Abstract
Pancreatic islet microendothelium and β cells exhibit an interdependent physical and functional relationship. In this study, we analyzed the effect of chronic hyperglycemia on human pancreatic islet microendothelial cells as well as the involvement of the phosphatidylinositol 3-kinase/Akt and nephrin pathways, interleukin-1β, and nitric oxide production. In addition, whether 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors can reverse the response to high-glucose conditions was investigated. Proliferation of purified islet microendothelial cells cultured under hyperglycemic conditions (28 mmol/L glucose) decreased compared to that of normoglycemic cells (from 12.7% after 2 days to 47.7% after 30 days, P < 0.05). In parallel, apoptosis progressively increased from 7% after 2 days to 79% after 30 days in high glucose (P < 0.05) concomitant with an early increase of caspase-3 activity. Intermittent hyperglycemia induced greater apoptosis than sustained hyperglycemia. Apoptosis was accompanied by a reduced p-Akt/Akt ratio and inhibition of nephrin tyrosine phosphorylation. Pravastatin (1 μmol/L) decreased apoptosis induced by high glucose or oxidized LDL and increased Akt phosphorylation. Hyperglycemia significantly increased the production of the proinflammatory cytokine interleukin-1β and stimulated the expression of inducible nitric oxide synthase and the production of nitric oxide, possibly relevant to β cell mass and function. Thus, chronic hyperglycemia reduces islet microendothelial cell survival by inhibiting the serine-threonine kinase Akt pathway, and the effect of pravastatin on this pathway represents a potential tool to improve islet vascularization and, indirectly, islet function.
The pancreatic endocrine vasculature exhibits distinctive functional and structural features, which render them highly adapted to communicate with the underlying endocrine tissue in a cross-talk relationship. This notion stands against the background that the microvasculature has a key role at the interface between the vascular space and organ parenchymas and participates in numerous pathophysiological processes. Pancreatic islets are one of the most vascularized organs,1,2 and vascular endothelial growth factor-A secreted by the neighboring β cells is responsible for this strong vascularization and capillary fenestration from organogenesis to adult life.3
Recent studies indicate that the islet vasculature is likely to play a role in the physiology as well as in the disease of the pancreatic islets. Besides providing oxygen, nutrients, and secretory signals from other cells to endocrine cells,4 producing a number of vasoactive, angiogenic substances, cytokines, and growth factors,5 islet endothelium has been shown to induce insulin gene expression during endocrine tissue development,6 to affect adult β cell function and to promote β cell proliferation. These effects are mediated by secretion of unknown paracrine signals that may include the hepatocyte growth factor,7 collagen IV, and laminins 8,9. Importantly, the islet endothelium is involved in the rapid transendothelial release of secreted insulin into the circulation,2 and it is suggested to have a role in fine-tuning blood glucose sensing and regulation.3,10,11,12,13 Studies in mice with pancreatic deletion of vascular endothelial growth factor-A or in murine models of type 2 diabetes indicate that morphological changes in islet vasculature are accompanied by defective blood glucose levels and impaired glucose-stimulated insulin secretion, which play a key pathogenetic role in the development of diabetes.3,13,14 Such endothelial disruption and metabolic abnormalities might involve the transmembrane signaling protein nephrin, specifically expressed in human pancreatic islet microvascular endothelial cells (MECs).10
Collectively, these data indicate that a normal capillary network is essential for optimal β cell secretory function and blood glucose regulation. Several studies demonstrated that hyperglycemia induces early endothelial dysfunction on cultured micro- and macrovascular endothelium, characterized by changes in proliferation, barrier function, sensitivity to apoptosis and adhesion, and angiogenic and synthetic properties of endothelial cells.15,16,17,18,19,20 However, studies on islet-derived endothelial cells are lacking. In the light of the endothelial-endocrine axis within adult pancreatic islets, it is conceivable that hyperglycemia may induce alterations in islet endothelium, potentially contributing to the progressive reduction of β cell function and mass that characterizes the natural history of type 2 diabetes.21,22
In the present study, we analyzed the in vitro effects of acute and chronic hyperglycemia on human pancreatic islet MECs. The effects of hyperglycemia on cell survival, Akt and nephrin phosphorylation, and interleukin (IL)-1β and nitric oxide (NO) production were evaluated. In addition, we investigated whether the 3-hydroxy-3-methylglutaryl coenzyme A inhibitor pravastatin, which is known to modulate phosphatidylinositol 3-kinase (PI3K)/Akt pathways and improve vascular function,23,24 may reverse the response of islet MECs to high-glucose conditions.
Materials and Methods
Islet Endothelial Cell Culture Conditions
Islet MECs were cultured onto endothelial cell attachment factor (Sigma Aldrich, Milano, Italy) coated tissue culture plates in endothelial basal medium with the EGM-bullet kit (Clonetics, San Diego, CA) containing 5.6 mmol/L glucose, with 20% fetal calf serum, 10 mmol/L l-glutamine, and antibiotics.25 Cells were grown until confluent, washed twice with Hanks’ balanced salt solution, and dispersed with trypsin/EDTA when subcultured in appropriate flasks or plates, depending on the experiment performed. When cultured under high-glucose conditions, complete medium was adjusted to 14 mmol/L or 28 mmol/L glucose (Sigma) to assess the diverse effects of glucose concentration and treatment duration. Medium was changed every 48 hours and experiments were always conducted in parallel with the physiological (5.6 mmol/L) concentration of glucose. All other experiments were performed using the glucose concentration of 28 mmol/L on the basis of these and previous studies.18,19,20
To evaluate the effect of intermittent hyperglycemia on islet MECs, cells were subjected to repeated cycles of 48 hours of growth in high glucose, alternating with 48 hours of growth in normal glucose. Staining for expression of endothelial marker von Willebrand’s factor by immunofluorescence technique was performed as described.10 For long-term culture in high-glucose conditions, an SV40-immortalized human cell line established from purified islet MECs was also used; these immortalized cells have been shown to retain their phenotypic and functional characteristics.25
Glucose uptake was assessed by a fluorimetric method, using a commercially available kit (Amplex Red glucose, Invitrogen, Milan, Italy) following the manufacturer’s instructions. Cell proliferation was assessed by trypan blue exclusion cell count and by measuring DNA synthesis by 5-bromo-2′-deoxyuridine incorporation colorimetric immunoassay, using a commercially available kit (cell proliferation ELISA, 5-bromo-2′-deoxyuridine assay, Roche Diagnostics, Mannheim, Germany), following the manufacturer’s instructions. Five separate experiments at different time points during different culture conditions were performed, each in triplicate. Data were expressed as percentage change between cells grown at 28 mmol/L glucose versus cells grown at 5.6 mmol/L glucose (mean Abs450 nm islet MECs in high glucose/mean Abs450 nm MECs in physiological glucose X-100).
