Abstract
Ndr kinases, such as Neurospora crassa COT1, are important for cell differentiation and polar morphogenesis, yet their input signals as well as their integration into a cellular signaling context are still elusive. Here, we identify the cot-1 suppressor gul-4 as mak-2 and show that mutants of the gul-4/mak-2 mitogen-activated protein (MAP) kinase pathway suppress cot-1 phenotypes along with a concomitant reduction in protein kinase A (PKA) activity. Furthermore, mak-2 pathway defects are partially overcome in a cot-1 background and are associated with increased MAK1 MAPK signaling. A comparative characterization of N. crassa MAPKs revealed that they act as three distinct modules during vegetative growth and asexual development. In addition, common functions of MAK1 and MAK2 signaling during maintenance of cell-wall integrity distinguished the two ERK-type pathways from the p38-type OS2 osmosensing pathway. In contrast to separate functions during vegetative growth, the concerted activity of the three MAPK pathways is essential for cell fusion and for the subsequent formation of multicellular structures that are required for sexual development. Taken together, our data indicate a functional link between COT1 and MAPK signaling in regulating filamentous growth, hyphal fusion, and sexual development.
APICAL tip extension is the hallmark of filamentous fungi, and fungal hyphae share, along with neurons and pollen tubes, the distinction of being among the most highly polarized cells found (Palanivelu and Preuss 2000; Borkovich et al. 2004; Harris 2006). Polarized growth is a complex multifactorial property, which is coordinated by numerous signals. These pathways, such as the cAMP-dependent protein kinase A (PKA), the mitogen-activated protein kinase (MAPK), or the nuclear Dbf2-related (Ndr) kinase pathways, are highly conserved and regulate numerous aspects of growth and development, including cell proliferation, differentiation, motility, and survival, among many others (Lewis et al. 1998; Lengeler et al. 2000; Hergovich et al. 2006). In fungal systems, they are important for maintaining polarity, pathogenicity, and development (Xu 2000; D'Souza and Heitman 2001; Monge et al. 2006; Xu et al. 2007).
MAPKs are modular signaling units composed of three-tiered kinase cascades, in which a series of three protein kinases phosphorylate and activate one another (Qi and Elion 2005). Frequently, a fourth kinase of the Ste20/PAK group acts upstream of the MAPK-signaling pathways [therefore also called MAPKKKK (Dan et al. 2001)]. Numerous reports have revealed that distinct MAPK pathways are tightly regulated by cross-communication with each other and other signaling pathways (summarized in Lengeler et al. 2000; Stork and Schmitt 2002). Both the functional modules of each MAPK pathway and the interplay between the different signaling routes are best understood in the unicellular ascomycete Saccharomyces cerevisiae and summarized in several recent reviews (Madhani and Fink 1998; Lengeler et al. 2000; Pan et al. 2000; Bahn et al. 2007). In the budding yeast, the MAPKs constitute five partially overlapping pathways regulating mating, filamentation, cell integrity, response to high osmolarity, and ascospore formation.
In filamentous fungi that undergo highly complex and multicellular developmental phases [e.g., Neurospora crassa has been shown to differentiate into at least 28 different cell types (Bistis et al. 2003)], the situation is much less clear. Three basic MAPK modules have been identified, but, so far, only the kinase cascade homologous to the S. cerevisiae osmosensing/stress pathway has been fully characterized in the filamentous ascomycetes N. crassa and Aspergillus nidulans (Zhang et al. 2002; Fujimura et al. 2003; Jones et al. 2007; Noguchi et al. 2007).
Osmostress signaling in N. crassa is transduced through the OS1 histidine kinase to the OS4, OS5, and OS2 MAPK cascade. os mutants are unable to grow on high-osmolarity media and are resistant to phenylpyrrole fungicides. Furthermore, lysis and increased pigmentation of asexually derived spores (macroconidia) and female sterility due to the lack of protoperithecia has been reported, yet the cellular or developmental defects involved have not been analyzed in depth (Zhang et al. 2002; Fujimura et al. 2003; Jones et al. 2007; Noguchi et al. 2007). A. nidulans HOG pathway mutants are similarly growth inhibited under high-osmolarity conditions and are sensitive to oxidative stress (Kawasaki et al. 2002; Furukawa et al. 2005). In contrast to the yeast HOG pathway, which depends on two upstream osmosensing branches [the Sln1p transmembrane hybrid-type histidine kinase and a putative seven-transmembrane osmosensor kinase (Maeda et al. 1995; Posas and Saito 1998)], activation of this pathway in A. nidulans and N. crassa depends solely on the two-component signaling system (Furukawa et al. 2005; Noguchi et al. 2007).
Several MAPK components homologous to the yeast pheromone/filamentation pathway have been found in N. crassa. The MAPKKK NRC1 was first identified as a repressor of the conidiation program, but was later shown to be also involved in hyphal fusion and in the activation of the MAPK MAK2 (Kothe and Free 1998; Pandey et al. 2004; Li et al. 2005). Mutants in mak-2 and pp-1 (the downstream transcription factor homologous to yeast Ste12p that is activated by the MAPK Fus3p/Kss1p) display reduced growth rates, the inability to undergo hyphal fusion, shortened aerial hyphae formation, and derepressed conidiation. Furthermore, they fail to develop protoperithecia, and ascospores carrying null mutations of either gene are autonomous lethal (Pandey et al. 2004; Li et al. 2005). A similar pleiotrophic phenotype has been observed in SteC MAPKKK mutants in A. nidulans, which result in slower growth rates, more branched hyphae, altered conidiophore morphology, inhibition of heterokaryon formation, and inhibited sexual development (Wei et al. 2003). Additional homologs of budding yeast Fus3p/Kss1p have been characterized in several pathogenic fungi and have been shown to play key roles in appressorium formation and host colonization (Xu 2000).
Even though mutants in the MAPK homologous to yeast Slt2 have been generated in A. nidulans, and in several phytopathogenic fungi (Xu et al. 1998; Bussink and Osmani 1999; Hou et al. 2002; Kojima et al. 2002; Mey et al. 2002), information concerning this third MAPK pathway in filamentous fungi is still limited. Common phenotypes of Slt2-like kinase mutants included altered cell walls and defects in conidial germination (which could be remedied by high-osmolarity media) and autolysis in central areas of the colony, suggesting the involvement of a cell-integrity-type MAPK pathway in filamentous fungi. Furthermore, the Fusarium graminearum Slt2 homolog MGV1 is required for female fertility, heterokaryon formation, and plant infection (Hou et al. 2002).
The functional analysis of Ndr kinases has gained much interest in recent years. They are important for normal cell differentiation and polar morphogenesis in various organisms, yet their specific functions are still elusive (Yarden et al. 1992; Geng et al. 2000; Racki et al. 2000; Zallen et al. 2000; summarized in Hergovich et al. 2006). An interesting connection between Ste20/PAK (= MAPKKKK) and Ndr kinase signaling was provided through the analysis of the Schizosaccharomyces pombe Ndr kinase mutant orb-6 (Verde et al. 1998). orb-6 and pak-1 share similar phenotypes, double mutants are synthetically lethal, and the overexpression of ORB6 in pak-1 partially suppressed the pak-1 defect, suggesting that PAK1 acts upstream of ORB6. Furthermore, members of the MST2 and MST3 groups of Ste20 kinases have recently been described as upstream regulators of Ndr kinases (Nelson et al. 2003; Kanai et al. 2005; Stegert et al. 2005; Emoto et al. 2006; Praskova et al. 2008).
The MST3 and Ndr kinases POD6 and COT1 of N. crassa are essential for hyphal tip extension and coordinated branch formation. Both kinases have been shown to interact, and they share common suppressors and are localized in a kinesin/dynein-dependent manner (Seiler et al. 2006). We have provided evidence indicating that COT1/POD6 and PKA act in parallel pathways that regulate polarity formation in a positive or negative manner, respectively, in N. crassa (Seiler et al. 2006). However, the input and outcome components of the Ndr kinase network as well as its integration into a cellular signaling context have not been described in any system. This information is critical for elucidation of the mechanistic involvement of Ndr kinases in cell growth and polarity.
