Summary
Essential hypertension has devastating effects on the brain, being the major cause of stroke and a leading cause of dementia. Hypertension alters the structure of cerebral blood vessels and disrupts intricate vasoregulatory mechanisms that assure an adequate blood supply to the brain. These alterations threaten the cerebral blood supply and increase the susceptibility of the brain to ischemic injury as well as Alzheimer’s disease. This review focuses on the mechanisms by which hypertension disrupts cerebral blood vessels, highlighting recent advances and outstanding issues.
Introduction
Hypertension is one of the major health problems of the western world. Hypertension, defined as elevations in blood pressure above 140 mmHg systolic or 90 mmHg diastolic, afflicts 25% of the general population and is the premier risk factor for serious diseases affecting brain, heart and kidneys (Messerli et al., 2007). Although much is known about the mechanisms controlling blood pressure, a specific cause for hypertension can be ascertained only in a minority of patients. Therefore, in most instances a cause for the elevated blood pressure cannot be found, hence the term “essential hypertension”. Fortunately, a wide variety of treatments are available to lower blood pressure, and their use has reduced the disease burden caused by hypertension (Messerli et al., 2007). Furthermore, a better understanding is emerging of how hypertension induces damage in susceptible organs, raising the possibility of organ-specific therapies (Messerli et al., 2007).
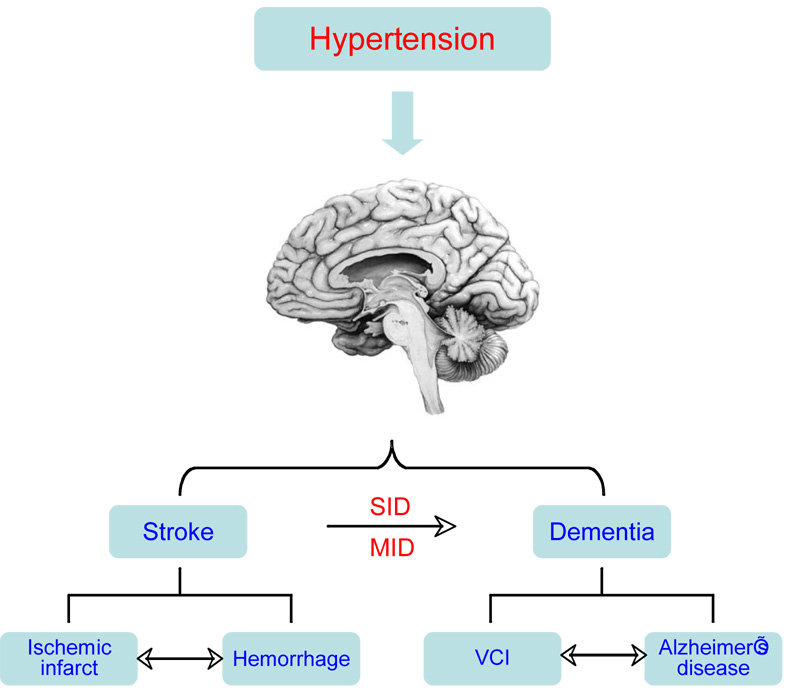
The brain is a major target of the deleterious effects of hypertension and is responsible for a large portion of the related mortality and morbidity (Dahlof, 2007). Hypertension is the number one risk factor for stroke and is a leading cause of cognitive decline and dementia (Dahlof, 2007) (fig. 1). There is a linear relationship between blood pressure and stroke mortality, and in patients with treated hypertension a 1 mmHg increase in systolic blood pressure increases stroke deaths by 2% (Palmer et al., 1992). Furthermore, hypertension is a powerful risk factor for Alzheimer’s disease (AD) (fig. 1), the most common cause of dementia in the elderly (Kelley and Petersen, 2007). Recent advances in neurovascular regulation and in the pathobiology of hypertension have led to a deeper understanding of how hypertension disrupts the cerebral blood supply. These new findings provide the opportunity for the present reappraisal of the cerebrovascular effects of hypertension.
Figure 1. Hypertension, stroke and dementia.
Hypertension has a key role in two major brain pathologies: stroke and dementia. Stroke can result from occlusion of a major cerebral artery (ischemic stroke) or rupture of intracerebral arterioles (hemorrhage). Hypertension also causes rupture of berry aneurysms of the circle of Willis leading to bleeding into the subarachnoid space (subarachnoid hemorrhage). Ischemia can lead to hemorrhage by rupture of ischemic vessels or extravasation of blood from leaky blood vessels. Conversely, hemorrhage can lead to ischemia by compressing the surrounding areas and reducing local blood flow. Vascular cognitive impairment (VCI) is caused by occlusion of small arterioles in the subcortical white matter, which interrupt neural connections subserving cognition and memory (Chui, 2007). A single stroke can lead to dementia by interrupting circuits involved in memory and cognition, such as the midline thalamus (strategic infarct dementia; SID). Multiple strokes can cause dementia by producing cumulative brain damage (multi infarct dementia; MID). Hypertension is a risk factor for Alzheimer’s disease (AD), a progressive dementia caused by accumulation of amyloid-β (Staessen et al., 2007). While vascular dementia and AD were traditionally considered separate entities, recent evidence suggests that they share common and interacting pathogenic factors (Iadecola, 2004).
The cerebral blood supply
The intracranial cerebral arteries take off from the circle of Willis at the base of the brain and give rise to progressively smaller vessels traveling on the brain surface. These surface vessels, termed pial arteries, branch out into smaller vessels, which penetrate into the substance of the brain and give rise to arterioles and capillaries. Like elsewhere in the body, brain blood vessels are lined with endothelial cell. Arteries and arterioles have one or more layers of smooth muscle cells (myocytes), contractile cells that regulate vascular diameter. In capillaries, myocytes are replaced by pericytes. Cerebral arteries and arterioles are innervated by nerve fibers arising from cranial autonomic and sensory ganglia (Iadecola and Nedergaard, 2007). Smaller arterioles (≤100µm) and capillaries are fully enveloped by the end feet processes of astrocytes (Iadecola and Nedergaard, 2007). Owing to the blood-brain barrier (BBB), cerebral capillaries are impermeable to most blood-borne substances (Zlokovic, 2008). Unlike other organs, extraparenchymal arteries and arterioles account for 2/3 of the vascular resistance, while intracerebral arterioles and capillaries account for the remaining 1/3 (Faraci and Heistad, 1990). Therefore, vessels residing outside the brain have the greatest impact on parenchymal blood flow.
Adaptive responses of the cerebral circulation
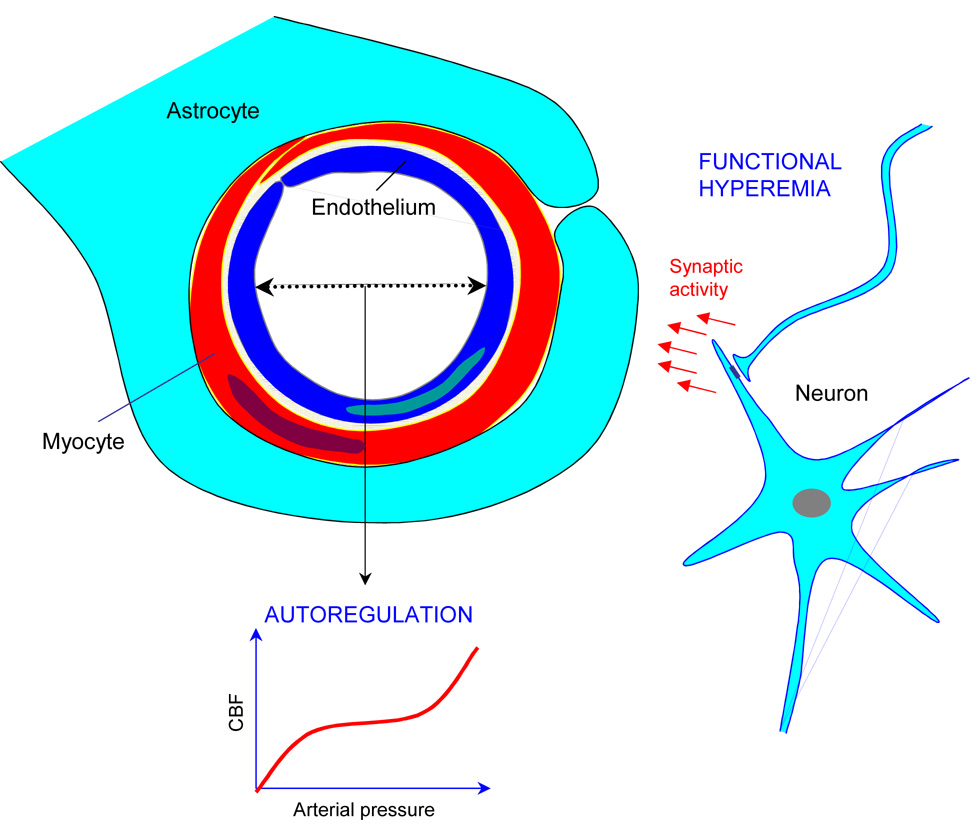
The brain has limited fuel reserves and its integrity depends on a continuous supply of oxygen and energy substrates delivered through blood flow. Thus, cerebral blood vessels are endowed with adaptive mechanisms that assure that the brain receives an adequate amount of blood at all times (fig. 2). The major control mechanisms relevant to the cerebrovascular effects of hypertension are briefly examined below.
