Abstract
In Xenopus oocytes, the water permeability of AQP0 (Pf) increases with removal of external calcium, an effect that is mediated by cytoplasmic calmodulin (CaM) bound to the C terminus of AQP0. To investigate the effects of serine phosphorylation on CaM-mediated Ca2+ regulation of Pf, we tested the effects of kinase activation, CaM inhibition, and a series of mutations in the C terminus CaM binding site. Calcium regulation of AQP0 Pf manifests four distinct phenotypes: Group 1, with high Pf upon removal of external Ca2+ (wild-type, S229N, R233A, S235A, S235K, K238A, and R241E); Group 2, with high Pf in elevated (5 mm) external Ca2+ (S235D and R241A); Group 3, with high Pf and no Ca2+ regulation (S229D, S231N, S231D, S235N, and S235N/I236S); and Group 4, with low Pf and no Ca2+ regulation (protein kinase A and protein kinase C activators, S229D/S235D and S235N/I236S). Within each group, we tested whether CaM binding mediates the phenotype, as shown previously for wild-type AQP0. In the presence of calmidazolium, a CaM inhibitor, S235D showed high Pf and no Ca2+ regulation, suggesting that S235D still binds CaM. Contrarily, S229D showed a decrease in recruitment of CaM, suggesting that S229D is unable to bind CaM. Taken together, our results suggest a model in which CaM acts as an inhibitor of AQP0 Pf. CaM binding is associated with a low Pf state, and a lack of CaM binding is associated with a high Pf state. Pathological conditions of inappropriate phosphorylation or calcium/CaM regulation could induce Pf changes contributing to the development of a cataract.
In the equatorial region of the lens, metabolically active epithelial cells differentiate into less active fiber cells and form the cortical region of the lens. In the core of the lens, mature fiber cells lack nuclei and organelles (1-3). Mature lens fiber cells are characterized by slow protein turnover and increasing post-translational modifications such as proteolytic cleavage, glycation, and phosphorylation. In the cortex of the lens, there is an elevated level of phosphorylated proteins, demonstrating the significance of phosphorylation in lens differentiation (3-5). Gap junctions, water channels, Na+/K+ pumps, and Ca2+, K+, Na+, and Cl- channels act in concert to maintain cellular homeostasis, possibly by generating an intrinsic lens circulation (6-9). Such a proposed intrinsic circulation would move nutrients from the aqueous humor to core fiber cells and waste products away from the central part of the lens toward the surface.
AQP0,4 the water channel of lens fiber cells, is essential for normal lens structure development and for maintaining lens transparency and focusing power. Lenses of AQP0 knock-out mice (AQP0-/-) present congenital cataracts, fail to develop normal structure, and lose 80% of their water permeability (10-13). When AQP0 and AQP0-LTR (a naturally occurring C-terminal mutant of AQP0) are expressed together in transgenic mice, the lenses show incomplete fiber cell development. In vitro expression of wild-type AQP0 and AQP0-LTR in Xenopus oocyte showed that AQP0 is a functional water channel in the presence of AQP0-LTR protein but lacks calcium regulation (14, 15). The elimination of Ca2+ regulation of water permeability could be a contributing cause of the aberrant lens development of AQP0-LTR transgenic mice.
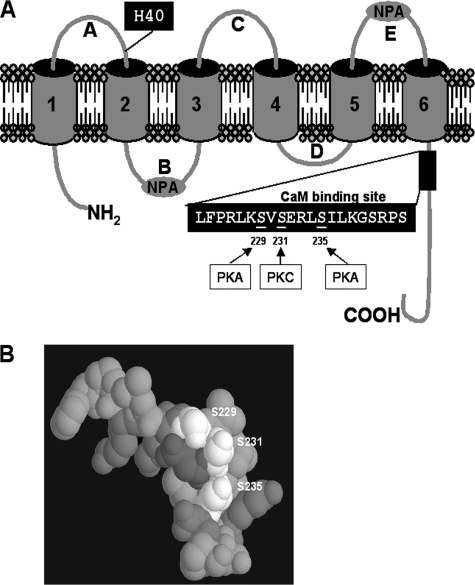
The water permeability (Pf) of AQP0 is regulated by pH, Zn2+, Ni2+, and Ca2+ (16-18). In the Xenopus oocyte expression system, shifting the pH from 7.4 to 6.5 or lowering the physiological external Ca2+ from 2 to 0 mm increases AQP0 water permeability. The calcium effect is mediated through CaM bound to the C terminus of AQP0 (16, 17). In lens vesicle preparation, AQP0 Pf increases with acid pH and 5 mm Ca2+ (19). The authors suggested that the difference between oocytes and lens vesicles results (5 mm calcium instead of 0 mm) could be explained by a difference in affinity for apo-CaM and calcium/CaM, due to a different lipid composition of the membranes. In a normal lens, phosphorylation of the C terminus of AQP0 is up-regulated in the cortex, and the phosphorylation prediction data base flags the serines at positions 229, 231, and 235 as consensus PKA and PKC phosphorylation sites (Fig. 1A) (4, 20, 21). These serines indeed show 10-15% of phosphorylation in vivo in normal lens cortex (4). At position 235, the level of serine phosphorylation increases from the outer to the inner cortex. In the CaM binding site (residues 223-243) (22), the high-resolution structure of the C-terminal end shows that the target serines form an exposed ridge (Fig. 1B) (23, 24), and the negative charges induced by phosphorylation likely alter the interaction with CaM and thus modulate calcium regulation. Rose et al. (26) have shown that a phosphorylated AQP0 C terminus peptide binds CaM more weakly than the unphosphorylated form, and we (16) have shown that CaM inhibitors increase the water permeability of AQP0 in Xenopus oocytes. It is thus plausible that phosphorylation weakens CaM binding just as CaM inhibitors do.