In experimental conditions using 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, statins, cells, were incubated with oxidized LDL (oxLDL), 100 μg/ml, for 24 hours at 37°C, as a proapoptotic and Akt-dephosphorylating stimulus.26 For preparation of oxLDL, 5 mg/ml LDL were mixed with 5 μmol/L CuSO4, incubated for 18 hours at 37°C and oxidation was evaluated as previously described.26
To evaluate the effects of statins on apoptosis, whether induced by high-glucose conditions or by oxLDL, and on Akt and nephrin dephosphorylation, islet MECs were treated with increasing doses of pravastatin (Calbiochem, Darmstadt, Germany) (0.1, 0.5, 1 μmol/L), on the basis of previous reports showing that the hydrophilic pravastatin at high concentration did not induce apoptosis compared to the lipophilic simvastatin.23,26 Three different conditions were assayed: pravastatin was given daily, or started after 4 days of high-glucose culture, or added overnight before cell collection.
Detection of Apoptosis
Apoptosis was evaluated in time course experiments at 3- to 6-day intervals by a photometric enzyme immunoassay measuring mono- and oligonucleosomes in the cytoplasmic fraction of cell lysates as an index of DNA fragmentation, which is, in turn, an early marker of apoptosis, using a commercially available kit (cell death detection ELISAPLUS, Roche), following the manufacturer’s instructions. Further, islet MECs were also subjected to terminal deoxynucleotidyl transferase dUTP nick-end labeling assay analysis (ApopTag, Intergen Company, Purchase, NY), using vincristine (0.3 μg/ml) as a positive control for the induction of apoptosis. Cells were seeded onto 96-well plates, washed in phosphate-buffered saline (PBS), fixed in 1% paraformaldehyde in PBS, pH 7.4, incubated with TdT enzyme and digoxigenin-dNTP, washed in PBS, and counterstained with anti-digoxigenin- fluorescein isothiocyanate antibody and with propidium iodide (1 μg/ml) in PBS. The fluorescein isothiocyanate-labeled DNA fragments in the apoptotic cells were visualized by inverted UV microscopy. Cells were counted by digital analysis (Windows MicroImage, version 3.4 CASTI Imaging, Venecia, I) of images obtained using a video camera (Leica DC100); positive apoptotic cells were expressed as a percentage of the total cells counted in 10× inverted microscope fields.
To confirm that apoptosis was occurring, activation of the caspase family was assessed by a caspase-3 colorimetric activity assay kit (Chemicon International, Temecula, CA), following the manufacturer’s instructions. Five to eight separate experiments at different time points during different culture conditions were performed, each in triplicate.
Immunoprecipitation and Western Blot Analyses
Islet MECs, subjected to different experimental conditions, were lysed at 4°C for 1 hour in lysis buffer.10 For the detection of nephrin contained within lipid raft microdomains the lysis buffer was supplemented with 20 mmol/L CHAPS 3-[(3-cholamidopropyl)-dimethilammonio-1-propanesulfonate] (Sigma). After centrifugation of the lysates at 15,000 × g, samples were normalized to 50 μg/sample in 20 μl and resolved by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions and transferred to nitrocellulose. Membranes were blocked and incubated with mouse monoclonal anti-phosphorylated Akt (p-Akt) Ab (Cell Signaling Technology, Beverly, MA) (1:1000) or with mouse monoclonal anti-Akt Ab (Upstate Biotechnology, Lake Placid, NY) (1:2000) overnight at 4°C. For nephrin detection, membranes were incubated with GP-N1 and GP-N2 polyclonal Ab (Progen Biotechnik GmbH, Heidelberg, Germany) (1:500) overnight at 4°C. Blots were then probed with peroxidase-conjugated goat anti-mouse IgG (1:5000) (Pierce, Rockford, IL) or protein A (Amersham, Buckinghamshire, UK) for 1 hour at room temperature and developed with chemiluminescence reagents (ECL, Amersham). Immortalized podocytes served as control cells. To detect phosphorylated nephrin, cell lysates were immunoprecipitated with an anti-nephrin IgG Ab, cross-linked to Sepharose-protein A, as described.27
Three separate experiments, each in duplicate for each experimental condition, were performed. Data were expressed as p-Akt/Akt ratio and as phosphorylated nephrin following densitometric analysis of WB bands. Staining for the expression of nephrin was performed by immunofluorescence technique and detected using guinea pig anti-nephrin polyclonal GP-N1 and GP-N2 Ab, as described.10
Further, the phosphorylation status of Akt was monitored by an ELISA assay, using a commercially available kit (cellular activation of signaling ELISA, CASE, Superarray Bioscience Corporation, DBA, Milan, Italy). Briefly, cells were seeded into a 96-well plate and fixed in 4% paraformaldehyde. One-half of the wells were treated with the anti-phosphoprotein specific primary antibody (1:150) and the other half with the anti-panprotein specific primary Ab (1:200) overnight at 4°C. Cells were then incubated with the secondary Ab (1:160) recognizing Akt regardless of its activation state for 1 hour at room temperature, and the amount of bound Ab was determined using a developing solution and an ELISA plate reader. The absorbance readings at 450 nm were normalized to relative cell number as determined by a cell staining solution. This assay was also used in experiments aiming to evaluate whether the effects of statin involved PI3K. In these experiments, islet MECs were treated with two unrelated PI3K pharmacological inhibitors, wortmannin (0.1 μmol/L) and LY294002 (10 μmol/L). Three to five experiments were performed for each experimental condition.
IL-1β, Nitric Oxide Synthases, and NO Detection
Cell culture supernatants were collected before each subculture and medium exchange, centrifuged and stored at −80°C. IL-1β was measured in duplicate by quantitative sandwich enzyme immunoassay (R&D Systems, Abingdon, UK), according to the manufacturer’s instructions. Color intensity was read at the appropriate wavelength on a microplate reader (Bio-Rad, Hercules, CA). Detection limit of the assay was 3.9 pg/ml.
For immunofluorescence detection of constitutive expression of nitric oxide synthase (eNOS) and inducible NOS (iNOS), cells were seeded onto eight-well chamber slides (Nalgene Nunc International, Rochester, NY) and cultured for 72 hours in normal or high glucose to subconfluence. Cells were fixed in 4% paraformaldehyde and permeabilized using 1% paraformaldehyde and 0.5% Triton X-100 for 10 minutes. After washing with PBS (0.25% bovine serum albumin), cells were then incubated with anti-human eNOS or with anti-human iNOS mAb (Transduction Laboratories, BD, Milan, Italy) (1:100) overnight at 4°C. Cells were washed and subsequently incubated with fluorescein isothiocyanate-conjugated anti-mouse IgG (Dako) for 1 hour at room temperature. After washing the slides were mounted in Vectashield H-1000 mounting medium (Vector Laboratories, Burlingame, CA) and examined by UV microscopy and digital images obtained using a low-light video camera. NOSs were also detected by WB using the same mAbs to probe the membranes and as described above.
