Abstract
Unexplained intrauterine growth restriction of the fetus (IUGR) results from impaired placental development, frequently associated with maternal malperfusion. Some cases are complicated further by preeclampsia (PE+IUGR). Here, we provide the first evidence that placental protein synthesis inhibition and endoplasmic reticulum (ER) stress play key roles in IUGR pathophysiology. Increased phosphorylation of eukaryotic initiation factor 2α suggests suppression of translation initiation in IUGR placentas, with a further increase in PE+IUGR cases. Consequently, AKT levels were reduced at the protein, but not mRNA, level. Additionally, levels of other proteins in the AKT-mammalian target of rapamycin pathway were decreased, and there was associated dephosphorylation of 4E-binding protein 1 and activation of glycogen synthase kinase 3β. Cyclin D1 and the eukaryotic initiation factor 2B epsilon subunit were also down-regulated, providing additional evidence for this placental phenotype. The central role of AKT signaling in placental growth regulation was confirmed in Akt1 null mice, which display IUGR. In addition, we demonstrated ultrastructural and molecular evidence of ER stress in human IUGR and PE+IUGR placentas, providing a potential mechanism for eukaryotic initiation factor 2α phosphorylation. In confirmation, induction of low-grade ER stress in trophoblast-like cell lines reduced cellular proliferation. PE+IUGR placentas showed elevated ER stress with the additional expression of the pro-apoptotic protein C/EBP-homologous protein/growth arrest and DNA damage 153. These findings may account for the increased microparticulate placental debris in the maternal circulation of these cases, leading to endothelial cell activation and impairing placental development.
Intrauterine growth restriction (IUGR), defined as failure of a fetus to reach its genetic growth potential, affects 4 to 7% of births. It can occur in isolation or in association with maternal hypertensive disorders, such as preeclampsia (PE+IUGR), and remains a leading cause of perinatal morbidity and mortality.1 As the interface between a mother and her fetus, the placenta is critical for fetal nutrition. Thus, during human pregnancy, reduced placental growth precedes fetal IUGR.2,3 There are many potential causes of human IUGR, including maternal smoking, undernutrition, infection, or congenital malformations, but the majority of cases remain unexplained. Nonetheless, these are frequently associated with deficient conversion of the maternal spiral arteries supplying the placenta.4,5 We recently proposed that this failure results in excessive spontaneous constriction of the arteries, exposing the placenta to low-grade repetitive ischemia-reperfusion (I/R) injury.6 I/R is a powerful generator of ROS (reactive oxygen species), and oxidative stress is increased in IUGR placentas, and further so in PE+IUGR placentas.7
I/R also depletes intracellular ATP concentrations. One of the consequences of I/R is induction of endoplasmic reticulum (ER) stress. The ER serves many specialized functions in the cell including synthesis, folding and transport of membrane and secretory proteins, and sequestration of calcium ions (Ca2+). The mechanisms underlying I/R activation of ER stress have been explored in other systems, especially the brain.8 Both depletion of ATP and generation of ROS reduce Ca2+ storage within the ER compartment by inhibition of ATP-dependent sarcoplasmic/endoplasmic reticulum Ca2+-ATPases.8,9 In the ER lumen, loss of Ca2+ homeostasis and low ATP concentrations suppress ATP and Ca2+-dependent posttranslational modifications, including disulfide bond formation, N-linked glycosylation and controlled proteolysis. As a result misfolded proteins accumulate, provoking ER stress response pathways or the unfolded protein response.10,11,12
