Abstract
Acute humoral rejection (AHR), which occurs in up to 8% of kidney transplant recipients, is a significant cause of renal allograft dysfunction and loss. More efficacious treatment modalities are needed to eliminate or curtail alloantibody production and its deleterious effects on the kidney. The availability of animal models mimicking human AHR is essential to understand its pathophysiology and develop new treatment strategies. Using a mouse kidney transplant model, we demonstrate that presensitization of recipients with donor skin grafts results in rejection of subsequent renal allografts. All presensitized mice developed renal failure 8.6 ± 4.3 days after engraftment, with serum creatinine values near 100 μmol/dl. Graft histology revealed mild, diffuse, interstitial, mononuclear cell infiltrates; prominent peritubular capillary inflammatory cell margination; patchy interstitial hemorrhage; interstitial edema; and focal glomerular fibrin deposition. Complement (C3d) deposition was diffuse and prominent in peritubular capillaries. Serum analysis demonstrated high levels of circulating alloantibodies with broad cross-reactivity to many MHC haplotypes. The clinical setting and histological findings of our model strongly resemble AHR, which is frequently associated with cellular rejection, a situation commonly encountered in human renal allograft recipients. This animal model provides a valuable tool to study the pathogenesis of AHR, its relationship to cellular alloimmunity, its contribution to graft injury, and the effects of various potential therapeutic interventions.
Recent advances have enabled transplant physicians to more precisely define the contribution of alloantibodies to acute renal allograft dysfunction after transplantation. Although improvements in acute rejection prophylaxis after kidney transplantation have drastically reduced the incidence of acute cellular rejection, the prophylaxis for, and treatment of, alloantibody-mediated acute humoral rejection (AHR) remains in its infancy. AHR in human renal allografts is characterized by a variety of histopathological changes and the presence of diffuse C4d deposition along the peritubular capillaries (PTCs). AHR is associated with a poor clinical outcome1,2,3,4,5,6,7; therefore, new treatment modalities are needed to avoid antibody-mediated graft damage associated with AHR.
In 2003, the seventh Banff Conference on Allograft Pathology and the National Conference to Assess Antibody-Mediated Rejection in Solid Organ Transplantation defined the current criteria for AHR.7,8 These criteria include i) the presence of donor-specific antibodies, ii) the diffuse deposition of C4d in the PTCs as detected by immunohistochemistry, iii) histological evidence of tissue injury, and iv) clinical evidence of graft dysfunction. It has been reported that up to 8% of renal transplant patients experience AHR, which includes a majority of cases that are resistant to traditional therapy for acute rejection.2,3,9 Additionally, AHR is frequently found to co-exist with acute cellular rejection.3,9 Future improvements in patient treatment could be expedited by the careful study of appropriate experimental models.
In vivo experimental models of AHR in murine organ allograft recipients have been developed by our group and others.10,11,12 We have recently shown that CCR5-deficient recipients of cardiac10 and renal allografts12 generate high titers of donor-reactive alloantibodies that are sufficient to directly mediate acute antibody-mediated graft rejection. Studies by others involving passively transferred alloantibodies into immunoglobulin-deficient recipients have demonstrated a role for both complement fixing and nonfixing donor-reactive antibodies in mediating graft damage.11,13 Rejection mechanisms in murine kidney allografts have been characterized in naïve recipients in which acute cellular rejection precedes but overlaps with the slower developing AHR.14
Pre-existing alloantibodies attributable to blood transfusion, pregnancy, or previous transplantation are present in ∼25% of the US population.15 These alloantibodies are a significant impediment to transplantation in many instances. One method used to allow such patients to be transplanted involves antibody removal from the peripheral circulation before transplantation, a method known as desensitization. This method is resource intensive, expensive, and not uniformly successful. Additionally, a rebound in antibody production after transplantation is common. This rebound frequently results in AHR (incidence between 25 to 45%) usually leading to acute renal dysfunction and parenchymal damage, and occasionally ending in immediate or early graft loss.16 Clinical efforts at reversing AHR and limiting the resultant parenchymal damage are limited in efficacy because of a lack of understanding of the underlying mechanisms. In this report we describe an experimental kidney transplant model, in the setting of pre-existing antibodies, with predominantly histological features similar to those observed in renal transplant patients experiencing AHR. Use of this experimental model will be valuable in furthering our understanding of the pathophysiology of AHR and in developing immunotherapies targeted for the prevention of AHR.
Materials and Methods
Mice
C57BL/6 (H-2b) and DBA/2 (H-2d) were obtained from either Simonsen Laboratories, Inc. (Gilroy, CA) or Harlan (Indianapolis, IN). All mice were housed and treated in accordance with Animal Care Guidelines established by the National Institutes of Health and The Ohio State University.
Presensitization via Skin Grafting
Skin allografts were performed using abdominal skin from donor mice (DBA/2 or FVB/N). Square full-thickness grafts (∼8 × 10 mm) were placed on the graft beds prepared on the recipient’s flank (C57BL/6). The graft was covered with a protective bandage for 7 days. Rejection was considered to occur at the point when the grafts exhibited dark discoloration, scabbing, and necrotic degeneration. Fourteen to twenty-one days after skin allograft transplantation, presensitized recipients subsequently received renal grafts as described below.
Murine Kidney Transplantation
A total of 24 presensitized mice and 12 nonsensitized mice served as renal allograft recipients and were studied in this report. Murine kidney transplantation was performed as described by Zhang and colleagues.17 Briefly, the donor left kidney was isolated by ligating and dividing the adrenal and testicular vessels with microsuture. The aorta and inferior vena cava were mobilized at their junction, with the left renal artery and vein. The aorta was ligated below the renal vessel. An elliptical patch of bladder containing the left ureterovesical junction was excised. The graft was perfused in situ with 0.2 to 0.4 ml of cold, heparinized Ringer’s lactate. Finally, the kidney with vascular supply and ureter attached to the bladder patch were harvested en bloc. Presensitized (n = 24) and nonsensitized (n = 12) mice served as recipients of renal allografts. The recipient’s right native kidney was removed immediately before transplantation. The infrarenal aorta and inferior vena cava were carefully isolated and cross-clamped. An end-to-side anastomosis between the donor renal vein and the recipient inferior vena cava was performed using continuous 10-0 sutures. The arterial anastomosis between the donor aortic cuff and recipient aorta is performed in the same manner as the venous anastomosis except that only two or three stitches are required for each side because the aortic diameter is smaller than the vein. After successful anastomosis the kidney graft perfused instantly. Urinary reconstruction was then performed by a bladder-to-bladder anastomosis. The left native kidney was removed on day 5 after renal transplantation. Kidney graft survival was followed by daily examinations of overall animal health and creatinine checks. For reference, a nontransplanted, normal mouse has a creatinine level of ∼20 μmol/L. Renal allograft rejection was considered when the mouse showed signs of illness and the creatinine level was elevated to ∼100 μmol/L at which time grafts were harvested for histopathology analysis.
