Abstract
We used an antibody to choline acetyltransferase (ChAT) to label cholinergic cells in guinea pig brainstem. ChAT-immunoreactive (ChAT-IR) cells comprise several prominent groups, including the pedunculopontine tegmental nucleus, laterodorsal tegmental nucleus, and parabigeminal nucleus, as well as the cranial nerve somatic motor and parasympathetic nuclei. Additional concentrations are present in the parabrachial nuclei and superior colliculus.
Among auditory nuclei, the majority of ChAT-IR cells are in the superior olive, particularly in and around the lateral superior olive, the ventral nucleus of the trapezoid body and the superior paraolivary nucleus. A discrete group of ChAT-IR cells is located in the sagulum, and additional cells are scattered in the nucleus of the brachium of the inferior colliculus. A group of ChAT-IR cells lies dorsal to the dorsal nucleus of the lateral lemniscus. A few ChAT-IR cells are found in the cochlear nucleus and the ventral nucleus of the lateral lemniscus.
The distribution of cholinergic cells in guinea pigs is largely similar to that of other species; differences occur mainly in cell groups that have few ChAT-IR cells. The results provide a basis for further studies to characterize the connections of these cholinergic groups.
Keywords: Acetylcholine, pedunculopontine tegmental nucleus, laterodorsal tegmental nucleus, ChAT
Acetylcholine acts on many brainstem structures. Its affects are varied, and related to its origins. Three nuclei, the pedunculopontine tegmental nucleus (PPT), laterodorsal tegmental nucleus (LDT), and parabigeminal nucleus (PBG), are traditionally identified as the main sources of cholinergic projections to other brainstem nuclei and to the thalamus. The PBG projects to the superior colliculus and visual thalamus (e.g. Jiang et al., 1996; Wilson et al., 1995). The PPT and LDT have widespread projections, the best known of which are those to the dorsal thalamus (e.g., Woolf and Butcher, 1986; Steriade et al., 1988; Oakman et al., 1999).
Recent studies have begun to reveal the role of acetylcholine in sensory processing in brainstem nuclei (e.g., Fernández de Sevilla et al., 2006; Simons et al., 2006; Uteshev and Smith, 2006). In the auditory system, processing of sounds is affected by cholinergic projections from the superior olive to the cochlea and cochlear nucleus (Guinan, 1996; Mulders et al., 2003). A few studies have identified cholinergic effects in other brainstem auditory nuclei, such as the inferior colliculus (e.g., Habbicht and Vater, 1996), but the underlying circuitry is unknown. An important step in characterizing these circuits is to identify the source(s) of cholinergic input. This in turn requires a detailed description of cholinergic nuclei in the species under study.
The purpose of the present study is to describe the distribution of cholinergic cells in the brainstem of guinea pigs, a species widely used in auditory research. We identify cholinergic cells with antibodies to choline acetyltransferase (ChAT), the synthetic enzyme for acetylcholine and a specific marker of cholinergic cells (Levey and Wainer, 1982; Armstrong et al., 1983; German et al. 1985; Maley et al., 1988). This approach has been used in a variety of species and a general mammalian pattern has emerged (Woolf, 1991). Despite the overall similarities, there are differences that, at the least, must be attended when analyzing cholinergic circuitry. The present report provides a basis for future studies of cholinergic circuitry in guinea pig brainstem.
Experimental Procedures
Animals
All procedures were performed in accordance with the Institutional Animal Care and Use Committee and NIH guidelines. Seventeen albino (Charles River; Wilmington, MA) and ten pigmented (Elm Hill; Chelmsford, MA) adult guinea pigs weighing 296-602 grams were used. Efforts were made to minimize suffering and the number of animals used.
Perfusion
Each animal was given an overdose of sodium pentobarbital (440mg/kg ip). After cessation of breathing and loss of withdrawal reflex, the animal was perfused through the left ventricle with Tyrode's solution followed by 700 ml fixative (4% paraformaldehyde, with or without 0.2% picric acid and/or 0.1 – 0.5% glutaraldehyde) in 0.1M phosphate buffer, pH 7.4. For brains that were to be frozen for sectioning, the fixative was divided into two parts with 10% sucrose added to the second volume. The brain was removed and stored in fixative (with 30% sucrose for frozen sectioning) for 4-24 hours at 4°C.
Immunohistochemistry
After removal of cerebral cortex and cerebellum, the brainstem was cut on a Vibratome or frozen and cut on a sliding microtome into 40-50 μm sections in the transverse or sagittal plane. Sections were collected in six series. One series was mounted on slides and stained with thionin for cytoarchitectonic identification of nuclei. Two to four additional series were stained with antibodies against choline acetyltransferase (ChAT) to identify cholinergic cells. We tried several ChAT antibodies, including Chemicon AB144P, Chemicon AB5042, Chemicon AB1582, ImmunoStar 20093, and the ChAT antibody described in Schemann et al., (1993)1. One antibody, Chemicon AB144P, gave the most intense staining and we chose it to complete these experiments.
Sections were treated in 0.4% Triton X-100 in PBS (0.9% NaCl in 0.01M phosphate buffer) for 30 minutes (all steps at room temperature unless noted). After three five-minute washes in PBS, the tissue was treated with 20% normal rabbit serum (NRS) with 0.1% Triton X-100 in PBS for 1 hour. Goat anti-ChAT polyclonal antibody (Chemicon AB 144P) was applied with 0.1% Triton X-100 and 1% NRS in PBS for 24-72 hours at 4°C. The concentration of primary antibody varied from 1:100 to 1:400. Following three five-minute washes in PBS, the tissue was incubated for one hour with a secondary antibody, (biotinylated rabbit anti-goat IgG, BA-5000, Vector Lab), at a 1:100 concentration with 1% NRS in PBS. Following three five-minute washes in PBS, the sections were incubated in avidin-biotin-peroxidase (Vectastain ABC kit, Vector), then rinsed and stained with diaminobenzidine (DAB) with nickel enhancement (Adams, 1981). Reaction times were determined empirically. The sections were mounted on gelatin-coated slides and allowed to dry. The sections were dehydrated, cleared, and then coverslipped with DPX (Aldrich Chemical Co., St. Louis, MO).
