Abstract
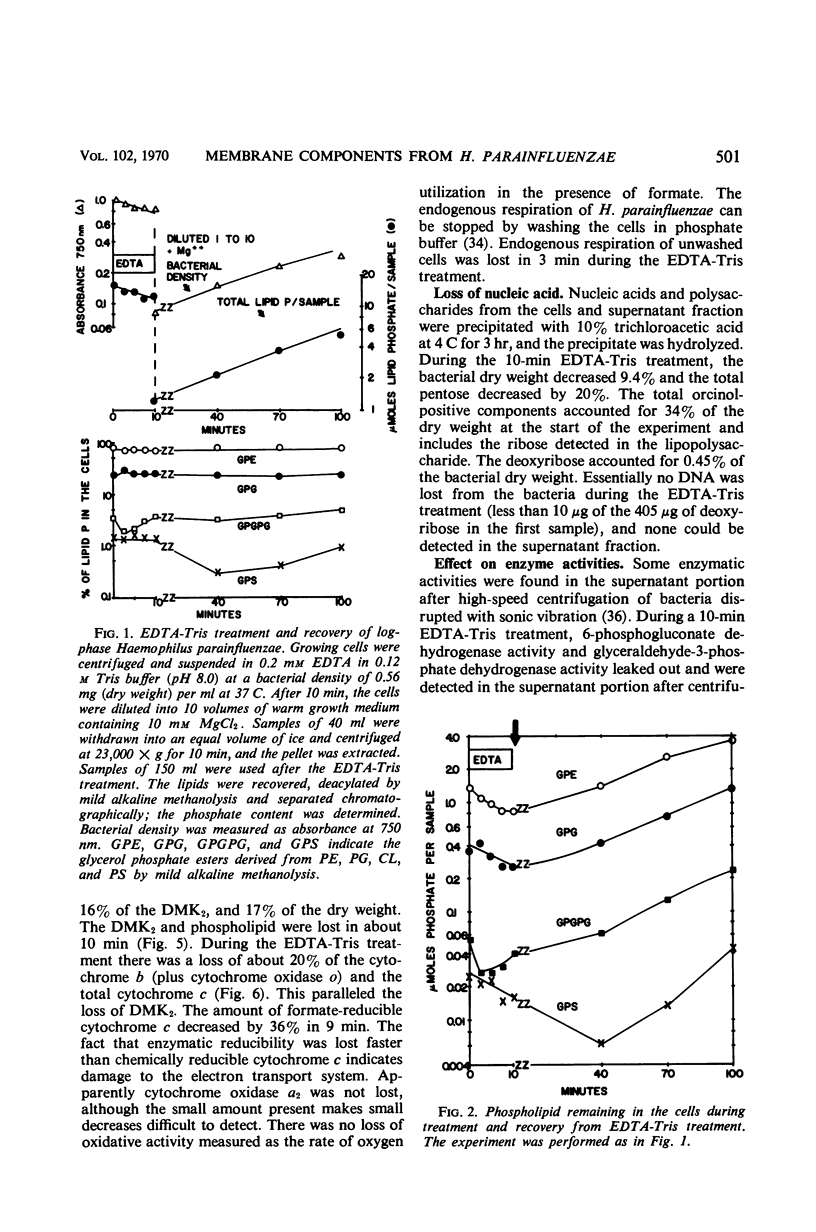
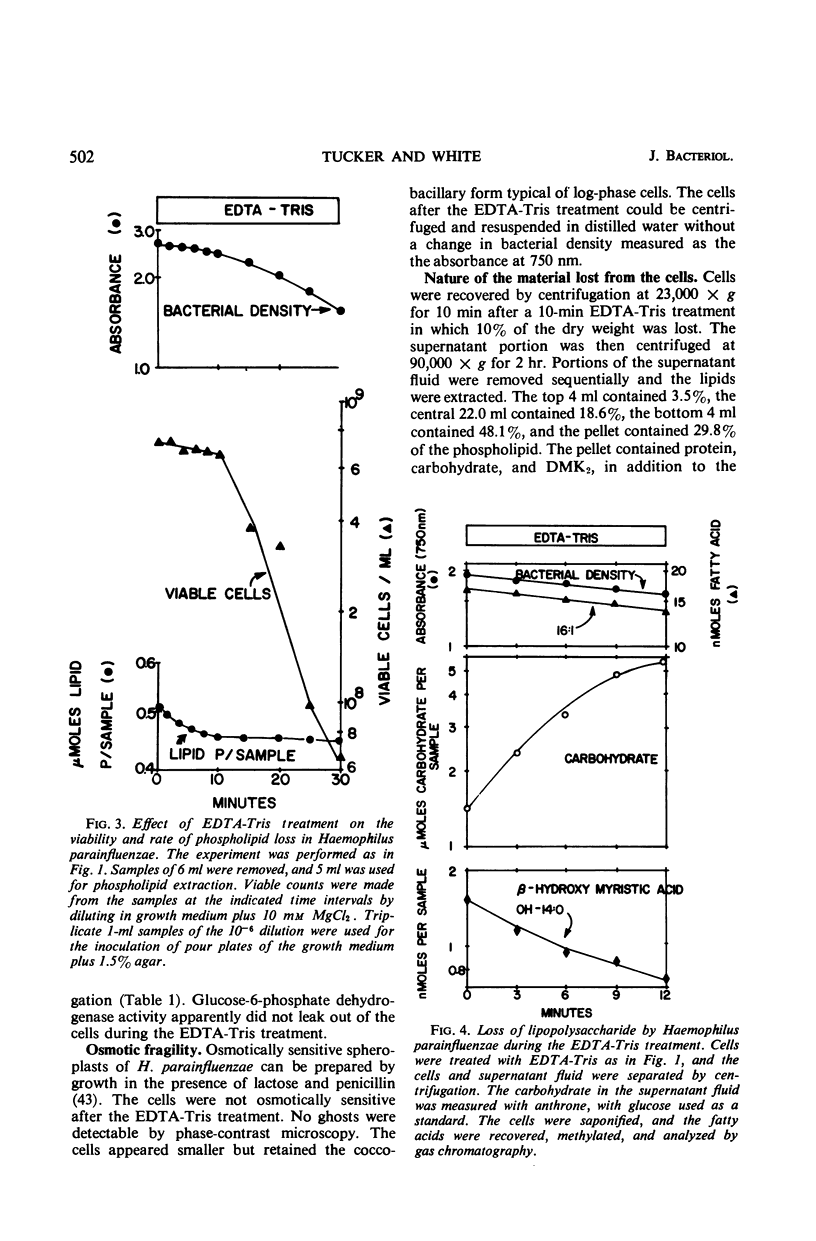
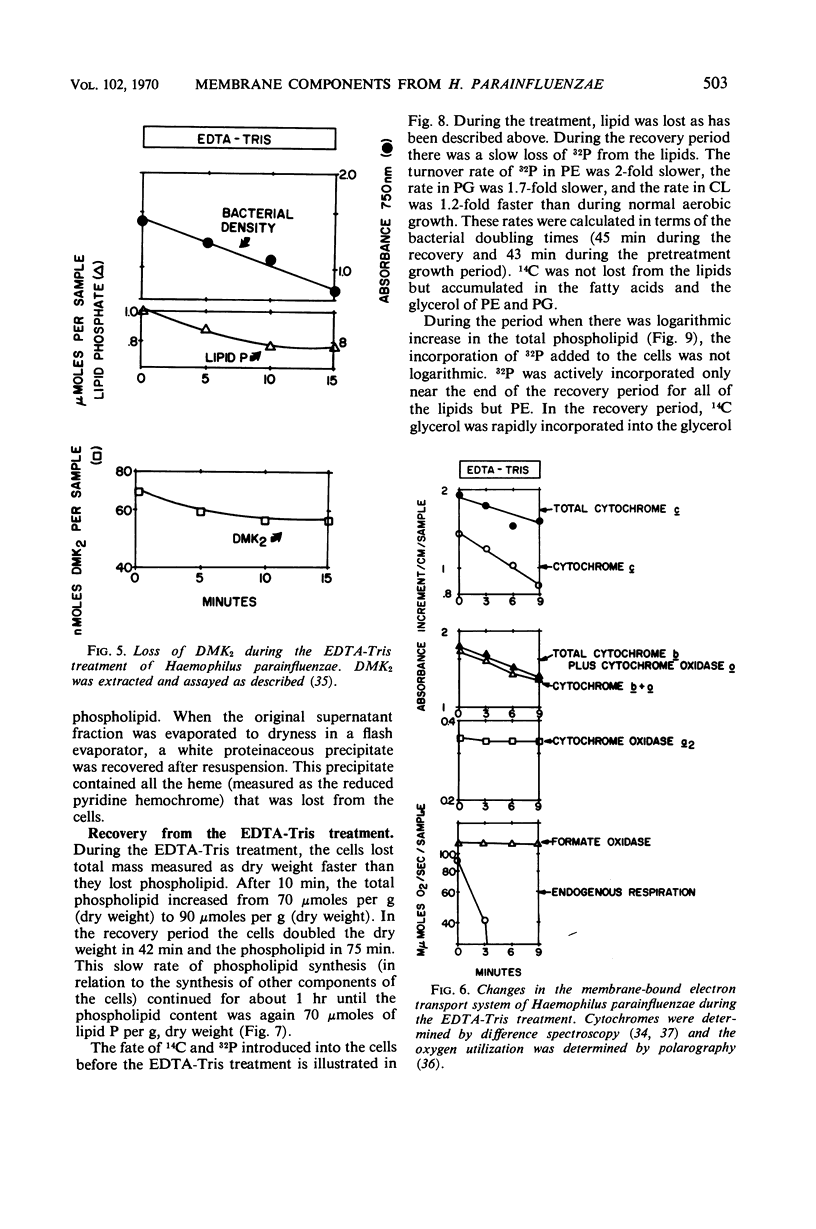
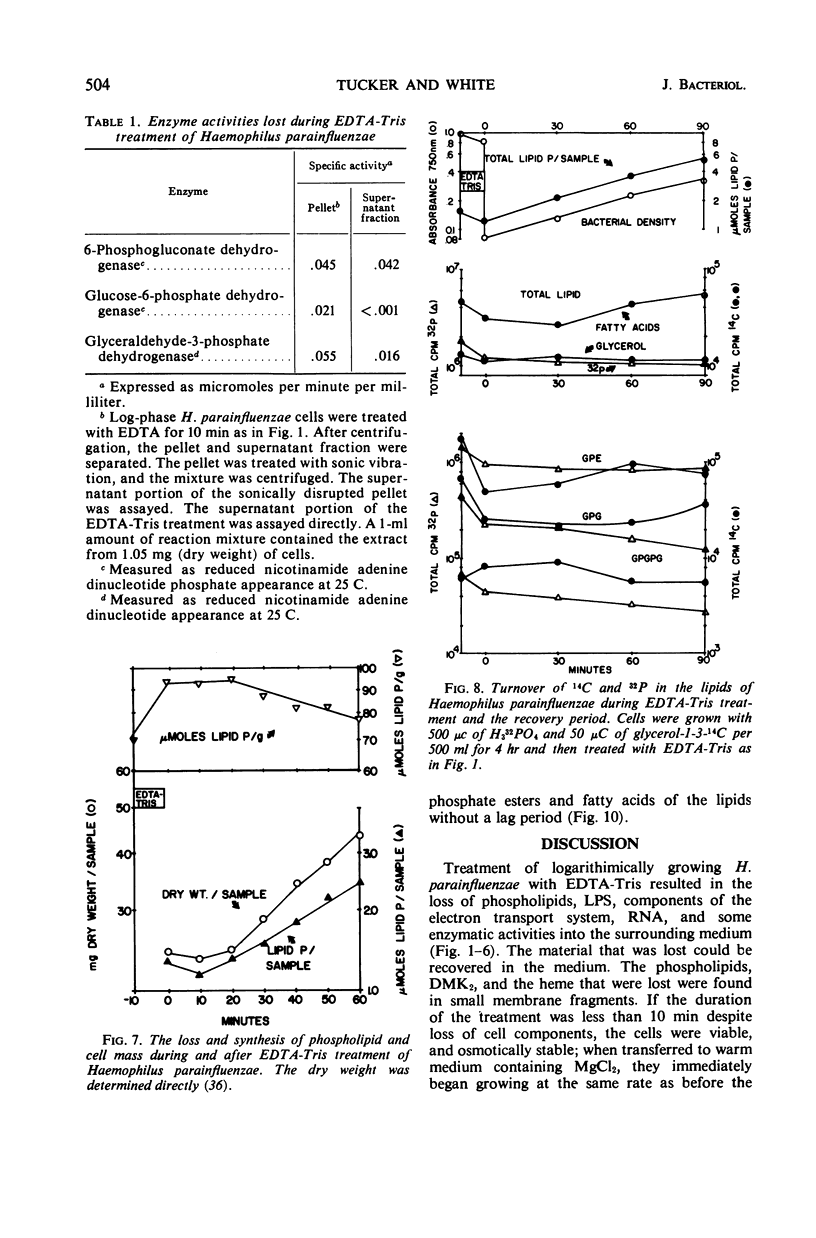
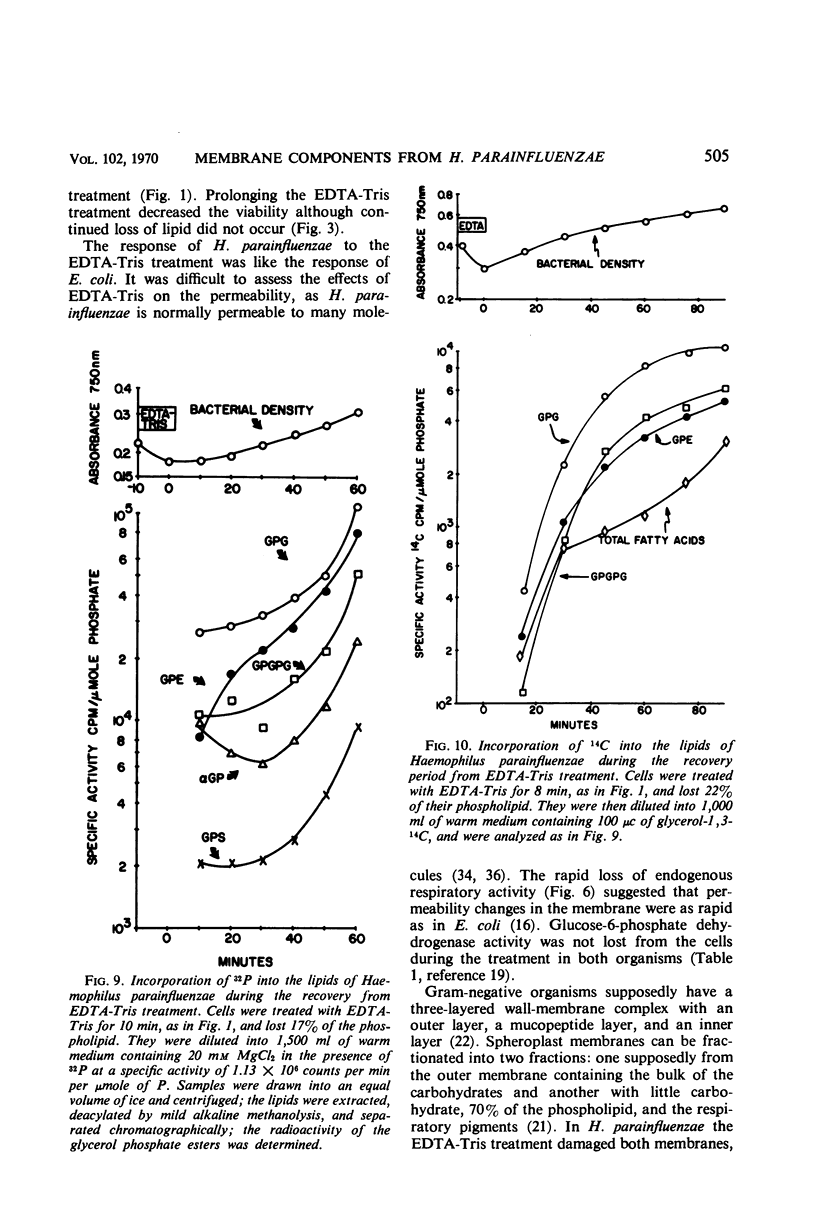
Logarithmically growing Haemophilus parainfluenzae lost 15 to 20% of the phospholipids, demethyl vitamin K2, cytochrome b, and cytochrome c, and 50% of the lipopolysaccharide when incubated in ethylenediaminetetraacetic acid (EDTA)-tris-(hydroxymethyl)aminomethane (Tris) for 10 min. This loss of membrane components occurred without loss in viability, and the lost components were recovered as membrane fragments in the surrounding buffer. The phospholipids recovered in the membrane fragments had a slightly lower specific activity than the phospholipids in the residue. Lysis of a portion of the cells could not account for the release of membrane components, as the cells lost neither glucose-6-phosphate dehydrogenase activity not deoxyribonucleic acid. The treated cells were osmotically stable and contained the same proportions of the individual phospholipids as pretreatment cells. Prolongation of the EDTA-Tris treatment did not induce further loss of phospholipid or demethyl vitamin K2, but caused a decrease in viability. If the cells