Abstract
Homologous recombination between dispersed repeated sequences is important in shaping eukaryotic genome structure, and such ectopic interactions are affected by repeat size and sequence identity. A transformation-based, gap-repair assay was used to examine the effect of 2% sequence divergence on the efficiency of mitotic double-strand break repair templated by chromosomal sequences in yeast. Because the repaired plasmid could either remain autonomous or integrate into the genome, the effect of sequence divergence on the crossover–noncrossover (CO–NCO) outcome was also examined. Finally, proteins important for regulating the CO–NCO outcome and for enforcing identity requirements during recombination were examined by transforming appropriate mutant strains. Results demonstrate that the basic CO–NCO outcome is regulated by the Rad1-Rad10 endonuclease and the Sgs1 and Srs2 helicases, that sequence divergence impedes CO to a much greater extent than NCO events, that an intact mismatch repair system is required for the discriminating identical and nonidentical repair templates, and that the Sgs1 and Srs2 helicases play additional, antirecombination roles when the interacting sequences are not identical.
RECOMBINATION is a high-fidelity process that copies information from one DNA duplex to repair single- or double-strand discontinuities in another DNA duplex (reviewed by Krogh and Symington 2004). Repair events involve either the unidirectional transfer of information between duplexes or the reciprocal exchange of information, which will be referred to here as noncrossover (NCO) and crossover (CO) events, respectively. In classic models of homologous recombination, NCO and CO events derive from alternative cleavage of a common intermediate known as a Holliday junction (HJ), which corresponds to the point where the single strands of the interacting duplexes switch pairing partners. In more recent recombination models, however, some NCO events proceed through an intermediate that cannot be processed into a CO. The template for recombinational repair is typically a homologous chromosome or sister chromatid, but because recombination is a homology-driven process, it also can engage repetitive sequences dispersed throughout the genome. Such ectopic interactions are important for shaping genome structure, with NCO events likely driving the concerted evolution of multigene families and COs leading to various types of genome rearrangements. In mitotic studies using model recombination substrates in the yeast Saccharomyces cerevisiae, the rate of ectopic interactions is directly proportional to repeat size (Ahn et al. 1988; Hayden and Byers 1992; Jinks-Robertson et al. 1993; Inbar et al. 2000) and inversely proportional to the level of sequence divergence (Datta et al. 1997; Chen and Jinks-Robertson 1998).
The major barrier to recombination between diverged sequences in yeast derives from antirecombination activity of the mismatch repair (MMR) system (reviewed in Surtees et al. 2004), which is best known for its roles in removing DNA replication errors and repairing mismatches in meiotic recombination intermediates (reviewed in Harfe and Jinks-Robertson 2000; Kunkel and Erie 2005). There are three major yeast complexes involved in mismatch removal and in antirecombination: MutSα, MutSβ, and MutLα composed of Msh2-Msh6, Msh2-Msh3, and Mlh1-Pms1, respectively. MutSα and MutSβ bind directly to mismatches, while MutLα couples MutSα/β-mediated mismatch recognition to the appropriate downstream processing steps, the precise mechanism(s) of which remain obscure. Additional proteins implicated in the repair of mismatches include the Rad1-Rad10 endonuclease (Kirkpatrick and Petes 1997), the Exo1 exonuclease (Tishkoff et al. 1997), and the PCNA sliding clamp (Johnson et al. 1996; Umar et al. 1996). Rad1-Rad10 and Exo1 are also important in antirecombination (Nicholson et al. 2000), but PCNA plays little, if any, role in this process (Stone et al. 2008). In contrast, the helicase Sgs1 is important in antirecombination (Myung et al. 2001; Spell and Jinks-Robertson 2004), but has no known role in the repair of mismatches. Thus, although there are similarities in the MMR-directed editing of replication and recombination intermediates, there are genetic and presumably mechanistic differences as well.
In yeast, chromosomal sequences can serve as a template for the faithful repair of a linear, gap-containing plasmid and such gap-repair reactions were instrumental in the development of the double-strand break (DSB) repair model of recombination (Szostak et al. 1983). Of particular significance was the observation that the repaired plasmid either remains autonomous or integrates into the host genome (Orr-Weaver and Szostak 1983), outcomes presumed to reflect cleavage of a Holliday junction intermediate to generate NCO or CO products, respectively. Subsequent studies have confirmed that plasmid-based repair assays generally recapitulate the genetic requirements and features of DSB-initiated chromosomal recombination (Bartsch et al. 2000) and hence are a useful model for studying basic recombination processes. In the experiments reported here, a gap-repair assay was used to examine the regulation of mitotic recombination fidelity, with an emphasis on how sequence divergence affects the CO–NCO decision in different genetic backgrounds. These studies support roles for the Srs2 and Sgs1 helicases, the Rad1-Rad10 endonuclease, and MutSβ in regulating the CO–NCO outcome, confirm that the regulation of recombination fidelity depends on activity of the MMR machinery, and demonstrate that sequence divergence impedes CO events to a much greater extent than NCO events.
MATERIALS AND METHODS
Media and growth conditions:
Yeast strains were grown nonselectively in YEP (1% Bacto-yeast extract, 2% Bacto peptone), supplemented with 2% glucose and 500 μg/ml adenine hemisulfate (YEPD). For selection of strains containing the kan or hph marker, YEPD was supplemented to 200 μg/ml with geneticin or to 300 μg/ml with hygromycin B, respectively. For selective growth, synthetic complete (SC) (Sherman 1991) medium contained 2% glucose and all but the one relevant amino acid or base (e.g., SC −his for the selection of His+ recombinants). Canavanine-resistant colonies were selected on SC −arg medium supplemented with 60 μg/ml l-canavanine. Ura− segregants were selected on SC plates supplemented with 0.1% 5-flouroorotic acid (US Biological). All growth was at 30°.
Plasmids:
A 938-bp fragment containing the 663 bp HIS3 ORF together with 199 bp of upstream and 76 bp of downstream sequence was PCR amplified from pSR22, a pUC7 derivative containing a 1.7-kb BamHI genomic HIS3 fragment. Following treatment with T4 DNA polymerase and T4 kinase, the fragment was inserted at the SmaI site of the LEU2-CEN vector pRS315 (Sikorski and Hieter 1989). The resulting plasmids pSR515 and pSR516 contain the HIS3 gene in the opposite and same orientation, respectively, as the vector lacZ gene. The HIS3 gene of these plasmids contains <10 bp of identity to the his3Δ200 allele at the endogenous HIS3 locus.
The HIS3-18 allele of pSR612 contains 18 silent, randomly distributed mutations (see supplemental Figure 1) and is 98% identical to the standard HIS3 gene. The mutations were introduced by subjecting pSR515 to sequential rounds of site-directed mutagenesis using the Chameleon double-stranded, site-directed mutagenesis kit (Stratagene). All mutations were confirmed by sequencing, and pSR612 was able to fully complement the His− phenotype of a his3Δ200 strain.
pSR800 contains a 3′-truncated, but otherwise wild-type (WT), his3 allele inserted into the CAN1 coding sequence. Because this allele contains no polymorphisms, it is referred to as his3-0,Δ3′ to distinguish it from the similarly truncated allele that contains the engineered silent changes (his3-18,Δ3′). The can1∷his3Δ3′ allele was constructed as follows. First, the smallest KpnI fragment of pSR515 was deleted, thereby truncating HIS3 at an internal KpnI site and eliminating the last 11 amino acids of the encoded protein. An 834-bp BamHI-KpnI fragment from the resulting plasmid (pSR798) was then treated with T4 DNA polymerase and inserted at the MscI site of pSR797. pSR797 is a pUC9 derivative containing an 1122-bp CAN1 fragment (+21 to +1141 of the 1773 nt CAN1 ORF) inserted at the SmaI site of the vector polylinker. The resulting plasmid pSR800 contains the his3-0,Δ3′ allele in an orientation opposite to that of the CAN1 sequences. pSR801 contains the can1∷his3-18,Δ3′ allele and was constructed in the same manner as pSR800, but starting from pSR612.
pSR840 contains the his3ΔBgl allele and was used as the template for producing the linear “gapped vector” PCR fragment for transformation assays. pSR840 was derived by first inserting a SacI-SalI fragment containing the full-length HIS3 gene (from pSR516) into SacI/SalI-digested pRS306, an integrating URA3 vector (Sikorski and Hieter 1989). The internal 60-bp BglII fragment was then deleted to generate the his3ΔBgl allele. Finally, a Klenow-treated HinfI-EcoO109 fragment containing the ARS4 replication origin of pRS315 (Sikorski and Hieter 1989) was ligated to AatII-digested plasmid that had been treated with T4 DNA polymerase.
