Abstract
To evaluate changes during the development of proteinuria, podocyte morphology and protein expression were evaluated in spontaneously proteinuric, Dahl salt-sensitive (Dahl SS) rats. Dahl SS rats on a low-salt diet were compared with spontaneously hypertensive rats (SHR) at age 2, 4, 6, 8, and 10 weeks. Blood pressure, urinary protein excretion, urinary albumin excretion, and podocyte morphology were evaluated. In addition, the expression of 11 podocyte-related proteins was determined by analyzing protein and mRNA levels. In Dahl SS rats, proteinuria became evident around week 5, increasing thereafter. SHR rats remained non-proteinuric. Dahl SS rats showed widespread foot process effacement at 10 weeks. At ≤8 weeks, expression and distribution of the podocyte proteins was similar between the two strains, except for the protein podoplanin. At 4 weeks, podoplanin began decreasing in the glomeruli of Dahl SS rats in a focal and segmental fashion. Podoplanin loss increased progressively and correlated with albuminuria (r = 0.8, P < 0.001). Double labeling experiments revealed increased expression of the podocyte stress marker desmin in glomerular areas where podoplanin was lost. Dahl SS rats did not show podoplanin gene mutations or decreased mRNA expression. Thus, podocyte morphology and the expression and distribution of most podocyte-specific proteins were normal in young Dahl SS rats, despite marked proteinuria. Our study suggests that decreased expression of podoplanin plays a role in the decrease of glomerular permselectivity.
The permselectivity of the glomerular filtration barrier restricts passage of proteins into Bowman’s space. Loss of permselectivity leads to proteinuria, which is common in renal diseases of diverse origin. Proteinuria is related to the progression of renal and cardiovascular disease.1,2,3 Although the details of glomerular filtration remain unknown, it is clear that the glomerular visceral epithelial cell or podocyte is an important component of the glomerular filtration barrier. Damage to podocytes is frequently involved in the pathogenesis of glomerular diseases.4
Podocyte damage can be the result of changes in individual podocyte-associated proteins. Examples include mutations in the genes that encode nephrin, podocin, CD2AP, and α-actinin-4.5,6,7,8 Such mutations can result in both congenital and hereditary forms of glomerular dysfunction. Podocyte damage also appears to be of pathogenic importance in acquired diseases. For example, in diabetic nephropathy and IgA nephropathy, podocyte loss is related to disease severity.9,10,11 Similarly, animal models have shown that loss of podocytes is related to the development of proteinuria and glomerulosclerosis.12,13,14,15
A change in podocyte morphology often accompanies proteinuria. In the normal glomerulus, the podocyte has an arborized phenotype, and its terminal branches or foot processes cover the outer wall of the glomerular capillaries. In proteinuric states, this morphology is typically lost as the podocyte converts to a flatter epithelial cell, a process referred to as “foot process effacement.” In foot process effacement, the cytoskeleton that normally supports the delicate architecture of the foot processes is condensed at the basal side of the flattened podocytes.16
We previously studied the mRNA and protein expression of several podocyte-associated proteins in acquired proteinuric diseases.17 Based on the results of that study, we hypothesized that the changes in expression of podocyte proteins represented a compensatory reaction of the podocyte to the occurrence of proteinuria. Accordingly, we wanted to investigate how the expression of podocyte-associated proteins is regulated during the development of proteinuria, and how changes in expression are related to podocyte morphology. We studied proteins that have been shown to be involved in congenital and hereditary nephrotic syndromes, as well as podocyte proteins that have been studied in animal models. Among these is the glycoprotein podoplanin, of which the expression was previously found to change in the puromycin aminonucleoside nephrosis model of the rat.18 Studying human kidney biopsy samples does not allow the rigorous evaluation of changes over time. In this study, we therefore used the Dahl salt-sensitive rat (Dahl SS) proteinuric model19 to evaluate changes in podocyte morphology and expression of podocyte-associated proteins during the development of proteinuria.
Materials and Methods
Animals and Study Design
We compared the spontaneously proteinuric Dahl SS rat strain with non-proteinuric spontaneous hypertensive (SHR) rats. Male Dahl SS and SHR rats were obtained from colonies at the Freie Universität Berlin as previously reported.19 All animals were fed a low-salt diet containing 0.2% NaCl by weight; on this diet, Dahl SS rats develop mild spontaneous hypertension comparable to SHR rats. To study changes in expression of podocyte-associated proteins during the development of proteinuria, groups of rats (n = 5 to 8 rats per group) were studied at 2, 4, 6, 8, and 10 weeks of age. Experiments were performed in accordance with institutional guidelines.
Urinalysis and Blood Pressure Measurements
Urinalysis was performed in each rat. Rats that were 4 weeks of age or older were placed in metabolic cages for a 24-hour period. In the 2-week-old rats, a urine sample was obtained by bladder punction before the perfusion procedure described below. Urinary albumin excretion was subsequently determined by enzyme-linked immunosorbent assay.20 Urinary protein excretion rates were determined using the Bradford method. In rats of 6 weeks of age or older, systolic blood pressure was determined using the tail-cuff method, as described previously.20 Three blood pressure measurements were performed each day on two consecutive days.
Perfusion, Tissue Preservation, and Isolation of Glomeruli
Rats were anesthetized using intraperitoneal injection of ketamine/xylazine. After the abdomen was opened, the aorta was ligated below the diaphragm and both kidneys were perfused with PBS. After clamping the vascular pole, the right kidney was removed. For electron microscopy, small pieces of the cortex of the right kidney were immersion-fixed for 24 hours in 0.1M glutaraldehyde containing cacodylate and were processed according to standard procedures. For histology and immunohistochemistry, part of the remaining right kidney was embedded in paraffin, and the other part was snap frozen and stored at −80°C.
The left kidney was used for isolation of glomeruli. Because the standard differential sieving method is not suitable for isolation of glomeruli in very young animals, we used the magnetic retraction method to isolate glomeruli from all animals.21 Briefly, the left kidney was perfused with sonicated 1.25% suspension of Fe3O4 (Sigma-Aldrich Chemicals, Zwijndrecht, The Netherlands) in PBS, removed, and placed in ice-cold PBS. The kidney was then cut into pieces and pressed through a 106-μm (weeks 2 and 4) or 150-μm (weeks 6 to 10) mesh filter. The glomeruli were isolated by holding the suspension to a magnet. The fluid was discarded, and the glomeruli were resuspended in fresh PBS. After repeating this procedure two times, glomeruli were pelleted and stored at −80°C until RNA extraction.
RNA isolation, cDNA Synthesis, and Real-Time PCR
RNA was isolated from the glomeruli using the TRIzol (Invitrogen) method. Total RNA (0.5 μg) was reverse transcribed into cDNA using Avian Myeloblastosis Virus reverse transcriptase (Roche Diagnostics).
Primers (Isogen Bioscience) for 14 podocyte-expressed genes were designed using BeaconDesigner 4.0 software (PREMIER Biosoft International). To prevent genomic contamination, all primers, except those for the synaptopodin gene that contains only one exon, were chosen to span at least one splice-site. Primers sequences are listed in Table 1. Real-time PCR was performed using an iCycler real-time PCR machine with iCyclerIQ 3.1 software (Bio-Rad laboratories). Sybrgreen was used as the fluorescent dye. The mean expression levels of three housekeeping genes (Hmbs, Tbp, and Hprt) were used to correct for variations in the quantity of input cDNA.
Table 1.
