Abstract
The all-female worker caste of the honeybee (Apis mellifera) is effectively barren in that workers refrain from laying eggs in the presence of a fecund queen. The mechanism by which workers switch off their ovaries in queenright colonies is pheromonally cued, but there is genetically based variation among individuals: some workers have high thresholds for ovary activation, while for others the response threshold is lower. Genetic variation for threshold response by workers to ovary-suppressing cues is most evident in “anarchist” colonies in which mutant patrilines have a proportion of workers that activate their ovaries and lay eggs, despite the presence of a queen. In this study we use a selected anarchist line to create a backcross queenright colony that segregated for high and low levels of ovary activation. We used 191 informative microsatellite loci, covering all 16 linkage groups to identify QTL for ovary activation and test the hypothesis that anarchy is recessively inherited. We reject this hypothesis, but identify four QTL that together explain ∼25% of the phenotypic variance for ovary activation in our mapping population. They provide the first molecular evidence for the existence of quantitative loci that influence selfish cheating behavior in a social animal.
IN societies where individuals act to benefit other members of the society at a cost to their own direct fitness, there is a selective advantage for individuals that “cheat,” reaping the benefits of group living while avoiding the cost of contributing personally. Where genes influence cooperation among individuals, single mutations at key loci may permit selfish behavior to arise that advantages the carrier, but reduces the fitness of the group. Thus, identifying mutations for cheating behavior provides the opportunity to characterize the genetic architecture of cooperation—a key goal of sociogenomics (Robinson et al. 2005). However, while cheating phenotypes have been identified and studied in some microorganisms (Haig 1996; Ennis et al. 2000; Velicer et al. 2000; Queller et al. 2003; Foster et al. 2004), no equivalent cheater mutants have been characterized in higher eukaryotes (Gilbert et al. 2007).
Reproductive division of labor, in which members of the worker caste have reduced reproductive capacity or are even sterile, is one of the defining features of eusocial insects (Wilson 1971). The honeybee (Apis mellifera) typically lives in colonies composed of a single fecund female (the queen), tens of thousands of female workers, and several hundred male drones. The resident queen is the mother of all drones and workers. The drones arise from unfertilized eggs and are thus haploid. Queens and workers, by contrast, arise from fertilized eggs and are diploid (Winston 1987).
In wild-type honeybee colonies, workers do not typically activate their ovaries when a queen is present. Pheromones play an important role in signaling to workers that queen and brood are present (Jay 1970; Jay and Nelson 1973; Winston and Slessor 1998). After a queen's death the workers no longer receive this signal, and up to 30% will activate their ovaries and produce eggs (Barron and Oldroyd 2001). Furthermore, there is genetic variation in the propensity for individual workers to activate their ovaries following queen death or when workers otherwise fail to receive the signal that maintains their effective sterility (Robinson et al. 1990; Martin et al. 2004).
Oldroyd et al. (1994) discovered a naturally occurring “anarchistic” colony, in which all males sampled were offspring of workers instead of the queen. Microsatellite DNA analysis of workers from this colony revealed the queen had mated with at least 12 males, producing 12 subfamilies of workers. Furthermore, microsatellite analysis showed that 49 of the 50 males examined were offspring of workers from a single subfamily—the grandsons of just one of the males involved in the mating. Subsequently, two other naturally occurring anarchist colonies were identified, one in Australia (Montague and Oldroyd 1998) and the other in the United Kingdom (Châline et al. 2002). In these colonies also, the males were offspring of just one or a small number of patrilines.
Recurrent selection for worker reproduction in a line derived from the colony identified by Montague and Oldroyd (1998) established a line in which up to 40% of 12-day-old workers have eggs in their ovarioles despite the presence of a fecund queen (Barron et al. 2001; Thompson et al. 2006). The significant association between worker ovary activation and patriline in naturally occurring anarchistic colonies and the strong response to selection for reproductive behavior by workers indicate a strong genetic basis for the anarchy phenotype (Barron et al. 2001). Because anarchist workers not only show higher rates of ovary activation than wild-type workers in queenless colonies, but also display significant levels of activation in queenright colonies, we hypothesized that ovary activation is under the control of a single genetic regulatory pathway that suppresses ovary activation when appropriate environmental (pheromonal) cues are present. In anarchist workers, we speculated that a putative single mutation (An) in this pathway has resulted in a partial loss of response to the normal ovary-suppressing signal emitted from queen and brood (Barron et al. 2001), allowing some workers to selfishly activate their ovaries in the presence of the queen.