Yeast strains:
Transformation with PstI/PvuII-digested pSR800 or pSR801 was used to replace the CAN1 allele in SJR328 (MATα ade2-101oc his3Δ200 ura3-Nhe lys2ΔRV∷hisG leu2-R Gal+; Chen and Jinks-Robertson 1999) with the can1∷his3-0,Δ3′ or can1∷his3-18,Δ3′ allele, respectively. Following selection of transformants on SC −arg +CAN, replacements were confirmed by PCR. SJR1500 contains the can1∷his3-0,Δ3′ allele, while SJR1501 contains the can1∷his3-18,Δ3′ allele.
Repair-defective derivatives of SJR1500 and SJR1501 were constructed by targeted gene deletion. The MSH2 gene was deleted by one-step gene replacement using AatII/XbaI-digested pΔmsh2 (msh2Δ∷hisG-URA3-hisG plasmid; Earley and Crouse 1998). Following the selection of Ura+ transformants, YEPD-purified colonies were patched to 5-FOA medium to select for loss of URA3 and one copy of hisG. SJR1476 and SJR1477 are the resulting msh2Δ∷hisG derivatives of SJR1500 and SJR1501, respectively. msh6Δ∷hisG (SJR2047 and SJR2048), msh3Δ∷hisG (SJR2054 and SJR2055), or rad1∷hisG derivatives (SJR2111 and SJR2112) of SJR1500 and SJR1501 were similarly constructed using SacI/EcoRI-digested pBUH-msh6∷hisG-URA3 (Kramer et al. 1996), EcoRI-digested pEN33 (Datta et al. 1996) or SalI/EcoRI-digested pR1.6 (Higgins et al. 1983), respectively. The pms1Δ derivatives of SJR1500 and SJR1501 (SJR2147 and SJR2148, respectively) were constructed by standard two-step allele replacement using BstXI-digested pJH523 (Kramer et al. 1989). The mlh1Δ∷kanMX4 (SJR2156 and SJR2157), sgs1Δ∷kanMX4 (SJR2122 and SJR2163), and srs2Δ∷kanMX4 (SJR2123 and SJR2160) derivatives of SJR1500 and SJR1501 were constructed by transformation with PCR deletion cassettes generated using pFA6-kanMX4 (Wach et al. 1994) as a template. Presence of the relevant mutant allele and absence of the corresponding WT allele were confirmed by PCR.
Gap-repair experiments:
The fragment used for gap-repair assays was produced by PCR amplification of pSR840. Primers HisBglIIF (5′-CTCTTGCGAGATGATCCCGC) and HisBglIIR (5′-ACCACCGCTCTGGAAAGTGCC), which anneal directly adjacent to the single BglII site that marks the 60-bp deletion, were used to amplify a 5.7-kb fragment with Taq Plus Precision polymerase (Stratagene). After the PCR reaction, the template pSR840 DNA was destroyed using the methylation-sensitive enzyme DpnI. To correct for variation in transformation efficiency, the PCR product was mixed with uncut control plasmid (pRS315, a CEN-LEU2 vector; Sikorski and Hieter 1989) in a 20:1 weight ratio.
A high-efficiency transformation protocol was used (Gietz and Woods 2002), with the following modifications. To minimize the culture-to-culture variation seen with some of the repair-defective strains, five colonies were pooled to inoculate 5 ml of liquid YEPD. After overnight growth, 1 ml was transferred to a prewarmed flask containing 50 ml YEPD, and the flask was incubated on a rotary shaker at 200 rpm for 3 hr. Cells were harvested by centrifugation at room temperature, washed twice, and resuspended in 360 μl of H2O. Twenty-five-microliter samples of the cell suspension were pelleted by centrifugation, the supernatant was removed, and 120 μl of PEG 3350 (50%, w/v) were layered over the pellets. Sixty microliters of freshly prepared transformation mix [18 μl of 1 m LiAc; 5 μl of 10 mg/ml boiled salmon sperm carrier DNA; 17 μl H2O; 20 μl of a gapped vector/control plasmid mix (40 ng + 2 ng)] were added and the tubes were vortexed for 1 min. Following incubation at 42° for 1 hr, cells were pelleted, resuspended in 550 μl of sterile water, and vortexed for 1 min. One-hundred-microliter aliquots were plated on SC −his plates to select recombinants and on SC −leu plates to determine transformation efficiency. For each experiment, four replicates of each transformation were performed and each experiment was repeated at least once. Colonies were counted after 3 days of growth on the selective media and gap-repair frequency was calculated as the ratio of His+ transformants to Leu+ transformants.
The proportions of NCOs and COs among the gap-repair events in each strain were determined by directly patching ∼50 His+ transformants from each of at least 5 independent transformations onto YEPD. Patches were replica plated to 5-FOA medium and those with full growth after 2 days were scored as NCO events; patches with no growth or only a few papillae were scored as CO events. Random genomic DNAs were analyzed by PCR to confirm the accuracy of the NCO–CO assignment.
RESULTS
A gap-repair system for assaying homologous and homeologous recombination:
The goal of the current study was to determine whether sequence divergence has similar inhibitory effects on mitotic crossover (CO) and noncrossover (NCO) events, and if so, to identify the gene products involved in this regulation. Because our earlier studies demonstrated that a single potential mismatch is sufficient to trigger the mitotic antirecombination activity of the yeast MMR system (Datta et al. 1997), it was essential to use 100%-identical sequences as a control against which to gauge the effects of sequence divergence. Genetic assays designed to simultaneously detect both NCO and CO events typically rely on the transfer of WT sequence to correct an auxotrophic allele, thereby precluding absolute identity between the interacting sequences. Such identity was achieved in the current study by using a transformation-based, gap-repair assay in which the template for the gap-filling reaction is a truncated, but otherwise WT, allele. Recombination between the two mutant alleles—extrachromosomal gapped and chromosomal truncated—produces a selectable, WT allele on the plasmid. If the gapped plasmid is furthermore capable either of autonomous replication or of integrating into the genome, it is straightforward to distinguish NCO from CO events, respectively (Orr-Weaver and Szostak 1983).
The HIS3 gene was used as the basis for developing the gap-repair assay and was placed on a vector containing the URA3 gene and an origin of replication (ARS), but no centromeric (CEN) sequence (Figure 1). This plasmid was then used as a PCR template to generate a linear (gapped) transformation fragment (see materials and methods). To obtain an identical (“homologous”) repair template, a mutant his3 allele missing the C-terminal 11 amino acids (his3-0,Δ3′) was inserted into the CAN1 locus of a haploid strain containing a deletion of the endogenous HIS3 coding sequence. To obtain a 98%-identical (“homeologous”) repair template, 18 silent mutations were engineered into the coding sequence of the truncated his3 allele (his3-18,Δ3′). The his3-0,Δ3′ and his3-18,Δ3′ strains (or appropriate mutant derivatives) were then transformed in parallel using the PCR-generated fragment. Variations in transformation efficiencies in different genetic backgrounds (Bartsch et al. 2000; Haghnazari and Heyer 2004) were corrected for by mixing an intact LEU2/ARS/CEN plasmid (pRS315; Sikorski and Hieter 1989) with the linear fragment prior to transformation. His+ and Leu+ transformants were selected separately, and the efficiency of gap repair was calculated as the ratio of His+ to Leu+ transformants. The stability of the URA3 marker on the repaired plasmid was then assessed by transferring His+ transformants to medium containing 5-FOA. A stable Ura+ phenotype is diagnostic of a chromosomal URA3 gene and hence plasmid integration at the CAN1 locus (CO event), whereas an unstable Ura+ phenotype is conferred when the URA3 gene is on an autonomous plasmid (NCO event). The primary data obtained following the transformation of WT, msh2, msh3, msh6, mlh1, pms1, rad1, srs2, or sgs1 strains containing either the his3-0,Δ3′ or his3-18,Δ3′ allele are presented in Table 1.
