Dear Editors:
Our group has shown through microarray data analysis that the gene expression pattern of Down Syndrome (DS) placentas differs from that of euploid control placentas [1]. One gene of interest that was over-expressed in DS placentas in microarray, Northern blot and quantitative real time PCR (qPCR) analyses was keratin 8 (KRT8). Keratins are the largest subgroup of the intermediate filaments. KRT8, along with keratin 18, is an intermediate filament that is associated with differentiation to the trophoectodermal layer of the blastocyst [2]. In trophoblast cells, the keratin cytoskeleton appears to be important in maintaining structural integrity of these cells and therefore, maybe important in intrauterine growth restriction and spontaneous abortion [3]. In addition to its role as a structural protein and as a protein involved in differentiation, KRT8 functions in signal transduction and potentially in apoptosis.
There is evidence that KRT8 modulates tumor necrosis factor (TNF) and fas mediated cell death [4]. Jaquemar et al. showed that KRT8 deficient embryos were exquisitely sensitive to apoptosis [5]. We have therefore evaluated the relationship between KRT8 and the TNF related apoptotic pathway in DS placentas as compared to euploid placentas.
In order to control for any possible effect of mode of delivery on differential gene expression, all placentas used in this work were dilatation and extraction specimens. Also, all placentas in the study were second trimester specimens, ranging in gestational age from 17 to 24 weeks. Gestational age was based upon last menstrual period, ultrasound and autopsy following pregnancy termination. Aneuploid and euploid placentas were individually gestational age-matched, meaning for every aneuploid placenta there was at least one normal control placenta of the same gestational age +/− two weeks and vice versa. Finally, the difference in the gestational ages in the two groups is not statistically significant.
Karyotype was confirmed by either amniocentesis or placental biopsy. All steps, from tissue harvesting to band karyotyping, were performed according to standard protocols established. Karyotypes were confirmed by an experienced clinical cytogeneticist. Although the presence of confined placental mosaicism can not be completely ruled out, generous placental biopsies comprised of large areas of chorionic villous parenchyma and generous amnionic fluid samples were collected for karyotyping, minimizing the risk of mosaicism. Furthermore, mosaicism has been found to occur in only 1% of the aneuploid cases at our center.
For placental biopsies, placental chorionic villous tissue blocks were extensively washed in either RNAlater@ (Invitrogen) or phosphate buffered saline before analysis. In addition, the purity of the samples was confirmed by histologic examination of the tissue sections, which revealed only fetal chorionic villi and no maternal basal plate or decidual vasculature present. For amniocentesis specimens, sample purity was verified, following clinical cytogenetics standard protocols. All measures were taken to prevent contamination. Only samples that met strict criteria were included in our study and were analyzed.
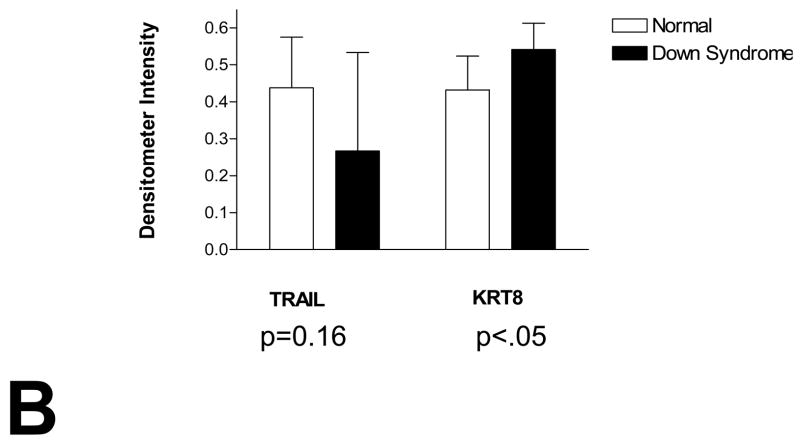
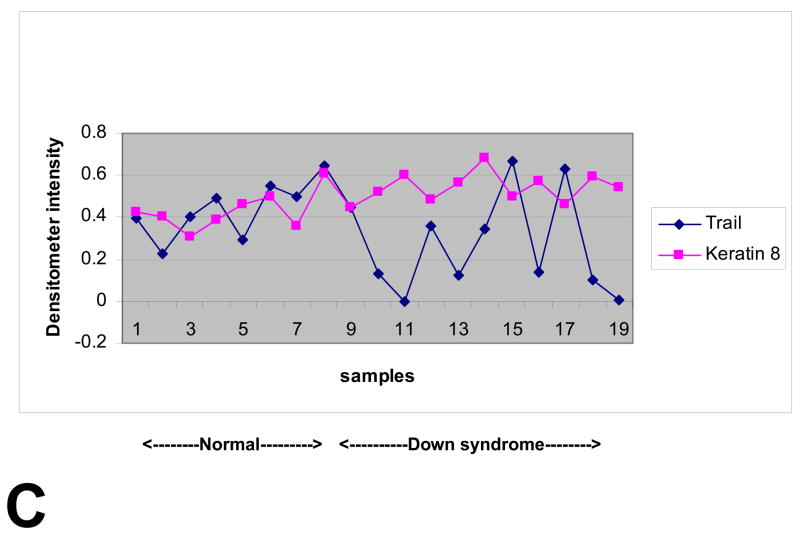
Quantitative PCR demonstrated that TNF-related apoptosis-inducing ligand (TRAIL) was significantly over-expressed in T21 placentas compared to controls, while TNF-α and the three TRAIL receptors evaluated were not (Table 1). The Western blot analysis of the normal and DS placental samples is shown in Figure 1a. Densitometry analysis is shown in Figure 1b. A significant difference in KRT8 expression is seen between these two groups. (p = .008) There is not a significant difference in TRAIL expression between DS and normal placental specimens (p = 0.16) A marked inverse relationship between KRT8 and TRAIL is seen in 8 out of the 11 DS specimens, whereas no such relationship is seen in the normal controls (Figure 1c.)
