Abstract
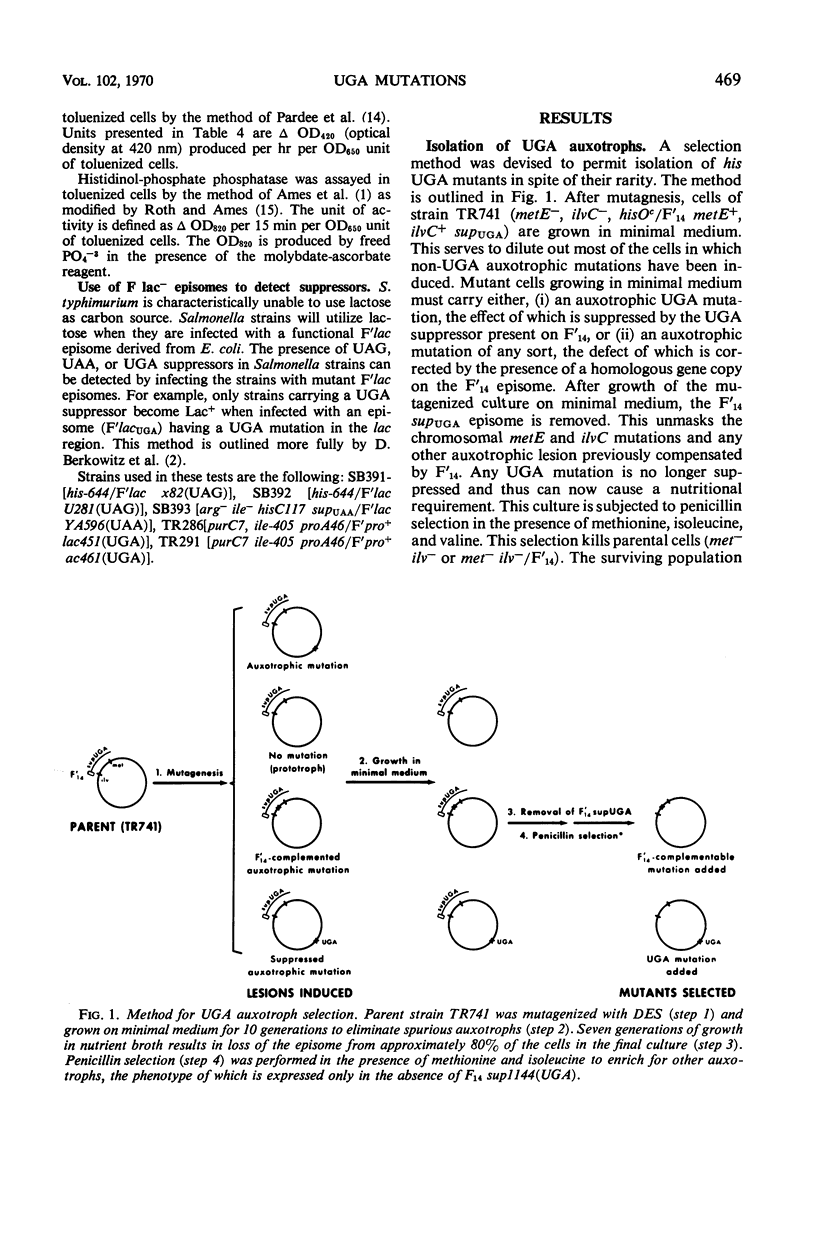
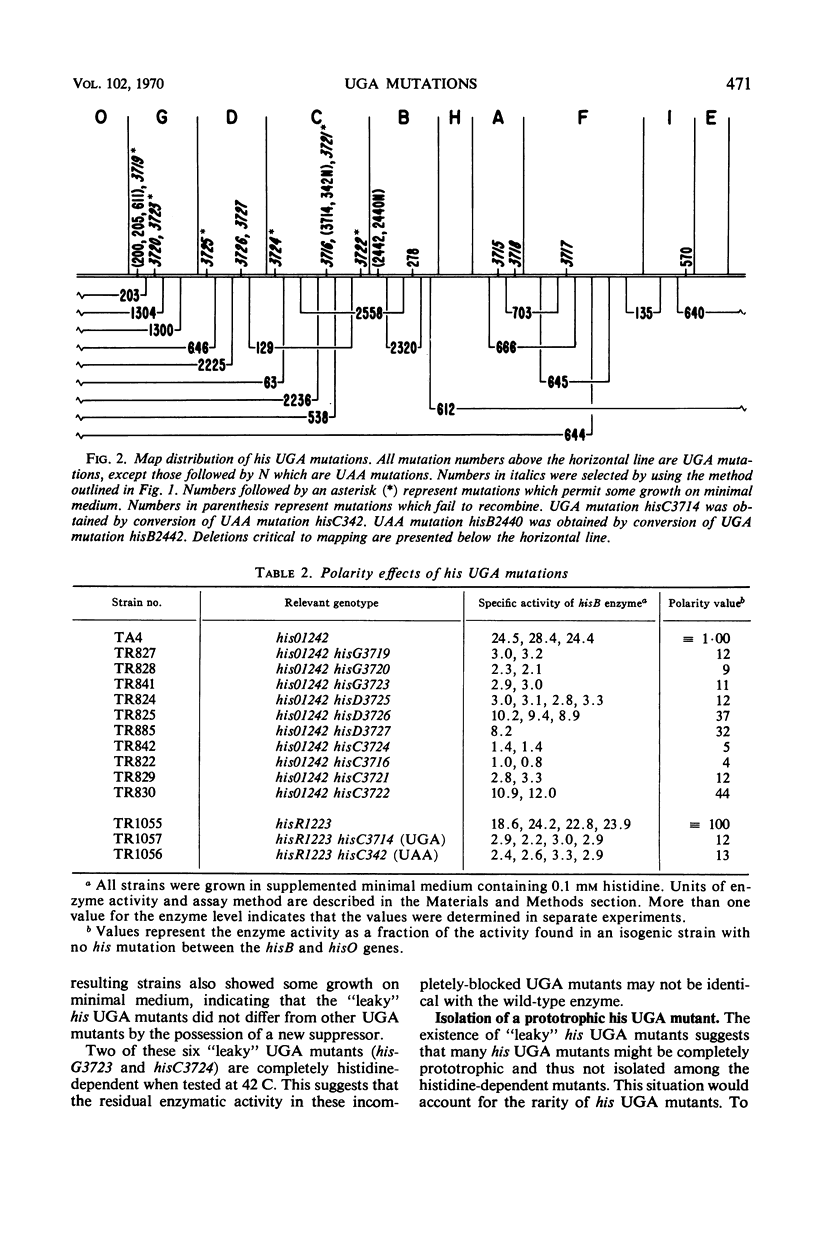
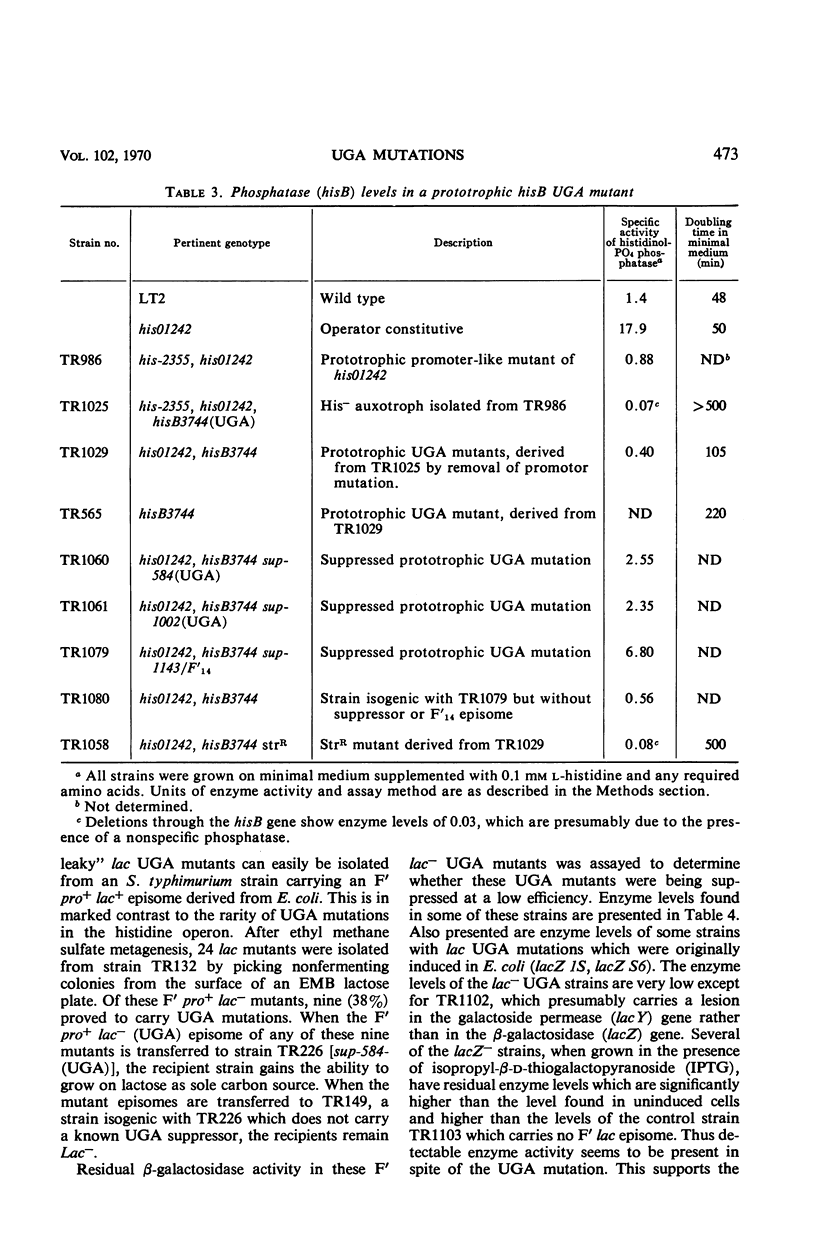
Salmonella typhimurium strain LT-2 carries a weak UGA suppressor activity. This activity prevents the detection of some UGA mutants as auxotrophs and probably accounts for the rarity of his UGA mutants in this strain. A selection method is described which permits the isolation of these rare his UGA mutants. Map distribution of his UGA mutations is normal, and their polarity effects are indistinguishable from the polarity effects of amber and ochre mutations at similar locations. Isolation and properties of a prototrophic his UGA mutant are described. UGA mutants are common among lac mutants isolated from Salmonella strains carrying an F′lac episome. Apparently the suppressor activity is insufficient to prevent detection of lac UGA mutants. It is not yet clear whether the suppressor activity plays an important role in normal cell physiology.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., HARTMAN P. E., JACOB F. Chromosomal alterations affecting the regulation of histidine biosynthetic enzymes in Salmonella. J Mol Biol. 1963 Jul;7:23–42. doi: 10.1016/s0022-2836(63)80016-9. [DOI] [PubMed] [Google Scholar]