Primers
| Name | Symbol | mRNA sequence | Forward | Reverse | Amplicon size |
|---|---|---|---|---|---|
| Actinin alpha 4 | Actn4 | NM_031675 | 5′-GGCTATGAAGAATGGCTGCTGAATG-3′ | 5′-AGGGTGGCTGTCTCGTAGTCC-3′ | 151 |
| CD2-associated protein | Cd2ap | NM_181475 | 5′-TCCAGCGAATCAGCACCTACG-3′ | 5′-CACTCCACCAGCCTTCTTCTACC-3′ | 197 |
| Dystroglycan | Dag1 | XM_343483 | 5′-GATGGCACGGCTGTTGTTGG-3′ | 5′-GCACTCACTGAGATGTAATGGACAC-3′ | 199 |
| Ezrin | Vil2 | NM_019357 | 5′-CTGGACGACCGTAACGAGGAG-3′ | 5′-CTTGCCGCATGTTCTCATTGTG-3′ | 156 |
| Glepp1 | Ptpro | NM_017336 | 5′-TGGATGGTCGTGGCAGAAGG-3′ | 5′-TGGAGGCAGGCTAAGGATGG-3′ | 107 |
| Hsp-27 | Hspb1 | NM_031970 | 5′-AAGGAAGGCGTGGTGGAGATC-3′ | 5′-ACCTGGAGGGAGCGTGTATTTC-3′ | 101 |
| Megalin | Lrp2 | NM_030827 | 5′-CACGACCGCTGCTTACAACTG-3′ | 5′-GATGGCATGGCACCGATTCAC-3′ | 128 |
| Neph1 | Kirrel1 | NM_207606 | 5′-GGATGGCGGTAAGGTGGAGTG-3′ | 5′-CGTTATTGATGGTGAGAGTGGACAG-3′ | 163 |
| Nephrin | Nphs1 | NM_022628 | 5′-CGTCAGCATCAGCAGCAACC-3′ | 5′-AGCCACATCTTCCAGCCTCTC-3′ | 72 |
| Podocalyxin | Podxl | NM_138848 | 5′-GGCTGTGTTTGAACTGCTGAAGG-3′ | 5′-ACGATGGTGATGATGAGAGGAAGG-3′ | 135 |
| Podocin | Nphs2 | NM_130828 | 5′-TGGACTCAGTGACCTGTGTTTGG-3′ | 5′-CAGCAATCACCCGCACTTTGG-3′ | 138 |
| Podoplanin | Pdpn | NM_019358 | 5′-CCAGCCACTCCACGGACAAG-3′ | 5′-GGGTCACTACAGCCAAGCCATC-3′ | 101 |
| Ras homolog gene family, member A | Rhoa | NM_057132 | 5′-GCACACAAGGCGGGAGTTAG-3′ | 5′-CGTCTTTGGTCTTTGCTGAACAC-3′ | 121 |
| Synaptopodin | Synpo | NM_021695 | 5′-AGCCTAAGGTGACGCCGAATC-3′ | 5′-TCTCTGCCTCCGCTTCTCATC-3′ | 70 |
| Wilms tumor 1 | Wt1 | NM_031534 | 5′-TGTGACTTCAAGGACTGCGAGAG-3′ | 5′-GGTGTGGGTCTTCAGGTGGTC-3′ | 143 |
| Hypoxanthine guanine phosphoribosyl transferase | Hprt | NM_012583 | 5′-GGCTATAAGTTCTTTGCTGACCTG-3′ | 5′-AACTTTTATGTCCCCCGTTGA-3′ | 138 |
| TATA box binding protein | Tbp | NM_001004198 | 5′-ACCGTGAATCTTGGCTGTAAACTTG-3′ | 5′-GCAGTTGTTCGTGGCTCTCTTATTC-3′ | 122 |
| Hydroxymethylbilane synthase | Hmbs | NM_013168 | 5′-TGAAGGATGTGCCTACCATACTACC-3′ | 5′-GCAAGGTTTCCAGGGTCTTTCC-3′ | 123 |
Immunohistochemistry, Immunofluorescence, and Morphometrics
Three-micron paraffin sections were deparaffinized, rehydrated, and used for immunostaining after appropriate antigen retrieval procedures. Three-micron cryosections were used untreated or after fixation with acetone/ethanol for 5 minutes and 100% ethanol for 10 minutes.
Endogenous peroxidase activity was blocked for 15 minutes in 0.1% H2O2 in water. After washing with PBS, sections were incubated with primary antibodies diluted in 1% bovine serum albumen in PBS for 2 hours. Details regarding antibodies, fixation, and antigen retrieval are listed in Table 2. After 1 hour incubation with the secondary antibody, the slides were developed with diaminobenzidine. Slides were counterstained with hematoxylin, dehydrated, and mounted. To minimize variations in intensity, staining for each antibody was performed on all sections during one session.
Table 2.
Antibodies
| Epitope | Cryo/paraffin | Antigen retrieval | Primary antibody, dilution, and source | Secondary antibody | Reference |
|---|---|---|---|---|---|
| α3 integrin | Cryo | None | Rabbit anti-α3 integrin, 1:800, Chemicon cat# AB1920 | Anti-rabbit Envision* | |
| α-actinin-4 | Cryo | None | Rabbit polyclonal antibody 6A3, 1:4000 | Anti-rabbit Envision | 8 |
| α-dystroglycan | Paraffin | Citrate† | Mouse mAb VIA4-1, 1:150, Upstate cat# 05-298 | Anti-mouse Envision | |
| Desmin | Paraffin/cryo | Tris/EDTA‡ | Mouse mAb clone 33, 1:750 | Anti-mouse Envision, Alexa | |
| Ezrin | Paraffin | Tris/EDTA | Mouse mAb, 1:100 | Anti-mouse Envision | |
| Nephrin (extra-cellular domain) | Cryo | None | Mouse mAb 5-1-6, 1:200 | Anti-mouse Envision | 51 |
| Podocalyxin | Paraffin | Proteinase K§ | Mouse mAb 5A, 1:300 | Anti-mouse Envision | 52 |
| Podocin (C-terminal part) | Paraffin | Proteinase K | Rabbit polyclonal antibody P35, 1:3000 | Anti-rabbit Envision | 53 |
| Podoplanin | Paraffin/cryo | Proteinase K | Rabbit polyclonal anti-rat podoplanin, 1:3000 | Anti-rabbit Envision, Alexa | 54 |
| Synaptopodin | Paraffin | Citrate | Mouse mAb G1D4, 1:4, Progen cat# 65194 | Anti-mouse Envision | |
| Wilms tumor 1 | Paraffin | Tris/EDTA | Mouse mAb WLM04, 1:50, Abcam cat# ab3236 | Anti-mouse Envision |
Envision: DakoCytomation, Glostrup, Denmark, Cat# K4001 (anti-mouse Envision HRP) and K4003 (anti-rabbit Envision HRP).
Citrate: 0.1 mol/L citrate buffer, pH 6.0.
Tris/EDTA: 0.1 mol/L Tris/EDTA, pH 9.0
Proteinase K: DakoCytomation, Glostrup Denmark, Cat# S3020.
Podoplanin staining was evaluated on a semiquantitative scale: 0, normal intensity and pseudo-linear staining pattern; 1, loss of staining <25% of the glomerular surface or a granular staining pattern; 2, loss of staining involving 25 to 75% of the glomerular surface; and 3, loss of staining in >75% of the glomerular surface.
Podoplanin and desmin were costained on paraffin sections and cryosections using the appropriate Alexa 466- and 536-conjugated secondary antibodies. Staining was evaluated with a Zeiss LSM 510 confocal microscope.