Figure 1. Expression of KRT8 and TRAIL in human placenta.
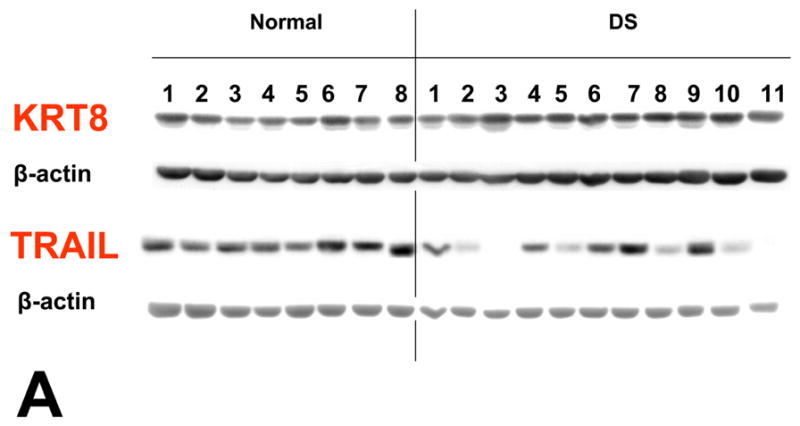
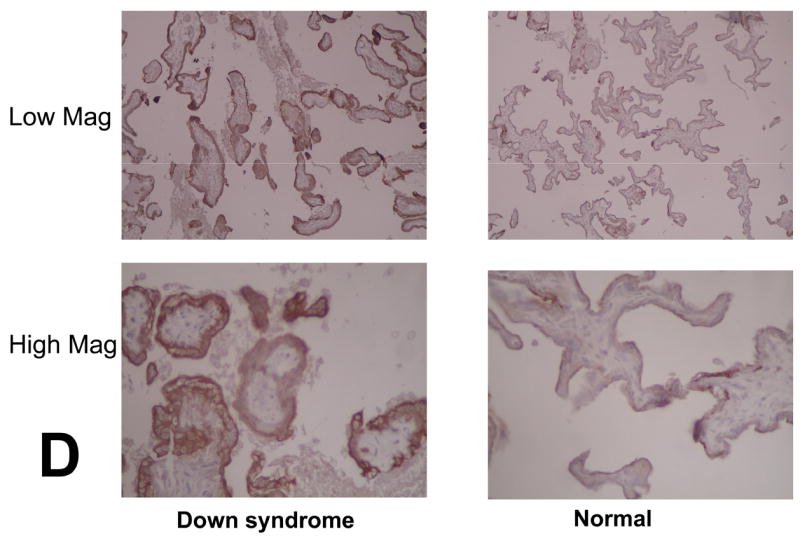
Each lane was loaded with 30 μg of proteins extracted from placental chorionic villi and immunoblots were reacted with antibodies directed against KRT8, TRAIL and β-actin (A). Levels of expression were determined by densitometry after normalization to β-actin (B). A marked inverse relationship between KRT8 and TRAIL is seen in 8 out of the 11 DS specimens, whereas no such relationship is seen in the normal controls (C). Immunostaining of KRT8 and TRAIL was performed on paraffin embedded placental sections from DS and controls. Slides were incubated overnight at 4° C with KRT8 (Santa Cruz) primary antibody. Staining was visualized using a Vectastain ABC kit (Vector Laboratories, Inc, Burlingame, CA) and diaminobenzidene (Sigma) as substrate (D).
