Abstract
Cocoa was shown to inhibit chemically induced carcinogenesis in animals and exert antioxidant activity in humans. However, the molecular mechanisms of the chemopreventive potential of cocoa and its active ingredient(s) remain unknown. Here we report that cocoa procyanidins inhibit neoplastic cell transformation by suppressing the kinase activity of mitogen-activated protein kinase kinase (MEK). A cocoa procyanidin fraction (CPF) and procyanidin B2 at 5 μg/ml and 40 μm, respectively, inhibited 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced neoplastic transformation of JB6 P+ mouse epidermal (JB6 P+) cells by 47 and 93%, respectively. The TPA-induced promoter activity and expression of cyclooxygenase-2, which is involved in tumor promotion and inflammation, were dose-dependently inhibited by CPF or procyanidin B2. The activation of activator protein-1 and nuclear factor-κB induced by TPA was also attenuated by CPF or procyanidin B2. The TPA-induced phosphorylation of MEK, extracellular signal-regulated kinase, and p90 ribosomal s6 kinase was suppressed by CPF or procyanidin B2. In vitro and ex vivo kinase assay data demonstrated that CPF or procyanidin B2 inhibited the kinase activity of MEK1 and directly bound with MEK1. CPF or procyanidin B2 suppressed JB6 P+ cell transformation induced by epidermal growth factor or H-Ras, both of which are known to be involved in MEK/ERK signal activation. In contrast, theobromine (up to 80 μm) had no effect on TPA-induced transformation, cyclooxygenase-2 expression, the transactivation of activator protein-1 or nuclear factor-κB, or MEK. Notably, procyanidin B2 exerted stronger inhibitory effects compared with PD098059 (a well known pharmacological inhibitor of MEK) on MEK1 activity and neoplastic cell transformation.
Considerable attention has focused on identifying various chemopreventive phytochemicals found in tea, fruits, vegetables, and red wines, which are commonly consumed in our daily diets. Others and we have demonstrated that epigallocatechin gallate (a polyphenol) and caffeine (a methylxanthine compound) in green tea exert strong chemopreventive effects in cell culture and animal models (1–5). Chocolate and cocoa are made from cacao (Theobroma cacao L.), which contains a large amount of procyanidins (see Fig. 1A) and theobromine (3,7-dimethylxanthine, see Fig. 1B). Procyanidins are the major flavonoids found in cocoa and can be expected to be strong antioxidants based on their structures. Procyanidin oligomers reportedly constitute 12–48% of the dry weight of the cocoa bean, and the contents of monomeric and polymeric procyanidins comprise almost 1400 mg per 100 g of cocoa liquor (6). Theobromine is the main methylxanthine compound found in cocoa, constituting 2–3% of the dry weight of the cocoa bean, and dark chocolate contains 474–1038 mg of theobromine per 100-g portion (7, 8). Therefore, cocoa beans contain much less theobromine than procyanidins. Accumulated data provide evidence that the consumption of cocoa or dark chocolate can decrease the burden and efficacy of epigenetic carcinogens (9, 10). However, the underlying molecular mechanisms and molecular target(s) for the potential chemopreventive effects of cocoa and its active ingredient(s) remain unknown.
FIGURE 1.
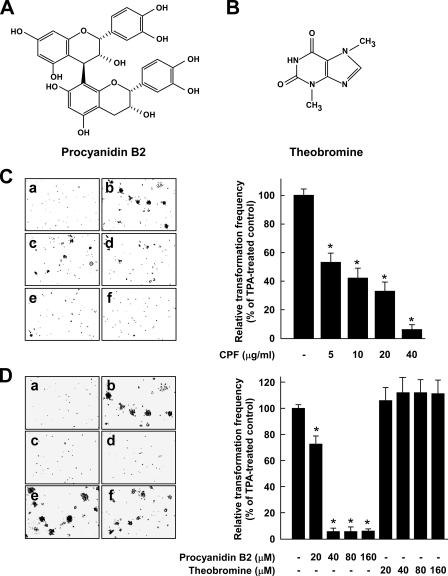
Effects of CPF, procyanidin B2, or theobromine on TPA-induced neoplastic transformation of JB6 P+ cells. Chemical structures of procyanidin B2 (A) and theobromine (B) are shown. C, CPF inhibits TPA-induced cell transformation. JB6 P+ cells were treated as described under “Experimental Procedures,” and colonies were counted 14 days later: a, untreated control; b, TPA alone; c, TPA and 5 μg/ml CPF; d, TPA and 10 μg/ml CPF; e, TPA and 20μg/ml CPF; f, TPA and 40μg/ml CPF. Cell colonies were counted under a microscope with the aid of Image-Pro Plus software (v.4). The effects of CPF on transformation of JB6 P+ cells are presented as the percent inhibition of cell transformation relative to TPA-stimulated cells in soft agar. D, procyanidin B2 (but not theobromine) inhibits TPA-induced cell transformation. JB6 P+ cells were treated as described under “Experimental Procedures,” and colonies were counted 14 days later: a, untreated control; b, TPA alone; c, TPA and 40 μm procyanidin B2; d, TPA and 80 μm procyanidin B2; e, TPA and 40 μm theobromine; f, TPA and 80 μm theobromine. Cell colonies were counted under a microscope with the aid of Image-Pro Plus software (v4). The effects of procyanidin B2 or theobromine on transformation of JB6 P+ cells in soft agar are presented as the percent inhibition of cell transformation relative to TPA-stimulated cells. For C and D, data are shown as means and S.D. values of three independent experiments. The asterisk (*) indicates a significant difference between groups treated with TPA and CPF, procyanidin B2, or theobromine and the group treated with TPA alone (p < 0.05).
Neoplastic transformation of cells is considered to be one of the major events occurring during carcinogenic processes. Multiple lines of evidence suggest the existence of a strong link between inflammation and carcinogenesis (11, 12). Elevated levels of cyclooxygenase-2 (COX-2)4; one of the key enzymes mediating inflammation) and prostaglandin E2 (a major inflammation-mediating product formed in a reaction catalyzed by COX-2) have been observed frequently in various types of transformed and cancerous cells (13, 14). This observation provides evidence indicating that COX-2 acts as a link between inflammation and carcinogenesis by its involvement in the tumor promotion stage. Previous studies demonstrating that COX-2 inhibitors suppress neoplastic transformation revealed that COX-2 overexpression might be one of the mechanisms underlying the induction of neoplastic transformation (15, 16). Therefore, inhibiting COX-2 expression and neoplastic transformation could be an effective strategy for delaying carcinogenesis.
Because the expression of COX-2 is primarily regulated by eukaryotic transcription factors such as nuclear factor (NF)-κB and activator protein (AP)-1, inhibition of AP-1 and/or NF-κB might lead to the suppression of cell transformation through the blocking of COX-2 expression (13, 17, 18). Stimulation of cells with various tumor promoters results in the activation of AP-1 and/or NF-κB by a series of upstream kinases, including those belonging to the mitogen-activated protein (MAP) kinase family. The MAP kinases include extracellular signal-regulated protein kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAP kinase. Although these three members exhibit some functional redundancy, ERK generally transmits signals initiated by tumor promoters such as 12-O-tetradecanoylphorbol-13-acetate (TPA), epidermal growth factor (EGF), Ras, or platelet-derived growth factor (2, 17–21). Mitogen-activated kinase kinase (MEK) is a dual-specificity protein kinase that phosphorylates the downstream target ERK on specific tyrosine and threonine residues. In particular, a diverse range of tumor promoters including TPA, H-Ras, and EGF perpetually activate the MEK/ERK signaling pathway and subsequently induce a more-aggressive cancer-like phenotype in cells such as anchorage-independent growth and elevated AP-1 and/or NF-κB activities (1, 20–22). These findings indicate that the MEK/ERK pathway plays an important role in the transformation of cells and development of tumors.
