Abstract
The DNA replication-related element binding factor (DREF) plays an important role in regulation of cell proliferation in Drosophila, binding to DRE and activating transcription of genes carrying this element in their promoter regions. Overexpression of DREF in eye imaginal discs induces a rough eye phenotype in adults, which can be suppressed by half dose reduction of the osa or moira (mor) genes encoding subunits of the BRM complex. This ATP-dependent chromatin remodeling complex is known to control gene expression and the cell cycle. In the 5′ flanking regions of the osa and mor genes, DRE and DRE-like sequences exist which contribute to their promoter activities. Expression levels and promoter activities of osa and mor are decreased in DREF knockdown cells and our results in vitro and in cultured cells indicate that transcription of osa and mor is regulated by the DRE/DREF regulatory pathway. In addition, mRNA levels of other BRM complex subunits and a target gene, string/cdc25, were found to be decreased by knockdown of DREF. These results indicate that DREF is involved in regulation of the BRM complex and thereby the cell cycle.
INTRODUCTION
Promoter regions of DNA replication-related genes contain a common regulatory sequence, 5′-TATCGATA, named the DNA replication-related element (DRE) (1). A specific DRE-binding factor (DREF) has been identified (2) and numerous studies have revealed that the DREF/DRE system is required for the expression of many genes involved in cell proliferation and the cell cycle (3). Computational analysis of Drosophila gene promoters revealed that DRE is one of the most prevalent motifs in core promoters (4). In fact immunostaining of polytene chromosomes with antiDREF antibody revealed that DREF binds to hundreds of interband regions on the polytene chromosomes (5). In addition SAGE analyses revealed that DRE exists in upstream regions of many genes expressed in proliferating cells in eye imaginal discs (6). RNAi-mediated knockdown of DREF in growing tissues also provided direct evidence that it is necessary for cell cycle and cell growth control (7,8).
It is reported that DREF is a component of the TATA box-binding protein-related factor 2 (TRF2) complex (9), which interacts with basal transcription machinery (10). This complex is directed to promoters of the PCNA and DNA polymerase α 180 kDa genes and upregulates their expression (9). DNA-binding activity of DREF is inhibited by dMi-2, which is the ATPase of an ATP-dependent chromatin remodeling complex (5). In addition, a homeodomein protein Dll interacts with the DNA-binding domain of DREF and prevents its DRE-binding activity (11).
Overexpression of the DREF in eye imaginal discs induces ectopic S phase, apoptosis and inhibited photoreceptor cell differentiation, resulting in a rough eye phenotype in adults. Enhancement of this phenotype was observed in crosses of DREF-overexpressing flies with Dll mutants. This observation combined with molecular and biochemical analyses suggested that Dll is a negative regulator of DREF (11,12).
By genetic screening of mutations that modify the rough eye phenotype, we searched for other genes that genetically interact with DREF. For example, the rough eye phenotype was suppressed by half dose reduction of the trithorax group genes brahma (brm), osa and moira (mor) (12) known to maintain expression patterns of homeotic genes (13). The three genes encode subunits of the BRM complex, a SWI/SNF class ATP-dependent chromatin remodeling complex in Drosophila, altering nucleosome structures to activate or repress gene transcription (14,15). BRM complexes can be subclassified into BAP and PBAP types depending on their signature subunits (16). OSA is specific to BAP, and Polybromo and BAP170 are specific to PBAP complex. BRM, MOR and five other proteins (Snr1, BAP111, BAP60, BAP55 and Actin) are shared with both complexes (16). Recent studies have revealed functional differences between BAP and PBAP in cell cycle regulation (17), only BAP promoting G2/M progression. BRM complexes also genetically interact with cell cycle regulators, E2F and cyclin E (18,19), suggesting a role in negatively controlling G1/S progression.
To clarify the relationship between DREF and BRM complexes, we searched for DRE or DRE-like sequences in 5′ flanking regions of brm, osa and mor, and found examples in the osa and mor genes. Binding of DREF to DRE in both genes could be demonstrated in vitro and in vivo and luciferase transient expression assays confirmed that DRE and DREF are important in their promoter activities. Furthermore, mRNA levels of osa and mor were reduced in DREF knockdown cells. These results indicate that transcription of osa and mor is regulated by the DRE/DREF regulatory pathway. In addition, mRNA levels of other BRM complex subunits and of its target gene, string/cdc25, were decreased by knockdown of DREF. These results taken together revealed that DREF is involved in regulation of BRM complexes and consequently the cell cycle.
MATERIALS AND METHODS
Fly strains
Fly strains were maintained at 25°C on standard food. Canton S flies were used as the wild-type strain. The UAS-dDREF transgenic fly line was described earlier (12) as was the transgenic fly line (line number 16) carrying GMR-GAL4 on the X chromosome (20,21). osa2/TM6B, P{PZ}osa00090/TM3 and mor1/TM6B were obtained from the Drosophila Genetic Resource Center.
Scanning electron microscopy
Adult flies were anesthetized, mounted on stages and observed under a VE-7800 (Keyence Inc., Osaka, Japan) scanning electron microscope in the high vacuum mode.
Oligonucleotides
For RT-PCR these primer pairs were chemically synthesized.
osa-F, 5′-CCCTGTCCCTGTCTTCTCAC
osa-R, 5′-GATGGAACACCGGTAACCAC
mor-F, 5′-CGACAAGGACGATGAAGAGG
mor-R, 5′-CGCTGATGATGATGTGGAAC
Rp49-F, 5′-GCTTCTGGTTTCCGGCAAGCTTCAAG
Rp49-R, 5′-GACCTCCAGCTCGCGCACGTTGTGCACCAGGAAC
DREF-F, 5′-GGCAATCTCCGTTGAATGACG
DREF-R, 5′-TTCACCTCCGAGAAGCCCTT
β-tubulin-F, 5′-AGTTCACCGCTATGTTCA
β-tubulin-R, 5′-CGCAAAACATTGATCGAG
brm-F, 5′-TGTGCAACCATCCGTTTATG
brm-R, 5′-GTCATGCATTGGGTCATCTG
snr1-F, 5′-ACAAGAAGTACCCGGGAATG
snr1-R, 5′-GCACATACTGTGGCTGCTTC
BAP55-F, 5′-GGACATCATTCCTTGCGAGT
BAP55-R, 5′-GGAACGGTCACGTAGTTGGT
BAP60-F, 5′-GTAGCAAAGATGTCGCAACG
BAP60-R, 5′-CCGGGAACAGGTTGAGTAGT
polybromo-F, 5′-CCTGAAACGGATCTTTACGC
polybromo-R, 5′-ATGTTCCGAAGTTCCTGGTG
BAP170-F, 5′-GCATCATCCATCCATCATCC
BAP170-R, 5′-GACGTCGTTACCACAACAGC
string-F, 5′-GTGGAGGAAAACAACTGCAG
string-R, 5′-TCCATGCTCATCAGTTCCAG
The following oligonucleotides were used for electrophoretic mobility shift assays as probes and competitors.