- Berkowitz D., Hushon J. M., Whitfield H. J., Jr, Roth J., Ames B. N. Procedure for identifying nonsense mutations. J Bacteriol. 1968 Jul;96(1):215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., Barnett L., Katz E. R., Crick F. H. UGA: a third nonsense triplet in the genetic code. Nature. 1967 Feb 4;213(5075):449–450. doi: 10.1038/213449a0. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Beaudet A., Nirenberg M. RNA codons and protein synthesis. 15. Dissimilar responses of mammalian and bacterial transfer RNA fractions to messenger RNA codons. J Mol Biol. 1968 Oct 14;37(1):99–118. doi: 10.1016/0022-2836(68)90076-4. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G. R., Klopotowski T., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium. IV. A positive selection for polar histidine-requiring mutants from histidine operator constitutive mutants. J Mol Biol. 1967 Nov 28;30(1):81–95. doi: 10.1016/0022-2836(67)90245-8. [DOI] [PubMed] [Google Scholar]
- Fink G. R., Martin R. G. Translation and polarity in the histidine operon. II. Polarity in the histidine operon. J Mol Biol. 1967 Nov 28;30(1):97–107. doi: 10.1016/0022-2836(67)90246-x. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Ishizawa M., Endo H. Su-II-specific restriction of amber suppression by mutation to streptomycin resistance. J Mol Biol. 1968 Apr 28;33(2):513–516. doi: 10.1016/0022-2836(68)90209-x. [DOI] [PubMed] [Google Scholar]
- LOPER J. C., GRABNAR M., STAHL R. C., HARTMAN Z., HARTMAN P. E. GENES AND PROTEINS INVOLVED IN HISTIDINE BIOSYNTHESIS IN SALMONELLA. Brookhaven Symp Biol. 1964 Dec;17:15–52. [PubMed] [Google Scholar]