were returned to the growth medium after 10 min, the cells immediately resumed growth at the pretreatment rate. During growth in the recovery period, the phospholipids increased logarithmically in the pretreatment rate. During growth in the recovery period, the phospholipids increased logarithmically in the pretreatment proportions, although there was a marked decrease in the turnover and a shift from the use of extracellular lipid precursors to the use of intracellular pools of precursors.
Full text
PDF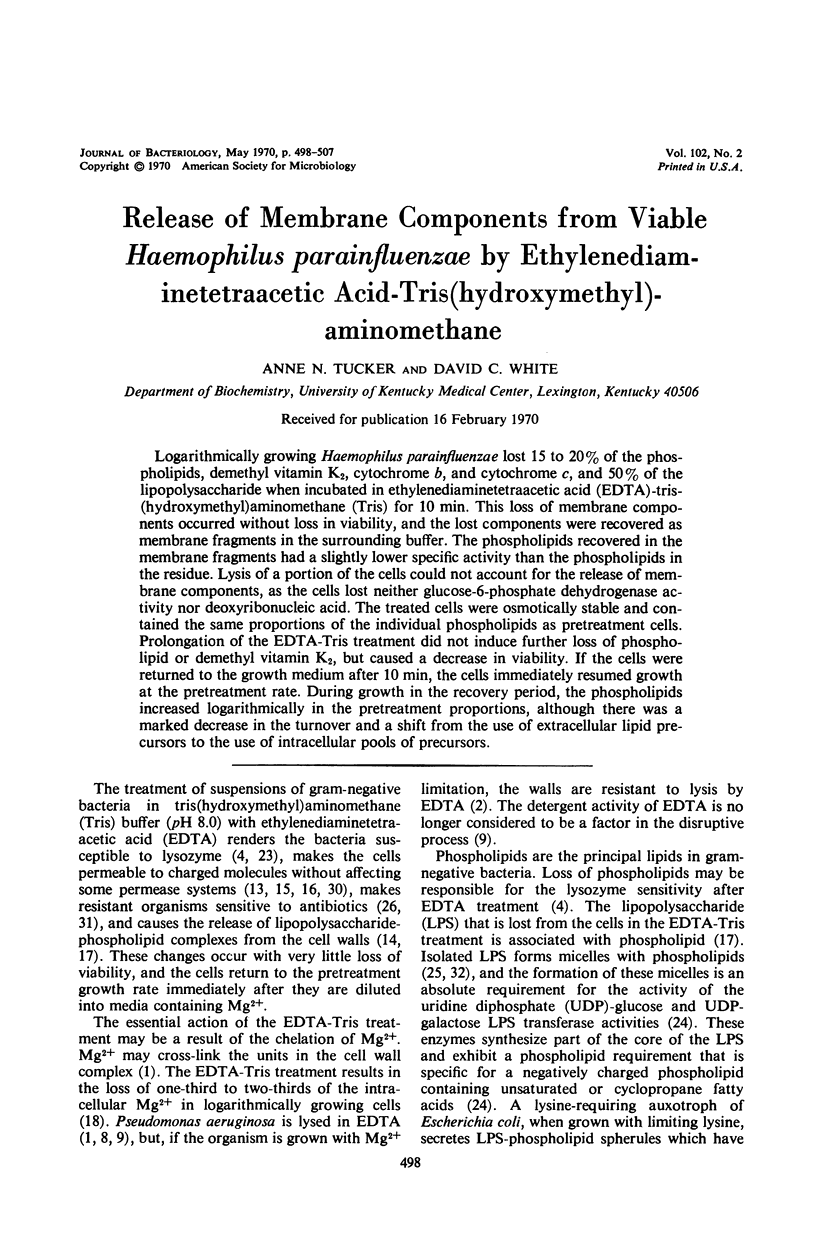




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbell M. A., Eagon R. G. Role of Multivalent Cations in the Organization, Structure, and Assembly of the Cell Wall of Pseudomonas aeruginosa. J Bacteriol. 1966 Aug;92(2):380–387. doi: 10.1128/jb.92.2.380-387.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON A. J., CARTER H. E. PURIFICATION AND CHARACTERIZATION OF THE LIPID A COMPONENT OF THE LIPOPOLYSACCHARIDES FROM ESCHERICHIA COLI. Biochemistry. 1964 Mar;3:411–418. doi: 10.1021/bi00891a018. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Melling J. Loss of sensitivity to EDTA by Pseudomonas aeruginosa grown under conditions of Mg-limitation. J Gen Microbiol. 1968 Dec;54(3):439–444. doi: 10.1099/00221287-54-3-439. [DOI] [PubMed] [Google Scholar]