osaDRE1,
5′-GCCAAATACTCGTACGTATCGATACTATCGTGCGTGCTACCCGC
osaDRE1 comp,
5′-GCGGGTAGCACGCACGATAGTATCGATACGTACGAGTATTTGGC
osaDRE2,
5′-CCCAGAGTAGCGAGAAATCGATATCGATGCCAACTCATTTTTGCGTC
osaDRE2 comp,
5′-GACGCAAAAATGAGTTGGCATCGATATCGATTTCTCGCTACTCTGGG
morDRE1,
5′-CGATAACGACTGTCACAACAACATCGATTTAAATACCATCTC
morDRE1 comp,
5′-GAGATGGTATTTAAATCGATGTTGTTGTGACAGTCGTTATCG
morDRE2,
5′-CAGTGGAGATAGCTGAAATATCGATAACGACTGTCACAACAACATCG
morDRE2 comp,
5′-CGATGTTGTTGTGACAGTCGTTATCGATATTTCAGCTATCTCCACTG
morDRE3,
5′-GCTCTCAAACTGTACAATCGATGCTGAGTTACTATAGCCGTACG
morDRE3 comp,
5′-CGTACGGCTATAGTAACTCAGCATCGATTGTACAGTTTGAGAGC
To carry out chromatin immunoprecipitation assays, the following oligonucleotides were used. The reverse primer to amplify morDRE1, 2 was mor3′HindIII as described below.
osaDRE1-F, 5′-CGAAACGAGCACCGATAATG
osaDRE1-R, 5′-ACGAAGAAGCGAGCCTGTAC
osaDRE2-F, 5′-GCACGCCGTTTAATTTGAGT
osaDRE2-R, 5′-CTGGCGAATTAGCGATATTTG
morDRE1,2-F, 5′-GCTTCCACCAACCAATAGGT
morDRE3-F, 5′-CTCATGCGCTCTGCATTTTC
morDRE3-R, 5′-CGAAAAGCAGTACCCAGTAC
brmDRE1-F, 5′-ACATGTCCCCCTACCAAACC
brmDRE1-R, 5′-CTGAGCTACTGTTTGTCCAG
brmDRE2-F, 5′-CGCAAACGTGTGGAATCTGC
brmDRE2-R, 5′-CCCGGATCACAAGTTCATCG
brmDRE3-F, 5′-GGATTGTTAACTACACCCAGC
brmDRE3-R, 5′-GAGGATACCCACAAGAATGAC
BAP55DRE-F, 5′-TAAGCGACCAAAGCGATAGG
BAP55DRE-R, 5′-CGCGAAAATACTTCCGTCTG
BAP60DRE-F, 5′-TGACCCACCAATTGTCTTCC
BAP60DRE-R, 5′-CAAACGCTCGATAGTTGCAG
BAP170DRE-F, 5′-CCAATGTAGATTGGATGCTG
BAP170DRE-R, 5′-ACAGCAGAGTTGCATTCACG
actin5C-F, 5′-CTCCATCATGAAGTGTGATGTG
actin5C-R, 5′-CGTACTCCTGCTTGGACGTC
Plasmid construction
To construct the plasmids p5′-415osawt-luc and p5′-894morwt-luc, PCR was performed using Drosophila genomic DNA as a template and the following primers in combination: osa5′KpnI, 5′-GGTACCGCCACGAATAAGGCTTTCTAG and osa3′HindIII, 5′-AAGCTTCTGCACAGGCTGCACATACT; mor5′KpnI, 5′-GGTACCTCTCGCTCTCTTCCTCTTCG and mor3′HindIII, 5′-AAGCTTAGCCAATAAACGCTGCGTTG. PCR products were digested with KpnI and HindIII and inserted between the KpnI and HindIII sites of the PGVB plasmid (Toyo Ink., Tokyo, Japan).
Mutant constructs were created using a QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) according to manufacturer's protocol. Oligonucleotide pairs carrying base substitutions in the region of interest were used as primers.
osaDRE1mut,
5′-GCCAAATACTCGTACGTcgCGAgcCTATCGTGCGTGCTACCCGC
osaDRE1mut comp,
5′-GCGGGTAGCACGCACGATAGgcTCGcgACGTACGAGTATTTGGC
osaDRE2αmut,
5′-CCCAGAGTAGCGAGAAATCGATcgCGAgGCCAACTCATTTTTGCGTC
osaDRE2αmut comp,
5′-GACGCAAAAATGAGTTGGCcTCGcgATCGATTTCTCGCTACTCTGGG
osaDRE2βmut,
5′-CCCAGAGTAGCGAGAAcgCGAgcTCGATGCCAACTCATTTTTGCGTC
osaDRE2βmut comp,
5′-GACGCAAAAATGAGTTGGCATCGAgcTCGcgTTCTCGCTACTCTGGG
osaDRE2αβmut,
5′-CCCAGAGTAGCGAGAAcgCGAgcgCGAgGCCAACTCATTTTTGCGTC
osaDRE2αβmut comp,
5′-GACGCAAAAATGAGTTGGCcTCGcgcTCGcgTTCTCGCTACTCTGGG
morDRE1mut,
5′-CGATAACGACTGTCACAACAACcgCGAgTTAAATACCATCTC
morDRE1mut comp,
5′-GAGATGGTATTTAAcTCGcgGTTGTTGTGACAGTCGTTATCG
morDRE2mut,
5′-CAGTGGAGATAGCTGAAATcgCGAgcACGACTGTCACAACAACATCG
morDRE2mut comp,
5′-CGATGTTGTTGTGACAGTCGTgcTCGcgATTTCAGCTATCTCCACTG
morDRE3mut,
5′-GCTCTCAAACTGTACAcgCGAgGCTGAGTTACTATAGCCGTACG
morDRE3mut comp,
5′-CGTACGGCTATAGTAACTCAGCcTCGcgTGTACAGTTTGAGAGC
Cell culture
Schneider (S2) cells were cultured in M3(BF) medium (22), supplemented with 10% fetal bovine serum (FBS) at 25°C in 5% CO2.
DNA transfection into cells and luciferase assays
Approximately 1 × 105 S2 cells were plated 24 h before transfection, then 500 ng of reporter plasmid and 1 ng of pAct5C-seapansy as an internal control were cotransfected into the cells using CellFectin reagent (Invitrogen). At 48 h after transfection, cells were harvested and luciferase activities were measured using the Dual-Luciferase Reporter assay system (Promega, Madison, WI, USA). All assays were performed within the range of linear relation of activity to incubation time and values were normalized to Renilla luciferase activity. Transfections were performed several times with at least two independent plasmid preparations.
For double-stranded RNA (dsRNA) interference experiments, 30 μg of DREF dsRNA or LacZ dsRNA in FBS-free M3(BF) medium were added to 1 × 106 S2 cells for 1 h. dsRNA-free incubation was conducted as a control for 1 h in FBS-free M3(BF). After the incubation, 4 v of M3(BF) medium containing 10% FBS were added. After 72 h of RNAi treatment, 1 × 105 cells were cotransfected with reporter genes, 2 μg of reporter plasmid and 1 ng of pAct5C-seapansy plasmids, with the aid of Cell-Fectin reagents (Invitrogen, Carlsbad, CA, USA). At 48 h after transfection, luciferase activity was measured as described above.
Western immunoblot analysis
S2 cells (1 × 106) were collected and lysed as described earlier (23). They were applied to 10% SDS–polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA) in transfer buffer (50 mM borate–NaOH, pH 9.0, 20% methanol). The blotted membranes were incubated with antiDREF monoclonal antibodies (2) at 1:10 000 dilution or antiTubulin IgG (Sigma, St Louis, MO, USA) at 1:5000 for 16 h at 4°C. The bound antibodies were detected with peroxidase-conjugated goat antimouse IgG and the ECL system (GE Healthcare, Little Chalfont, UK) according to the manufacturer's recommendations, and images were analyzed with a Lumivision Pro HSII image analyzer (Aisin Seiki, Aichi, Japan).