- Langridge J., Campbell J. H. Classification and intragenic position of mutations in the beta-galactosidase gene of Escherichia coli. Mol Gen Genet. 1969;103(4):339–347. doi: 10.1007/BF00383484. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Silbert D. F., Smith W. E., Whitfield H. J., Jr Polarity in the histidine operon. J Mol Biol. 1966 Nov 14;21(2):357–369. doi: 10.1016/0022-2836(66)90104-5. [DOI] [PubMed] [Google Scholar]
- Model P., Webster R. E., Zinder N. D. The UGA codon in vitro: chain termination and suppression. J Mol Biol. 1969 Jul 14;43(1):177–190. doi: 10.1016/0022-2836(69)90087-4. [DOI] [PubMed] [Google Scholar]
- Otsuji N., Aono H. Effect of mutation to streptomycin resistance on amber suppressor genes. J Bacteriol. 1968 Jul;96(1):43–50. doi: 10.1128/jb.96.1.43-50.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. R., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium II. Histidine regulatory mutants having altered histidyl-tRNA synthetase. J Mol Biol. 1966 Dec 28;22(2):325–333. doi: 10.1016/0022-2836(66)90135-5. [DOI] [PubMed] [Google Scholar]
- SANDERSON K. E., DEMEREC M. THE LINKAGE MAP OF SALMONELLA TYPHIMURIUM. Genetics. 1965 Jun;51:897–913. doi: 10.1093/genetics/51.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J. F., Fan D. P., Brenner S. A strong suppressor specific for UGA. Nature. 1967 Apr 29;214(5087):452–453. doi: 10.1038/214452a0. [DOI] [PubMed] [Google Scholar]