Figure 2. The neurovascular unit and adaptive responses of the cerebral circulation.
Neurons, astrocytes, myocytes and endothelial cells act in concert to maintain an adequate cerebral perfusion. Functional hyperemia increases CBF when neural activity increases. Cerebrovascular autoregulation maintains CBF stable during variations in arterial pressure within a certain range. Although not shown in this figure, larger cerebral arteries also contribute to autoregulation (see text). Endothelial cells release potent vasodilatator and constrictor agents in response to chemical and mechanical signals.
Functional hyperemia
The cerebral blood supply is regionally heterogeneous, reflecting the varying energetic needs of different brain regions. When a brain region is activated, cerebral blood flow (CBF) in that particular region increases, a phenomenon termed functional hyperemia (see (Iadecola and Nedergaard, 2007) for a review). Accumulating evidence suggests that neurons, astrocytes and vascular cells release a multitude of vasoactive agents that act in concert to produce vasodilatation of local arterioles during neural activity. These agents include mainly nitric oxide (NO), carbon monoxide, prostanoids, cytochrome p450 metabolites, adenosine, and K+ ions (Iadecola and Nedergaard, 2007). The vasodilatation of local arterioles is accompanied by vasodilatation of upstream pial arteries that supply the activated area. The coordinated vasodilatation of intraparenchymal arterioles and pial arterioles is essential for increasing CBF efficiently, and may involve intercellular communication between vascular cells or astrocytes (Iadecola and Nedergaard, 2007).
Cerebrovascular autoregulation
Cerebrovascular autoregulation renders CBF independent of changes in arterial pressure within a certain range, about 60–150 mmHg mean arterial pressure (Cipolla, 2007; Paulson et al., 1990)(fig. 2, fig. 4A). Arterial pressure varies markedly during normal daily activities (Mancia et al., 1988) and these changes may lead to potentially dangerous increases or decreases in CBF. To counteract the effects of blood pressure variations on CBF, cerebral arterioles adjust their resistance according to intravascular pressure. Thus, arterioles constrict when the pressure increases and relax when the pressure decreases. Autoregulation is related to the ability of arterial myocytes to constrict when intravascular pressure rises (myogenic response) (Brayden et al., 2008). The myogenic response stems from the fact that an increase in intravascular pressure depolarizes arterial myocytes, leading to Ca2+ influx and vasoconstriction (Brayden et al., 2008). In addition to Ca2+ influx, Ca2+ sensitization of the smooth muscle contractile apparatus via protein kinase C (PKC) and Rho kinase also plays a role (Brayden et al., 2008; Chrissobolis and Sobey, 2006).
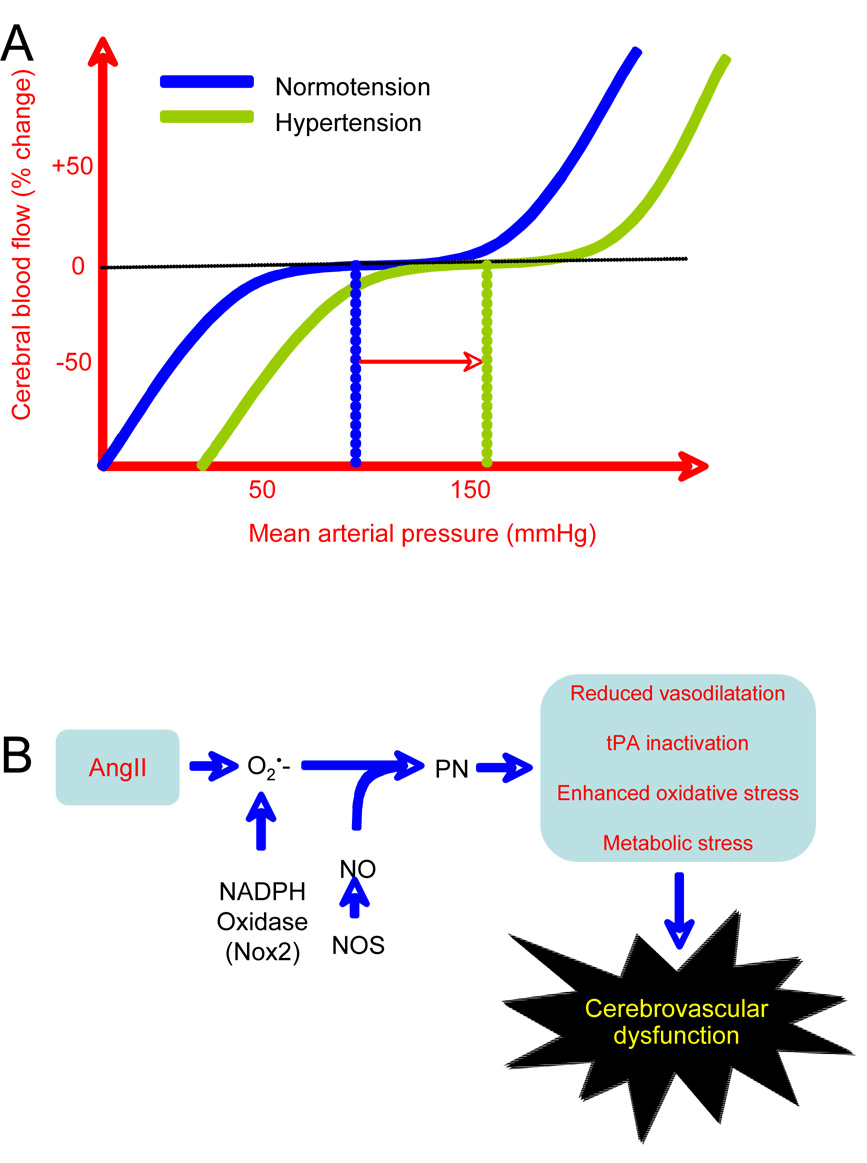
Figure 4.
A. Cerebrovascular autoregulation: CBF is maintained relatively constant despite changes in arterial pressure within a certain range (60 to 150 mmHg mean arterial pressure). Hypertension shifts the curve to the right, so that higher levels of blood pressure are needed to maintain CBF in the autoregulated range. B. Peroxynitrite (PN) production mediates cerebrovascular dysfunction by AngII: PN formed from NADPH-derived superoxide and NO induces vascular dysfunction through different mechanisms (see (Pacher et al., 2007) for references): (1) PN opposes vasodilation by removing NO and by inhibiting prostacylin synthase, the enzyme that produces prostacyclin; (2) PN nitrates tPA and attenuates its proteolytic activity (Nielsen et al., 2004); (3) PN exacerbates oxidative stress by inhibiting SOD and by altering NOS to produce superoxide instead of NO (NOS uncoupling); (4) PN induces DNA damage leading to overactivation of the DNA repair enzyme poly(ADP)ribose polymerase resulting in NAD depletion and energy deficit; (5) PN inactivates mitochondrial enzymes and alcohol dehydrogenase, and can induce metabolic stress.