FIGURE 1.
Topology of AQP0. A, AQP0 has six transmembrane regions; A, C, and E loops are located on the extracellular side of the membrane. H40 is essential for pH regulation. B and D loops are on the intracellular side of the membrane. The two NPA boxes in loops B and E fold back and form the pore selectivity filter. The N and C termini are located on the intracellular side of the membrane. At the C terminus, the CaM binding site contains three phosphorylation sites. Serines 229 and 235 can be phosphorylated by PKA, and serine 231 can be phosphorylated by PKC. B, three serines (Ser229, Ser231, and Ser235) form a negative ridge along the surface of CaM binding helix (from the crystal structure of AQP0, Protein Data Bank code 2B6P).
To study the effects of phosphorylation on AQP0 water permeability, we used a combination of PKA and PKC activators, CaM inhibitor calmidazolium (CDZ), and AQP0 mutants that were designed to mimic or prevent phosphorylation of particular target serines and studied their effects on CaM-mediated Ca2+ regulation of Pf in the Xenopus oocyte expression system. Because differences in mutant expression might mask regulatory effects of interest, we developed an antibody against the extracellular E loop to test the expression of AQP0 in the presence of PKA and PKC activators and the expression of AQP0 mutants. We compared the association of a fluorescently labeled CaM (Alexa Fluor 488-conjugated CaM; CaM-488) with wild-type AQP0 to its association with the phosphorylation-mimicking mutant, S229D.
EXPERIMENTAL PROCEDURES
Site-directed Mutagenesis—Mutagenesis was performed using the Stratagene QuikChange XL site-directed mutagenesis kit (catalog no. 200516-5). The oligo-DNAs for the mutations were synthesized at Sigma Genosys, and the mutations were confirmed by sequencing at the University of Chicago Cancer Research Center DNA Sequencing Facility.
cRNA Preparation for Xenopus Oocytes—The expression construct for bovine AQP0 (pXβG-AQP0) was a kind gift from Peter Agre (Johns Hopkins, Baltimore, MD). Wild-type and mutated AQP0 cRNAs were transcribed in vitro using the T3 RNA polymerase (mMACHINE kit, Ambion, Inc.).
Xenopus Oocyte Swelling Assay—Female Xenopus laevis were anesthetized, and stage V and VI oocytes were prepared and injected with 10 ng of cRNA as described previously (16, 27). The oocytes were incubated in 100% ND96 buffer with the desired test Ca2+ concentration and pH for 5 min before the swelling assay. Swelling assays were performed at room temperature (20-21 °C) by transferring oocytes from a 200 mosm (100% ND96 in mm: 96 NaCl, 2 KCl, 5 HEPES, 1.8 CaCl2, 1 MgCl2, and 2.5 sodium pyruvate, pH 7.4) to a 70 mosm (30% ND96) solution adjusted to the desired calcium concentration and pH. Each data point is the average of at least nine measurements (three different batches of oocytes and three oocytes from each batch). Error bars are shown as ± S.E., and we performed a Student's t test to check the significance of differences of water permeability. All results with p values <10-6 are marked with an asterisk on the figures.
PKA and PKC Activation in Xenopus Oocytes—PKA and PKC activators were applied for 15 min before the swelling assays were performed (PKA activator mixture, 10 μm dibutyryl-cAMP; 25 μm forskolin; or PKC activator, 20 nm PMA) and were maintained during the swelling assays.