Controls
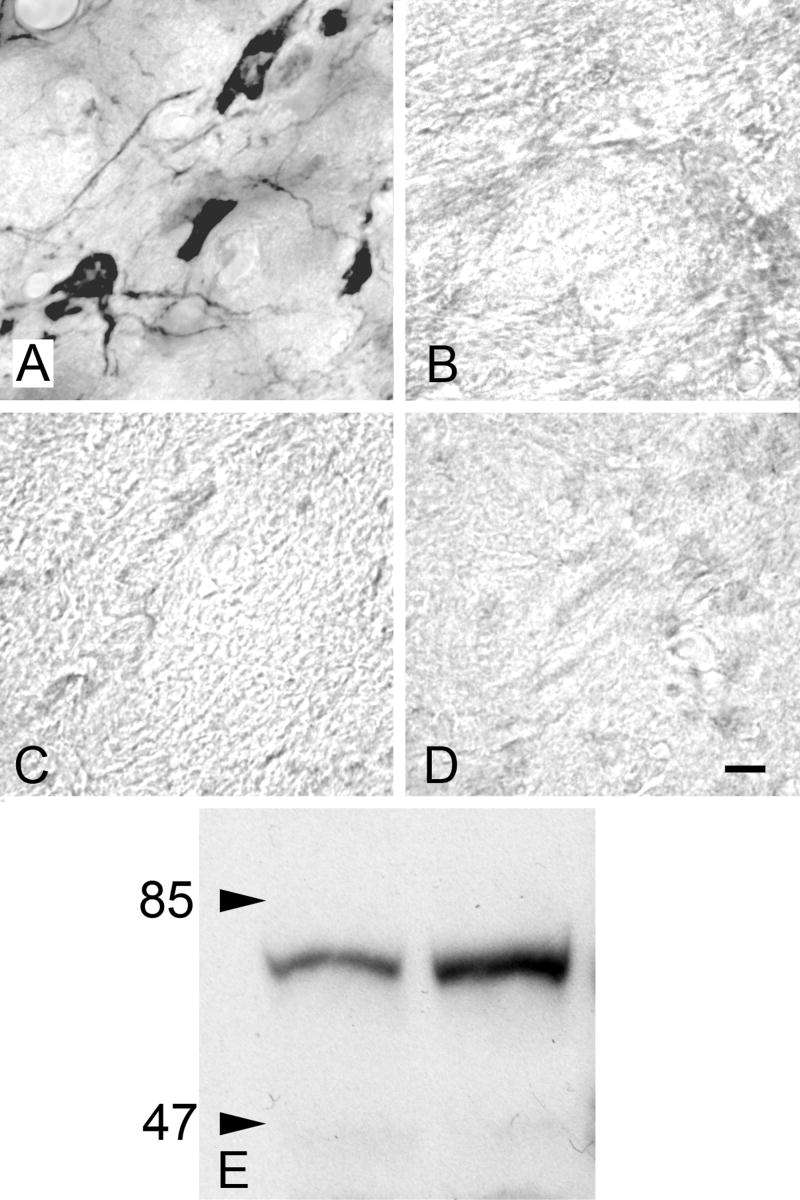
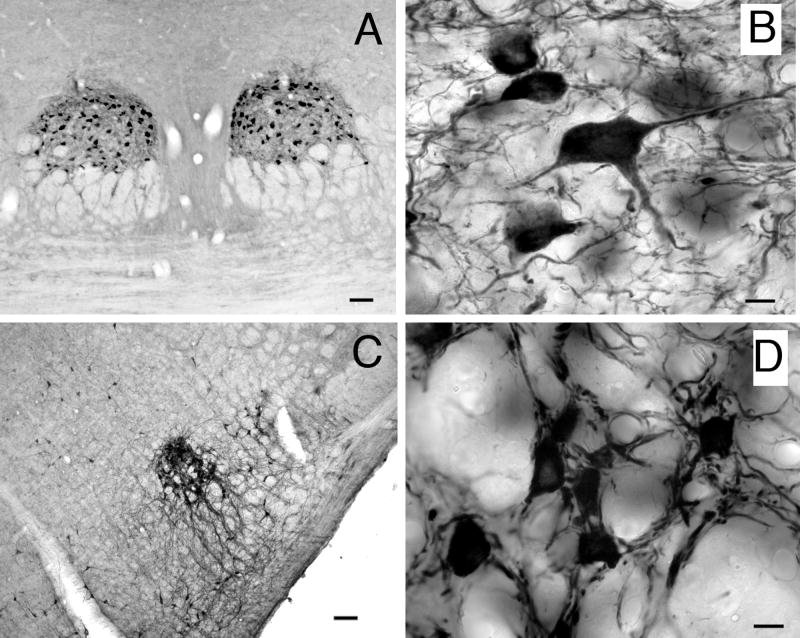
Controls were carried out with the Chemicon AB144P antibody. Staining was eliminated with preadsorption of the antibody with ChAT (Chemicon AG220) (Fig. 1A, B) or with omission of primary or secondary antibodies (Fig. 1A, C, D). Western blot analysis of brainstem tissue from two guinea pigs revealed a single band of staining (Fig. 1E).
Figure 1.
Immunohistochemistry controls. A) ChAT-IR staining in the pedunculopontine tegmental (PPT) nucleus. Absence of staining is demonstrated with B) preabsorption, C) primary antibody omission, and D) secondary antibody omission controls in nearby sections from the same animal. All images are from the PPT. Scale bar = 10 μm for A-D. E) Western blot analysis of brain tissue with ChAT antibody (Chemicon AB144P). Left lane: brainstem. Right lane: thalamus and forebrain. In each lane a single band is present at the expected 68-70 kDa molecular weight.
Data Analysis
Labeled cells were plotted throughout the brainstem using a Zeiss Axioplan II microscope and Neurolucida reconstruction system (MBF Bioscience, Williston, VT). Adjacent thionin-stained sections were used to identify nuclei. We used a rat brain atlas (Paxinos and Watson, 1998) to identify brainstem regions. In addition, we used papers that describe specific nuclei in guinea pig (e.g., Hackney et al., 1990; Schofield and Cant, 1991, 1997; Leonard et al., 1995). Adobe Illustrator was used to illustrate the plotted sections. Photomicrographs were obtained using a Zeiss Axioskop microscope and Magnafire digital camera (Optronics). Adobe Photoshop was used to add scale bars and labels, size and crop images, convert to grayscale, erase background outside tissue sections, and adjust brightness and contrast.
Results
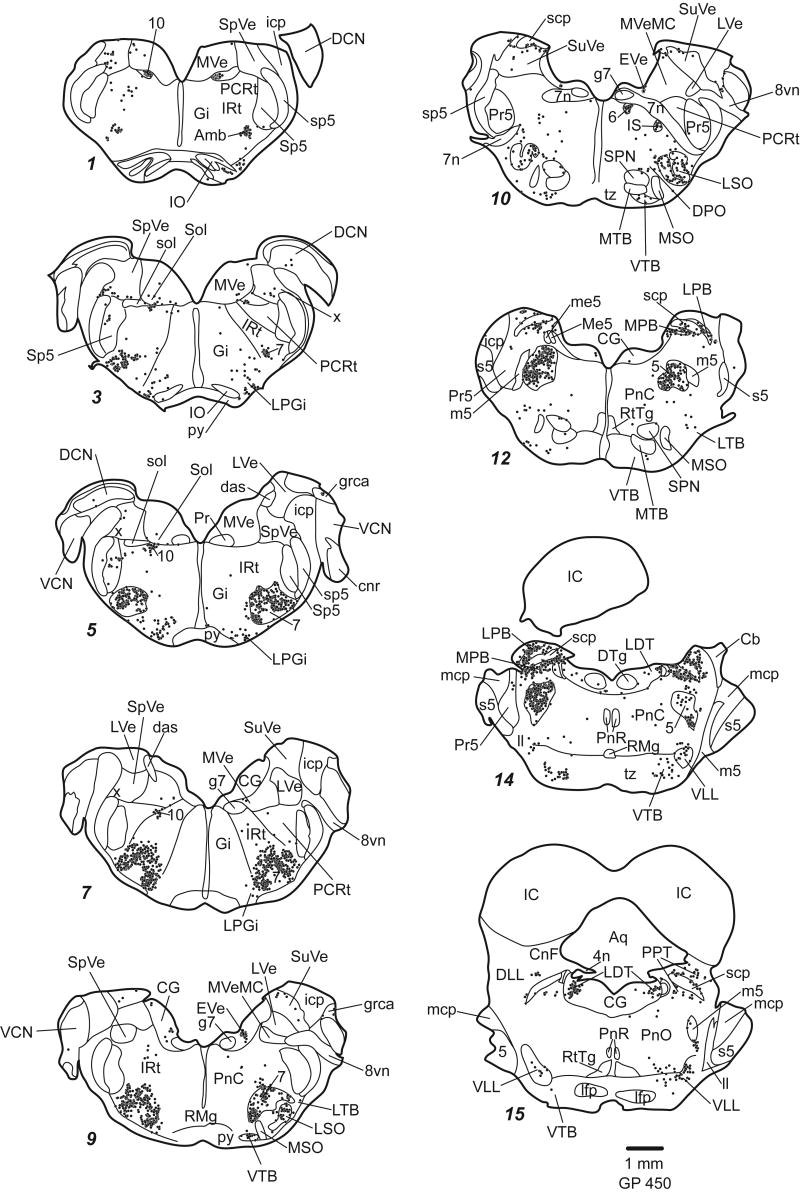
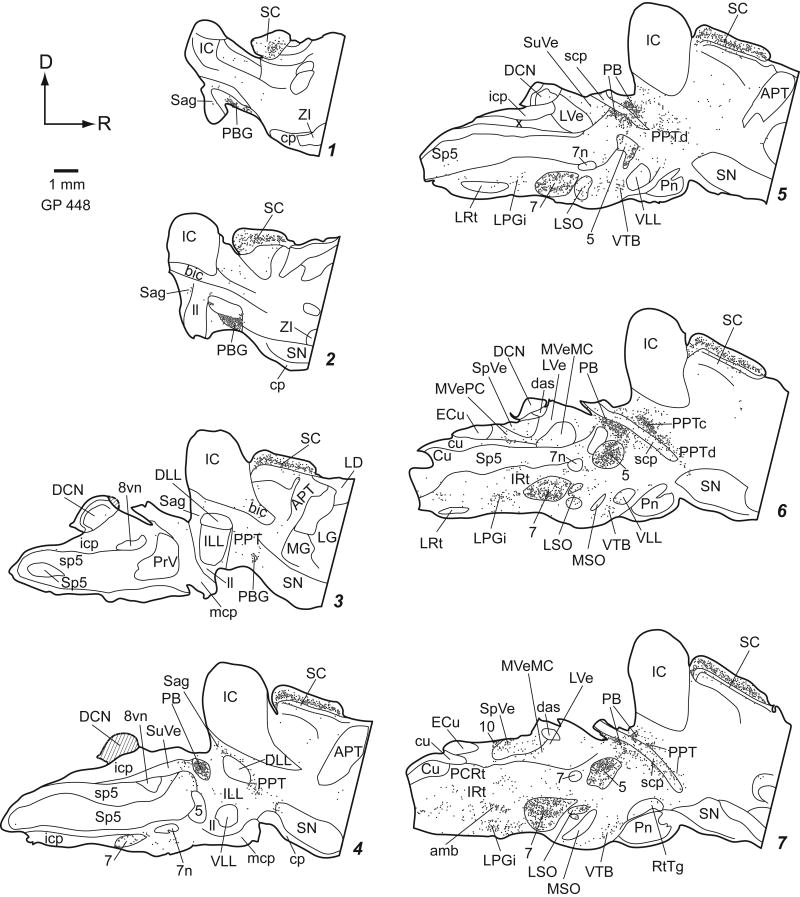
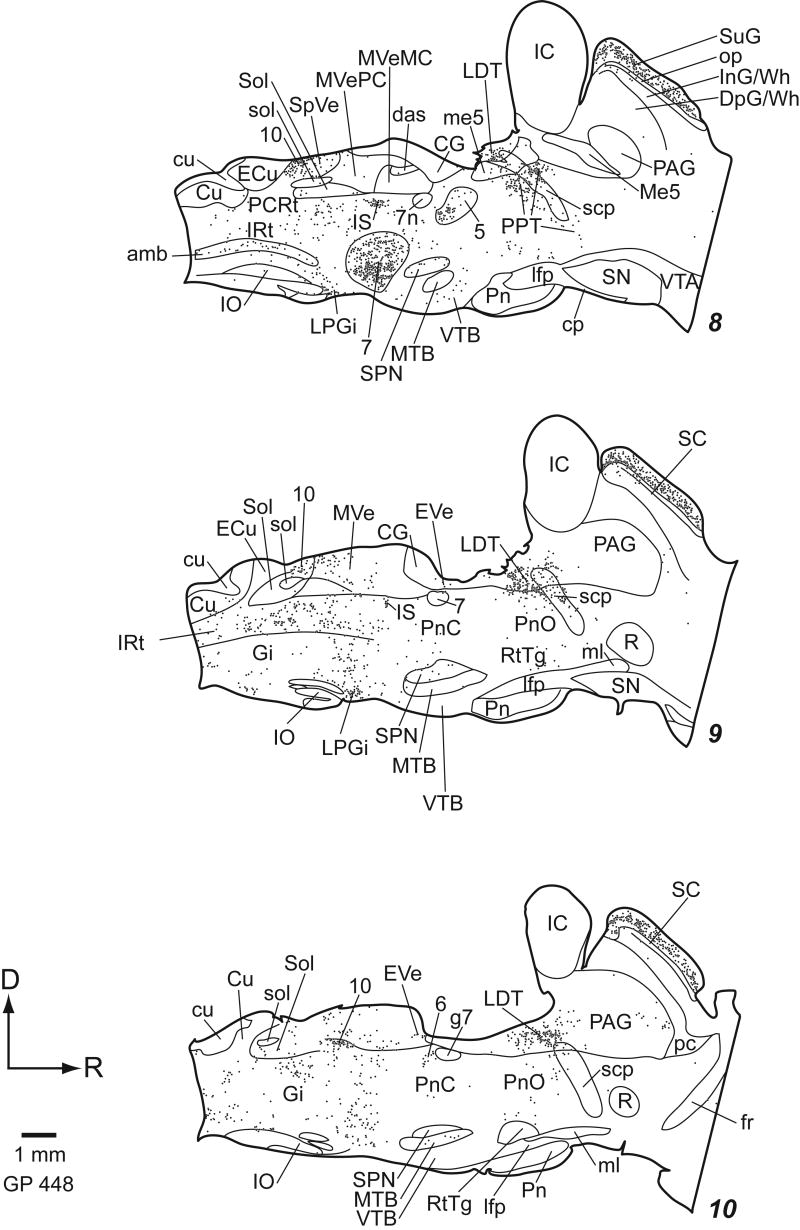