Electrophoretic mobility shift assays
Electrophoretic mobility shift assays were performed as reported previously (1), with minor modifications. Preparation of Kc cell nuclear extracts was described elsewhere (1). They were mixed with double-stranded 32P-labeled synthetic oligonucleotides (100 000 cpm) in a reaction buffer [15 mM HEPES, pH 7.6, 60 mM KCl, 0.1 mM EDTA, 1 mM DTT, 12% glycerol and 0.1 mg/ml poly(dI-dC)], and incubated for 15 min at 0°C. When necessary, unlabeled oligonucleotides were added as competitors at this step. DNA–protein complexes were electrophoretically resolved on 4% polyacrylamide gels in 50 mM Tris-borate, pH 8.3, 1 mM EDTA and 2.5% glycerol. The gels were dried and autoradiographed.
Electrophoretic mobility shift assays were also performed in the presence of antiDREF monoclonal antibody 1, antiDREF monoclonal antibody 4 (2) or normal mouse IgG as a control. Kc cell nuclear extracts were mixed with each antibody, incubated for 2 h at 0°C and added to mixtures containing 32P-labeled synthetic oligonucleotides (100 000 cpm). After 15 min incubation at 0°C, electrophoresis was carried out as described above.
Chromatin immunoprecipitation assays
We performed chromatin immunoprecipitation using a Chip Assay kits as recommended by the manufacturer (Upstate, Lake Placid, NY, USA) (24). Approximately 1 × 107 S2 cells were fixed in 1% formaldehyde at 37°C for 10 min and then quenched in 125 mM glycine for 5 min at 25°C. The cells were then washed in PBS containing protease inhibitors (1 mM PMSF, 1 μg/ml aprotinin and 1 μg/ml pepstatin A) and lysed in SDS lysis buffer. Lysates were sonicated to break DNA and the sonicated cell supernatants were diluted 10-fold in chip dilution buffer. Supernatant was precleared with Protein A Agarose/Salmon Sperm DNA (50% slurry) for 30 min at 4°C. After brief centrifugation, each supernatant was incubated with 1 μg of the rabbit IgG or antiDREF polyclonal antibody for 16 h at 4°C. Protein A Agarose/Salmon Sperm DNA (50% slurry) was added, with incubation for 1 h at 4°C. After washing, immunoprecipitated DNA was eluted with elution buffer (1% SDS, 0.1 M NaHCO3). Then the protein–DNA crosslinks were reversed by heating at 65°C for 4 h. After deproteinization with proteinase K, DNA was recovered by phenol–chloroform extraction and ethanol precipitation.
The immunoprecipitated DNA fragments were detected by quantitative real-time PCR using SYBR Premix Ex Taq (Takara, Kyoto, Japan) and the ABI PRISM® 7000 Sequence Detection System (Applied Biosystems, Tokyo, Japan). Primer pairs for PCR were described above.
Quantitative RT-PCR
S2 cells were exposed to 30 μg of dsRNA and at 5 days after dsRNA treatment total RNA was isolated using Trizol® Reagent (Invitrogen). The cDNA was prepared with a Takara high fidelity RNA PCR kit using the oligo dT primer (Takara) for PCR with SYBR Premix Ex Taq (Takara) and the ABI PRISM® 7000 Sequence Detection System (Applied Biosystems). Ct values were normalized to that of the Rp49 gene.
RESULTS
Half reduction of the osa or mor gene dose suppresses the DREF-induced rough eye phenotype
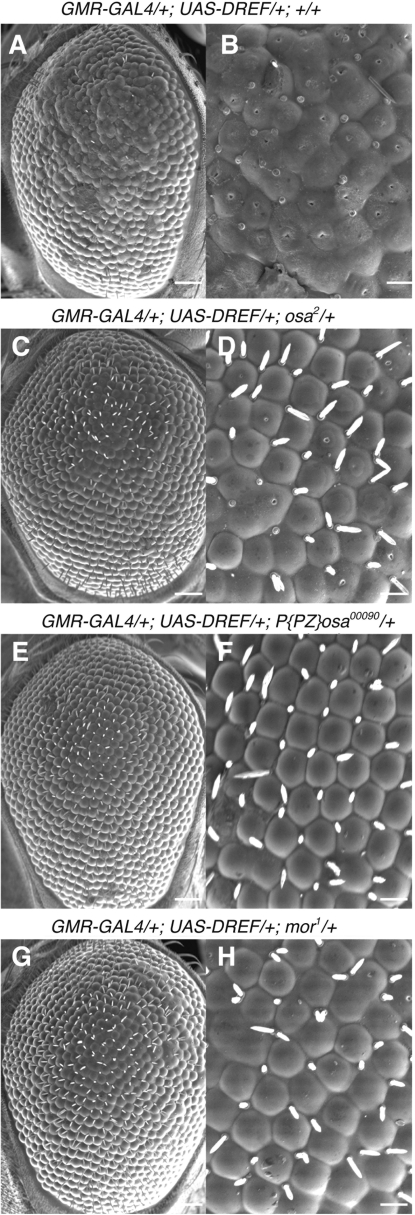
Overexpression of DREF-induced ectopic DNA synthesis and apoptosis, and inhibited photoreceptor cell differentiation in eye imaginal discs so that adult flies exhibited a severe rough eye phenotype (12) (Figure 1A and B), but their viability and fertility were normal. Therefore, we utilized these flies as a genetic tool for screening of mutations that can modify the rough eye phenotype (11,25–27).
Figure 1.
osa and mor genes genetically interact with DREF. Scanning electron micrographs of adult eyes. DREF overexpressing flies (GMR-GAL4/GMR-GAL4; UAS-DREF/UAS-DREF) were crossed with osa or mor mutant flies and developed at 28°C. (A, B) GMR-GAL4/+; UAS-DREF/+; +/+. (C, D) GMR-GAL4/+; UAS-DREF/+; osa2/+. (E, F) GMR-GAL4/+; UAS-DREF/+; P{PZ}osa00090/+. (G, H) GMR-GAL4/+; UAS-DREF/+; mor1/+. (A, C, E, G) Scale bars are for 50 μm. (B, D, F, H) Scale bars are for 12.5 μm.
Previously we reported that half dose reduction of the trithorax group genes, osa and mor suppressed the DREF-induced rough eye phenotype (12). Here, we further confirmed that the F1 progeny of crosses between the DREF overexpressing strain and osa2, osa00090 or mor1 mutant flies showed suppression of the rough eye phenotype (Figure 1C–H). Although their eyes were modestly rough, fused ommatidia were not apparent, suggesting that osa and mor are DREF target genes or positive regulators of the DRE/DREF regulatory pathway.
The 5′ flanking regions of the osa and mor genes contain DRE and DRE-like sequences
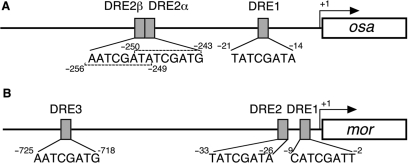
On the basis of our evidence that osa and mor might be DREF target genes, we searched for DRE or DRE-like sequences within 1.4 kb genomic regions from the transcription initiation sites of each of these genes. It has been described that DRE within such regions can upregulate transcription, a central 6 bp of DRE being sufficient for DREF to bind and activate the promoter (1,28).