The unfolded protein response attempts to restore ER homeostasis and involves activation of three highly conserved signaling pathways.9,13 The first aims to reduce the burden of new proteins entering the ER lumen through translational attenuation. This is achieved through phosphorylation of eukaryotic initiation factor (eIF)2α (P-eIF2α) by activation of PRKR-like endoplasmic reticulum kinase (PERK),14 and prevents binding of the initiator Met-tRNA to the ribosome.15 The second enhances the protein folding capacity by increasing ER chaperone proteins, such as glucose-regulated protein 78/binding immunoglobulin protein (GRP78) and glucose-regulated protein 94 heat shock protein 90 kDa beta 1(GRP94), and the folding enzymes, such as protein disulfide isomerase and peptidyl-prolyl isomerase. Expression of these proteins is regulated through activation of activating transcription factor 6 and X-box binding protein-1 (XBP-1), a second transcription factor that is a marker of ER stress especially in relation to hypoxia.13 The third pathway promotes degradation of the remaining unfolded or misfolded proteins through increased capacity of the cytosolic ubiquitin-proteosome system.16,17
However, when ER function is severely impaired, apoptosis is induced to eliminate damaged cells. Again, multiple pathways are involved, including increased expression of the transcription factor C/EBP homologous protein/growth arrest and DNA damage 153 (CHOP),18 which in turn suppresses expression of the anti-apoptotic gene, Bcl-2.19 There may also be activation of the Ire1-TRAF2-ASK1-MAP kinase pathway,20 and of ER-associated caspase-4 (in human)21 or caspase-12 (in mouse).22
Recently, we showed that I/R induces ER stress in the human trophoblast-like cell line, JEG-3 choriocarcinoma cells, and that this is associated with inhibition of protein synthesis.23 One of the consequences we observed was reduced concentrations of both phosphorylated and total AKT. AKT signaling plays a central role in the regulation of cell growth and proliferation by integrating the actions of growth factors and functioning as an ATP and amino acid sensor to fine tune protein synthesis to nutrient availability via control of the mammalian target of rapamycin (mTOR) pathway and glycogen synthase kinase 3 (GSK-3) activity, respectively.24,25,26,27 In the mouse homozygous disruption of the Akt1 gene causes IUGR.28
mTOR signaling regulates a number of components of the translational machinery. AKT acts as an upstream kinase that controls mTOR activity via two distinct mechanisms. Firstly, AKT can phosphorylate mTOR directly at serine 244829 although the importance of this is contentious.30 Secondly, AKT phosphorylates tuberous sclerosis complex (TSC)2/tuberin at threonine 1462.31 TSC2 is one component of a dimeric complex that also contains TSC1/hamartin. In its unphosphorylated state it is thought to serve as a GTPase activating protein for the small G protein, ras homolog enriched in brain (Rheb). Rheb, in its GTP-bound state, activates mTOR.32,33
In mammalian cells, mTOR exists in at least in two distinct complexes; mTOR complex 1 (mTORC1) containing the partner protein regulatory associated protein of mTOR (raptor), and mTORC2, containing the partner rapamycin-insensitive companion of mTOR (rictor). It is mTORC1 that regulates protein translation, and it may act via at least three mechanisms. First, activation of mTORC1 promotes cap-dependent translation initiation through phosphorylation of 4E binding protein 1 (4E-BP1), preventing its complexing with eukaryotic initiation factor 4E (eIF4E).26 Second, mTORC1 also modulates initiation through the phosphorylation of multiple serine residues in the C terminus of eIF4G providing an additional mechanism for fine tuning the protein synthesis rate.34 Finally, the ribosomal protein S6 kinase 1 (S6K1, also known as p70RSK), but not S6 kinase 2 (S6K2, also known as p90RSK), is a substrate of mTORC1. S6Ks phosphorylate eukaryotic elongation factor 2 kinase (eEF2K), which in turn phosphorylates and inactivates eEF2, preventing translation elongation.35 In contrast, mTORC2 does not involve any direct role in controlling the protein translation. However, its function seems to provide positive feedback by maintaining AKT activity through phosphorylation at serine 473.36 Multiallelic disruption of rictor, the key component in mTORC2, induces embryonic lethality at E11, resulting from impaired AKT activity.37