- COLOBERT L. Etude de la lyse de salmonelles pathogènes provoquée par le lysozyme, après délipidation partielle de la paroi externe. Ann Inst Pasteur (Paris) 1958 Aug;95(2):156–167. [PubMed] [Google Scholar]
- ELBEIN A. D., HEATH E. C. THE BIOSYNTHESIS OF CELL WALL LIPOPOLYSACCHARIDE IN ESCHERICHIA COLI. I. THE BIOCHEMICAL PROPERTIES OF A URIDINE DIPHOSPHATE GALACTOSE 4-EPIMERASELESS MUTANT. J Biol Chem. 1965 May;240:1919–1925. [PubMed] [Google Scholar]
- GROLLMAN A. P., OSBORN M. J. O-PHOSPHORYLETHANOLAMINE: A COMPONENT OF LIPOPOLYSACCHARIDE IN CERTAIN GRAM-NEGATIVE BACTERIA. Biochemistry. 1964 Oct;3:1571–1574. doi: 10.1021/bi00898a031. [DOI] [PubMed] [Google Scholar]
- Gray G. W., Wilkinson S. G. The effect of ethylenediaminetetra-acetic acid on the cell walls of some gram-negative bacteria. J Gen Microbiol. 1965 Jun;39(3):385–399. doi: 10.1099/00221287-39-3-385. [DOI] [PubMed] [Google Scholar]
- JELLINICK P. H. Enzymic oxidation of stilboestrol labelled with carbon-14. Nature. 1960 Apr 9;186:157–158. doi: 10.1038/186157a0. [DOI] [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. I. METABOLISM OF PHOSPHOLIPIDS IN ESCHERICHIA COLI B. J Biol Chem. 1963 Sep;238:2919–2922. [PubMed] [Google Scholar]
