Abstract
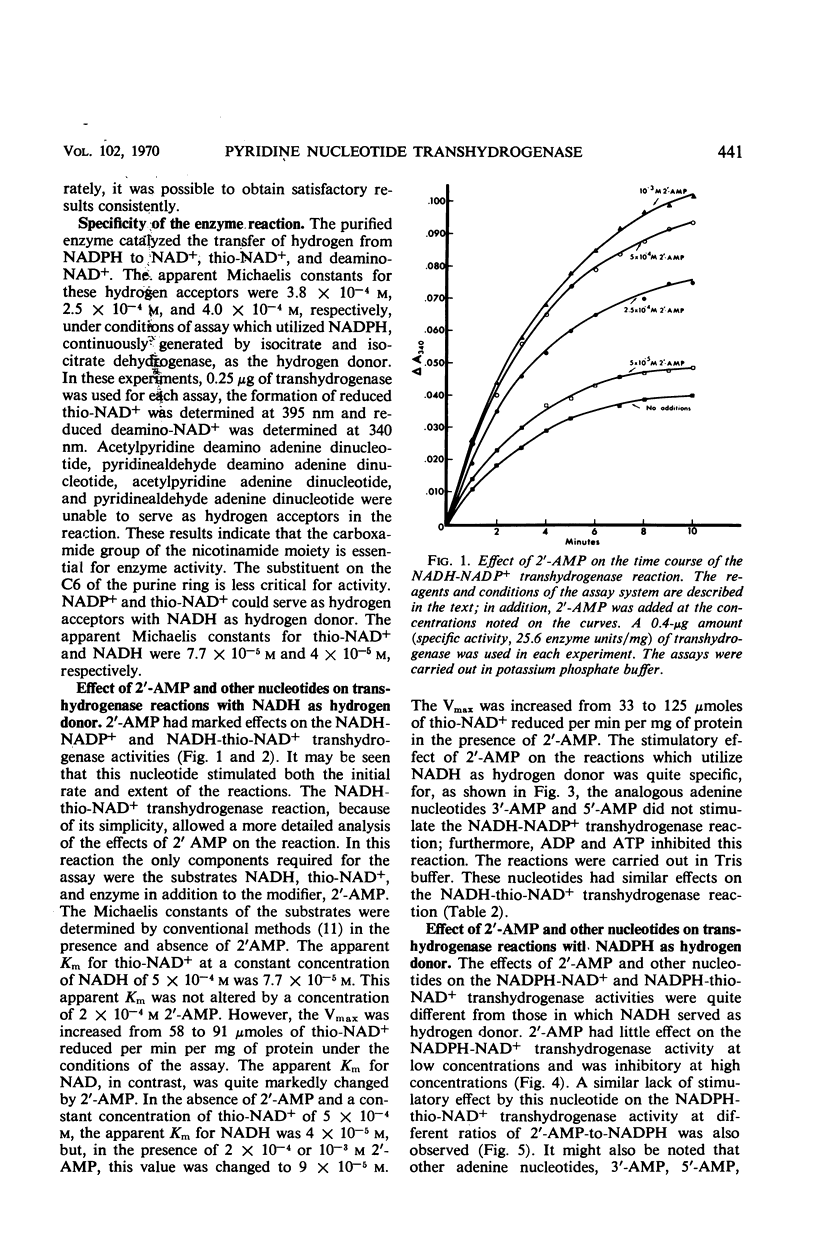
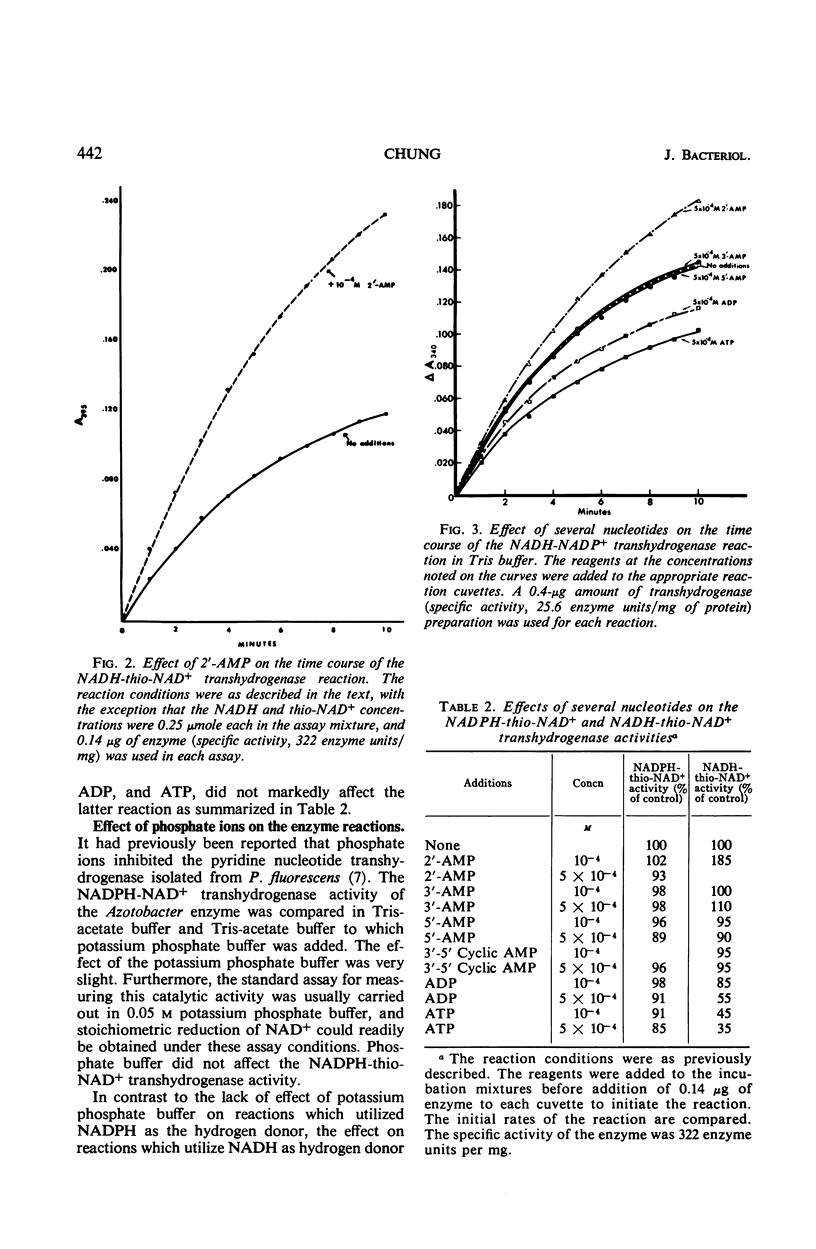
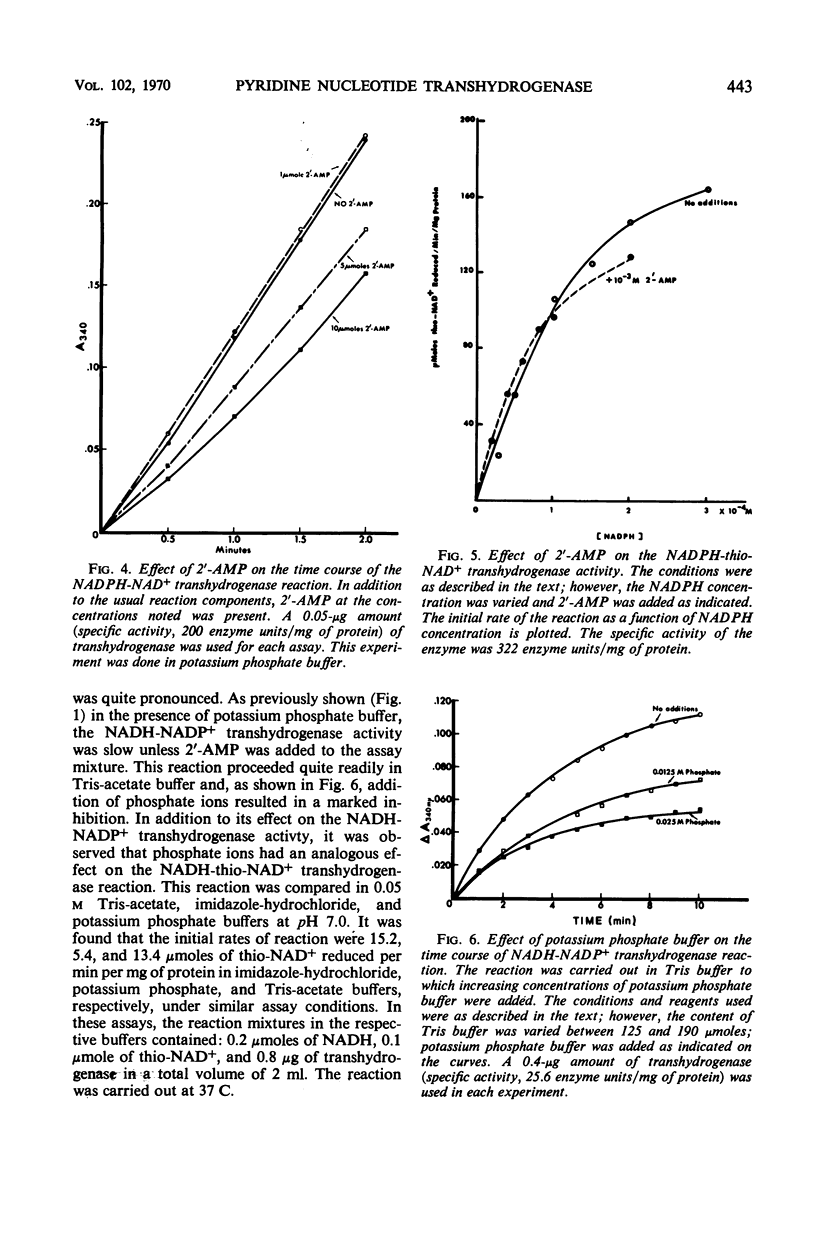
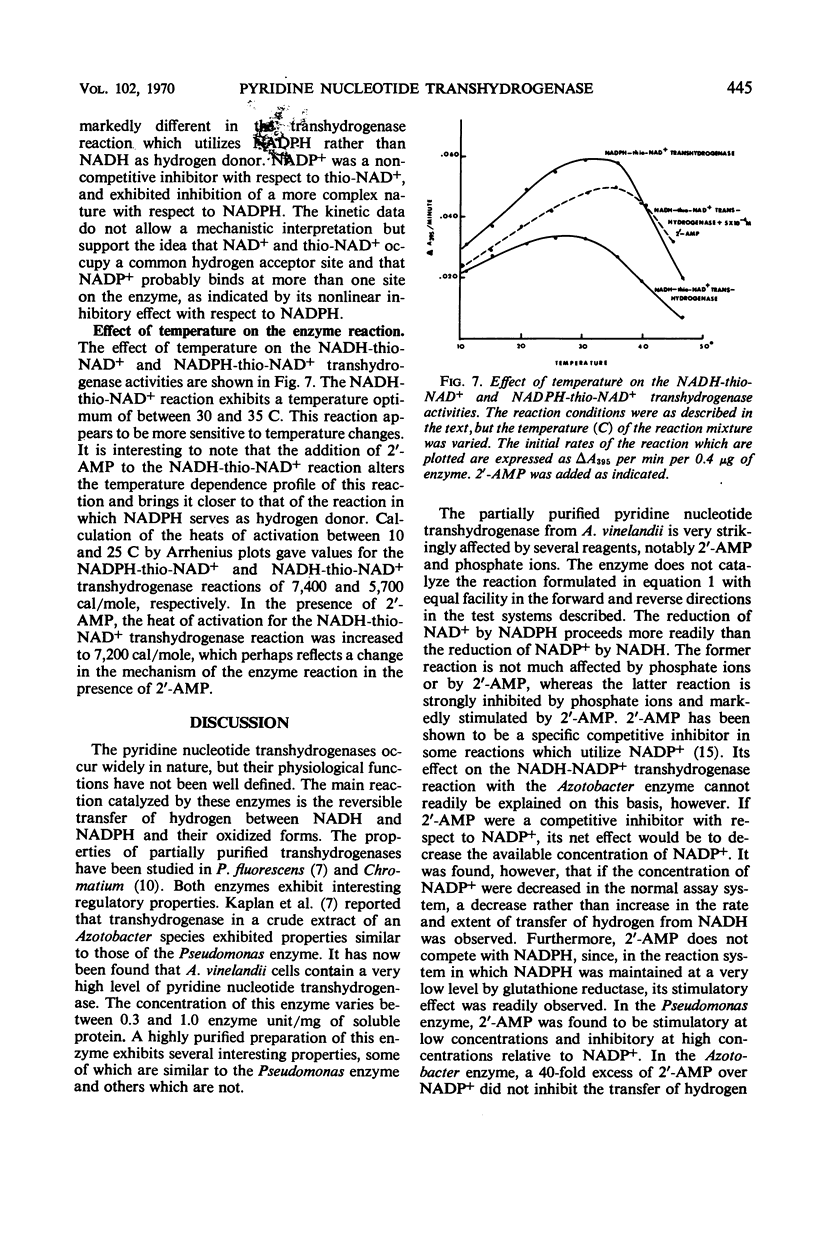
A method is described for the partial purification of pyridine nucleotide transhydrogenase from Azotobacter vinelandii (ATCC 9104) cells. The most highly purified preparation catalyzes the reduction of 300 μmoles of nicotinamide adenine dinucleotide (NAD+) per min per mg of protein under the assay conditions employed. The enzyme catalyzes the reduction of NAD+, deamino-NAD+, and thio-NAD+ with reduced nicotinamide adenine dinucleotide phosphate (NADPH) as hydrogen donor, and the reduction of nicotinamide adenine dinucleotide phosphate (NADP+) and thio-NAD+ with reduced NAD (NADH) as hydrogen donor. The reduction of acetylpyridine AD+, pyridinealdehyde AD+, acetylpyridine deamino AD+, and pyridinealdehydedeamino AD+ with NADPH as hydrogen donor was not catalyzed. The enzyme catalyzes the transfer of hydrogen more readily from NADPH than from NADH with different hydrogen acceptors. The