TPA has been shown to promote two-stage skin carcinogenesis and potently stimulate COX-2 expression in various cell lines (17, 23). The JB6 mouse epidermal cell system of clonal genetic variants, which are promotion sensitive (P+) or promotion resistant (P–), allows the study of genetic susceptibility to transformation, promotion, and progression at the molecular level. Especially, in JB6 P+ cells, TPA induces the formation of large, tumorigenic, and anchorage-independent colonies in soft agar (17, 24, 25). In the present study we elucidate the mechanism underlying the antitumorigenic effects of cocoa and its effective compounds on TPA-induced neoplastic transformation using JB6 P+ cell models. Here we report that cocoa procyanidin B2 (but not theobromine) is a potent inhibitor of the kinase activity of MEK, and this inhibition subsequently suppresses neoplastic transformation and COX-2 expression.
EXPERIMENTAL PROCEDURES
Chemicals—Eagle's minimum essential medium (MEM), basal medium Eagle (BME), gentamicin, and l-glutamine were purchased from Invitrogen; fetal bovine serum (FBS) was obtained from Gemini Bio-Products (Calabasas, CA); TPA and EGF were purchased from Sigma. The antibodies against phosphorylated MEK (Ser-217/221), phosphorylated ERK (Thr-202/Tyr-204), total ERK, phosphorylated p90RSK (Thr-359/Ser-363), total p90RSK, phosphorylated JNK (Thr-183/Tyr-185), and total JNK were purchased from Cell Signaling Biotechnology (Beverly, MA). The antibodies against COX-2 and total MEK1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The antibody against β-actin was obtained from Sigma. The MEK1 assay kit was purchased from Upstate Biotechnology (Lake Placid, NY). Polyvinylidene fluoride, CNBr-Sepharose 4B, glutathione-Sepharose 4B, [γ-32P]ATP, and a chemiluminescence detection kit were purchased from Amersham Biosciences, and the protein assay kit was purchased from Bio-Rad. G418 and the luciferase assay substrate were purchased from Promega (Madison, WI).
Preparation of Cocoa Procyanidin Fraction (CPF)—A cocoa procyanidin was extracted from commercial cocoa powder (50 g) with 500 ml of 50% (v/v) aqueous ethanol under reflux for 6 h. After the extraction, the solution was filtered twice to collect the extract. The collected cocoa extract was loaded onto a styrene-based adsorption resin column (60 × 450 mm; HP-20, Mitsubishi, Japan), washed with 20% (v/v) aqueous ethanol, and then eluted with 60% (v/v) aqueous ethanol. The eluted CPF was concentrated at 50 °C under reduced pressure, frozen, and dried.
Cell Culture—The JB6 P+ mouse epidermal cell line or H-Ras-transformed JB6 mouse epidermal cell line was cultured in monolayers at 37 °C in a 5% CO2 incubator in MEM containing 5% FBS, 2 mml-glutamine, and 25 μg/ml gentamicin. The JB6 P+ mouse epidermal cell line stably transfected with COX-2 luciferase reporter plasmid was a kind gift from Dr. Chauanshu Huang (School of Medicine, New York University). The JB6 mouse epidermal cell lines were stably transfected with an AP-1, NF-κB, or COX-2 luciferase reporter plasmid and maintained in MEM supplemented with 5% FBS containing 200 g/ml G418.
Anchorage-independent Transformation Assay—The effects of CPF or individual cocoa compounds on TPA- or EGF-induced cell transformation were investigated in JB6 P+ cells. Cells (8 × 103/ml) were exposed to TPA or EGF with or without CPF or cocoa compounds in 1 ml of 0.33% BME agar containing 10% FBS or in 3.5 ml of 0.5% BME agar containing 10% FBS. The effects of CPF or cocoa compounds on H-Ras-induced cell transformation were investigated in H-Ras-transformed JB6 cells. The H-Ras-transformed cells (8 × 103/ml) were incubated with or without CPF or cocoa compounds in 1 ml of 0.33% BME agar containing 10% FBS or in 3.5 ml of 0.5% BME agar containing 10% FBS. The cultures were maintained at 37 °C in a 5% CO2 incubator for 14 days, at which time the cell colonies were counted under a microscope with the aid of Image-Pro Plus software (v.4; Media Cybernetics, Silver Spring, MD).
Luciferase Assay for COX-2, AP-1, or NF-κB Transactivation—Confluent monolayers of JB6 P+ cells stably transfected with COX-2, AP-1, or NF-κB luciferase reporter plasmid were trypsinized, and 8 × 103 viable cells suspended in 100 μl of 5% FBS MEM were added to each well of a 96-well plate. Plates were incubated at 37 °C in a humidified atmosphere of 5% CO2. When cells reached 80–90% confluence, they were starved by culturing them in 0.1% FBS-MEM for an additional 24 h. The cells were then treated for 1 h with CPF (0–40 μg/ml) and then exposed to 20 ng/ml TPA for 24 h. After treatment, cells were disrupted with 100 μl of lysis buffer (0.1 M potassium phosphate buffer (pH 7.8), 1% Triton X-100, 1 mm dithiothreitol (DTT), and 2 mm EDTA), and the luciferase activity was measured using a luminometer (Luminoskan Ascent, Thermo Electron, Helsinki, Finland).
Reporter Gene Assay—For reporter gene assays, transient transfections were carried out using jetPEI (Qbiogene) and JB6 P+ cells. An AP-1 luciferase reporter plasmid was cotransfected with a pcDNA3 vector or constitutively active-MEK1 and 20 ng of a β-galactosidase-expressing plasmid. The β-galactosidase-expressing plasmid was cotransfected for normalizing the transfection efficiency. Then, 24 h post-transfection, cells were washed and then starved for 24 h in 0.1% FBS/MEM, and cell lysates were then prepared 24 h after treatment with procyanidin B2 (0–80 μm). Luciferase and β-galactosidase activities were measured using the Luminoskan Ascent (Thermo Electron) and Multiskan MS (Labsystems), respectively.
Western Blotting—After the cells (1.5 × 106) were cultured in a 10-cm dish for 48 h, they were starved in serum-free medium for an additional 24 h to eliminate the influence of FBS on the activation of kinases. The cells were then treated with CPF (0–20 μg/ml) for 1 h before being exposed to 20 ng/ml TPA for different times. The harvested cells were disrupted, and the supernatant fractions were boiled for 5 min. The protein concentration was determined using a DC protein assay kit (Bio-Rad) as described in the manufacturer's manual. Lysate protein (20 μg) was subjected to 10% SDS-PAGE and electrophoretically transferred to a polyvinylidene fluoride membrane. After blotting, the membrane was incubated with the specific primary antibody at 4 °C overnight. Protein bands were visualized by a chemiluminescence detection kit after hybridization with the horseradish peroxidase-conjugated secondary antibody. The relative amounts of proteins associated with specific antibodies were quantified using Scion Image (NIH, Bethesda, MD).