Oocyte Membrane Isolation and Immunoblot Analysis—Total membranes of 10 oocytes were prepared with the ProteoExtract® native membrane protein extraction kit (Calbiochem) according the manufacturer's directions. Half an oocyte of each sample was loaded and run on a 4-12% SDS NuPAGE gel (Invitrogen) and then electrotransferred to nitrocellulose. The blot was blocked with 3% goat serum and 1% bovine serum albumin in phosphate-buffered saline and then incubated with anti-AQP0 antibody (a 1:500 dilution of a polyclonal rabbit antiserum raised against the external E loop of AQP0 (amino acids from 184-202, N-MNPARSFAPAILTRNFSNHW-C)). Then it was washed three times (phosphate-buffered saline with 0.1% Tween) and incubated for 1 h at room temperature with a peroxidase-conjugated anti-rabbit IgG. Finally, it was washed three times again and visualized using the SuperSignal West Pico rabbit IgG detection system (Pierce).
Calmodulin Inhibitor Treatment—Oocytes were soaked in 5 μm CDZ for 30 min in the dark. CDZ was maintained during the swelling assays with 2 mm Ca2+.
Detection of CaM-488 in Xenopus Oocytes—Xenopus oocytes exhibiting a Pf of 30-35 μm/s for the wild type and a Pf of 60-65 μm/s for S229D mutant were selected for these experiments. The oocytes were injected (5 ng/oocyte, 0.3 μm approximate concentration) with CaM-488 (Molecular Probes, catalog no. C13844) 12-24 h after AQP0 WT or mutant cRNA injection. Two days after AQP0 WT or mutant cRNA injection, water permeability was measured, and the oocytes were fixed in 4% paraformaldehyde in artificial sea water (460 mm NaCl, 10.4 mm KCl, 55 mm MgCl2, 11 mm CaCl2, 15 mm HEPES, pH 7.8, and 400 mm sucrose). For confocal microscopy, the oocytes were placed in a holding chamber to position them with the equator perpendicular to the plane of imaging. The oocytes were imaged at 488 nm excitation (emission filter 500-530 nm, 25% laser intensity, and 1 Airy unit) to detect CaM-488 at the oocyte membrane. Cross-sectional images of oocytes were taken and quantified by image analysis software (LSM510Meta).
RESULTS
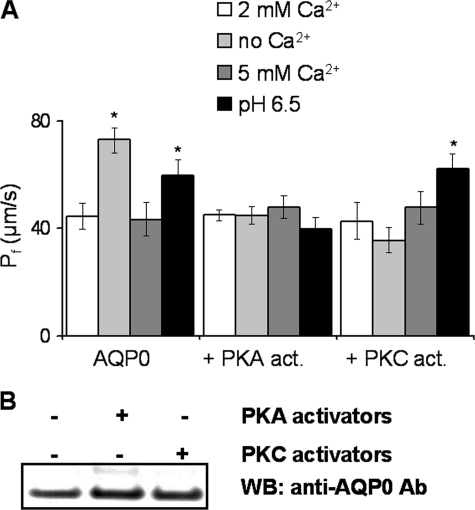
Phosphorylation by PKA or PKC Activators Alters Ca2+ Regulation of AQP0—Both PKA and PKC activators eliminated the calcium regulation of AQP0 Pf in Xenopus oocytes (Fig. 2A). In the presence of the PKA activator mixture, the water permeability of AQP0 was comparable with that of AQP0 in untreated oocytes, but its Ca2+ regulation and pH 6.5-induced Pf increase were abolished. In the presence of PMA (a PKC activator), the Ca2+ modulation of Pf was abolished, but the pH 6.5 Pf increase remained intact.
FIGURE 2.
Phosphorylation of AQP0 alters pH and Ca2+ modulation of water permeability. A, the oocytes (n = 12-18 in three separate experiments) were treated with PKA activators (act.) or with PKC activator. AQP0 Pf increased in pH 6.5 or no-added Ca2+ medium. Both PKA and PKC activators eliminated the Ca2+ regulation of AQP0 Pf. The pH response of AQP0 was eliminated by PKA activators. *, p values < 10-6. B, Western blot (WB) of AQP0 from non-treated (-) and PKA- and PKC-treated (+) Xenopus oocytes. The Western blot shows AQP0 staining with the extracellular E loop antibody (Ab).
We used our antibody directed against the extracellular E loop (residues 184-202) of AQP0 to confirm that the expression levels of AQP0 at the plasma membrane were not altered by incubation with PKA or PKC activators (Fig. 2B). In summary, the Ca2+ regulation of AQP0 water permeability was lost upon either PKA or PKC phosphorylation of AQP0, and PKA phosphorylation also abolished the increase in water permeability induced by pH 6.5, whereas PKC phosphorylation did not.