CD8 Depletion
CD8 T cells were depleted in presensitized recipients (n = 4) by treatment with a 100 μg mixture of CD8 monoclonal antibodies (TIB 105 and YTS 169) on days −3, −2, −1, +5, and +10 relative to renal allograft transplantation. CD8 depletion was confirmed by peripheral blood mononuclear cell flow analysis on the day of renal transplantation and at the time of rejection.
Creatinine Determination
Quantitative whole blood creatinine levels were determined using an I-Stat portable clinical analyzer (Heska Corp., Fort Collins, CO). Conventional units (mg/dl) were converted to SI units by multiplying the conventional units by 88.4. The concentration of creatinine is expressed in μmol/L.
Histological Examination of Renal Tissue
Renal tissues were excised and fixed in 10% neutral buffered formalin, dehydrated in upgraded ethanol (70%, 95%, and 100%), and embedded in paraffin. For histological analysis, 2-μm sections were mounted on glass slides and stained with hematoxylin and eosin (H&E), trichrome, and in selected cases periodic acid-Schiff. Histological changes were evaluated according to the Banff scoring criteria18,19 on a scale of 0 to 3. If changes were minimal but not absent, the score of 0.5 was applied.
Immunofluorescence
For C3d, renal tissues were excised, embedded in OCT compound (Sakura Finetek Inc., Torrance, CA), and snap-frozen in liquid nitrogen. Three-μm cryostat sections were placed in cold acetone for 10 minutes and then allowed to air-dry. Sections were then washed in phosphate-buffered saline (PBS) and blocked with serum-free protein block (DAKO, Carpinteria, CA) for 1 hour. Fluorescein isothiocyanate-conjugated polyclonal rabbit anti-human antibody to C3d (DAKO) was applied for 1 hour in a dilution of 1:20. Sections were washed with PBS and coverslipped with glycerol-Tris aqueous mounting medium. In three rejected kidneys, direct immunofluorescence for fibrinogen and IgG was performed. The fluorescein isothiocyanate-labeled antibodies to fibrinogen (rabbit polyclonal, DAKO) and IgG (goat anti-mouse; Beckman Coulter, Fullerton, CA) were used in a dilution of 1:20 and 1:50, respectively.
Characterization of Infiltrating Inflammatory Cells
Immunostains for T cells (polyclonal rabbit anti-human CD3, DAKO), B cells (monoclonal rat anti-mouse-B220/biotinylated; BD Biosciences, San Jose, CA), and macrophages (polyclonal rat anti-mouse monocytes/macrophage marker F4/80; Serotec, Kidlington, Oxford, UK) were performed using routine immunoperoxidase methodology on paraffin sections in seven selected animals with antibody-mediated rejection. Briefly, after deparaffinization, the sections were pretreated with DAKO target retrieval solution in a commercially available vegetable steamer. After 20 minutes of cooling, the sections were treated with 3% H2O2 solution for 5 minutes. In addition to the peroxidase block, sections stained for CD3 and for F4/80 were additionally blocked with 10% normal goat serum. The primary antibodies were used in the after dilutions: i) anti-CD3, 1:200; ii) B220, 1:35; iii) F4/80, 1: 25. Secondary antibodies were goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) in 1:200 dilution for CD3 and goat anti-rat IgG (Serotec) in 1:25 dilution for F4/80. The monoclonal rat anti-mouse B-cell antibody is biotinylated; therefore a secondary antibody is not needed. Reactions were visualized using ABC Elite kit (Vector Laboratories) and DAB+ (DAKO). The number of cells, positive with each antibody, was counted separately in at least 10 consecutive high-power fields (HPFs) (×40 objective) in the renal cortex. The HPFs were chosen in a way that they covered the entire thickness of renal cortex from the capsule to the corticomedullary junction. Cells in the renal cortical tubulointerstitium were counted, which included the PTCs. Infiltrating glomerular cell populations were determined by counting the CD3-, F4/80-, and B220-positive cells in at least 20 glomeruli. The number of infiltrating cells per glomerulus was calculated for the individual cell populations.
Electron Microscopy
Two kidneys collected at day 5 and day 8 after transplant with classic morphological findings of antibody-mediated rejection (including diffuse PTC C3d staining and the characteristic light microscopic findings) were selected for ultrastructural examination. Normal DBA mouse kidneys served as control. Tissue from the renal cortex was cut into 1-mm cubes and immersion-fixed in 3% buffered glutaraldehyde immediately after euthanasia. The tissues were routinely processed for electron microscopy using osmium tetroxide after fixation, Epon embedding, and uranyl acetate and lead citrate contrasting. Semithin sections were stained with methylene blue basic fuchsin and representative areas were selected for electron microscopy. Ultrathin sections were examined with an EM 900 transmission electron microscope (Zeiss, Thornwood, NY).
Circulating Alloantibody Analysis
The presence of donor-reactive antibodies was determined by the ability of sera to bind to DBA/2 splenocytes. Binding was detected by flow cytometry, using fluorescein isothiocyanate-conjugated goat anti-mouse IgG (γ-chain-specific) (Pierce Chemical Co., Rockford, IL), rat anti-mouse IgG1, rat anti-mouse IgG2a, or rat anti-mouse IgG2b (BD Biosciences) detection antibodies. Antibody binding was measured as the mean fluorescence intensity (linear values) on a 4-decade scale using DBA/2 splenocytes as targets for Ig binding. Background fluorescence was determined in control experiments for each subtype by taking the mean fluorescence intensity value plus three standard deviations obtained from binding of five naïve C57BL/6 sera. Negative staining controls included splenocytes plus each detection antibody with mean fluorescence intensity values ranging from 9 to 31. In experiments to determine cross-reactivity of alloantibodies to other H-2 haplotypes, splenocytes from FVB/N (H-2q) and CBA/J (H-2k) were used as targets for binding of serum from presensitized renal allograft rejectors (n = 5). For further control, sera were also tested for reactivity to BALB/c (H-2d), C57BL/6 (H-2b), and BALB.B (H-2b) splenocytes.