In Vitro MEK1 Kinase Assay—The in vitro kinase assays were performed in accordance with the instructions provided by Upstate Biotechnology. In brief, every reaction contained 20 μl of assay dilution buffer (20 mm MOPS, pH 7.2), 25 mm β-glycerol phosphate, 5 mm EGTA, 1 mm sodium orthovanadate (Na3VO4), and 1 mm DTT) and a magnesium-ATP mixture buffer. For MEK1, inactive ERK2 substrate peptide (1 μg) was also included. A 4-μl aliquot was removed from the reaction mixture, which contained 20 μg of myelin basic protein substrate peptide and 10 μl of diluted [γ-32P]ATP solution, and incubated at 30 °C for 30 min. This mixture was incubated for an additional 10 min at 30 °C, and then 25-μl aliquots were transferred onto p81 filter paper and washed 3 times with 0.75% phosphoric acid for 5 min per wash followed with acetone once for 2 min. The radioactive incorporation was determined using a scintillation counter (LS6500, Beckman Coulter, Fullerton, CA). The effects of CPF, procyanidin B2, and theobromine were evaluated by separately incubating each compound with the reaction mixtures at 30 °C for 30 min. Each experiment was performed three times.
Ex Vivo MEK1 Immunoprecipitation and Kinase Assay—JB6 P+ cells were cultured to 80% confluence and then starved in 0.1% FBS, MEM for 24 h at 37 °C. Cells were either treated or not treated with CPF, procyanidin B2, or theobromine for 1 h, then treated with 20 ng/ml TPA for 30 min, disrupted with lysis buffer (20 mm Tris-HCl (pH 7.4), 1 mm EDTA, 150 mm NaCl, 1 mm EGTA, 1% Triton X-100, 1 mm β-glycerophosphate, 1 mg/ml leupeptin, 1 mm Na3VO4, and 1 mm phenylmethylsulfonyl fluoride), and finally centrifuged at 19,000 × g for 10 min in a microcentrifuge. The lysates containing 500 μg of protein were used for immunoprecipitation with an antibody against MEK1 and then incubated at 4 °C overnight. Protein A/G Plusagarose beads were then added, and the mixture was continuously rotated for another 3 h at 4 °C. The beads were washed 3 times with kinase buffer (20 mm MOPS (pH 7.2), 25 mm β-glycerol phosphate, 5 mm EGTA, 1 mm Na3VO4, and 1 mm DTT) and then resuspended in 20 μl of 1 × kinase buffer supplemented with 1 μg of inactive ERK2 and incubated for an additional 30 min at 30 °C. Then myelin basic protein (20 μg) and 10 μl of diluted [γ-32P]ATP solution were added, and the mixture was incubated for 10 min at 30 °C. A 20-μl aliquot was transferred onto p81 filter paper and washed 3 times with 0.75% phosphoric acid for 5 min per wash followed by 1 wash with acetone for 2 min. The radioactive incorporation was determined using a scintillation counter. Each experiment was performed three times.
Ex Vivo Pulldown Assay—A JB6 P+ cellular supernatant fraction (500 μg) was incubated with CPF-Sepharose 4B (or Sepharose 4B alone as a control) beads (100 μl, 50% slurry) in reaction buffer (50 mm Tris (pH 7.5), 5 mm EDTA, 150 mm NaCl, 1 mm DTT, 0.01% Nonidet P-40, 2 μg/ml bovine serum albumin, 0.02 mm phenylmethylsulfonyl fluoride, and 1× protease inhibitor mixture). After incubation with gentle rocking overnight at 4 °C, the beads were washed 5 times with buffer (50 mm Tris (pH 7.5), 5 mm EDTA, 150 mm NaCl, 1 mm DTT, 0.01% Nonidet P-40, and 0.02 mm phenylmethylsulfonyl fluoride), and proteins bound to the beads were analyzed by immunoblotting.
ATP and CPF (or Procyanidin B2) Competition Assay—Briefly, 0.2 μg of active MEK1 was incubated with 100 μl of CPF (or procyanidin B2)-Sepharose 4B or 100 μl of Sepharose 4B alone in reaction buffer (see the previous section) for 12 h at 4 °C, and ATP was added at either 10 or 100 μm to a final volume of 500 μl for 30 min. The samples were washed, and then proteins were detected by Western blotting.
Statistical Analysis—When applicable, data are expressed as mean and S.D. values, and Student's t test was used for single statistical comparisons. A probability value of p < 0.05 was used as the criterion for statistical significance.
RESULTS
CPF or Procyanidin B2 Inhibits TPA-induced Neoplastic Transformation of JB6 P+ Cells, Whereas Theobromine Has No Effect—CPF was obtained from commercial cocoa powder and was found to contain 413 mg/g of epicatechin-equivalent flavonoids. We first examined the inhibitory effects of CPF on TPA-induced neoplastic transformation of JB6 P+ cells. Based on the numbers of cell colonies, treatment with CPF significantly suppressed TPA-induced neoplastic transformation of JB6 P+ cells in a dose-dependent manner (Fig. 1C, left panel), with CPF at 5 μg/ml inhibiting TPA-induced cell transformation by 47% (Fig. 1C, right panel). To identify the effective compounds responsible for this inhibitory effect on cell transformation by CPF, we next examined the effects of procyanidin B2 or theobromine on TPA-induced neoplastic transformation of JB6 P+ cells. Based on the numbers of cell colonies, treatment with procyanidin B2 significantly inhibited the TPA-induced neoplastic transformation of JB6 P+ cells (Fig. 1D, left panel) with procyanidin B2 at 40 μm inhibiting TPA-induced cell transformation by 93% (Fig. 1D, right panel). In contrast, theobromine at up to 160 μm had no effect on TPA-induced neoplastic transformation. The inhibition by CPF or procyanidin B2 was not caused by cytotoxicity because a 3-(4,5-dimethylthiazol-2-yl)-5-(carboxymethoxy-phenyl)-2-(4-sulfonyl)-2H-tetrazolium assay indicated that the effective concentration range for inhibiting cell transformation did not affect the viability of JB6 P+ cells (data not shown).
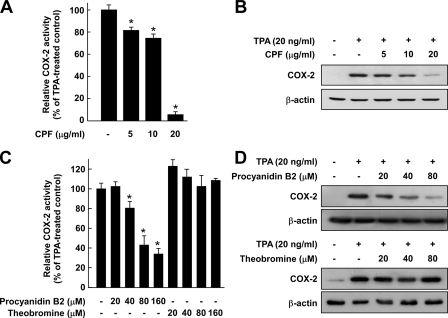
CPF or Procyanidin B2, but Not Theobromine, Suppresses TPA-induced COX-2 Promoter Activity and Protein Expression in JB6 P+ Cells—A close relationship between chronic inflammation and cancer might be explained by the involvement of COX-2, which is a major enzyme triggering the inflammatory response and also inducing tumor promotion and progression stages, especially neoplastic transformation (12, 26). Therefore, we evaluated whether CPF affects TPA-induced up-regulation of COX-2. A luciferase assay showed that CPF inhibited the TPA-induced COX-2 promoter activity in JB6 cells stably transfected with a COX-2 luciferase reporter plasmid (Fig. 2A). CPF also inhibited the TPA-induced COX-2 protein expression in a dose-dependent manner (Fig. 2B). Together these results indicated that CPF regulates TPA-induced COX-2 up-regulation at the transcriptional level. We next determined the ability of procyanidin B2 or theobromine to inhibit TPA-induced COX-2 promoter activity and protein expression. Similar to CPF, procyanidin B2 dose-dependently attenuated TPA-induced COX-2 promoter activity and COX-2 protein expression (Figs. 2, C and D), whereas theobromine at up to 80 μm had no effect (Figs. 2, C and D). These results indicated that oligomeric procyanidins may contribute to the antitumor-promoting activity of cocoa.
FIGURE 2.
