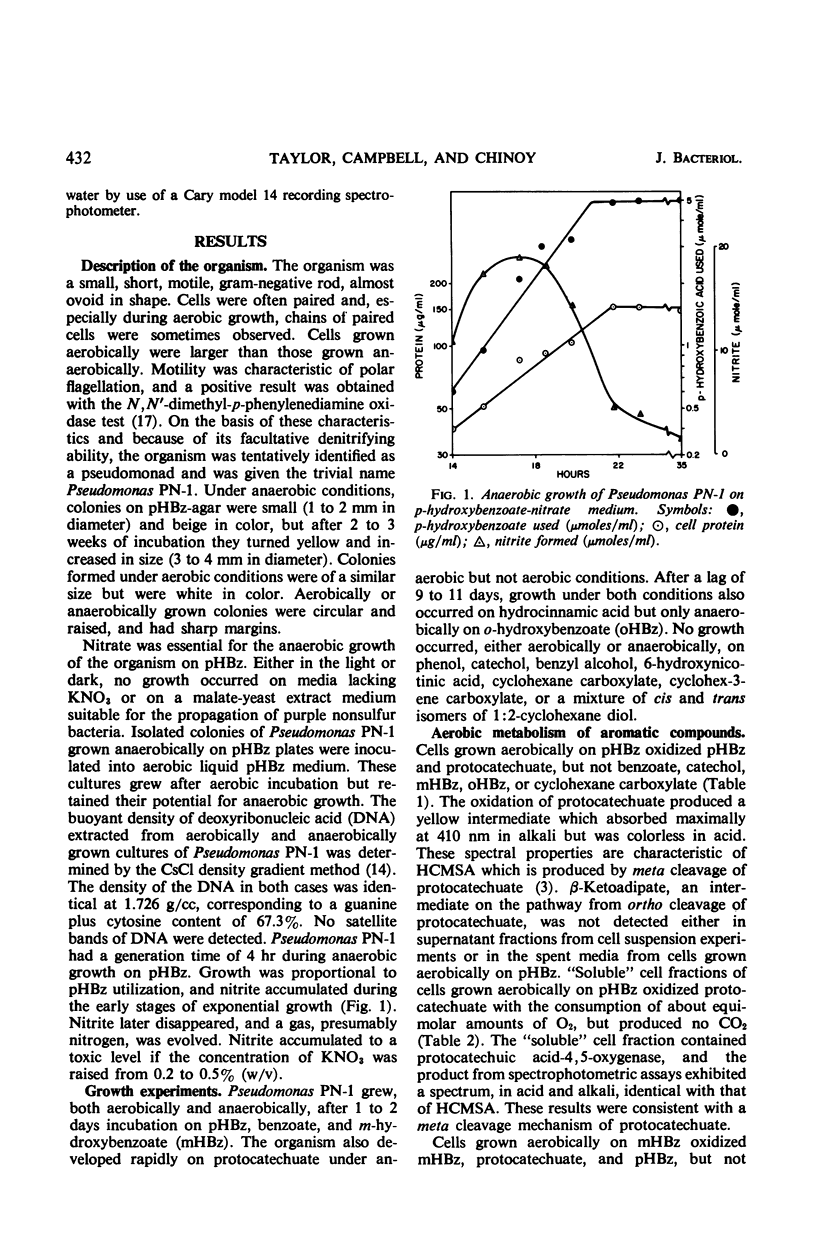
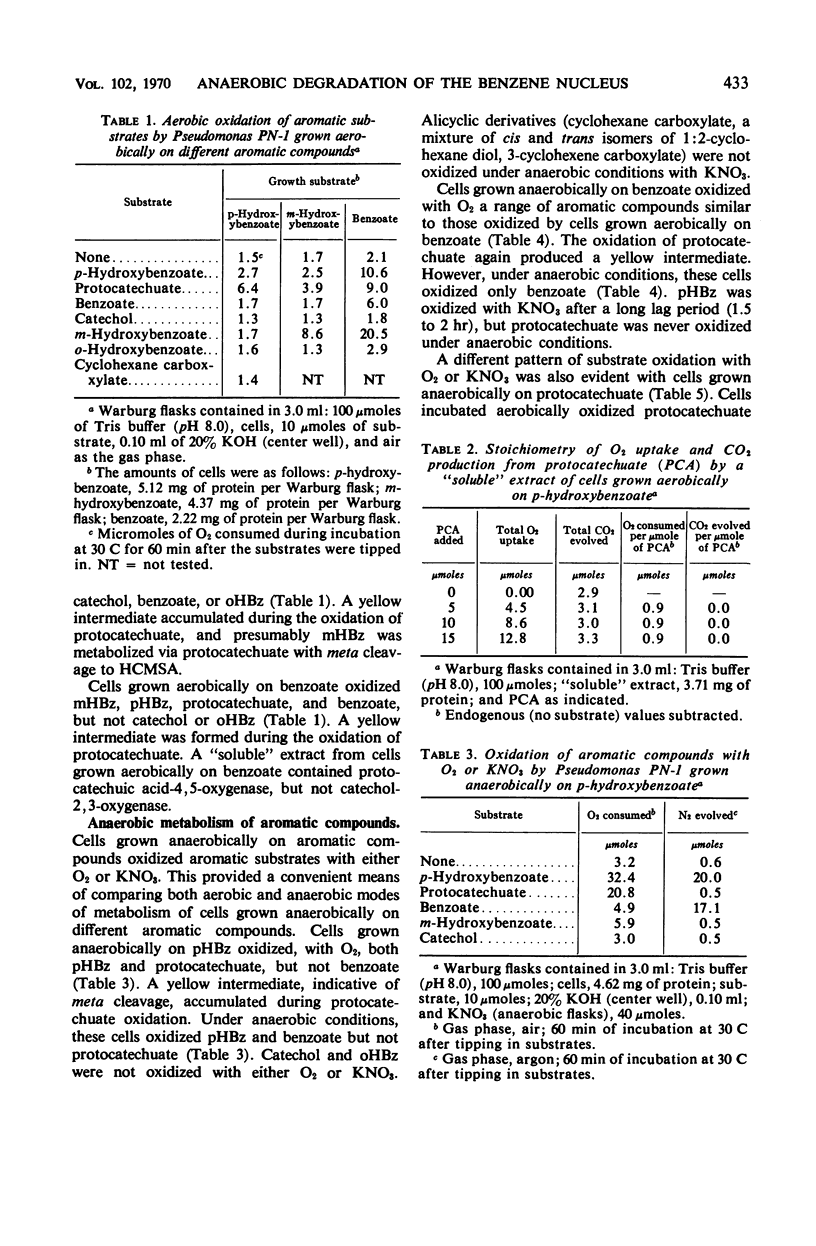
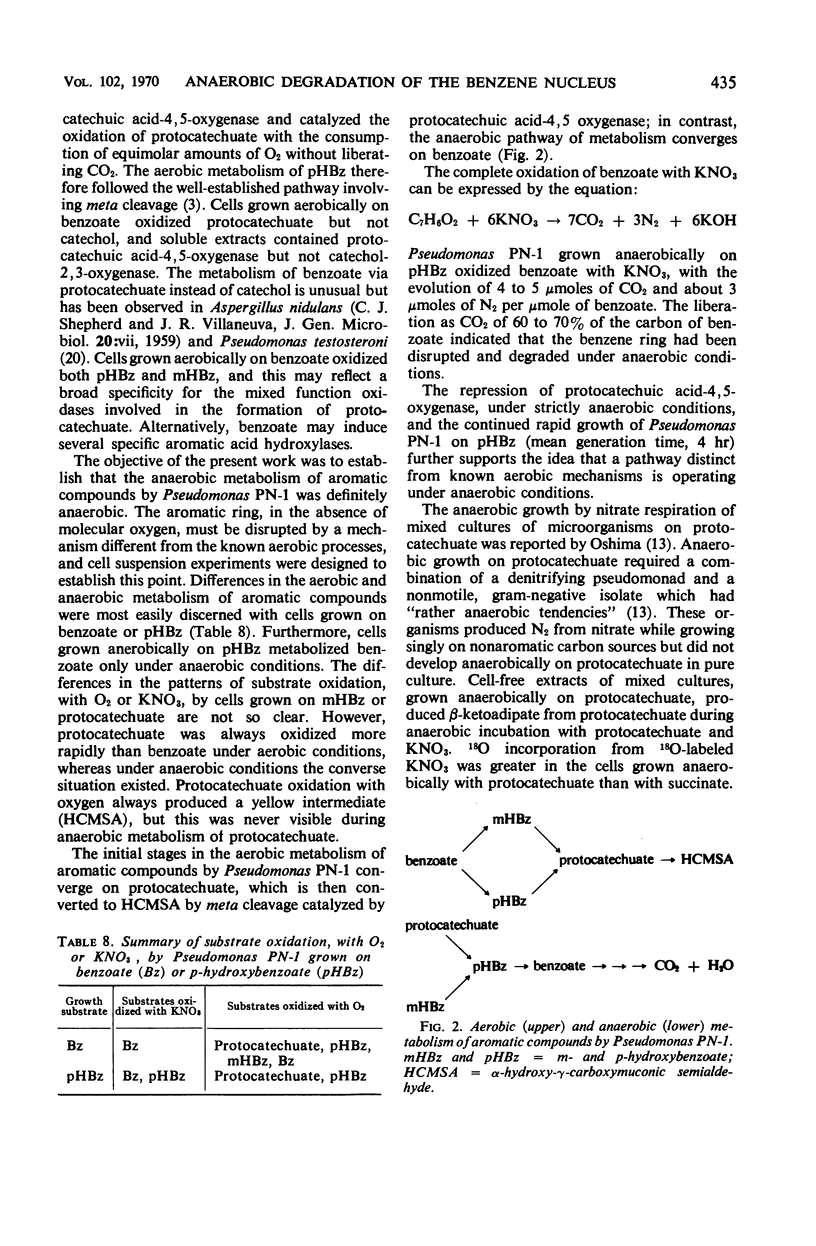
Abstract
A bacterium was isolated by elective culture with p-hydroxybenzoate as substrate and nitrate as electron acceptor. It grew either aerobically or anaerobically, by nitrate respiration, on a range of aromatic compounds. The organism was identified as a pseudomonad and was given the trivial name Pseudomonas PN-1. Benzoate and p-hydroxybenzoate were metabolized aerobically via protocatechuate, followed by meta cleavage catalyzed by protocatechuic acid-4,5-oxygenase, to yield α-hydroxy-γ-carboxymuconic semialdehyde. Pseudomonas PN-1 grew rapidly on p-hydroxybenzoate under strictly anaerobic conditions, provided nitrate was present, even though protocatechuic acid-4,5-oxygenase was repressed. Suspensions of cells grown anaerobically on p-hydroxybenzoate oxidized benzoate with nitrate and produced 4 to 5 μmoles of CO2 per μmole of benzoate added; these cells did not oxidize benzoate aerobically. The patterns of the oxidation of aromatic substrates with oxygen or nitrate by cells grown aerobically or anaerobically on different aromatic compounds indicated that benzoate rather than protocatechuate was a key intermediate in the early stages of anaerobic metabolism. It was concluded that the pathway for the anaerobic breakdown of the aromatic ring is different and quite