Endothelial regulation
Cerebral endothelial cells exert powerful effects on vascular tone. by releasing vasodilators (NO, prostacylin, bradykinin, etc.) and vasoconstrictors (endothelin, endothelium-derived constrictor factor, etc.) (Andresen et al., 2006). Endothelium-derived vasoactive factors participate in the maintenance of resting vascular tone and may play a role in coordinating that vasodilatation of intraparenchymal arterioles with that of upstream pial arteries, and in local adjustments of flow in response to mechanical forces (Andresen et al., 2006; Iadecola, 2004).
Hypertension alters the structure of cerebral blood vessels
Atherosclerosis and lipohyalinosis
Hypertension promotes formation of atherosclerotic plaques in cerebral arteries and arterioles, which may lead to arterial occlusions and ischemic injury (Dahlof, 2007; Lammie, 2002)(fig. 3). In addition, hypertension induces fibrinoid necrosis (lipohyalinosis) of penetrating arteries and arterioles supplying the white matter, resulting in small white matter infarcts (lacunes) or brain hemorrhage (Lammie, 2002) (fig. 3).
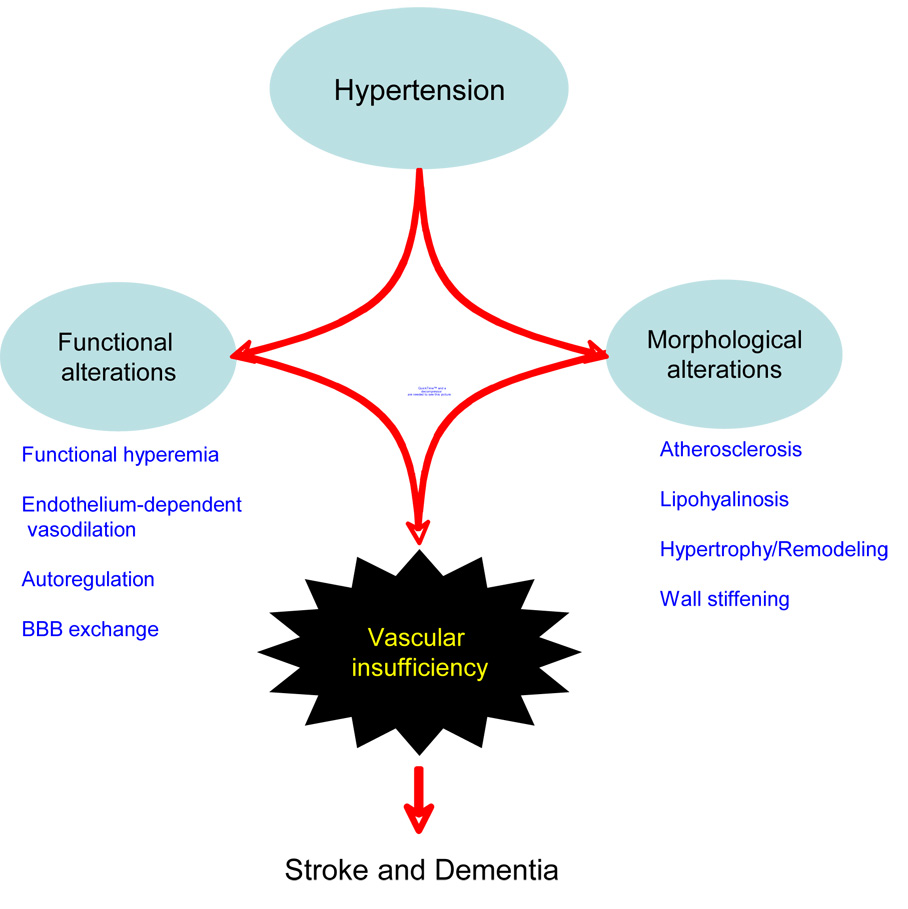
Figure 3. Effect of hypertension on the cerebral blood vessels.
Hypertension induces structural and functional alterations in cerebral blood vessels, which compromise the blood supply to the brain and increase the risk of stroke and dementia. See text for details.
Hypertrophy, remodeling and stiffening
Hypertension induces adaptive changes in systemic and cerebral arteries known as hypertrophic and eutrophic remodeling. In hypertrophic remodeling smooth muscle cells undergo hypertrophy or hyperplasia, and grow inward encroaching into the lumen of the artery. This process increases the wall thickness and reduces the lumen of the vessel (Baumbach and Heistad, 1988). In eutrophic remodeling smooth muscle cells undergo a rearrangement that leads to a reduction of the vessel lumen without changes in total vascular mass or wall thickness (Baumbach and Heistad, 1988). Hypertension also leads to vascular stiffening, a process that increases collagen content and rigidity of the vessel wall (Baumbach and Heistad, 1988).
Several factors contribute to hypertrophy in cerebral arteries and arterioles. The sympathetic perivascular innervation, which exerts a trophic effect on the vascular wall, is required for the development of cerebrovascular hypertrophy (Baumbach et al., 1989). Furthermore, mechanical effects of the elevated intravascular pressure on the vascular wall play a role through growth factors, oxidative stress and NO (Harrison et al., 2006; O'Callaghan and Williams, 2000). Reduced availability of NO, an agent with antiproliferative activity, leads to hyperthophy, as indicated by the vascular growth observed with NOS inhibition or in eNOS-null mice (Table). Interestingly, in eNOS-null mice, which have elevated blood pressure, hyperthrophy persists even if the increase in cerebrovascular pressure is prevented by ligation of the ipsilateral carotid artery (Baumbach et al., 2004). Furthermore, in mice lacking the homocysteine metabolism enzyme cystationine-β synthase or the ROS scavenger enzyme Cu-Zn-superoxide dismutase hypertrophy develops in the absence of hypertension (Table). Therefore, hypertrophy can occur without elevations in intravascular pressure, indicating that mechanical effects on the vascular wall are not an absolute requirement.
Table.
Cerebrovascular remodeling and hypertrophy in mouse models relevant to hypertension
| Mouse model | Relevant phenotype | Hypertrophy | Remodeling | Reference |
|---|---|---|---|---|
| BPH-2 strain | Renin-independent HTN | + | − | (Baumbach et al., 2003) |
| L-NAME treatment | NO-deficiency HTN | + | − | (Baumbach et al., 2004) |
| eNOS−/− | Endothelial NO deficiency HTN | + | − | (Baumbach et al., 2004) |
| SOD+/− | Increased oxidative stress | + | − | (Baumbach et al., 2006) |
| CBS+/− | Increased plasma homocysteine | + | − | (Baumbach et al., 2002) |
| R+/A+ transgenics | AngII-dependent HTN | + | + | (Baumbach et al., 2003) |
| PPAR-γ dom/neg in smooth muscle | Vascular dysregulation HTN | + | + | (Halabi et al., 2008) |
Abbreviations: CBS, cystationine beta synthase; eNOS, endothelial nitric oxide synthase; HTN, hypertension; L-NAME, L-arginine methyl ester; PPARγ, peroxisome proliferator-activated receptor-γ; R+/A+, renin and angiotensinogen; SOD, superoxide dismutase.
