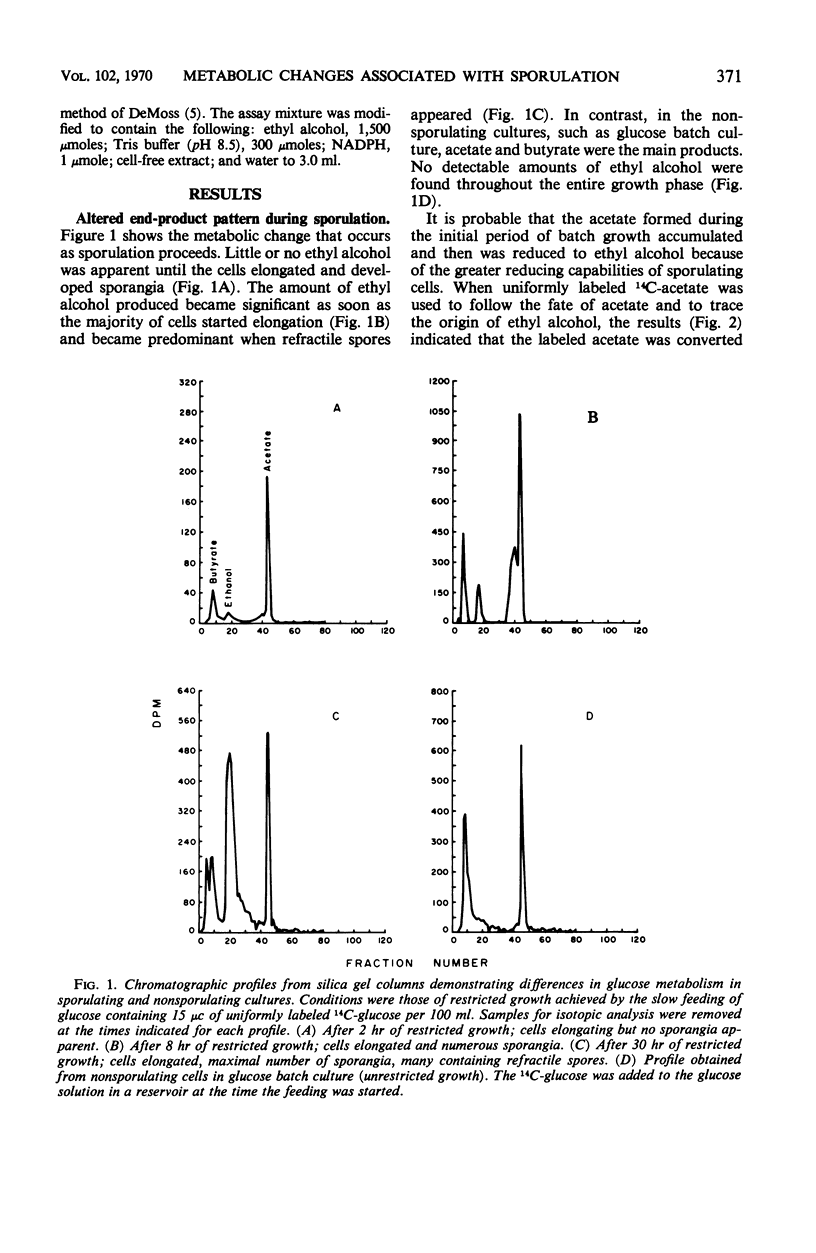
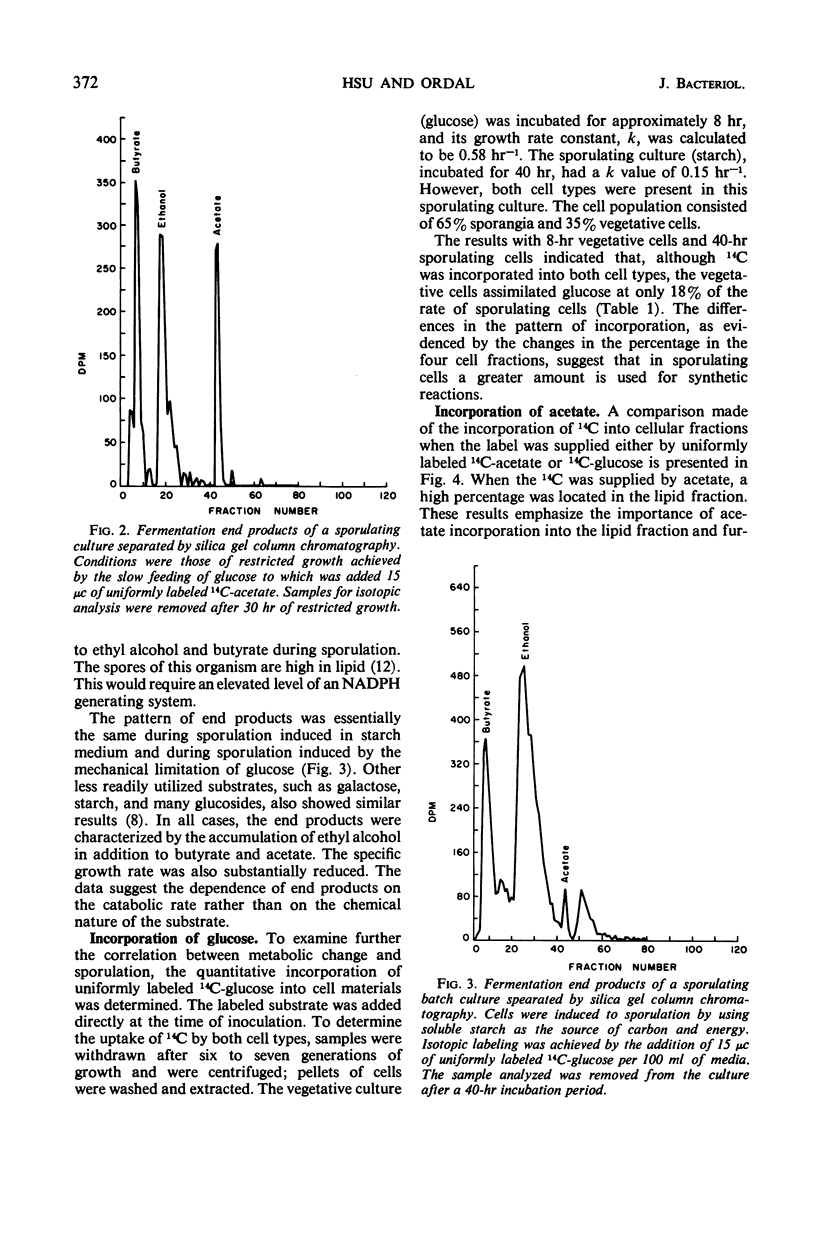
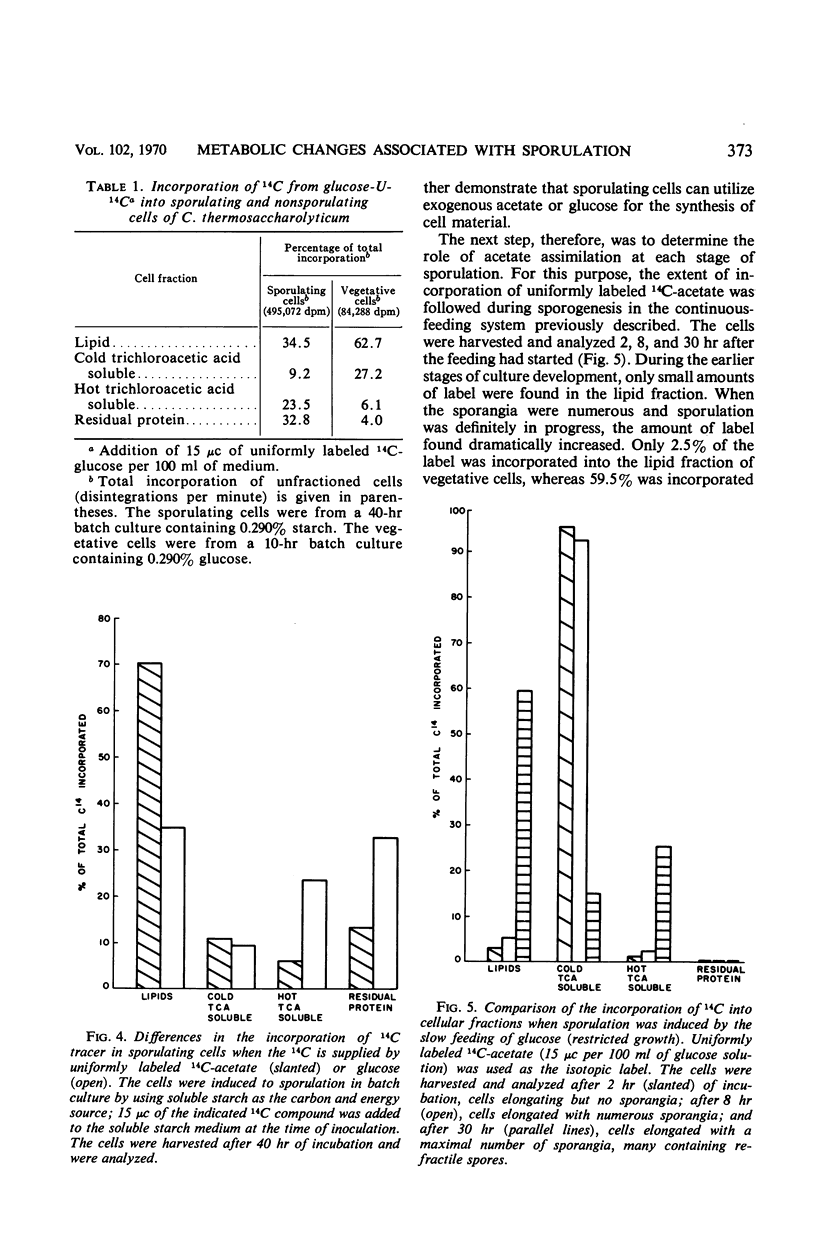
Cultures of Clostridium thermosaccharolyticum, under conditions of restricted growth achieved by slow feeding of glucose, showed a high degree of sporulation. Analysis of the end products showed an accumulation of ethyl alcohol in addition to butyrate and acetate, whereas, in the nonsporulating cultures, acetate and butyrate were the principal products. Incorporation of uniformly labeled 14C-glucose by sporulating cells was three to four times higher than by nonsporulating cells. The efficiency of acetate assimilation into the lipid fraction of sporulating cells was at least two times higher than that of glucose. When starch was used as the carbon source, the growth rate was reduced; sporulation occurred, and the end products and carbon distribution were similar. Alcohol dehydrogenase, glucose-6-phosphate dehydrogenase, and 6-phosphogluconate dehydrogenase were preferentially formed by sporulating cells. In vegetative cells, the formation of these enzymes was repressed if the glucose concentration in the medium was increased. The change