distinct from the aerobic pathway. Mechanisms for the anaerobic degradation of the benzene nucleus by Pseudomonas PN-1 are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLARK F. M., FINA L. R. The anaerobic decomposition of benzoic acid during methane fermentation. Arch Biochem Biophys. 1952 Mar;36(1):26–32. doi: 10.1016/0003-9861(52)90374-3. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., EVANS W. C., RIBBONS D. W. New pathways in the oxidative metabolism of aromatic compounds by microorganisms. Nature. 1960 Nov 12;188:560–566. doi: 10.1038/188560a0. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Evans W. C. The metabolism of aromatic compounds by Rhodopseudomonas palustris. A new, reductive, method of aromatic ring metabolism. Biochem J. 1969 Jul;113(3):525–536. doi: 10.1042/bj1130525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS W. C. THE MICROBIOLOGICAL DEGRADATION OF AROMATIC COMPOUNDS. J Gen Microbiol. 1963 Aug;32:177–184. doi: 10.1099/00221287-32-2-177. [DOI] [PubMed] [Google Scholar]
- FINA L. R., FISKIN A. M. The anaerobic decomposition of benzoic acid during methane fermentation. II. Fate of carbons one and seven. Arch Biochem Biophys. 1960 Dec;91:163–165. doi: 10.1016/0003-9861(60)90483-5. [DOI] [PubMed] [Google Scholar]