Angiotensin II (AngII) has emerged as a key factor in the mechanisms of cerebrovascular remodeling (Schiffrin and Touyz, 2004). Treatment of spontaneously-hypertensive rats with angiotensin converting enzyme inhibitors attenuates remodeling independently of effects on blood pressure (Chillon and Baumbach, 1999). Hypertensive mice overexpressing human renin and angiotensinogen (R+/A+) exhibit both hypertrophy and remodeling, whereas BPH-2 mice, in which hypertension is not related to AngII, exhibit only hypertrophy (Table). ROS participate in the remodeling of cerebral blood vessels induced by AngII (Schiffrin and Touyz, 2004). ROS promote smooth muscle cell proliferation and initiate remodeling of the extracellular matrix via activation of matrix metalloproteases (MMP) (Flamant et al., 2007). Extracellular matrix proteins play a critical role in hypertrophy, remodeling and stiffening. AngII-induced hypertension is associated with activation of MMP and breakdown of matrix proteins (Flamant et al., 2007). Antagonists of the integrin ανβ3 prevent arterial remodeling in mesenteric arteries of renin overexpressing rats (Heerkens et al., 2007). In addition, mice lacking elastin-1 develop vascular stiffening and hypertension (Faury et al., 2003). Similarly, deficiency of the vascular matrix protein emilin-1 leads to increased vascular resistance and hypertension (Zacchigna et al., 2006). Therefore, mechanical, neural and humoral factors contribute to the changes in cerebrovascular wall structure and composition induced by hypertension.
Hypertrophy and remodeling are adaptive responses aimed at reducing stress on the vessel wall and protecting downstream microvessels from the effect of increased pressure (Baumbach and Heistad, 1988; Laurent et al., 2005). Failure of this protective mechanism leads to BBB alterations, cerebral edema and cerebrovascular pathology. Thus, ablation of perivascular sympathetic nerves early in life prevents cerebrovascular hypertrophy in stroke-prone spontaneously hypertensive rats and promotes the development of cerebrovascular lesions (Sadoshima et al., 1983). Similarly, the cerebrovascular alterations associated with eclampsia may be related to the lack of hypertrophy or remodeling during pregnancy (Cipolla, 2007). On the other hand, remodeling of systemic or cerebral vessels is potentially damaging because it reduces the vessel’s lumen and increases vascular resistance resulting in a greater propensity for vascular insufficiency (Barry, 1985; Mathiassen et al., 2007) (see next section). Arterial stiffening is also deleterious because it leads to increases in pulse pressure, a good predictor of stroke and cognitive impairment (Benjo et al., 2007; Waldstein et al., 2008).
The relationships among stiffening, hypertrophy and remodeling are poorly understood (Laurent et al., 2005). Cerebral arterioles undergoing hypertrophy or remodeling have reduced stiffening (Baumbach et al., 2003; Chillon and Baumbach, 1999). However, if hypertension is sustained the changes in vascular wall composition may lead to reduced distensibility and stiffening (Izzard et al., 2006). Therefore, duration and magnitude of the blood pressure elevation, as well as vessel size are important determinants of the alteration in the vascular wall induced by hypertension.
In summary, hypertension has profound effects on the structure of cerebral blood vessels. While atherosclerosis and lipohyalinosis may lead to vascular occlusions, rearrangement of the cellular architecture and changes in the composition of the vascular wall alters the mechanical and hemodynamic properties of the vessels. The functional implications of these structural changes are examined in the next section.
Hypertension alters resting CBF and disrupts adaptive responses of the cerebral circulation
Alterations in resting CBF and functional hyperemia
Cross sectional studies of CBF in hypertensive patients have usually reported unchanged or slightly reduced resting CBF, reflecting an autoregulatory increase in cerebrovascular resistance to counteract the increase in perfusion pressure and keep CBF constant (Jennings et al., 2005; Kety et al., 1948; Matsushita et al., 1994). However, longitudinal studies have shown reductions in CBF in selected brain regions (Beason-Held et al., 2007). The CBF reductions often precede cerebrovascular symptoms or white matter lesions (O'Sullivan et al., 2002). CBF reductions have also been observed in stroke-prone spontaneously hypertensive rats just prior to developing brain infarcts (Mies et al., 1999). The CBF reductions are likely to result from a combination of factors, including aging, reduced brain activity, vascular narrowing, as well as increased vascular tone secondary to endothelial dysfunction (Barry, 1985; Mentis et al., 1994; Park et al., 2007). Hypertension also alters functional hyperemia. The increase in CBF induced by brain activation is attenuated in patients with chronic hypertension (Jennings et al., 2005). The cerebrovascular dysfunction correlates with cognitive deficits (Jennings et al., 2005). In mice in which AngII was administered systemically for 30 min or 7 days, the increase in CBF induced by activation of the somatosensory cortex by stimulation of the facial whiskers is markedly attenuated (Kazama et al., 2003). The attenuation is not dependent on the elevation in blood pressure, because application of AngII to the somatosensory cortex attenuates functional hyperemia without inducing hypertension (Kazama et al., 2003). Therefore, the effects of AngII on functional hyperemia can occur independently of elevations in blood pressure.
Endothelial dysfunction
Hypertension alters endothelium-dependent relaxation of cerebral blood vessels. The increase in CBF produced by neocortical application of endothelium-dependent vasodilators is attenuated in spontaneously hypertensive rats, in R+/A+ mice or in wild type mice infused systemically with AngII (Didion et al., 2000; Kazama et al., 2003; Yang et al., 1991). In contrast, responses mediated by the smooth muscle relaxants adenosine and nitroprusside are not attenuated (Didion and Faraci, 2003; Kazama et al., 2003), indicating that the deficit of vasodilation is not due to a non-specific reduction in vascular reactivity or to smooth muscle dysfunction. Altered responses to acetylcholine have also been reported in the vertebral artery of hypertensive patients (Charpie et al., 1996).
Impairment of autoregulation
Hypertension alters cerebrovascular autoregulation leading to a shift of the pressure-flow curve to the right (fig. 4A) (Paulson et al., 1990). Consequently, in hypertension higher perfusion pressures are needed to maintain the same level of CBF (fig. 4A). Studies using transcranial Doppler to measure middle cerebral artery flow velocity have shown that transient changes in blood pressure still elicit compensatory flow adjustments (Traon et al., 2002), indicating that the ability of cerebral vessels to adapt to dynamic pressure changes may be preserved despite the shift of the pressure-flow curve. The shift in autoregulation is related to the increase in myogenic tone induced by an increase in Ca2+ sensitivity of myocytes (Chrissobolis and Sobey, 2006). Remodeling and hypertrophy also contribute to the shift in autoregulation by reducing the vascular lumen and increasing cerebrovascular resistance (Barry, 1985; Baumbach and Heistad, 1988). Alterations in autoregulation increase the susceptibility of the brain to cerebral ischemia when blood pressure drops because cerebral blood vessels fail to compensate for the reduction in perfusion pressure (Immink et al., 2004). The periventricular white matter is most susceptible to ischemic damage because it is located at the boundary between two arterial territories: penetrating arteries reaching down from the brain surface and basal ganglia arteries arising from the base of the brain (De Reuck, 1971). In hypertensive patients, the severity of periventricular white matter injury, or leukoaraiosis, correlates with the magnitude of autoregulatory dysfunction (Matsushita et al., 1994) and with cognitive impairment as well (Chui, 2007). Impaired autoregulation also leads to more severe ischemia after arterial occlusion. Thus, middle cerebral artery occlusion in spontaneously hypertensive rats leads to larger infarcts than in normotensive rats (Nishimura et al., 2000).
To summarize, the alterations in functional hyperemia, autoregulation and endothelial function induced by hypertension act in concert with the structural alterations of cerebral blood vessels to reduce the compensatory capacity of the cerebral circulation and increases the susceptibility of the brain to vascular insufficiency (fig. 3).
Oxidative stress is a critical factor in the cerebrovascular effects of hypertension
Increasing evidence suggests that oxidative stress is involved in the deleterious effects of hypertension. In humans, systemic markers of oxidative stress are increases in essential hypertension, renovascular hypertension, malignant hypertension and preeclampsia (Touyz, 2004). Oxidative stress in brain and cerebral blood vessels plays a central role in the mechanisms of hypertension and its cerebrovascular effects. In models of hypertension, ROS production is elevated in central autonomic regions implicated in blood pressure control (see (Peterson et al., 2006) for a review). ROS production within these brain regions contributes to the neurohumoral changes that drive the hypertension (Peterson et al., 2006). In addition, hypertension induces oxidative stress in cerebral blood vessels. In models of AngII-induced hypertension, ROS production increases in large and small cerebral vessels, including pial arterioles (Didion et al., 2005; Girouard et al., 2006, 2007; Kazama et al., 2004). Free radical scavengers prevent the effects of hypertension on functional hyperemia and endothelium-dependent responses, indicating that the cerebrovascular dysfunction is mediated by ROS (Didion et al., 2005; Girouard et al., 2006; Kazama et al., 2004).