- Schwartz N. M. Revertant instability in Escherichia coli. II. Genetic studies. Genetics. 1967 Nov;57(3):505–512. doi: 10.1093/genetics/57.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E., Tompkins R., Caskey T., Nirenberg M. Release factors differing in specificity for terminator codons. Proc Natl Acad Sci U S A. 1968 Oct;61(2):768–774. doi: 10.1073/pnas.61.2.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Levine M. A phage P22 gene controlling integration of prophage. Virology. 1967 Feb;31(2):207–216. doi: 10.1016/0042-6822(67)90164-x. [DOI] [PubMed] [Google Scholar]
- Söll D., Ohtsuka E., Jones D. S., Lohrmann R., Hayatsu H., Nishimura S., Khorana H. G. Studies on polynucleotides, XLIX. Stimulation of the binding of aminoacyl-sRNA's to ribosomes by ribotrinucleotides and a survey of codon assignments for 20 amino acids. Proc Natl Acad Sci U S A. 1965 Nov;54(5):1378–1385. doi: 10.1073/pnas.54.5.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Voll M. J. Translation and polarity in the histidine operon. 3. The isolation of prototrophic polar mutations. J Mol Biol. 1967 Nov 28;30(1):109–124. doi: 10.1016/0022-2836(67)90247-1. [DOI] [PubMed] [Google Scholar]
- Whitfield H. J., Jr, Martin R. G., Ames B. N. Classification of aminotransferase (C gene) mutants in the histidine operon. J Mol Biol. 1966 Nov 14;21(2):335–355. doi: 10.1016/0022-2836(66)90103-3. [DOI] [PubMed] [Google Scholar]