Figure 1.—
The gap-repair assay. The lengths of homology that flank the gap on the 5′ and 3′ sides are ∼600 bp and 160 bp, respectively. See text for further explanation of the system.
TABLE 1.
Gap-repair efficiencies using 100- vs. 98%-identical chromosomal donor sequences in different genetic backgrounds
| Strain | Relevant genotype | Substrate identity (%) | His+/Leu+ frequency | CO proportion | CO/NCO |
|---|---|---|---|---|---|
| SJR1500 | WT | 100 | 1.6 ± 0.08 | 0.53 ± 0.031 | 1.1 |
| SJR1501 | WT | 98 | 0.66 ± 0.035 | 0.17 ± 0.016 | 0.20 |
| SJR1476 | msh2 | 100 | 0.83 ± 0.045 | 0.44 ± 0.054 | 0.79 |
| SJR1477 | msh2 | 98 | 0.57 ± 0.085 | 0.24 ± 0.032 | 0.32 |
| SJR2054 | msh3 | 100 | 0.97 ± 0.052 | 0.21 ± 0.044 | 0.27 |
| SJR2055 | msh3 | 98 | 0.53 ± 0.031 | 0.066 ± 0.020 | 0.075 |
| SJR2047 | msh6 | 100 | 1.7 ± 0.11 | 0.55 ± 0.029 | 1.2 |
| SJR2048 | msh6 | 98 | 1.6 ± 0.089 | 0.56 ± 0.042 | 1.3 |
| SJR2156 | mlh1 | 100 | 1.6 ± 0.032 | 0.61 ± 0.034 | 1.6 |
| SJR2157 | mlh1 | 98 | 1.6 ± 0.092 | 0.54 ± 0.059 | 1.2 |
| SJR2147 | pms1 | 100 | 1.6 ± 0.11 | 0.55 ± 0.037 | 1.2 |
| SJR2148 | pms1 | 98 | 1.2 ± 0.12 | 0.42 ± 0.019 | 0.72 |
| SJR2111 | rad1 | 100 | 1.0 ± 0.044 | 0.052 ± 0.018 | 0.053 |
| SJR2112 | rad1 | 98 | 0.49 ± 0.017 | 0.020 ± 0.024 | 0.020 |
| SJR2122 | sgs1 | 100 | 1.2 ± 0.065 | 0.87 ± 0.023 | 6.7 |
| SJR2163 | sgs1 | 98 | 0.60 ± 0.018 | 0.76 ± 0.089 | 3.2 |
| SJR2123 | srs2 | 100 | 0.75 ± 0.013 | 0.67 ± 0.11 | 2.0 |
| SJR2160 | srs2 | 98 | 0.32 ± 0.019 | 0.38 ± 0.064 | 0.61 |
The mean and standard deviation of the ratio of His+ to Leu+ transformants and of the proportion of CO events obtained in independent transformations is indicated. NCO, noncrossover; CO, crossover.
Genetic control of homologous gap repair:
Comparison of the gap-repair efficiency in WT vs. mutant strains containing the chromosomal his3-0,Δ3′ allele allows one to ascertain the general effects of the corresponding gene products on homologous gap repair (HGR). These results are presented in Figure 2, where the efficiency of gap repair in each mutant background was normalized to that obtained in the WT strain (i.e., the His+/Leu+ ratio of the WT strain was set to 1.0). The levels of NCO vs. CO events in each background were calculated by multiplying the relative gap-repair efficiency by the percentage of unstable vs. stable transformants, respectively. In the WT strain, the repaired plasmid was integrated into the yeast genome in about half of the His+ transformants (47% NCOs and 53% COs).
Figure 2.—
The genetic control of homologous gap repair. The total gap-repair efficiency using an identical chromosomal template (the his3-0,Δ3′ allele) in each strain background was measured as the ratio of His+ to Leu+ transformants. These ratios were then normalized to that obtained in the WT strain. The open and shaded areas within each bar correspond to the NCO and CO efficiencies, respectively, and were obtained by multiplying the total (normalized) efficiency by the proportion of the relevant event. The standard deviation of the total efficiency in each strain is indicated.
In terms of their effects on the total efficiency of gap repair between identical substrates, the MMR proteins fell into two distinct groups. The efficiency was not detectably altered in the msh6, pms1, or mlh1 background, but there was an approximately twofold decrease in an msh2 or msh3 mutant. In the case of Msh3, its loss had no effect on the frequency of NCOs, but reduced CO events fourfold; Msh2 loss was associated with approximately twofold decreases in both NCO and CO events. The NCO–CO distribution in the msh2 mutant was significantly different from that in the msh3 mutant (P < 0.001). Given that the only known function for Msh3 is as part of the MutSβ complex with Msh2, it was surprising that msh2 and msh3 mutants did not exhibit similar levels of NCO and CO events between identical repeats. It is possible that either Msh2 alone or the Msh2-Msh6 complex, which might be more abundant in the absence of Msh3 (Drummond et al. 1997), has subtle effects on recombination.
The role of MutSβ during gap repair most likely reflects an accessory role during the Rad1-Rad10-dependent processing of branched recombination intermediates (see Surtees and Alani 2006). The Rad1-Rad10 complex is best known for its essential function during the incision step of nucleotide excision repair, where it nicks at the junction of single- and double-stranded DNA (Prakash and Prakash 2000). During recombination, Rad1-Rad10 removes nonhomologous 3′ tails from recombination intermediates (Fishman-Lobell and Haber 1992), stimulates deletion events between direct repeats (Saparbaev et al. 1996), facilitates “ends-in” plasmid-chromosome CO events (Schiestl and Prakash 1988; Symington et al. 2000), and increases the efficiency of “ends-out” targeted gene replacement (Langston and Symington 2005). Consistent with previous results, a rad1 mutant exhibited a specific, 16-fold decrease in COs in our gap-repair assay, which translated into a 2-fold reduction in the total gap-repair efficiency. The requirement of Rad1, and by inference the Rad1-Rad10 complex, for >90% of COs associated with gap repair suggests a key role for this protein in the formation of Holliday junction-containing intermediates (see discussion).
In addition to analyzing the roles of the MutSα, MutSβ, MutLα, and Rad1-Rad10 complexes in gap repair, we also examined the effects of the Srs2 and Sgs1 helicases. While mutants defective in either helicase exhibit a spontaneous hyperrecombination phenotype (see Fabre et al. 2002), srs2 mutants exhibit a hypo-rec phenotype when recombination is initiated with a DSB (Aylon et al. 2003; Ira et al. 2003). With regard to both spontaneous and DSB-initiated recombination, loss of either Srs2 or Sgs1 shifts the distribution of recombinants toward more CO events (Ira et al. 2003; Robert et al. 2006). In the gap-repair assay there was a 2-fold decrease in the overall transformation efficiency in an srs2 background, and a subtle, 25% decrease in an sgs1 mutant. While both NCO and CO events were reduced in the srs2 mutant, there was a greater reduction in NCO than in CO recombinants (3-fold and 1.7-fold, respectively), resulting in a 2-fold bias for CO events. In the sgs1 mutant, there was a much more striking shift in the distribution of CO relative to NCO events, with almost 90% of the gap-repaired plasmids integrating into the genome. This strong CO bias can be attributed to a 5-fold reduction in NCOs, some of which may have been converted into CO events.