Staining was similar regardless of fixation protocol or animal pigmentation (albino or pigmented). The general pattern of staining was similar for all the ChAT antibodies we tried. In general, the antibodies stained cell bodies and often dendrites. Large cholinergic axon bundles such as the facial nerve were usually stained, but axons in other areas were rarely stained. Figures 2 (transverse plots) and 3 (parasagittal plots) show the distribution of ChAT-immunoreactive (ChAT-IR) cells. Clusters of stained cells are numerous, while additional ChAT-IR cells are scattered sparsely across many nuclei. We first describe the traditionally named cholinergic groups and the cranial nerve nuclei. Next we describe other discrete groups of ChAT-IR cells, including ChAT-IR cells in auditory nuclei. Finally we discuss areas with more diffuse distributions of ChAT-IR cells.
Figure 2.
Plots of ChAT-IR cells in transverse sections. Each dot represents one ChAT-IR cell. Sections are 50 μm thick. Each section is numbered from caudal to rostral and every whole number increase in section number represents a spacing of 300 μm between sections. Not every section is shown.
Traditionally-named cholinergic nuclei
ChAT-IR cells are prominent in the PPT and LDT (Fig. 4) and PBG (Fig. 5). The cells are generally smaller than those in the cranial nerve motor nuclei but larger than almost all other ChAT-IR cells in the brainstem.
Figure 4.
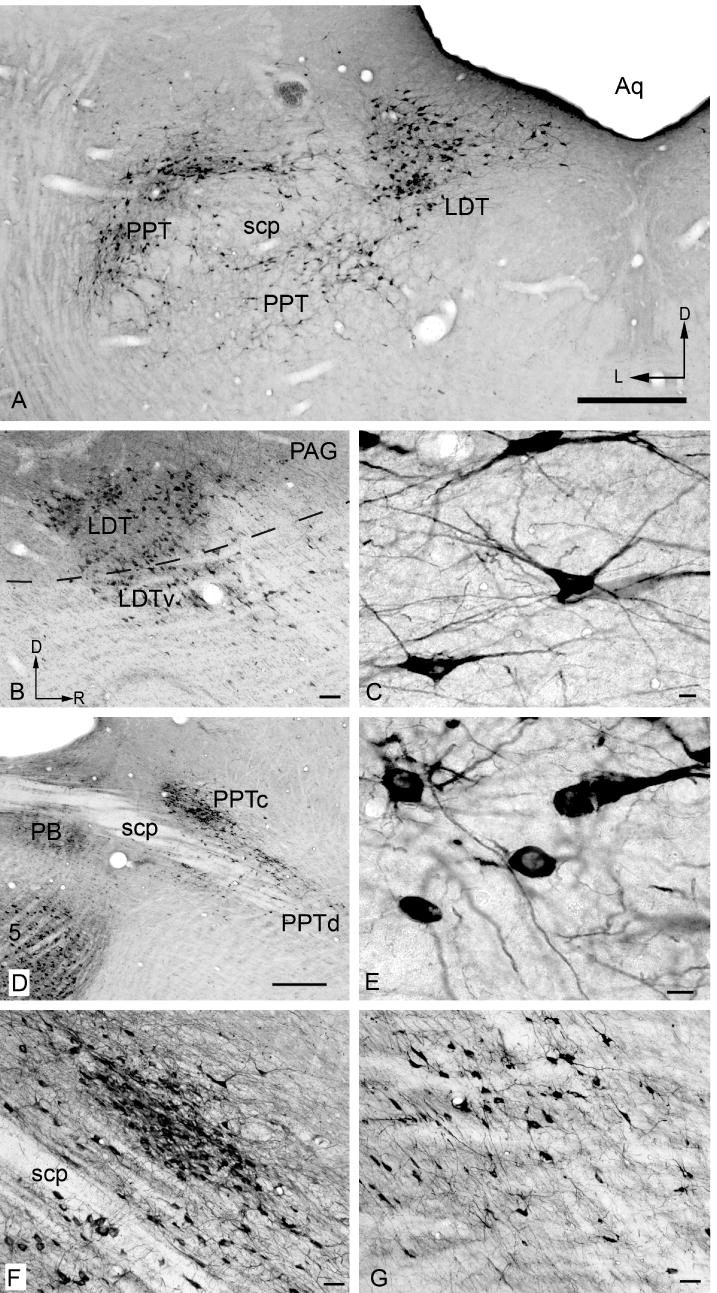
Photomicrographs showing ChAT-IR cells in traditionally-named cholinergic nuclei. A) pedunculopontine (PPT) and laterodorsal tegmental (LDT) nuclei (compare with Fig. 2, approx. section 16). D - Dorsal; L - lateral. B) Parasagittal section showing LDT and its ventral subdivision (LDTv), located ventral to the border of the periaqueductal gray (dotted line). Dorsal is up and rostral to the right in this and subsequent panels. C) LDT cells. D) Parasagittal section showing the relationship between the parabrachial nuclei (PB), the compact part of PPT (PPTc) and the dissipated part of PPT PPTd). E) PPTd cells. F) compact PPT. G) dissipated PPT. Scale bar = 0.5 mm for A, D; 100 μm for B; 10 μm for C, E; and 50 μm for F, G.