NADPH Oxidase as a source of ROS
There are several potential sources of ROS in cerebrovascular cells, including mitochondrial enzymes, xanthine/xanthine oxidase, and NOS uncoupling, a condition in which NOS produces superoxide instead of NO (Faraci, 2006). However, the enzyme NADPH oxidase has emerged as a major source of the ROS mediating cerebrovascular dysfunction. NADPH oxidase is a multiunit enzyme initially discovered in phagocytes that is also present in vascular cells and is particularly enriched in cerebral blood vessels (Miller et al., 2005). The enzyme is comprised of membrane bound (p22phox and Nox) and cytoplasmic subunits (p47phox, p67phox) and requires the small GTPase Rac for its activation (Bedard and Krause, 2007). Nox is present in 5 homologues, Nox1 through 5, and is the catalytic site of the enzyme (Bedard and Krause, 2007). Nox1, 2 and 4 are present in cerebral blood vessels (Ago et al., 2005; Kazama et al., 2004; Miller et al., 2005). Activation of AngII AT1 type receptors leads to activation of phospholipase C, which increases cytoplasmic Ca2+ and activates PKC (Bedard and Krause, 2007). PKC, in turn, phosphorylates p47phox leading to the assembly of the enzyme and ROS production (Bedard and Krause, 2007). NADPH oxidase-derived ROS have been implicated in the cerebrovascular dysfunction induced by AngII. Mice lacking Nox2 do not exhibit cerebrovascular oxidative stress and are protected from the alterations in endothelium-dependent relaxation and functional hyperemia induced by AngII (Girouard et al., 2006; Kazama et al., 2004). Furthermore, a peptide inhibiting the assembly of NADPH oxidase or a pharmacological inhibitor of this enzyme blocks the ROS production and cerebrovascular dysfunction induced by AngII (Didion and Faraci, 2003; Girouard et al., 2006).
Peroxynitrite and cerebrovascular dysfunction
One prominent pathway through which ROS alter vascular regulation involves formation of peroxynitrite, the product of the reaction between NO and the radical superoxide (Pacher et al., 2007). Peroxynitrite exerts powerful biological effects by inducing DNA damage and lipid peroxidation, and by altering protein function through interaction with transition metal centers, tyrosine nitration and cysteine oxidation (Pacher et al., 2007). Peroxynitrite can have deleterious effects on cerebral blood vessels (Faraci, 2006)(fig. 4B). Recent evidence suggests that peroxynitrite is critical for the cerebrovascular dysfunction induced by AngII. Systemic administration of AngII produces marked nitration of cerebral arterioles, which depends on NO and NADPH oxidase-derived superoxide (Girouard et al., 2007). Inhibition of nitration with a peroxynitrite decomposition catalyst or with the peroxynitrite inactivator uric acid prevents the cerebrovascular dysfunction induced by AngII (Girouard et al., 2007). The observation that AngII does not induce vascular nitration and cerebrovascular dysfunction in eNOS-null mice points to the endothelium as the main source of NO responsible for peroxynitrite formation (Girouard et al., 2007). Another pathway through which AngII could induce vascular dysfunction involves the tissue plasminogen activator (tPA). In addition to its role in intravascular fibrinolysis, tPA is a powerful neuromodulator, which is critical for NMDA receptor signaling (Samson and Medcalf, 2006). tPA contributes to functional hyperemia by regulating the coupling between NMDA receptor activity and neuronal NO production (Park et al., 2008a). AngII counteracts the biological effect of tPA by upregulating the expression of its endogenous inhibitor plasminogen activator inhibitor-1 (PAI-1) (Chen and Feener, 2004). In addition, peroxynitrite attenuates tPA proteolytic activity (Nielsen et al., 2004), providing an additional pathway through which AngII-induced peroxynitrite could impair vascular regulation (fig. 4B).
In summary, ROS are key mediators of the cerebrovascular effects of hypertension. They participate in the structural remodeling of cerebral blood vessels and in the functional alterations induced by hypertension. Some of these effects are mediated by vascular nitrosative stress induced by peroxynitrite derived from NADPH oxidase-derived superoxide and NO.
Hypertension and Alzheimer’s disease
AD has traditionally been considered a neurodegenerative condition caused by neuronal dysfunction resulting from brain accumulation of amyloid-β (amyloid plaques) and neuronal cytoskeletal abnormalities (neurofibrillary tangles) (Kelley and Petersen, 2007). However, epidemiological, pathological and experimental evidence suggests that vascular factors, including hypertension, play a significant role in the pathogenesis of AD (see (Iadecola, 2004) for a review). In particular, midlife hypertension increases the risk of AD later in life and accelerates the progression of AD (see (Skoog and Gustafson, 2006) for a review). Furthermore, brain atrophy, amyloid plaques and neurofibrillary tangles are increased in the brain of patients with a history of midlife hypertension (Skoog and Gustafson, 2006). AD patients have white matter lesions resembling those associated with hypertension, and if these lesions coexist with amyloid plaques they produce cognitive deficits greater than those predicted based on the AD pathology alone (Sheng et al., 2007; Snowdon et al., 1997). A proportion of patients with AD also have ischemic brain lesions and AD patients have more severe atherosclerosis in cerebral arteries than non demented controls (Roher et al., 2003; Skoog and Gustafson, 2006). Studies in mouse models of AD have demonstrated that amyloid-β disrupts the function of cerebral blood vessels (Iadecola, 2004). Amyloid-β attenuates functional hyperemia and endothelium-dependent responses (Iadecola et al., 1999; Niwa et al., 2000), and impairs cerebrovascular autoregulation (Niwa et al., 2002). Interestingly, the mechanisms of amyloid-β-induced cerebrovascular dysfunction are similar to those of AngII and involve NADPH oxidase-derived ROS. Thus, mice lacking Nox2 are protected from the deleterious cerebrovascular and cognitive effects of brain amyloid-β accumulation (Park et al., 2008b). Hypertension could promote AD by increasing the production of the amyloid-β peptide. This possibility is supported by the finding that cerebral hypoxia-ischemia, which can occur with hypertension, facilitates the cleavage of amyloid-β from its precursor protein and increase the brain’s amyloid-β burden (Sun et al., 2006). Furthermore, the brain deposition of circulating amyloid-β is increased in models of hypertension, suggesting another mechanism by which hypertension could increase brain amyloid-β (Gentile et al., 2008). However, in the years immediately preceding the onset of dementia blood pressure begins to decrease in AD patients and continues to decline during the course of the illness (Skoog and Gustafson, 2006). Because autoregulation is impaired by amyloid-β, hypotension is likely to cause cerebral hypoperfusion, which, in turn, can enhance amyloid-β cleavage and deposition (Bennett et al., 2000).
The biological bases of the reciprocal interaction between AD and hypertension remain unclear. It is unlikely that AD pathology, i.e., plaques and tangles, causes the elevation in arterial pressure, because hypertension occurs decades before the onset of symptoms. Although AD has a long presymptomatic period, this preclinical phase is not likely span over several decades (Kelley and Petersen, 2007). Therefore, hypertension may precede AD pathology and, as such, could have a role in the pathogenesis of the disease. On the other hand, the reduction in arterial pressure that occurs when AD is fully developed is more likely to be related to pathological changes in central autonomic nuclei controlling blood pressure, such as the C1 area of the rostral ventrolateral medulla (Burke et al., 1994). The reduction in physical activity, dehydration and malnutrition associated with severe dementia could also contribute to the decrease in blood pressure (Skoog and Gustafson, 2006). Therefore, while hypertension may promote the development of amyloid plaques early in the course of the disease, the pathological changes induced by AD lead to a reduction in arterial pressure in the late phase. The hypotension, in turns, may produce hypoxia-ischemia, which acts synergistically with AD pathology to worsen the dementia.