Statistical Analysis
Antibody results were evaluated by unpaired Student’s t-test. Differences between data of experimental groups were considered significant if the analysis yielded P values less than 0.05.
Results
Skin-Presensitized C57BL/6 Recipients Reject Renal Allografts
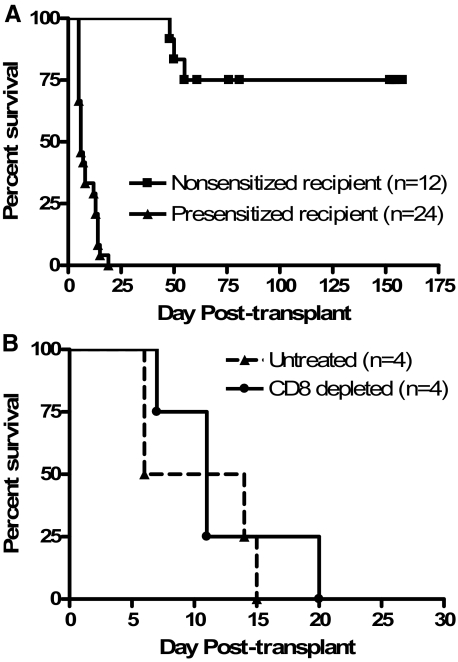
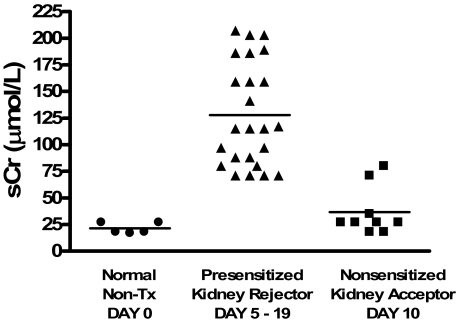
Previous studies by our group have demonstrated that C57BL/6 mice spontaneously accept DBA/2 renal allografts with good graft function beyond 60 days after transplant.20 In this study, C57BL/6 recipients were presensitized to DBA/2 alloantigens by transplantation with fully MHC-mismatched DBA/2 skin allografts, which were rejected within 11 days after transplantation (mean skin allograft survival = 11.3 ± 1.7 days). Fourteen to twenty-one days after DBA/2 skin grafting, presensitized recipient mice received donor-matched DBA/2 renal allografts. Recipient native kidneys were removed 5 days after renal allograft transplantation, thus renal function after native nephrectomy was maintained solely by the allograft. As shown in Figure 1A, all presensitized recipients (n = 24) rejected renal allografts within 19 days after transplantation (mean renal allograft survival 8.6 ± 4.3 days). Rejection was determined when the recipients displayed ill health and elevated creatinine levels confirmed graft dysfunction (Figure 2). In contrast, 75% of nonsensitized recipients (recipients receiving only renal allografts without sensitization via previous skin grafting, n = 12) survived greater than 60 days and some survived to day 150 after transplant with good graft function (Figures 1 and 2). Serum creatinine levels were significantly higher in presensitized recipients compared to nonsensitized recipients tested at a similar time after transplant (P < 0.0001) (Figure 2). Control experiments demonstrated that presensitized recipients accept C57BL/6 renal isografts longer than 60 days with good graft function (data not shown).
Figure 1.
Renal allograft rejection by skin-presensitized C57BL/6 recipients. A: C57BL/6 mice were either presensitized by DBA/2 skin graft rejection (▴, n = 24) or left nonsensitized (▪, n = 12) and then received DBA/2 renal allografts. B: Presensitized recipients were either left untreated (▴, n = 4) or were depleted of CD8 T cells (•, n = 4) at the time of renal allograft transplantation. Renal allograft rejection was considered at the time animals displayed signs of ill health and creatinine levels greater than 70 μmol/L.
Figure 2.
Creatinine levels in skin-presensitized versus nonsensitized renal allograft recipients. Whole blood was collected and analyzed for creatinine levels in presensitized mice (n = 24) at the time of rejection and in nonsensitized mice (n = 10) 10 days after transplant. The mean creatinine level in the serum of presensitized mice was 127.8 ± 48.6 compared to 36.7 ± 7.6 in nonsensitized mice.
Histological Evidence of Predominantly AHR
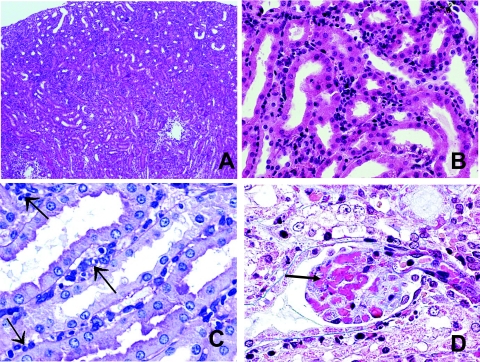
On average by 11 days after transplant, presensitized renal allograft recipients had developed severe graft dysfunction as evidenced by significantly elevated serum creatinine (Figure 2). Allografts were removed at this time and prepared for histological analysis to determine the nature of the immunologically-mediated allograft damage. The morphological findings are summarized in Table 1. As shown in Figure 3, renal allograft tissues displayed significant pathology including mild diffuse interstitial mononuclear cell infiltrate with minimal tubulitis (Figure 3A), prominent margination of inflammatory cells within the PTCs (Figure 3B) patchy interstitial hemorrhage and diffuse interstitial edema (Figure 3C), and presence of some amorphous material (probably fibrin with focal fuchsinophilic staining) in the glomerular capillaries (Figure 3D). Widely open glomerular capillaries were rarely seen; most of them were occluded by cells (swollen endothelial cells and inflammatory cells—see following paragraph) and/or amorphous material. Most arteries did not show evidence of inflammation indicative of acute arteritis; mild intimal arteritis was seen in 4 of 12 light microscopically examined kidneys. The animals revealed mild changes of cellular rejection (mild to moderate interstitial inflammation, mild tubulitis, and mild endarteritis) In contrast, renal allografts from nonsensitized recipients exhibited only mild histological changes with cuffing of leukocytes around the arteries and small aggregates of mononuclear cells scattered within the cortex. These grafts show no evidence of tissue destruction, endothelialitis, or tubulitis.20
Table 1.