Figure 5.
Low (A) and high (B) magnification photomicrographs of ChAT-IR cells in the parabigeminal nucleus. Transverse sections. Scale bar = 50 μm for A; 10 μm for B.
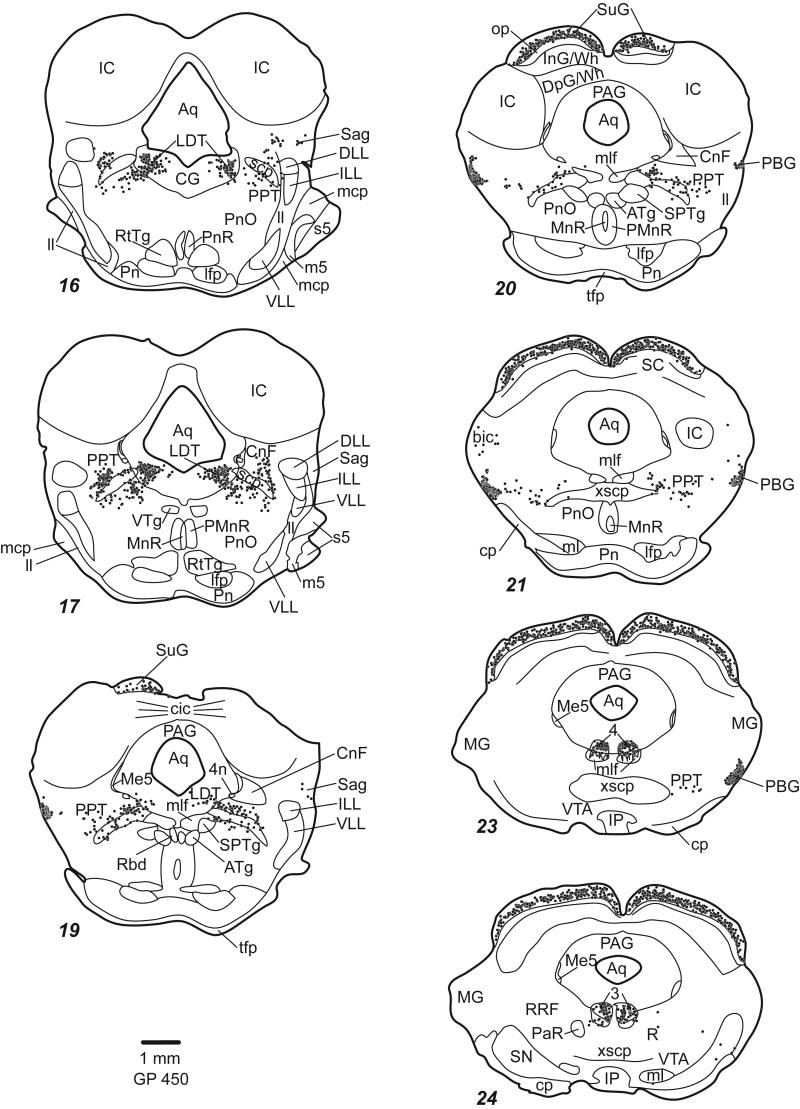
The PPT extends through the midbrain from caudo-dorsal tegmentum (surrounding the superior cerebellar peduncle [scp]) to the rostro-ventral tegmentum bordering the substantia nigra (Fig. 2 sections 15-23; Fig. 3 sections 3-8). The PPT can be divided into “compact” and “dissipated” regions (Leonard et al., 1995). The compact region contains tightly clustered ChAT-IR cells and is centered dorsal to the scp (Fig. 4D, F). The larger part of the nucleus is the dissipated region, which contains less tightly clustered cells, and extends ventral and medial to the scp (Fig. 4D, G).
Figure 3.
Plots of ChAT-IR cells in parasagittal sections. Conventions as in Figure 2. Cross hatching (sections 3 & 4) indicate tissue damage.
The LDT is located largely within the periaqueductal gray except rostrally, where it extends slightly beyond the border of the central gray (ventral LDT; Leonard, 1995) (Fig. 2 sections 14-19; Fig. 3 sections 8-10; Fig. 4B).
Cranial Nerve Motor Nuclei
The cranial nerve motor nuclei have densely packed ChAT-IR cells that are generally larger than most other ChAT-IR cells in the brainstem (Fig. 6).
Figure 6.
Low and high magnification photomicrographs of ChAT-IR cells in representative cranial nerve nuclei. A, B) trochlear nuclei, C, D) nucleus ambiguus. Transverse sections. Scale bar = 100 μm for A, C; 10 μm for B, D.
Other discrete clusters of ChAT-IR cells
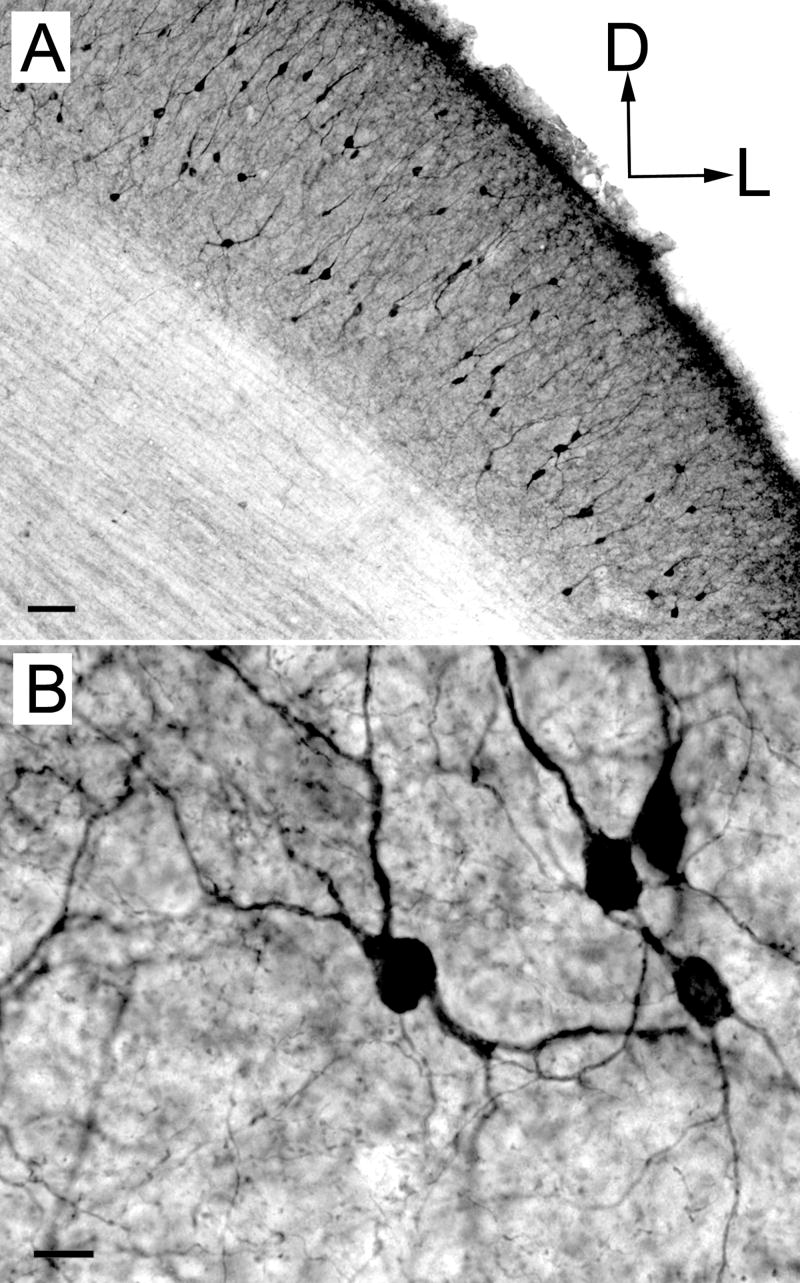
Other discrete groups of ChAT-IR cells are found near the scp. The medial and lateral parabrachial nuclei have a dense collection of darkly stained ChAT-IR cells (Fig. 2, sections 12-14; Fig. 3, section 7; Fig. 4D). Another substantial collection of ChAT-IR cells is found in the superficial gray layer of the superior colliculus (Fig. 7).