Challenges and opportunities
Great progress has been made in understanding the biological bases of hypertension and the mechanisms underlying its powerful effects on the brain circulation. Although these developments have unveiled new avenues for the treatment of the devastating effects of hypertension on the brain, they also have raised new questions that remain to be addressed. For example, the effects of blood pressure control on stroke risk are well documented and emerging data suggest that controlling blood pressure reverses some of the effects of hypertension on cerebral blood vessels and ameliorates cognition (Peila et al., 2006). Therefore, treatment of elevated blood pressure remains the mainstay of preventive approaches to protect the brain and the other organs from hypertension (Dahlof, 2007; Messerli et al., 2007). In addition, considering the role of hypertension in amyloid-β neuropathology, controlling hypertension may be valuable for AD prevention as well (Skoog and Gustafson, 2006). However, questions remain about the “ideal” blood pressure that should be achieved in hypertensive patients. The linear relationship between stroke mortality and blood pressure suggests that the lower the value the better the outcome, but excessive lowering of diastolic blood pressure may increase the incidence of periventricular white matter lesions (van Dijk et al., 2004). This is clearly an area that requires further investigation.
Another question concerns whether all blood pressure lowering agents are equivalent or whether specific agents may afford brain protection beyond that achieved by their antihypertensive effect. In clinical trials, angiotensin converting enzyme inhibitors or AT1 receptor antagonists conferred greater protection against stroke or dementia than treatment with other agents despite comparable blood pressure lowering (Dahlof, 2007; Messerli et al., 2007). This finding is consistent with the experimental observation that some of the cerebrovascular effects of AngII are independent of the blood pressure elevation (Chillon and Baumbach, 1999; Kazama et al., 2003), raising the possibility that the deleterious effects of AngII on the cerebral circulation are not secondary to the effect of this peptide on systemic blood pressure. However, some studies have failed to observe a benefit of agents targeting the renin-angiotensin system versus other antihypertensive treatments (Messerli et al., 2007; Staessen et al., 2007). Therefore, more data are needed on the effects of antagonists of the renin-angiotensin system and other antihypertensive agents in the prevention of the cerebral complications of hypertension. Interestingly, the AT1 receptor antagonist valsartan reduces amyloid plaques in a mouse model of AD and improves behavioral performance without altering arterial pressure (Wang et al., 2007). Furthermore, Rho kinase inhibitors and statins could also have a role in the treatment of the deleterious effects of hypertension on the brain (Chrissobolis and Sobey, 2006). Like AT1 receptor inhibitors, these agents are attractive because they also have neuroprotective effects against ischemia and amyloid-β pathology (Chrissobolis and Sobey, 2006; Mueller et al., 2005). Therefore, the choice of the antihypertensive agents used may also be important in the prevention of stroke and dementia.
Animal studies have demonstrated that ROS have a prominent role in the pathogenic effects of hypertension on the brain and other organs, yet free radical scavengers have failed to provide a benefit in cardiovascular diseases and stroke (Griendling and FitzGerald, 2003). The finding that NADPH oxidase is the major source of the ROS involved in the cerebrovascular effects of hypertension suggests that treatments targeting this enzyme would be effective. However, in light of the role of NADPH oxidase in immune defense, chronic inhibition of NADPH oxidase is not a viable strategy. Thus, approaches to selectively inhibit NADPH oxidase-dependent ROS production in vascular cells need to be developed. A greater understanding of the cellular and molecular features of vascular NADPH oxidase is needed to develop pharmacological strategies for its selective inhibition.
The fact that PPARγ may serve as an endogenous cerebrovasoprotective system (Halabi et al., 2008), suggests that PPARγ activators, such as thiazolidinediones used in the treatment of type 2 diabetes, could have therapeutic potential in the deleterious effects of hypertension. However, recent meta-analyses indicate that PPARγ activators increase the risk of cardiovascular events (Nissen and Wolski, 2007). Therefore, the role of PPARγ in cardiovascular and cerebrovascular diseases needs further scrutiny.
Better animal models of essential hypertension are needed to address some questions still outstanding (Lerman et al., 2005). In particular, models that allow investigators to introduce genetic manipulations in a temporally defined and cell specific fashion would be highly desirable. Furthermore, models that combine other vascular risk factors, such as aging, gender, diabetes, hyperhomocysteinemia, hyperlipidemia, etc., would be useful by reflecting more closely the context in which the human disease occurs.
Acknowledgments
Supported by National Institute of Health grants HL18974 (CI), HL63887 (RLD), and HL84624 (RLD), and by American Heart Association grant 050114N (RLD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Costantino Iadecola, Division of Neurobiology, Department of Neurology and Neuroscience, Weill Cornell Medical College, New York, NY.
Robin L. Davisson, Department of Biomedical Sciences, College of Veterinary Medicine, Cornell University, Ithaca, NY, and Department of Cell and Developmental Biology, Weill Cornell Medical College, New York, NY
References
- Ago T, Kitazono T, Kuroda J, Kumai Y, Kamouchi M, Ooboshi H, Wakisaka M, Kawahara T, Rokutan K, Ibayashi S, et al. NAD(P)H oxidases in rat basilar arterial endothelial cells. Stroke. 2005;36:1040–1046. doi: 10.1161/01.STR.0000163111.05825.0b. [DOI] [PubMed] [Google Scholar]
- Andresen J, Shafi NI, Bryan RM., Jr Endothelial influences on cerebrovascular tone. J Appl Physiol. 2006;100:318–327. doi: 10.1152/japplphysiol.00937.2005. [DOI] [PubMed] [Google Scholar]
- Barry DI. Cerebral blood flow in hypertension. J Cardiovasc Pharmacol. 1985;7 Suppl 2:S94–S98. doi: 10.1097/00005344-198507002-00018. [DOI] [PubMed] [Google Scholar]
- Baumbach GL, Didion SP, Faraci FM. Hypertrophy of cerebral arterioles in mice deficient in expression of the gene for CuZn superoxide dismutase. Stroke. 2006;37:1850–1855. doi: 10.1161/01.STR.0000227236.84546.5a. [DOI] [PubMed] [Google Scholar]
- Baumbach GL, Heistad DD. Cerebral circulation in chronic arterial hypertension. Hypertension. 1988;12:89–95. doi: 10.1161/01.hyp.12.2.89. [DOI] [PubMed] [Google Scholar]
- Baumbach GL, Heistad DD, Siems JE. Effect of sympathetic nerves on composition and distensibility of cerebral arterioles in rats. J Physiol. 1989;416:123–140. doi: 10.1113/jphysiol.1989.sp017753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbach GL, Sigmund CD, Bottiglieri T, Lentz SR. Structure of cerebral arterioles in cystathionine beta-synthase-deficient mice. Circ Res. 2002;91:931–937. doi: 10.1161/01.res.0000041408.64867.1d. [DOI] [PubMed] [Google Scholar]
- Baumbach GL, Sigmund CD, Faraci FM. Cerebral arteriolar structure in mice overexpressing human renin and angiotensinogen. Hypertension. 2003;41:50–55. doi: 10.1161/01.hyp.0000042427.05390.5c. [DOI] [PubMed] [Google Scholar]