in enzyme activity appeared to be related to a morphological change in the cells and indicated an altered metabolic pattern for sporulating cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- Campbell M. F., Ordal Z. J. Inhibition of sporulation of Clostridium thermosaccharolyticum. Appl Microbiol. 1968 Dec;16(12):1949–1951. doi: 10.1128/am.16.12.1949-1951.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., FOSTER S. M. The formation of ethanol in Escherichia coli. Biochim Biophys Acta. 1956 Nov;22(2):253–265. doi: 10.1016/0006-3002(56)90148-2. [DOI] [PubMed] [Google Scholar]
- DEMOSS R. D. A triphosphopyridine nucleotide dependent alcohol dehydrogenase from Leuconostoc mesenteroides. J Bacteriol. 1954 Aug;68(2):252–257. doi: 10.1128/jb.68.2.252-257.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu E. J., Ordal Z. J. Sporulation of Clostridium thermosaccharolyticum under conditions of restricted growth. J Bacteriol. 1969 Mar;97(3):1511–1512. doi: 10.1128/jb.97.3.1511-1512.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu E. J., Ordal Z. J. Sporulation of Clostridium thermosaccharolyticum. Appl Microbiol. 1969 Nov;18(5):958–960. doi: 10.1128/am.18.5.958-960.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEBECK M. E., KLEIN H. P. Substrates for Myxococcus virescens with special reference to eubacterial fractions. J Gen Microbiol. 1956 Apr;14(2):281–289. doi: 10.1099/00221287-14-2-281. [DOI] [PubMed] [Google Scholar]
- Lee C. K., Ordal Z. J. Regulatory effect of pyruvate on the glucose metabolism of Clostridium thermosaccharolyticum. J Bacteriol. 1967 Sep;94(3):530–536. doi: 10.1128/jb.94.3.530-536.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pheil C. G., Ordal Z. J. Fatty acid composition of spores of the "thermophilic anaerobes". J Bacteriol. 1967 May;93(5):1727–1728. doi: 10.1128/jb.93.5.1727-1728.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pheil C. G., Ordal Z. J. Sporulation of the "thermophilic anaerobes". Appl Microbiol. 1967 Jul;15(4):893–898. doi: 10.1128/am.15.4.893-898.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjolander N. O. Studies on Anaerobic Bacteria: XII. The Fermentation Products of Clostridium Thermosaccharolyticum. J Bacteriol. 1937 Oct;34(4):419–428. doi: 10.1128/jb.34.4.419-428.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]