The cell-permeable NO reactive dye DAF2-DA (Alexis Italia, Vinci, Italy) was used to examine intracellular production of NO under normal or high-glucose culture conditions, as described.28 NO was determined as nitrite concentration in culture supernatants by diazotization reaction, using NaNO2 as standard, as described.29
Statistical Analysis
5-Bromo-2′-deoxyuridine incorporation, apoptosis (ie, optical density for DNA fragmentation assay and percentage of apoptotic cell for terminal deoxynucleotidyl transferase dUTP nick-end labeling assay), levels of p-Akt/Akt expression, and levels of IL-1β and NO between cells grown in different glucose concentrations were compared using the Mann-Whitney U-test. Data were analyzed using the SPSS statistical package (SPSS, Chicago, IL), and P values less than 0.05 were considered significant. Due to batch-to-batch and interassay variations, in some experimental data are represented as percentages of variation (means ± SD) of the results obtained in 5.6 mmol/L glucose conditions within each experiment, unless otherwise stated.
Results
Cell Growth and Apoptosis
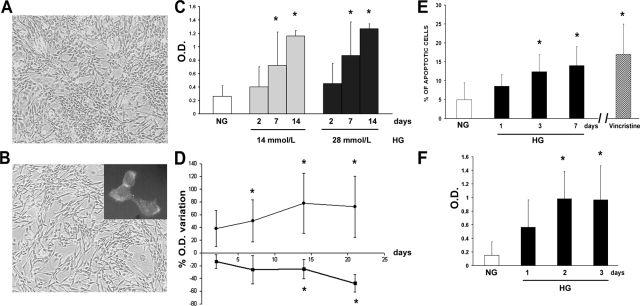
Under hyperglycemic culture conditions, islet MECs maintained the same morphological aspect of normal glucose counterparts (Figure 1, A and B) and the endothelial characteristics as assessed by detection of von Willebrand’s factor (Figure 1B, inset). Time course experiments showed that apoptosis, assessed by DNA fragmentation, was significantly higher in islet MECs cultured in 14 or 28 mmol/L glucose concentrations than in normal glucose starting from day 7 of culture. No significant difference was observed at day 2 of high-glucose culture nor between 14 and 28 mmol/L glucose concentrations (Figure 1C). Therefore, all subsequent experiments were performed using 28 mmol/L concentration. Glucose incorporation was increased, fluctuating from an increase of approximately 60 to 250% compared to that in normoglycemic conditions without showing particular temporal trends.
Figure 1.
Morphological aspects, proliferation, and apoptosis of islet MECs cultured in high glucose. Representative micrograph of islet MECs in normal (A) and high-glucose (B) culture. Original magnification, ×100. In high glucose, cells retain endothelial cell characteristics, ie, positive cytoplasm staining for von Willebrand’s factor, assessed by immunofluorescence (inset in B). C: Apoptosis, assessed as DNA fragmentation, of islet MECs cultured for 2, 7, or 14 days in 14 (gray columns) or 28 mmol/L glucose (black columns) compared to parallel culture in normal glucose (white column). D: Percentage of optical density variation of apoptosis, assessed by DNA fragmentation (closed circle) and proliferation, assessed as 5-bromo-2′-deoxyuridine incorporation (closed square) of cells in 28 mmol/L glucose, compared to cell in normal glucose (taken as 0). E: Percentage of apoptotic islet MECs subjected to terminal deoxynucleotidyl transferase dUTP nick-end labeling assay after culture in normal (NG) or in 28 mmol/L glucose (HG). Cells were incubated with vincristine (0.3 μg/ml) overnight as control. F: Assessment of caspase-3 activity in islet MECs incubated for up to 3 days in normal (NG) or 28 mmol/L glucose (HG). Data are expressed as means ± SD of five different experiments for each time point. *P < 0.05 compared to normal glucose culture.
After 1 week culture in 28 mmol/L glucose, the number of viable cells detected by trypan blue exclusion was reduced to 65 ± 11% (n = 5) in respect to cells cultured in physiological concentration of glucose. In time course experiments, DNA synthesis assessed by 5-bromo-2′-deoxyuridine incorporation was progressively reduced in islet MECs grown in high glucose compared to physiological concentrations, decreasing from 13.5 ± 11% after 48 hours to 48 ± 14% after approximately 30 days of culture compared to normal glucose conditions (Figure 1D). The mean ± SD optical densities were 0.34 ± 0.1 in normal glucose, 0.27 ± 0.12 after 48 hours, 0.25 ± 0.13 after 7 days, 0.18 ± 0.04 after 14 days, and 0.12 ± 0.04 after 30 days in high glucose (P > 0.05 until 7 days and P < 0.05 afterward versus normal glucose conditions).
Apoptosis assessed by DNA fragmentation progressively increased in islet MECs grown in high glucose, ranging from an increase of 33 ± 28% after 48 hours up to an increase of 73 ± 48% after 30 days of culture compared to normal glucose conditions (Figure 1D). The mean ± SD optical densities were 0.28 ± 0.1 in normal glucose, 0.43 ± 0.3 after 48 hours, 0.44 ± 0.2 after 7 days, 0.6 ± 0.4 after 14 days, and 0.74 ± 0.5 after 30 days in high glucose (P > 0.05 at 48 hours and P < 0.05 afterward versus normal glucose conditions). Primary islet MECS in short-term experiments exhibited similar behavior in proliferation and apoptosis assays (data not shown).
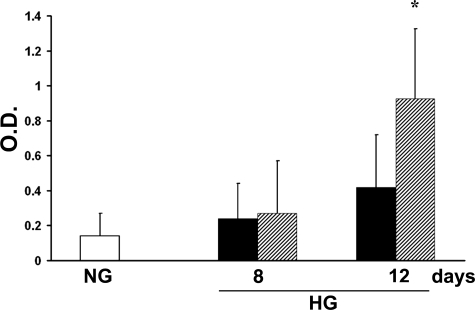
The percentage of terminal deoxynucleotidyl transferase dUTP nick-end labeling-positive cells was similar (5 to 7%) in islet MECs incubated for 24 hours in normal or high-glucose conditions; by 72 hours, however, a significantly greater percentage of cells in high glucose were apoptotic (14 ± 5%, P < 0.05), as shown in Figure 1E. An increase in caspase-3 activity, which is thought to be an early signal of apoptosis, was evident in islet MECs incubated with high glucose, with the highest results in the first 72 hours showing approximately a mean increase of activity of 115% (P < 0.05 versus normal glucose) (Figure 1F). In experimental conditions with intermittent hyperglycemia, islet MECs showed greater apoptosis, detected as DNA fragmentation, compared to parallel culture of sustained hyperglycemia (after 12 days of culture, means ± SD optical densities: 0.14 ± 0.1 for normal glucose, 0.42 ± 0.3 for sustained hyperglycemia, and 0.95 ± 0.4 for intermittent hyperglycemia, P < 0.05) (Figure 2).