Comparison of anarchist workers over the 10 years of selection for this character reveals an increasing frequency of individuals showing ovary activation, from ∼9% (Oldroyd and Osborne 1999) to nearly 40% (Thompson et al. 2006). This directional response to selection on the mutant line may be explained by the presence of additional loci of small to medium effect that influence the propensity of workers to activate their ovaries, in this case when the pathway is already mutant and no longer fully suppressing ovary activation, i.e., when it is showing the anarchist phenotype.
The genes that regulate worker ovary activation in honeybees and other social insects are key to the evolution of cooperation among workers, enabling this helper caste to subsume its individual reproduction into the reproductive output of the whole colony. Such “genes for altruism” have been frequently postulated (e.g., Hamilton 1972; Dawkins 1989) but molecular-genetic evidence for their existence has to date proved elusive. The phenomenon of the anarchistic phenotype in honeybees provides one opportunity for the discovery of genes for altruism: anarchists are likely mutant along any pathway that normally promotes cooperation among workers. If so, then finding this mutation could reveal how cheater mutants arise from within reproductively altruistic systems and how altruism (in honeybees) works at a molecular level. Although anarchic colonies are rare, their existence is consistent with the expectation for cheaters to occasionally arise out of the persistent reproductive conflict between each worker's direct fitness potential and the fitness of the worker collective (Ratnieks 1988).
Oldroyd and Osborne (1999) performed a crossing experiment in which wild-type queens were inseminated with either anarchist worker-laid males or a mix of both anarchist worker-laid males and wild-type queen-laid males. These crosses produced a range of reproductive phenotypes among worker offspring (Oldroyd and Osborne 1999). In some colonies only workers fathered by anarchist males had activated ovaries, while in others there was no correlation between paternity and ovary activation. This inconsistent pattern suggests a strong G × E interaction for the anarchy phenotype and further suggests that the postulated anarchy locus controlling worker reproduction in the anarchist line is recessive to the wild-type allele.
Here we report an attempt to confirm the hypothesis that anarchy is recessive and to locate the postulated An locus, using QTL mapping on an anarchist backcross. All microsatellite markers and the linkage map were obtained from the honeybee genome project (Solignac et al. 2007).
MATERIALS AND METHODS
Genetic line and crosses:
The anarchist line has been maintained for ∼10 generations from a single colony identified from the field in 1995 (Montague and Oldroyd 1998). Selection for anarchy in each generation has led to an average annual increase of 2% in the frequency of ovary activation in colonies (Oldroyd and Osborne 1999; Barron and Oldroyd 2001; Oldroyd et al. 2001; Hoover et al. 2005a; Thompson et al. 2006).
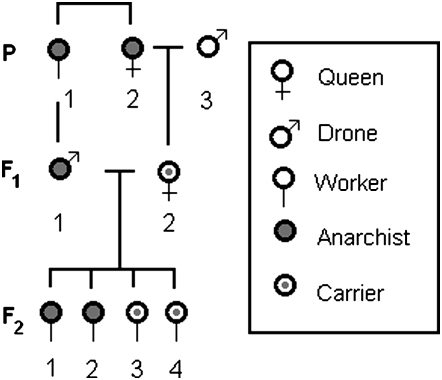
For our mapping population we used a backcross design (Figure 1), consisting of a hybrid queen (the daughter of an anarchist queen and wild-type drone) artificially inseminated by a single worker-laid drone. Assuming a recessive mode of inheritance, offspring from this backcross will segregate into two phenotypic classes: anarchist (ovaries activated) and wild type (ovaries not activated), depending on the maternal allele inherited.
Figure 1.—
Backcross design for QTL mapping population. Queen P2 and worker P1 were reared from the anarchy line. Drone P3 was collected from a wild-type colony that (typically) did not show any signs of worker-laid brood. A presumably heterozygous F1 queen F12 was mated to an F1 anarchist drone F11 to give the F2 generation of workers. The F2 generation was used as the mapping population.