In the 5′ flanking region of the osa gene, one DRE and two DRE-like sequences were identified, and named DRE1 (−14 to −21), DRE2α (−243 to −250) and DRE2β (−249 to −256) (Figure 2A). DRE1 was found to perfectly match the 8 bp DRE sequence, 5′-TATCGATA, while DRE2α and DRE2β each, matched 7 out of 8 bp.
Figure 2.
DRE and DRE-like sequences in the 5′ flanking regions of the osa and mor genes. Transcription initiation site is numbered as +1. (A) A schematic representation of DREs in the osa gene. (B) A schematic representation of DREs in the mor gene.
In the promoter region of the mor gene, one DRE and two DRE-like sequences were found. They were named DRE1 (−2 to −9), DRE2 (−26 to −33) and DRE3 (−718 to −725) (Figure 2B), DRE2 perfectly matching the consensus DRE sequence and DRE1 and DRE3 showing identity in 6 out of 8 bp. Since all of these sites were detected in located at positions within 1.4 kb of the transcription initiation site, they are good candidate transcriptional regulatory sites for the osa and mor genes.
Roles of DRE sites in osa and mor gene promoter activities
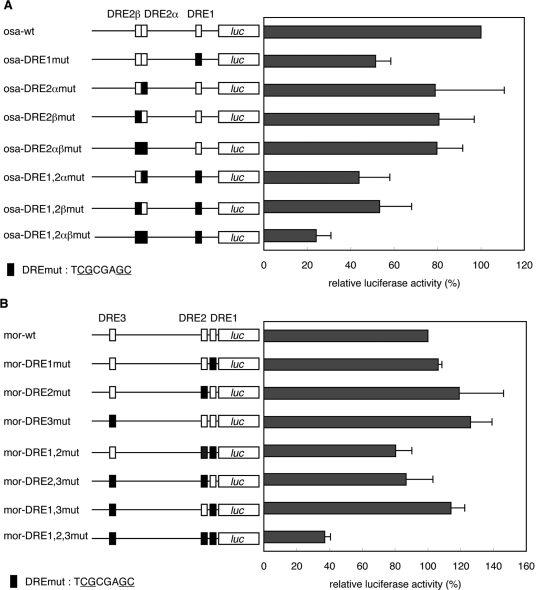
To examine the roles of the DREs in osa promoter activity, we carried out luciferase transient expression assays in cultured Drosophila S2 cells. We constructed a osa promoter–luciferase fusion plasmid and derivatives carrying mutations in one or more of DRE1, DRE2α and DRE2β. These plasmids were transfected into S2 cells and luciferase activities were measured. Mutation in DRE1 (osa-DRE1mut) reduced the promoter activity by 49% relative to that of the wild-type promoter. Mutations in DRE2α (osa-DRE2αmut) and DRE2β (osa-DRE2βmut) reduced the promoter activity by 21 and 19%, respectively. Mutations in both DRE2α and DRE2β (osa-DRE2αβmut) reduced it by 20%. Mutations in all DREs (osa-DRE1 and 2αβmut) reduced the promoter activity by 76% (Figure 3A). These results indicate that DRE1 mainly acts for activation of the osa promoter while DRE2 might have a supporting role.
Figure 3.
Effects of mutations in DRE sites in the 5′ flanking region of the osa and mor genes on their promoter activities. osa or mor promoter–luciferase fusion plasmids were transfected into S2 cells. Luciferase activities are expressed relative to wild-type osa (A) and mor (B) promoters. Mean values with standard deviations from three independent transfections are shown. The white box, wild-type DRE; the black box, mutant DRE.
The roles of DREs in mor promoter activity were examined in a similar way. Mutations in any one of three DREs alone exerted no effects on mor promoter activity and only slight reduction was noted with mutations in any two of the three. However, when all three DREs were mutated simultaneously, promoter activity was decreased by 73% (Figure 3B). These results revealed that all DREs can contribute to mor promoter activation and any one appears to be sufficient for activity.
Effects of knockdown of the DREF gene on osa and mor gene promoter activity
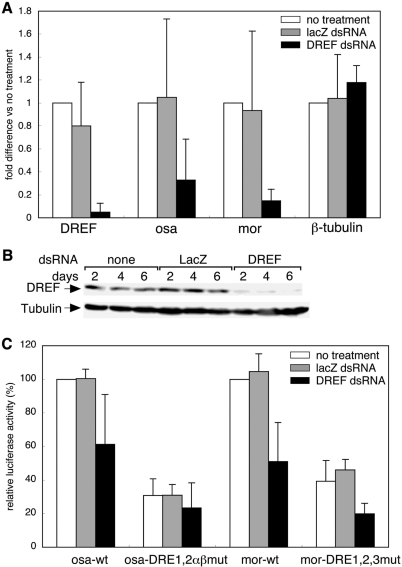
Endogenous osa and mor gene expression in RNAi-mediated DREF knockdown cells was examined to demonstrate that osa and mor are DREF target genes. Total RNAs from dsRNA-treated S2 cells were isolated and quantitative RT-PCR was carried out to measure the level of mRNAs. The DREF mRNA level was reduced by 95% in DREFdsRNA-treated cells, but was not changed in LacZdsRNA-treated cells (Figure 4A). Under these conditions, the level of osa mRNA was decreased to 32% and that of mor mRNA to 14% relative to those with no RNAi treatment (Figure 4A). The β-tubulin gene was used as a negative control and its expression was not affected by DREFdsRNA treatment (Figure 4A).
Figure 4.
DREFdsRNA treatment affects mRNA levels and promoter activities of osa and mor. (A) cDNAs were prepared from total RNA isolated from dsRNA-treated S2 cells and levels of DREF, osa and mor mRNAs were measured by quantitative RT-PCR. Fold differences against the amplification with no treatment are shown with standard deviations from three independent dsRNA treatments. β-tubulin was used as a negative control. (B) Cell extracts were analyzed by western blotting with antiDREF antibodies. (C) Promoter activities of osa and mor in dsRNA-treated cells. Luciferase activities relative to that of wild-type promoter in non-dsRNA-treated cells are shown with standard deviations from five independent transfections.
To further investigate the requirement of DREF for osa and mor promoter activity, we performed luciferase transient expression in DREF knockdown cells. DREFdsRNA or LacZdsRNA were added to S2 cells, and reduction of DREF protein was confirmed by western blotting. DREF protein was not detectable at 2, 4 and 6 days after DREFdsRNA treatment, while no change was evident in mock and LacZdsRNA-treated cells (Figure 4B). The luciferase reporter plasmids were transfected into S2 cells at 3 days after RNAi treatment, and luciferase activities were determined. In DREFdsRNA-treated cells, the wild-type osa promoter activity was decreased to 61% and mor promoter activity to 51% of those in non-dsRNA treatment cells. The promoter activities of both genes were not changed in LacZdsRNA-treated cells (Figure 4C).
To further confirm dependence of promoter activation by DREF on DREs, we measured promoter activities of all DRE-mutant promoters of both osa and mor genes. Although we expected that the promoter activities of DRE mutants would not change in DREF knockdown cells, osa and mor promoter activities were slightly but significantly decreased in DREFdsRNA-treated cells (Figure 4C). These results suggest that DREF not only directly but also indirectly regulates the osa and mor gene transcription. It may be possible that some DREF target gene products activate the osa and mor gene transcription.