For morphometric analysis of the size of the glomeruli and the number of podocytes, we used slides stained for WT-1, a podocyte-specific transcription factor that is used to identify and count podocytes in tissue sections.22 Slides stained for WT-1 were evaluated with a Zeiss Axioplan microscope equipped with a Sony DXC-950P 3CCD color camera (Sony Corporation, Tokyo, Japan). Ten randomly chosen regions of the outer glomerular cortex were photographed at ×200 magnification. The surface area of all glomeruli in the photographs was determined using ImageJ 1.34 software (National Institutes of Health, http://rsb.info.nih.gov/ij). The number of WT-1 positive nuclei in each glomerular cross section was counted using the same software. The number of podocytes per glomerulus and the glomerular volume per podocyte were calculated from these measurements.22
Periodic acid-Schiff staining was performed to determine changes in morphology.
Sequencing
To check for genetic differences between the Dahl SS and SHR rats, we sequenced the full-length podoplanin cDNA in a representative rat from each strain. The sequences of the primers used are available on request.
Laser Capture Microscopy
We used laser capture microscopy to determine whether there were differences in podoplanin mRNA expression in glomeruli that showed a difference in podoplanin protein expression. Four-micron cryosections were obtained from four 8-week-old Dahl SS rats. One section was used for immunohistochemical staining for podoplanin to identify glomeruli that had either completely lost or retained podoplanin expression. The cross-section of the same glomerulus was identified in the sequential unstained section and isolated by laser capture microdissection using a PALM Laser-MicroBeam system (Wolfratshausen, Germany). RNA was isolated from the isolated glomeruli, and quantitative measurements of the expression of podoplanin and RhoA mRNA were performed as described above.
Electron Microscopy and Immunoelectron Microscopy
Small pieces of the cortex were fixed in 1.5% glutaraldehyde and 1% paraformaldehyde for 24 hours and then stored in 0.1 M cacodylate buffer with 6% sucrose. After postfixation in 1% reduced osmium in 0.1 M cacodylate buffer, the samples were dehydrated and embedded in EPON. Ultrathin sections were cut using a Leica Ultracut microtome and mounted on uncoated copper grids. Sections were contrasted with uranyl acetate and lead citrate before evaluation with a JEOL JEM-1011 electron microscope equipped with a digital camera.
Random pictures of 2 to 3 glomeruli were taken in two rats per group using ×15,000 magnification. Using image analysis, we determined the extent of foot process effacement by measuring the average foot process width, as described previously.17
For immunoelectron microscopy, small pieces of the cortex from 8-week-old Dahl SS rats were fixed in 4% paraformaldehyde in 0.1 M PBS and stored in 0.1% paraformaldehyde in 0.1 M PBS. Immunogold labeling was performed on ultrathin frozen sections as described previously.18
Statistical Analysis
Data are reported as the mean ± SD. Analysis of variance with a Least Significant Difference posthoc correction was used to test for differences between groups. Correlations were tested using Pearson’s or Spearman’s tests, where appropriate. P < 0.05 was considered significant.
Results
Proteinuria and Blood Pressure
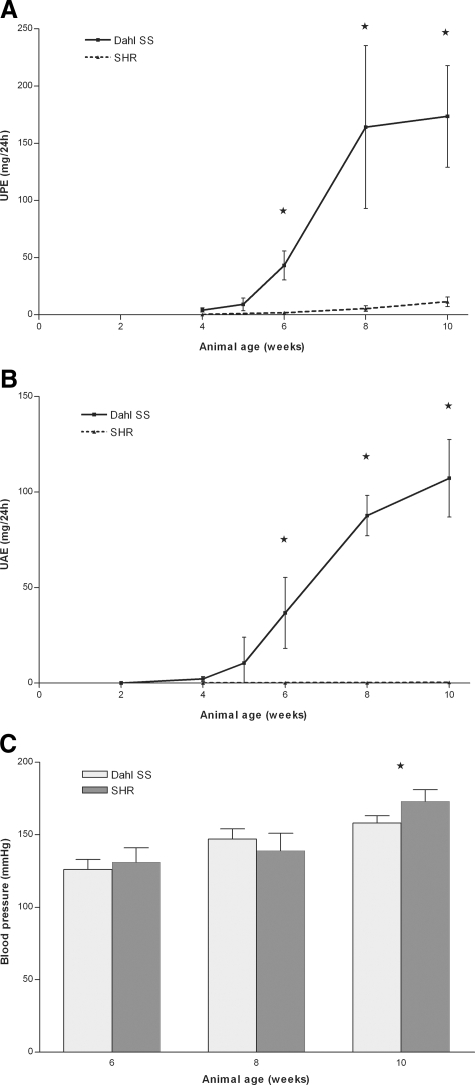
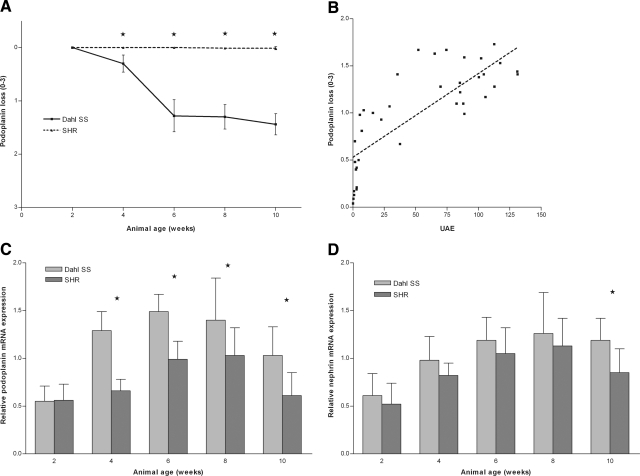
Starting at 4 weeks, proteinuria was increasingly evident in Dahl SS rats. As expected, SHR rats remained non-proteinuric throughout the time course of the study (Figure 1, A and B). Both rats developed hypertension starting at the age of 8 weeks (Figure 1C). The blood pressure of the 10-week old SHR rats was significantly higher than that of Dahl SS rats of the same age (P < 0.05).
Figure 1.
Urinary protein excretion (A), urinary albumin excretion (B), and blood pressure (C) in Dahl SS and SHR rats. As expected, SHR rats remained non-proteinuric throughout the time course of the study. Dahl SS rats developed proteinuria and albuminuria between 4 and 6 weeks of age (A and B). In Dahl SS rats, measurements were taken at an additional time point, 5 weeks of age, to obtain a more detailed view of the early phase of proteinuria development in these rats. Both rat strains developed hypertension starting at week 8. At 10 weeks of age, the blood pressure of SHR rats was significantly higher than that of Dahl SS rats (C). An asterisk indicates P < 0.05.