Biological material:
We introduced the focal queen (individual F12 in Figure 1) into a standard Langstroth hive containing a colony comprising eight frames of honey and queen-laid brood. When the host colony's progeny were eventually replaced by progeny from our focal queen (after 2 months), we removed a single comb of emerging brood and incubated it overnight (34.5°). The following day we paint marked (Posca Poster Pens, Mitsubishi Pencil Co.) ∼1000 newly emerged workers and reintroduced them into their colony of origin. After 14 days, 595 marked workers were recaptured and snap frozen on dry ice. We then dissected individual worker abdomens and scored their ovaries according to the degree of development (after Dade 1977). Specifically, we scored ovaries on an arbitrary five-point scale, 0 indicating that ovarioles (the finger-like projections at the distal end of each ovary) were thin and lacking any sign of activation, and 4 indicating that at least one ovary had a mature (oval-shaped) ovum. Scores within this range reflect intermediate stages of ovary activation. For the purposes of this study and to allow comparisons with previous work, workers with a score of 2–4 were considered to have “activated” ovaries.
From the total number of individuals scored from our backcross population (n = 595) we selectively genotyped the 96 individuals with the lowest numerical score (0) and 96 individuals with the highest numerical scores (2–4). This subset of individuals represents the maximum contrast in ovary activation phenotypes. DNA was extracted from these 192 individuals by grinding one hind leg on dry ice, followed by addition of 0.5 ml Chelex solution (5%) and boiling for 15 min (Walsh et al. 1991). After centrifugation (13,000 × g, 10 min), we added proteinase K (1 μg) to the DNA-containing supernatant and incubated it at 37° for 3 hr and then at 80° for 15 min to deactivate the enzyme. The solution was diluted 1:1 in sterile H2O and stored at 4° prior to use in PCR.
We genotyped all 192 individuals at 417 microsatellite loci (Solignac et al. 2007), distributed across all 16 linkage groups. For “touchdown” PCR genotyping (whereby Ta decreased from 58° to 52° in single-degree increments, with five cycles per degree), we used fluorescently labeled tags (Pet, Fam, Ned, and Vic; Applied Biosystems, Foster City, CA) at a final concentration of 0.25 μm (PET/FAM/NED/VIC-CCTGGCGACTCCTGGAG), with 0.0165 μm tagged reverse primers (CCTGGCGACTCCTGGAG-RevPrimer) and 0.25 μm forward primer. We electrophoresed amplified products using a 3130xl Analyzer (Applied Biosystems) and subsequently visualized and acquired individual genotypes at each locus using the associated software (Genemapper Software 3.7, Applied Biosystems).
Genetic linkage map:
We assembled markers into linkage groups using the positions and intermarker distances published in version AmelMap3 of the honeybee genome assembly (Solignac et al. 2007). We assessed the likelihood of marker order for each of our linkage groups, using the Map-Manager QTX (Manly et al. 2001) “ripple” function. The marker order of the most likely map deviated from the published map (Solignac et al. 2007) at only 11 loci, and map choice did not change the results of the interval mapping analysis (below).
Statistical analysis:
To determine the association of any QTL to individual markers we used a regression analysis of marker genotype on ovary score, as implemented in the computer program Map-Manager QTX (Manly et al. 2001). This initial approach is map independent and was primarily to search for evidence of QTL outside the region screened using interval mapping. To minimize type-I errors associated with testing over multiple loci, we adjusted the associated P-values to maintain a false discovery rate (FDR) of 5% (Q value = 0.05; Benjamini and Hochberg 1995; Verhoeven et al. 2005).
Subsequently, Map-Manager QTX was used to perform interval mapping, using the Kosambi mapping function. We identified QTL for each linkage group by first calculating significance thresholds using permutation (Churchill and Doerge 1994) to determine the P-value for the highest LOD score in each linkage group. These P-values (Table 1) were then ranked and assessed for significance at a 5% FDR across all linkage groups.
TABLE 1.
Empirically determined P-values for the highest LOD score recorded in each linkage group
| LGa | LODb | P-valuec | 5% thresholde |
|---|---|---|---|
| 1 | 2.7 | 0.024d | 2.0 |
| 2 | 0.9 | 0.57 | 2.1 |
| 3 | 1.6 | 0.13 | 2.0 |
| 4 | 0.5 | 0.9 | 1.5 |
| 5 | 1.2 | 0.25 | 2.0 |
| 6 | 2.0 | 0.057 | 2.0 |
| 7 | 2.6 | 0.017d | 2.1 |
| 8 | 1.1 | 0.33 | 2.0 |
| 9 | 1.7 | 0.11 | 2.0 |
| 10 | 0.5 | 0.84 | 1.9 |
| 11 | 1.2 | 0.25 | 1.9 |
| 12 | 0.9 | 0.38 | 2.0 |
| 13 | 2.3 | 0.035d | 2.0 |
| 14 | 1.1 | 0.3 | 2.1 |
| 15 | 2.9 | 0.0049d | 2.4 |
| 16 | 0.5 | 0.46 | 2.0 |
Linkage group (LG) number refers to the NCBI assigned chromosome number in AmelMap_4 (Solignac et al. 2007).