- Baumbach GL, Sigmund CD, Faraci FM. Structure of cerebral arterioles in mice deficient in expression of the gene for endothelial nitric oxide synthase. Circ Res. 2004;95:822–829. doi: 10.1161/01.RES.0000146279.11923.14. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Moghekar A, Zonderman AB, Kraut MA, Resnick SM. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke. 2007;38:1766–1773. doi: 10.1161/STROKEAHA.106.477109. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Benjo A, Thompson RE, Fine D, Hogue CW, Alejo D, Kaw A, Gerstenblith G, Shah A, Berkowitz DE, Nyhan D. Pulse pressure is an age-independent predictor of stroke development after cardiac surgery. Hypertension. 2007;50:630–635. doi: 10.1161/HYPERTENSIONAHA.107.095513. [DOI] [PubMed] [Google Scholar]
- Bennett SA, Pappas BA, Stevens WD, Davidson CM, Fortin T, Chen J. Cleavage of amyloid precursor protein elicited by chronic cerebral hypoperfusion. Neurobiol Aging. 2000;21:207–214. doi: 10.1016/s0197-4580(00)00131-7. [DOI] [PubMed] [Google Scholar]
- Brayden JE, Earley S, Nelson MT, Reading S. Transient Receptor Potential (Trp) Channels, Vascular Tone and Autoregulation of Cerebral Blood Flow. Clin Exp Pharmacol Physiol. 2008 doi: 10.1111/j.1440-1681.2007.04855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke WJ, Galvin NJ, Chung HD, Stoff SA, Gillespie KN, Cataldo AM, Nixon RA. Degenerative changes in epinephrine tonic vasomotor neurons in Alzheimer's disease. Brain Res. 1994;661:35–42. doi: 10.1016/0006-8993(94)91177-0. [DOI] [PubMed] [Google Scholar]
- Charpie JR, Schreur KD, Papadopoulos SM, Webb RC. Acetylcholine induces contraction in vertebral arteries from treated hypertensive patients. Clin Exp Hypertens. 1996;18:87–99. doi: 10.3109/10641969609082609. [DOI] [PubMed] [Google Scholar]
- Chen HC, Feener EP. MEK1,2 response element mediates angiotensin II-stimulated plasminogen activator inhibitor-1 promoter activation. Blood. 2004;103:2636–2644. doi: 10.1182/blood-2003-05-1737. [DOI] [PubMed] [Google Scholar]
- Chillon JM, Baumbach GL. Effects of an angiotensin-converting enzyme inhibitor and a beta-blocker on cerebral arterioles in rats. Hypertension. 1999;33:856–861. doi: 10.1161/01.hyp.33.3.856. [DOI] [PubMed] [Google Scholar]
- Chrissobolis S, Sobey CG. Recent evidence for an involvement of rho-kinase in cerebral vascular disease. Stroke. 2006;37:2174–2180. doi: 10.1161/01.STR.0000231647.41578.df. [DOI] [PubMed] [Google Scholar]
- Chui HC. Subcortical ischemic vascular dementia. Neurol Clin. 2007;25:717–740. doi: 10.1016/j.ncl.2007.04.003. vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ. Cerebrovascular function in pregnancy and eclampsia. Hypertension. 2007;50:14–24. doi: 10.1161/HYPERTENSIONAHA.106.079442. [DOI] [PubMed] [Google Scholar]
- Dahlof B. Prevention of stroke in patients with hypertension. Am J Cardiol. 2007;100:17J–24J. doi: 10.1016/j.amjcard.2007.05.010. [DOI] [PubMed] [Google Scholar]
- De Reuck J. The human periventricular arterial blood supply and the anatomy of cerebral infarctions. Eur Neurol. 1971;5:321–334. doi: 10.1159/000114088. [DOI] [PubMed] [Google Scholar]
- Didion SP, Faraci FM. Angiotensin II produces superoxide-mediated impairment of endothelial function in cerebral arterioles. Stroke. 2003;34:2038–2042. doi: 10.1161/01.STR.0000081225.46324.AA. [DOI] [PubMed] [Google Scholar]
- Didion SP, Kinzenbaw DA, Faraci FM. Critical role for CuZn-superoxide dismutase in preventing angiotensin II-induced endothelial dysfunction. Hypertension. 2005;46:1147–1153. doi: 10.1161/01.HYP.0000187532.80697.15. [DOI] [PubMed] [Google Scholar]
- Didion SP, Sigmund CD, Faraci FM. Impaired endothelial function in transgenic mice expressing both human renin and human angiotensinogen. Stroke. 2000;31:760–764. doi: 10.1161/01.str.31.3.760. [DOI] [PubMed] [Google Scholar]
- Faraci FM. Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol. 2006;100:739–743. doi: 10.1152/japplphysiol.01044.2005. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad HH. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, et al. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest. 2003;112:1419–1428. doi: 10.1172/JCI19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamant M, Placier S, Dubroca C, Esposito B, Lopes I, Chatziantoniou C, Tedgui A, Dussaule JC, Lehoux S. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension. 2007;50:212–218. doi: 10.1161/HYPERTENSIONAHA.107.089631. [DOI] [PubMed] [Google Scholar]
- Gentile MT, Poulet R, Pardo AD, Cifelli G, Maffei A, Vecchione C, Passarelli F, Landolfi A, Carullo P, Lembo G. beta-Amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiol Aging in press. 2008 doi: 10.1016/j.neurobiolaging.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through nox-2-derived radicals. Arterioscler Thromb Vasc Biol. 2006;26:826–832. doi: 10.1161/01.ATV.0000205849.22807.6e. [DOI] [PubMed] [Google Scholar]
- Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2007;27:303–309. doi: 10.1161/01.ATV.0000253885.41509.25. [DOI] [PubMed] [Google Scholar]
- Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, Sigmund CD. Interference with PPARgamma Function in Smooth Muscle Causes Vascular Dysfunction and Hypertension. Cell Metab. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med. 2006;259:351–363. doi: 10.1111/j.1365-2796.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- Heerkens EH, Izzard AS, Heagerty AM. Integrins, vascular remodeling, and hypertension. Hypertension. 2007;49:1–4. doi: 10.1161/01.HYP.0000252753.63224.3b. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, Younkin S, Borchelt DR, Hsiao KK, Carlson GA. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2:157–161. doi: 10.1038/5715. [DOI] [PubMed] [Google Scholar]
- Immink RV, van den Born BJ, van Montfrans GA, Koopmans RP, Karemaker JM, van Lieshout JJ. Impaired cerebral autoregulation in patients with malignant hypertension. Circulation. 2004;110:2241–2245. doi: 10.1161/01.CIR.0000144472.08647.40. [DOI] [PubMed] [Google Scholar]
- Izzard AS, Horton S, Heerkens EH, Shaw L, Heagerty AM. Middle cerebral artery structure and distensibility during developing and established phases of hypertension in the spontaneously hypertensive rat. J Hypertens. 2006;24:875–880. doi: 10.1097/01.hjh.0000222757.54111.06. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Muldoon MF, Ryan C, Price JC, Greer P, Sutton-Tyrrell K, van der Veen FM, Meltzer CC. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005;64:1358–1365. doi: 10.1212/01.WNL.0000158283.28251.3C. [DOI] [PubMed] [Google Scholar]
- Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH-oxidase-derived radicals. Circ Res. 2004;95:1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- Kazama K, Wang G, Frys K, Anrather J, Iadecola C. Angiotensin II attenuates functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2003;285:H1890–H1899. doi: 10.1152/ajpheart.00464.2003. [DOI] [PubMed] [Google Scholar]
- Kelley BJ, Petersen RC. Alzheimer's disease and mild cognitive impairment. Neurol Clin. 2007;25:577–609. doi: 10.1016/j.ncl.2007.03.008. v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety SS, Hafkenschiel JH, Jeffers WA, Leopold IH, Shenkin HA. The Blood Flow, Vascular Resistance, and Oxygen Consumption of the Brain in Essential Hypertension. J Clin Invest. 1948;27:511–514. doi: 10.1172/JCI101998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammie GA. Hypertensive cerebral small vessel disease and stroke. Brain Pathol. 2002;12:358–370. doi: 10.1111/j.1750-3639.2002.tb00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P, Lacolley P. Structural and genetic bases of arterial stiffness. Hypertension. 2005;45:1050–1055. doi: 10.1161/01.HYP.0000164580.39991.3d. [DOI] [PubMed] [Google Scholar]