- Guyer M., Hegeman G. Evidence for a reductive pathway for the anaerobic metabolism of benzoate. J Bacteriol. 1969 Sep;99(3):906–907. doi: 10.1128/jb.99.3.906-907.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. The metabolism of p-hydroxybenzoate by Rhodopseudomonas palustris and its regulation. Arch Mikrobiol. 1967;59(1):143–148. doi: 10.1007/BF00406325. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nottingham P. M., Hungate R. E. Methanogenic fermentation of benzoate. J Bacteriol. 1969 Jun;98(3):1170–1172. doi: 10.1128/jb.98.3.1170-1172.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTON L. N., STANIER R. Y. MECHANISM OF BETA-KETOADIPATE FORMATION BY BACTERIA. Nature. 1964 Dec 26;204:1279–1283. doi: 10.1038/2041279a0. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Hoare D. S. Acetate assimilation by Nitrobacter agilis in relation to its "obligate autotrophy". J Bacteriol. 1968 Mar;95(3):844–855. doi: 10.1128/jb.95.3.844-855.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y. The Oxidation of Aromatic Compounds by Fluorescent Pseudomonads. J Bacteriol. 1948 Apr;55(4):477–494. doi: 10.1128/jb.55.4.477-494.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISHNIAC W., SANTER M. The thiobacilli. Bacteriol Rev. 1957 Sep;21(3):195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelis M. L., Palleroni N. J., Stanier R. Y. The metabolism of aromatic acids by Pseudomonas testosteroni and P. acidovorans. Arch Mikrobiol. 1967;59(1):302–314. doi: 10.1007/BF00406344. [DOI] [PubMed] [Google Scholar]