Refers to the highest LOD score calculated in the linkage group using interval mapping.
Calculated empirically through 10,000 permutations (Churchill and Doerge 1994).
Significant with a false discovery rate of 5%.
LOD threshold for 5% significance level across the linkage group, calculated by permutation (10,000 iterations).
RESULTS
Worker ovary activation scores spanned the full range, from 0 to 4 (Table 2). A total of 119 workers (20%) were classified as having active ovaries.
TABLE 2.
Percentage of ovary activation scores for 14-day-old workers (n = 595)
| Ovary activation score | % of workers classified |
|---|---|
| 0 | 48 |
| 1 | 32 |
| 2 | 11 |
| 3 | 6 |
| 4 | 3 |
Ovary activation was scored on an integer scale from 0 to 4, on the basis of the degree of ovary activation (see materials and methods). Workers with a score of 2, 3, or 4 were considered to have “active” ovaries.
From 417 markers, 191 showed allelic variation between the two parents (individuals P1 and P3 in Figure 1). These informative markers produced a near-saturated linkage map, whereby 95.7% of the genome was within 20 cM of at least one marker. A full 93.1% of the genome was delimited by markers <50 cM apart. All 16 honeybee chromosomes were represented in our map (not shown), with the average distance between markers being 22.4 cM with a standard deviation of 11.83 cM.
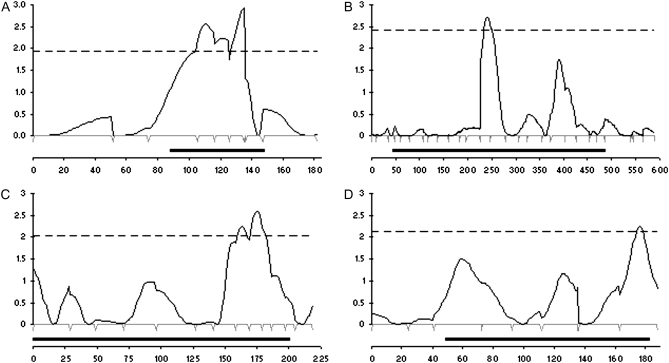
Interval mapping revealed four significant QTL, one each on linkage groups 15, 1, 7, and 13 (in order of greatest effect, Figure 2). We label these QTL OvA1, OvA2, OvA3, and OvA4, respectively, and we estimate they account for 8, 6, 6, and 5% of the total phenotypic variation (Table 3).
Figure 2.—
Interval maps of the four linkage groups containing significant QTL at 5% false discovery rate. LOD score (y-axis) was calculated at 1-cM intervals across each linkage group (x-axis). The 5% empirical threshold is indicated by the dashed line. Ninety-five percent confidence intervals are shown by solid bars. Microsatellite marker positions are indicated by peaks just below each x-axis. Maps A, B, C, and D show linkage groups 15, 1, 7, and 13 and QTL OvA1, OvA2, OvA3, and OvA4, respectively.
TABLE 3.
Significant quantitative trait loci for ovary activation identified through interval mapping
| QTL | Linkage group | Closest marker | P-value | QTL effect | Effect size (%) |
|---|---|---|---|---|---|
| OvA1 | 15 | Am253 | 0.0049 | +0.92 | 8 |
| OvA2 | 7 | SV272 | 0.017 | −0.80 | 6 |
| OvA3 | 1 | SV084 | 0.024 | −0.84 | 6 |
| OvA4 | 13 | Am074 | 0.035 | −1.24 | 5 |
A negative QTL effect indicates that workers carrying the allele inherited from the anarchist queen have a higher average ovary activation score than workers carrying the allele inherited from the wild-type drone.