transfer of hydrogen from NADH to NADP+ and thio-NAD+ was markedly stimulated by 2′-adenosine monophosphate (2′-AMP) and inhibited by adenosine diphosphate (ADP), adenosine triphosphate (ATP), and phosphate ions. The transfer of hydrogen from NADPH to NAD+ was only slightly affected by phosphate ions and 2′-AMP, except at very high concentrations of the latter reagent. In addition, the transfer of hydrogen from NADPH to thio-NAD+ was only slightly influenced by 2′-AMP, ADP, ATP, and other nucleotides. The kinetics of the transhydrogenase reactions which utilized thio-NAD+ as hydrogen acceptor and NADH or NADPH as hydrogen donor were studied in some detail. The results suggest that there are distinct binding sites for NADH and NAD+ and perhaps a third regulator site for NADP+ or 2′-AMP. The heats of activation for the transhydrogenase reactions were determined. The properties of this enzyme are compared with those of other partially purified transhydrogenases with respect to the regulatory functions of 2′-AMP and other nucleotides on the direction of flow of hydrogen between NAD+ and NADP+.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- COLOWICK S. P., KAPLAN N. O., NEUFELD E. F., CIOTTI M. M. Pyridine nucleotide transhydrogenase. I. Indirect evidence for the reaction and purification of the enzyme. J Biol Chem. 1952 Mar;195(1):95–105. [PubMed] [Google Scholar]
- DANIELSON L., ERNSTER L. Demonstration of a mitochondrial energy-dependent pyridine nucleotide transhydrogenase reaction. Biochem Biophys Res Commun. 1963 Jan 18;10:91–96. doi: 10.1016/0006-291x(63)90274-2. [DOI] [PubMed] [Google Scholar]
- KAPLAN N. O., COLOWICK S. P., NEUFELD E. F., CIOTTI M. M. Pyridine nucleotide transhydrogenase. IV. Effect of adenylic acid a on the bacterial transhydrogenases. J Biol Chem. 1953 Nov;205(1):17–29. [PubMed] [Google Scholar]
- KAPLAN N. O., COLOWICK S. P., NEUFELD E. F. Pyridine nucleotide transhydrogenase. II. Direct evidence for and mechanism of the transhydrogenase reaction. J Biol Chem. 1952 Mar;195(1):107–119. [PubMed] [Google Scholar]