DREF binds to the DRE sequence in vitro
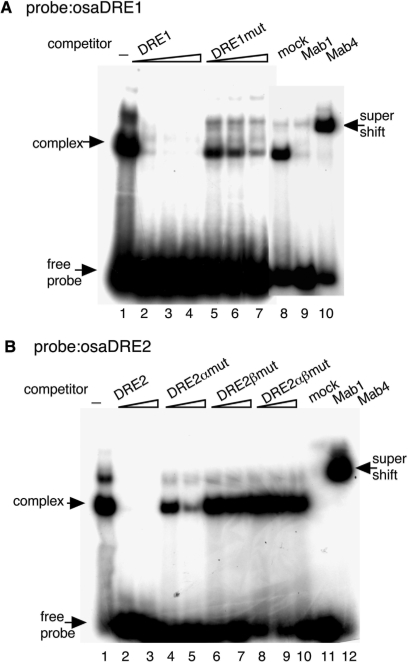
Binding of DREF to DRE is essential for transcriptional activation. We therefore investigated whether DREF has binding ability to the DRE sequences in the osa and mor promoter regions by electrophoretic mobility shift assays. 32P labeled DRE-containing oligonucleotides were mixed with Drosophila Kc cell nuclear extracts and DNA–protein complexes were detected with oligonucleotides containing osaDRE1 (Figure 5A) or osaDRE2 (Figure 5B). On addition of non-labeled DRE oliognucleotides as competitors, shifted bands were diminished (Figure 5A and B). Addition of a competitor carrying mutations in each DRE did not affect the complex formation with osaDRE1 and osaDRE2 (Figure 5A and B). The shifted band with osaDRE1 was decreased by the addition of a 100-fold excess of DRE1mut competitor, while those with osaDRE2 were not, suggesting the DRE1mut competitor oligonucleotide to possess an additional DREF-binding site. To confirm that the DRE–protein complex contains DREF, we added antiDREF monoclonal antibodies to the binding reaction. The addition of antiDREF 1, which binds to the DNA-binding domain of DREF (2), inhibited the complex formation with osaDRE1 and osaDRE2 (Figure 5A and B). Furthermore, the band with osaDRE1 or osaDRE2 was supershifted by addition of antiDREF 4 (2) (Figure 5A and B). These results indicate that DREF indeed form complexes with osaDRE1 and osaDRE2.
Figure 5.
Complex formation between osa DREs and Kc cell nuclear extracts. 32P-labeled double-stranded oligonucleotides osaDRE1 (A) and osaDRE2 (B) were incubated with Kc cell nuclear extracts in the presence of the indicated competitor oligonucleotides or antiDREF monoclonal antibodies. The amounts of competitors were 25-, 50- or 100-fold molar ratios for the osaDRE1 probe (A) and 100- or 400-fold molar ratios for the osaDRE2 probe (B). Mock, normal mouse IgG; Mab1, antiDREF monoclonal antibody 1; Mab4, antiDREF monoclonal antibody 4.
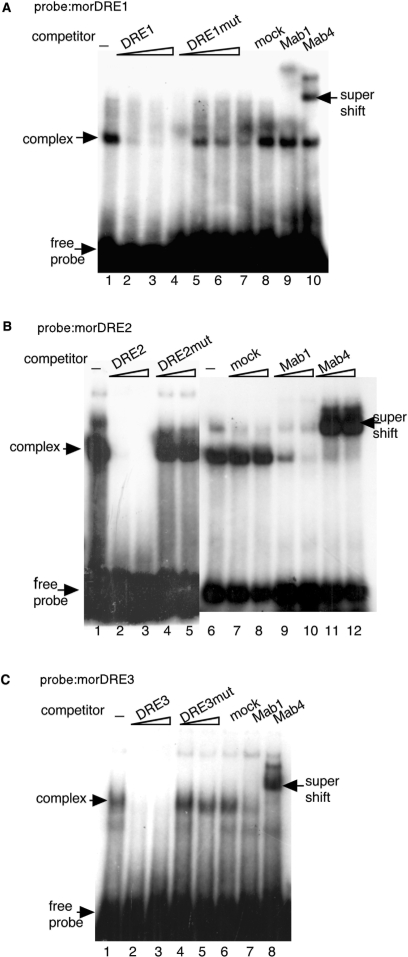
We also examined whether DREF can bind to DREs of the mor gene in the similar way. DNA–protein complexes were detected with the oligonucleotides morDRE1, morDRE2 and morDRE3 as probes (Figure 6A–C). Since more nuclear extract was required to detect the DNA–protein complex with morDRE1, DREF appears to have lower affinity for morDRE1 than for the others. Each of the shifted bands disappeared on adding non-labeled DRE oliognucleotides as competitors, while competitor oligonucleotides carrying mutations in the DRE sequences exerted no effect (Figure 6A–C). To further verify the existence of DREF in the complex with morDRE oligonucleotides, antiDREF monoclonal antibodies were added to the binding reaction. The addition of the antiDREF 1 inhibited the complex formation with morDRE2 and morDRE3 (Figure 6B and C). The shifted bands with morDRE2 and morDRE3 were supershifted by adding antiDREF 4 (Figure 6B and C). These results indicate that DREF form complexes with the oligonucleotides morDRE2 and morDRE3.
Figure 6.
Complex formation between mor DREs and Kc cell nuclear extracts. 32P-labeled double-stranded oligonucleotides morDRE1 (A), morDRE2 (B) and morDRE3 (C) were incubated with Kc cell nuclear extracts in the presence of the indicated competitor oligonucleotides or antiDREF monoclonal antibodies. The amounts of competitors were 25-, 50- or 100-fold molar ratios for the morDRE1 probe (A) and 100- or 400-fold molar ratios for the morDRE2 (B) and morDRE3 probes (C). Mock, normal mouse IgG; Mab1, antiDREF monoclonal antibody 1; Mab4, antiDREF monoclonal antibody 4.
Although addition of antiDREF 1 to the binding reaction only marginally affected the complex formation with morDRE1, the shifted band was at least partially supershifted by adding antiDREF 4 (Figure 6A). These results suggest that not only DREF but also other protein(s) can form complexes with morDRE1.
DREF binds to the genomic regions containing DRE and DRE-like sequences of the osa and mor in vivo
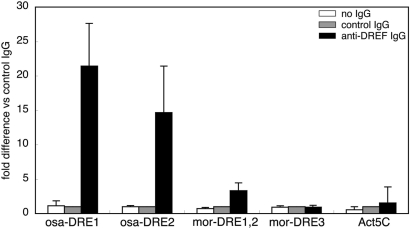
Based on the observation that DREF has ability to bind to osa and mor DRE sequences in vitro, we further examined DREF binding under cellular conditions by chromatin immunoprecipitation assays using affinity purified antiDREF polyclonal antibodies. osaDRE1 and osaDRE2 could be considerably amplified from the immunoprecipitates with antiDREF IgG. Amplification of osaDRE1 was 21-fold and that of osaDRE2 was 14-fold compared with amplification from the immunoprecipitates with control IgG, while amplification of Act5C genomic region was only 1.5-fold (Figure 7). Thus, we concluded that DREF binds to the osaDRE1- and osaDRE2-containing genomic region in cultured cells.
Figure 7.
Binding of DREF to the DRE-containing genomic regions of the osa and mor genes. Crosslinked chromatin of S2 cells was immunoprecipitated with either antiDREF IgG or control rabbit IgG. The genomic regions containing osaDRE1, osaDRE2, morDRE1, 2 or morDRE3 were amplified by real-time PCR and compared with the amplification from the immunoprecipitates with the control IgG.