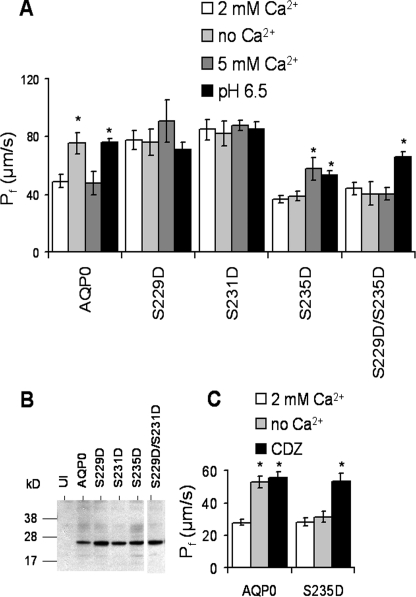
Pseudophosphorylation of Key Serines in the CaM Binding Site Determines Pf and Ca2+ Regulation of Pf—The three C-terminal serines (Ser229, Ser231, and Ser235) of AQP0 are phosphorylated in the normal lens (4), and the pattern of phosphorylation in the CaM binding domain of AQP0 (Fig. 1B) is likely a critical determinant of CaM binding and Ca2+ regulation of AQP0 Pf. To investigate this further, we created pseudophosphorylated mutants of AQP0 (S229D, S231D, S235D, and S229D/S235D) and tested their effect on the water permeability and on the Ca2+ regulation of Pf (Fig. 3A). Because the response to Ca2+ and pH depends on whether a particular mutant is in a high or low Pf state under standard conditions, it is critical to have experimental criteria defining “high” and “low” Pf. We consider AQP0 or its mutant to be in a low Pf state if a 10-ng cRNA expression results in a Pf with a value of 30-50 μm/s at 2 mm Ca2+, pH 7.4, and can be further increased by a factor of 1.5-2 in pH 6.5 test solution reaching a maximum value of 60-80 μm/s. We consider a mutant to be in a high Pf state when 10-ng cRNA expression results in a Pf value of 60-80 μm/s at 2 mm Ca2+, pH 7.4, and pH 6.5 produces no further increase. Two mutations (S229D and S231D) each produced a high Pf channel phenotype insensitive to calcium or pH. The S235D mutation showed a unique phenotype in which Pf was in the low state and in which the calcium response shifted from increased Pf in 0 mm Ca2+ to increased Pf in 5 mm Ca2+. The S235D mutation mimicked the Ca2+ sensitivity measured in lens vesicles (19). This mutant also retained the pH 6.5-induced increase in Pf of wild-type AQP0. We constructed an additional double mutant, S229D/S235D, mimicking presumptive PKA phosphorylation. The addition of two negative charges eliminated the Ca2+ regulation, but the water permeability still increased at pH 6.5. Western blots of these mutants (Fig. 3B) confirm that expression levels of pseudophosphorylated AQP0s were comparable with that of wild-type AQP0, as expected from the magnitudes of the induced water permeability. The involvement of CaM in 5 mm Ca2+ sensitivity was tested by incubating the S235D mutant with CDZ, a CaM inhibitor that binds with high affinity to CaM. Fig. 3C shows that Pf of AQP0 and S235D was increased in the presence of CDZ, indicating that CaM is involved in 5 mm Ca2+ sensitivity. In summary, phosphorylation of three key serines located at the CaM binding site determines the water permeability and its Ca2+ sensitivity of AQP0.
FIGURE 3.
Aspartic acid mutations for serine in the CaM binding domain of AQP0 alter Pf and calcium sensitivity. A, the water permeability of AQP0 can be increased by 0 mm Ca2+ or with pH 6.5. The extracellular 5 mm Ca2+ had no effect on Pf. When aspartic acid replaced serine (pseudophosphorylation) at the 229 or 231 position, the Pf was already in maximum conductivity state, and neither 0 nor 5 mm Ca2+ or pH 6.5 increased Pf further. The S235D mutant showed 5 mm Ca2+ and pH 6.5 sensitivity. The double mutant, S229D/S235D, lost its Ca2+ regulation, but the pH 6.5 regulation remained intact. B, Western blots of mutant AQP0s from Xenopus oocyte membranes are shown. The Western blot shows the predicted molecular weight when wild-type and mutant AQP0s were expressed in Xenopus oocytes. The AQP0 and its mutants were visualized with the external loop antibody. C, CDZ (5 μm) elevates the Pf of WT and S235D at 2 mm Ca2+. *, p values < 10-6. Error bars are shown as ± S.E.