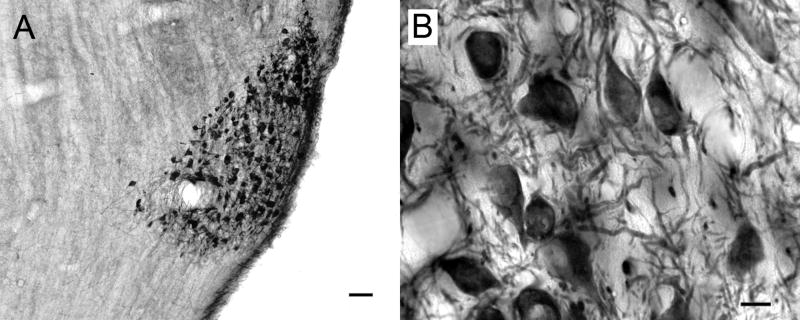
Figure 7.
Low (A) and high (B) magnification photomicrographs of ChAT-IR cells in the superficial gray layer of the superior colliculus. Transverse sections. Scale bars = 50 μm for A, 10 μm for B.
Auditory Nuclei
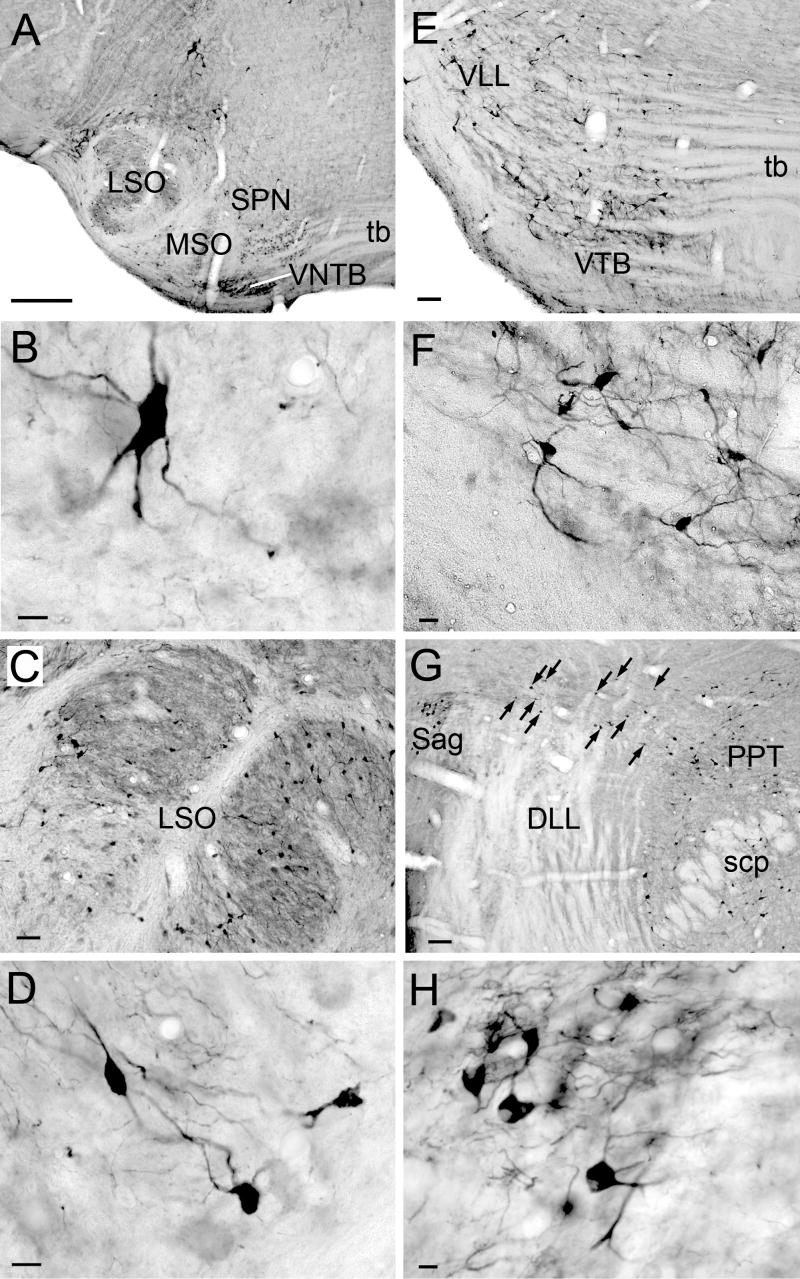
Within the auditory system, there are several structures with ChAT-IR cells (Fig. 8). In the superior olivary complex, ChAT-IR cells are present in and around the lateral superior olive, and in most periolivary nuclei, especially the ventral nucleus of the trapezoid body and the superior paraolivary nucleus (Fig. 8A-F). In the lateral lemniscus, labeled cells are scattered in the ventral nucleus of the lateral lemniscus (Fig. 8E), particularly in the ventral division. Labeled cells are not present in the dorsal nucleus of the lateral lemniscus (DLL), but are found medial, lateral and dorsal to it (Fig. 8G). The medial cells are part of PPT. The cluster lateral to the DLL is within the sagulum (Fig. 8H). Dorsal to the DLL, ChAT-IR cells form a “bridge” that stretches from the sagulum to the PPT (Fig. 8G, arrows). The ChAT-IR cells in the bridge are smaller than those in PPT or sagulum. Scattered ChAT-IR cells are present in the nucleus of the brachium of the IC (e.g. Fig. 2, section 21; Fig 3, section 2). A few scattered labeled cells are present in the deep layer of dorsal cochlear nucleus, on or near the medial margin of the ventral cochlear nucleus and in the granule cell area. In a few cases, the IC contained a few labeled cells.
Figure 8.
Photomicrographs of ChAT-IR cells in auditory nuclei A) Major nuclei of the superior olivary complex: lateral superior olivary nucleus (LSO), medial nucleus of the trapezoid body (MTB), medial superior olivary nucleus (MSO), superior paraolivary nucleus (SPN), and ventral nucleus of the trapezoid body (VTB). B) SPN cell. C, D) LSO cells. E) VTB and ventral nucleus of lateral lemniscus. F) VTB cells. G) Cells in the sagulum (Sag) lateral to DLL, in the PPT medial to DLL, and forming a “bridge” (arrows) stretching from sagulum to PPT dorsal to the DLL. H) Sagulum cells. Transverse sections. Scale bar = 100 μm for A, E, G; 50 μm for C; 20 μm for F; and 10 μm for B, D, H.
Cranial Nerve Sensory Nuclei
Scattered ChAT-IR cells are present in the spinal nucleus of the trigeminal nerve (Fig. 2, section 5; Fig. 3, sections 5, 6). Scattered ChAT-IR cells are also present in the medial and superior vestibular nuclei and, in smaller numbers, in the spinal vestibular nucleus (Fig. 2, sections 1-10; Fig. 3, sections 3-10). No ChAT-IR cells are seen in the lateral vestibular nucleus.
The nucleus of the solitary tract contains ChAT-IR cells (Fig. 2, sections 3-5; Fig. 3, sections 8-10). These cells are adjacent to cells in the dorsal motor nucleus of the vagus; the latter cells are larger and more densely packed than those in nucleus of the solitary tract (Ruggiero et al., 1990).
Reticular and tegmental areas
There are scattered ChAT-IR cells in many reticular formation nuclei and some other tegmental areas. Lateral to the genu of the seventh nerve is a small, distinct cluster of ChAT-IR cells (“EVe”, Fig. 2, sections 9, 10); these are probably the vestibular “efferents”, which project to the vestibular component of the inner ear (Strutz, 1982). The cuneiform nucleus borders the PPT (Fig. 2, sections 16-17); the ChAT-IR cells are similar in the two nuclei, and it is difficult to determine whether the ones within the cuneiform nucleus form a separate group. ChAT-IR cells are present in the nucleus prepositus hypoglossi. The dorsolateral periaqueductal gray and external cuneate nucleus each have a clustering of ChAT-IR cells that is less dense than the motor cranial nerve nuclei and the named cholinergic nuclei.