Besides regulating mTOR activity, AKT can also influence protein translation indirectly through GSK-3. AKT phosphorylates both GSK-3 isoforms (α, serine 21, and β, serine 9) at their N termini, inhibiting their activities. eIF2Bε (eukaryotic initiation factor 2B subunit epsilon) is substrate of GSK-3, and is crucial for the regeneration of active eIF2.GTP from its inactive eIF2.GDP form in the initiation step of translation machinery.38 Phosphorylation inhibits its activity,39 and will be promoted by low AKT levels. Loss of function mutations in the epsilon and beta subunits of eIF2B result in a reduction of protein synthesis, and are the major cause of a human inherited genetic disease termed ‘vanishing white matter.’ Interestingly, IUGR is associated with this disease.40,41
We hypothesize, therefore, that deficient conversion of the spiral arteries causes a low-grade I/R injury to the placenta that activates phosphorylation of eIF2α. Subsequent inhibition of protein translation leads to reduced AKT signaling, suppressing activity in the mTOR and GSK-3 pathways and further reinforcing attenuation of protein synthesis through modulation of components of the translation initiation and elongation machinery, including eIF2Bε, 4E-BP1, and eEF2K. Activation of P-eIF2α in the pathological placentas may occur through induction of ER stress secondary to oxidative stress. Evidence of ER stress includes increases in the proteins, GRP94 and C/EBP homologous protein (CHOP), an increase in the spliced variant of Xbp-1 mRNA, and dilated ER cis ternae. Ultimately, reduced concentrations of cell cycle regulatory proteins such as cyclin D1 would result in slowing of cell proliferation. This is confirmed by induction of low-grade ER stress through I/R in choriocarcinoma cells. If maintained over a period of weeks or months in utero we predict these effects would have a dramatic impact on placental, and hence fetal, growth.
Materials and Methods
Sample Collection
Samples were collected with informed written consent of the patients and Local Ethical Committee approvals. All placentas were delivered by elective caesarean section from non-laboring singleton pregnancies. IUGR was diagnosed ultrasonically when abdominal circumference measurements were below the 10th percentile,42 and confirmed at birth by a neonatal weight below the 10th percentile.43 Severity of IUGR was evaluated by Doppler velocimetry of the umbilical artery and by fetal heart rate, as previously described.44 Of the six IUGR cases, three had normal umbilical artery Doppler velocimetry and normal fetal heart rate, one had absent end diastolic blood flow of the umbilical artery but normal fetal heart rate and two had abnormal umbilical arterial Doppler velocimetry and abnormal fetal heart rate. On the maternal side, four displayed abnormal uterine artery Doppler blood flow velocimetry, confirming deficient conversion of the spiral arteries.
Preeclampsia (PE+IUGR) was defined as the onset of gestational hypertension and proteinuria after 20 weeks of gestation, and the cases examined also included all of the criteria for IUGR. Hypertension was defined as two or more recordings of a diastolic blood pressure of 90 mmHg or more taken at least 4 hours apart. Proteinuria was taken as the excretion of 300 mg protein or more over a 24-hour period. Of the six PE+IUGR cases, one had normal umbilical artery Doppler velocimetry and normal fetal heart rate, one had absent end diastolic blood flow of the umbilical artery but normal fetal heart rate, and four had abnormal umbilical arterial Doppler velocimetry and abnormal fetal heart rate.
Controls were from normotensive term pregnancies delivering appropriate for gestational age fetuses that displayed no abnormality on routine scans.
In all pregnancies gestational age was calculated from the last menstrual period and confirmed by routine ultrasonography at 11 to 12 weeks of gestation. Exclusion criteria for both controls and IUGR were: fetal chromosomal abnormalities, pre- and postnatal malformations or phenotypic anomalies, and maternal diseases predisposing to IUGR, such as autoimmune diseases, thrombophilic conditions, and diabetes.
Cell Culture
The human choriocarcinoma JEG-3 cells were cultured as previously described.23 Human choriocarcinoma JAR cells were a gift from Dr. Ashley Moffett and were grown in RPMI 1640 medium (Invitrogen Ltd, Paisley, UK) supplemented with 5% heat-inactivated fetal bovine serum (Invitrogen), penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37°C in a 5% CO2 atmosphere.