Effects of sequence divergence on gap repair in a WT background:
The effects of 2% sequence divergence on gap repair in a WT background are presented in Figure 3. The homeologous gap-repair (HeGR) efficiency was reduced 2.4-fold relative to that between homologous sequences. Whereas the HGR events were evenly distributed between NCO and CO events, only 17% of the HeGR events were of the CO type. This translates into a 7.3-fold reduction in homeologous relative to homologous CO events, but only a 1.4-fold reduction in NCO events. Thus, at least in the gap-repair assay used here, low levels of sequence divergence have a strong inhibitory effect only on the maturation of recombination intermediates into CO products. The observation that the reduction of homeologous COs was not accompanied by a compensatory gain in NCOs suggests that not all repair events can be simply diverted from a CO to a NCO pathway.
Figure 3.—
Homologous and homeologous gap repair (HGR and HeGR, respectively) in a WT strain. Strains containing a homologous or homeologous repair template (the his3-0,Δ3′ or his3-18,Δ3′ allele, respectively) were transformed with the gapped plasmid and the total (NCO + CO) repair efficiency was measured as the ratio of His+ to Leu+ colonies. These ratios were normalized to that obtained with the homologous, 100%-identical substrates; the standard deviations of the total efficiencies are indicated. NCO and CO efficiencies were obtained by multiplying the total (normalized) efficiency by the proportion of the relevant event. Open and shaded bars correspond to HGR and HeGR efficiencies, respectively.
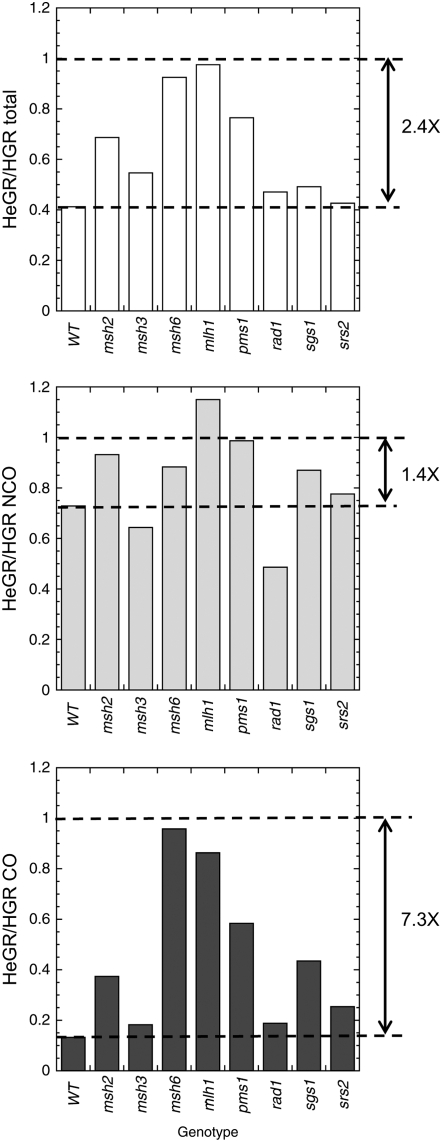
Regulation of homeologous gap repair:
The roles of individual proteins in regulating the fidelity of recombination, and thereby reducing interactions between diverged sequences, were examined by transforming the gapped plasmid into an msh2, msh3, msh6, mlh1, pms1, rad1, srs2, or sgs1 strain. To correct for nonspecific effects of a given protein on the recombination process (e.g., its loss conferring a general hyper-rec phenotype or altering the CO–NCO distribution) the ratio of total HeGR to HGR was calculated in each strain background, as well as the ratio for NCO and CO events (Figure 4). The smaller the HeGR/HGR ratio, the greater the inhibitory effect of sequence divergence on the recombination process being examined; a ratio of 1.0 indicates equivalent efficiencies of homologous and homeologous recombination. For the WT strain, the baseline HeGR/HGR ratios were 0.41, 0.73, and 0.13 for total, NCO, and CO events, respectively. Relative to these baseline WT values, an increase in the HeGR/HGR ratio in a given mutant background indicates a relaxation of homology requirements and, therefore, a role of the corresponding protein in enforcing recombination fidelity.
Figure 4.—
Effect of sequence divergence on gap repair in different genetic backgrounds. The HeGR efficiency (His+/Leu+) ratio was normalized to the HGR efficiency obtained in the same genetic background. The NCO and CO efficiencies were determined by multiplying the total repair efficiency by the proportion of the relevant event and these efficiencies were used to calculate the corresponding HeGR/HGR ratio. If sequence divergence has no effect on repair efficiency, then HeGR/HGR = 1. An HeGR/HRG ratio <1 indicates that sequence divergence inhibits repair; the smaller the ratio, the greater the inhibition. The double arrowheads to the right indicate the magnitude of the inhibition in the WT background.
All of the potential mismatches generated during repair of the gapped plasmid are base–base mismatches and hence should primarily be detected by the MutSα complex. In accord with this prediction, the total or CO HeGR/HGR ratios changed little, if any, in an msh3 mutant, but increased to ∼1.0 in an msh6 or mlh1 mutant. The absence of an effect of sequence divergence on gap repair in the msh6 or mlh1 mutant is consistent with antirecombination activity being derived solely from the MMR machinery. In contrast to the msh6 and mlh1 mutants, the total HeGR/HGR ratio increased to 0.69 and the CO ratio to only 0.38 in an msh2 mutant; the transformation data in Table 1 indicate that these ratios indeed reflect a persistent reduction of homeologous recombination in the absence of Msh2. We suggest that this might reflect the role of MutSβ in stabilizing the substrate of the Rad1-Rad10 endonuclease, a role that could become more important when the interacting sequences are not identical. Finally, it should be noted that the HeGR/HGR ratio in the pms1 mutant was only 0.77; the data in Table 1 again suggest a persistent inhibition of homeologous gap repair when Pms1 is absent but not when Mlh1 is absent. One possibility is that in the absence of Pms1, Mlh1 might partner with either Mlh2 or Mlh3 (Wang et al. 1999) to carry out a low level of antirecombination.
In the HGR assay, loss of Rad1 was associated with a strong reduction in the proportion of CO events, while loss of Sgs1 or Srs2 elevated the proportion of COs (Table 1 and Figure 2). Additional roles for these proteins in the regulation of recombination fidelity should be revealed as an increase in the HeGR/HGR ratio for CO events in the appropriate mutant strains. Loss of either Sgs1 or Srs2 was associated with an increase in the HeGR/HGR ratio; there was a 3.3-fold increase in the CO ratio in the sgs1 mutant, and a smaller, 1.9-fold increase in the srs2 mutant. A mismatch-related antirecombination role for Sgs1 has been previously observed in both spontaneous and DSB-induced recombination assays (Myung et al. 2001; Spell and Jinks-Robertson 2004; Sugawara et al. 2004), but this is the first indication that there may be a similar activity associated with Srs2. In a rad1 background, there was no obvious change in the HeGR/HGR ratio, although an effect would have been difficult to detect given the very strong dependence of COs on the presence of Rad1. We note that this is in contrast to the clear role for the Rad1-Rad10 complex in limiting recombination between diverged, chromosomal inverted-repeat substrates (Nicholson et al. 2000).
DISCUSSION
The genetic regulation of mitotic recombination fidelity was examined by transforming a gapped plasmid into strains containing either a 100%-identical homologous or a 98%-identical homeologous chromosomal repair template. A key feature of the gap-repair system used here is that it allows a distinction to be made between NCO and CO events; this was not possible with the inverted-repeat (IR) assay we previously used (Chen and Jinks-Robertson 1998). An additional advantage of a gap-repair system is that both the position and nature of the initiating lesion are known, whereas the IR assay only detects randomly initiated events. The major results obtained using the gap-repair assay and discussed further below are that (1) the Rad1-Rad10 endonuclease strongly promotes CO events, (2) sequence divergence affects CO much more than NCO events, (3) the negative effect of sequence divergence on recombination requires MutS- and MutL-like complexes to similar extents, and (4) the Sgs1 and Srs2 helicases have roles in enforcing recombination identity requirements as well as in regulating the CO–NCO outcome.