Figure 2.
Effects of intermittent hyperglycemia on apoptosis. Apoptosis, assessed as DNA fragmentation, of islet MECs when subjected to repeated cycles of 48-hour growth in high glucose (shaded columns) compared to parallel culture of normal glucose (white column) and sustained hyperglycemia (black columns). Data are expressed as means ± SD of three different experiments. *P < 0.05 compared to normal glucose culture and sustained hyperglycemia.
Expression of Phosphorylated Akt and Nephrin
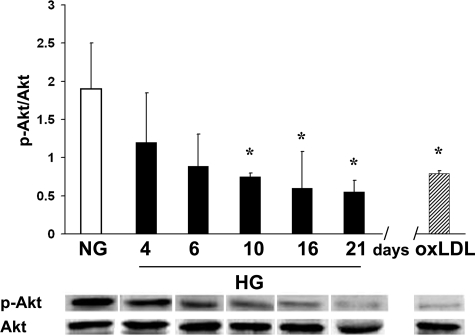
Analysis of the Akt-dependent survival pathway by WB indicated that high-glucose conditions induced dephosphorylation of Akt, as demonstrated by the significant reduction of the p-Akt/Akt ratio (mean ratio 1.9 ± 0.6 for normal glucose, 0.8 ± 0.3 for all high-glucose cultures, P < 0.05). The p-Akt/Akt ratio exhibited a trend of progressive decrease with the duration of high-glucose culture, as shown in Figure 3. Similarly, oxLDL induced dephosphorylation of Akt of islet MECs.
Figure 3.
Effects of hyperglycemia and oxLDL on Akt phosphorylation. Progressive reduction of p-Akt/Akt ratio was evaluated as densitometric analysis of WB bands in islet MECs cultured for different times in high glucose (black columns) compared to normal glucose (white column). Similarly, oxLDL treatment (100 μg/ml) induced reduction of p-Akt/Akt ratio (shaded column). Data are expressed as means ± SD of three different experiments for each time point. *P < 0.05 compared to normal glucose culture.
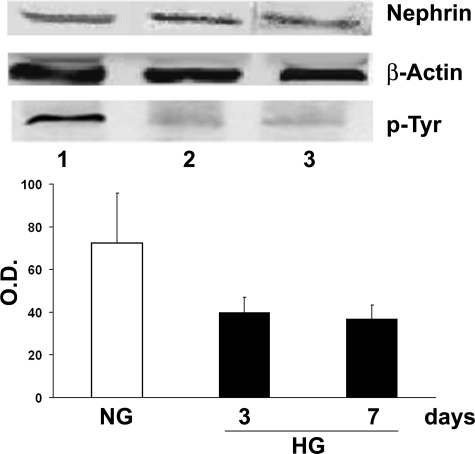
By immunofluorescence microscopy on confluent monolayers of islet MECs in high glucose nephrin was detectable on the cell surface without evidence of nephrin loss or redistribution compared to normal glucose conditions, confirming previous observations10 (data not shown). However, WB analysis of the immunoprecipitates showed that hyperglycemia, although it did not reduce the expression of nephrin, inhibited its tyrosine phosphorylation (Figure 4), which has been shown to be a critical step in nephrin-induced signaling.30
Figure 4.
Effects of hyperglycemia on nephrin and phosphorylated nephrin. Expression of nephrin was detected by WB analysis as a band of approximately 160 kd. Nephrin immunoprecipitates of cell lysates were submitted to WB and probed with anti-phosphotyrosine (anti-p-Tyr) Ab. Densitometric analysis of WB bands was performed on Tyr-phosphorylated nephrin. Islet MECs were cultured in normal (lane 1 and NG) or 3 day (lane 2) and 7 day (lane 3) high glucose (HG). Data are expressed as means ± SD of three different experiments for each time point.
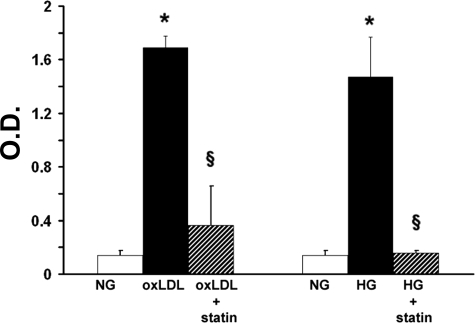
Pravastatin Inhibits the Effects of High Glucose and oxLDL on Islet MEC Apoptosis and Akt Activation
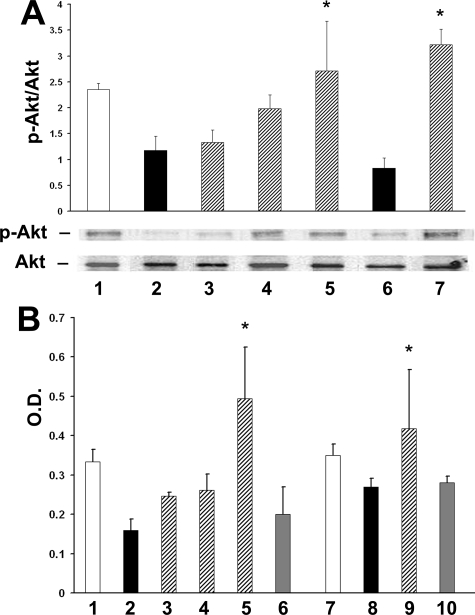
Exposure to the 3-hydroxy-3-methylglutaryl coenzyme A inhibitor pravastatin significantly inhibited apoptosis of islet MECs chronically (8 days) exposed to high glucose, and apoptosis was induced by 100 μg/ml oxLDL (Figure 5). The effect of pravastatin was evident at the daily dose of 1 μmol/L, with the lower concentration not having a significant effect (data not shown). The reduced apoptosis detected with pravastatin treatment was accompanied by an increase in the p-Akt/Akt ratio (Figure 6A). This effect increased with treatment duration, with an increase of the p-Akt/Akt ratio of 12% for 1 day of pravastatin treatment, 70% increase for 3 days treatment, and 130% increase for daily treatment when the cells were cultured for 8 days in high glucose (mean ratio 2.35 ± 0.12 for normal and 1.17 ± 0.2 for high-glucose culture, respectively, and 2.1 ± 0.7 for all statin treatment regimes, P < 0.05).