DISCUSSION
This study has not found evidence to support the hypothesis that anarchy is recessively inherited, but has identified four quantitative trait loci that influence ovary activation rates in queenright honeybee workers. These are the first QTL for cheating to be mapped in a eusocial insect. We reject our initial model of inheritance based on the number, direction, and magnitude of QTL effects identified in our map. This is because, under the assumption of a recessive mutation (Oldroyd and Osborne 1999; Barron et al. 2001), we expect to find a single QTL of large effect. However, we found multiple QTL that together account for only 25% of total phenotypic variation. Furthermore, the wild-type allele from one QTL increased ovary activation (OvA1; Table 3), rather than decreasing it, as was expected. Given the high saturation of our linkage map (95.7% of the genome lies within 20 cM of at least one marker), it is unlikely that a single recessive mutation accounting for the majority of phenotypic variation would remain undetected following our open-ended screen. We therefore suggest that the An locus exhibits a more complex mode of inheritance and propose a new hypothesis, that the anarchy allele is dominant to its wild-type counterpart. This hypothesis remains consistent with the conditional expression of the anarchy phenotype following an anarchist × wild-type cross (Oldroyd and Osborne 1999), provided that we allow for incomplete penetrance. Analysis of ovary activation in the reciprocal backcross compared to that used in this study would likely prove useful in understanding the mode of inheritance of this trait and is the subject of current research in our laboratory.
Under a dominant model, all workers in our backcross population would be either An/An or An/an (cf. Figure 1) and would therefore be genetically predisposed to ovary activation. However, we observed only 20% of workers with active ovaries, indicating a low phenotypic penetrance for anarchy. The QTL identified in the present study are therefore likely to be secondary modifiers of the dominant but incompletely penetrating anarchy phenotype: these loci influence the likelihood that an anarchist worker already predisposed at the major locus will activate her ovaries.
The role of our QTL in wild-type workers is not yet known and cannot be assessed from the current study. However, we suggest a role similar to that in anarchists in that it influences a worker's propensity to activate her ovaries in the absence of pheromonal suppression. In anarchists, this absence of suppression results from the as-yet-unmapped An mutation or loss of queen, whereas in wild types it results solely from loss of the queen. In our mapping population, the allele for high ovary activation was inherited from the anarchist line in three of the four QTL (OvA2-4), as would be expected given the continuous selection for anarchistic alleles since 1998.
Candidate genes:
The strongest QTL identified, OvA1, explains 8% of variance in ovary activation scores and contains 86 candidate genes within its 95% confidence interval (Figure 2A). On the basis of a functional annotation from Gene Ontology terms (http://www.geneontology.org/), we highlight four genes of particular interest.
OvA1 dopamine receptor type D2:
The neurotransmitter dopamine has been found to mediate a wide range of honeybee behaviors, including hygienic (Spivak et al. 2003) and foraging (Barron et al. 2002, 2007; Schulz et al. 2002) behavior in workers. Dombroski et al. (2003) showed that when queenless workers are fed a diet supplemented with dopamine the workers show increased levels of ovary activation. Thus genes associated with dopamine activity may be important to ovary activation. OvA1 encompasses a dopamine receptor type D2 gene (Dop2). This gene may therefore be associated with a change in responsiveness to dopamine in workers already cued to activate their ovaries.
Odorant receptors:
Ovary activation is in part mediated by pheromones emitted from mature larvae that signal workers to refrain from ovary activation. Brood pheromones are less effective at inhibiting ovary activation in the anarchist line than in wild types (Oldroyd and Osborne 1999; Barron and Oldroyd 2001; Hoover et al. 2005b). It is therefore possible that odorant-binding protein 9 (Obp9) or the gene similar to putative odorant receptor 13a (LOC72693) is involved in the detection of brood pheromones and thereby plays a role in the regulation of ovary activation. Similarly, the gene neuronal nicotinic acetylcholine Apisα7-2 subunit (GB17254) is expressed in brain regions associated with olfactory learning, as well as being expressed in the antennal lobes of workers (Thany et al. 2005; Jones et al. 2006). It may also be associated with response to brood signals.
Differentially expressed loci:
Thompson et al. (2006) used a cDNA microarray of honeybee brain ESTs to identify loci that are differentially expressed between 4-day-old wild-type and anarchist workers. Of ∼5500 genes screened, three were significantly differentially expressed. These loci are candidates for the regulation of worker sterility (Thompson et al. 2006), but are not associated with any of our OvA-series QTL. However, among the secondary candidates reported from that study, three (GB17541, GB13621, and GB14785) are located within the genomic regions of OvA1, OvA2, and OvA3, respectively. An additional two secondary candidates (BI511564 and BI509796) are associated with OvA2 and OvA4, respectively. These five genes, first identified from the microarray screen, are therefore of interest in the context of the current study.