In discussing the implications of results reported here, it is important to consider them in the context of current models of DSB repair (for a review, see Krogh and Symington 2004). As shown in Figure 5, the ends of a broken or gapped molecule are first resected to produce 3′ single-stranded tails that are incorporated into Rad51 nucleoprotein filaments. Following the invasion of a homologous duplex by a nucleoprotein filament and displacement of a D-loop, the invading 3′ end is used to prime new DNA synthesis (step A). When DNA synthesis proceeds past the other side of the DSB/gap, the unengaged 3′ end can be “captured” by annealing to the displaced D-loop, generating an intermediate with a double HJ (step B). Double HJs are a key element of the classic DSB repair model of recombination (Szostak et al. 1983), and their mode of resolution can generate either a CO or NCO product (steps D–E). Enzymatic cleavage of both junctions in the same direction (both horizontally or both vertically) produces NCOs (step D), while cleavage in different directions (one junction horizontally and the other vertically) produces COs (step E). Instead of HJ resolution by direct cleavage, the two junctions can migrate toward each other, with the resulting hemicatenated molecules being resolved by topoisomerase activity (step F). This latter mode of resolution yields only NCO products and is thought to require the Sgs1 helicase and Top3 in yeast (Ira et al. 2003). While capture of the unengaged end by an intact D-loop leads to formation of a double HJ, cleavage of the D-loop at its base results in the formation of a single HJ (step C). A partial dependence of COs on the Rad1 endonuclease in some assays suggests that such cleavage can facilitate D-loop capture and/or HJ formation (Symington et al. 2000). As with a double HJ, the direction of cleaving a single HJ determines the NCO or CO outcome; cleavage of the initially exchanged or nonexchanged strands yields a NCO or CO product, respectively. The Sgs1 helicase might also be expected to generate NCOs by reverse branch migration of a single HJ intermediate. In contrast to the dissolution of double HJs, however, no accompanying topoisomerase activity would be needed to resolve a single HJ. Finally, as an alternative to second-end capture and HJ formation, collapse of the D-loop will free the extended 3′ end to pair with the unengaged, single-stranded tail on the other side of the DSB/gap (step G). This latter type of recombination is referred to as synthesis-dependent strand annealing (SDSA) and yields only NCO products (Paques and Haber 1999). The observed temporal separation of NCO and CO events (Allers and Lichten 2001; Ira et al. 2003) is consistent with an SDSA pathway that does not involve HJ resolution. In yeast, SDSA appears to be enhanced by the Srs2 helicase (Ira et al. 2003), which recently has been shown to dismantle Rad51-containing D-loops in vitro (Dupaigne et al. 2008). Although not applicable to the current gap-repair assay, single-strand annealing (SSA) is a final DSB repair mechanism that specifically deletes the region between direct repeats and has been used to examine the regulation of recombination fidelity (Sugawara et al. 2004).
Figure 5.—
Models for recombinational repair of a gapped plasmid using chromosomal DNA as a template. Plasmid and chromosomal DNA are indicated as black and red lines, respectively. Arrowheads represent 3′ ends, and dotted lines correspond to newly-synthesized DNA. The colors of the dotted lines correspond to that of the template. Heteroduplex DNA forms adjacent to the original gap and is depicted as paired black and red lines. Details of the models are given in the text.
Genetic control of gap repair between identical sequences:
In a WT background with identical substrates, approximately one-half of the repaired plasmids were integrated into the yeast genome. Neither the gap-repair efficiency nor the distribution of NCO–CO products was altered in msh6, mlh1, or pms1 mutants, indicating that the MutSα and MutLα complexes are not involved in recombination between identical sequences. In contrast, there was a specific, 4-fold decrease in CO events in an msh3 mutant, implicating MutSβ in the processing of recombination intermediates. Plasmid integration was decreased even more (16-fold) in a rad1 strain, consistent with MutSβ playing an accessory role by stabilizing the relevant Rad1-Rad10 substrate (Surtees and Alani 2006). The strong dependence of COs on Rad1 is consistent with the suggestion that Rad1-Rad10 mediated D-loop cleavage promotes second-end capture to stabilize an HJ intermediate (Symington et al. 2000). If this is the case, then most COs in this system likely derive from a single rather than a double HJ intermediate. Although there appears to be a consistent role of Rad1-Rad10 in promoting COs in plasmid-based DSB/gap repair assays (Schiestl and Prakash 1988; Bartsch et al. 2000), it should be noted that its effect during the repair of an HO-induced chromosomal DSB has been variable (Ira et al. 2003; Nicholson et al. 2006). This variability could be related to the lengths of the homology that flank the DSB, which would limit the extent and hence stability of heteroduplex intermediates. Finally, the decrease in the total efficiency of gap repair in msh3 or rad1 mutants suggests either that the Rad1-Rad10 complex plays a role in the alternative SDSA pathway as well or that some D-loop intermediates are dead-end products. A possible role of Rad1-Rad10 in SDSA might be in the removal of 3′ ends that have replicated past the region of plasmid-chromosome homology, an activity that has also been attributed to the Mus81-Mms4 complex (De Los Santos et al. 2001; Fabre et al. 2002).
In spontaneous recombination assays, elimination of either Sgs1 or Srs2 results in a hyper-rec phenotype. Sgs1 is thought to reduce the accumulation of recombination-initiating lesions (Fabre et al. 2002) while Srs2 is thought to antagonize the formation of Rad51 nucleoprotein filaments (Krejci et al. 2003; Veaute et al. 2003). If the primary inhibitory role of Srs2 derives from its ability to strip Rad51 from nucleoprotein filaments before the initial strand invasion occurs, then it is not clear why this negative role should be limited only to spontaneously initiated events. An interesting possibility is that Srs2 efficiently disrupts filaments formed within single-stranded gaps, which may initiate most spontaneous recombination (Lettier et al. 2006), but is relatively inefficient at removing Rad51 from the free 3′ tails formed at the ends of DSBs. This would be consistent with Srs2 “channeling” damage-containing gaps into a postreplication repair pathway (error-prone translesion DNA synthesis or error-free template switching) rather than into the Rad51-dependent homologous recombination pathway (reviewed by Wu and Hickson 2006). In the case of a DSB, neither template switching nor gap filling by the translesion synthesis pathway would be a viable repair option.
The provision of initiating DSBs has revealed additional, recombination-promoting roles of Sgs1 and Srs2, with the corresponding mutant strains exhibiting reduced repair of chromosomal DSBs (Aylon et al. 2003; Ira et al. 2003). Consistent with these results, we observed 50 or 25% reductions in total gap-repair efficiency in an srs2 or sgs1 background, respectively. In the case of the srs2 mutant, the reduction in total gap repair may reflect the requirement of Srs2 for efficient recovery from checkpoint-mediated cell-cycle arrest following successful DSB repair (Vaze et al. 2002). A final phenotype of sgs1 or srs2 mutants is an increased mitotic CO/NCO ratio for both spontaneous (Robert et al. 2006) and HO-initiated events (Ira et al. 2003; Lo et al. 2006). A similar effect was evident in our gap-repair system, where the CO/NCO ratio was 1.1, 6.7, and 2.0 in WT, sgs1, and srs2 strains, respectively (Table 1). It has been suggested that the elevated CO/NCO ratio in srs2 mutants reflects a less efficient dismantling of D-loops (Dupaigne et al. 2008) and hence loss of the NCO-specific SDSA pathway (Figure 5; Ira et al. 2003). The reduction in total gap repair in an srs2 mutant further suggests that the SDSA pathway is not completely interchangeable with the HJ pathways; that is, not all of the persistent D-loops necessarily lead to the formation of HJs. Finally, the occurrence of more COs than NCOs in the srs2 mutant, where the non-SDSA pathways dominate, implies that the cleavage of HJs does not occur randomly, but rather in a manner that favors COs.