- KAPLAN N. O., COLOWICK S. P., NEUFELD E. F. Pyridine nucleotide transhydrogenase. III. Animal tissue transhydrogenases. J Biol Chem. 1953 Nov;205(1):1–15. [PubMed] [Google Scholar]
- KEISTER D. L., SAN PIETRO A., STOLZENBACH F. E. Pyridine nucleotide transhydrogenase from spinach. I. Purification and properties. J Biol Chem. 1960 Oct;235:2989–2996. [PubMed] [Google Scholar]
- Keister D. L., Hemmes R. B. Pyridine nucleotide transhydrogenase from Chromatium. J Biol Chem. 1966 Jun 25;241(12):2820–2825. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAPSON L. W., GODDARD D. R. Reduction of glutathione by co-enzyme II. Nature. 1951 Jun 16;167(4259):975–976. doi: 10.1038/167975a0. [DOI] [PubMed] [Google Scholar]
- MIZE C. E., LANGDON R. G. Hepatic glutathione reductase. I. Purification and general kinetic properties. J Biol Chem. 1962 May;237:1589–1595. [PubMed] [Google Scholar]
- NEUFELD E. F., KAPLAN N. O., COLOWICK S. P. Effect of adenine nucleotides on reactions involving triphosphopyridine nucleotide. Biochim Biophys Acta. 1955 Aug;17(4):525–535. doi: 10.1016/0006-3002(55)90415-7. [DOI] [PubMed] [Google Scholar]