ChAT-IR cells are scattered very diffusely in the gigantocellular nucleus, dorsal tegmental nucleus, nucleus X, pontine reticular nucleus, lateral paragigantocellular nucleus, intermediate reticular nucleus and raphe nuclei. The parvicellular reticular nucleus, reticulotegmental nucleus of the pons, and ventral tegmental nucleus appear devoid of ChAT-IR cells.
Discussion
The present study describes the distribution of ChAT-IR cells in guinea pig brainstem. Previous studies in guinea pigs are limited to a short description by Maley et al., (1988), who described ChAT-IR cells in the cranial nerve motor nuclei, parabrachial region, superior olive and lateral reticular nucleus. Our results confirm the earlier study and extend it with details on many additional brainstem regions. Before further comparing our results with those of previous studies, we consider several technical issues associated with immunohistochemical identification of presumptive cholinergic cells.
Technical Issues
ChAT is considered a specific marker for cholinergic cells (Levey and Wainer, 1982; Armstrong et al., 1983; German et al. 1985; Maley et al., 1988). We tried several ChAT antibodies. While the staining varied in intensity, the locations of stained cells were similar, supporting the conclusion that the stained cells were cholinergic. We continued our study with the antibody that gave the most intense staining in preliminary studies. This antibody successfully stains cholinergic axons in guinea pig peripheral nervous system (Hoover et al., 2004). Our controls validate the use of this antibody in guinea pig central nervous system. Our results are consistent with those described previously in guinea pigs (Maley, et al., 1988, who used a different antibody). In addition, we observed staining in “expected” areas such as the cranial nerve motor and parasympathetic nuclei and in the major cholinergic nuclei of the brainstem (e.g., PPT) and forebrain (e.g., striatum; data not shown). We conclude that the staining shown here is highly likely to be associated with cholinergic cells.
Comparisons with other studies
Major cholinergic nuclei
ChAT-IR cells are routinely found in the cranial nerve motor and parasympathetic nuclei. Two additional nuclei that are regularly identified as cholinergic are the PPT and LDT. These nuclei are conserved across species. They are best known as the source of cholinergic input to the thalamus (e.g. Woolf & Butcher, 1986; Steriade et al., 1988; Oakman et al., 1999). We have shown in preliminary studies that ChAT-IR cells in the PPT and LDT project to the thalamus in guinea pigs (Motts and Schofield, 2006).
The parabigeminal nucleus (PBG) is another nucleus that consistently contains ChAT-IR cells across species. It provides cholinergic input to the superior colliculus and visual parts of the thalamus (e.g. Jiang et al., 1996; Wilson et al., 1995). The present results suggest that the major cholinergic nuclei – PPT, LDT and PBG and the motor and parasympathetic nuclei – are similar in guinea pigs and other species.
Other cholinergic nuclei
Many other brainstem areas contain ChAT-IR cells. Table 1 summarizes results across species. The final section discusses some of the apparent variation. ChAT-IR cells are found in a number of auditory nuclei. Most numerous are the cells in the superior olivary complex. Many of these cells are part of the olivocochlear system (Altschuler et al., 1984; Robertson et al., 1987, Tokunaga, 1988; Warr, 1992; Warr et al., 2002) others may project to the cochlear nucleus (Sherriff and Henderson, 1994). The sagulum and, to a lesser extent, the nucleus of the brachium of the inferior colliculus, contain ChAT-IR cells. Both nuclei project to the auditory thalamus, and the sagulum also projects to the inferior colliculus. The contributions of cholinergic cells to these projections is unknown. In other species, the sagulum comprises two or more subdivisions. The ChAT-IR cells may be restricted to a portion of the nucleus, but this awaits identification of subdivisions in guinea pigs. The ChAT-IR cells in the ventral nucleus of the lateral lemniscus may project to the cochlea, as a small number of “olivocochlear” cells have been described in this nucleus in guinea pigs (Robertson et al., 1987). In addition, ChAT-IR cells were observed in areas near the dorsal nucleus of the lateral lemniscus and, in smaller numbers, in the cochlear nucleus. The projections of the ChAT-IR cells in these areas are unknown. No ChAT-IR cells were observed in the medial superior olivary nucleus or dorsal and intermediate nuclei of the lateral lemniscus.
Table 1. Species differences in ChAT staining in brainstem structures.
| Guinea pig1-2 | Rat3-5 | Cat6-9 | Ferret10 | Baboon11 | Monkey12-13 | Human14-15 | |
|---|---|---|---|---|---|---|---|
| Region | |||||||
| Sensory (non-auditory) cranial nerve nuclei | |||||||
| external cuneate nu. | xx | y | o | ||||
| spinal V | x | y | |||||
| nu. of the solitary tract | xxxx | y | y | o | |||
| superior vestibular nu. | xx | y | o | o | |||
| lateral vestibular nu. | o | o | o | o | x | ||
| medial vestibular nu. | xx | y | y | o | |||
| spinal vestibular nu. | x | y | y | ||||
| OTHER | |||||||
| cuneiform nu. | y | o | x/o | ||||
| Kölliker-Fuse nu. | y | y | y | ||||
| locus coeruleus | y | y | x | ||||
| nu. prepositus hypoglossi | x | y | y | xxx | |||
| parabrachial nu. | xxx | y | y | y | o | xx | |
| raphe magnus nu. | y/o | y | o | o | o | ||
| raphe obscurus nu. | y/o | y | o | o | |||
| superior colliculus | xxx | y/o | y | o | |||
| AUDITORY GROUPS | |||||||
| dorsal cochlear nu. | x | y | |||||
| ventral cochlear nu. | x | ||||||
| Superior olivary complex | x | ||||||
| LSO | xx | y | y | ||||
| MSO | o | y | |||||
| periolivary nu. | xx | y | y | ||||
| nuclei of lateral lemniscus | x | y | y | o | x | ||
| inferior colliculus | o | o | o | ||||
| nucleus of the brachium of the inferior colliculus | xx | ||||||
| sagulum | xxx | ||||||
| RETICULAR FORMATION | |||||||
| caudal pontine reticular nu. | y | ||||||
| gigantocellular reticular nu. | x | y | y | o | xxx | ||
| intermediate reticular nu. | x | y | |||||
| lateral paragigantocellular nu. | x | y | |||||
| lateral reticular nu. | x | y | y | x | |||
| magnocellular tegmental field | y | o | |||||
| oral pontine reticular nu. | x | y | |||||
| paramedian reticular nu. | y | ||||||
| parvocellular reticular nu. | y | y | |||||
| reticular nu. medulla pars ventralis | y |
Summary of ChAT-IR stained cell bodies across species. Symbols: “y” indicates the presence of ChAT-IR cells; “o” indicates lack of ChAT-IR cells; a blank box indicates staining in the structure was not described in the study. In guinea pig and human, the number of labeled cells in a nucleus was rated on a 4 “x” scale, with one x meaning relatively few cells and four x's meaning many labeled cells. The motor and parasympathetic nuclei of the cranial nerves and the “traditionally-named” cholinergic nuclei (PPT, LDT, PBG) are not listed because they were considered positive in all species studied. Only those nuclei that were mentioned in two or more studies are listed. Symbols separated by a “/” indicate that different findings were present in different studies. * Source:
current study;
Mesulam et al., 1984; 1998;
Cholinergic cells are common in many sensory nuclei (Table 1). The superior colliculus (SC) is also noteworthy. In guinea pigs, intensely-stained ChAT-IR cells are very numerous in the superficial gray layer of the SC. Scattered ChAT-IR cells have been noted in the superficial gray layer of the cat SC (Vincent and Reiner, 1987). Similar cells are both ChAT-IR and AChE-positive in guinea pigs (present data, Schnurr et al., 1992), but are apparently absent in other species. The significance of this difference is unclear.
Finally, ChAT-IR cells are scattered among many nuclei of the reticular formation. Some of the variation apparent in Table 1 may be due to differences in terminology or identification of the nuclei. This is particularly likely with reticular formation nuclei, which are often poorly differentiated, and also with nuclei such as the medial and lateral parabrachial nuclei. The latter nuclei are prominently stained in guinea pigs and apparently in some other species (Table 1). However, they are contiguous with the PPT, and the term parabrachial (or “peribrachial”) is sometimes applied to these groups in different ways.