The identification of QTL and corresponding candidate genes as “modifiers of ovary activation” is a step toward uncovering molecular pathways influencing the expression of cheating behavior in animal societies. Our use of the FDR is appropriate because it controls both type-I and -II errors, substantially increasing the power to detect QTL without compromising the familywise error rate (Benjamini and Hochberg 1995). Because this method requires all tests to be statistically independent (Benjamini and Hochberg 1995; Chen and Storey 2006) we have applied it to the uncorrected P-values associated with the single highest LOD score on each linkage group (N = 16), rather than to the P-value for each (nonindependent) mapping interval within groups. The dependency of intervals within linkage groups was taken into account by using permutation (Churchill and Doerge 1994).
Genotype × environment interactions:
Quantification of QTL effects can be complicated by the presence of genotype × environment interactions, which have been suggested in previous studies of anarchy (Oldroyd et al. 1999; Barron and Oldroyd 2001). Worker ovary activation has been shown to be more inhibited by the presence of wild-type brood than by anarchist brood (Oldroyd et al. 2001). The backcross workers in our experiment were exposed to signals from both homozygous and heterozygous anarchist pupae, potentially resulting in an inhibitory signal from the brood that was stronger than a pure anarchist colony, but weaker than from wild-type brood. Therefore, the small percentage of workers with activated ovaries observed in our backcross compared to studies of pure anarchist colonies (20% vs. 38%; e.g., Thompson et al. 2006) could be explained in part by the segregation of ovary activation loci (i.e., OvA) combined with an increased inhibitory signal from the brood.
It should be noted that the level of ovary activation observed is still three orders of magnitude greater than that expected in wild-type colonies (Ratnieks 1993). Furthermore, by genotyping individuals with the most extreme ovary activation scores, the frequency of workers with active ovaries in our mapping population was magnified to 50%.
Further considerations:
An alternative genetic model to dominance is one in which many small loci contribute to the phenotype additively. However, as the largest significant additive effect we identified inherited from the anarchist line was a mere 6%, it would require each of the original anarchist colonies (Oldroyd et al. 1994; Montague and Oldroyd 1998; Châline et al. 2002) to have inherited very large numbers of loci to produce the large differences in ovary activation rates between wild-type workers and anarchists. It is therefore more probable that there exist a small number of QTL of large dominant effect.
The four QTL identified in our study explain ∼25% of the phenotypic variance in our backcross. If anarchy is dominant, then further testing would be necessary to determine if there are more QTL that contribute to variation in anarchists' ovary activation or whether the rest is due to G × E interactions.
Conclusions:
This study represents the first QTL mapping project in honeybees to utilize the complete microsatellite-based linkage map and gives further corroboration to the positioning of these markers on the 16 linkage groups. More importantly, we have shown for the first time that QTL affecting worker sterility via ovary activation in honeybees can be statistically detected. These QTL suggest a number of candidate genes that influence cheating behavior—the first for a social animal.
Further, we modify our initial hypothesis for a single recessive gene that controls ovary activation (sensu Barron et al. 2001) to that of a single locus of moderate to high dominance, combined with incomplete penetrance. If correct, then a future QTL study of the reciprocal backcross to that used here should see the An locus segregate directly with ovary activation in queenright workers. The anarchistic line therefore remains an ideal model for the continued exploration of the evolution of cooperation among social insects at the molecular level.
Acknowledgments
We thank M. Solignac for advice and J. Lim, J. Paar, and M. Duncan for assistance. Funding for this project was provided by an Australian Research Council grant to B.P.O.