The described differences between the MAPK pathways in various filamentous fungi and yeasts highlight the need for a comparative analysis of MAPK modules during vegetative growth and the multiple developmental decisions made in a filamentous fungus. Here, we describe three MAPK cascades, which function as distinct modules during vegetative growth of N. crassa, but whose joint activity is necessary for hyphal fusion and the development of complex multicellular sexual structures. Furthermore, we provide evidence for cross talk between COT1 and the MAK1 and MAK2 pathways.
MATERIALS AND METHODS
Strains, media, and growth conditions:
General genetic procedures and media used in the handling of N. crassa have been described (Davis and Deserres 1970) or are available through the Fungal Genetic Stock Center (http://www.fgsc.net), with the exception of genetic crosses, which were performed on 2% cornmeal agar (Sigma) supplemented with 0.1% glucose. This complex, low-nitrogen-containing media increased the success rate of crosses with strains that are difficult or impossible to cross on standard synthetic crossing media such as gul-4 and most MAPK mutants, and the hygR and cot-1 markers segregate perfectly in crosses that produce viable spores. Also, the terminal phenotype of mutants defective in sexual reproduction could be determined in a more reliable manner on this media compared to synthetic crossing media (Muller et al. 1995). Strains were grown in either liquid or solid (supplemented with 1.5% agar) Vogel's minimal media with 2% (w/v) sucrose, unless otherwise stated. When required, 5 μm KT5720, 500 μm Br-cAMP, or 5 mg/ml lysing enzymes, all purchased from Sigma, were added. Gradient plates contained solid Vogel's minimal media with 1% sucrose (w/v) and 1% sorbose (w/v) to restrict the radial growth rate. Inhibitors were added at 50°, the plates slanted during the solidification of the agar and then overlaid with an equal volume of the same medium lacking additives in horizontal position, and incubated for 1 day to allow equal diffusion of the additive. To induce stress-dependent MAPK signaling, H2O2 (7 mm) or NaCl (1 m) were added to liquid cultures of the relevant strains 2 hr prior to harvesting. Stress induction by temperature shift was achieved by germinating the strains for 15 hr on cellophane-covered agar plates, followed by a shift to 37° for 10 hr. For protein extraction, the mycelial sheet was peeled off the cellophane and plunged into liquid nitrogen.
The gul-4/mak-2 complementation construct was generated by amplifying the mak-2 ORF using the primers 2393-Not-5′ (ATC GGC GGC CGC CAT GAG CAG CGC ACA AAG AGG CG) and 52393-Not-3′ (ATC GGC GGC CGC TCA CCT CAT AAT CTC CTG GTA GAT C) designed to introduce NotI restriction sites. The NotI-digested PCR product was cloned into the expression vector pEHN1nat (kindly provided by Stephanie Poeggler), which allowed the expression of mak-2 via A. nidulans gpd promotor and trpC terminator sequences. DNA-mediated transformation of N. crassa protoplasts was carried out as described (Vollmer and Yanofsky 1986). The nourseothricin concentration was adjusted to 30 μg/ml to select for transformants.
Strains used in this study are listed in Table 1 (see also McCluskey 2003). gul-4 was mapped by introducing the auxotrophic markers arg-10, arg-11, and met-7 into the cot-1(ts) background and subsequently crossing the obtained double mutants with gul-4;cot-1(ts). Progeny were plated on Vogel's minimal media containing 0.005% sucrose and 2% sorbose at 25°, overlaid with Vogel's minimal media containing 2% sucrose after 2 days, and incubated for an additional 5–10 days at 37°. The ratio between cot-1 and cot-1;gul-4 progeny was scored by stereomicroscopy and indicated the linkage of gul-4 with the auxotrophic marker.
TABLE 1.
N. crassa strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| Wild type | 74-OR23-1A | FGSC 987 |
| cot-1(ts) | cot-1(C102t) | FGSC 4066 |
| gul-4;pe;fl;cot-1;inl | gul-4 pe fl cot-1 inl | FGSC 1173 |
| cot-1;gul-4 | gul-4 cot-1 | This study |
| Δos-4 (heterokaryon) | hph∷os-4Δ bar∷mus-51 + bar∷mus-51 | FGSC 11479 |
| Δos-4 (microconidia) | hph∷os-4Δ bar∷mus-51 | This study |
| Δnrc-1 (heterokaryon) | hph∷nrc-1Δ bar∷mus-51 + bar∷mus-51 | FGSC 11466 |
| Δnrc-1 | hph∷nrc-1 | This study |
| Δmik-1 | hph∷mik-1Δ | FGSC 11326 |
| Δos-5 (heterokaryon) | hph∷os-5Δ bar∷mus-51 + bar∷mus-51 | FGSC 11480 |
| Δos-5 | hph∷os-5Δ | This study |
| Δmek-2 (heterokaryon) | hph∷mek-2Δ bar∷mus-51 + bar∷mus-51 | FGSC 11481 |
| Δmek-2 (microconidia) | hph∷mek-2Δ bar∷mus-51 | This study |
| Δmek-1 | hph∷mek-1Δ | FGSC 11318 |
| Δos-2 | hph∷os-2Δ | FGSC 11436 |
| Δmak-2 | hph∷mak-2Δ | Li et al. (2005) |
| Δmak-1 | hph∷mak-1Δ | FGSC 11321 |
| Δnrc-1;cot-1(ts) | hph∷nrc-1 cot-1(C102t) | This study |
| Δos-5;cot-1(ts) | hph∷os-5Δ cot-1(C102t) | This study |
| Δmek-1;cot-1(ts) | hph∷mek-1Δ cot-1(C102t) | This study |
| Δos-2;cot-1(ts) | hph∷os-2Δ cot-1(C102t) | This study |
| Δmak-2;cot-1(ts) | hph∷mak-2Δ cot-1(C102t) | This study |
| Δmak-1;cot-1(ts) | hph∷mak-1Δ cot-1(C102t) | This study |
| mcb(14-4) | mcb(14-4) | Seiler and Plamann (2003) |
| Δmak-2;mcb(14-4) | hph∷mak-1Δ mcb(14-4) | This study |
Protein extraction, immunoblotting, and PKA activity measurement:
Western blot analysis was performed as previously described (Gorovits and Yarden 2003). Briefly, N. crassa mycelial samples were frozen in liquid nitrogen, pulverized, and suspended in lysis buffer [1 m sorbitol, 10 mm HEPES (pH 7.5), 5 mm EDTA, 5 mm EGTA, 5 mm NaF, 0.1 m KCl, 0.2% Triton X-100, and complete protease inhibitor mixture (Roche Applied Science)]. The samples were homogenized by 10 strokes of pestle A in a Dounce homogenizer. The homogenates were centrifuged for 40 min at 10,000 × g and the supernatant recovered and stored at −70° until analysis. Proteins were separated by 7.5 or 10% SDS–PAGE and subsequently blotted onto nitrocellulose membranes. Antibodies used throughout this study included anti-COT1 (Gorovits et al. 1999), anti-PhosphoMAPK (Cell Signaling Technology), monoclonal 9E10 anti-cMYC (Santa Cruz), and goat peroxidase-coupled secondary antibody (Amersham Biosciences).
PKA assays were performed as previously described (Ziv et al. 2008) with minor modifications. Specifically, 106 conidia/ml were shaken for 11 hr in prewarmed (36°) Vogel's sucrose minimal medium. The cultures were harvested by centrifugation (10 min, 3000 × g, 4°) and immediately assayed for PKA activity. Differences in kemptide phosphorylation were determined by densitometry and subjected to paired two-sample t-test analyses.
Microscopy:
Samples were viewed with an ORCA ER digital camera (Hamamatsu) mounted on an Axiovert S100 microscope (Zeiss). Image acquisition was done using the Openlab 5.01 software (Improvision) and images were further processed using Photoshop CS2 (Adobe). Low-magnification documentation of fungal hyphae or colonies was performed with an SZX12 stereomicroscope (Olympus) and a PS30 camera (Kappa).