Amplification of the region containing morDRE1 and 2 from the immunoprecipitates with antiDREF IgG was 3.3-fold in comparison with amplification of Act5C gene locus, but morDRE3 was amplified only 0.9-fold (Figure 7). We therefore conclude that DREF does not bind to the genomic region containing morDRE3 in vivo. It should be noted that morDRE3 matches only six out of the eight bases canonical DRE and appeared to have only a weak affinity for DREF in the electrophoretic mobility shift assay in vitro (Figure 6C).
Knockdown of DREF in S2 cells reduces expression of several genes encoding components of BRM complexes
In the 5′ flanking regions of the genes coding for BAP55, BAP60 and BAP170, DRE or DRE-like sequences were found. Although no DRE was found in the 5′ flanking region of brm, its second intron contains three DRE-like sequences, suggesting that these sequences might act as enhancer elements. The snr1, BAP111 and polybromo genes carry no DRE or DRE-like sequences in their 5′ flanking regions or introns (Table 1). These findings suggest that DREF plays a role in activating the transcription of several genes encoding subunits of both BAP and PBAP complexes.
Table 1.
DRE or DRE-like sequences in and around the genes coding for subunits of BRM complexes
| Gene | DRE or DRE-like | Position | |
|---|---|---|---|
| BAP | brm | 5′-cATCGATA | +881 to +874 |
| PBAP | 5′-cATCGATg | +2208 to +2201 | |
| 5′-cATCGATg | +7120 to +7113 | ||
| mor | 5′-cATCGATt | −9 to −2 | |
| 5′-TATCGATA | −33 to −26 | ||
| 5′-aATCGATg | −725 to −718 | ||
| BAP55 | 5′-aATCGATA | −39 to −32 | |
| 5′-aATCGATA | −91 to −84 | ||
| BAP60 | 5′-TATCGATA | −209 to −202 | |
| 5′-gATCGATA | −277 to −270 | ||
| snr1 | None | – | |
| BAP111 | None | – | |
| BAP | osa | 5′-TATCGATA | −21 to −14 |
| 5′-TATCGATg | −250 to −243 | ||
| 5′-aATCGATA | −256 to −249 | ||
| PBAP | BAP170 | 5′-TATCGATA | −16 to −9 |
| 5′-cATCGATA | −29 to −22 | ||
| 5′-aATCGATt | −442 to −435 | ||
| Polybromo | None | – |
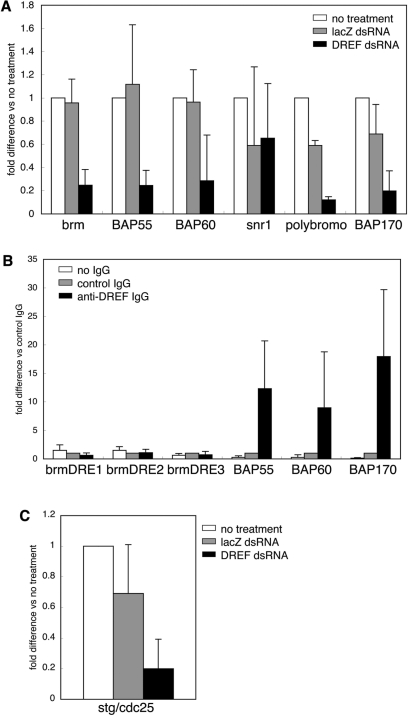
To examine whether DREF affects expression of these genes, mRNA levels of brm, BAP55, BAP60, snr1, polybromo and BAP170 in DREF knockdown cells were measured by quantitative RT-PCR. The brm expression level in DREFdsRNA-treated cells was reduced to 25% relative to non-RNAi-treated cells. The mRNA levels for BAP55 and BAP60 were decreased to 24 and 29%, respectively (Figure 8A). PBAP-specific polybromo and BAP170 were also decreased to 12 and 20%, respectively (Figure 8A). DREF is assumed to control PBAP expression as well as BAP. Expression of the snr1 gene was not affected by knockdown of DREF (Figure 8A).
Figure 8.
Effects of DREF knockdown on mRNA levels of other BRM complex components. (A) Total RNA was isolated from S2 cells treated with dsRNA and cDNA was prepared. mRNA levels were determined by quantitative RT-PCR. Fold differences versus no dsRNA treatment are shown as mean values with standard deviations from three independent dsRNA transfections. (B) DRE-containing regions were amplified from immunoprecipitates with antiDREF IgG. (C) mRNA level of stg in DREFdsRNA-treated cells.
To investigate whether DREF directly regulates brm, BAP55, BAP60 and BAP170, we carried out chromatin immunoprecipitation assays. Genomic regions containing brmDRE1 (+881 to +874), brmDRE2 (+2208 to +2201) and brmDRE3 (+7120 to +7113) were not amplified from immunoprecipitates with antiDREF IgG (Figure 8B). It is therefore not likely that these DREs in the second intron of the brm gene act as enhancer elements. DRE-containing regions of BAP55, BAP60 and BAP170 were amplified by 9-, 12- and 18-fold, respectively (Figure 8B). Therefore, DREF appears to bind to DREs in these genes and directly activates BAP55, BAP60 and BAP170 transcription.
It is reported that the OSA-containing BAP complex is necessary for G2/M progression through string (stg)/cdc25 promoter activation (17). We therefore examined mRNA levels of stg in DREF knockdown cells. Quantitative RT-PCR revealed 80% decrease (Figure 8C).
DISCUSSION
In this study, we demonstrated that both osa and mor are DREF target genes. Thus osa and mor promoters exhibited decreased activities when carrying mutations in their DREs and after knockdown of DREF in cultured cells. In addition, levels of osa and mor mRNAs were reduced in DREF knockdown cells. Third, DREF can bind to DREs of osa and mor in vitro, and binding of DREF to the genomic regions containing DREs of both genes was observed in cultured cells. These results showed that DRE and DREF are important for osa and mor promoter activation. Promoters having mutations in all DREs of both osa and mor genes, however, still retained some activity. It is therefore possible that another element(s) and/or unknown factor(s) regulated by DREF are involved in osa and mor transcriptional activation. The observed rescue of the DREF-induced rough eye phenotype by a reduction in the osa and moira gene dosage is consistent with the idea that the osa and moira gene transcription is activated by DREF. However, we cannot exclude the possibility that the rescue could also be affected by a mechanism involving protein–protein interactions between DREF and BAP/PBAP at the promoters of cell cycle-regulated genes. Further analyses are necessary to address this point.
Both osa and mor encode components of the BRM complex (16,29–31), which is a SWI/SNF type ATP-dependent chromatin remodeling complex conserved from yeast to human (14), with two forms, BAP and PBAP. OSA is a signature subunit of BAP, while PBAP contains Polybromo and BAP170 in its place (16). Localization patterns of OSA and Polybromo on polytene chromosomes differ, though several sites overlap (16). Whole-genome expression analysis also demonstrated that BAP and PBAP differentially regulate gene expression (17). For example, OSA negatively regulates expression of the Wingless-target genes and the achaete/scute gene (32,33). OSA, Polybromo and BAP170 are all required for function of BRM complex (17). It is thought that OSA functions in recruitment of BAP to its target genes. MOR, a subunit common to both BAP and PBAP, is presumed to be essential for complex integrity, since its absence results in degradation of both forms (17). SRG3, which is a homolog of MOR in mammals, also acts for complex stabilization by protecting against proteasomal degradation (34). Therefore, OSA and MOR are essential subunits for function and stabilization of BRM complexes and DREF may control integrity of the BRM complex through activating osa and mor gene expression.