References
- Barron, A. B., and B. P. Oldroyd, 2001. Social regulation of ovary activation in ‘anarchistic’ honey-bees (Apis mellifera). Behav. Ecol. Sociobiol. 49 214–219. [Google Scholar]
- Barron, A. B., B. P. Oldroyd and F. L. W. Ratnieks, 2001. Worker reproduction in honey-bees (Apis) and the anarchic syndrome: a review. Behav. Ecol. Sociobiol. 50 199–208. [Google Scholar]
- Barron, A. B., D. J. Schulz and G. E. Robinson, 2002. Octopamine modulates responsiveness to foraging-related stimuli in honey bees (Apis mellifera). J. Comp. Physiol. A 188 603–610. [DOI] [PubMed] [Google Scholar]
- Barron, A. B., R. Maleszka, R. K. van der Meer and G. E. Robinson, 2007. Octopamine modulates honey bee dance behavior. Proc. Natl. Acad. Sci. USA 104(5): 1703–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini, Y., and Y. Hochberg, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 57 289–300. [Google Scholar]
- Châline, N., F. L. W. Ratnieks and T. Bourke, 2002. Anarchy in the UK: detailed genetic analysis of worker reproduction in a naturally-occurring British anarchistic honeybee, Apis mellifera, colony using DNA microsatellites. Mol. Ecol. 11 1795–1803. [DOI] [PubMed] [Google Scholar]
- Chen, L., and J. D. Storey, 2006. Relaxed significance criteria for linkage analysis. Genetics 137 2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill, G. A., and R. W. Doerge, 1994. Empirical threshold values for quantitative trait mapping. Genetics 138 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dade, H. A., 1977. Anatomy and Dissection of the Honeybee. International Bee Research Association, London.
- Dawkins, R., 1989. The Selfish Gene. Oxford University Press, London/New York/Oxford.
- Dombroski, T. C. D., Z. L. P. Simões and M. M. G. Bitondi, 2003. Dietary dopamine causes ovary activation in queenless Apis mellifera workers. Apidologie 34 281–289. [Google Scholar]
- Ennis, H. L., D. N. Dao, S. U. Pukatzki and R. H. Kessin, 2000. Dictyostelium amoebae lacking an F-box protein form spores rather than stalk in chimeras with wild type. Proc. Natl. Acad. Sci. USA 97 3292–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, K. R., G. Shaulsky, J. E. Strassmann, D. C. Queller and C. R. L. Thompson, 2004. Pleiotropy as a mechanism to stabilise cooperation. Nature 431 693–696. [DOI] [PubMed] [Google Scholar]
- Gilbert, O., K. R. Foster, N. Mehdiabadi, J. E. Strassmann and D. C. Queller, 2007. High relatedness maintains multicellular cooperation in a social amoeba by controlling cheater mutants. Proc. Natl. Acad. Sci. USA 104 8913–8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig, D., 1996. Gestational drive and the green-bearded placenta. Proc. Natl. Acad. Sci. USA 93 6547–6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, W. D., 1972. Altruism and related phenomena, mainly in social insects. Annu. Rev. Ecol. Syst. 3 193–232. [Google Scholar]
- Hoover, S. E. R., B. P. Oldroyd, T. C. Wossler and M. L. Winston, 2005. a Anarchistic queen honey bees have normal queen mandibular pheromones. Insectes Soc. 52 6–10. [Google Scholar]
- Hoover, S. E. R., M. L. Winston and B. P. Oldroyd, 2005. b Retinue attraction and ovary activation: responses of wild type and anarchistic honey bees (Apis mellifera) to queen pheromones. Behav. Ecol. Sociobiol. 59 278–284. [Google Scholar]
- Jay, S. C., 1970. The effect of various combinations of immature queen and worker bees on the ovary development of worker honeybees in colonies with and without queens. Can. J. Zool. 48 169–173. [Google Scholar]
- Jay, S. C., and E. V. Nelson, 1973. The effects of laying worker honeybees (Apis mellifera L.) and their brood on the ovary development of other worker honeybees. Can. J. Zool. 51 629–632. [Google Scholar]
- Jones, A. K., V. Raymond-Delpech, S. H. Thany, M. Gauthier and D. B. Sattelle, 2006. The nicotinic acetylcholine receptor gene family of the honey bee, Apis mellifera. Genome Res. 16 1422–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly, K. F., R. H. Cudmore Jr. and J. M. Meer, 2001. Map Manager QTX, cross-platform software for genetic mapping. Mamm. Genome 12 930–932. [DOI] [PubMed] [Google Scholar]