RESULTS
Mutants of the MAK2 MAP kinase pathway suppress cot-1 growth defects:
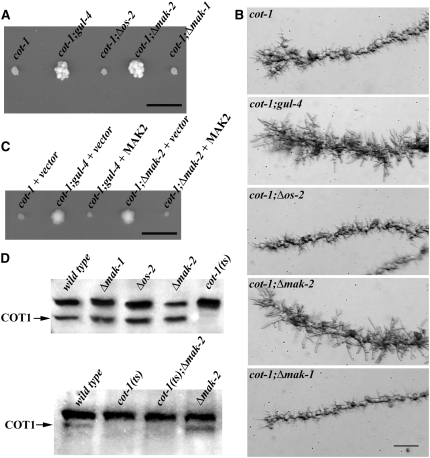
The phenotypic characteristics of the conditional cot-1(ts) mutant, which forms tight colonies with growth-arrested needle-shaped hyphal tips when germinated at restrictive temperature, facilitates the easy identification of cot-1 suppressors. This efficient procedure makes N. crassa ideal for the genetic dissection of Ndr signaling. Several mutants designated “gulliver” that act as modifiers of the compact cot-1(ts) morphology at restrictive temperature have been described (Terenzi and Reissig 1967; Bruno et al. 1996b; Seiler et al. 2006). gul-4 has been mapped to nic-3 (17%) on linkage group VII (Perkins et al. 2001). Using additional auxotropic markers, we determined that gul-4 is closely linked with arg-10, arg-11, and met-7 (<1%, <1%, and <0.1% recombination frequencies, respectively). This information and the available genome sequence identified several candidate genes for gul-4. By sequencing potential ORFs as well as their 5′ and 3′ untranslated regions, we identified a 12-bp insertion (CAA CAA CAA CAA) in the mak-2 promotor at position −270/271 upstream of the start ATG as a potential cause for the suppression of cot-1(ts). To test if gul-4 is allelic to mak-2, we generated a cot-1(ts);Δmak-2 double mutant. When tested at restrictive temperature, the Δmak-2 deletion partially suppressed the cot-1(ts) defect in a manner identical to that observed in the original gul-4 background (Figure 1A; Southern blot analyses confirming the genetic nature of the double mutants generated throughout this report are available as supplemental Figure 1). Microscopic analysis of the hyphal apex revealed that, in contrast to the extension-arrested pointed tips of cot-1(ts) grown at restrictive temperature, the cot-1(ts);gul-4 and cot-1(ts);Δmak-2 strains generated a dome-shaped apex, typical of a normal (although slow) growing tip (Figure 1B). The presence of a tight genetic linkage between mak-2 and gul-4 was made evident by the analysis of crosses between cot-1(ts);gul-4 and cot-1;Δmak-2. Of >2000 progeny screened, no cot-1(ts);gul+ strains were obtained. To confirm that gul-4 is allelic to mak-2, we expressed MAK2 in gul-4 and Δmak-2 and found that it complemented the growth defects of both mutants (data not shown). Furthermore, when we expressed MAK2 in cot-1(ts);gul-4 and cot-1(ts);Δmak-2, the suppression of the cot-1(ts) growth defect was abolished at the restrictive temperature (Figure 1C).
Figure 1.—
gul-4/Δmak-2 strains suppress the cot-1(ts) growth defects. (A) The indicated strains were germinated and grown on minimal media plates for 3 days at 37°. Note the increased colony diameters of cot-1(ts);gul-4 and cot-1(ts);Δmak-2 compared to cot-1(ts). Bar, 1 cm. (B) Results of temperature-shift experiments, in which strains grown at 25° and shifted to 37° for 8 hr illustrate pointed growth-arrested tips of cot-1(ts), cot-1(ts);Δos-2, and cot-1(ts);Δmak-1, while dome-shaped slow-growing apices are visible in cot-1(ts),gul-4 and cot-1(ts);Δmak-2. Bar, 20 μm. (C) The indicated strains were transformed with mak-2 expression vector or the empty vector as control and grown on minimal media plates supplemented with 30 μg/ml nourseothricin for 3 days at 37°. Bar, 1 cm. (D) Western blot analysis of cell extracts probed with anti-COT1 antibodies indicate that deleting any of the three MAPKs does not affect COT1 expression (top) and that the gulliver-like suppression of the cot-1(1) phenotype by Δmak-2 at restrictive temperature is independent of the presence of the COT1 67-kDa band (arrow on bottom).
To determine if the suppression of cot-1(ts) is specific to the MAK2 MAPK pathway, we generated double mutants of cot-1(ts) with loss-of-function mutants in os-2 and mak-1, the other two MAPK genes present in the N. crassa genome (Borkovich et al. 2004). When we introduced the three MAPK mutations into the cot-1(ts) background, only Δmak-2 suppressed the cot-1(ts) growth defects, indicating a specific interaction between COT1 and MAK2 kinase signaling (Figure 1, A and B).
Western analyses were performed to determine if deletion of any one of the three MAPKs affected the pattern of COT1 expression (Figure 1D). The typical 67-kDa COT1 band was clearly evident in protein extracts of all three MAPK mutants. Furthermore, loss of MAK2 function in cot-1(ts);Δmak-2 did not confer quantitative or qualitative alterations in the COT1 protein expression pattern, indicating that the improved growth of cot-1(ts) by deleting mak-2 was not dependent on the presence of COT1. On the basis of these results, we concluded that COT1 and MAK2 act in independent pathways and that the suppression of the cot-1(ts) defect was indirect.
Deletion of mak2 is accompanied by a reduction in PKA activity:
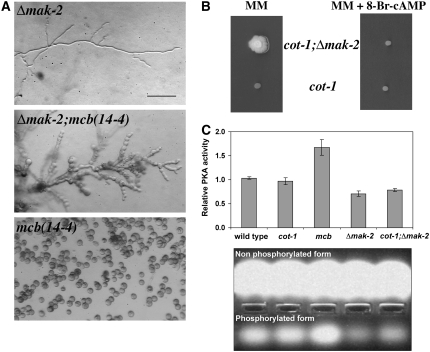
The suppression of cot-1(ts) by Δmak-2 resembled the previously described environmental suppression of cot-1(ts) and pod-6(ts) by external stresses (Gorovits and Yarden 2003; Seiler et al. 2006). As environmental suppression of both kinases was correlated with reduced PKA activity levels, we analyzed PKA activity in the Δmak-2 strain and found several lines of evidence for reduced PKA activity. mcb is a temperature-sensitive mutant defective in the regulatory subunit of PKA, which displays elevated PKA activity levels at restrictive temperature, resulting in apolar growth and irregular chains of spherical cells (Bruno et al. 1996a; Seiler et al. 2006; Ziv et al. 2008). Genetic analysis of a Δmak-2;mcb(14-4) double mutant demonstrated that Δmak-2;mcb(14-4) grew slower than the parental strains at permissive temperature, suggesting a genetic interaction between MAK2 and PKA signaling. Nevertheless, the Δmak-2 background partially suppressed the polarity defect of mcb(14-4) at restrictive temperature, suggesting that PKA activity levels are reduced in Δmak-2 (Figure 2A). To test this hypothesis, we increased the cellular PKA activity in cot-1(ts);Δmak-2 grown at 37° by culturing the strain in the presence of 500 μm 8-Br-cAMP, which mimics increased levels of cAMP, and found that the suppressive effect of Δmak-2 on cot-1(ts) at restrictive temperature was abolished (Figure 2B), while it had only a minor effect on the growth rate of cot-1(ts) or wild type (data not shown). Finally, we directly measured PKA activity in Δmak-2 single and Δmak-2;cot-1(ts) double mutants and found that a significant (P < 0.001; paired two-sample t-test) reduction in PKA activity could be detected in these strains (Figure 2C). Several measurements (with independent cultures) detected a consistent 30–35% decrease in kinase activity in the Δmak-2 and cot-1(ts);Δmak-2 strains in comparison to wild type. An ∼70% increase (P < 0.001) in PKA activity was measured in the mcb(14-4) control, as expected (Ziv et al. 2008). Thus, we suggest that the suppression of cot-1 by the deletion of mak-2 is part of a bypass mechanism, which includes a reduction in PKA activity levels.