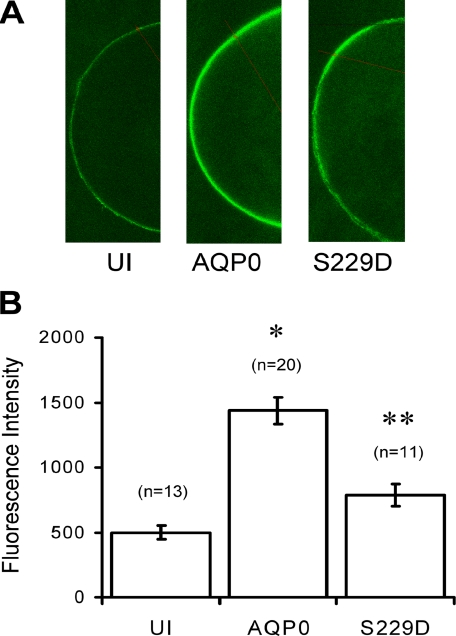
The Low Water Permeability State of AQP0 Is Associated with Bound CaM—Pseudophosphorylation of AQP0 in the CaM binding site creates two water permeability phenotypes: a low Pf state and a high Pf state. In response to reports of reduced CaM binding in the presence of phosphorylated peptides, we tested whether these phenotypes could arise from altered affinity of CaM binding. To do this, we measured the recruitment of fluorescent CaM, CaM-488, to the membrane by both low Pf (wild-type) and by the high Pf mutant S229D. To visualize localization of CaM binding, we injected CaM-488 into oocytes expressing either wild-type AQP0 or S229D mutant protein and measured membrane-associated fluorescence. Fig. 4A shows that CaM-488 is stable in Xenopus oocytes, and even without exogenous protein expression, there is background CaM-488 fluorescence at the oocyte membrane (UI panel). Endogenous CaM is in the μm range in Xenopus oocytes (25). When AQP0 was expressed in the presence of CaM-488, fluorescence at the membrane increased about 3-fold (AQP0 panel). When the S229D mutant was expressed in the presence of CaM-488, the increase in fluorescence at the oocyte membrane was about 1.6-fold more than uninjected oocytes. Fig. 4B shows the bar graphs of the average membrane fluorescence values. These experiments suggest that the low water permeability of AQP0 is strongly associated with CaM-488, whereas the high Pf S229D mutant weakly binds CaM-488, as expected from the peptide binding results of Rose et al. (26).
FIGURE 4.
AQP0 recruits CaM to the membrane. A, we expressed a low Pf (wild-type) AQP0 and a high Pf (S229D mutant) AQP0 in Xenopus oocytes in the presence of fluorescent CaM (CaM-488). Uninjected (UI) oocytes were injected only with CaM-488. B, the fluorescence intensities were measured in the equatorial region. *, p values < 10-8; **, not significantly different from UI oocytes. Error bars are shown as ± S.E.
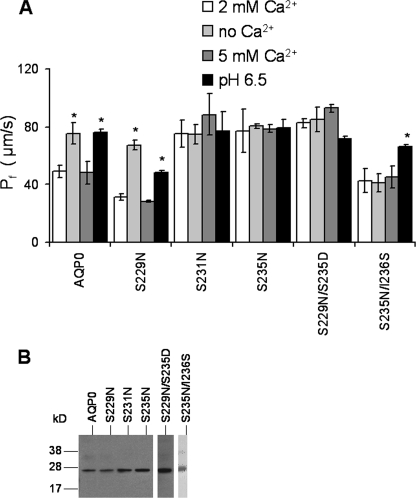
Replacement of Serines with Neutral Amino Acids Can Result in the Loss of Calcium Regulation—Position, charge, or size of the amino acids at the critical serine positions in the CaM binding site can alter the water permeability of AQP0 and its Ca2+ regulation. Substitution of asparagine for serine at position 229 had no effect on the calcium sensitivity, Pf, or pH sensitivity (Fig. 5A). However, the same substitution at Ser231 and Ser235 resulted in a high water permeability channel with a Pf between 78 and 85 μm/s and lacking sensitivity to either Ca2+ or pH. The double mutant, S229N/S235D, also exhibited a high Pf phenotype and lost both Ca2+ and pH regulation. A second double mutation, S235N/I236S, effectively shifted the position of the serine toward the C terminus and away from the “serine-ridge” line (Fig. 1B). This produced a low water permeability channel lacking Ca2+ regulation but with pH regulation intact. The Western blot of Fig. 5B shows that all mutants are expressed at approximately the same levels as wild-type AQP0. In particular, the mutant S229N, which has the same regulation as the wild type, had slightly lower water permeability than the wild type, probably accounted for by a slightly decreased expression level. It is important to note that none of the regulation differences between these mutants and wild type can be accounted for by differences in expression level. We additionally tested the role of three positive charges (Arg233, Lys238, and Arg241) in CaM binding (data not shown). Three of our four mutations (R233A, K238A, and R241E) did not change the modulation phenotype, and one (R241A) shifted the Ca2+ sensitivity toward 5 mm, similar to S235D (Table 1).
FIGURE 5.