- Knox K. W., Vesk M., Work E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1206–1217. doi: 10.1128/jb.92.4.1206-1217.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIVE L. ACTINOMYCIN SENSITIVITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Biochem Biophys Res Commun. 1965 Jan 4;18:13–17. doi: 10.1016/0006-291x(65)90874-0. [DOI] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- Lusk J. E., Williams R. J., Kennedy E. P. Magnesium and the growth of Escherichia coli. J Biol Chem. 1968 May 25;243(10):2618–2624. [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation by density gradient centrifugation of two types of membranes from spheroplast membrane of Escherichia coli K12. Biochim Biophys Acta. 1968 Jan 3;150(1):159–161. doi: 10.1016/0005-2736(68)90020-5. [DOI] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative organisms and the role of versene. Biochim Biophys Acta. 1958 Nov;30(2):225–232. doi: 10.1016/0006-3002(58)90044-1. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Horne R. W. Reassociation of purified lipopolysaccharide and phospholipid of the bacterial cell envelope: electron microscopic and monolayer studies. J Bacteriol. 1967 May;93(5):1705–1721. doi: 10.1128/jb.93.5.1705-1721.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Pearlman M. The role of cell envelope phospholipid in the enzymatic synthesis of bacterial lipopolysaccharide. Structural requirements of the phospholipid molecule. J Biol Chem. 1966 Mar 25;241(6):1386–1392. [PubMed] [Google Scholar]
- Russell A. D. Effect of magnesium ions and ethylenediamine tetra-acetic acid on the activity of vancomycin against Escherichia coli and Staphylococcus aureus. J Appl Bacteriol. 1967 Aug;30(2):395–401. doi: 10.1111/j.1365-2672.1967.tb00314.x. [DOI] [PubMed] [Google Scholar]
- Sinclair P. R., White D. C. Effect of nitrate, fumarate, and oxygen on the formation of the membrane-bound electron transport system of Haemophilus parainfluenzae. J Bacteriol. 1970 Feb;101(2):365–372. doi: 10.1128/jb.101.2.365-372.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss J. G. Effects of organic cations on the gram-negative cell wall and their bactericidal activity with ethylenediaminetetra-acetate and surface active agents. J Gen Microbiol. 1967 Sep;48(3):391–400. doi: 10.1099/00221287-48-3-391. [DOI] [PubMed] [Google Scholar]