Figure 2.—
PKA activity is reduced in Δmak-2. (A) Morphology of Δmak-2; mcb(14-4) and mcb(14-4);Δmak-2 germinated for 12 hr at 37°. Bar, 20 μm. (B) Growth of cot-1(ts) and cot-1(ts);Δmak-2 on minimal media and media supplemented with 500 μm 8-Br-cAMP at restrictive temerature. (C) PKA activity in extracts of germinating conidia of wild type, cot-1(ts), mcb(14-4), Δmak-2, and cot-1(ts);Δmak-2, 11 hr post-inoculation, relative to wild type. Cultures were incubated in prewarmed liquid Vogel's minimal medium at 36° and were assayed for PKA activity. Data presented in the graph are means of at least four independent experiments with two replicates each. Standard errors are shown. (Bottom) A selected experiment demonstrating the nonphosphorylated and phosphorylated (indicating PKA activity) fluorescent Kemptide substrates, migrated to the anode and cathode of the agarose gel, respectively. The PepTag assays utilize fluorescent peptide substrates specific for PKA. Phosphorylation of the substrate by PKA alters the peptide's net charge from +1 to −1, allowing separation of the phosphorylated substrate from the nonphosphorylated on the agarose gel.
The three N. crassa MAP kinases act as three distinct modules during growth and development:
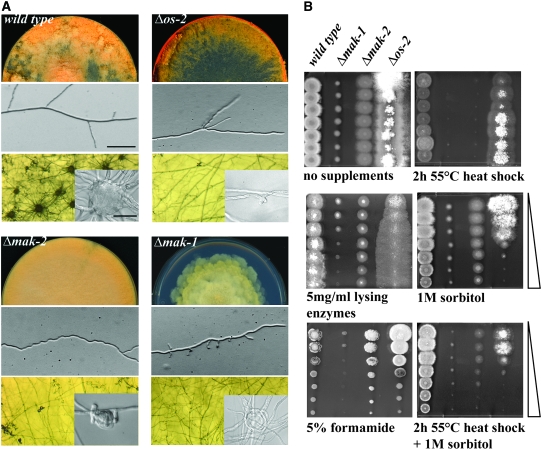
To further dissect the cross-communication between the MAK2 pathway and COT1 signaling, we carried out a comparative characterization of the three N. crassa MAPKs (Figure 3). Δos-2 displayed growth rates that were similar to wild type, but frequent dichotomous branching events suggested minor defects at the hyphal apex. The conidial lysis defect and its sensitivity to sorbitol have already been described (Zhang et al. 2002; Noguchi et al. 2007), and these phenotypes clearly distinguished Δos-2 from Δmak-1 and Δmak-2. Δmak-1 was the most drastically growth-impaired MAPK mutant with tip extension rates of <15% of wild type forming a rosetta-like colony (Figure 3A). The mutant was almost devoid of aerial hyphae and produced few conidia, and the conidial population was highly enriched with arthroconidia (86% compared with 5% in wild type). Abnormal and apolar branching events indicated a major defect during polarity establishment of newly formed branches. Polarity establishment was also affected during germination. Only 15% of Δmak-1 conidia produced germ tubes after 7 hr in liquid minimal medium in contrast to 85% of wild-type conidia. Δmak-1 and Δmak-2 exhibited a cell-wall defect, as protoplast production was approximately four- and twofold, respectively, enhanced in comparison to wild type in the presence of Novozyme. In addition, their growth behavior on plates containing 1% sucrose and 1% sorbose in the presence of a concentration gradient of lysing enzymes indicated that both Δmak-1 and Δmak-2 have altered cell walls, with Δmak-1 being more sensitive than Δmak-2 (Figure 3B; note that the effect of sorbose on tip extension and the cell wall is not compensated by the addition of lysing enzymes in a manner similar to the wild type and Δos-2 strains). In addition to their common cell-wall defect, the two strains displayed additional similarities such as their conidial sensitivity to high temperature, which could be overcome by the addition of 1 m sorbitol prior to the heat shock. In addition, Δmak-1 is sensitive to formamide, a general stress-inducing agent, which is readily taken up by fungi yet is not metabolized (Hampsey 1997). A unique defect of Δmak-2 is its highly irregular zig-zagging growth, which suggested Spitzenkörper positioning defects, but no altered sensitivity to the microtubule inhibitors Benomyl or Nocodazol were observed (data not shown).
Figure 3.—
Comparative characterization of the N. crassa MAP kinase mutants. (A) Colony morphology, asexual development, and hyphal morphology (top and middle, respectively; bar, 20 μm) of the indicated strains grown on minimal media plates. Sexual development (bottom) was induced by growth for 5 days on cornmeal agar. The insets illustrate the terminal morphology of the female reproductive structures (protoperithecia). Bar, 10 μm. (B) Growth of the three MAPK mutants on gradient plates supplemented with the indicated additives. Conidia (5 × 103) were inoculated for each spot. Wedges denote the compound gradient. To restrict the radial growth rates of the strains, all plates were supplemented with 1% sorbose in addition to the indicated additives.
Δmak-2 has been described as female sterile (Pandey et al. 2004; Li et al. 2005), but the exact developmental block in sexual development has not been reported. Inspection of Δmak-2 grown on cornmeal agar plates for 10 days revealed no mature and fertilization-competent protoperithecia (female sexual structures in N. crassa), but did reveal an ∼50-fold reduced number of protoperithecia-like structures in comparison to wild type (Figure 3A). Furthermore, the protoperithecia-like structures produced in the Δmak-2 strain were smaller, less developed, and nonfertile, but morphologically resembled immature protoperithecia of wild type (e.g., Poggeler and Kuck 2004; Poggeler et al. 2006). This indicated that loss of the MAK2 pathway function does not abolish the capability of initiating protoperithecia formation, but rather affects their abundance and, more importantly, their maturation into fertile structures. Interestingly, when we tested Δos-2 and Δmak-1, we found them to also be female sterile yet they produced no protoperithecia at all. Thus, the other two MAPK mutants were blocked at an earlier developmental stage. In Δmak-1, we observed only lasso-like structures embedded in the agar, suggesting failed attempts of hyphae to coil and fuse during ascogonia formation. In Δos-2, we detected the presence of small, curled side branches, typical of early stages during ascogonia formation, suggesting that both strains are blocked at, or even prior to, the initiation of ascogonia formation.
To better characterize the modularity of the upstream MAPKs (Galagan et al. 2003; Borkovich et al. 2004), we extended this analysis to include the respective MAPKK and MAPKKK components. Three distinct MAP kinase cascades were previously found by in silico analyses in several fungal genomes (Galagan et al. 2003; Borkovich et al. 2004) but a comparative functional characterization is still lacking. Several of the mutants provided by the genome project (Dunlap et al. 2007) were available only as heterokaryons and were therefore backcrossed to wild type to isolate homokaryotic deletions or, if crosses were not successful, the heterokaryons were colony purified several times and their homokaryotic status confirmed by Southern analysis (Table 1). A detailed phenotypic analysis of the mutants confirmed the phylogenetic comparison and supported the existence of three functional modules (Table 2), each consisting of a kinase, a kinase–kinase, and a kinase–kinase–kinase, each of which displayed identical phenotypes on the basis of growth rate, hyphal morphology, conidiation pattern, sexual development, and behavior with respect to inhibitors. The only exception was Δmik-1, which displayed a slightly better growth rate and produced more conidia than the respective MAPKK and MAPK mutants of the MAK1 pathway. Furthermore, double-mutant analysis of cot-1(ts) with the available MAPKK and MAPKKK mutants corroborated that the suppression of the cot-1 defect was specific for mak-2 pathway deletions (Table 2).
TABLE 2.
Phenotypic characteristics of N. crassa MAPK pathway single and respective cot-1;MAPK double mutants
| Growth ratesa
|
Vegetative fusion
|
Major hyphal defects of MAP kinase mutantsb | Female fertilityc
|
|||||
|---|---|---|---|---|---|---|---|---|
| Strain | mapk | cot-1(ts);mapk | mapk | cot-1(ts);mapk | Asexual development of MAP kinase mutantsb | mapk | cot-1(ts);mapk | |
| Wild type | 3.5 | Yes | Yes | |||||
| cot-1(ts) | 3.2 | Yes | Yes | |||||
| Osmosensing pathway | ||||||||
| Δos-4 (NCU03071) | 3.2 | NDd | No | NDd | ± Wild type; frequent tip splitting | Conidial lysis | No | NDd |
| Δos-5 (NCU00587) | 3.1 | 3.0 | No | No | No | No | ||
| Δos-2 (NCU07024) | 3.2 | 3.2 | No | No | No | No | ||
| Cell fusion/fertility pathway | ||||||||
| Δnrc-1 (NCU06182) | 1.2 | 2.5 | No | Yes | Highly irregular growth axisb | Reduced aerial hyphae and conidia formationb | No | Yes |
| Δmek-2 (NCU04612) | 1.1 | NDd | No | NDd | No | NDd | ||
| Δmak-2 (NCU02393) | 1.2 | 2.6 | No | Yes | No | Yes | ||
| Cell-wall integrity pathway | ||||||||
| Δmik-1 (NCU02234) | 0.6 | NDd | No | NDd | Polarity defect; branch formation abnormal | Arthroconidiation for mak-1 and mek-1 | No | NDd |
| Δmek-1 (NCU06419) | 0.5 | 0.6 | No | No | No | No | ||
| Δmak-1 (NCU11376) | 0.6 | 0.5 | No | No | No | No | ||
In centimeters/day at 20° (n = 3) as determined by radial hyphal growth experiments.