In conclusion, the distribution of ChAT-IR cells in guinea pig brainstem appears largely similar to that described in other species. There are several prominent cell groups, as well as scattered cells across many of the brainstem nuclei. The scattered nature will complicate efforts to characterize the cholinergic circuitry. The present results should complement such efforts in guinea pigs by providing a detailed description of the locations of presumptive cholinergic cells.
Acknowledgments
We gratefully acknowledge Dr. Raymond Papka and Dr. Kenneth Rosenthal for use of their laboratories for generation of the Western blot. We thank Dr. Papka for his generous gift of the Schemann ChAT antibody. We thank Dr. Diana Peterson for feedback on a previous version of this manuscript. In addition, we thank Jennifer Hafemeister and Ryan Schofield for expert technical assistance. Supported by NIH DC04391 and DC08463.
List of Abbreviation
- 3
oculomotor nucleus
- 4
trochlear nucleus
- 4n
4th nerve
- 5
motor nucleus of 5th nerve
- 6
abducens nucleus
- 7
facial nucleus
- 7n
facial nerve
- 8vn
vestibular root of 8th nerve
- 10
dorsal motor nucleus of the vagus
- Amb
nucleus ambiguus
- APT
anterior pretectal nucleus
- Aq
aqueduct
- ATg
anterior tegmental nucleus
- bic
brachium of the inferior colliculus
- Cb
cerebellum
- CG
central gray
- ChAT
choline acetyltransferase
- ChAT-IR
choline acetyltransferase immunoreactive
- cic
commissure of inferior colliculus
- CnF
cuneiform nucleus
- cnr
cochlear nerve root
- cp
cerebral peduncle
- Cu
cuneate nucleus
- cu
cuneate fasciculus
- DAB
diaminobenzidine
- das
dorsal acoustic stria
- DCN
dorsal cochlear nucleus
- DLL
dorsal nucleus of lateral lemniscus
- DpG/Wh
deep gray/white layers of superior colliculus
- DPO
dorsal periolivary nucleus
- DTg
dorsal tegmental nucleus
- ECu
external cuneate nucleus
- EVe
vestibular efferents
- fr
fasciculus retroflexus
- g7
genu of facial nerve
- Gi
gigantocellular reticular nucleus
- grca
granule cell area
- IC
inferior colliculus
- icp
inferior cerebellar peduncle
- ILL
intermediate nucleus of lateral lemniscus
- InG/W
intermediate gray/white layers of superior colliculus
- IO
inferior olive
- IP
interpeduncular nucleus
- ip
intraperitoneal
- IRt
intermediate reticular nucleus
- IS
inferior salivatory nucleus
- LD
laterodorsal thalamic nucleus
- LDT
laterodorsal tegmental nucleus
- lfp
longitudinal fasciculus of pons
- LG
lateral geniculate nucleus
- ll
lateral lemniscus
- LPB
lateral parabrachial nucleus
- LPGi
lateral paragigantocellular reticular nucleus
- LRt
lateral reticular nucleus
- LSO
lateral superior olive
- LTB
lateral nucleus of trapezoid body
- LVe
lateral vestibular nucleus
- m5
motor root of 5th nerve
- mcp
middle cerebellar peduncle
- Me5
mesencephalic nucleus of 5th nerve
- me5
mesencephalic tract of 5th nerve
- MG
medial geniculate body
- ml
medial lemniscus
- mlf
medial longitudinal fasciculus
- MnR
median raphe nucleus
- MPB
medial parabrachial nucleus
- MSO
medial superior olive
- MTB
medial nucleus of trapezoid body
- MVe
medial vestibular nucleus
- MVeMC
medial vestibular nucleus, magnocellular
- MVePC
medial vestibular nucleus, parvicellular
- NRS
normal rabbit serum
- op
optic nerve layer of superior colliculus
- PAG
periaqueductal gray
- PaR
pararubral nucleus
- PB
parabrachial nucleus
- PBG
parabigeminal nucleus
- PBS
phosphate buffered saline
- pc
posterior commissure
- PCRt
parvicellular reticular nucleus
- PMnR
paramedian raphe nucleus
- Pn
pontine nuclei
- PnC
pontine reticular nucleus, caudal
- PnO
pontine reticular nucleus, oral
- PnR
pontine raphe nucleus
- PPT
pedunculopontine tegmental nucleus
- PPTc
pedunculopontine tegmental nucleus, compact region
- PPTd
pedunculopontine tegmental nucleus, dissipated region
- Pr
prepositus nucleus
- Pr5
principle sensory 5 nucleus
- py
pyramidal tract
- R
red nucleus
- Rbd
rhabdoid nucleus
- RMg
raphe magnus nucleus
- RRF
retrorubral field
- RtTg
reticulotegmental nucleus of the pons
- s5
sensory root of 5th nerve
- Sag
sagulum
- SC
superior colliculus
- scp
superior cerebellar peduncle
- SN
substantia nigra
- Sol
nucleus of the solitary tract
- sol
solitary tract
- sp5
spinal trigeminal tract
- Sp5
spinal trigeminal nucleus
- SPN
superior paraolivary nucleus
- SPTg
subpeduncular tegmental nucleus
- SpVe
spinal vestibular nucleus
- SuG
superficial gray layer of superior colliculus
- SuVe
superior vestibular nucleus
- tfp
transverse fibers of the pons
- tz
trapezoid body
- VCN
ventral cochlear nucleus
- VLL
ventral nucleus of lateral lemniscus
- VTA
ventral tegmental area
- VTB
ventral nucleus of trapezoid body
- VTg
ventral tegmental nucleus
- X
nucleus X
- xscp
decussation of superior cerebellar peduncle
- ZI
zona incerta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Schemann ChAT was a gift from Dr. Raymond Papka.
References
- Adams JC. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981;29:775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, Fex J, Parakkal M, Eckenstein F. Colocalization of enkephalin-like and choline acetyltransferase-like immunoreactivities in olivocochlear neurons of the guinea pig. J Histochem Cytochem. 1984;32:839–843. doi: 10.1177/32.8.6379037. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol. 1983;216:53–68. doi: 10.1002/cne.902160106. [DOI] [PubMed] [Google Scholar]
- Fernández de Sevilla D, Rodrigo-Angulo M, Nuñez A, Buño W. Cholinergic modulation of synaptic transmission and postsynaptic excitability in the rat gracilis dorsal column nucleus. J Neurosci. 2006;26:4015–4025. doi: 10.1523/JNEUROSCI.5489-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Bruce G, Hersh LB. Immunohistochemical staining of cholinergic neurons in the human brain using a polyclonal antibody to human choline acetyltransferase. Neurosci Lett. 1985;61:1–5. doi: 10.1016/0304-3940(85)90391-x. [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr . Physiology of olivocochlear efferents. In: Dallos P, et al., editors. The Cochlea. Vol. 8. New York: Springer-Verlag; 1996. pp. 435–502. [Google Scholar]
- Habbicht H, Vater M. A microiontophoretic study of acetylcholine effects in the inferior colliculus of horseshoe bats: implications for a modulatory role. Brain Res. 1996;724:169–179. doi: 10.1016/0006-8993(96)00224-7. [DOI] [PubMed] [Google Scholar]
- Hackney CM, Osen KK, Kolston J. Anatomy of the cochlear nuclear complex of guinea pig. Anat Embryol. 1990;182:123–149. doi: 10.1007/BF00174013. [DOI] [PubMed] [Google Scholar]
- Henderson Z. Overlap in the distribution of cholinergic and catecholaminergic neurons in the upper brainstem of the ferret. J Comp Neurol. 1987;265:581–592. doi: 10.1002/cne.902650409. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Mainville LS, Jones BE. Distribution of cholinergic, GABAergic and serotonergic neurons in the medial medullary reticular formation and their projections studied by cytotoxic lesions in the cat. Neuroscience. 1994;62:1155–1178. doi: 10.1016/0306-4522(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Shimizu T. Organization of choline acetyltransferase-containing structures in the cranial nerve motor nuclei and spinal cord of the monkey. Brain Res. 1998;779:96–103. doi: 10.1016/s0006-8993(97)01090-1. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Houtani T, Ueyama T, Sugimoto T. Distribution and cerebellar projections of cholinergic and corticotropin-releasing factor-containing neurons in the caudal vestibular nuclear complex and adjacent brainstem structures. Neuroscience. 1992;49:635–651. doi: 10.1016/0306-4522(92)90233-r. [DOI] [PubMed] [Google Scholar]