- Lerman LO, Chade AR, Sica V, Napoli C. Animal models of hypertension: an overview. J Lab Clin Med. 2005;146:160–173. doi: 10.1016/j.lab.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Mancia G, Parati G, Albini F, Villani A. Circadian blood pressure variations and their impact on disease. J Cardiovasc Pharmacol. 1988;12 Suppl 7:S11–S17. doi: 10.1097/00005344-198812007-00003. [DOI] [PubMed] [Google Scholar]
- Mathiassen ON, Buus NH, Sihm I, Thybo NK, Morn B, Schroeder AP, Thygesen K, Aalkjaer C, Lederballe O, Mulvany MJ, et al. Small artery structure is an independent predictor of cardiovascular events in essential hypertension. J Hypertens. 2007;25:1021–1026. doi: 10.1097/HJH.0b013e32805bf8ed. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Kuriyama Y, Nagatsuka K, Nakamura M, Sawada T, Omae T. Periventricular white matter lucency and cerebral blood flow autoregulation in hypertensive patients. Hypertension. 1994;23:565–568. doi: 10.1161/01.hyp.23.5.565. [DOI] [PubMed] [Google Scholar]
- Mentis MJ, Salerno J, Horwitz B, Grady C, Schapiro MB, Murphy DG, Rapoport SI. Reduction of functional neuronal connectivity in long-term treated hypertension. Stroke. 1994;25:601–607. doi: 10.1161/01.str.25.3.601. [DOI] [PubMed] [Google Scholar]
- Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370:591–603. doi: 10.1016/S0140-6736(07)61299-9. [DOI] [PubMed] [Google Scholar]
- Mies G, Hermann D, Ganten U, Hossmann KA. Hemodynamics and metabolism in stroke-prone spontaneously hypertensive rats before manifestation of brain infarcts. J Cereb Blood Flow Metab. 1999;19:1238–1246. doi: 10.1097/00004647-199911000-00008. [DOI] [PubMed] [Google Scholar]
- Miller AA, Drummond GR, Schmidt HH, Sobey CG. NADPH oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ Res. 2005;97:1055–1062. doi: 10.1161/01.RES.0000189301.10217.87. [DOI] [PubMed] [Google Scholar]
- Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4:387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- Nielsen VG, Crow JP, Zhou F, Parks DA. Peroxynitrite inactivates tissue plasminogen activator. Anesth Analg. 2004;98:1312–1317. doi: 10.1213/01.ane.0000111105.38836.f6. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Ito T, Saavedra JM. Angiotensin II AT(1) blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke. 2000;31:2478–2486. doi: 10.1161/01.str.31.10.2478. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol. 2002;283:H315–H323. doi: 10.1152/ajpheart.00022.2002. [DOI] [PubMed] [Google Scholar]
- Niwa K, Younkin L, Ebeling C, Turner SK, Westaway D, Younkin S, Ashe KH, Carlson GA, Iadecola C. Abeta 1–40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci U S A. 2000;97:9735–9740. doi: 10.1073/pnas.97.17.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan CJ, Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: role of TGF-beta(1) Hypertension. 2000;36:319–324. doi: 10.1161/01.hyp.36.3.319. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Lythgoe DJ, Pereira AC, Summers PE, Jarosz JM, Williams SC, Markus HS. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology. 2002;59:321–326. doi: 10.1212/wnl.59.3.321. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AJ, Bulpitt CJ, Fletcher AE, Beevers DG, Coles EC, Ledingham JG, O'Riordan PW, Petrie JC, Rajagopalan BE, Webster J, et al. Relation between blood pressure and stroke mortality. Hypertension. 1992;20:601–605. doi: 10.1161/01.hyp.20.5.601. [DOI] [PubMed] [Google Scholar]
- Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- Park L, Gallo EF, Anrather J, Wang G, Norris EH, Paul J, Strickland S, Iadecola C. Key role of tissue plasminogen activator in neurovascular coupling. Proc Natl Acad Sci U S A. 2008a;105:1073–1078. doi: 10.1073/pnas.0708823105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A. 2008b;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:162–192. [PubMed] [Google Scholar]
- Peila R, White LR, Masaki K, Petrovitch H, Launer LJ. Reducing the Risk of Dementia. Efficacy of Long-Term Treatment of Hypertension. Stroke. 2006;37:1165–1170. doi: 10.1161/01.STR.0000217653.01615.93. [DOI] [PubMed] [Google Scholar]
- Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep. 2006;8:232–241. doi: 10.1007/s11906-006-0056-1. [DOI] [PubMed] [Google Scholar]
- Roher AE, Esh, Kokjohn Ta, Kalback, Luehrs Dc, Seward Jd, Sue, et al. Circle of Willis Atherosclerosis Is a Risk Factor for Sporadic Alzheimer's Disease. Arterioscler Thromb Vasc Biol. 2003;23:2055–2062. doi: 10.1161/01.ATV.0000095973.42032.44. [DOI] [PubMed] [Google Scholar]
- Sadoshima S, Busija DW, Heistad DD. Mechanisms of protection against stroke in stroke-prone spontaneously hypertensive rats. Am J Physiol. 1983;244:H406–H412. doi: 10.1152/ajpheart.1983.244.3.H406. [DOI] [PubMed] [Google Scholar]
- Samson AL, Medcalf RL. Tissue-type plasminogen activator: a multifaceted modulator of neurotransmission and synaptic plasticity. Neuron. 2006;50:673–678. doi: 10.1016/j.neuron.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL, Touyz RM. From bedside to bench to bedside: role of renin-angiotensin-aldosterone system in remodeling of resistance arteries in hypertension. Am J Physiol Heart Circ Physiol. 2004;287:H435–H446. doi: 10.1152/ajpheart.00262.2004. [DOI] [PubMed] [Google Scholar]
- Sheng B, Cheng LF, Law CB, Li HL, Yeung KM, Lau KK. Coexisting cerebral infarction in Alzheimer's disease is associated with fast dementia progression: applying the National Institute for Neurological Disorders and Stroke/Association Internationale pour la Recherche et l'Enseignement en Neurosciences Neuroimaging Criteria in Alzheimer's Disease with Concomitant Cerebral Infarction. J Am Geriatr Soc. 2007;55:918–922. doi: 10.1111/j.1532-5415.2007.01171.x. [DOI] [PubMed] [Google Scholar]
- Skoog I, Gustafson D. Update on hypertension and Alzheimer's disease. Neurol Res. 2006;28:605–611. doi: 10.1179/016164106X130506. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama. 1997;277:813–817. [PubMed] [Google Scholar]
- Staessen JA, Richart T, Birkenhager WH. Less atherosclerosis and lower blood pressure for a meaningful life perspective with more brain. Hypertension. 2007;49:389–400. doi: 10.1161/01.HYP.0000258151.00728.d8. [DOI] [PubMed] [Google Scholar]
- Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, Song W. Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: what is the clinical significance? Hypertension. 2004;44:248–252. doi: 10.1161/01.HYP.0000138070.47616.9d. [DOI] [PubMed] [Google Scholar]
- Traon AP, Costes-Salon MC, Galinier M, Fourcade J, Larrue V. Dynamics of cerebral blood flow autoregulation in hypertensive patients. J Neurol Sci. 2002;195:139–144. doi: 10.1016/s0022-510x(02)00010-2. [DOI] [PubMed] [Google Scholar]
- van Dijk EJ, Breteler MM, Schmidt R, Berger K, Nilsson LG, Oudkerk M, Pajak A, Sans S, de Ridder M, Dufouil C, et al. The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension. 2004;44:625–630. doi: 10.1161/01.HYP.0000145857.98904.20. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Chen L, Zhao Z, Zhao W, Qian X, Humala N, Seror I, Bartholomew S, Rosendorff C, et al. Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J Clin Invest. 2007;117:3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ST, Mayhan WG, Faraci FM, Heistad DD. Endothelium-dependent responses of cerebral blood vessels during chronic hypertension. Hypertension. 1991;17:612–618. doi: 10.1161/01.hyp.17.5.612. [DOI] [PubMed] [Google Scholar]
- Zacchigna L, Vecchione C, Notte A, Cordenonsi M, Dupont S, Maretto S, Cifelli G, Ferrari A, Maffei A, Fabbro C, et al. Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell. 2006;124:929–942. doi: 10.1016/j.cell.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]