Glomerular Volume, Podocyte Number, and Light Microscopy
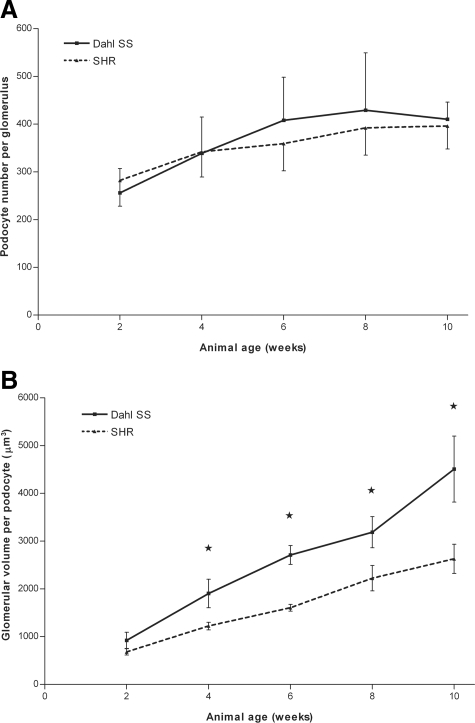
The number of podocytes per glomerulus, as determined using WT-1 staining, was not different between the two rat strains (Figure 2A). The glomerular volume was higher in Dahl SS rats than in SHR rats. The glomerular volume per podocyte22 was also higher in Dahl SS rats (Figure 2B); this difference was statistically significant from 4 weeks of age onwards. Fibrotic changes in glomeruli or in the interstitium were not observed at any time point.
Figure 2.
Number of podocytes per glomerulus (A) and glomerular volume per podocyte (B) in Dahl SS and SHR rats. The total number of podocytes per glomerulus increased over time in both Dahl SS and SHR rats, and remained stable from 6 to 8 weeks onwards at 350 to 400 podocytes per glomerulus. Although there was no difference in absolute number of podocytes per glomerulus, the glomerular volume per podocyte was higher in Dahl SS compared with SHR rats. The glomerular volume per podocyte is an index used to indicate the glomerular volume that one podocyte ‘serves’ and is calculated as the mean glomerular volume divided by the mean number of podocytes per glomerulus. This difference was statistically significant from week 4 onward. An asterisk indicates P < 0.05.
Electron Microscopy
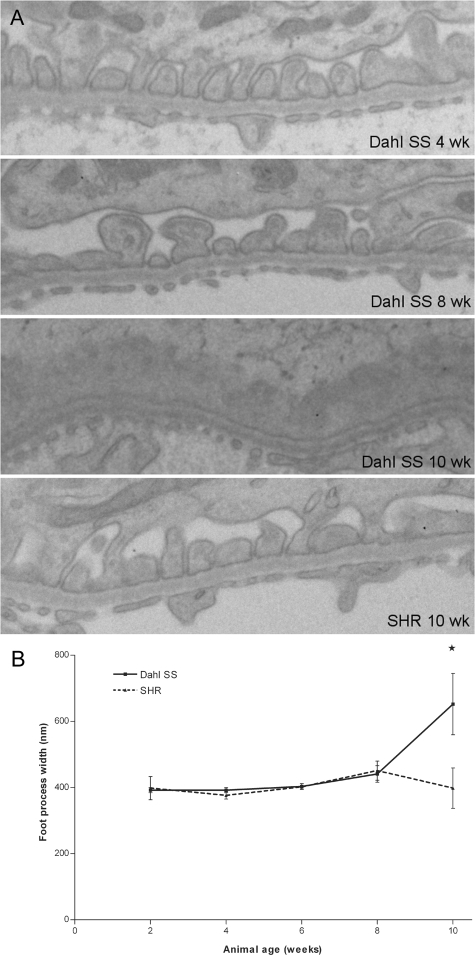
Throughout the first 8 weeks of life, both the Dahl SS and the SHR rats generally showed normal ultrastructural glomerular morphology. In 6- and 8-week-old Dahl SS rats, subtle coarsening of the foot processes was occasionally seen, together with protein droplets in the podocyte cell bodies and major processes (Figure 3A). Ten-week-old Dahl SS rats showed more widespread foot process effacement. Quantification of the mean foot process width underscored the normal ultrastructure of the podocyte in the first 8 weeks of life (Figure 3B). Detachment of podocytes from the glomerular basement membrane was not seen at any time point, and alterations in the glomerular basement membrane morphology were not observed.
Figure 3.
Electron microscopy of podocytes in Dahl SS and SHR rats (A), and evaluation of the mean foot process width (B). In 4-week-old Dahl SS rats (a, top panel), the podocyte ultrastructure was normal, showing regularly spaced foot processes. Occasionally, protein droplets were observed in podocyte major processes and cell bodies (arrowheads). At 8 weeks, subtle coarsening of the foot processes was observed sporadically in Dahl SS rats (A, second panel). At 10 weeks of age, widespread foot process effacement was observed in segmental areas of the glomerulus, with condensation of the actin cytoskeleton at the basal site of the effaced processes (arrow). Microvillous transformation of podocytes was observed frequently at this time point. In contrast, the podocytes of SHR rats had normal ultrastructure throughout the time course studied (A, bottom panel). Original magnification: ×15,000. Quantification of the mean foot process width showed significant effacement only in the 10-week-old Dahl SS rats (B). An asterisk indicates P < 0.05.
Protein Expression
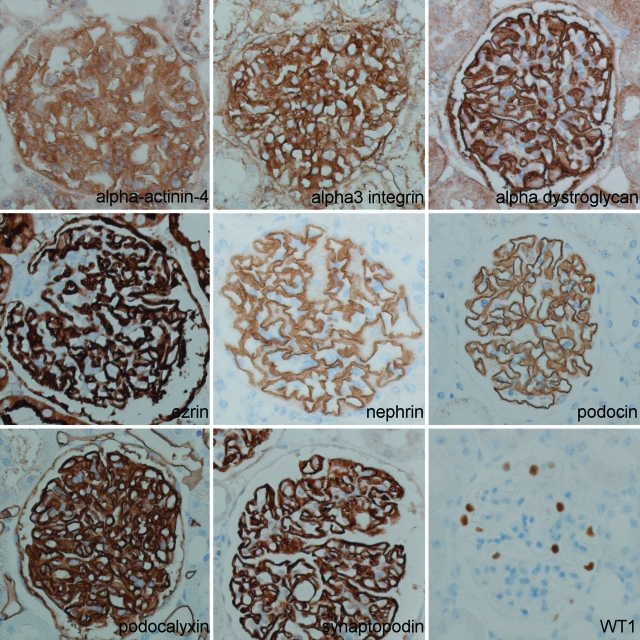
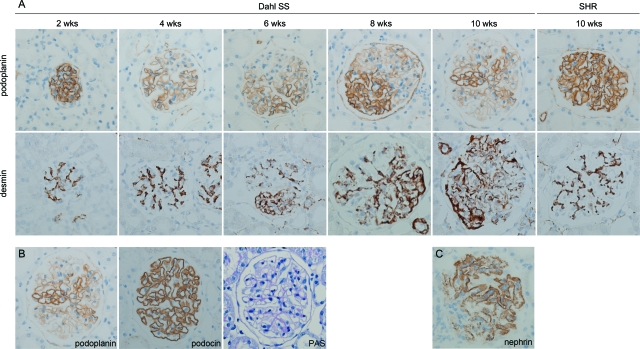
Immunohistochemistry showed that expression of α3-integrin, α-actinin-4, α-dystroglycan, ezrin, podocalyxin, podocin, and synaptopodin, was similar at all time points in both rat strains (Figure 4). Expression of the nephrin protein showed a normal pseudo-linear staining pattern throughout the first 8 weeks of life; however, by 10 weeks of age, expression became more granular in a focal and segmental fashion in Dahl SS rats (Figure 5C). In contrast to all other proteins studied, and only in Dahl SS rats, expression of the podoplanin protein decreased in a focal and segmental fashion. The decrease in podoplanin expression started as early as week 4 and increased with age (Figure 5A and Figure 6A). There was a strong correlation between development of albuminuria and loss of podoplanin protein expression (r = 0.8, P < 0.001, Figure 6B). The loss of podoplanin protein expression was not related to changes in glomerular morphology, and did not coincide with changes in expression of other podocyte-associated proteins (Figure 5B). Podoplanin expression in endothelial cells in intrarenal lymphatic vessels was not diminished.