Summary of Morphological Findings in Renal Allografts of Presensitized Animals
| Case no. | Post-transplantation day | Glomerulus thrombosis | Diffuse inflammatory infiltrate | Perivascular inflammatory cell infiltrate | Tubulitis | Interstitial edema | Interstitial hemorrhage |
|---|---|---|---|---|---|---|---|
| 1 | 5 | 1+ | 1 to 2+ | 2+ | 0.5+ | 0.5+ | 1+ focal |
| 2 | 14 | 1+ | 2 | 2 | 0.5+ | 0.5+ | 1+ focal |
| 3 | 13 | 1+ | 2 to 3+ | 2+ | 1+ | 1 to 2+ | 1+ focal |
| 4 | 19 | 1+ | 2 to 3+ | 2+ | 1+ | 1+ | 1+ focal |
| 5 | 5 | 0 | 1 to 2+ | 1+ | 0.5+ | 0.5+ | 0 |
| 6 | 14 | 2+ | 1 to 2+ | 2+ | 1+ | 1 to 2+ | 1+ focal |
| 7* | 5 | 2+ | 1+ | 1+ | 0.5 | 1+ | 3+ |
| 8 | 13 | 1+ | 1+ | 1 to 2+ | 0.5 | 1 to 2+ | 0.5 focal |
| 9 | 19 | 2+ | 1 to 2+ | 2+ | 1+ | 1+ | 1+ focal |
| 10 | 14 | 2+ | 1 to 2+ | 2+ | 1+ | 1+ | 1+ focal |
| 11 | 5 | 1+ | 1+ | 1+ | 0.5 | 1+ | 0 |
| 12 | 8 | 2+ | 1+ | 1+ | 0.5 | 1+ | 0 |
| (Continued) |
Histological changes were evaluated according to the semiquantitative Banff scoring criteria: 0, absent; 1+, mild; 2+, moderate; 3+, prominent. If changes were minimal but not absent, the score of 0.5 was applied.
Partial necrosis. Diff: Diffuse.
Table 1.
(Continued)
| Peritubular capillary margination | Acute tubular necrosis | Interstitial fibrosis | Tubular atrophy | Intimal arteritis | Arterial thrombosis | Intimal thickening | Peritubular capillary C3d |
|---|---|---|---|---|---|---|---|
| 3+ | 2+ | 0 | 0 | 0 | 0 | 0 | Diff |
| 3+ | 2+ | 0 | 0 | 0 | 0 | 0 | Diff |
| 3+ | 2+ | 1+ | 0.5+ | 0.5+ | 0 | 0 | Diff |
| 3+ | 2+ | 1+ | 0.5+ | 0 | 0 | 0 | Diff |
| 3+ | 2 to 3+ | 0 | 0 | 0 | 0 | 0 | Diff |
| 3+ | 2 to 3+ | 0 | 0 | 0 | 0 | 0 | Diff |
| 3+ | 3+ | 0 | 0 | 0 | 3 | 0 | Negative |
| 3+ | 2 to 3+ | 0 | 0 | 0.5 | 0 | 0 | Diff |
| 3+ | 2+ | 0.5 | 0 | 1+ | 0 | 0 | Diff |
| 3+ | 2+ | 0 | 0 | 0.5 | 0.5 | 0 | Diff |
| 3+ | 3+ | 0 | 0 | 0 | 0 | 0 | Diff |
| 3+ | 3+ | 0 | 0 | 0 | 0 | 0 | Diff |
Figure 3.
Histological characteristics of rejected renal allografts in presensitized C57BL/6 recipients. Renal allografts were harvested at the time of rejection. A: Low magnification shows mild diffuse interstitial inflammation (H&E). B: Most of the inflammatory cells appear to localize to the PTCs. Note the absence of tubulitis (H&E). C: Higher magnification reveals that the inflammatory cells are not infiltrating tubules. Many of the inflammatory cells are in the PTCs (arrows) (periodic acid-Schiff). Left arrows point to interstitial edema, which was a common finding (H&E). D: Glomerular capillaries filled by fuchsinophilc (red) amorphous material, probably representing fibrin (Masson’s trichrome). Original magnifications: ×40 (A); ×400 (B); ×600 (C, D).
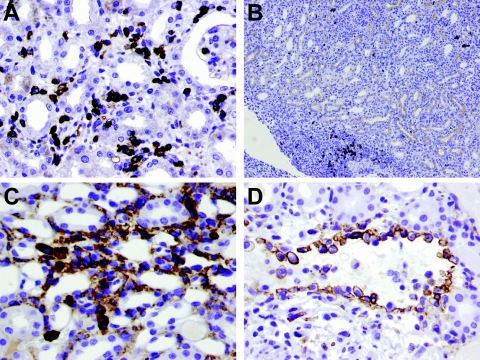
Infiltrating inflammatory cells within the rejecting renal allografts were characterized by immunohistochemistry (Figure 4). The majority of the cells in the PTCs and interstitium were T cells (Figure 4A). Monocytes/macrophages were also abundant, particularly among marginating inflammatory cells in the PTCs and venules (Figure 4, C and D). B cells were scarce and, if seen, occurred primarily in perivascular areas (Figure 4B). The number of interstitial (including PTCs) T cells, macrophages/monocytes, and B cells was 190.5 ± 45.6 per HPF, 110.2 ± 33.6 per HPF, and 8.4 ± 3.8 per HPF, respectively. Scattered polymorphonuclear leukocytes were also noted in the PTCs but they were substantially less prominent than T cells or monocytes/macrophages. The inflammatory cells infiltrating glomeruli were mainly T cells (6.6 ± 4.0 per glomerulus). Few glomerular macrophages (0.07 ± 0.11 per glomerulus) and B cells (0.31 ± 0.16 per glomerulus) were observed.
Figure 4.
Characterization of inflammatory infiltrate of rejected renal allografts in presensitized C57BL/6 recipients. A: Most of the infiltrating inflammatory cells were T cells (anti-CD3, immunoperoxidase). B: Only rare B cells were seen in the interstitium; B cells were mainly present in perivascular clusters (B220 antibody, immunoperoxidase). C: Macrophages/monocytes were present in the PTCs and interstitium (F4/80 antibody, immunoperoxidase). D: Large numbers of monocytes/macrophages marginating along the endothelium of a vein (F4/80 antibody, immunoperoxidase). Original magnifications: ×400 (A, C, D); ×40 (B).