Neutral charge mutations in the CaM binding site alter the water permeability and calcium sensitivity of AQP0. A, S229N did not cause change in Pf or its calcium sensitivity. S231N, S235N, and S229N/S235S double mutants are in a high Pf state that does not respond to pH 6.5 or to 0 or 5 mm Ca2+. The double mutant S235N/I236S showed low Pf with no Ca2+ regulation, but the pH regulation remained intact. *, p values < 10-6. B, Western blots of mutant AQP0s from Xenopus oocyte membranes are shown. The Western blot shows the predicted molecular weight when wild-type and mutant AQP0s were expressed in Xenopus oocytes. The AQP0 and its mutants were visualized with the external E loop AQP0 antibody. Error bars are shown as ± S.E.
TABLE 1.
AQP0 has four calcium regulation phenotypes
Mutations in the CaM binding site give rise to four phenotypes according to water permeability and Ca2+ response.
| Group 1a | Group 2b | Group 3c | Group 4d |
|---|---|---|---|
| WT | |||
| S229N | S229D | ||
| R233A | S235D | S231N | S229D/S235D |
| S235A | R241A | S231D | |
| S235K | S235N | S235N/I236S | |
| K238A | S229N/S235D | ||
| R241E |
Low Pf at 2 mm Ca2+ and high Pf at 0 mm Ca2+.
Low Pf at 2 mm Ca2+ and high Pf at 5 mm Ca2+.
Locked in high Pf and no Ca2+ regulation.
Locked in low Pf and no Ca2+ regulation.
DISCUSSION
In this study, we have shown that the pattern of phosphorylation at the C terminus of AQP0 profoundly influences its water permeability and its regulation by calcium. Calcium regulation of AQP0 is mediated by CaM, and modifications of key residues in the CaM binding site alter the calcium regulation and water permeability. The elevated levels of phosphorylated proteins in the lens cortex region signal the significance of coordinated regulation in the differentiating fiber cells (4, 28-31). We demonstrate here for the first time that AQP0 modified by phosphorylation exhibits four calcium regulation phenotypes. Our results suggest a model in which CaM binding inhibits AQP0 Pf.
AQP0 Exhibits Four Calcium Regulation Phenotypes—The phenotypes of 15 mutations are summarized in Table 1 based on water permeability and Ca2+ sensitivity. The mutations segregate into four groups: Group 1, high Pf with removal of external Ca2+ (WT, S229N, R233A, S235A, S235K, K238A, and R241E); Group 2, high Pf with 5 mm external Ca2+ (S235D and R241A); Group 3, high Pf and no Ca2+ regulation (S229D, S231N, S231D, S235N, and S235N/I236S); and Group 4, low Pf and no Ca2+ regulation (S229D/S235D and S235N/I236S). Our results show that the three serine positions are not equivalent for regulation of Pf and, according to our model, CaM binding. For example, at position 229, the addition of a negative charge (S229D) shifts the phenotype to Group 3, whereas S229N retains wild-type modulation. In contrast, at position 231, an increase in charge or size results in high Pf and a loss of Ca2+ regulation (Group 3). Mutations at position 235 exhibit three different phenotypes that cannot be explained simply by size or charge of the substituted amino acid (see discussion below).
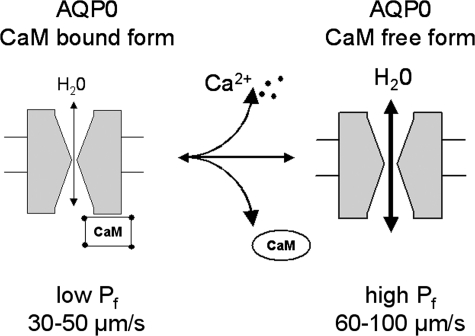
A Model for CaM as an Inhibitor of AQP0 Water Permeability—A body of evidence from functional studies demonstrates that AQP0 water permeability is regulated extrinsically by changes in pH or calcium concentration (16, 18, 19). For regulation by calcium, most of the effects can be accounted for by a simple two-state model in which the Pf of AQP0 can transition from low to high when CaM unbinds from the C terminus (Fig. 6). Under physiological conditions in the presence of Ca2+, when CaM is bound to the C terminus of wild-type AQP0, Pf is low. When Ca2+ is removed from the external solution, the Ca2+ concentration at the oocyte membrane plummets, leading to the release of CaM from the C terminus of AQP0. CaM-free AQP0 would then enter into the high Pf state. Because the phosphorylation sites are not equivalent in their effects, CaM release can apparently be effected in two ways. At position 229, the negative charge induced by pseudophosphorylation induces the high Pf state, presumably by weakening interactions between CaM and the C terminus binding site. In addition, many of the mutations shown probably alter the binding affinity or the binding site of the C terminus in some fashion. Indeed, we directly demonstrated that this is so for the high Pf mutant S229D (Fig. 4). At positions 231 and 235, an increase in amino acid size or charge (S231N and S235N) induces the high Pf phenotype, leading to the prediction that these mutations would also release CaM because of structural changes in the binding site. This model accounts for nearly all of our observations, summarized in Table 1.