No differences in hyphal morphology and asexual development were observed for the single and respective cot-1 double mutants grown at 25° except for a better condidation rate of the cot-1;mak-2 and cot-1;nrc-1 double mutants compared to mak-2 and nrc-1.
Protoperithecia formation after 7 days at room temperature on 2% cornmeal agar supplemented with 0.1% glucose and viable ascospore formation when fertilized with wild-type conidia.
Not determined, as we were not able to obtain viable hygromycin-resistant ascospores in crosses with wild type or cot-1 as the female partner.
An increase in MAK1 activity in a cot-1 background bypasses the mak-2 pathway defects:
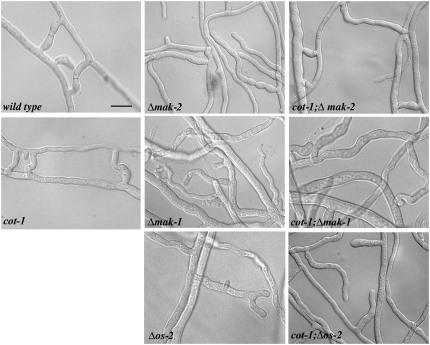
In a more detailed analysis of the MAPK deletions and in comparison with respective cot-1(ts) double mutants, we determined that cot-1(ts);Δmak-2 and cot-1(ts);Δnrc-1 double mutants had an intermediate growth rate when compared to that of the parental strains when grown at permissive conditions (Figure 4A; Table 2). Additional mak-2 pathway defects, such as their shortened aerial hyphae, the derepression of their conidial production, and the female sterility, were also suppressed in the cot-1(ts) background (Figure 4, B and C). As Δmak-2 and Δnrc-1 have been described as hyphal fusion defective mutants (Pandey et al. 2004), we also tested if cot-1(ts) has any effect on the fusion of vegetative hyphae. cot-1(ts) grown at permissive temperature is fusion competent, and we did not observe any qualitative differences when compared with wild type. When we analyzed the cot-1(ts);Δmak-2 and cot-1(ts);Δnrc-1 double mutants, we observed a suppression of the fusion defect of the mak-2 pathway deletions (Figure 5; Table 2). The resulting interconnected, syncycial mycelium could increase the efficiency of nutrient flow and organelle distribution throughout the colony. This, in turn, may explain the increased growth rate, the enhanced formation of aerial hyphae, the better conidiation rates, and the restored female fertility of the double mutants in comparison to the mak-2 pathway deletions.
Figure 4.—
mak-2 pathway defects are suppressed when COT1 activity is reduced. (A) cot-1(ts);Δmak-2 grown at 25° in race tubes has an intermediate tip-extension rate (A) and generates intermediate amounts of aerial hyphae and conidia (B) when compared to the parental strains. (C) Time course of protoperithecia formation by cot-1(ts);Δmak-2. Bars, 100 μm (5 and 10 days overview), 10 μm (5-day inset), and 25 μm (10-day inset).
Figure 5.—
Hyphal fusion is dependent on the three MAP kinase modules. Microscopic analysis of the indicated strains grown for 2 days on minimal media plates at 25°. Note that the three MAPK mutants show extended cell–cell contacts, but no distinct fusion bridges, which are clearly visible in wild type, cot-1(ts), and cot-1(ts);Δmak-2. Bar, 5 μm.
To confirm that this cot-1-dependent suppression is specific for the MAK2 pathway, we analyzed the involvement of the other two MAPK modules in the hyphal fusion process. When we tested Δos-2 and Δmak-1, we found that both mutants were also defective in vegetative fusion, but determined that the cot-1(ts);Δos-2 and the cot-1(ts);Δmak-1 double mutants did not regain their fusion competence (Figure 5). We also tested the remaining MAPKK and MAPKKK deletions and found them to be fusion defective. Thus, the cot-1(ts)-dependent suppression was specific for mak-2 pathway components (Table 2). Taken together, these data indicate that the activity of all three MAPK pathways is essential for hyphal fusion. However, on the basis of the specificity of cot-1(ts) suppression of mak-2 pathway deletion strains, this also indicates the presence of different mechanistic functions of the three MAP pathways during cell fusion.
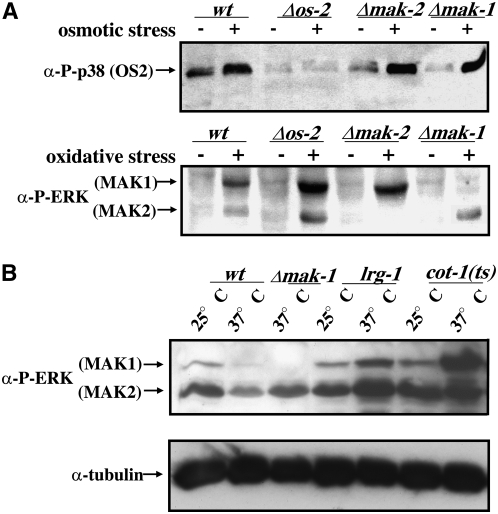
The characterization of the MAPK mutants has revealed phenotypic similarities between the mak-1 and mak-2 pathway deletion strains, indicating a potential functional overlap between the two signaling cascades. Therefore, the loss of one pathway may affect the MAPK activity of one or two of the others. We tested the activity of the three MAPKs and found it to increase under various stress conditions, as determined by the use of phospho-specific antibodies against activated MAPKs (Figure 6A, top). Nevertheless, we detected a similar phospho-activation pattern in the two remaining MAPK pathways when one MAPK was deleted, suggesting that there is no compensatory activation of the other MAPK pathways under normal stress-sensing conditions (Figure 6A, bottom). However, in cot-1(ts), we detected a marked increase of MAK1 phosphorylation as measured 8 hr after the shift to restrictive temperatures while MAK2 activity remained constant (Figure 6B). As mentioned before, cot-1(ts); Δmak-1 pathway double mutants did not display any synthetic characteristics. Thus, these results identified COT1 as a potential negative regulator of MAK1 activity.
Figure 6.—
MAK1 activity is increased in cot-1(ts). (A) Total soluble protein (100 μg/lane) was extracted from the indicated strains grown in the presence or absence of stress inducers (1 m NaCl, 7 mm H2O2). The blot was probed with anti-phospho-ERK (α-P-ERK) and anti-phospho-p38 (α-P-p38) antibodies to detect activated MAK1, MAK2, and OS2 kinase. (B) For the temperature-shift experiments, total soluble protein (50 μg/lane) of the indicated strains grown at 25° and shifted to 37° for 12 hr was extracted and the blot was probed with anti-phospho-ERK (α-P-ERK) antibody (top). To confirm equal loading, the blot was stripped and reprobed with α-tubulin antibody (bottom). lrg-1 is an unrelated temperature-sensitive hyperbranching mutant used as a control.
DISCUSSION
Molecular understanding of fungal morphogenesis is still a major challenge. Phylogenetic analyses and the comparison of S. cerevisiae morphogenetic data with the limited results from various filamentous asco- and basidiomycetes have established that a core set of “polarity factors,” including the existence of most signal transduction pathway components, are conserved between unicellular and filamentous fungi (Borkovich et al. 2004). Nevertheless, it is becoming increasingly evident that differences in the wiring of these conserved components and the presence of additional proteins that are absent in unicellular fungi result in dramatically different morphogenetic outcomes that range from unicellular to true filamentous growth and multicellular differentiation.