BAP and PBAP share seven subunits, BRM, MOR, Snr1, BAP111, BAP60, BAP55 and Actin (16). BRM is a catalytic subunit harboring the ATPase domain (35) and it was previously reported that reduction of the brm gene dose suppressed the DREF-induced rough eye phenotype (12). We also found the mRNA level of brm to be decreased in DREF knockdown cells. However, DRE-like sequences in the second intron, do not appear to function as regulatory elements, since DREF does not bind to the genomic region containing these sites in vivo. DREF may therefore indirectly control brm gene expression.
The genes coding for BAP55 and BAP60, common subunits for BAP and PBAP, also contain DRE or DRE-like sequences in their 5′ flanking regions and are affected by DREF knockdown. DREF binds to the genomic regions containing their DREs in vivo and it is, therefore, possible that BAP55 and BAP60 are directly regulated by DREF. Furthermore, the PBAP-specific subunit BAP170 carries a DRE in its 5′ flanking region. Reduction of mRNA levels of osa, polybromo and BAP170 in DREF knockdown cells also is evidence that DREF contributes to the transcriptional regulation of both BAP and PBAP complexes. Therefore, DREF may regulate expression of genes coding for most subunits for both BAP and PBAP complexes and influence expression of many genes through chromatin remodeling.
It is reported that OSA-containing BAP complexes are necessary for G2/M progression through stg promoter activation while PBAP complexes are not (17). stg encodes a CDC25 phosphatase, which is required for G2/M progression (36). It is well known that DREF predominantly regulates the transcription of DNA replication-related genes (1,25,37,38). Reduced stg mRNA has been reported in DREF-eliminated cells (8) and we also observed reduction of stg mRNA levels in DREF knockdown cells, as with brm, osa and mor. In addition to regulation of S phase entry, DREF thus appears to play an important role in G2/M transition by activating the BAP complex to promote cell cycling. We found two DRE-like sequences in the stg gene upstream region, −219 to −212 (5′-aATCGATg) and −591 to −584 (5′-TATCGATt). Therefore, DREF could regulate stg gene expression directly via binding to DRE-like and/or indirectly via activation of genes coding for BAP complexes. Further analysis is necessary to distinguish these possibilities.
BRM complexes are thought to inhibit S phase entry and mutations of brm, osa and mor suppress the rough eye phenotype induced by E2F/DP/p35 overexpression (18). The rough eye phenotype of a cyclin E hypomorphic mutant was also suppressed by BRM complex mutation through increase in the S phase (19). Therefore, BRM complexes appear to negatively regulate S phase entry, while DREF activates E2F gene transcription and promotes G1/S progression (39). Although osa is ubiquitously expressed in eye imaginal discs, it is most intensely expressed anterior to the morphogenic furrow where cells enter the G1 phase (40). Similarly, DREF is strongly expressed in this region (12). It is conceivable that DREF simultaneously activates both positive and negative regulators of G1/S progression. This kind of regulation may be necessary for fine tuning of cell cycle progression to inhibit excess S phase induction.
ACKNOWLEDGEMENTS
We thank Dr M. Moore for comments on the English language in the manuscript. This study was partially supported by grants from the KIT. Funding to pay the Open Access publication charges for this article was provided by KIT.
Conflict of interest statement. None declared.
REFERENCES
- 1.Hirose F, Yamaguchi M, Handa H, Inomata Y, Matsukage A. Novel 8-base pair sequence (Drosophila DNA replication-related element) and specific binding factor involved in the expression of Drosophila genes for DNA polymerase α and proliferating cell nuclear antigen. J. Biol. Chem. 1993;268:2092–2099. [PubMed] [Google Scholar]
- 2.Hirose F, Yamaguchi M, Kuroda K, Omori A, Hachiya T, Ikeda M, Nishimoto Y, Matsukage A. Isolation and characterization of cDNA for DREF, a promoter-activating factor for Drosophila DNA replication-related genes. J. Biol. Chem. 1996;271:3930–3937. doi: 10.1074/jbc.271.7.3930. [DOI] [PubMed] [Google Scholar]
- 3.Matsukage A, Hirose F, Yoo MA, Yamaguchi M. The DRE/DREF transcriptional regulatory system: a master key for cell proliferation. Biochim. Biophys. Acta. 2008;1779:81–89. doi: 10.1016/j.bbagrm.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Ohler U, Liao GC, Niemann H, Rubin GM. Computational analysis of core promoters in the Drosophila genome. Genome Biol. 2002;3:1–12. doi: 10.1186/gb-2002-3-12-research0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirose F, Ohshima N, Kwon E-J, Yoshida H, Yamaguchi M. Drosophila Mi-2 negatively regulates dDREF by inhibiting its DNA-binding activity. Mol. Cell. Biol. 2002;22:5182–5193. doi: 10.1128/MCB.22.14.5182-5193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jasper H, Benes V, Atzberger A, Sauer S, Ansorge W, Bohmann D. A genomic switch at the transition from cell proliferation to terminal differentiation in the Drosophila eye. Dev. Cell. 2002;3:511–521. doi: 10.1016/s1534-5807(02)00297-6. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida H, Kwon E, Hirose F, Otsuki K, Yamada M, Yamaguchi M. DREF is required for EGFR signalling during Drosophila wing vein development. Genes Cells. 2004;9:935–944. doi: 10.1111/j.1365-2443.2004.00775.x. [DOI] [PubMed] [Google Scholar]
- 8.Hyun J, Jasper H, Bohmann D. DREF is required for efficient growth and cell cycle progression in Drosophila imaginal discs. Mol. Cell. Biol. 2005;25:5590–5598. doi: 10.1128/MCB.25.13.5590-5598.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hochheimer A, Zhou S, Zheng S, Holmes MC, Tjian R. TRF2 associates with DREF and directs promoter-selective gene expression in Drosophila. Nature. 2002;420:439–445. doi: 10.1038/nature01167. [DOI] [PubMed] [Google Scholar]
- 10.Rabenstein MD, Zhou S, Lis JT, Tjian R. TATA box-binding protein (TBP)-related factor 2 (TRF2), a third member of the TBP family. Proc. Natl Acad. Sci. USA. 1999;96:4791–4796. doi: 10.1073/pnas.96.9.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi Y, Kato M, Seto H, Yamaguchi M. Drosophila Distal-less negatively regulates dDREF by inhibiting its DNA binding activity. Biochim. Biophys. Acta. 2006;1759:359–366. doi: 10.1016/j.bbaexp.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Hirose F, Ohshima N, Shiraki M, Inoue HY, Taguchi O, Nishi Y, Matsukage A, Yamaguchi M. Ectopic expression of DREF induced DNA synthesis, apoptosis, and unusual morphogenesis in the Drosophila eye imaginal disc: possible interaction with Polycomb and trithorax group proteins. Mol. Cell. Biol. 2001;21:7231–7242. doi: 10.1128/MCB.21.21.7231-7242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennison JA, Tamkun JW. Dosage-dependent modifiers of Polycomb and Antennapedia mutations in Drosophila. Proc. Natl Acad. Sci. USA. 1988;85:8136–8140. doi: 10.1073/pnas.85.21.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohrmann L, Verrijzer CP. Composition and functional specificity of SWI2/SNF2 class chromatin remodeling complexes. Biochim. Biophys. Acta. 2005;1681:59–73. doi: 10.1016/j.bbaexp.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Bouazoune K, Brehm A. ATP-dependent chromatin remodeling complexes in Drosophila. Chromosome Res. 2006;14:433–449. doi: 10.1007/s10577-006-1067-0. [DOI] [PubMed] [Google Scholar]