FIGURE 6.
A model for CaM as an inhibitor of AQP0 water permeability. The cartoon showed a two-state model in which the Pf of AQP0 can transition from low to high when CaM unbinds from the C terminus.
Effects of Two Mutations That Are Not Accounted for by This Model—First, the double mutant S229D/S235D presumably cannot bind CaM but is nevertheless in a low permeability state unresponsive to calcium concentration changes. We suggest that this mutant is in the low permeability state because altered charge on the C terminus induces an auto-inhibitory state, possibly because of an interaction between the N and C termini or within the C terminus. Second, the S235D mutant increases its water permeability either in the presence of 5 mm Ca2+ or in the presence of CDZ (Fig. 3, A and C). According to in vitro CaM binding affinity assays, phosphorylation of serine 235 decreases the binding affinity (26). According to our results on S235D, CaM would still bind AQP0 perhaps in a secondary site with an altered configuration as was seen in in vitro cross-linking experiments (26). Alternatively, elevating Ca2+ to 5 mm may have effects not at all related to CaM binding, for example by increasing phosphorylation or calcium binding to an unknown intrinsic AQP0 site.
Relation to Other Models of Pf Regulation—Although structural analysis of AQP0 has failed to reveal any gating mechanism, it seems likely that addition of a regulatory protein, in this case CaM, can locally alter the properties of the pore. We previously suggested that acid pH modulates the orientation of water molecules by histidine residues near the outer vestibule and that Ca2+, through the mediation of CaM, modulates their orientation in the inner vestibular site (16). We then showed that zinc induces a functional cooperativity among AQP0 subunits, presumably by inducing a conformational change of the entire tetramer (18). Furthermore, Varadaraj et al. (19) suggested that the C terminus of AQP0, with a net of four positive charges and nine hydrophobic amino acids, is also likely to bind to the inside surface of the plasma membrane. Thus, membrane charge effects may also exert an influence on the interaction between CaM and AQP0.
Implications for Lens Physiology—We have demonstrated unequivocal regulation of AQP0 water permeability in the oocyte expression system, a result that suggests that regulation of AQP0 water permeability is probably essential for normal function of the lens. In vivo evidence that this is so is compelling but somewhat indirect. The effect of the CatFr (cataract Fraser) mouse strain mutation on AQP0 water permeability can account for the unexpectedly severe cataract in the CatFr WT heterozygote and for the limited rescue of the cataract in a transgenic expressing AQP0 on the CatFr background (15). Coexpression of the defective AQP0-LTR abolishes the Ca2+ regulation of AQP0 water permeability in oocytes and probably also in the CatFr transgenic, suggesting that it is the loss of calcium regulation that accounts for the severity of the phenotype. Additional circumstantial evidence for the likely importance of calcium regulation of water permeability comes from a naturally occurring mutation in the CaM binding domain of AQP0, R233K. This mutation, found in six generations of a Chinese family, produces a binocular congenital cataract (32). Thus it seems very likely that calcium regulation of water permeability, particularly as modulated by CaM and C terminus phosphorylation, is critical in maintaining lens transparency. In pathological conditions, when phosphorylation may be hyperactive, inappropriate water permeability without calcium regulation could contribute to the development of a cataract.
Acknowledgments
We thank Dr. Michael Cahalan for critical readings of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant EY 5661 (to J. E. H.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: AQP0, aquaporin zero; Pf, water permeability; CaM, calmodulin; CDZ, calmidazolium; PKA, protein kinase A; PKC, protein kinase C; WT, wild-type.