Figure 5.
Pravastatin inhibits the effects of hyperglycemia and oxLDL on islet MEC apoptosis. Effects of 1 μmol/L pravastatin daily treatment (shaded columns) on apoptosis, assessed by DNA fragmentation, of islet MECs treated with oxLDL (100 μg/ml) (black columns) or cultured for 7 days in high glucose (black columns). Data are expressed as means ± SD of five separate experiments. *P < 0.05 versus normal glucose culture; §P < 0.05 versus oxLDL treatment or high-glucose culture.
Figure 6.
Pravastatin prevents the reduction of Akt phosphorylation. A: Densitometric analysis of p-Akt/Akt ratio assessed by WB analysis in normal glucose culture (1); 7-day culture in high glucose (2); treatment with 1 μmol/L pravastatin for 1 day (3), 3 days (4), and 7 days (5); treatment with oxLDL, 100 μg/ml (6); treatment with oxLDL and pravastatin (7). *P < 0.05 versus high-glucose culture and versus oxLDL treatment. B: The effects of pravastatin on p-Akt were confirmed by an ELISA assay in the following conditions: normal glucose culture (1 and 7); 7-day culture in high glucose (2); treatment with 1 μmol/L pravastatin for 1 day (3), 3 days (4), and 7 days (5); treatment with oxLDL, 100 μg/ml (8); treatment with oxLDL and pravastatin (9). A significant reduction of Akt phosphorylation was induced by simultaneous treatment with pravastatin and wortmannin and LY294002 PI3K inhibitors of islet MECs cultured in high glucose (6) or treated with oxLDL (10). Data are expressed as means ± SD of three to five different experiments. *P < 0.05 versus high-glucose culture and versus statin plus PI3K inhibitors.
These results were confirmed by an Akt ELISA assay, which revealed a similar trend of the p-Akt/Akt ratio induced by high glucose, by oxLDL, and by pravastatin treatment (Figure 6B). The protective effect of pravastatin on Akt phosphorylation was reduced by overnight treatment of islet MECs with wortmannin and LY294002, suggesting that this effect was at least in part dependent on the activation of PI3K (Figure 6B).
Levels of IL-1β, NO, and NOS Expression
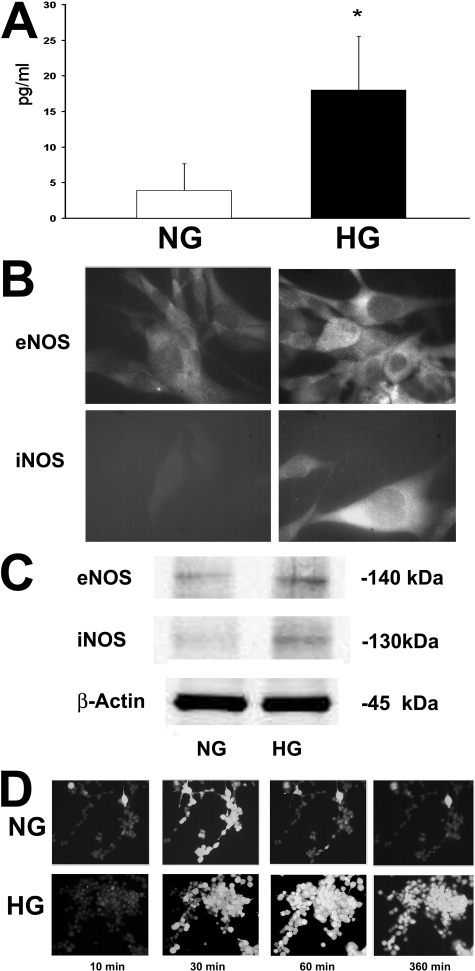
Production of IL-1β, measured by ELISA on cell-free supernatants, was increased by 2 weeks of high-glucose culture (mean 17.3 ± 7.6 pg/ml) compared to levels in supernatants of normal glucose culture (3.9 ± 3.7 pg/ml) (P < 0.05) (Figure 7A). By immunofluorescence staining, eNOS expression was detected in islet MECs in both culture conditions, while iNOS expression was detected only in cells grown in high glucose (Figure 7B). Similarly by WB analysis, only lysates of islet MECs in high glucose showed iNOS expression, whereas eNOS was expressed in normal glucose and up-regulated in high glucose (Figure 7C).
Figure 7.
Endothelial IL-1β production and expression of NO synthases. A: Mean value ± SD of IL-1β in cell-free supernatants of islet MECS in normal (white column) and high (black column) glucose during 2 weeks of culture. *P < 0.05. B: Representative immunofluorescence micrographs of islet MECs stained with anti-eNOS and anti-iNOS Abs. By immunofluorescence eNOS was detectable in islet MECs in normal and high-glucose culture, whereas iNOS was detectable only in cells in high-glucose culture. Original magnification, ×400. Five experiments were performed with similar results. C: Representative WB analysis of eNOS and iNOS expression. Lysates of islet MECs in normal glucose expressed eNOS but not iNOS, whereas lysates of islet MECs in high glucose showed up-regulation of eNOS and expression of iNOS. D: Representative micrographs of time lapse fluorescent microscopy of NO-dependent generation of fluorescence by DAF2-DA in islet MECs. In normal glucose, only a few islet MECs synthesize NO, becoming detectable in the field after 10 minutes, with fluorescence peak after 30 minutes followed by a rapid decrease. In high glucose, a progressive increase in the lightening of individual cells and fluorescence intensity was observed, with a peak approximately at 60 minutes; lightening persisted for up to 6 to 8 hours and decreased slowly. As positive control, cells were stimulated with l-arginine (10−3 mol/L) and the number of positive cells increased progressively up to 15 minutes, to decrease within 30 minutes. The specificity of fluorescence was assessed by the abrogation of NO fluorescence in the presence of Nω-nitro-l-arginine methyl ester (10−3 mol/L) (not shown).
NO synthesis by islet MECs was investigated as the detection of DAF-2 DA fluorescence in a system of time lapse cinematography. As cells synthesize NO, they become detectable in the field. Under basal conditions, some cells were detectable with a fluorescence peak after 30 minutes followed by a rapid decrease; culture in high glucose induced a progressive increase in individual cells and in NO-dependent fluorescence intensity, persisting for many hours (Figure 7D). Mean values for repeated measurements of the stable NO oxidation product nitrite were significantly higher in supernatants of cells in high than in normal glucose (normal glucose, 3.5 ± 1.5 nmol/L; 24 hours in high glucose, 5.2 ± 1.8 nmol/L, P < 0.05; 2–7 days in high glucose, 38.9 ± 20 nmol/L, P < 0.005).