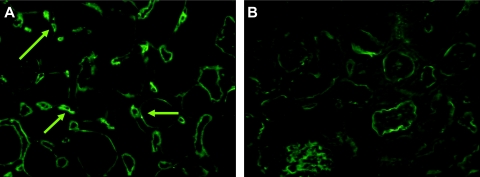
Immunofluorescent staining for the complement split product C3d was performed on frozen sections of renal allografts for evidence of deposition within the PTCs. Rejected renal allografts from presensitized recipients had diffuse, moderate to severe C3d deposition in the PTCs (Figure 5A). Grafts from nonsensitized recipients collected at day 10 after transplant were devoid of staining within the PTCs but had positive staining within the basement membranes of the tubules, a site in renal tissues known to nonspecifically stain (Figure 5B). Immunofluorescence with antibodies to fibrinogen and IgG revealed smudgy segmental glomerular fibrinogen staining in some glomeruli and mild granular mesangial IgG fluorescence (data not shown). The significance of the mild mesangial IgG staining is unclear because electron-dense immune-type deposits were not seen by electron microscopy (see below). Glomerular capillary or PTC IgG staining was not seen. Electron microscopy was performed on rejected renal allografts from presensitized recipients to further characterize the histological damage (Figure 6).
Figure 5.
Immunofluorescence for C3d. A: Diffuse strong PTC C3d staining within rejected renal allografts of presensitized recipients. Arrows point to the positive staining in the PTCs. B: PTC C3d staining is absent in renal allografts of nonsensitized recipients collected at day 10 after transplant. There is tubular basement membrane C3d staining, which is probably a common nonspecific finding and was seen in most animals. Indirect immunofluorescence. Original magnifications, ×400.
Figure 6.
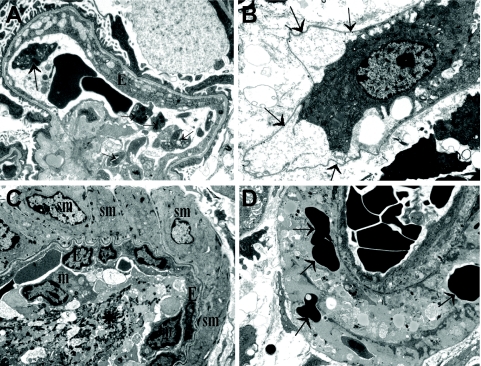
Ultrastructural findings. A: A glomerular capillary with swollen endothelium (E). Note the platelets in the lumen (arrows). B: A swollen PTC endothelial cell is lifting off from the underlying basement membrane (arrows). C: Platelets, admixed with fibrin (asterisk) and inflammatory cells fill the lumen of this small artery. Note that the smooth muscle cells (sm) are intact. E, endothelia; m, monocyte. D: A small artery with necrotic smooth muscle cells. Note that RBCs (arrows) are present in the necrotic/degenerated smooth muscle layer. Uranyl acetate-lead citrate (all images). Original magnifications: ×4,400 (A, B); ×3,000 (C, D).
Glomeruli
Endothelial swelling with loss of endothelial fenestration was evident. Segmental lifting off of the endothelium from the glomerular basement membrane and subsequent mild electron-lucent subendothelial widening was seen. Occasional platelet aggregates were present. Inflammatory cells, mainly lymphocytes and monocytes, were attached to the glomerular endothelium. The mesangial matrix appeared loose, edematous, and electron lucent with signs of mesangiolysis. Occasional red blood cells were seen in the loose mesangial matrix. Podocyte foot process effacement appeared mild. The glomerular basement membrane showed segmental wrinkling but otherwise it was unremarkable.
PTCs
Endothelial swelling and focal lifting off of the endothelium from the underlying basement membrane were evident. Occasional platelet aggregates were seen in the lumen and inflammatory cells (mainly monocytes) were attached to the endothelium. The PTC basement membrane was unremarkable with no lamellation.
Small Arteries/Arterioles
Smooth muscle cell necrosis and degeneration was evident in some small arteries and arterioles. Fragmented red blood cells were focally seen among the degenerating smooth muscle cells and in the subendothelial space. The endothelium was swollen and platelet aggregates admixed with fibrin (fibrin thrombi) were encountered in a few small arteries.
Tubules
Degenerative epithelial changes and tubular epithelial necrosis with sloughed off necrotic epithelial cells in tubular lumina was a common finding.
Circulating Donor-Reactive Antibodies
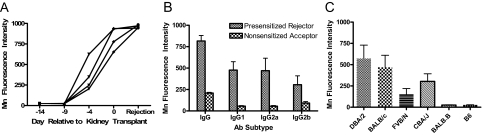
Alloantibody analysis was performed by testing reactivity of recipient sera to donor splenocytes analyzed by flow cytometry. The temporal development of donor-reactive alloantibodies was determined by collecting serum from recipients (n = 4) on the day of skin transplantation (d-14 relative to kidney transplantation), after 5 days (d-9), after 10 days (d-4), on the day of kidney transplant (day 0), and at the time of severe renal allograft dysfunction (rejection). As shown in Figure 7A, high levels of donor-reactive alloantibodies are generated by skin allograft recipients within 14 days after transplant, the time of kidney transplantation. Further analysis of the donor-reactive IgG antibodies present at the time of renal allograft rejection demonstrated titers ∼25-fold higher than those found in the sera of nonsensitized recipients collected at day 10 after transplant (data not shown). In addition, multicolor flow cytometry indicated these alloantibodies bind to donor-derived, but not recipient-derived, T cells, and are presumed to be MHC class I-specific. These data document the presence of presumptive MHC class I-reactive antibodies but do not exclude the possibility that MHC class II-reactive antibodies or tissue-specific antibodies are present and/or operative in this model.
Figure 7.
Alloantibody development in presensitized renal allograft recipients. A: Sera were collected from four individual presensitized recipients on the day of skin transplantation (d-14 relative to kidney transplantation), after 5 days (d-9), after 10 days (d-4), on the day of kidney transplant (day 0), and at the time of severe renal allograft dysfunction (rejection), diluted 1:16 and then tested for reactivity to donor splenocytes. B: Sera from presensitized recipients (n = 8) collected at the time of rejection and nonsensitized recipients (n = 5) collected at day 10 after transplant were diluted 1:16 and assessed for DBA/2-reactive IgG, IgG1, IgG2a, and IgG2b antibodies by flow cytometry. Antibody binding was measured as the mean fluorescence intensity (linear values). C: Sera from presensitized recipients (n = 5) collected at the time of rejection was diluted 1:20 and assessed for reactivity to DBA/2 (donor, H-2d), BALB/c (H-2d), FVB/N (H-2q), CBA/J (H-2k), BALB.B (H-2b), and C57BL/6 (recipient, H-2b) splenocytes by flow cytometry.