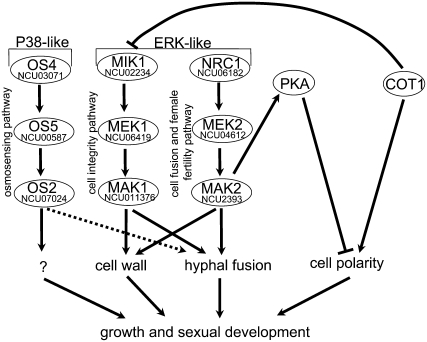
The recent advent of available genome sequences for several filamentous fungi (Galagan et al. 2003, 2005; Dean et al. 2005) has provided the MAPK toolbox present in filamentous ascomycetes. In this report, we comparatively characterized the nine components of three MAPK modules of N. crassa and provided evidence that they act as three distinct modules during vegetative growth and asexual development, but also that the joined activity of the three pathways is required for hyphal fusion and for the formation of more complex multicellular structures necessary to undergo sexual development. Furthermore, we suggest a partial overlap of MAK1- and MAK2-dependent signaling for maintaining the functions of the cell wall on the basis of the shared phenotypes and similar sensitivities against cell-wall drugs, which distinguished the two ERK-type MAPK routes from the p38-type OS2-dependent osmosensing pathway (Figure 7).
Figure 7.—
Model summarizing the components and functions of the three N. crassa MAPK modules and cross-communication between COT1, MAP kinase, and PKA signaling pathways. Details are discussed in the text.
Despite their common phenotype as female sterile mutants, we observed distinct terminal phenotypes of the MAPK mutants during the development of female reproductive structures. Thus, the three MAPK pathways seem to act by different mechanisms in regulating sexual development. The function of the MAK2 pathway was not necessary for the initial steps during the formation of ascogonia, but was required for the maturation of young protoperithecia. In contrast, mutants in the other two pathways are blocked prior to the formation of ascogonia. The coiling of Δmak-1 may indicate defects in cell–cell contact formation due to an altered cell wall or may suggest cell–cell signaling defects, while in Δos-2 we observed only small, bent side branches, suggesting that even the initial attempts of hyphal curling during ascogonia formation are defective. On the basis of the relative late block in the formation of female reproductive structures in mak-2 pathway deletions, we speculate that the MAK2 pathway is an integral part of sexual development and that blocking either of the other two pathways impairs the sexual cycle by preventing the initiation of fruiting-body development or as part of the pleiotropic consequences of their inactivation.
We found it interesting that all mutants described here and in the literature that lack female reproductive structures are also cell-fusion defective. This is best documented in mutants characterized in N. crassa (Wilson and Dempsey 1999; Perkins et al. 2001; Xiang et al. 2002; Fleissner et al. 2005) and the closely related fungus Sordaria macrospora (Poggeler and Kuck 2004; Engh et al. 2007), but was also observed in A. nidulans (Wei et al. 2003). The hypothesis that hyphal fusion is functionally linked with sexual fruiting-body formation is also supported by our characterization of the suppression of the mak-2 pathway by cot-1(ts): the lack or delay of hyphal fusion correlated with defects in the formation of protoperithecia. Furthermore, hyphal fusion has been shown to occur in the fruiting bodies of basidiomycete species (Williams 1985). However, it is currently still unclear whether hyphal fusion is a prerequisite for the formation of female reproductive structures (Glass et al. 2004; Poggeler et al. 2006).
Our genetic analysis suggests that the MAK1, MAK2, and COT1 signaling pathways in N. crassa are linked (Figure 7). This is best characterized by the gulliver-type suppression of the cot-1(ts) growth defects at restrictive conditions observed in mutants that harbor mak-2 pathway deletions. We have recently presented evidence indicating that inhibiting PKA activity can suppress the cot-1(ts) phenotype (Gorovits and Yarden 2003; Seiler et al. 2006). Here, we demonstrate that the loss of MAK2 activity can also partially suppress the cot-1(ts) phenotype. It is tempting to speculate that the observed reduction in PKA activity in Δmak-2 may be involved in the suppression mechanism, thus establishing a potential MAK2-PKA interaction in N. crassa. Extensive literature supports the occurrence of direct cross talk between PKA and MAPK signaling in various organisms (Mosch et al. 1999; Lengeler et al. 2000; Pan et al. 2000; Stork and Schmitt 2002). However, cross talk between these two pathways is generally directed from PKA toward the MAPK pathway and not vice versa. One of the few examples of MAPK-to-PKA signaling is the phosphorylation of the phospho-diesterase RegA by Erk2 in Dictyostelium discoideum that results in the degradation of the cAMP-specific diesterase and thereby the activation of PKA (Loomis 1998; Mohanty et al. 2001). Alternatively, a common upstream link between MAPK and PKA (e.g., via the small GTPase RAS) may be responsible for coordinating the activity intensities of the MAPK and PKA pathways in a manner that confers the observed phenotypes. If this is the case, additional gulliver-type suppressors may serve as a tool to further define the MAK2/PKA pathways in N. crassa.
Another example of the link between COT1 and MAPK signaling is the suppression of mak-2 pathway defects by cot-1(ts). A candidate component of this link is MAK1, whose activity was increased in cot-1(ts). On the basis of the phenotypic similarities of mak-1 and mak-2 pathway deletions, we suggest that both pathways have partially overlapping functions and that the increase in phospho-MAK1 in cot-1(ts) can compensate, at least in part, for the loss of mak-2 pathway functions. An interesting open question is, Why is this compensation mechanism specific for Δmak-2? One possible explanation may be that the primary interaction between COT1 and MAPKs is via MAK1. This is supported by studies in yeasts and animals indicating the presence of a link between Ndr kinases and Rho-type GTPase. Genetic data in S. cerevisiae suggest that the COT1 homolog Cbk1p may negatively regulate the small GTPase Rho1p, which in turn activates the cell-wall integrity pathway that is most similar to the N. crassa MAK1 pathway (Versele and Thevelein 2001; Jorgensen et al. 2002; Schneper et al. 2004). A physical interaction has also been shown to exist between the Ndr kinase ORB6 and the Rho-GTPase-activating protein RGA4 in fission yeast (Das et al. 2007). An indication that this connection may be conserved between fungi and animals has been provided by studies in Drosophila melanogaster and Caenorhabditis elegans, which also describe genetic interactions between Ndr kinases and RhoA (Zallen et al. 2000; Emoto et al. 2004). Thus, the connections among COT1, MAK1, and MAK2 signaling during hyphal growth may provide insights into the regulation of morphogenesis in other highly polar cells such as neurons or pollen tubes.
Acknowledgments
We thank Stephanie Poeggeler for kindly providing us with the plasmid pEHN1nat. This research project was financially supported by the German Bundesland of Lower Saxony and the Volkswagen Foundation (S.S. and O.Y.), by the Deutsche Forschungsgemeinschaft (DFG) through the DFG Research Center of Molecular Physiology of the Brain and the DFG priority program “Cell Polarity” (S.S.), and by The Israel Science Foundation (O.Y.).