- WHITE D. C. Cytochrome and catalase patterns during growth of Haemophilus parainfluenzae. J Bacteriol. 1962 Apr;83:851–859. doi: 10.1128/jb.83.4.851-859.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C. SYNTHESIS OF 2-DEMETHYL VITAMIN K2 AND THE CYTOCHROME SYSTEM IN HAEMOPHILUS. J Bacteriol. 1965 Feb;89:299–305. doi: 10.1128/jb.89.2.299-305.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M. M., Rothfield L. The reassociation of lipopolysaccharide, phospholipid, and transferase enzymes of the bacterial cell envelope. Isolation of binary and ternary complexes. J Biol Chem. 1968 Mar 25;243(6):1320–1328. [PubMed] [Google Scholar]
- Weiser R., Asscher A. W., Wimpenny J. In vitro reversal of antibiotic resistance by ethylenediamine tetraacetic acid. Nature. 1968 Sep 28;219(5161):1365–1366. doi: 10.1038/2191365a0. [DOI] [PubMed] [Google Scholar]
- White D. C., Cox R. H. Indentification and localization of the fatty acids in Haemophilus parainfluenzae. J Bacteriol. 1967 Mar;93(3):1079–1088. doi: 10.1128/jb.93.3.1079-1088.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. Effect of glucose on the formation of the membrane-bound electron transport system in Haemophilus parainfluenzae. J Bacteriol. 1967 Feb;93(2):567–573. doi: 10.1128/jb.93.2.567-573.