Figure 4.
Immunohistochemistry of podocyte proteins. Despite marked proteinuria, 10-week-old Dahl SS rats showed normal expression of α-actinin-4, α3 integrin, α-dystroglycan, ezrin, nephrin, podocin, podocalyxin, synaptopodin, and WT1. Original magnification: ×400.
Figure 5.
Podoplanin protein expression in Dahl SS glomeruli was progressively lost in a focal and segmental fashion. Loss of podoplanin protein expression was first seen in 4-week-old Dahl SS rats and increased thereafter. In contrast, podoplanin protein expression remained normal in SHR rats throughout the time course of the study. The upper row of images in A shows podoplanin staining in Dahl SS rats at the indicated time points and in a 10-week-old SHR rat. The lower row of images in A shows desmin staining in Dahl SS rats at the indicated time points and in a 10-week-old SHR rat. Starting at 6 weeks, desmin expression was visible in the extramesangial areas of Dahl SS glomeruli. Expression level increased with age. No change in desmin expression was observed in aging SHR rats. Sequential sections of the kidney of a 10-week-old Dahl SS rat stained with anti-rat podoplanin antibodies, anti-podocin antibodies, and with PAS show that loss of podoplanin is not related to morphological alterations observed by light microscopy or to alterations in podocin expression (B). Nephrin expression was diminished sporadically in segmental parts of glomeruli, but only in 10-week-old Dahl SS rats (C). Original magnification: ×400.
Figure 6.
Loss of podoplanin protein expression was determined using a semiquantitative method. The loss of podoplanin protein staining in Dahl SS samples was statistically significant from week 4 onward (A). The extent of loss of podoplanin expression was positively correlated with urinary albumin excretion rates (r = 0.8, P < 0.001) B: In contrast, there was a slight but significant increase in podoplanin mRNA expression levels in Dahl SS rats compared with SHR rats at weeks 4 to 10 (C). Nephrin mRNA expression was increased only in 10-week-old Dahl SS rats (D). An asterisk indicates P < 0.05.
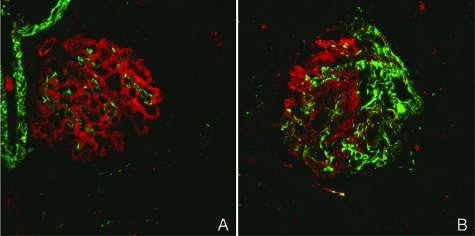
Expression of the intermediate filament desmin can be used as a marker of podocyte stress or damage in rat podocytes. Indeed, we found an increase in desmin protein expression during the time course (Figure 5A). When desmin and podoplanin were costained, we found that desmin expression was absent in glomeruli that retained podoplanin expression. In contrast, segmental loss of podoplanin was mirrored by an increase in desmin expression in that particular segment (Figure 7). Desmin expression seemed to lag behind podoplanin loss.
Figure 7.
Podoplanin (red) and desmin (green) were costained in kidney sections from 8-week-old Dahl SS rats. Normal glomeruli show desmin expression in renal blood vessels and glomerular mesangium and podoplanin expression in podocytes. Desmin and podoplanin did not colocalize (A). In glomeruli that showed segmental loss of podoplanin expression, increased desmin expression was observed in the podoplanin-negative areas (B). Original magnification: ×630.
mRNA Expression
We also investigated the expression of 14 podocyte-expressed genes at the mRNA level (Table 1), revealing varied expression patterns. In both strains, the expression of podocyte-specific genes was lowest at the early time points. At 4 weeks of age, the podoplanin and ezrin genes were more highly expressed in Dahl SS than in SHR rats. From 4 to 10 weeks, the podoplanin mRNA expression levels were significantly increased in Dahl SS rats compared with SHR rats (Figure 6C), mirroring the decreased podoplanin protein expression in Dahl SS at these time points (Figure 5A). Other genes (NEPH-1, podocin, synaptopodin, α-actinin-4) showed late up-regulation (at 8 to 10 weeks) of mRNA expression in Dahl SS rats as compared with SHR rats.
Only at 10 weeks were nephrin mRNA expression levels significantly elevated in Dahl SS rats compared with SHR rats (P < 0.05, Figure 6D), again mirroring the nephrin protein staining results in Dahl SS rats at this time point (Figure 5C). Despite marked proteinuria, the pivotal podocyte protein transcription factor WT-1 was not differentially regulated (data not shown).
Sequencing and Laser Capture Microdissection
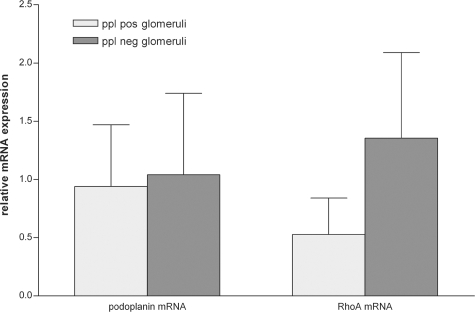
Sequencing the full-length podoplanin cDNA in a representative Dahl SS and SHR rat did not reveal mutations in the Dahl SS podoplanin gene. There was no difference in podoplanin mRNA expression in glomeruli that had completely lost podoplanin protein expression versus those that had retained normal podoplanin expression (Figure 8). Podoplanin has been linked to the activity of the small GTPase RhoA,23 so we evaluated whether such a link existed in glomeruli. We found that RhoA mRNA levels were increased in sections of glomeruli that lacked podoplanin expression (Figure 8), although this did not reach statistical significance.
Figure 8.
In 8-week-old Dahl SS rats, podoplanin mRNA expression was not different in glomeruli that showed extensive loss of podoplanin protein (ppl) expression compared with glomeruli that had retained podoplanin expression. RhoA mRNA expression was higher in glomeruli that no longer expressed the podoplanin protein, although this difference did not reach statistical significance.
Immunoelectron Microscopy
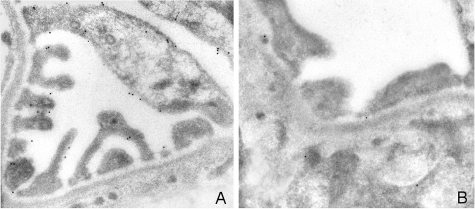
Immunogold labeling of podoplanin in 8-week-old Dahl SS rats revealed that podoplanin was extensively expressed in areas that showed no foot process effacement. Conversely, foot process effacement was accompanied by a loss of podoplanin expression (Figure 9).
Figure 9.
Immunogold electron microscopic analysis of podoplanin labeling in 8-week-old Dahl SS rats. Podoplanin labeling was most prominent in glomerular areas that showed no foot process effacement (A). Podoplanin labeling was weak or absent in areas in which foot process effacement was observed (B).
Discussion
In this study, spontaneous development of proteinuria in Dahl SS rats preceded changes in podocyte morphology and changes in expression of most of the observed podocyte-specific proteins. In contrast, focal and segmental loss of the podoplanin protein accompanied proteinuria. The early loss of podoplanin expression did not coincide with changes in the expression of other podocyte proteins and could not be explained by mutations in the podoplanin gene or by decreased transcription.