Electron Microscopy
Samples from normal and IUGR placentas were fixed immediately after delivery in 2.5% glutaraldehyde in 0.1M cacodylate buffer for 4 hours, postfixed in 1% osmium tetroxide and then embedded in Araldite resin. The placentas were from non-smokers who delivered at term by caesarean section. Thin sections were stained with lead citrate and uranyl acetate, and viewed in a Philips CM100 microscope (Eindhoven, The Netherlands).
Western Blot
Western blotting analysis of protein expression and kinase phosphorylation was preformed as previously described.23 β-actin or Ponceau red staining was used to normalize protein loading. All phospho-specific and other antibodies were from Cell Signaling Technology (Danvers, MA) except where specifically mentioned. Anti-β actin antibody was from Sigma-Aldrich (Poole, UK). Anti-C/EBP- homologous protein/growth arrest and DNA damage 153, phospho-eIF2Bε (Ser539), and eIF2Bε antibodies were from Abcam (Cambridge, UK).
RT-PCR Analysis of Xbp-1 mRNA Splicing
The assay was performed as previously described.23
Quantitative Real-Time RT-PCR Analysis
The assay was conducted as previously described.23
Pulse 35S-Methionine Labeling
JEG cells were incubated for 21 hours in the presence or absence of 75 μmol/L salubrinal (ChemBridge Corporation, San Diego, CA, USA) or 10 μg/ml cycloheximide (Sigma). Cells were then washed and incubated with cysteine and methionine-free RPMI 1640 medium (Sigma) for 10 minutes in the presence of inhibitors twice before being pulsed with [35S]methionine by incubation with cysteine and methionine-free RPMI 1640 medium (Sigma) containing translation grade [35S]methionine (Amersham Biotech, Munich, Germany) and cysteine in the presence of inhibitors for 1 hour. After extensive washing with RPMI 1640 medium, cells were lysed in lysis buffer (refers to Western blotting). Equal amounts of protein from different samples were resolved using SDS-polyacrylamide gel electrophoresis. The gel was stained with 0.25% (w/v) Coomasie Brilliant Blue R 250 to show an equal protein loading between the samples, and the dried gel was exposed to a phosphorimager screen (Molecular Dynamics, Sunnyvale, CA, USA). Image was captured and analyzed using ImageQuant software (Molecular Dynamics).
Akt1−/− Mice
These were prepared as described.28 Placentas were collected at E18.5, weighed and snap-frozen in liquid nitrogen before analysis.
Measurement of Cell Proliferation
Cells were counted using a hemocytometer. All 18 fields from two grids, each containing nine squares were counted, and two duplicates were used in each condition.
Statistical Analysis
Differences between means were tested using either two-tailed Student’s t-test, or nonparametric Mann Whitney-U-test, with P < 0.05 being considered significant.
Results
Increased Phosphorylation of eIF2α Inhibits Protein Synthesis and Reduces Cell Proliferation in Pathologic Placentas
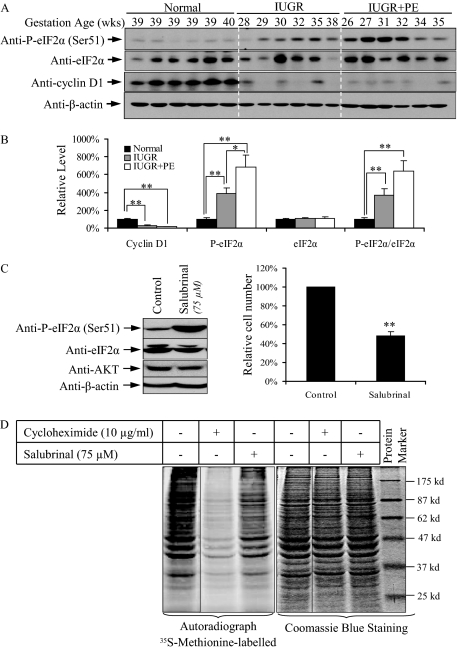
Samples from six normotensive IUGR, six PE+IUGR, and six normal placentas, all delivered by elective caesarean section, were studied. Their gestational ages were; IUGR 28 to 38 weeks, PE+IUGR 26 to 35 weeks, and controls ∼39 weeks (Table 1). Phosphorylation of eIF2α at Ser51 was increased in the IUGR and PE+IUGR placentas by 3.9 and 6.9 times, respectively, whereas eIF2α remained relatively constant compared to non-pathological controls (Figure 1, A and B). The levels in IUGR and PE+IUGR placentas also differed significantly. Phosphorylation of eIF2α is associated with protein synthesis inhibition (PSI), which can lower the levels of cell cycle regulatory proteins.45,46 We found an approximately 75% and 85% reduction in cyclin D1 protein, in the IUGR and PE+IUGR placentas, respectively (Figure 1, A and B).