Effects of CPF, procyanidin B2, or theobromine on TPA-induced COX-2 promoter activity and protein expression. A, CPF inhibits TPA-induced COX-2 promoter activity. B, CPF suppresses TPA-induced COX-2 protein expression. C, procyanidin B2 (but not theobromine) inhibits TPA-induced COX-2 promoter activity. D, procyanidin B2 (but not theobromine) inhibits TPA-induced COX-2 protein expression. For the luciferase assay, JB6 cells stably transfected with a COX-2 luciferase reporter were cultured as described under “Experimental Procedures.” The cells were starved in 0.1% FBS, MEM and then either treated or not treated with CPF, procyanidin B2, or theobromine at the indicated concentrations for 1 h before being exposed to 20 ng/ml TPA for 24 h. The luciferase activity was assayed, and COX-2 promoter activity is expressed as the percent inhibition relative to cells treated with TPA alone. For the Western blot analysis, cells were pretreated with CPF, procyanidin B2, or theobromine at the indicated concentrations for 1 h, then stimulated with 20 ng/ml TPA and harvested 4 h later. The COX-2 expression was determined by Western blot analysis as described under “Experimental Procedures” using specific antibodies against the corresponding total proteins. For A and C, data are presented as the mean ± S.D. values of COX-2 luciferase activity calculated from three separate experiments. The asterisk (*) indicates a significant difference between groups treated with TPA and CPF, procyanidin B2, or theobromine and the group treated with TPA alone (p < 0.05).
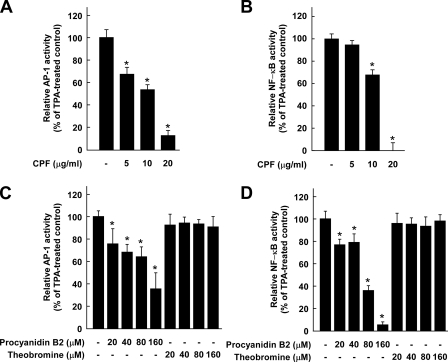
CPF or Procyanidin B2 Attenuates TPA-induced AP-1 and NF-κB Transactivation in JB6 P+ Cells, Whereas Theobromine Has No Effect—The activation of AP-1 and/or NF-κB is considered to play a major role in tumor promoter-induced neoplastic transformation and up-regulation of COX-2 in JB6 P+ cells (21, 27). To determine whether the repression of transformation and COX-2 expression by CPF involves inhibition of the activities of AP-1 and NF-κB, we measured AP-1 and NF-κB transactivation using JB6 cell lines stably transfected with an AP-1 or NF-κB luciferase plasmid. CPF inhibited the TPA-induced transactivation of AP-1 and NF-κB in a dose-dependent manner (Figs. 3, A and B), which suggests that the inhibition of cell transformation and COX-2 expression by CPF is mediated by suppressing the activities of AP-1 and NF-κB. We next investigated whether procyanidin B2 acts by the same inhibitory mechanisms as CPF. Consistent with the above results, procyanidin B2 significantly attenuated TPA-induced transactivation of AP-1 and NF-κB, whereas theobromine at up to 160 μm had no effect (Figs. 3, C and D).
FIGURE 3.
Effects of CPF, procyanidin B2, or theobromine on TPA-induced AP-1 or NF-κB activation. A, CPF inhibits TPA-induced AP-1 transactivation. B, CPF inhibits TPA-induced NF-κB transactivation. C, procyanidin B2 inhibits TPA-induced AP-1 transactivation, whereas theobromine has no effect. D, procyanidin B2 inhibits TPA-induced NF-κB transactivation, whereas theobromine has no effect. For the luciferase assay, JB6 cells stably transfected with an AP-1 or NF-κB luciferase reporter were cultured as described under “Experimental Procedures.” The cells were starved in 0.1% FBS, MEM and then either treated or not treated with CPF, procyanidin B2, or theobromine at the indicated concentrations for 1 h before being exposed to 20 ng/ml TPA for 24 h. Luciferase activity was assayed, and AP-1 or NF-κB activity is expressed as the percent inhibition relative to cells treated with TPA alone. Data are presented as the mean ± S.D. values of AP-1 or NF-κB luciferase activity calculated from three independent experiments. The asterisk (*) indicates a significant difference between groups treated with TPA and CPF, procyanidin B2, or theobromine and the group treated with TPA alone (p < 0.05).
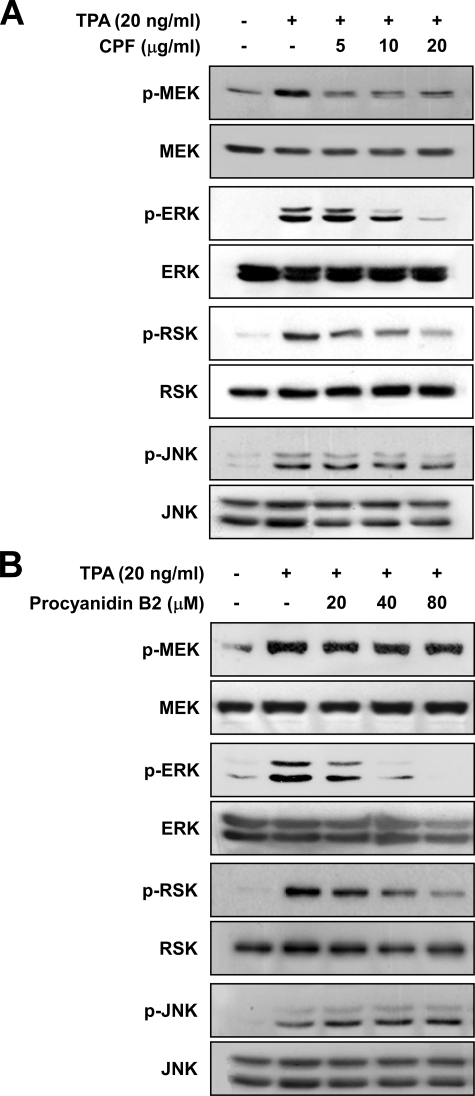
CPF or Procyanidin B2 Suppresses TPA-induced Phosphorylation of MEK, ERK, and p90RSK in JB6 P+ Cells—Previous studies have shown that the MEK/ERK signaling pathway is strongly involved in TPA-induced neoplastic transformation of JB6 P+ cells (21, 28). Thus, we next investigated the effect of CPF on the MEK/ERK signaling pathway. CPF strongly suppressed TPA-induced phosphorylation of MEK, ERK, and p90RSK in JB6 P+ cells (Fig. 4A). Previous studies have shown that JNK is also involved in TPA-induced AP-1 activation and neoplastic transformation of JB6 P+ cells (29). We found that CPF did not inhibit JNK phosphorylation induced by TPA in JB6 P+ cells (Fig. 4A). Similar to CPF, procyanidin B2 inhibited the TPA-induced phosphorylation of ERK and p90RSK but not JNK (Fig. 4B). These results suggested that CPF and procyanidin B2 might suppress the phosphorylation of ERK and p90RSK through the inhibition of MEK activity.
FIGURE 4.
Effects of CPF or procyanidin B2 on TPA-induced phosphorylation of MEK, ERK, p90RSK, and JNK. A and B, CPF or procyanidin B2 inhibits TPA-induced phosphorylation (p) of MEK, ERK, and p90RSK but not JNK. Cells were treated with CPF or procyanidin B2 at the indicated concentrations for 1 h, then stimulated with 20 ng/ml TPA and harvested 15 min later (phosphorylated MEK, ERK, and p90RSK) or 6 h later (phosphorylated JNK). The levels of phosphorylated and total MEK, ERK, p90RSK, and JNK proteins were then determined by Western blot analysis as described under “Experimental Procedures” using specific antibodies against the corresponding phosphorylated or total proteins. Data are representative of three independent experiments that gave similar results.