References
- Bahn, Y. S., C. Xue, A. Idnurm, J. C. Rutherford, J. Heitman et al., 2007. Sensing the environment: lessons from fungi. Nat. Rev. Microbiol. 5 57–69. [DOI] [PubMed] [Google Scholar]
- Bistis, G. N., D. D. Perkins and N. D. Read, 2003. Different cell types in Neurospora crassa. Fungal Genet. Newsl. 50 17–19. [Google Scholar]
- Borkovich, K. A., L. A. Alex, O. Yarden, M. Freitag, G. E. Turner et al., 2004. Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol. Mol. Biol. Rev. 68 1–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno, K. S., R. Aramayo, P. F. Minke, R. L. Metzenberg and M. Plamann, 1996. a Loss of growth polarity and mislocalization of septa in a Neurospora mutant altered in the regulatory subunit of cAMP-dependent protein kinase. EMBO J. 15 5772–5782. [PMC free article] [PubMed] [Google Scholar]
- Bruno, K. S., J. H. Tinsley, P. F. Minke and M. Plamann, 1996. b Genetic interactions among cytoplasmic dynein, dynactin, and nuclear distribution mutants of Neurospora crassa. Proc. Natl. Acad. Sci. USA 93 4775–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussink, H. J., and S. A. Osmani, 1999. A mitogen-activated protein kinase (MPKA) is involved in polarized growth in the filamentous fungus, Aspergillus nidulans. FEMS Microbiol. Lett. 173 117–125. [DOI] [PubMed] [Google Scholar]
- Dan, I., N. M. Watanabe and A. Kusumi, 2001. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 11 220–230. [DOI] [PubMed] [Google Scholar]
- Das, M., D. J. Wiley, S. Medina, H. A. Vincent, M. Larrea et al., 2007. Regulation of cell diameter, For3p localization, and cell symmetry by fission yeast Rho-GAP Rga4p. Mol. Biol. Cell 18 2090–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, R. D., and F. J. DeSerres, 1970. Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 17 79–143. [Google Scholar]
- Dean, R. A., N. J. Talbot, D. J. Ebbole, M. L. Farman, T. K. Mitchell et al., 2005. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 434 980–986. [DOI] [PubMed] [Google Scholar]
- D'Souza, C. A., and J. Heitman, 2001. Conserved cAMP signaling cascades regulate fungal development and virulence. FEMS Microbiol. Rev. 25 349–364. [DOI] [PubMed] [Google Scholar]
- Dunlap, J. C., K. A. Borkovich, M. R. Henn, G. E. Turner, M. S. Sachs et al., 2007. Enabling a community to dissect an organism: overview of the Neurospora functional genomics project. Adv. Genet. 57 49–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto, K., Y. He, B. Ye, W. B. Grueber, P. N. Adler et al., 2004. Control of dendritic branching and tiling by the Tricornered-kinase/Furry signaling pathway in Drosophila sensory neurons. Cell 119 245–256. [DOI] [PubMed] [Google Scholar]
- Emoto, K., J. Z. Parrish, L. Y. Jan and Y. N. Jan, 2006. The tumour suppressor Hippo acts with the NDR kinases in dendritic tiling and maintenance. Nature 443 210–213. [DOI] [PubMed] [Google Scholar]
- Engh, I., C. Wurtz, K. Witzel-Schlomp, H. Y. Zhang, B. Hoff et al., 2007. The WW domain protein PRO40 is required for fungal fertility and associates with Woronin bodies. Eukaryot. Cell 6 831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner, A., S. Sarkar, D. J. Jacobson, M. G. Roca, N. D. Read et al., 2005. The so locus is required for vegetative cell fusion and postfertilization events in Neurospora crassa. Eukaryot. Cell 4 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura, M., N. Ochiai, M. Oshima, T. Motoyama, A. Ichiishi et al., 2003. Putative homologs of SSK22 MAPKK kinase and PBS2 MAPK kinase of Saccharomyces cerevisiae encoded by os-4 and os-5 genes for osmotic sensitivity and fungicide resistance in Neurospora crassa. Biosci. Biotechnol. Biochem. 67 186–191. [DOI] [PubMed] [Google Scholar]
- Furukawa, K., Y. Hoshi, T. Maeda, T. Nakajima and K. Abe, 2005. Aspergillus nidulans HOG pathway is activated only by two-component signalling pathway in response to osmotic stress. Mol. Microbiol. 56 1246–1261. [DOI] [PubMed] [Google Scholar]
- Galagan, J. E., S. E. Calvo, K. A. Borkovich, E. U. Selker, N. D. Read et al., 2003. The genome sequence of the filamentous fungus Neurospora crassa. Nature 422 859–868. [DOI] [PubMed] [Google Scholar]
- Galagan, J. E., S. E. Calvo, C. Cuomo, L. J. Ma, J. R. Wortman et al., 2005. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438 1105–1115. [DOI] [PubMed] [Google Scholar]
- Geng, W., B. He, M. Wang and P. N. Adler, 2000. The tricornered gene, which is required for the integrity of epidermal cell extensions, encodes the Drosophila nuclear DBF2-related kinase. Genetics 156 1817–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, N. L., C. Rasmussen, M. G. Roca and N. D. Read, 2004. Hyphal homing, fusion and mycelial interconnectedness. Trends Microbiol. 12 135–141. [DOI] [PubMed] [Google Scholar]
- Gorovits, R., and O. Yarden, 2003. Environmental suppression of Neurospora crassa cot-1 hyperbranching: a link between COT1 kinase and stress sensing. Eukaryot. Cell 2 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovits, R., O. Propheta, M. Kolot, V. Dombradi and O. Yarden, 1999. A mutation within the catalytic domain of COT1 kinase confers changes in the presence of two COT1 isoforms and in Ser/Thr protein kinase and phosphatase activities in Neurospora crassa. Fungal Genet. Biol. 27 264–274. [DOI] [PubMed] [Google Scholar]
- Hampsey, M., 1997. A review of phenotypes in Saccharomyces cerevisiae. Yeast 13 1099–1133. [DOI] [PubMed] [Google Scholar]
- Harris, S. D., 2006. Cell polarity in filamentous fungi: shaping the mold. Int. Rev. Cytol. 251 41–77. [DOI] [PubMed] [Google Scholar]
- Hergovich, A., M. R. Stegert, D. Schmitz and B. A. Hemmings, 2006. NDR kinases regulate essential cell processes from yeast to humans. Nat. Rev. Mol. Cell Biol. 7 253–264. [DOI] [PubMed] [Google Scholar]
- Hou, Z., C. Xue, Y. Peng, T. Katan, H. C. Kistler et al., 2002. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol. Plant Microbe Interact. 15 1119–1127. [DOI] [PubMed] [Google Scholar]
- Jones, C. A., S. E. Greer-Phillips and K. A. Borkovich, 2007. The response regulator RRG-1 functions upstream of a mitogen-activated protein kinase pathway impacting asexual development, female fertility, osmotic stress, and fungicide resistance in Neurospora crassa. Mol. Biol. Cell 18 2123–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen, P., B. Nelson, M. D. Robinson, Y. Chen, B. Andrews et al., 2002. High-resolution genetic mapping with ordered arrays of Saccharomyces cerevisiae deletion mutants. Genetics 162 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai, M., K. Kume, K. Miyahara, K. Sakai, K. Nakamura et al., 2005. Fission yeast MO25 protein is localized at SPB and septum and is essential for cell morphogenesis. EMBO J. 24 3012–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki, L., O. Sanchez, K. Shiozaki and J. Aguirre, 2002. SakA MAP kinase is involved in stress signal transduction, sexual development and spore viability in Aspergillus nidulans. Mol. Microbiol. 45 1153–1163. [DOI] [PubMed] [Google Scholar]
- Kojima, K., T. Kikuchi, Y. Takano, E. Oshiro and T. Okuno, 2002. The mitogen-activated protein kinase gene MAF1 is essential for the early differentiation phase of appressorium formation in Colletotrichum lagenarium. Mol. Plant Microbe Interact. 15 1268–1276. [DOI] [PubMed] [Google Scholar]
- Kothe, G. O., and S. J. Free, 1998. The isolation and characterization of nrc-1 and nrc-2, two genes encoding protein kinases that control growth and development in Neurospora crassa. Genetics 149 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W. C. Shen et al., 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64 746–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, T. S., P. S. Shapiro and N. G. Ahn, 1998. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74 49–139. [DOI] [PubMed] [Google Scholar]
- Li, D., P. Bobrowicz, H. H. Wilkinson and D. J. Ebbole, 2005. A mitogen-activated protein kinase pathway essential for mating and contributing to vegetative growth in Neurospora crassa. Genetics 170 1091–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis, W. F., 1998. Role of PKA in the timing of developmental events in Dictyostelium cells. Microbiol. Mol. Biol. Rev. 62 684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhani, H. D., and G. R. Fink, 1998. The riddle of MAP kinase signaling specificity. Trends Genet. 14 151–155. [DOI] [PubMed] [Google Scholar]