- 16.Mohrmann L, Langenberg K, Krijgsveld J, Kal AJ, Heck AJ, Verrijzer CP. Differential targeting of two distinct SWI/SNF-related Drosophila chromatin-remodeling complexes. Mol. Cell. Biol. 2004;24:3077–3088. doi: 10.1128/MCB.24.8.3077-3088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moshkin YM, Mohrmann L, van Ijcken WF, Verrijzer CP. Functional differentiation of SWI/SNF remodelers in transcription and cell cycle control. Mol. Cell. Biol. 2007;27:651–661. doi: 10.1128/MCB.01257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staehling-Hampton K, Ciampa PJ, Brook A, Dyson N. A genetic screen for modifiers of E2F in Drosophila melanogaster. Genetics. 1999;153:275–287. doi: 10.1093/genetics/153.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brumby AM, Zraly CB, Horsfield JA, Secombe J, Saint R, Dingwall AK, Richardson H. Drosophila cyclin E interacts with components of the Brahma complex. EMBO J. 2002;21:3377–3389. doi: 10.1093/emboj/cdf334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi Y, Hirose F, Matsukage A, Yamaguchi M. Identification of three conserved regions in the DREF transcription factors from Drosophila melanogaster and Drosophila virilis. Nucleic Acids Res. 1999;27:510–516. doi: 10.1093/nar/27.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaguchi M, Hirose F, Inoue YH, Shiraki M, Hayashi Y, Nishi Y, Matsukage A. Ectopic expression of human p53 inhibits entry into S phase and induces apoptosis in the Drosophila eye imaginal disc. Oncogene. 1999;18:6767–6775. doi: 10.1038/sj.onc.1203113. [DOI] [PubMed] [Google Scholar]
- 22.Cross DP, Sang JH. Cell culture of individual Drosophila embryos. I. Development of wild type cultures. J. Embryol. Exp. Morphol. 1978;45:161–172. [PubMed] [Google Scholar]
- 23.Kwon EJ, Park HS, Kim YS, Oh EJ, Nishida Y, Matsukage A, Yoo MA, Yamaguchi M. Transcriptional regulation of the Drosophila raf proto-oncogene by Drosophila STAT during development and in immune response. J. Biol. Chem. 2000;275:19824–19830. doi: 10.1074/jbc.M001114200. [DOI] [PubMed] [Google Scholar]
- 24.Luo RX, Postigo AA, Dean DC. Rb interacts with histone deacetylase to repress transcription. Cell. 1998;92:463–473. doi: 10.1016/s0092-8674(00)80940-x. [DOI] [PubMed] [Google Scholar]
- 25.Okudaira K, Ohno K, Yoshida H, Asano M, Hirose F, Yamaguchi M. Transcriptional regulation of the Drosophila orc2 gene by the DREF pathway. Biochim. Biophys. Acta. 2005;1732:23–30. doi: 10.1016/j.bbaexp.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Phuong Thao DT, Ida H, Yoshida H, Yamaguchi M. Identification of the Drosophila skpA gene as a novel target of the transcription factor DREF. Exp. Cell Res. 2006;312:3641–3650. doi: 10.1016/j.yexcr.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Ida H, Yoshida H, Nakamura K, Yamaguchi M. Identification of the Drosophila eIF4A gene as a target of the DREF transcription factor. Exp. Cell Res. 2007;313:4208–4220. doi: 10.1016/j.yexcr.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi M, Hayashi Y, Nishimoto Y, Hirose F, Matsukage A. A nucleotide sequence essential for the function of DRE, a common promoter element for Drosophila DNA replication-related genes. J. Biol. Chem. 1995;270:15808–15814. doi: 10.1074/jbc.270.26.15808. [DOI] [PubMed] [Google Scholar]
- 29.Papoulas O, Beek SJ, Moseley SL, McCallum CM, Sarte M, Shearn A, Tamkun JW. The Drosophila trithorax group proteins BRM, ASH1 and ASH2 are subunits of distinct protein complexes. Development. 1998;125:3955–3968. doi: 10.1242/dev.125.20.3955. [DOI] [PubMed] [Google Scholar]
- 30.Crosby MA, Miller C, Alon T, Watson KL, Verrijzer CP, Goldman-Levi R, Zak NB. The trithorax group gene moira encodes a brahma-associated putative chromatin-remodeling factor in Drosophila melanogaster. Mol. Cell. Biol. 1999;19:1159–1170. doi: 10.1128/mcb.19.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins RT, Furukawa T, Tanese N, Treisman JE. Osa associates with the Brahma chromatin remodeling complex and promotes the activation of some target genes. EMBO J. 1999;18:7029–7040. doi: 10.1093/emboj/18.24.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins RT, Treisman JE. Osa-containing Brahma chromatin remodeling complexes are required for the repression of wingless target genes. Genes Dev. 2000;14:3140–3152. doi: 10.1101/gad.854300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heitzler P, Vanolst L, Biryukova I, Ramain P. Enhancer-promoter communication mediated by chip during Pannier-driven proneural patterning is regulated by Osa. Genes Dev. 2003;17:591–596. doi: 10.1101/gad.255703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sohn DH, Lee KY, Lee C, Oh J, Chung H, Jeon SH, Seong RH. SRG3 interacts directly with the major components of the SWI/SNF chromatin remodeling complex and protects them from proteasomal degradation. J. Biol. Chem. 2007;282:10614–10624. doi: 10.1074/jbc.M610563200. [DOI] [PubMed] [Google Scholar]
- 35.Elfring LK, Deuring R, McCallum CM, Peterson CL, Tamkun JW. Identification and characterization of Drosophila relatives of the yeast transcriptional activator SNF2/SWI2. Mol. Cell. Biol. 1994;14:2225–2234. doi: 10.1128/mcb.14.4.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edgar BA, O’Farrell PH. Genetic control of cell division patterns in the Drosophila embryo. Cell. 1989;57:177–187. doi: 10.1016/0092-8674(89)90183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi Y, Yamaguchi M, Hirose F, Cotterill S, Kobayashi J, Miyajima S, Matsukage A. DNA replication-related elements cooperate to enhance promoter activity of the Drosophila DNA polymerase α 73-kDa subunit gene. J. Biol. Chem. 1996;271:14541–14547. doi: 10.1074/jbc.271.24.14541. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchiya A, Inoue YH, Ida H, Kawase Y, Okudaira K, Ohno K, Yoshida H, Yamaguchi M. Transcriptional regulation of the Drosophila rfc1 gene by the DRE-DREF pathway. FEBS J. 2007;274:1818–1832. doi: 10.1111/j.1742-4658.2007.05730.x. [DOI] [PubMed] [Google Scholar]
- 39.Sawado T, Hirose F, Takahashi Y, Sasaki T, Shinomiya T, Sakaguchi K, Matsukage A, Yamaguchi M. The DNA replication-related element (DRE)/DRE-binding factor system is a transcriptional regulator of the Drosophila E2F gene. J. Biol. Chem. 1998;273:26042–26051. doi: 10.1074/jbc.273.40.26042. [DOI] [PubMed] [Google Scholar]
- 40.Treisman JE, Luk A, Rubin GM, Heberlein U. eyelid antagonizes wingless signaling during Drosophila development and has homology to the Bright family of DNA-binding proteins. Genes Dev. 1997;11:1949–1962. doi: 10.1101/gad.11.15.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]