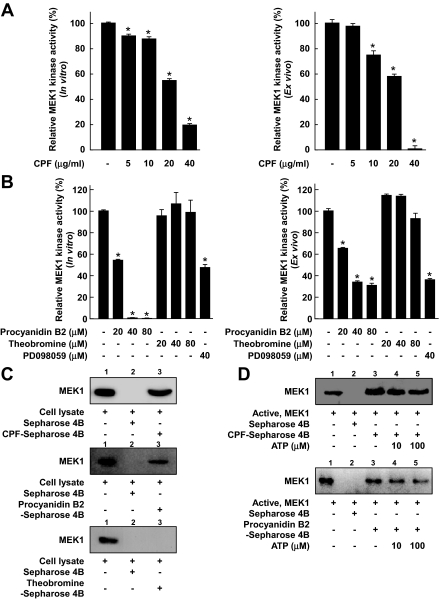
CPF or Procyanidin B2, but Not Theobromine, Specifically Inhibits MEK1 Kinase Activity—To investigate whether MEK1 might be a molecular target of CPF for the inhibition of cell transformation, we next examined the effect of CPF on the kinase activity of MEK1. We found that CPF inhibited both active MEK1 in vitro (Fig. 5A, left panel) and TPA-induced MEK1 activity ex vivo (Fig. 5A, right panel). We next investigated whether the molecular basis of the inhibition of cell transformation and COX-2 expression by procyanidin B2 is also involved in the inhibition of MEK1 activity by CPF. In vitro and ex vivo MEK1 assays revealed that procyanidin B2 (but not theobromine) inhibited MEK kinase activity (Fig. 5B) and that the extent of inhibition was much greater than that of PD098059 (a commercial MEK inhibitor) at the same concentration. Additionally, to confirm whether procyanidin B2 can affect the other kinases, we also examined the effects of procyanidin B2 on the kinase activities of ERK2, JNK1, and RSK2. Procyanidin B2 had no effect on these kinase activities in vitro (data not shown). These results indicated that MEK1 is an important molecular target of CPF or procyanidin B2 for the inhibition of cell transformation, and our results support the possibility that procyanidin B2 is one of the major effective compounds in cocoa that is responsible for the inhibition of cell transformation.
FIGURE 5.
Effects of CPF, procyanidin B2, or theobromine on the kinase activity of MEK1 and direct binding with MEK1. A, CPF inhibits the kinase activity of MEK1. B, procyanidin B2 inhibits MEK1 activity, whereas theobromine has no effect. An in vitro MEK1 assay was performed as described under “Experimental Procedures”, and the kinase activity is expressed as the percent inhibition relative to untreated control MEK1 activity. For the ex vivo MEK1 assay, cells were pretreated with CPF, procyanidin B2, theobromine, or PD098059 at the indicated concentrations for 1 h and then stimulated with 20 ng/ml TPA for 30 min. Cells were harvested, and immunoprecipitation and an ex vivo MEK1 kinase assay were performed as described under “Experimental Procedures.” The kinase activity is expressed as the percent inhibition relative to cells treated with TPA only. The average 32P count was determined from three independent experiments, and data are presented as mean ± S.D. values. The asterisk (*) indicates a significant decrease in kinase activity compared between groups treated with active MEK1 and CPF, procyanidin B2, theobromine or PD098059 and the group treated with active MEK1 alone (in vitro kinase assays) or TPA alone (ex vivo kinase assays; p < 0.05). C, CPF or procyanidin B2 (but not theobromine) specifically binds with MEK1 ex vivo. The MEK1-CPF (upper panel), MEK1-procyanidin B2 (middle panel), and MEK1-theobromine (lower panel) binding ex vivo was assessed by immunoblotting using a specific antibody against MEK1. First lane (input control), whole-cell lysates from JB6 P+ cells; second lane (control), a lysate of JB6 P+ cells precipitated with Sepharose 4B as described under “Experimental Procedures”; third lane, whole-cell lysate from JB6 P+ cells precipitated by CPF-Sepharose 4B (upper panel), procyanidin B2–Sepharose 4B (middle panel), or theobromine-Sepharose 4B (lower panel) as described under “Experimental Procedures”. D, CPF or procyanidin B2 does not compete with ATP in binding with MEK1. Active MEK1 (2 μg) was incubated with ATP at different concentrations (0, 10, or 100 μm) and 50 μl of CPF (top panel) or procyanidin B2-Sepharose 4B (bottom panel) or 50 μl of Sepharose 4B (as a negative control) in reaction buffer to a final volume of 500 μl. The mixtures were incubated at 4 °C overnight with shaking. After washing, the pulled-down proteins were detected by Western blotting. Lane 2 (negative control), MEK1 does not bind with Sepharose 4B; lane 3 (positive control), MEK1 binding with CPF-Sepharose 4B (upper panel) or procyanidin B2-Sepharose 4B (lower panel); lanes 4 and 5, increasing the amount of ATP has no effect on CPF binding with MEK1.
CPF or Procyanidin B2 (but Not Theobromine) Specifically Binds with MEK1 but Does Not Compete with ATP for Binding with MEK1—The results above indicated that the inhibition of cell transformation by CPF or procyanidin B2 involves the suppression of MEK1 activity and subsequent inhibition of downstream signaling pathways. To determine whether CPF exerts its effects by directly interacting with MEK1, we performed an ex vivo pulldown assay using CPF-Sepharose 4B beads and JB6 P+ cell lysates. After being treated with TPA for 30 min, cell lysates were collected and subjected to SDS-PAGE. MEK1 was observed in CPF-Sepharose 4B beads (Fig. 5C, upper panel, third lane) but not in Sepharose-4B-only beads (Fig. 5C, upper panel, second lane). The data reveal that CPF can directly bind with MEK1 and subsequently inhibit the activation of MEK and downstream signals. Procyanidin B2 also binds with MEK1 (Fig. 5C, middle panel, third lane), whereas theobromine does not directly interact with MEK1 (Fig. 5C, lower panel, third lane). The first lane in Fig. 5C indicates that only cell lysates were loaded as a marker, thereby ensuring that the detected bands were only related to the MEK1 protein itself. Moreover, ATP did not compete with CPF or procyanidin B2 for binding with MEK1, as indicated by the binding of CPF (Fig. 5D, top panel) or procyanidin B2 (Fig. 5D, bottom panel) with MEK1 did not decrease with increasing amounts of ATP. Therefore, these results suggested that CPF or procyanidin B2 inhibits MEK1 activity through direct binding.
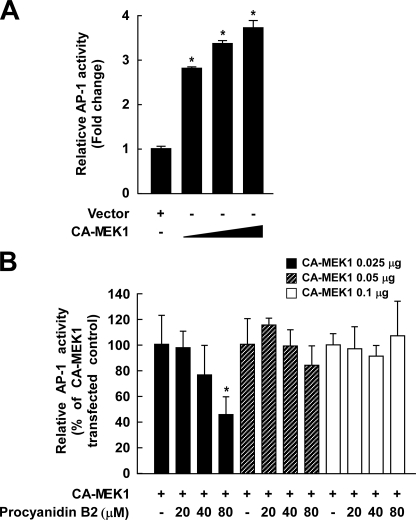
Procyanidin B2 Inhibition of TPA-induced AP-1 Activation Is Dependent on MEK1—To determine whether procyanidin B2 specifically inhibits MEK1 kinase activity, we investigated the effect of procyanidin B2 on AP-1-luciferase-transfected JB6 cells that were also transfected with constitutively active-MEK1. Results indicated that overexpression of MEK1 increases the transcriptional activity of AP-1 (Fig. 6A), and overexpression of MEK1 attenuated procyanidin B2 inhibition of TPA-induced AP-1 activation (Fig. 6B). These data further support procyanidin B2 specificity for targeting MEK1.
FIGURE 6.