Table 1.
Clinical Characteristics of the Study Participants
| Normal | IUGR | IUGR+PE | |
|---|---|---|---|
| Gestation age (weeks) | 39 ± 0.6 | 32 ± 3.5** | 31 ± 3.3** |
| Fetal weight (gram) | 3615 ± 417 | 1045 ± 465** | 1034 ± 495** |
| Placental weight (gram) | 629 ± 91 | 223 ± 42** | 178 ± 59** |
| Umbilical artery Doppler | Normal = 6 | Normal = 3 | Normal = 1 |
| PEDF = 2 | PEDF = 4 | ||
| AREDF = 1 | AREDF = 1 |
PEDF = Abnormal but present end diastolic blood flow; AREDF = Absent or reversed end diastolic blood flow.
Data are mean ± SD;
P < 0.001.
Figure 1.
Increased P-eIF2α at Ser51 inhibits protein synthesis and reduces cell proliferation in pathological placentas. A: Western blot showing increased P-eIF2α (Ser51) with constant levels of eIF2α, and reduced cyclin D1 in both IUGR and PE+IUGR placentas. B: Densitometry of bands expressed relative to normal controls (100%). Phosphorylation status is presented as the ratio between phosphorylated and total protein, both normalized to β-actin. Data are mean ± SEM for six placentas per group. C: Salubrinal treatment slows cell proliferation in JEG-3 cells. Cell numbers were compared to the untreated control (100%). D: Protein translation is halted under eIF2α phosphorylation. Cells were exposed for 1 hour to [35S]methionine before protein extraction. Whole cell lysates were used to show an equal input of protein. Data are mean ± SEM for three independent experiments. **P < 0.01.
To test whether P-eIF2α reduces proliferation of trophoblast cells two trophoblast-like cell lines, JEG-3 and JAR cells, were incubated with salubrinal, an inhibitor of eIF2α dephosphorylation.47 Incubation of JEG-3 cells with salubrinal increased P-eIF2α at Ser51 (Figure 1C, left panel), and cell number reduced by approximately 48% after 48 hour (Figure 1C, right graph). Double staining with propidium iodide and Hoechst 33348 revealed no increase in cell death compared to untreated cultures (data not shown). Similar results were obtained using the JAR cells (data not shown).
To examine whether the salubrinal reduced cell proliferation through PSI, a 35S-labeled methionine pulse experiment was preformed. JEG-3 cells were pretreated with either salubrinal or cycloheximide for 21 hours before incubation in medium containing 35S-methionine for 1 hour. The autoradiograph revealed that salubrinal induced general protein synthesis inhibition. Densitometric analysis showed a 22% suppression of global protein translation, while cycloheximide, a positive control, attenuated it by over 53% (Figure 1D).
These data confirmed an increase in P-eIF2α in pathological placentas, and that this is sufficient to reduce trophoblastic cell proliferation in vitro through suppression of protein translation.