We explored whether a dominant IgG subclass of donor-reactive antibody was generated by presensitized renal allograft rejectors (n = 8). Comparison was made to antibody binding measured in sera from nonsensitized renal allograft acceptors (n = 5) collected 10 days after transplant. All sera were diluted 1:16 and tested for anti-donor IgG, IgG1, IgG2a, and IgG2b antibody subtypes using donor splenocytes as targets. As shown in Figure 7B, at the time of renal allograft rejection, presensitized recipients generate high levels of donor-reactive IgG antibodies with no significant predominance of tested IgG subclasses. Nonsensitized renal allograft acceptors generate significantly lower levels of anti-donor IgG antibodies than presensitized kidney rejectors (P = 0.01), again with no significant IgG subclass predominance.
Alloantibodies in the sera of presensitized renal allograft rejectors were tested for donor specificity by testing their ability to bind donor splenocytes in comparison to splenocytes from other H-2 haplotypes. As shown in Figure 7C, sera from presensitized renal allograft rejectors were reactive to donor DBA/2 (H-2d) as well as BALB/c (H-2d), FVB/N (H-2q), and CBA/J (H-2k) splenocytes. Thus alloantibodies generated in this model are broadly cross-reactive to strains of diverse H-2 haplotypes. Reactivity was not observed in recipient C57BL/6 (H-2b) splenocytes or BALB.B (H-2b) splenocytes that differ genetically only in minor histocompatibility antigens. Interestingly MHC cross-reactive alloantibodies are also observed in nonsensitized renal allograft recipients without rejection negating a causal relationship between this characteristic and rejection (data not shown). We cannot rule out qualitative differences in the alloantibodies and are developing assays to examine the alloantibody specificities in more detail.
CD8 T-Cell Depletion Studies
To determine whether acute cellular rejection mediated by CD8+ T cells is an operative effector mechanism contributing to renal allograft destruction in this model, presensitized recipients were depleted of CD8+ T cells days −3, −2, −1, and +5 relative to renal transplantation. As shown in Figure 1B, depletion of CD8+ T cells had no effect on renal allograft survival by presensitized recipients (mean renal allograft survival, 12.3 ± 5.5 days). These data indicate that graft destruction in this model is not mediated by CD8+ T cells; however, these data do not exclude a role for other possible mediators of cellular rejection including B cells, macrophages, and CD4+ T cells.
Discussion
AHR is increasingly being recognized as a significant cause of renal allograft dysfunction and loss. The ability to routinely detect the presence of complement deposition on renal allograft PTC endothelium has recently enabled transplant physicians to more accurately detect and diagnose alloantibody-mediated acute graft dysfunction. Still, the exact mechanism of alloantibody-mediated graft damage remains poorly understood. To address these issues, we developed an experimental model of antibody-mediated rejection using a mouse kidney transplant model. In previous studies we have shown that C57BL/6 mice spontaneously accept fully MHC-mismatched DBA/2 renal allografts with good renal function beyond 60 days after transplant with prominent leukocyte graft infiltration but no evidence of tissue destruction.20 Despite the presence of low to moderate levels of alloantibodies in the serum of renal allograft acceptors, complement is not deposited in the grafts out to 150 days after transplant (unpublished observation). In the studies reported here, skin presensitization induced renal allograft rejection, presumably because of the presence of circulating preformed antibodies. We observed that none of the animals developed hyperacute rejection; however, within 5 to 19 days, presensitized C57BL/6 recipients rejected DBA/2 renal allografts, concomitant with the generation of high levels of circulating donor-reactive antibodies, and the graft tissue displayed histological changes similar to those seen in AHR in human renal allografts.
In addition to AHR, rejecting allografts displayed histological features of acute cellular rejection as well (some interstitial inflammatory cell infiltrate, mild tubulitis, mild intimal arteritis). This also parallels human AHR because in human renal allograft biopsies, taken from patients with AHR, changes of acute cellular rejection are commonly seen.3,7,21 In our center, 65% of renal allograft biopsies with AHR also show some evidence of acute cellular rejection. However, in these mixed humoral and cellular rejection cases the histological changes of cellular rejection are usually relatively mild and it is believed that in most patients the dominating pathomechanism, responsible for the graft dysfunction is AHR, although this remains a matter of conjecture. In the experimental model described herein, despite a mild cellular infiltration present within the graft interstitium, depletion studies demonstrated no significant effector role for CD8+ T cells in graft dysfunction and subsequent loss. Cellular rejection may be promoted by various subsets of leukocytes including T cells,22 macrophages,22 and even B cells23 and plasma cells.24 Although our CD8 depletion data excludes cellular rejection involving CD8 cells, it does not rule out a contribution by these cell populations.
Deposition of the complement split product C4d in the PTCs has been shown to be a reliable marker of AHR in human renal allografts.1,2,3,7 It is evident that in a substantial proportion of C4d-positive human renal allograft biopsies, C3d is also diffusely present in the PTCs.25 At the time these studies were undertaken, antibodies reactive to mouse C4d were not commercially available, thus murine C3d-reactive antibodies were used to detect complement deposition in rejecting mouse renal allografts. Indirect immunofluorescence shows that there is diffuse, strong PTC C3d staining in grafts from presensitized recipients, which is absent in grafts from nonsensitized recipients. Although we cannot exclude the possibility that the alternative pathway of complement activation contributes to the presence of C3d deposition in presensitized recipients, its absence in the nonpresensitized recipients makes this possibility unlikely.
Although complement deposition is a hallmark of clinical AHR, there are other histological changes that are less specific but commonly seen in human renal biopsies with antibody-mediated rejection. Such changes include peritubular and glomerular capillary margination of inflammatory cells, glomerular capillary and arteriolar fibrin thrombi, endothelial/vascular necrosis, and interstitial edema and patchy interstitial hemorrhage.1,3,6,7,8,26 The composition of inflammatory cell infiltrate in the PTCs (mainly T cells and macrophages, admixed with some polymorphonuclear leukocytes) is also quite similar to that seen in many human renal allograft biopsies with AHR.8,26 Thus, the animal model described herein displays the salient features commonly associated with AHR in human renal allografts. The paucity of glomerular monocytes/macrophages (F4/80-positive cells) in our model is intriguing and different from that seen in human AHR. At the present time we do not know the significance of this finding.