As an explanation for the elevated CO/NCO ratio in sgs1 mutants, it has been suggested that Sgs1 promotes a topoisomerase-mediated mode of dHJ resolution that yields only NCOs (Wu and Hickson 2003); in its absence the only option would be the enzymatic cleavage of both HJs (see Ira et al. 2003). Because COs in the current gap-repair assay are strongly Rad1 dependent, we assume that most are generated through a single HJ intermediate. The loss of 80% of the NCO events in the sgs1 mutant implies that Sgs1 might also dismantle single HJs and that, at least in our system, the major route of generating NCOs may be via an HJ-containing pathway rather than the SDSA pathway. Furthermore, the very high CO/NCO ratio in the sgs1 mutant again indicates that HJ cleavage in this system generates predominantly CO products. Although there may be subtle differences, the roles of Srs2 and Sgs1 in our gap-repair assay are generally consistent with those inferred previously (Ira et al. 2003), providing additional support that transformation-associated gap repair accurately mimics the repair of chromosomal DSBs in yeast.
Sequence divergence differentially affects CO and NCO events:
One of the most striking findings in the current study is that sequence divergence impedes CO events to a much greater extent than NCO events. In the gap-repair system used here, 2% sequence divergence reduced CO events approximately sevenfold, but NCOs less than twofold. This difference is easiest to reconcile if sequence divergence exerts its primary effect subsequent to the initial strand invasion step that is common to all pathways in Figure 5; otherwise, one would expect CO and NCO events to be similarly affected. It is possible, for example, that mismatches are detected and trigger antirecombination only after extension of the invading 3′ end has been initiated. As long as the end extends far enough to pair with the single-stranded tail on the other side of the DSB/gap (a 60-bp gap in the assay used here), SDSA would not be affected. An alternative explanation is that HJ formation (i.e., second-end capture) requires more extensive heteroduplex formation than does SDSA, in which case CO intermediates would contain more of the mismatches that inhibit recombination. Finally, potential mismatches may not differentially affect the SDSA and HJ pathways, but rather may alter the mode of HJ resolution, specifically biasing the outcome toward more NCO events. The presence of mismatches could either favor the noncrossover mode of HJ cleavage or could promote the dissolution of HJs by Sgs1. In terms of biological significance, the differential control of NCO and CO events would have the net effect of allowing a nonidentical sequence to be used as a repair template for a broken chromosome, while at the same time limiting potentially deleterious rearrangements due to interactions between dispersed repeats.
The differential effect of sequence divergence on CO and NCO events has not been previously reported, and there are several reasons why an effect may not have been evident. First, some assays are capable of detecting only NCO or only CO events (e.g., Nicholson et al. 2006), which obviously precludes the detection of differential effects. Second, there may be inherent differences in the mismatch sensitivity of spontaneously initiated vs. DSB-induced recombination (see below). Third, it is possible that a differential effect is evident only when heteroduplex extension is limited by the lengths of the interacting sequences, as in the gap-repair assay used here, or when there is some critical level of divergence between the interacting sequences. Finally, the detection of a differential effect requires that one be able to directly compare homologous and homeologous recombination. In virtually all other assays, the homologous control substrates contain one or more potential mismatches. Exceptions include our previous studies using IR substrates, where CO and NCO cannot be distinguished (Chen and Jinks-Robertson 1998) and the HO-initiated system of Nicholson et al. (2006), which only detects COs.
In our earlier studies using 350-bp chromosomal IR substrates, recombination was exquisitely sensitive to potential mismatches, with a level of sequence divergence comparable to that used here (2%) reducing recombination ∼50-fold (Datta et al. 1997). We suggest that this reflects either a basic difference in plasmid–chromosome vs. chromosome–chromosome recombination (e.g., recombination may occur before the plasmid DNA becomes organized into chromatin), a difference in the initiating event (single-strand gap vs. DSB) or a cell cycle-related timing issue (Nicholson et al. 2006).
The MMR system regulates recombination fidelity during gap repair:
In the current gap-repair assay, the CO events were not inhibited by sequence homeology in msh6 or mlh1 mutants. These data indicate that, as in other types of recombination assays (Selva et al. 1995; Datta et al. 1997; Sugawara et al. 2004), the regulation of recombination fidelity derives primarily from activity of the MMR system. In contrast to assays used previously, however, where antirecombination was only partially dependent on MutLα (Chen and Jinks-Robertson 1999; Nicholson et al. 2000; Spell and Jinks-Robertson 2003; Sugawara et al. 2004), the contributions of MutS and MutL homologs appear to be equivalent in the gap-repair system. The variable requirement for MutLα during mismatch-triggered antirecombination could reflect basic differences in the underlying recombination process and/or antirecombination mechanism. There are, in principle, several distinct steps at which the MMR system could exert antirecombination activity (reviewed by Surtees et al. 2004). Mismatch detection could block or retard strand exchange, it could interfere with branch migration that extends heteroduplex DNA, or it could decrease the stability of recombination intermediates (e.g., trigger reverse branch migration).
The roles of the Sgs1 and Srs2 helicases in the fidelity of gap repair:
In vitro data suggest that the primary recombination-related role of the Srs2 helicase is to dismantle Rad51 nucleoprotein filaments (Krejci et al. 2003; Veaute et al. 2003) or D-loops (Dupaigne et al. 2008), while that of the Sgs1 is in the dissolution of Holliday junctions (see Ira et al. 2003). An in vivo relevance of the Sgs1-mediated branch migration has been recently questioned, however, as the helicase activity of Sgs1 does not appear to be required for the mitotic CO–NCO decision in yeast (Lo et al. 2006). As in previous studies using either IR substrates (Myung et al. 2001; Spell and Jinks-Robertson 2004) or an HO-initiated SSA assay (Sugawara et al. 2004), we observed a role for Sgs1 in limiting gap repair between diverged substrates. As in the IR assay, the Sgs1 requirement during gap repair was only partial. Although the CO-specific effect of sequence divergence in the gap-repair assay would be consistent with the HJs being the primary target for Sgs1-mediated antirecombination, HJs are not an intermediate in SSA, where the antirecombination activities of MMR proteins and Sgs1 appear to be equivalent (Sugawara et al. 2004). One possibility is that mismatch-containing annealed strands are the relevant target for Sgs1; strand annealing not only generates the key intermediate in the SSA pathway, but also is relevant to second-end interactions during both SDSA and HJ formation. The requirement for the helicase activity of Sgs1 in the regulation of recombination fidelity (Spell and Jinks-Robertson 2004), but not necessarily in HJ resolution (Lo et al. 2006), also would be consistent with a non-HJ intermediate being the primary target of the mismatch-related Sgs1 antirecombination activity.
Unexpectedly, the HeGR/HGR ratio for CO events was elevated in an srs2 mutant, suggesting that there may be an MMR-related role for Srs2 in modulating the fidelity of recombination in the gap-repair system. This is in contrast to the IR and SSA systems, where elimination of Srs2 did not differentially affect homologous and homeologous recombination (Spell and Jinks-Robertson 2004; Sugawara et al. 2004). While the mismatch-related antirecombination activity of Sgs1 could result from interaction with Msh6 and/or Mlh1 (Pedrazzi et al. 2001, 2003), no interactions of Srs2 with MMR proteins have been reported. One possibility is that the presence of MMR proteins retards the formation and/or extension of the initial Rad51-dependent strand-invasion intermediate (Worth et al. 1994), thereby making it a more efficient target for dismantling by Srs2.