Effects of procyanidin B2 on AP-1 JB6 cells transfected with constitutively active-MEK1. A, overexpression of MEK1 increases the transcriptional activity of AP-1. The JB6 mouse epidermal cell line transfected with an AP-1 luciferase reporter plasmid was cotransfected with a pcDNA3 vector or constitutively active-MEK1. Then, 24 h post-transfection the transfectants were assayed as described under “Experimental Procedures.” B, overexpression of MEK1 attenuates procyanidin B2 inhibition of AP-1 activation. The AP-1 JB6 mouse epidermal cell line transfected with constitutively active (CA)-MEK1 was pretreated with procyanidin B2 for 24 h at the concentrations indicated. Luciferase activity is expressed as relative AP-1 activity (% of constitutively active-MEK1 transfected control). Data from three independent experiments are averaged and presented as the means ± S.D. The asterisk (*) indicates a significant difference between the groups, treated and untreated with procyanidin B2 (p < 0.05).
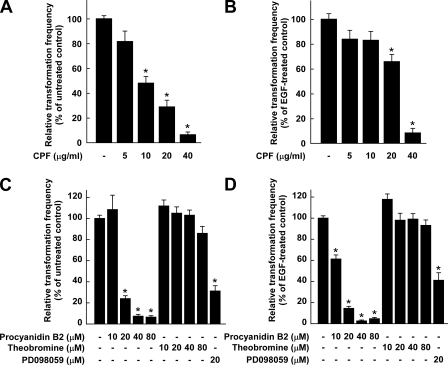
CPF or Procyanidin B2 Suppresses Either H-Ras- or EGF-induced Neoplastic Transformation of JB6 P+ Cells, Whereas Theobromine Has No Effect—Our previous studies showed that H-Ras and EGF activated the growth-signal pathway involving MEK and ERK protein kinases, leading to anchorage-independent growth of JB6 P+ cells (2, 22). In the present study we examined the potential inhibitory activities of CPF on H-Ras- or EGF-induced cell transformations. Based on the number of cell colonies, CPF at 40 μg/ml inhibited H-Ras- or EGF-induced neoplastic cell transformation by 94 or 88%, respectively (Fig. 7, A and B). These findings provide evidence indicating that CPF suppresses cell transformation mainly by targeting the MEK/ERK signaling pathway. We next examined the potential inhibitory activities of procyanidin B2 or theobromine on either H-Ras- or EGF-induced cell transformation. Based on the number of cell colonies, procyanidin B2 at 20 μm inhibited H-Ras- or EGF-induced cell transformation by 76 or 86%, respectively, whereas theobromine at up to 80 μm had no significant effect (Figs. 7, C and D). Moreover, the inhibition of H-Ras- or EGF-induced cell transformation by procyanidin B2 was more potent than that of PD098059 at the same concentration. Together these findings provide evidence showing that procyanidin from cocoa suppresses cell transformation mainly by targeting the MEK/ERK signaling pathway.
FIGURE 7.
Effects of CPF, procyanidin B2, or theobromine on H-Ras and EGF-induced cell transformation. A, CPF inhibits H-Ras-induced transformation of JB6 P+ cells. B, CPF inhibits EGF-induced transformation of JB6 P+ cells. C, Procyanidin B2 (but not theobromine) inhibits H-Ras-induced transformation of JB6 P+ cells. D, Procyanidin B2 (but not theobromine) inhibits EGF-induced cell transformation of JB6 P+ cells. H-Ras- and EGF-induced cell transformation assays were performed as described under “Experimental Procedures”, and colonies were counted 14 days later under a microscope with the aid of Image-Pro Plus software (v.4). The effects of CPF, procyanidin B2, theobromine, or PD098059 on transformation of JB6 P+ cells in soft agar are presented as the percent inhibition of cell transformation relative to H-Ras- or EGF-stimulated cells. Data are presented as mean ± S.D. values of the percent inhibition as determined from 3 independent experiments. For A and C, The asterisk (*) indicates a significant difference between groups treated with CPF, procyanidin B2, theobromine, or PD098059, and the untreated control group (p < 0.05). For B and D, The asterisk (*) indicates a significant difference between groups treated with EGF and procyanidin B2, theobromine, or PD098059, and the group treated with EGF alone (for all p < 0.05).
DISCUSSION
Naturally occurring dietary phytochemicals originating from plant-based foodstuffs can exert potential chemopreventive effects in carcinogenesis, particularly at the promotion stage (13, 17). Cocoa and chocolate are excellent dietary sources of polyphenols, including the procyanidins, methylxanthines, and theobromine (30). A previous study indicated that on a per-serving basis, the intake of polyphenols would be higher for the consumption of cocoa than for the consumption of tea or red wine (31). In the present study we found that CPF or procyanidin B2 inhibited the TPA-induced transformation of JB6 P+ cells in a dose-dependent manner, whereas theobromine at up to 80 μm had no significant effect (Fig. 1). Previous studies showed that cocoa polyphenols exert antiproliferative effects in different cell types (32, 33). Our previous studies demonstrated that cocoa extracts protected against oxidative stress-induced inhibition of gap-junction intercellular communication (33) and TPA-induced COX-2 protein expression in mouse skin (34). Moreover, recent studies have shown that orally administered cocoa powder or cocoa liquor inhibits chemically induced carcinogenesis in experimental animals (9, 10). Our results, therefore, support the chemopreventive potential of cocoa procyanidins.
The accumulated data provide evidence for a strong link between inflammation and cancer (for a recent review, see Refs. 11 and 12). Knock-out of COX-2 results in reduced tumor formation and progression, and epidemiologic studies and animal experiments have demonstrated that nonsteroidal antiinflammatory drugs reduce the incidence of colorectal carcinoma (35). A previous study demonstrated that overexpression of COX-2 in MCF-7 cells shortens the doubling time and augments the number of cells during the phase of exponential growth as well as increasing the number of colonies formed in soft agar (36). Exposure to prostaglandin E2 also increases the rate of colony formation in colon cancer cells but only in COX-2-expressing cell lines (37). Recent studies have revealed that COX-2 expression promotes neoplastic transformation of JB6 P+ cells, indicating that COX-2 acts as a link between inflammation and carcinogenesis in the tumor promotion stage (26). Thus, COX-2 has been recognized as a molecular target of many chemopreventive as well as anti-inflammatory agents. In the present study we found that CPF or procyanidin B2 (but not theobromine) blocked TPA-induced COX-2 expression and promoter activity in JB6 P+ cells (Fig. 2). Thus, inhibition of COX-2 by cocoa procyanidin may contribute to its antitumorpromoting activity.
The AP-1 and NF-κB signal transduction pathways are considered to be important in tumor promoter-induced transformation and the development of tumors (17, 18, 20). Recent studies have shown that the expression of COX-2 is primarily regulated by AP-1 and NF-κB, and accumulating evidence indicates that the inhibition of COX-2 promoter activity exerts antitumor-promoting effects due to the blocking of the transcriptional activities of AP-1 and NF-κB (17, 18, 20). The present results suggest that CPF or procyanidin B2 (but not theobromine) suppressed TPA-induced neoplastic transformation and COX-2 expression by blocking AP-1 and NF-κB activation in JB6 P+ cells (Fig. 3). AP-1 and NF-κB are at least partially activated by a series of upstream kinases including those belonging to the MAP kinase family. The MAP kinase signaling pathways are critical for activating AP-1 and NF-κB in response to a wide variety of extracellular stimuli including TPA, growth factors, cytokines, arsenic, and UV radiation (17, 18, 38). The first members of this family to be characterized were the ERK proteins, and inhibition of ERK activity by a dominant-negative ERK2 or a MEK1 inhibitor, PD098059, blocked TPA- or EGF-induced AP-1 transactivation and cell transformation (22). The activated form of ERK can phosphorylate and activate transcription factors, thereby altering the expression of COX-2. The MEK/ERK signaling pathway, therefore, represents a promising target for pharmacological interventions in carcinogenesis (39). To study the molecular basis of the inhibition of AP-1 and NF-κB activation by CPF, we hypothesized that their activities could be altered by upstream kinases in the ERK signaling pathway. Our data indicated that CPF or procyanidin B2 blocks activation of the ERK signaling pathway. CPF or procyanidin B2 inhibited the TPA-induced phosphorylation of ERK and p90RSK in JB6 P+ cells (Fig. 4), with this being linked to the suppression of neoplastic transformation and AP-1 and NF-κB activities. In addition, previous studies have shown that JNK activation is involved in JB6 P+ cell transformation induced by TPA (29). In the present study we found that CPF or procyanidin B2 did not inhibit TPA-induced JNK phosphorylation in these cells (Fig. 4).