Discussion
Pancreatic islet microendothelium exhibits unique structural and functional features in an interdependent physical and functional relationship with the neighboring β cells.5,31 The present study is the first to highlight that glucose toxicity is not solely restricted to β cells within the endocrine pancreas. Sustained hyperglycemia progressively affects cellular survival and proliferation, and it increases apoptosis of cultured islet MECs. We detected early apoptosis after 24 to 48 hours in high glucose both by DNA fragmentation and activation of the caspase family, representing the first step of apoptosis in eukaryotic cells.32,33 The triggering of caspase-3 has been established as the downstream executor of high-glucose-induced apoptosis in human umbilical vein endothelial cells via reactive oxygen species.18 Notably, apoptosis was amplified by intermittent high-glucose conditions, in line with direct and indirect evidence that glucose excursions carry an independent risk for cardiovascular disease.34 Experimental data indicate that acute hyperglycemia, such as postprandial blood glucose, can exert deleterious effects on the arterial wall through mechanisms including oxidative stress, endothelial dysfunction, and activation of the coagulation cascade.35
The metabolic mechanisms by which hyperglycemia initiates apoptosis in vascular endothelium are incompletely understood. These mechanisms include oxidative stress, increased intracellular Ca2+, mitochondrial dysfunction, changes in intracellular fatty acid metabolism, and impaired phosphorylation of the protein kinase Akt.20 The Akt signaling pathway plays a pivotal role in preventing apoptosis in a variety of settings36 and, in particular, Akt activation is crucial for the ability of factors such as insulin, insulin-like growth factor-1, and vascular endothelial growth factor to inhibit apoptosis in cultured endothelium.37 Recent data highlight its role also in insulin-mediated glucose transport and pancreatic β cell mass and function.38,39
In the present study we observed that islet MECs under conditions of sustained hyperglycemia showed progressively reduced phosphorylation of Akt, suggesting an interference with the pathway(s) involved in Akt activation. Apoptosis of islet MECs and reduced Akt phosphorylation were induced also by treatment with oxLDL, known to affect the behavior of other endothelial cells40 and to play a major role in the pathogenesis of atherosclerosis.41 Concomitantly, in our study, hyperglycemia down-regulated the tyrosine phosphorylated form of the transmembrane protein nephrin without affecting its cellular expression or distribution. It is known that nephrin, once phosphorylated, associates with PI3K and activates the multifunctional Akt-dependent pathways.30 Therefore, hyperglycemia-induced apoptosis of islet endothelium likely involves the nephrin-mediated signaling cascade, as shown for human podocyte survival.26,42 In fact, nephrin appears to be more than a determinant of cellular ultrastructure, which, similarly to other adhesion molecules, might function in intracellular signal transduction. In addition, phosphorylation of the tyrosine sites (by Src family kinases) within the intracytoplasmic C terminal domain of nephrin activates mitogen-activated protein kinase p38 and jun kinase (JNK) and thereby the transcription factor activating protein-1 (AP-1). AP-1 modulates a variety of cellular programs, including proliferation, differentiation, and apoptosis.30,43
Recent studies have linked the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, statins, and Akt, since Akt is a biological target for statin action in endothelial cells.23,26 Testing whether statins may reverse the response of islet MECs to oxLDL and hyperglycemia, we demonstrated that pravastatin treatment inhibited apoptosis and increased Akt phosphorylation. Akt is a major effector of the PI3K survival signaling pathway.44 In the present study, we observed that the protective effects of statin were blocked by treatment with pharmacological inhibitors of PI3K, thus suggesting the involvement of the PI3K/Akt-dependent pathway was impaired by hyperglycemia or oxLDL. These findings are in line with previous data showing that in endothelial cells, statins rapidly promote the activation of PI3K/Akt pathways, mediating cell survival, NO synthesis and migration, and improve vascular function independently from their lipid-lowering effects.23
Moreover, the present report highlights that the effects of sustained hyperglycemia on islet vasculature could impair β cell function. The natural history of type 2 diabetes is in fact characterized by progressive increase in glucose levels, which has been claimed to be due to progressive reduction of β cells function and mass.21,22,45 The mechanisms causing this loss are still debated. Further, a major defect of insulin secretory function is found in all forms of diabetes,46 and sustained hyperglycemia has a detrimental impact on angiogenesis, growth, and function of transplanted islets.47,48 In this study we detected increased production of the proinflammatory cytokine IL-1β by islet MECs under hyperglycemic conditions. Previous studies demonstrated that human β cells are a potential source of glucose-induced IL-1β independently of any viral or immune-mediated process.49 IL-1β has been shown to impair insulin release in human islets and to induce Fas expression enabling Fas-mediated apoptosis, thus implicating an inflammatory process in the pathogenesis of glucotoxicity in the diabetic condition.45,50 Hyperglycemia also induced expression of the enzyme inducible NOS in islet MECs and increased NO production. This is in line with data in animal models indicating that islet microendothelium has a unique phenotype also in terms of expression of NO synthases, since their activities are closely regulated by glucose concentration,51 suggesting an organ-specific control of NO formation. The role of NO in islet cytotoxicity is well established29,52; its role in the physiology of insulin release, although controversial, indicates that NO could directly impair insulin release.53
At variance with other vascular endothelial cells, islet MECs express the barrier and signaling protein nephrin. Human and murine studies on glomerular filtration apparatus where nephrin is specifically located indicate that nephrin, when dephosphorylated, is associated with increased podocyte apoptosis and altered function.42,54 Nephrin dephosphorylation induced by hyperglycemia could therefore also impair islet vasculature function in addition to the effects on the PI3K-Akt pathway.
Due to the interplay between islet endothelium and β cells,1,2,31 the apoptotic effects of a diabetic milieu on islet MECs may carry additional relevant consequences to the endocrine cells. Among the islet vasculature functions serving the β cells there is, in fact, promotion of β cell proliferation.7,8,9 It is now accepted that postnatal β cell mass is dynamic and can increase, both in function and mass, to compensate for added demand, by replication and/or neogenesis.55 It remains to be elucidated how the islet microvasculature participates in sensing the environment of the islets and generates signals to affect adult islet endocrine function.
In conclusion, the data presented offer an insight into the pathological processes taking place within pancreatic endothelium in hyperglycemia. In this condition, in virtue of their participation in the endocrine tissue development and physiopathology, islet endothelial cells represent a target and an effector of hyperglycemic condition, actively contributing to progressive islet dysfunction. Statin treatment, by inhibiting apoptosis, represents a therapeutic tool to improve islet vascularization and, indirectly, islet function. Further investigations to understand the comprehensive role of endothelium in islet physiology and pathology are warranted.