- Jiang ZD, King AJ, Moore DR. Topographic organization of projection from the parabigeminal nucleus to the superior colliculus in the ferret revealed with fluorescent latex microspheres. Brain Res. 1996;743:217–232. doi: 10.1016/s0006-8993(96)01042-6. [DOI] [PubMed] [Google Scholar]
- Jones BE, Beaudet A. Distribution of acetylcholine and catecholamine neurons in the cat brainstem: a choline acetyltransferase and tyrosine hydroxylase immunohistochemical study. J Comp Neurol. 1987;261:15–32. doi: 10.1002/cne.902610103. [DOI] [PubMed] [Google Scholar]
- Leonard CS, Kerman I, Blaha G, Taveras E, Taylor B. Interdigitation of nitric oxide synthase-, tyrosine hydroxylase-, and serotonin-containing neurons in and around the laterodorsal and pedunculopontine tegmental nuclei of the guinea pig. J Comp Neurol. 1995;362:411–432. doi: 10.1002/cne.903620309. [DOI] [PubMed] [Google Scholar]
- Levey AI, Wainer BH. Cross-species and intraspecies reactivities of monoclonal antibodies against choline acetyltransferase. Brain Res. 1982;234:469–473. doi: 10.1016/0006-8993(82)90889-7. [DOI] [PubMed] [Google Scholar]
- Maley BE, Frick ML, Levey AI, Wainer BH, Elde RP. Immunohistochemistry of choline acetyltransferase in the guinea pig brain. Neurosci Lett. 1988;84:137–142. doi: 10.1016/0304-3940(88)90397-7. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Neuroscience. 1984;12:669–686. doi: 10.1016/0306-4522(84)90163-5. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mash D, Hersh L, Bothwell M, Geula C. Cholinergic innervation of the human striatum, globus pallidus, subthalamic nucleus, substantia nigra, and red nucleus. J Comp Neurol. 1992;323:252–268. doi: 10.1002/cne.903230209. [DOI] [PubMed] [Google Scholar]
- Mizukawa K, McGeer PL, Tago H, Peng JH, McGeer EG, Kimura H. The cholinergic system of the human hindbrain studied by choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Brain Res. 1986;379:39–55. doi: 10.1016/0006-8993(86)90253-2. [DOI] [PubMed] [Google Scholar]
- Motts SD, Schofield BR. Program No 543.20. 2006 Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience; 2006. Cholinergic projections to thalamus and inferior colliculus in guinea pigs. Online. [Google Scholar]
- Mulders WH, Paolini AG, Needham K, Robertson D. Olivocochlear collaterals evoke excitatory effects in onset neurones of the rat cochlear nucleus. Hear Res. 2003;176:113–121. doi: 10.1016/s0378-5955(02)00750-5. [DOI] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Cozzari C, Hartman BK. Characterization of the extent of pontomesencephalic cholinergic neurons' projections to the thalamus: comparison with projections to midbrain dopaminergic groups. Neuroscience. 1999;94:529–547. doi: 10.1016/s0306-4522(99)00307-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; Boston: 1998. [DOI] [PubMed] [Google Scholar]
- Robertson D, Cole KS, Corbett K. Quantitative estimate of bilaterally projecting medial olivocochlear neurones in the guinea pig brainstem. Hear Res. 1987;27:177–181. doi: 10.1016/0378-5955(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Giuliano R, Anwar M, Stornetta R, Reis DJ. Anatomical substrates of cholinergic-autonomic regulation in the rat. J Comp Neurol. 1990;292:1–53. doi: 10.1002/cne.902920102. [DOI] [PubMed] [Google Scholar]
- Satoh K, Armstrong DM, Fibiger HC. A comparison of the distribution of central cholinergic neurons as demonstrated by acetylcholinesterase pharmacohistochemistry and choline acetyltransferase immunohistochemistry. Brain Res Bull. 1983;11:693–720. doi: 10.1016/0361-9230(83)90013-8. [DOI] [PubMed] [Google Scholar]
- Satoh K, Fibiger HC. Distribution of central cholinergic neurons in the baboon (Papio papio). II. A topographic atlas correlated with catecholamine neurons. J Comp Neurol. 1985;236:215–233. doi: 10.1002/cne.902360206. [DOI] [PubMed] [Google Scholar]
- Schemann M, Sann H, Schaaf C, Mader M. Identification of cholinergic neurons in enteric nervous system by antibodies against choline acetyltransferase. Am J Physiol. 1993;265:G1005–1009. doi: 10.1152/ajpgi.1993.265.5.G1005. [DOI] [PubMed] [Google Scholar]
- Schnurr B, Spatz WB, Illing RB. Similarities and differences between cholinergic systems in the superior colliculus of guinea pig and rat. Exp Brain Res. 1992;90:291–296. doi: 10.1007/BF00227240. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Cant NB. Organization of the superior olivary complex in the guinea pig. I. Cytoarchitecture, cytochrome oxidase histochemistry, and dendritic morphology. J Comp Neurol. 1991;314:645–670. doi: 10.1002/cne.903140403. [DOI] [PubMed] [Google Scholar]
- Schofield BR, Cant NB. Ventral nucleus of the lateral lemniscus in guinea pigs: cytoarchitecture and inputs from the cochlear nucleus. J Comp Neurol. 1997;379:363–385. doi: 10.1002/(sici)1096-9861(19970317)379:3<363::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Sherriff FE, Henderson Z. Cholinergic neurons in the ventral trapezoid nucleus project to the cochlear nuclei in the rat. Neuroscience. 1994;58:627–633. doi: 10.1016/0306-4522(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Simons CT, Boucher Y, Carstens MI, Carstens E. Nicotine suppression of gustatory responses of neurons in the nucleus of the solitary tract. J Neurophysiol. 2006;96:1877–1886. doi: 10.1152/jn.00345.2006. [DOI] [PubMed] [Google Scholar]
- Steriade M, Paré D, Parent A, Smith Y. Projections of cholinergic and non-cholinergic neurons of the brainstem core to relay and associational thalamic nuclei in the cat and macaque monkey. Neuroscience. 1988;25:47–67. doi: 10.1016/0306-4522(88)90006-1. [DOI] [PubMed] [Google Scholar]
- Strutz J. The origin of efferent vestibular fibres in the guinea pig. A horseradish peroxidase study. Acta Otolaryngol. 1982;94:299–305. doi: 10.3109/00016488209128917. [DOI] [PubMed] [Google Scholar]
- Tago H, McGeer PL, McGeer EG, Akiyama H, Hersh LB. Distribution of choline acetyltransferase immunopositive structures in the rat brainstem. Brain Res. 1989;495:271–297. doi: 10.1016/0006-8993(89)90221-7. [DOI] [PubMed] [Google Scholar]
- Tatehata T, Shiosaka S, Wanaka A, Rao ZR, Tohyama M. Immunocytochemical localization of the choline acetyltransferase containing neuron system in the rat lower brain stem. J Hirnforsch. 1987;28:707–716. [PubMed] [Google Scholar]
- Tokunaga A. Superior olivary and lateral lemniscal neurons projecting to the cochlea in the guinea pig. Neurosci Res. 1988;6:20–30. doi: 10.1016/0168-0102(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Smith DV. Cholinergic modulation of neurons in the gustatory region of the nucleus of the solitary tract. Brain Res. 2006;1084:38–53. doi: 10.1016/j.brainres.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Reiner PB. The immunohistochemical localization of choline acetyltransferase in the cat brain. Brain Res Bull. 1987;18:371–415. doi: 10.1016/0361-9230(87)90015-3. [DOI] [PubMed] [Google Scholar]
- Warr WB. Organization of olivocochlear efferent systems in mammals. In: Webster DB, et al., editors. The Mammalian Auditory Pathway: Neuroanatomy. Vol. 1. New York NY: Springer-Verlag; 1992. pp. 410–448. [Google Scholar]
- Warr W, Beck Boche J, Ye Y, Kim DO. Organization of olivocochlear neurons in the cat studied with the retrograde tracer cholera toxin-B. J Assoc Res Otolaryngol. 2002;3:457–478. doi: 10.1007/s10162-002-2046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JR, Hendrickson AE, Sherk H, Tigges J. Sources of subcortical afferents to the macaque's dorsal lateral geniculate nucleus. Anat Rec. 1995;242:566–574. doi: 10.1002/ar.1092420413. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Research Bulletin. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]