Immunohistochemical staining revealed that KRT8 and TRAIL are both expressed in the outermost portion of the apical membranes of placental chorionic villous syncytiotrophoblast cells. In the DS samples, KRT8 immunohistochemical staining is more prominent when compared to normal controls (Figure 1d). There is not a significant difference in TRAIL expression (not shown).
The external death receptor pathway, mediated by the TNF family of genes, has garnered particular attention, as this pathway has been implicated in mammalian immune function and has consequently helped elucidate our understanding of immune privilege at the maternal-fetal interface. Similar to the way that apoptosis of immune cells protects against autoimmune disorders, the external pathway appears to provide protection of the fetal/placental semiallograft from maternal immune attack [6]. Corresponding TNF receptor (TNFR) superfamily members have also been identified in placenta, such as TNFR1 (which binds TNFα and LTα) and Fas (which binds FasL). TRAIL binds to DR4 and DR5, and can also bind to decoy receptors DcR1 and DcR2. Decoy receptors have non-existent or truncated death domains and thereby do not typically result in apoptosis [7]. As a result, there may be critical levels of ligands and/or receptors that make cells sensitive or resistant to cell death.
The inverse relationship between KRT8 and TRAIL in 8 of our 11 DS specimens may be explained by a negative feedback mechanism. TRAIL induced apoptosis results in an elevated level of KRT8. KRT8, in turn, controls apoptosis by down-regulating TRAIL. An interesting caveat is that this inverse relationship was not present in all individual samples, as seen in Figure 1c, which may explain the individual variation in DS fetuses with respect to viability. KRT8 has been shown to be critical in the development of mouse embryos beyond mid-gestation. This relationship may be one possible mechanism to explain why some DS fetuses survive to term and some do not.
Our results confirm the recent findings of other investigators that apoptosis appears to be an inconsistent finding in aneuploid placentae. Wright et al. [8] studied DS placentae and the cytotrophoblast differentiation pathway that leads to uterine invasion. Of note, they showed some Trisomy 21 cell samples exhibiting significantly increased apoptosis and other samples appearing normal. Subtle interplay between proteins in the external apoptotic pathway may help explain not only the underlying cellular regulation in aneuploid placentae, but also such striking variation between samples. Future studies in this area may help elucidate why many DS pregnancies end in fetal demise, while others survive to term.
Acknowledgments
This work was supported by National Institute of Child Health and Human Development grants 5R03HD40342-2 and K12HD001255.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gross SJ, Ferreira JC, Morrow B, Dar P, Funke B, Khabele D, et al. Gene expression profile of trisomy 21 placentas: a potential approach for designing noninvasive techniques of prenatal diagnosis. Am J Obstet Gynecol. 2002;187:457–462. doi: 10.1067/mob.2002.123542. [DOI] [PubMed] [Google Scholar]
- 2.Brulet P, Babinet C, Kemler R, Jacob F. Monoclonal antibodies against trophectoderm-specific markers during mouse blastocyst formation. Proc Natl Acad Sci USA. 1980;77:4413–4417. doi: 10.1073/pnas.77.7.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson ED. 2005 Trophoblast Research Award Lecture: Defects in the Keratin Cytoskeleton Disrupt Normal Murine Placental Development and Trophoblast Cell Function. Placenta. 2007;28:S111–115. doi: 10.1016/j.placenta.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Oshima RG. Apoptosis and keratin intermediate filaments. Cell Death Differ. 2002;9:486–492. doi: 10.1038/sj.cdd.4400988. [DOI] [PubMed] [Google Scholar]
- 5.Jaquemar D, Kupriyanov S, Wankell M, Avis J, Benirschke K, Baribault H, et al. Keratin 8 protection of placental barrier function. J Cell Biol. 2003;161:749–756. doi: 10.1083/jcb.200210004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jerzak M, Bischof P. Apoptosis in the first trimester human placenta: the role in maintaining immune privilege at the marternal-foetal interface and in the trophoblast remodeling. Eur J Obstet Gynecol Reprod Biol. 2002;100:138–142. doi: 10.1016/s0301-2115(01)00431-6. [DOI] [PubMed] [Google Scholar]
- 7.Phillips TA, Ni J, Hunt JS. Death-inducing rumour necrosis factor (TNF) superfamily ligands and receptors are transcribed in human placentae, cytotrophoblasts, placental macrophages and placental cell lines. Placenta. 2001;22:663–672. doi: 10.1053/plac.2001.0703. [DOI] [PubMed] [Google Scholar]
- 8.Wright A, Zhou Y, Weier JF, Caceres E, Kapidzic M, Tabata T, Kahn M, Nash C, Fisher SJ. Trisomy 21 is associated with variable defects in cytotrophoblast differentiation along the invasive pathway. Am J Med Genet. 2004;130A:354–364. doi: 10.1002/ajmg.a.30254. [DOI] [PubMed] [Google Scholar]