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Extraction, characterization, and cellular localization of the lipids of Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1854–1867. doi: 10.1128/jb.94.6.1854-1867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. Lipid composition of the electron transport membrane of Haemophilus parainfluenzae. J Bacteriol. 1968 Oct;96(4):1159–1170. doi: 10.1128/jb.96.4.1159-1170.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. The obligatory involvement of the electron transport system in the catabolic metabolism of Haemophilus parainfluenzae. Antonie Van Leeuwenhoek. 1966;32(2):139–158. doi: 10.1007/BF02097454. [DOI] [PubMed] [Google Scholar]
- White D. C., Tucker A. N. Phospholipid metabolism during bacterial growth. J Lipid Res. 1969 Mar;10(2):220–233. [PubMed] [Google Scholar]
- White D. C., Tucker A. N. Phospholipid metabolism during changes in the proportions of membrane-bound respiratory pigments in Haemophilus parainfluenzae. J Bacteriol. 1969 Jan;97(1):199–209. doi: 10.1128/jb.97.1.199-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. A., White D. C. Formation of a functional electron transport system during growth of penicillin-induced spheroplasts of Haemophilus parainfluenzae. J Bacteriol. 1966 Mar;91(3):1356–1362. doi: 10.1128/jb.91.3.1356-1362.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