Footnotes
Address reprint requests to Dr. Maria M. Zanone, Dipartimento di Medicina Interna, Corso Dogliotti 14, 10126 Torino, Italy. E-mail: mmz@libero.it.
Supported by Regione Piemonte, Ricerca Sanitaria Finalizzata 2004 (1634/27.01), and by Università degli Studi di Torino, fondi 2006.
References
- Bonner-Weir S, Go VLW, Dimagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA. The pancreas. Biology, pathobiology and disease. New York: Raven Press,; The microvasculature of the pancreas, with special emphasis on that of the islets of Langerhans. Anatomy and functional implications. 1993:pp 759–768. [Google Scholar]
- Konstantinova I, Lammert E. Microvascular development: learning from pancreatic islets. Bioessays. 2004;26:1069–1075. doi: 10.1002/bies.20105. [DOI] [PubMed] [Google Scholar]
- Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- Sakamoto C, Goldfine ID, Roach E, Williams JA. Localization of saturable CCK binding sites in rat pancreatic islets by light and electron microscope autoradiography. Diabetes. 1985;34:390–394. doi: 10.2337/diab.34.4.390. [DOI] [PubMed] [Google Scholar]
- Olsson R, Carlsson PO. The pancreatic islet endothelial cell: emerging roles in islet function and disease. Int J Biochem Cell Biol. 2006;38:492–497. doi: 10.1016/j.biocel.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147:2315–2324. doi: 10.1210/en.2005-0997. [DOI] [PubMed] [Google Scholar]
- Kaido T, Yebra M, Cirulli V, Montgomery AM. Regulation of human beta-cell adhesion, motility, and insulin secretion by collagen IV and its receptor alpha1beta1. J Biol Chem. 2004;279:53762–53769. doi: 10.1074/jbc.M411202200. [DOI] [PubMed] [Google Scholar]
- Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fässler R, Gu G, Gerber HP, Ferrara N, Melton DA, Lammert E. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Zanone MM, Favaro E, Doublier S, Lozanoska-Ochser B, Deregibus MC, Greening J, Huang GC, Klein N, Cavallo Perin P, Peakman M, Camussi G. Expression of nephrin by human pancreatic islet endothelial cells. Diabetologia. 2005;48:1789–1797. doi: 10.1007/s00125-005-1865-5. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S. Regulation of pancreatic beta-cell mass in vivo. Recent Prog Horm Res. 1994;49:91–104. doi: 10.1016/b978-0-12-571149-4.50008-8. [DOI] [PubMed] [Google Scholar]
- Treutelaar MK, Skidmore JM, Dias-Leme CL, Hara M, Zhang L, Simeone D, Martin DM, Burant CF. Nestin-lineage cells contribute to the microvasculature but not endocrine cells of the islet. Diabetes. 2003;52:2503–2512. doi: 10.2337/diabetes.52.10.2503. [DOI] [PubMed] [Google Scholar]
- Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, Baldwin HS, Nicholson W, Bader DM, Jetton T, Gannon M, Powers AC. Pancreatic islet production of vascular endothelial growth factor–a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang L, Meshinchi S, Dias-Leme C, Raffin D, Johnson JD, Treutelaar MK, Burant CF. Islet microvasculature in islet hyperplasia and failure in a model of type 2 diabetes. Diabetes. 2006;55:2965–2973. doi: 10.2337/db06-0733. [DOI] [PubMed] [Google Scholar]
- Lorenzi M, Cagliero E. Pathobiology of endothelial and other vascular cells in diabetes mellitus. Call for data. Diabetes. 1991;40:653–659. doi: 10.2337/diab.40.6.653. [DOI] [PubMed] [Google Scholar]
- Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwart BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- Goligorsky MS, Chen J, Brodsky S. Workshop: endothelial cell dysfunction leading to diabetic nephropathy : focus on nitric oxide. Hypertension. 2001;37(part 2):744–748. doi: 10.1161/01.hyp.37.2.744. [DOI] [PubMed] [Google Scholar]
- Ho FM, Liu SH, Liau CS, Huang PJ, Lin-Shiau SY. High glucose-induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH(2)-terminal kinase and caspase-3. Circulation. 2000;101:2618–2624. doi: 10.1161/01.cir.101.22.2618. [DOI] [PubMed] [Google Scholar]
- Baumgartner-Parzer SM, Wagner L, Pettermann M, Grillari J, Gessl A, Waldhäusl W. High-glucose-triggered apoptosis in cultured endothelial cells. Diabetes. 1995;44:1323–1327. doi: 10.2337/diab.44.11.1323. [DOI] [PubMed] [Google Scholar]
- Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- Donath MY, Halban PA. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47:581–589. doi: 10.1007/s00125-004-1336-4. [DOI] [PubMed] [Google Scholar]
- Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53 Suppl 3:S16–S21. doi: 10.2337/diabetes.53.suppl_3.s16. [DOI] [PubMed] [Google Scholar]
- Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, Sessa WC, Walsh K. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–1010. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halcox JP, Deanfield JE. Beyond the laboratory: clinical implications for statin pleiotropy. Circulation. 2004;109:42–48. doi: 10.1161/01.CIR.0000129500.29229.92. [DOI] [PubMed] [Google Scholar]
- Favaro E, Bottelli A, Lozanoska-Ochser B, Ferioli E, Huang GC, Klein N, Chiaravalli A, Perin PC, Camussi G, Peakman M, Conaldi PG, Zanone MM. Primary and immortalised human pancreatic islet endothelial cells: phenotypic and immunological characterisation. Diabetologia. 2005;48:2552–2562. doi: 10.1007/s00125-005-0008-3. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Deregibus MC, Fonsato V, Doublier S, Spatola T, Procida S, Di Carlo F, Camussi G. Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J Am Soc Nephrol. 2005;16:1936–1947. doi: 10.1681/ASN.2004080629. [DOI] [PubMed] [Google Scholar]
- Deregibus MC, Cantaluppi V, Doublier S, Brizzi MF, Deambrosis I, Albini A, Camussi G. HIV-1-Tat protein activates phosphatidylinositol 3-kinase/AKT survival pathways in Kaposi’s sarcoma cells. J Biol Chem. 2002;77:25195–25202. doi: 10.1074/jbc.M200921200. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Mariano F, Migliori M, Camussi G. Nitric oxide/platelet activating factor cross-talk in mesangial cells modulates the interaction with leukocytes. Kidney Int. 2002;62:1322–1331. doi: 10.1111/j.1523-1755.2002.kid589.x. [DOI] [PubMed] [Google Scholar]