Proteinuria is an important risk factor for the progression of renal disease,1,2 and understanding its pathogenesis may help reduce the burden of chronic renal disease. Elucidation of the molecular basis of congenital and hereditary proteinuric diseases has contributed significantly to our understanding of glomerular function in health and disease. We previously studied the expression of podocyte-associated proteins in human acquired proteinuric diseases, and found that the mRNA expression of these was up-regulated, whereas the protein expression was down-regulated. These changes correlated with the extent of foot process effacement. Based on these results, we hypothesized that the changes in expression represented a compensatory reaction of the podocyte to damage.17 In the current study, we used a rat model to determine how the observed changes may have developed over time.
Proteinuria is often, but not always, accompanied by podocyte foot process effacement. A remarkable finding in the current study was that foot process effacement early after the development of proteinuria was subtle, and the difference in mean foot process width between SHR and Dahl SS rats only became statistically significant at 10 weeks of age. There are several reports in the literature that support this temporal uncoupling of proteinuria and foot process effacement: similar observations were made in young proteinuric Dahl SS rats,19 and in other spontaneous proteinuric rat strains24 as well as in experimental models.25,26 Some reports have indicated that in humans, the extent of foot process effacement is not related to the severity of proteinuria.27,28 Others have reported patients and families with nephrotic syndromes that did not show foot process effacement.29,30,31
Our observations in the spontaneously proteinuric rat model studied here are in agreement with the pattern we found in humans in which the mRNA expression levels of several podocyte proteins were up-regulated during the development of proteinuria. For example, only at time points at which proteinuria was present in combination with more widespread foot process effacement (ie, at week 10) changes in nephrin protein expression were seen, paralleled by increased nephrin mRNA expression levels (Figures 5C and 6D). Although the etiology of proteinuria in the Dahl SS rat is probably different from that in human disease, these findings support the hypothesis that changes in nephrin expression are secondary to proteinuria development and foot process effacement.
In remarkable contrast to the general pattern of most of the podocyte-associated proteins studied, the glycoprotein podoplanin showed changes in expression with a clear temporal relationship to the development of proteinuria. Increasing loss of glomerular podoplanin expression was highly correlated with urinary albumin excretion (r = 0.8, P < 0.001, Figure 6B). Observation of rats up to 6 weeks of age showed that the onset of decreased glomerular podoplanin expression preceded detectable morphological alterations in the podocytes. At later time points (from 8 weeks onwards), when proteinuria had already progressed appreciably, immunoelectron microscopy showed that foot process effacement coincided with the loss of podoplanin; in contrast, areas in which podoplanin expression was retained showed normal ultrastructural morphology (Figure 9). The semiquantitative nature of the immunoelectron microscopy experiments did not allow us to draw conclusions with regard to more subtle changes in podoplanin expression in areas without foot process effacement. The expression of the slit diaphragm-associated proteins nephrin and podocin appeared unchanged up until weeks 8 (nephrin) and 10 (podocin) of age and was not related to the expression of podoplanin (Figure 5), suggesting that loss of podoplanin expression was not related to changes in the slit diaphragm.
Expression of podoplanin in the glomerulus was first reported by Breiteneder-Geleff et al, who described a 43-kDa glycoprotein that was specifically down-regulated in puromycin aminonucleoside nephrosis in the rat.18 Since down-regulation of this protein was related to foot process effacement, it was named podoplanin. The relationship between foot process effacement and podoplanin expression was further substantiated by the observation that injection of antibodies directed against a specific podoplanin epitope caused reversible proteinuria and foot process effacement, but only if divalent IgG antibodies were used.32 Homologs of podoplanin (T1-α, P2.26, and E-11) are expressed in other cell types, including type I alveolar cells in the lung, keratinocytes, and osteoblasts.33,34,35 In recent years, podoplanin has received more interest as a marker of lymphatic endothelial cells36 and various tumors.37
The mechanism underlying the involvement of podoplanin in glomerular pathology has remained unclear. In the current study, we found that focal and segmental loss of podoplanin protein coincided with the occurrence of proteinuria. The down-regulation of podoplanin at the protein level in Dahl SS rats could not be explained by decreased mRNA expression, which was actually increased during the development of proteinuria compared with non-proteinuric SHR rats. In addition, sequencing of the full-length Dahl SS podoplanin cDNA did not reveal any differences between Dahl SS and SHR rats that could explain the down-regulation of podoplanin protein in Dahl SS rats.
Although the exact extra- and intracellular interactions of podoplanin are currently unknown, several studies have indicated that podoplanin is involved in cell motility and actin cytoskeleton modeling. Ectopic expression of podoplanin in cultured cells increases the formation of cell extensions as well as increasing cell adhesiveness and migration.38,39 Furthermore, podoplanin expression in MCF7 cells induces filopodia formation, loss of stress fibers, and relocalization of actin to the newly formed filopodia; relocalization is mediated by inhibition of RhoA.23 Studies indicate that podoplanin and ezrin colocalize in subcellular compartments,23,39 and one study found that podoplanin and ezrin could be coimmunoprecipitated.39 Although the details of the interaction remain to be elucidated, these studies suggest a role for podoplanin in actin cytoskeleton modeling. In our study, ezrin did not show an altered expression pattern. However, immunohistochemical staining may not detect protein modifications such as phosphorylation.
How could down-regulation of this protein with actin-modeling properties relate to the development of proteinuria? The temporal relationship between podoplanin down-regulation and development of proteinuria does not necessarily imply a causal involvement of podoplanin dysregulation in the pathogenesis of proteinuria. However, in the current model, because no marked regulation of the expression of other podocyte proteins has been observed, it is most likely that podoplanin expression is specifically regulated. In puromycin aminonucleoside nephrosis in the rat, podoplanin is also down-regulated.18 This suggests that either down-regulation of podoplanin causes proteinuria, or that the expression of podoplanin is actively down-regulated by the podocyte in response to proteinuria. Until now, it has not been possible to study the effect of the absence of podoplanin in glomeruli, since podoplanin knockout mice have an embryonic lethal phenotype.38 However, injection of divalent anti-podoplanin IgG antibodies in rats induces rapid foot process effacement and proteinuria,32 indicating that changes in podoplanin can indeed be the initial step in the development of proteinuria. We recently found that podoplanin protein expression is also selectively lost in proteinuric Munich Wistar Frömter rats.40 This may indicate that loss of podoplanin is a more general phenomenon in proteinuria in rats.
It has been suggested that foot process effacement and formation of stress fibers are reactions of the podocyte to prevent dilation of capillaries and further leakage of proteins.41 Recently, Morigi et al showed that following protein load, podocytes reorganize their cytoskeleton in a RhoA-dependent manner.42 Similarly, Zhang et al reported that complement-mediated injury of cultured podocytes increases RhoA activity, resulting in a change in cellular morphology that mimics foot process effacement.43 Thus, the events that take place during foot process effacement (ie, stress fiber formation and foot process effacement in a RhoA-dependent fashion) seem to be the reverse of those brought about by the actin remodeling properties of podoplanin (prevention of stress fiber formation, filopodia formation, and RhoA inhibition). The loss of podoplanin we observed thus suggests a scenario in which the podocyte actively remodels its shape and down-regulates proteins, including podoplanin, which normally would promote foot process formation. Indeed, we found that RhoA mRNA levels were up-regulated in microdissected glomeruli that showed a loss of podoplanin protein expression compared with those that had retained podoplanin (Figure 8).
The extracellular domain of podoplanin is presumably negatively charged.32 Given the predominant localization on the apical membrane of the foot processes, it has been suggested that podoplanin may have a function comparable to that of podocalyxin, another negatively charged protein that is thought to serve as a spacer molecule between adjacent foot processes.32,44 Loss of podocalyxin is associated with foot process effacement,45 and it could be hypothesized that loss of podoplanin has similar consequences.