- Maeda, T., M. Takekawa and H. Saito, 1995. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science 269 554–558. [DOI] [PubMed] [Google Scholar]
- McCluskey, K., 2003. The Fungal Genetics Stock Center: from molds to molecules. Adv. Appl. Microbiol. 52 245–262. [DOI] [PubMed] [Google Scholar]
- Mey, G., K. Held, J. Scheffer, K. B. Tenberge and P. Tudzynski, 2002. CPMK2, an SLT2-homologous mitogen-activated protein (MAP) kinase, is essential for pathogenesis of Claviceps purpurea on rye: evidence for a second conserved pathogenesis-related MAP kinase cascade in phytopathogenic fungi. Mol. Microbiol. 46 305–318. [DOI] [PubMed] [Google Scholar]
- Mohanty, S., S. Lee, N. Yadava, M. J. Dealy, R. S. Johnson et al., 2001. Regulated protein degradation controls PKA function and cell-type differentiation in Dictyostelium. Genes Dev. 15 1435–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge, R. A., E. Roman, C. Nombela and J. Pla, 2006. The MAP kinase signal transduction network in Candida albicans. Microbiology 152 905–912. [DOI] [PubMed] [Google Scholar]
- Mosch, H. U., E. Kubler, S. Krappmann, G. R. Fink and G. H. Braus, 1999. Crosstalk between the Ras2p-controlled mitogen-activated protein kinase and cAMP pathways during invasive growth of Saccharomyces cerevisiae. Mol. Biol. Cell 10 1325–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, F., D. Krüger, E. Sattlegger, B. Hoffmann, P. Ballario et al., 1995. The cpc-2 gene of Neurospora crassa encodes a protein entirely composed of WD-repeat segments that is involved in general amino acid control and female fertility. Mol. Gen. Genet. 248 162–173. [DOI] [PubMed] [Google Scholar]
- Nelson, B., C. Kurischko, J. Horecka, M. Mody, P. Nair et al., 2003. RAM: a conserved signaling network that regulates Ace2p transcriptional activity and polarized morphogenesis. Mol. Biol. Cell 14 3782–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi, R., S. Banno, R. Ichikawa, F. Fukumori, A. Ichiishi et al., 2007. Identification of OS-2 MAP kinase-dependent genes induced in response to osmotic stress, antifungal agent fludioxonil, and heat shock in Neurospora crassa. Fungal Genet. Biol. 44 208–218. [DOI] [PubMed] [Google Scholar]
- Palanivelu, R., and D. Preuss, 2000. Pollen tube targeting and axon guidance: parallels in tip growth mechanisms. Trends Cell Biol. 10 517–524. [DOI] [PubMed] [Google Scholar]
- Pan, X., T. Harashima and J. Heitman, 2000. Signal transduction cascades regulating pseudohyphal differentiation of Saccharomyces cerevisiae. Curr. Opin. Microbiol. 3 567–572. [DOI] [PubMed] [Google Scholar]
- Pandey, A., M. G. Roca, N. D. Read and N. L. Glass, 2004. Role of a mitogen-activated protein kinase pathway during conidial germination and hyphal fusion in Neurospora crassa. Eukaryot. Cell 3 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins, D. D., A. Radford and M. S. Sachs, 2001. The Neurospora Compendium. Academic Press, New York/London/San Diego.
- Poggeler, S., and U. Kuck, 2004. A WD40 repeat protein regulates fungal cell differentiation and can be replaced functionally by the mammalian homologue striatin. Eukaryot. Cell 3 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggeler, S., M. Nowrousian and U. Kuck, 2006. Fruiting-body development in Ascomycetes, pp. 325–355 in The Mycota I, edited by U. Kües and R. Fischer. Springer-Verlag, Heidelberg, Germany.
- Posas, F., and H. Saito, 1998. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J. 17 1385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praskova, M., F. Xia and J. Avruch, 2008. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr. Biol. 18 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, M., and E. A. Elion, 2005. MAP kinase pathways. J. Cell Sci. 118 3569–3572. [DOI] [PubMed] [Google Scholar]
- Racki, W. J., A. M. Becam, F. Nasr and C. J. Herbert, 2000. Cbk1p, a protein similar to the human myotonic dystrophy kinase, is essential for normal morphogenesis in Saccharomyces cerevisiae. EMBO J. 19 4524–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneper, L., A. Krauss, R. Miyamoto, S. Fang and J. R. Broach, 2004. The Ras/protein kinase A pathway acts in parallel with the Mob2/Cbk1 pathway to effect cell cycle progression and proper bud site selection. Eukaryot. Cell 3 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler, S., N. Vogt, C. Ziv, R. Gorovits and O. Yarden, 2006. The STE20/germinal center kinase POD6 interacts with the NDR kinase COT1 and is involved in polar tip extension in Neurospora crassa. Mol. Biol. Cell 17 4080–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegert, M. R., A. Hergovich, R. Tamaskovic, S. J. Bichsel and B. A. Hemmings, 2005. Regulation of NDR protein kinase by hydrophobic motif phosphorylation mediated by the mammalian Ste20-like kinase MST3. Mol. Cell. Biol. 25 11019–11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork, P. J., and J. M. Schmitt, 2002. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 12 258–266. [DOI] [PubMed] [Google Scholar]
- Terenzi, H. F., and J. L. Reissig, 1967. Modifiers of the cot gene in Neurospora: the gulliver mutants. Genetics 56 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde, F., D. J. Wiley and P. Nurse, 1998. Fission yeast orb6, a ser/thr protein kinase related to mammalian rho kinase and myotonic dystrophy kinase, is required for maintenance of cell polarity and coordinates cell morphogenesis with the cell cycle. Proc. Natl. Acad. Sci. USA 95 7526–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versele, M., and J. M. Thevelein, 2001. Lre1 affects chitinase expression, trehalose accumulation and heat resistance through inhibition of the Cbk1 protein kinase in Saccharomyces cerevisiae. Mol. Microbiol. 41 1311–1326. [DOI] [PubMed] [Google Scholar]
- Vollmer, S. J., and C. Yanofsky, 1986. Efficient cloning of genes of Neurospora crassa. Proc. Natl. Acad. Sci. USA 83 4869–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, H., N. Requena and R. Fischer, 2003. The MAPKK kinase SteC regulates conidiophore morphology and is essential for heterokaryon formation and sexual development in the homothallic fungus Aspergillus nidulans. Mol. Microbiol. 47 1577–1588. [DOI] [PubMed] [Google Scholar]
- Williams, M. A. J., 1985. Ultrastructural aspects of fruit body differentiation in Flammulina velutipes, pp. 429–450 in Developmental Biology of Higher Fungi, edited by D. Moore. Cambridge University Press, Cambridge, UK.
- Wilson, J. F., and J. A. Dempsey, 1999. A hyphal fusion mutant in Neurospora crassa. Fungal Genet. Newsl. 46 31. [Google Scholar]
- Xiang, Q., C. Rasmussen and N. L. Glass, 2002. The ham-2 locus, encoding a putative transmembrane protein, is required for hyphal fusion in Neurospora crassa. Genetics 160 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. R., 2000. Map kinases in fungal pathogens. Fungal Genet. Biol. 31 137–152. [DOI] [PubMed] [Google Scholar]
- Xu, J. R., C. J. Staiger and J. E. Hamer, 1998. Inactivation of the mitogen-activated protein kinase Mps1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. Proc. Natl. Acad. Sci. USA 95 12713–12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. R., X. Zhao and R. A. Dean, 2007. From genes to genomes: a new paradigm for studying fungal pathogenesis in Magnaporthe oryzae. Adv. Genet. 57 175–218. [DOI] [PubMed] [Google Scholar]
- Yarden, O., M. Plamann, D. J. Ebbole and C. Yanofsky, 1992. cot-1, a gene required for hyphal elongation in Neurospora crassa, encodes a protein kinase. EMBO J. 11 2159–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen, J. A., E. L. Peckol, D. M. Tobin and C. I. Bargmann, 2000. Neuronal cell shape and neurite initiation are regulated by the Ndr kinase SAX-1, a member of the Orb6/COT-1/warts serine/threonine kinase family. Mol. Biol. Cell 11 3177–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., R. Lamm, C. Pillonel, S. Lam and J. R. Xu, 2002. Osmoregulation and fungicide resistance: the Neurospora crassa os-2 gene encodes a HOG1 mitogen-activated protein kinase homologue. Appl. Environ. Microbiol. 68 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv, C., R. Gorovits and O. Yarden, 2008. Carbon source affects PKA-dependent polarity of Neurospora crassa in a CRE-1-dependent and independent manner. Fungal Genet. Biol. 45 103–116. [DOI] [PubMed] [Google Scholar]