Ultrastructural studies in AHR are scant.27 Overall, our ultrastructural findings confirm that the renal vasculature (peritubular and glomerular capillaries as well as small arteries and arterioles), is the primary site of injury in the present model. Many ultrastructural changes, including the platelet aggregates, fibrin, endothelial damage, fragmented red blood cells in the vascular wall, suggest a low-level thrombotic microangiopathy (TMA). Some degree of glomerular thrombosis was seen in many animal kidneys by light microscopy as well; however, obvious arterial thrombosis was noted only in 2 of 10 examined mouse allografts. Thrombotic microangiopathy is not an unusual complication of human AHR. In our and others’ experience, thrombotic microangiopathy is particularly common in presensitized patients who receive a renal allograft after desensitization.28 The ultrastructural findings in our model are similar to those described in human AHR.27
Characterization of the alloantibodies generated under conditions leading to AHR can render insight into their nature and thus the pathophysiology of their damaging effects. We confirm that there is no relative difference between presensitized and nonsensitized recipients with regard to either donor-reactive alloantibody IgG subset composition or MHC cross-reactivity. Thus we cannot explain the lack of graft destruction in the nonpresensitized recipients based on the absence of complement- fixing IgG subtype production. However, our data do indicate a significant augmentation in IgG levels in presensitized versus nonsensitized recipients. Based on these data we postulate that for presensitized versus nonsensitized recipients, an increase in antibody affinity parallels the increased levels and that this accounts for, or contributes to, the observed graft damage. We observed that presensitized recipients undergoing renal allograft rejection generate alloantibodies that are broadly cross-reactive with MHC haplotypes. Importantly, however, these cross-reactive alloantibodies are also observed in nonsensitized renal allograft recipients without rejection negating a causal relationship between this characteristic and rejection. It is important to note that broadly cross-reactive MHC-specific antibodies are commonly observed in patients undergoing renal allograft rejection.29 We cannot exclude qualitative differences in the alloantibodies and are developing assays to examine the alloantibody specificities in more detail. Further characterization of the alloantibodies generated in this model will provide insight regarding their deleterious effects and assist in developing treatment strategies to interfere with their generation and function.
In summary, presensitization by skin graft rejection results in immunological deviation from the usual spontaneous graft acceptance to early, severe graft dysfunction and animal demise associated with significantly elevated anti-donor antibody levels. Based on the report of the National Conference to Assess Antibody-Mediated Rejection in Solid Organ Transplantation,8 the recommended criteria for humoral rejection in human renal allografts include the following: i) clinical evidence of graft dysfunction, ii) histological evidence of tissue injury, iii) C4d (and/or C3d/IgG) deposition in PTCs, and iv) evidence of anti-HLA or other donor-specific antibodies in the recipient. All these criteria are evident in our model. Features of mild acute cellular rejection were common in the animals, consistent with the known clinical scenario. These parallel findings between our model and the criteria established for AHR indicate that this simple mouse model may provide an excellent tool to explore the pathogenesis and immunological events involved in human renal allograft rejection. This model of AHR provides several unique advantages over existing models including: i) it does not involve exogenous immunosuppression and thus perturbation with the natural immunological mechanisms can be avoided; ii) high titers of donor-specific (and perhaps organ-specific) antibodies are generated that can be used for passive transfer experiments; iii) recipient mice are on a C57BL/6 background, which provides an opportunity to explore the development of rejection in a wide variety of knockout mice lacking specific molecules that may be involved in the process; and iv) the potential involvement of T cells can be explored. The development of this valuable model has the potential to advance understanding in several key areas including local and systemic immunological events that result in alloantibody elaboration, mechanisms of alloantibody-mediated graft damage, and effective treatment strategies to interfere with these processes.
Acknowledgments
We thank Patrick Adams, Bree Heestand, and Jonathon Campbell for their excellent technical support in the development of the flow cytometric analysis of alloantibodies; Cherri Bott for her expert technical support with immunohistochemistry; and Misty Bear, Christina Sass, and Doug Hurak for their assistance in monitoring the murine transplant recipients.
Footnotes
Address reprint requests to Tibor Nadasdy, M.D., Department of Pathology, M015 Starling Loving Hall, 320 W. 10th Ave., Columbus OH 43210. E-mail: tibor.nadasdy@osumc.edu.
Supported in part by the National Institutes of Health (grants RO1-AI053094 to C.G.O. and T.N. and PO1-HL070294 to C.G.O., R.P.P., and T.N.).
References
- Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, Cosimi AB, Colvin RB. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999;10:2208–2214. doi: 10.1681/ASN.V10102208. [DOI] [PubMed] [Google Scholar]
- Crespo M, Pascual M, Tolkoff-Rubin N, Mauiyyedi S, Collins AB, Fitzpatrick D, Farrell ML, Williams WW, Delmonico FL, Cosimi AB, Colvin RB, Saidman SL. Acute humoral rejection in renal allograft recipients: I. Incidence, serology and clinical characteristics. Transplantation. 2001;71:652–658. doi: 10.1097/00007890-200103150-00013. [DOI] [PubMed] [Google Scholar]
- Mauiyyedi S, Crespo M, Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Tolkoff-Rubin NE, Williams WW, Delmonico FL, Cosimi AB, Colvin RB. Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol. 2002;13:779–787. doi: 10.1681/ASN.V133779. [DOI] [PubMed] [Google Scholar]
- Böhmig GA, Exner M, Watschinger B, Regele H. Acute humoral renal allograft rejection. Curr Opin Urol. 2002;12:95–99. doi: 10.1097/00042307-200203000-00003. [DOI] [PubMed] [Google Scholar]