As the name implies, the Dahl SS rat is known for developing severe hypertension when raised on a high-salt diet. The development of hypertension is delayed and diminished when the rats are fed a low-salt diet, as in the current study. Although development of hypertension aggravates the rate of disease progression, several lines of evidence suggest that the development of proteinuria is uncoupled from the development of hypertension. First, proteinuria in Dahl SS rats develops before the onset of hypertension,46 starting from about 4 weeks of age. Furthermore, micropuncture experiments indicate that proteinuria is not explained by increased intraglomerular pressure.46 From a genetic point of view, development of proteinuria is independent of the development of hypertension.19,47 Recently, Nagase et al reported that in Dahl SS rats, podocytes are injured only on salt loading, as judged by de novo expression of desmin and B7–1 and down-regulation of nephrin protein expression.48 The authors suggested that podocyte injury might underlie the development of proteinuria in these rats. We, too, observed desmin expression in the podocytes of Dahl SS rats in the absence of salt loading, most notably in glomerular segments that showed a loss of podoplanin protein expression. However, this does not necessarily implicate podocyte damage as the cause of proteinuria in the Dahl SS rat. Desmin protein expression may also reflect other injury, such as increased mechanical strain as a result of the increased glomerular volume covered per podocyte.14 Furthermore, increased protein trafficking through the glomerular filtration barrier may itself be harmful for podocytes and may promote expression of podocyte stress markers.42,49,50
In conclusion, we found that in the spontaneously proteinuric Dahl SS rat, significant proteinuria was detected several weeks before widespread changes in podocyte morphology were observed. Moreover, segmental loss of podoplanin protein expression accompanied proteinuria and preceded widespread podocyte alterations.
Acknowledgments
We thank Dr. Schweikert and Dr. Beggs for the anti-α-actinin-4 antibody, Dr. Antignac for the anti-podocin antibody, and Dr. Mittienen for the anti-podocalyxin antibody. We thank Peter Neeskens and Frans Prins for their help with the electron microscopy studies.
Footnotes
Address reprint requests to Klaas Koop, M.D., Department of Pathology, Leiden University Medical Center, Building 1, L1-Q, PO BOX 9600, 2300 RC Leiden, The Netherlands. E-mail: k.koop@lumc.nl.
References
- Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, Marcantoni C, de Jong PE, de Zeeuw D, Shahinfar S, Ruggenenti P, Remuzzi G, Levey AS. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int. 2001;60:1131–1140. doi: 10.1046/j.1523-1755.2001.0600031131.x. [DOI] [PubMed] [Google Scholar]
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974–2984. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- De Zeeuw D. Albuminuria, not only a cardiovascular/renal risk marker, but also a target for treatment? Kidney Int. 2004;66:S2–S6. doi: 10.1111/j.1523-1755.2004.09201.x. [DOI] [PubMed] [Google Scholar]
- Shankland SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;70:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, Unanue ER, Shaw AS. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science. 2003;300:1298–1300. doi: 10.1126/science.1081068. [DOI] [PubMed] [Google Scholar]
- Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR. Mutations in ACTN4, encoding α-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24:251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffes MW, Schmidt D, McCrery R, Basgen JM. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001;59:2104–2113. doi: 10.1046/j.1523-1755.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- Lemley KV, Lafayette RA, Safai M, Derby G, Blouch K, Squarer A, Myers BD. Podocytopenia and disease severity in IgA nephropathy. Kidney Int. 2002;61:1475–1485. doi: 10.1046/j.1523-1755.2002.00269.x. [DOI] [PubMed] [Google Scholar]
- Macconi D, Bonomelli M, Benigni A, Plati T, Sangalli F, Longaretti L, Conti S, Kawachi H, Hill P, Remuzzi G, Remuzzi A. Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am J Pathol. 2006;168:42–54. doi: 10.2353/ajpath.2006.050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 2001;60:957–968. doi: 10.1046/j.1523-1755.2001.060003957.x. [DOI] [PubMed] [Google Scholar]
- Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: prevention by calorie restriction. J Am Soc Nephrol. 2005;16:2953–2966. doi: 10.1681/ASN.2005050488. [DOI] [PubMed] [Google Scholar]
- Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat Genet. 2000;24:333–335. doi: 10.1038/74139. [DOI] [PubMed] [Google Scholar]
- Koop K, Eikmans M, Baelde HJ, Kawachi H, De Heer E, Paul LC, Bruijn JA. Expression of podocyte-associated molecules in acquired human kidney diseases. J Am Soc Nephrol. 2003;14:2063–2071. doi: 10.1097/01.asn.0000078803.53165.c9. [DOI] [PubMed] [Google Scholar]
- Breiteneder-Geleff S, Matsui K, Soleiman A, Meraner P, Poczewski H, Kalt R, Schaffner G, Kerjaschki D. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol. 1997;151:1141–1152. [PMC free article] [PubMed] [Google Scholar]
- Poyan MA, Siegel AK, Kossmehl P, Schulz A, Plehm R, de Bruijn JA, De Heer E, Kreutz R. Early onset albuminuria in Dahl rats is a polygenetic trait that is independent from salt loading. Physiol Genomics. 2003;14:209–216. doi: 10.1152/physiolgenomics.00053.2003. [DOI] [PubMed] [Google Scholar]
- Kreutz R, Kovacevic L, Schulz A, Rothermund L, Ketteler M, Paul M. Effect of high NaCl diet on spontaneous hypertension in a genetic rat model with reduced nephron number. J Hypertens. 2000;18:777–782. doi: 10.1097/00004872-200018060-00017. [DOI] [PubMed] [Google Scholar]
- Baelde JJ, Bergijk EC, Hoedemaeker PJ, De Heer E, Bruijn JA. Optimal method for RNA extraction from mouse glomeruli. Nephrol Dial Transplant. 1994;9:304–308. [PubMed] [Google Scholar]
- Sanden SK, Wiggins JE, Goyal M, Riggs LK, Wiggins RC. Evaluation of a thick and thin section method for estimation of podocyte number, glomerular volume, and glomerular volume per podocyte in rat kidney with Wilms’ tumor-1 protein used as a podocyte nuclear marker. J Am Soc Nephrol. 2003;14:2484–2493. doi: 10.1097/01.asn.0000089829.45296.7c. [DOI] [PubMed] [Google Scholar]
- Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–272. doi: 10.1016/j.ccr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Macconi D, Ghilardi M, Bonassi ME, Mohamed EI, Abbate M, Colombi F, Remuzzi G, Remuzzi A. Effect of angiotensin-converting enzyme inhibition on glomerular basement membrane permeability and distribution of zonula occludens-1 in MWF rats. J Am Soc Nephrol. 2000;11:477–489. doi: 10.1681/ASN.V113477. [DOI] [PubMed] [Google Scholar]
- Orikasa M, Matsui K, Oite T, Shimizu F. Massive proteinuria induced in rats by a single intravenous injection of a monoclonal antibody. J Immunol. 1988;141:807–814. [PubMed] [Google Scholar]