Polyphenols are well known antioxidants, but they may also make other contributions to improve health. Recent research has highlighted several important mechanisms of polyphenols that are related to their ability to bind to diverse proteins including cellular kinases, thereby influencing gene expression and cell signaling (2, 17, 40–42). Components of the ERK pathway such as MEK are considered to be promising targets for chemopreventive strategies due to their converging functions. MEK is another important pathway component which catalyzes the phosphorylation of ERK on both a threonine residue (Thr-183) and a tyrosine residue (Tyr-185) (43). No substrate for MEK1/2 has been reported other than ERK (44), and this specificity coupled with a unique ability to phosphorylate both tyrosine and threonine residues indicates that this kinase is essential for integrating signals into the MAP kinase pathway. Several lines of evidence suggest that MEK plays a key role in the transformation of cells and development of tumors. MEK has a critical role in transmitting signals initiated by tumor promoters such as TPA, EGF, and platelet-derived growth factor (17, 19). Additionally, a mutant H-ras gene perpetually activates the MEK/ERK signaling pathway and drives cells to develop a more aggressive cancer-like phenotype, such as anchorage-independent growth (1, 2, 45). The constitutive activation of MEK1 results in cellular transformation, whereas a small-molecule inhibitor of MEK is capable of inhibiting transformation and tumor growth in both cell culture and mouse models (19, 39). In the present study CPF or procyanidin B2 significantly inhibited the activity of MEK (Figs. 5, A and B). On the other hand, procyanidin B2 did not inhibit ERK2, JNK1, or RSK2 kinase activities in vitro (data not shown).
The present study showed that ATP did not compete with CPF for binding with MEK1 (Fig. 5C). Similar results were found in experiments with procyanidin B2. MEK is known to have a unique inhibitor binding pocket adjacent to but distinct from the ATP binding site, and this accounts for the selectivity of well known MEK inhibitors, including PD098059 (46, 47). Because CPF or procyanidin B2 also does not compete with ATP for binding with MEK1, their inhibition of MEK1 might be highly selective. Moreover, overexpression of MEK1 attenuated procyanidin B2 inhibition of TPA-induced AP-1 activation (Figs. 6, A and B). Existing data suggested that the relevant mechanisms of cancer prevention attributed to procyanidin B2 are related to the direct inhibition of MEK1 kinase activity.
Further investigation revealed that CPF or procyanidin B2 inhibited not only TPA-induced but also H-Ras- or EGF-induced neoplastic transformation (Fig. 7), and these results support the finding that CPF or procyanidin B2 selectively inhibits the MEK/ERK/RSK signaling pathway regardless of the types of inducers stimulating this pathway.
Cocoa contains a variety of procyanidins, most of which are oligomeric compounds. Previous studies analyzing the percentage content of procyanidin in Brazilian cocoa beans revealed that procyanidin dimers make up only about 10% of total polyphenols, with procyanidins from trimers to decamers making up about 80% (7). Therefore, the strong inhibition of the activity of MEK1 by CPF might be attributable to oligomeric procyanidins other than the procyanidin B2 dimer. Moreover, previous reviews have revealed that targeting a single signal transduction pathway might not be sufficient for the successful inhibition of abnormal signal activation and suggested a combination of multiple signal inhibitors as another promising strategy for treating cancer (44). Therefore, because CPF can target more than one kinase (e.g. MEK1, as revealed in this study), it might represent a molecule with potent antitumor-promoting effects.
In summary, either CPF or procyanidin B2 inhibits tumor promoter-induced neoplastic transformation of JB6 P+ cells. This inhibition is mediated mainly through the blocking of the MEK/ERK/p90RSK signaling pathway and subsequent suppression of AP-1 and NF-κB activities. CPF or procyanidin B2 strongly inhibits MEK1 activity through binding with MEK1 without competing with ATP. These results together suggest that MEK1 is a potent molecular target for the suppression of neoplastic transformation by CPF or procyanidin B2 and that the chemopreventive effects of cocoa are mainly attributable to the procyanidins rather than to theobromine.
Supplementary Material
Acknowledgments
We thank Andria Hansen for secretarial assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants CA120388, CA111536, CA88961, and CA81064. This work was also supported by the Hormel Foundation and by grants from the BioGreen21 Program (20070301-034-042), Rural Development Administration, the Korea Science and Engineering Foundation (R01-2007-000-11957-0), and the Ministry of Science and Technology, Republic of Korea. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
Footnotes
The abbreviations used are: COX-2, cyclooxygenase-2; MOPS, 3-(N-morpholino)propanesulfonic acid; ERK, extracellular signal-regulated protein kinase; JNK, c-Jun N-terminal kinase; RSK, ribosomal S6 kinase; MAP, mitogen-activated protein; MEK, MAP kinase kinase; CPF, cocoa procyanidin fraction; TPA, 12-O-tetradecanoylphorbol-13-acetate; NF, nuclear factor; AP, activator protein; EGF, epidermal growth factor; MEM, minimum essential medium; BME, basal medium Eagle; FBS, fetal bovine serum; DTT, dithiothreitol.