- Martin, C. J., B. P. Oldroyd and M. Beekman, 2004. Differential reproductive success among subfamilies in queenless honey bee colonies (Apis mellifera L.). Behav. Ecol. Sociobiol. 56 42–49. [Google Scholar]
- Montague, C. E., and B. P. Oldroyd, 1998. The evolution of worker sterility in honey bees: an investigation into a behavioral mutant causing a failure of worker policing. Evolution 52 1408–1415. [DOI] [PubMed] [Google Scholar]
- Oldroyd, B. P., and K. E. Osborne, 1999. The evolution of worker sterility in honeybees: the genetic basis of failure of worker policing. Proc. R. Soc. Lond. Ser. B 266 1335–1339. [Google Scholar]
- Oldroyd, B. P., A. J. Smolenski, J.-M. Cornuet and R. H. Crozier, 1994. Anarchy in the beehive. Nature 371 749. [Google Scholar]
- Oldroyd, B. P., L. Halling and T. E. Rinderer, 1999. Development and behaviour of anarchistic honeybees. Proc. R. Soc. Lond. Ser. B 266 1875–1878. [Google Scholar]
- Oldroyd, B. P., T. C. Wossler and F. L. W. Ratnieks, 2001. Regulation of ovary activation in worker honey-bees (Apis mellifera): larval signal production and adult response thresholds differ between anarchistic and wild-type bees. Behav. Ecol. Sociobiol. 50 366–377. [Google Scholar]
- Queller, D. C., E. Ponte, S. Bazzaro and J. E. Strassmann, 2003. Single-gene greenbeard effects in the social amoeba Dictyostelium discoideum. Science 299 105–106. [DOI] [PubMed] [Google Scholar]
- Ratnieks, F. L. W., 1988. Reproductive harmony via mutual policing by workers in eusocial Hymenoptera. Am. Nat. 132 217–236. [Google Scholar]
- Ratnieks, F. L. W., 1993. Egg-laying, egg-removal, and ovary development by workers in queenright honey bee colonies. Behav. Ecol. Sociobiol. 32 191–198. [Google Scholar]
- Robinson, G. E., R. E. Page and M. K. Fondrk, 1990. Intracolonial behavior in worker oviposition, oophagy, and larval care in queenless honey-bee colonies. Behav. Ecol. Sociobiol. 26 315–323. [Google Scholar]
- Robinson, G. E., C. M. Grozinger and C. W. Whitfield, 2005. Sociogenomics: social life in molecular terms. Nat. Rev. Genet. 6 257–270. [DOI] [PubMed] [Google Scholar]
- Schulz, D. J., A. B. Barron and G. E. Robinson, 2002. A role for octopamine in honey bee division of labor. Brain Behav. Evol. 60 350–359. [DOI] [PubMed] [Google Scholar]
- Solignac, M., F. Mougel, D. Vautrin, M. Monnerot and J.-M. Cornuet, 2007. A third-generation microsatellite-based linkage map of the honey bee, Apis mellifera, and its comparison with the sequence-based physical map. Genome Biol. 8 R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak, M., R. Masterman, R. Ross and K. A. Mesce, 2003. Hygienic behavior in the honey bee (Apis mellifera L.) and the modulatory role of octopamine. J. Neurobiol. 55 341–354. [DOI] [PubMed] [Google Scholar]
- Thany, S. H., M. Crozatier, V. Raymond-Delpech, M. Gauthier and G. Lenaers, 2005. Apisa2, Apisa7-1 and Apisa7-2: three new neuronal nicotinic acetylcholine receptor α-subunits in the honeybee brain. Genome Res. 16 1422–1430. [DOI] [PubMed] [Google Scholar]
- Thompson, G. J., R. Kucharski, R. Maleszka and B. P. Oldroyd, 2006. Towards a molecular definition of worker sterility: differential gene expression and reproductive plasticity in honey bees. Insect Mol. Biol. 15 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer, G. J., L. Kroos and R. E. Lenski, 2000. Developmental cheating in the social bacterium Myxococcus xanthus. Nature 404 598–601. [DOI] [PubMed] [Google Scholar]
- Verhoeven, K. J. F., K. L. Simonsen and L. M. McIntyre, 2005. Implementing false discovery rate control: increasing your power. Oikos 108 643–647. [Google Scholar]
- Walsh, P. S., D. A. Metzger and R. Higuchi, 1991. Chelex (R)100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10 507.. [PubMed] [Google Scholar]
- Wilson, E. O., 1971. The Insect Societies. Harvard University Press, Cambridge, MA.
- Winston, M. L., 1987. The Biology of the Honey Bee. Harvard University Press, Cambridge, MA.
- Winston, M. L., and K. N. Slessor, 1998. Honey bee primer pheromones and colony organization: gaps in our knowledge. Apidologie 29 81–95. [Google Scholar]