- Jarad G, Cunningham J, Shaw AS, Miner JH. Proteinuria precedes podocyte abnormalities inLamb2 mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest. 2006;116:2272–2279. doi: 10.1172/JCI28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg JG, van den Bergh Weerman MA, Assmann KJ, Weening JJ, Florquin S. Podocyte foot process effacement is not correlated with the level of proteinuria in human glomerulopathies. Kidney Int. 2004;66:1901–1906. doi: 10.1111/j.1523-1755.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- Lahdenkari AT, Lounatmaa K, Patrakka J, Holmberg C, Wartiovaara J, Kestila M, Koskimies O, Jalanko H. Podocytes are firmly attached to glomerular basement membrane in kidneys with heavy proteinuria. J Am Soc Nephrol. 2004;15:2611–2618. doi: 10.1097/01.ASN.0000139478.03463.D9. [DOI] [PubMed] [Google Scholar]
- Branten AJ, van den BJ, Jansen JL, Assmann KJ, Wetzels JF. Familial nephropathy differing from minimal change nephropathy and focal glomerulosclerosis. Kidney Int. 2001;59:693–701. doi: 10.1046/j.1523-1755.2001.059002693.x. [DOI] [PubMed] [Google Scholar]
- Good KS, O'Brien K, Schulman G, Kerjaschki D, Fogo AB. Unexplained nephrotic-range proteinuria in a 38-year-old man: a case of “no change disease.”. Am J Kidney Dis. 2004;43:933–938. doi: 10.1053/j.ajkd.2003.06.006. [DOI] [PubMed] [Google Scholar]
- Seefeldt T, Bohman SO, Jorgen H, Gundersen HJ, Maunsbach AB, Petersen VP, Olsen S. Quantitative relationship between glomerular foot process width and proteinuria in glomerulonephritis. Lab Invest. 1981;44:541–546. [PubMed] [Google Scholar]
- Matsui K, Breiteneder-Geleff S, Kerjaschki D. Epitope-specific antibodies to the 43-kD glomerular membrane protein podoplanin cause proteinuria and rapid flattening of podocytes. J Am Soc Nephrol. 1998;9:2013–2026. doi: 10.1681/ASN.V9112013. [DOI] [PubMed] [Google Scholar]
- Rishi AK, Joyce-Brady M, Fisher J, Dobbs LG, Floros J, VanderSpek J, Brody JS, Williams MC. Cloning, characterization, and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev Biol. 1995;167:294–306. doi: 10.1006/dbio.1995.1024. [DOI] [PubMed] [Google Scholar]
- Gandarillas A, Scholl FG, Benito N, Gamallo C, Quintanilla M. Induction of PA2.26, a cell-surface antigen expressed by active fibroblasts, in mouse epidermal keratinocytes during carcinogenesis. Mol Carcinog. 1997;20:10–18. doi: 10.1002/(sici)1098-2744(199709)20:1<10::aid-mc3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Wetterwald A, Hoffstetter W, Cecchini MG, Lanske B, Wagner C, Fleisch H, Atkinson M. Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone. 1996;18:125–132. doi: 10.1016/8756-3282(95)00457-2. [DOI] [PubMed] [Google Scholar]
- Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht V, Dadras SS, Johnson LA, Jackson DG, Hong YK, Detmar M. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol. 2005;166:913–921. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, Detmar M. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl FG, Gamallo C, Vilaró S, Quintanilla M. Identification of PA2.26 antigen as a novel cell-surface mucin-type glycoprotein that induces plasma membrane extensions and increased motility in keratinocytes. J Cell Sci. 1999;112(Pt 24):4601–4613. doi: 10.1242/jcs.112.24.4601. [DOI] [PubMed] [Google Scholar]
- Ijpelaar DH, Schulz A, Koop K, Schlesener M, Bruijn JA, Kerjaschki D, Kreutz R, de Heer E. Glomerular hypertrophy precedes albuminuria and segmental loss of podoplanin in podocytes in Munich-Wistar-Fromter rats. Am J Physiol Renal Physiol. 2008;294:F758–F767. doi: 10.1152/ajprenal.00457.2007. [DOI] [PubMed] [Google Scholar]
- Kriz W, Kretzler M, Provoost AP, Shirato I. Stability and leakiness: opposing challenges to the glomerulus. Kidney Int. 1996;49:1570–1574. doi: 10.1038/ki.1996.227. [DOI] [PubMed] [Google Scholar]
- Morigi M, Buelli S, Angioletti S, Zanchi C, Longaretti L, Zoja C, Galbusera M, Gastoldi S, Mundel P, Remuzzi G, Benigni A. In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: implication for permselective dysfunction of chronic nephropathies. Am J Pathol. 2005;166:1309–1320. doi: 10.1016/S0002-9440(10)62350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cybulsky AV, Aoudjit L, Zhu J, Li H, Lamarche-Vane N, Takano T. Role of Rho-GTPases in complement-mediated glomerular epithelial cell injury. Am J Physiol Renal Physiol. 2007;293:F148–F156. doi: 10.1152/ajprenal.00294.2006. [DOI] [PubMed] [Google Scholar]
- Takeda T, Go WY, Orlando RA, Farquhar MG. Expression of podocalyxin inhibits cell-cell adhesion and modifies junctional properties in Madin-Darby canine kidney cells. Mol Biol Cell. 2000;11:3219–3232. doi: 10.1091/mbc.11.9.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, McQuistan T, Orlando RA, Farquhar MG. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest. 2001;108:289–301. doi: 10.1172/JCI12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzel RB, Luft FC, Gao Y, Schnermann J, Briggs JP, Ganten D, Waldherr R, Schnabel E, Kriz W. Renal disease and the development of hypertension in salt-sensitive Dahl rats. Kidney Int. 1988;33:1119–1129. doi: 10.1038/ki.1988.120. [DOI] [PubMed] [Google Scholar]
- Garrett MR, Dene H, Rapp JP. Time-course genetic analysis of albuminuria in Dahl salt-sensitive rats on low-salt diet. J Am Soc Nephrol. 2003;14:1175–1187. doi: 10.1097/01.asn.0000060572.13794.58. [DOI] [PubMed] [Google Scholar]
- Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T. Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension. 2006;47:1084–1093. doi: 10.1161/01.HYP.0000222003.28517.99. [DOI] [PubMed] [Google Scholar]
- Abbate M, Zoja C, Morigi M, Rottoli D, Angioletti S, Tomasoni S, Zanchi C, Longaretti L, Donadelli R, Remuzzi G. Transforming growth factor-beta1 is up-regulated by podocytes in response to excess intraglomerular passage of proteins: a central pathway in progressive glomerulosclerosis. Am J Pathol. 2002;161:2179–2193. doi: 10.1016/s0002-9440(10)64495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- Topham PS, Kawachi H, Haydar SA, Chugh S, Addona TA, Charron KB, Holzman LB, Shia M, Shimizu F, Salant DJ. Nephritogenic mAb 5-1-6 is directed at the extracellular domain of rat nephrin. J Clin Invest. 1999;104:1559–1566. doi: 10.1172/JCI7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen A, Dekan G, Farquhar MG. Monoclonal antibodies against membrane proteins of the rat glomerulus. Immunochemical specificity and immunofluorescence distribution of the antigens. Am J Pathol. 1990;137:929–944. [PMC free article] [PubMed] [Google Scholar]
- Roselli S, Gribouval O, Boute N, Sich M, Benessy F, Attie T, Gubler MC, Antignac C. Podocin localizes in the kidney to the slit diaphragm area. Am J Pathol. 2002;160:131–139. doi: 10.1016/S0002-9440(10)64357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka Y, Koike H, Ikezumi Y, Ito Y, Oyanagi A, Gejyo F, Shimizu F, Kawachi H. Podocyte injuries exacerbate mesangial proliferative glomerulonephritis. Kidney Int. 2001;60:2192–2204. doi: 10.1046/j.1523-1755.2001.00047.x. [DOI] [PubMed] [Google Scholar]