References
- 1.Chung, J. Y., Huang, C., Meng, X., Dong, Z., and Yang, C. S. (1999) Cancer Res. 59 4610–4617 [PubMed] [Google Scholar]
- 2.Chung, J. Y., Park, J. O., Phyu, H., Dong, Z., and Yang, C. S. (2001) FASEB J. 15 2022–2024 [DOI] [PubMed] [Google Scholar]
- 3.Lu, Y. P., Lou, Y. R., Lin, Y., Shih, W. J., Huang, M. T., Yang, C. S., and Conney, A. H. (2001) Cancer Res. 61 5002–5009 [PubMed] [Google Scholar]
- 4.Lu, Y. P., Lou, Y. R., Xie, J. G., Peng, Q. Y., Liao, J., Yang, C. S., Huang, M. T., and Conney, A. H. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 12455–12460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He, Z., Ma, W. Y., Hashimoto, T., Bode, A. M., Yang, C. S., and Dong, Z. (2003) Cancer Res. 63 4396–4401 [PubMed] [Google Scholar]
- 6.Steinberg, F. M., Bearden, M. M., and Keen, C. L. (2003) J. Am. Diet Assoc. 103 215–223 [DOI] [PubMed] [Google Scholar]
- 7.Weisburger, J. H. (2001) Exp. Biol. Med. (Maywood) 226 891–897 [DOI] [PubMed] [Google Scholar]
- 8.Smit, H. J., Gaffan, E. A., and Rogers, P. J. (2004) Psychopharmacology (Berl.) 176 412–419 [DOI] [PubMed] [Google Scholar]
- 9.Yamagishi, M., Natsume, M., Osakabe, N., Nakamura, H., Furukawa, F., Imazawa, T., Nishikawa, A., and Hirose, M. (2002) Cancer Lett. 185 123–130 [DOI] [PubMed] [Google Scholar]
- 10.Yamagishi, M., Natsume, M., Osakabe, N., Okazaki, K., Furukawa, F., Imazawa, T., Nishikawa, A., and Hirose, M. (2003) Cancer Lett. 191 49–57 [DOI] [PubMed] [Google Scholar]
- 11.Balkwill, F., and Mantovani, A. (2001) Lancet 357 539–545 [DOI] [PubMed] [Google Scholar]
- 12.Coussens, L. M., and Werb, Z. (2002) Nature 420 860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee, K. W., and Lee, H. J. (2006) Biofactors 26 105–121 [DOI] [PubMed] [Google Scholar]
- 14.Pai, R., Soreghan, B., Szabo, I. L., Pavelka, M., Baatar, D., and Tarnawski, A. S. (2002) Nat. Med. 8 289–293 [DOI] [PubMed] [Google Scholar]
- 15.Wong, B. C., Jiang, X. H., Lin, M. C., Tu, S. P., Cui, J. T., Jiang, S. H., Wong, W. M., Yuen, M. F., Lam, S. K., and Kung, H. F. (2004) Gastroenterology 126 136–147 [DOI] [PubMed] [Google Scholar]
- 16.Liu, G., Ma, W. Y., Bode, A. M., Zhang, Y., and Dong, Z. (2003) J. Biol. Chem. 278 2124–2130 [DOI] [PubMed] [Google Scholar]
- 17.Bode, A. M., and Dong, Z. (2005) Prog. Nucleic Acid Res. Mol. Biol. 79 237–297 [DOI] [PubMed] [Google Scholar]
- 18.Hsu, T. C., Young, M. R., Cmarik, J., and Colburn, N. H. (2000) Free Radic. Biol. Med. 28 1338–1348 [DOI] [PubMed] [Google Scholar]
- 19.Cowley, S., Paterson, H., Kemp, P., and Marshall, C. J. (1994) Cell 77 841–852 [DOI] [PubMed] [Google Scholar]
- 20.Suzukawa, K., Weber, T. J., and Colburn, N. H. (2002) Environ. Health Perspect. 110 865–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, C., Ma, W. Y., Dawson, M. I., Rincon, M., Flavell, R. A., and Dong, Z. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 5826–5830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang, C., Ma, W. Y., Young, M. R., Colburn, N., and Dong, Z. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 156–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bode, A. M., and Dong, Z. (2000) Lancet Oncol. 1 181–188 [DOI] [PubMed] [Google Scholar]
- 24.Dong, Z., and Cmarik, J. L. (2002) Sci. STKE 2002, PL7. [DOI] [PubMed]
- 25.Dong, Z., Crawford, H. C., Lavrovsky, V., Taub, D., Watts, R., Matrisian, L. M., and Colburn, N. H. (1997) Mol. Carcinog. 19 204–212 [DOI] [PubMed] [Google Scholar]
- 26.Yan, Y., Li, J., Ouyang, W., Ma, Q., Hu, Y., Zhang, D., Ding, J., Qu, Q., Subbaramaiah, K., and Huang, C. (2006) J. Cell Sci. 119 2985–2994 [DOI] [PubMed] [Google Scholar]
- 27.Li, J. J., Westergaard, C., Ghosh, P., and Colburn, N. H. (1997) Cancer Res. 57 3569–3576 [PubMed] [Google Scholar]
- 28.Dong, Z., Birrer, M. J., Watts, R. G., Matrisian, L. M., and Colburn, N. H. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 609–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou, D. X., Kai, K., Li, J. J., Lin, S., Terahara, N., Wakamatsu, M., Fujii, M., Young, M. R., and Colburn, N. (2004) Carcinogenesis. 25 29–36 [DOI] [PubMed] [Google Scholar]
- 30.Miller, K. B., Stuart, D. A., Smith, N. L., Lee, C. Y., McHale, N. L., Flanagan, J. A., Ou, B., and Hurst, W. J. (2006) J. Agric. Food Chem. 54 4062–4068 [DOI] [PubMed] [Google Scholar]
- 31.Lee, K. W., Kim, Y. J., Lee, H. J., and Lee, C. Y. (2003) J. Agric. Food Chem. 51 7292–7295 [DOI] [PubMed] [Google Scholar]
- 32.Carnesecchi, S., Schneider, Y., Lazarus, S. A., Coehlo, D., Gosse, F., and Raul, F. (2002) Cancer Lett. 175 147–155 [DOI] [PubMed] [Google Scholar]
- 33.Lee, K. W., Hur, H. J., Lee, H. J., and Lee, C. Y. (2005) J. Agric. Food Chem. 53 1990–1995 [DOI] [PubMed] [Google Scholar]
- 34.Lee, K. W., Kundu, J. K., Kim, S. O., Chun, K. S., Lee, H. J., and Surh, Y. J. (2006) J. Nutr. 136 1150–1155 [DOI] [PubMed] [Google Scholar]
- 35.Chan, A. T., Ogino, S., and Fuchs, C. S. (2007) N. Engl. J. Med. 356 2131–2142 [DOI] [PubMed] [Google Scholar]
- 36.Prosperi, J. R., Mallery, S. R., Kigerl, K. A., Erfurt, A. A., and Robertson, F. M. (2004) Prostaglandins Other Lipid Mediat. 73 249–264 [DOI] [PubMed] [Google Scholar]
- 37.Sheng, H., Shao, J., Morrow, J. D., Beauchamp, R. D., and DuBois, R. N. (1998) Cancer Res. 58 362–366 [PubMed] [Google Scholar]
- 38.Surh, Y.-J. (2003) Nat. Rev. Cancer 3 768–780 [DOI] [PubMed] [Google Scholar]
- 39.Sebolt-Leopold, J. S., Dudley, D. T., Herrera, R., Becelaere, K., Wiland, A., Gowan, R. C., Tecle, H., Barrett, S. D., Bridges, A., Przybranowski, S., Leopold, W. R., and Saltiel, A. R. (1999) Nat. Med. 5 810–816 [DOI] [PubMed] [Google Scholar]
- 40.Ermakova, S., Choi, B. Y., Choi, H. S., Kang, B. S., Bode, A. M., and Dong, Z. (2005) J. Biol. Chem. 280 16882–16890 [DOI] [PubMed] [Google Scholar]
- 41.Ermakova, S. P., Kang, B. S., Choi, B. Y., Choi, H. S., Schuster, T. F., Ma, W. Y., Bode, A. M., and Dong, Z. (2006) Cancer Res. 66 9260–9269 [DOI] [PubMed] [Google Scholar]
- 42.Sang, S., Hou, Z., Lambert, J. D., and Yang, C. S. (2005) Antioxid. Redox Signal. 7 1704–1714 [DOI] [PubMed] [Google Scholar]
- 43.Anderson, N. G., Maller, J. L., Tonks, N. K., and Sturgill, T. W. (1990) Nature 343 651–653 [DOI] [PubMed] [Google Scholar]
- 44.Kohno, M., and Pouyssegur, J. (2006) Ann. Med. 38 200–211 [DOI] [PubMed] [Google Scholar]
- 45.Shapiro, P. (2002) Crit. Rev. Clin. Lab. Sci. 39 285–330 [DOI] [PubMed] [Google Scholar]
- 46.Dudley, D. T., Pang, L., Decker, S. J., Bridges, A. J., and Saltiel, A. R. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 7686–7689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts, P. J., and Der, C. J. (2007) Oncogene 26 3291–3310 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.