- Lederer SR, Kluth-Pepper B, Schneeberger H, Albert E, Land W, Feucht HE. Impact of humoral alloreactivity early after transplantation on the long-term survival of renal allografts. Kidney Int. 2001;59:334–341. doi: 10.1046/j.1523-1755.2001.00495.x. [DOI] [PubMed] [Google Scholar]
- Herzenberg AM, Gill JS, Djurdjev O, Magil AB. C4d deposition in acute rejection: an independent long-term prognostic factor. J Am Soc Nephrol. 2002;13:234–241. doi: 10.1681/ASN.V131234. [DOI] [PubMed] [Google Scholar]
- Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K. Antibody-mediated rejection criteria—an Addition to the Banff ’97 Classification of Renal Allograft Rejection. Am J Transplant. 2003;3:708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, Kupiec-Weglinski J, Matas A, Montgomery RA, Nickerson P, Platt JL, Rabb H, Thistlethwaite R, Tyan D, Delmonico FL. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4:1033–1041. doi: 10.1111/j.1600-6143.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- Moll SPM. Humoral rejection of organ allografts. Am J Transplant. 2005;5:2611–2618. doi: 10.1111/j.1600-6143.2005.01086.x. [DOI] [PubMed] [Google Scholar]
- Nozaki T, Amano H, Bickerstaff A, Orosz CG, Novick AC, Tanabe K, Fairchild RL. Antibody-mediated rejection of cardiac allografts in CCR5-deficient recipients. J Immunol. 2007;179:5238–5245. doi: 10.4049/jimmunol.179.8.5238. [DOI] [PubMed] [Google Scholar]
- Wasowska BA, Qian Z, Cangello DL, Behrens E, Van Tran K, Layton J, Sanfilippo F, Baldwin WM., III Passive transfer of alloantibodies restores acute cardiac rejection in IgKO mice. Transplantation. 2001;71:727–736. doi: 10.1097/00007890-200103270-00007. [DOI] [PubMed] [Google Scholar]
- Bickerstaff A, Nozaki T, Wang JJ, Pelletier R, Hadley G, Nadasdy G, Nadasdy T, Fairchild RL. Acute humoral rejection of renal allografts in CCR5(−/−) recipients. Am J Transplant. 2008;8:557–566. doi: 10.1111/j.1600-6143.2007.02125.x. [DOI] [PubMed] [Google Scholar]
- Rahimi S, Qian Z, Layton J, Fox-Talbot K, Baldwin WM, III, Wasowska BA. Non-complement- and complement-activating antibodies synergize to cause rejection of cardiac allografts. Am J Transplant. 2004;4:326–334. doi: 10.1111/j.1600-6143.2004.00334.x. [DOI] [PubMed] [Google Scholar]
- Jabs WJ, Sedlmeyer A, Ramassar V, Hidalgo LG, Urmson J, Afrouzian M, Zhu LF, Halloran PF. Heterogeneity in the evolution and mechanisms of the lesions of kidney allograft rejection in mice. Am J Transplant. 2003;3:1501–1509. doi: 10.1046/j.1600-6135.2003.00269.x. [DOI] [PubMed] [Google Scholar]
- Jackson AM, Zachary AA. The problem of transplanting the sensitized patient: whose problem is it? Front Biosci. 2008;13:1396–1412. doi: 10.2741/2770. [DOI] [PubMed] [Google Scholar]
- Stegall MD, Gloor J, Winters JL, Moore SB, Degoey S. A comparison of plasmapheresis versus high-dose IVIG desensitization in renal allograft recipients with high levels of donor-specific alloantibody. Am J Transplant. 2006;6:346–351. doi: 10.1111/j.1600-6143.2005.01178.x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Schlachta C, Duff J, Stiller C, Grant D, Zhong R. Improved techniques for kidney transplantation in mice. Microsurgery. 1995;16:103–109. doi: 10.1002/micr.1920160212. [DOI] [PubMed] [Google Scholar]
- Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Marcussen N, Mihatsch MJ, Nadasdy T, Nickerson P, Olsen TS, Papadimitriou JC, Randhawa PS, Rayner DC, Roberts I, Rose S, Rush D, Salinas-Madrigal L, Salomon DR, Sund S, Taskinen E, Trpkov K, Yamaguchi Y. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ. Banff ’05 meeting report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN′). Am J Transplant. 2007;7:518–526. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- Bickerstaff AA, Wang JJ, Pelletier RP, Orosz CG. Murine renal allografts: spontaneous acceptance is associated with regulated T cell-mediated immunity. J Immunol. 2001;167:4821–4827. doi: 10.4049/jimmunol.167.9.4821. [DOI] [PubMed] [Google Scholar]
- Al-Aly Z, Reddivari V, Moiz A, Balasubramanian G, Cortese CM, Salinas-Madrigal L, Bastani B. Renal allograft biopsies in the era of C4d staining: the need for change in the Banff classification system. Transpl Int. 2008;21:268–275. doi: 10.1111/j.1432-2277.2007.00604.x. [DOI] [PubMed] [Google Scholar]
- Hancock WW, Thomson NM, Atkins RC. Composition of interstitial cellular infiltrate identified by monoclonal antibodies in renal biopsies of rejecting human renal allografts. Transplantation. 1983;35:458–463. doi: 10.1097/00007890-198305000-00013. [DOI] [PubMed] [Google Scholar]
- Hippen BE, DeMattos A, Cook WJ, Kew CE, II, Gaston RS. Association of CD20+ infiltrates with poorer clinical outcomes in acute cellular rejection of renal allografts. Am J Transplant. 2005;5:2248–2252. doi: 10.1111/j.1600-6143.2005.01009.x. [DOI] [PubMed] [Google Scholar]
- Charney DA, Nadasdy T, Lo AW, Racusen LC. Plasma cell-rich acute renal allograft rejection. Transplantation. 1999;68:791–797. doi: 10.1097/00007890-199909270-00011. [DOI] [PubMed] [Google Scholar]
- Kuypers DR, Lerut E, Evenepoel P, Maes B, Vanrenterghem Y, Van Damme B. C3D deposition in peritubular capillaries indicates a variant of acute renal allograft rejection characterized by a worse clinical outcome. Transplantation. 2003;76:102–108. doi: 10.1097/01.TP.0000069040.16457.06. [DOI] [PubMed] [Google Scholar]
- Magil AB, Tinckam K. Monocytes and peritubular capillary C4d deposition in acute renal allograft rejection. Kidney Int. 2003;63:1888–1893. doi: 10.1046/j.1523-1755.2003.00921.x. [DOI] [PubMed] [Google Scholar]
- Lipták P, Kemeny E, Morvay Z, Szederkenyi E, Szenohradszky P, Marofka F, Toldi J, Exner M, Ivanyi B. Peritubular capillary damage in acute humoral rejection: an ultrastructural study on human renal allografts. Am J Transplant. 2005;5:2870–2876. doi: 10.1111/j.1600-6143.2005.01102.x. [DOI] [PubMed] [Google Scholar]
- Haas M, Rahman MH, Racusen LC, Kraus ES, Bagnasco SM, Segev DL, Simpkins CE, Warren DS, King KE, Zachary AA, Montgomery RA. C4d and C3d staining in biopsies of ABO- and HLA-incompatible renal allografts: correlation with histologic findings. Am J Transplant. 2006;6:1829–1840. doi: 10.1111/j.1600-6143.2006.01356.x. [DOI] [PubMed] [Google Scholar]
- Pei R, Lee JH, Shih NJ, Chen M, Terasaki PI. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation. 2003;75:43–49. doi: 10.1097/00007890-200301150-00008. [DOI] [PubMed] [Google Scholar]