- Steiner L, Kröncke K, Fehsel K, Kolb-Bachofen V. Endothelial cells as cytotoxic effector cells: cytokine-activated rat islet endothelial cells lyse syngeneic islet cells via nitric oxide. Diabetologia. 1997;40:150–155. doi: 10.1007/s001250050656. [DOI] [PubMed] [Google Scholar]
- Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, Egger L, Lecha RL, Borner C, Pavenstadt H, Shaw A, Walz G, Benzing T. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol. 2003;23:4917–4928. doi: 10.1128/MCB.23.14.4917-4928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanone MM, Favaro E, Camussi G. From endothelial to Beta cells: insights into pancreatic islet microendothelium. Curr Diabetes Rev. 2008;4:1–9. doi: 10.2174/157339908783502415. [DOI] [PubMed] [Google Scholar]
- Vaux DL, Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci. 1996;93:2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. The apoptotic cysteine protease CPP32. Int J Biochem Cell Biol. 1997;29:393–396. doi: 10.1016/s1357-2725(96)00146-x. [DOI] [PubMed] [Google Scholar]
- Bonora E. Postprandial peaks as a risk factor for cardiovascular disease: epidemiological perspectives. Int J Clin Pract Suppl. 2002;129:5–11. [PubMed] [Google Scholar]
- Båvenholm PN, Efendic S. Postprandial hyperglycaemia and vascular damage–the benefits of acarbose. Diab Vasc Dis Res. 2006;3:72–79. doi: 10.3132/dvdr.2006.017. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Jung F, Haendeler J, Goebel C, Zeiher AM, Dimmeler S. Growth factor-induced phosphoinositide 3-OH kinase/Akt phosphorylation in smooth muscle cells: induction of cell proliferation and inhibition of cell death. Cardiovasc Res. 2000;48:148–157. doi: 10.1016/s0008-6363(00)00152-8. [DOI] [PubMed] [Google Scholar]
- Bernal-Mizrachi E, Fatrai S, Johnson JD, Ohsugi M, Otani K, Han Z, Polonsky KS, Permutt MA. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J Clin Invest. 2004;114:928–936. doi: 10.1172/JCI20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elghazi L, Balcazar N, Bernal-Mizrachi E. Emerging role of protein kinase B/Akt signaling in pancreatic beta-cell mass and function. Int J Biochem Cell Biol. 2006;38:1578–163. doi: 10.1016/j.biocel.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Kita T, Kume N, Minami M, Hayashida K, Murayama T, Sano H, Moriwaki H, Kataoka H, Nishi E, Horiuchi H, Arai H, Yokode M. Role of oxidized LDL in atherosclerosis. Ann NY Acad Sci. 2001;947:199–205. doi: 10.1111/j.1749-6632.2001.tb03941.x. [DOI] [PubMed] [Google Scholar]
- Galle J, Heermeier K, Wanner C. Atherogenic lipoproteins, oxidative stress, and cell death. Kidney Int Suppl. 1999;71:S62–S65. doi: 10.1046/j.1523-1755.1999.07116.x. [DOI] [PubMed] [Google Scholar]
- Foster RR, Saleem MA, Mathieson PW, Bates DO, Harper SJ. Vascular endothelial growth factor and nephrin interact and reduce apoptosis in human podocytes. Am J Physiol Renal Physiol. 2005;288:F48–F57. doi: 10.1152/ajprenal.00146.2004. [DOI] [PubMed] [Google Scholar]
- Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Hawkins PT, Davidson K, Stephens LR. The role of PI3Ks in the regulation of the neutrophil NADPH oxidase. Biochem Soc Symp. 2007;74:59–67. doi: 10.1042/BSS0740059. [DOI] [PubMed] [Google Scholar]
- Maedler K, Spinas GA, Lehmann R, Sergeev P, Weber M, Fontana A, Kaiser N, Donath MY. Glucose induces beta-cell apoptosis via upregulation of the Fas receptor in human islets. Diabetes. 2001;50:1683–1690. doi: 10.2337/diabetes.50.8.1683. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr Beta-cells in type II diabetes mellitus. Diabetes. 1991;40:166–180. doi: 10.2337/diab.40.2.166. [DOI] [PubMed] [Google Scholar]
- Vasir B, Reitz P, Xu G, Sharma A, Bonner-Weir S, Weir GC. Effects of diabetes and hypoxia on gene markers of angiogenesis (HGF, cMET, uPA and uPAR. TGF-alpha, TGF-beta, bFGF and Vimentin) in cultured and transplanted rat islets. Diabetologia. 2000;43:763–772. doi: 10.1007/s001250051374. [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Hawkins YC, Lock J, Lebet J, Sharma A, Bonner-Weir S, Weir GC. Influence of diabetes on the loss of beta cell differentiation after islet transplantation in rats. Diabetologia. 2007;50:2117–2125. doi: 10.1007/s00125-007-0749-2. [DOI] [PubMed] [Google Scholar]
- Maedler K, Fontana A, Ris F, Sergeev P, Toso C, Oberholzer J, Lehmann R, Bachmann F, Tasinato A, Spinas GA, Halban PA, Donath MY. FLIP switches Fas-mediated glucose signaling in human pancreatic beta cells from apoptosis to cell replication. Proc Natl Acad Sci USA. 2002;99:8236–8241. doi: 10.1073/pnas.122686299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth AC, Williams GT, James RF, Scarpello JH, Morgan NG. Human islets of Langerhans express Fas ligand and undergo apoptosis in response to interleukin-1beta and Fas ligation. Diabetes. 1998;47:727–232. doi: 10.2337/diabetes.47.5.727. [DOI] [PubMed] [Google Scholar]
- Suschek C, Fehsel K, Kröncke KD, Sommer A, Kolb-Bachofen V. Primary cultures of rat islet capillary endothelial cells. Constitutive and cytokine-inducible macrophagelike nitric oxide synthases are expressed and activities regulated by glucose concentration. Am J Pathol. 1994;145:685–695. [PMC free article] [PubMed] [Google Scholar]
- Kroncke KD, Rodriguez ML, Kolb H, Kolb-Bachofen V. Cytotoxicity of activated rat macrophages against syngeneic islet cells is arginine-dependent, correlates with citrulline and nitrite concentrations and is identical to lysis by the nitric oxide donor nitroprusside. Diabetologia. 1993;36:17–24. doi: 10.1007/BF00399088. [DOI] [PubMed] [Google Scholar]
- Corbett JA, Sweetland MA, Wang JL, Lancaster JR, Jr, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci USA. 1993;90:1731–1735. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, Hamano Y, Charytan D, Cosgrove D, Kieran M, Sudhakar A, Kalluri R. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem. 2003;278:12605–12608. doi: 10.1074/jbc.C300012200. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Sharma A. Pancreatic stem cells. J Pathol. 2002;197:519–526. doi: 10.1002/path.1158. [DOI] [PubMed] [Google Scholar]