Advantages and limitations of a gap-repair assay:
Results obtained using gap-repair assays indicate that these assays faithfully recapitulate the repair of HO-induced chromosomal DSBs. A basic question that has yet to be fully resolved, however, is whether spontaneous mitotic recombination typically initiates with a DSB or with a single-strand nick/gap, although recent data support the latter (Lettier et al. 2006). The distinct fidelity differences observed with our IR system, where most events appear to involve sister chromatids (Chen and Jinks-Robertson 1998), vs. the gap-repair assay would be consistent with spontaneous recombination being primarily a gap-filling process. Regardless of the lesion that normally initiates mitotic recombination, however, it is clear that meiotic recombination initiates with Spo11-generated DSBs (Paques and Haber 1999). It is possible that mitotic DSB/gap repair systems more accurately reflect meiotic than mitotic mechanisms of recombination.
The experiments reported here have demonstrated that sequence divergence inhibits mitotic COs to a greater extent than NCOs. Examination of the associated gene conversion tracts may reveal why the former are more sensitive to potential mismatches. In addition, the sequencing of recombinants derived from the transformation of MMR-defective cells, in which heteroduplex intermediates persist, may provide an unprecedented view of DSB-repair intermediates and how specific proteins alter recombination mechanisms. Specifically, each of the pathways depicted in Figure 5 predicts different positions of heteroduplex DNA (hDNA). With SDSA, hDNA should only be present on the plasmid and only on one side of the original gap; with HJ cleavage, there should be hDNA on both sides of the gap, with a single hDNA tract associated with each his3 allele; and with Sgs1-mediated HJ dissolution, there again should be hDNA on both sides of the gap, but all hDNA should be associated with the plasmid-encoded HIS3 allele. Finally, the production of a gapped plasmid by PCR will allow the position and/or size of the initiating gap to be systematically varied by simply changing the positions of the PCR primers. The position of the gap within the region of homology and/or its size could affect the overall repair efficiency, the CO–NCO decision, or MMR-directed antirecombination.
Acknowledgments
We are grateful to Tom Petes, Lucas Argueso, and members of the Jinks-Robertson lab for helpful discussions and comments on the manuscript. This work was supported by grant GM-038464 from the National Institutes of Health.
This article is dedicated to the memory of Caroline Welz-Voegele, who died on September 12, 2007. During her 10 years with the Jinks-Robertson group, Caroline made numerous experimental and intellectual contributions, she was generous with her time and knowledge, and she served as a mentor and role model for all who passed through the lab. Caroline is greatly missed and fondly remembered by her friends and colleagues at Emory University and Duke University.
References
- Ahn, B.-Y., K. J. Dornfeld, T. J. Fagrelius and D. M. Livingston, 1988. Effect of limited homology on gene conversion in a Saccharomyces cerevisiae plasmid recombination system. Mol. Cell. Biol. 8 2442–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers, T., and M. Lichten, 2001. Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106 47–57. [DOI] [PubMed] [Google Scholar]
- Aylon, Y., B. Liefshitz, G. Bitan-Banin and M. Kupiec, 2003. Molecular dissection of mitotic recombination in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 23 1403–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch, S., L. E. Kang and L. S. Symington, 2000. RAD51 is required for the repair of plasmid double-stranded DNA gaps from either plasmid or chromosomal templates. Mol. Cell. Biol. 20 1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W., and S. Jinks-Robertson, 1998. Mismatch repair proteins regulate heteroduplex formation during mitotic recombination in yeast. Mol. Cell. Biol. 18 6525–6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W., and S. Jinks-Robertson, 1999. The role of the mismatch repair machinery in regulating mitotic and meiotic recombination between diverged sequences in yeast. Genetics 151 1299–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta, A., A. Adjiri, L. New, G. F. Crouse and S. Jinks-Robertson, 1996. Mitotic crossovers between diverged sequences are regulated by mismatch repair proteins in Saccharomyces cerevisiae. Mol. Cell. Biol. 16 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta, A., M. Hendrix, M. Lipsitch and S. Jinks-Robertson, 1997. Dual roles for DNA sequence identity and the mismatch repair system in the regulation of mitotic crossing-over in yeast. Proc. Natl. Acad. Sci. USA 94 9757–9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Santos, T., J. Loidl, B. Larkin and N. M. Hollingsworth, 2001. A role for MMS4 in the processing of recombination intermediates during meiosis in Saccharomyces cerevisiae. Genetics 159 1511–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond, J. T., J. Genschel, E. Wolf and P. Modrich, 1997. DHFR/MSH3 amplification in methotrexate-resistant cells alters the hMutSα/hMutSβ ratio and reduces the efficiency of base-base mismatch repair. Proc. Natl. Acad. Sci. USA 94 10144–10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupaigne, P., C. Le Breton, F. Fabre, S. Gangloff, E. Le Cam et al., 2008. The Srs2 helicase activity is stimulated by Rad51 filaments on dsDNA: implications for crossover incidence during mitotic recombination. Mol. Cell 29 243–254. [DOI] [PubMed] [Google Scholar]
- Earley, M. C., and G. F. Crouse, 1998. The role of mismatch repair in the prevention of base pair mutations in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 95 15487–15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre, F., A. Chan, W. D. Heyer and S. Gangloff, 2002. Alternate pathways involving Sgs1/Top3, Mus81/ Mms4, and Srs2 prevent formation of toxic recombination intermediates from single-stranded gaps created by DNA replication. Proc. Natl. Acad. Sci. USA 99 16887–16892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman-Lobell, J., and J. E. Haber, 1992. Removal of nonhomologous DNA ends in double-strand break recombination: the role of the yeast ultraviolet repair gene RAD1. Science 258 480–484. [DOI] [PubMed] [Google Scholar]
- Gietz, R. D., and R. A. Woods, 2002. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 350 87–96. [DOI] [PubMed] [Google Scholar]
- Haghnazari, E., and W. D. Heyer, 2004. The DNA damage checkpoint pathways exert multiple controls on the efficiency and outcome of the repair of a double-stranded DNA gap. Nucleic Acids Res. 32 4257–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe, B. D., and S. Jinks-Robertson, 2000. DNA mismatch repair and genetic instability. Annu. Rev. Genet. 34 359–399. [DOI] [PubMed] [Google Scholar]
- Hayden, M. S., and B. Byers, 1992. Minimal extent of homology required for completion of meiotic recombination in Saccharomyces cerevisiae. Dev. Genet. 13 498–514. [DOI] [PubMed] [Google Scholar]
- Higgins, D., S. Prakash, P. Reynolds, R. Polakowska, S. Weber et al., 1983. Isolation and characterization of the RAD3 gene of Saccharomyces cerevisiae and inviability of rad3 deletion mutants. Proc. Natl. Acad. Sci. USA 80 5680–5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar, O., B. Liefshitz, G. Bitan and M. Kupiec, 2000. The relationship between homology length and crossing over during the repair of a broken chromosome. J. Biol. Chem. 275 30833–30838. [DOI] [PubMed] [Google Scholar]
- Ira, G., A. Malkova, G. Liberi, M. Foiani and J. E. Haber, 2003. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell 115 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks-Robertson, S., M. Michelitch and S. Ramcharan, 1993. Substrate length requirements for efficient mitotic recombination in Saccharomyces cerevisiae. Mol. Cell. Biol. 13 3937–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, R. E., G. K. Kovvali, S. N. Guzder, N. S. Amin, C. Holm et al., 1996. Evidence for involvement of yeast proliferating cell nuclear antigen in DNA mismatch repair. J. Biol. Chem. 271 27987–27990. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick, D. T., and T. D. Petes, 1997. Repair of DNA loops involves DNA-mismatch and nucleotide-excision repair proteins. Nature 387 929–931. [DOI] [PubMed] [Google Scholar]