References
- 1.Smith, R. S., John, S. W. M., Nishina, P. M., and Sundberg, J. P. (eds) (2002) Systematic Evaluation of the Mouse Eye, CRC Press, Boca Raton, FL
- 2.Menko, A. S. (2002) Exp. Eye Res. 75 485-490 [DOI] [PubMed] [Google Scholar]
- 3.Hanson, S. R., Hasan, A., Smith, D. L., and Smith, J. B. (2000) Exp. Eye Res. 71 195-207 [DOI] [PubMed] [Google Scholar]
- 4.Ball, L. E., Garland, D. L., Crouch, R. K., and Schey, K. L. (2004) Biochemistry 43 9856-9865 [DOI] [PubMed] [Google Scholar]
- 5.Ervin, L. A., Ball, L. E., Crouch, R. K., and Schey, K. L. (2005) Investig. Ophthalmol. Vis. Sci. 46 627-635 [DOI] [PubMed] [Google Scholar]
- 6.Mathias, R. T., Kistler, J., and Donaldson, P. (2007) J. Membr. Biol. 216 1-16 [DOI] [PubMed] [Google Scholar]
- 7.Mathias, R. T., and Rae, J. L. (2004) Exp. Eye Res. 78 689-698 [DOI] [PubMed] [Google Scholar]
- 8.Donaldson, P., Kistler, J., and Mathias, R. T. (2001) News Physiol. Sci. 16 118-123 [DOI] [PubMed] [Google Scholar]
- 9.Mathias, R. T., Rae, J. L., and Baldo, G. J. (1997) Physiol. Rev. 77 21-50 [DOI] [PubMed] [Google Scholar]
- 10.Shiels, A., and Bassnett, S. (1996) Nat. Genet. 12 212-215 [DOI] [PubMed] [Google Scholar]
- 11.Francis, P., Berry, V., Bhattacharya, S., and Moore, A. (2000) Br. J. Ophthalmol. 84 1376-1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiels, A., Bassnett, S., Varadaraj, K., Mathias, R., Al Ghoul, K., Kuszak, J., Donoviel, D., Lilleberg, S., Friedrich, G., and Zambrowicz, B. (2001) Physiol. Genomics 7 179-186 [DOI] [PubMed] [Google Scholar]
- 13.Al Ghoul, K. J., Kirk, T., Kuszak, A. J., Zoltoski, R. K., Shiels, A., and Kuszak, J. R. (2003) Anat. Rec. A Discov. Mol. Cell Evol. Biol. 273 714-730 [DOI] [PubMed] [Google Scholar]
- 14.Shiels, A., Mackay, D., Bassnett, S., Al Ghoul, K., and Kuszak, J. (2000) FASEB J. 14 2207-2212 [DOI] [PubMed] [Google Scholar]
- 15.Kalman, K., Németh-Cahalan, K. L., Froger, A., and Hall, J. E. (2006) Biochim. Biophys. Acta 1758 1094-1099 [DOI] [PubMed] [Google Scholar]
- 16.Németh-Cahalan, K. L., and Hall, J. E. (2000) J. Biol. Chem. 275 6777-6782 [DOI] [PubMed] [Google Scholar]
- 17.Németh-Cahalan, K. L., Kalman, K., and Hall, J. E. (2004) J. Gen. Physiol. 123 573-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Németh-Cahalan, K. L., Kalman, K., Froger, A., and Hall, J. E. (2007) J. Gen. Physiol. 130 457-464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varadaraj, K., Kumari, S., Shiels, A., and Mathias, R. T. (2005) Investig. Ophthalmol. Vis. Sci. 46 1393-1402 [DOI] [PubMed] [Google Scholar]
- 20.Garland, D., and Russell, P. (1985) Proc. Natl. Acad. Sci. U. S. A. 82 653-657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, K. R., Lampe, P. D., Hur, K. C., Louis, C. F., and Johnson, R. G. (1986) J. Cell Biol. 102 1334-1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girsch, S. J., and Peracchia, C. (1991) Curr. Eye. Res. 10 839-849 [DOI] [PubMed] [Google Scholar]
- 23.Gonen, T., Cheng, Y., Sliz, P., Hiroaki, Y., Fujiyoshi, Y., Harrison, S. C., and Walz, T. (2005) Nature 438 633-638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harries, W. E., Akhavan, D., Miercke, L. J., Khademi, S., and Stroud, R. M. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 14045-14050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lan, L., Brereton, H., and Barritt, G. J. (1998) Biochem. J. 330 1149-1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rose, K. M., Wang, Z., Magrath, G. N., Hazard, E. S., Hildebrandt, J. D., and Schey, K. L. (2008) Biochemistry 47 339-347 [DOI] [PubMed] [Google Scholar]
- 27.Preston, G. M., Carroll, T. P., Guggino, W. B., and Agre, P. (1992) Science 256 385-387 [DOI] [PubMed] [Google Scholar]
- 28.Maddala, R., Skiba, N., and Vasantha, R. P. (2007) Differentiation 75 713-725 [DOI] [PubMed] [Google Scholar]
- 29.Iyengar, L., Wang, Q., Rasko, J. E., McAvoy, J. W., and Lovicu, F. J. (2007) Differentiation 75 662-668 [DOI] [PubMed] [Google Scholar]
- 30.Zampighi, G. A., Planells, A. M., Lin, D., and Takemoto, D. (2005) Investig. Ophthalmol. Vis. Sci. 46 3247-3255 [DOI] [PubMed] [Google Scholar]
- 31.Lin, D., Barnett, M., Lobell, S., Madgwick, D., Shanks, D., Willard, L., Zampighi, G. A., and Takemoto, D. J. (2006) J. Exp. Biol. 209 4371-4378 [DOI] [PubMed] [Google Scholar]
- 32.Lin, H., Hejtmancik, J. F., and Qi, Y. (2007) Mol. Vis. 13 1822-1827 [PubMed] [Google Scholar]