- Kramer, W., B. Fartmann and E. C. Ringbeck, 1996. Transcription of mutS and mutL-homologous genes in Saccharomyces cerevisiae during the cell cycle. Mol. Gen. Genet. 252 275–283. [DOI] [PubMed] [Google Scholar]
- Kramer, W., B. Kramer, M. S. Williamson and S. Fogel, 1989. Cloning and nucleotide sequence of DNA mismatch repair gene PMS1 from Saccharomyces cerevisiae: homology of PMS1 to procaryotic MutL and HexB. J. Bacteriol. 171 5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejci, L., S. Van Komen, Y. Li, J. Villemain, M. S. Reddy et al., 2003. DNA helicase Srs2 disrupts the Rad51 presynaptic filament. Nature 423 305–309. [DOI] [PubMed] [Google Scholar]
- Krogh, B. O., and L. S. Symington, 2004. Recombination proteins in yeast. Annu. Rev. Genet. 38 233–271. [DOI] [PubMed] [Google Scholar]
- Kunkel, T. A., and D. A. Erie, 2005. DNA mismatch repair. Annu. Rev. Biochem. 74 681–710. [DOI] [PubMed] [Google Scholar]
- Langston, L. D., and L. S. Symington, 2005. Opposing roles for DNA structure-specific proteins Rad1, Msh2, Msh3, and Sgs1 in yeast gene targeting. EMBO J. 24 2214–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettier, G., Q. Feng, A. A. de Mayolo, N. Erdeniz, R. J. Reid et al., 2006. The role of DNA double-strand breaks in spontaneous homologous recombination in S. cerevisiae. PLoS Genet. 2 1773–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo, Y. C., K. S. Paffett, O. Amit, J. A. Clikeman, R. Sterk et al., 2006. Sgs1 regulates gene conversion tract lengths and crossovers independently of its helicase activity. Mol. Cell. Biol. 26 4086–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung, K., A. Datta, C. Chen and R. D. Kolodner, 2001. SGS1, the Saccharomyces cerevisiae homologue of BLM and WRN, suppresses genome instability and homeologous recombination. Nat. Genet. 27 1–4. [DOI] [PubMed] [Google Scholar]
- Nicholson, A., R. M. Fabbri, J. W. Reeves and G. F. Crouse, 2006. The effects of mismatch repair and RAD1 genes on interchromosomal crossover recombination in Saccharomyces cerevisiae. Genetics 173 647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, A., M. Hendrix, S. Jinks-Robertson and G. F. Crouse, 2000. Regulation of mitotic homeologous recombination in yeast: functions of mismatch repair and nucleotide excision repair genes. Genetics 154 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver, T. L., and J. W. Szostak, 1983. Yeast recombination: the association between double-strand gap repair and crossing-over. Proc. Natl. Acad. Sci. USA 80 4417–4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paques, F., and J. E. Haber, 1999. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63 349–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzi, G., C. Perrera, H. Blaser, P. Kuster, G. Marra et al., 2001. Direct association of Bloom's syndrome gene product with the human mismatch repair protein MLH1. Nucleic Acids Res. 29 4378–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzi, G., C. Z. Bachrati, N. Selak, I. Studer, M. Petkovic et al., 2003. The Bloom's syndrome helicase interacts directly with the human DNA mismatch repair protein hMSH6. Biol. Chem. 384 1155–1164. [DOI] [PubMed] [Google Scholar]
- Prakash, S., and L. Prakash, 2000. Nucleotide excision repair in yeast. Mutat. Res. 451 13–24. [DOI] [PubMed] [Google Scholar]
- Robert, T., D. Dervins, F. Fabre and S. Gangloff, 2006. Mrc1 and Srs2 are major actors in the regulation of spontaneous crossover. EMBO J. 25 2837–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saparbaev, M., L. Prakash and S. Prakash, 1996. Requirement of mismatch repair genes MSH2 and MSH3 in RAD1–RAD10 pathway of mitotic recombination in Saccharomyces cerevisiae. Genetics 142 727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl, R. H., and S. Prakash, 1988. RAD1, an excision repair gene of Saccharomyces cerevisiae, is also involved in recombination. Mol. Cell. Biol. 8 3619–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selva, E. M., L. New, G. F. Crouse and R. S. Lahue, 1995. Mismatch correction acts as a barrier to homeologous recombination in Saccharomyces cerevisiae. Genetics 139 1175–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, F., 1991. Getting started with yeast. Meth. Enzymol. 194 3–20. [DOI] [PubMed] [Google Scholar]
- Sikorski, R. S., and P. Hieter, 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spell, R. M., and S. Jinks-Robertson, 2003. Role of mismatch repair in the fidelity of RAD51- and RAD59-dependent recombination in Saccharomyces cerevisiae. Genetics 165 1733–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spell, R. M., and S. Jinks-Robertson, 2004. Examination of the roles of the Sgs1 and Srs2 helicases in the enforcement of recombination fidelity in Saccharomyces cerevisiae. Genetics 168 1855–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, J., R. Gealy, T. Petes and S. Jinks-Robertson, 2008. Role of PCNA interactions in the mismatch repair-dependent processing of mitotic and meiotic recombination intermediates in yeast. Genetics 178 1221–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara, N., T. Goldfarb, B. Studamire, E. Alani and J. E. Haber, 2004. Heteroduplex rejection during single-strand annealing requires Sgs1 helicase and mismatch repair proteins Msh2 and Msh6 but not Pms1. Proc. Natl. Acad. Sci. USA 101 9315–9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees, J. A., and E. Alani, 2006. Mismatch repair factor MSH2–MSH3 binds and alters the conformation of branched DNA structures predicted to form during genetic recombination. J. Mol. Biol. 360 523–536. [DOI] [PubMed] [Google Scholar]
- Surtees, J. A., J. L. Argueso and E. Alani, 2004. Mismatch repair proteins: key regulators of genetic recombination. Cytogenet. Genome Res. 107 146–159. [DOI] [PubMed] [Google Scholar]
- Symington, L. S., L. E. Kang and S. Moreau, 2000. Alteration of gene conversion tract length and associated crossing over during plasmid gap repair in nuclease-deficient strains of Saccharomyces cerevisiae. Nucleic Acids Res. 28 4649–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak, J. W., T. L. Orr-Weaver, R. J. Rothstein and F. W. Stahl, 1983. The double-strand-break repair model for recombination. Cell 33 25–35. [DOI] [PubMed] [Google Scholar]
- Tishkoff, D. X., A. L. Boerger, P. Bertrand, N. Filosi, G. M. Gaida et al., 1997. Identification and characterization of Saccharomyces cerevisiae EXO1, a gene encoding an exonuclease that interacts with MSH2. Proc. Natl. Acad. Sci. USA 94 7487–7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umar, A., A. B. Buermeyer, J. A. Simon, D. C. Thomas, A. B. Clark et al., 1996. Requirement for PCNA in DNA mismatch repair at a step preceding DNA resynthesis. Cell 87 65–73. [DOI] [PubMed] [Google Scholar]
- Vaze, M. B., A. Pellicioli, S. E. Lee, G. Ira, G. Liberi et al., 2002. Recovery from checkpoint-mediated arrest after repair of a double-strand break requires Srs2 helicase. Mol. Cell 10 373–385. [DOI] [PubMed] [Google Scholar]
- Veaute, X., J. Jeusset, C. Soustelle, S. C. Kowalczykowski, E. Le Cam et al., 2003. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature 423 309–312. [DOI] [PubMed] [Google Scholar]
- Wach, A., A. Brachat, R. Pohlmann and P. Philippsen, 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10 1793–1808. [DOI] [PubMed] [Google Scholar]
- Wang, T.-F., N. Kleckner and N. Hunter, 1999. Functional specificity of MutL homologs in yeast: evidence for three Mlh1-based heterocomplexes with distinct roles during meiosis in recombination and mismatch correction. Proc. Natl. Acad. Sci. USA 96 13914–13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worth, L. J., S. Clark, M. Radman and P. Modrich, 1994. Mismatch repair proteins MutS and MutL inhibit RecA-catalyzed strand transfer between diverged DNAs. Proc. Natl. Acad. Sci. USA 91 3238–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L., and I. D. Hickson, 2003. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature 426 870–874. [DOI] [PubMed] [Google Scholar]
- Wu, L., and I. D. Hickson, 2006. DNA helicases required for homologous recombination and repair of damaged replication forks. Annu. Rev. Genet. 40 279–306. [DOI] [PubMed] [Google Scholar]