Loss of AKT Protein in IUGR Placentas Results from Protein Synthesis Inhibition
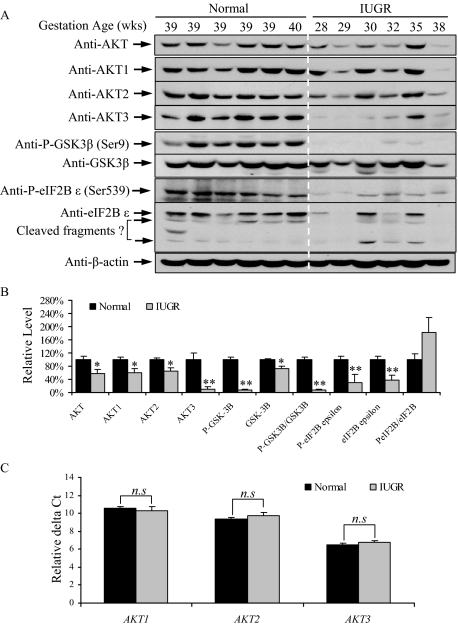
Increased P-eIF2α with salubrinal treatment also down-regulated AKT protein concentration in JEG-3 cells (Figure 1C, left panel). Therefore, it is of interest to examine AKT protein concentration in the IUGR placentas. We observed a 41% decrease in AKT total protein (Figure 2, A and B). AKT protein has three different isoforms, AKT1-3, and all isoforms were reduced in the IUGR placentas to different extents (Figure 2A). Both AKT1 and AKT2 fell by approximately 40%, while AKT3 was reduced by over 90% (Figure 2B). The reduction of AKT protein concentration was accompanied by a loss of its kinase activity, as indicated by a >90% reduction in phosphorylation of its down-stream target substrate, GSK-3β at Ser9 (Figure 2, A and B). We also observed a ∼30% reduction in GSK-3β protein.
Figure 2.
Protein synthesis inhibition induces loss of AKT-GSK-3β signaling in IUGR placentas. A: Western blotting revealed reduced AKT isoforms, P-GSK-3β, GSK-3β eIF2B ε subunit and P-eIF2Bε in IUGR placentas. B: Densitometry of band intensity in IUGR expressed relative to normal controls (100%). Phosphorylation status is presented as the ratio between phosphorylated and total protein, both normalized to β-actin. C: Quantitative real-time-PCR revealed no difference in transcript levels for the three AKT isoforms in IUGR. Data are mean ± SEM for six placentas per group. *P < 0.05, **P < 0.01, n.s indicates non-significant change.
One of GSK-3β downstream substrates is the eIF2Bε subunit. Phosphorylation of eIF2Bε at serine 539 reduces its guanine nucleotide-exchange activity, thereby suppressing translation initiation.48 The eIF2Bε antibody recognized three bands at 75 kDa, 73 kDa, and 50 kDa; the latter two are suggested by the manufacturer to be cleaved fragments of eIF2Bε (Figure 2A). We observed a greater than 60% reduction in the eIF2Bε 75 kDa band in the IUGR placentas (Figure 2B). The relative phosphorylation level of the eIF2Bε at Ser539 increased by approximately 1.8 times in the IUGR placentas, but this did not reach statistical significance due to high variability between samples. Nonetheless, the reduction in eIF2Bε protein alone would be sufficient to reduce eIF2B guanine nucleotide exchange activity.
To determine whether the loss of AKT proteins was the result of transcriptional suppression or through other mechanisms, transcript levels of all three AKT isoforms were quantified by real-time quantitative RT-PCR. No differences were found between IUGR and normal placentas (Figure 2C), confirming that loss of AKT proteins did not result from transcriptional inhibition. It is also unlikely to be the result of increased degradation as Hass and Sohn49 reported reduced proteosomal activity in IUGR and preeclamptic placentas. We recently demonstrated that P-eIF2α directly attenuates AKT protein translation in JEG-3 cells,23 suggesting that the loss of AKT proteins in IUGR placentas is most likely due to translational inhibition.
Reduction of mTOR Signaling Suppresses 4E-BP1 Phosphorylation but Not Activity of S6 Kinases
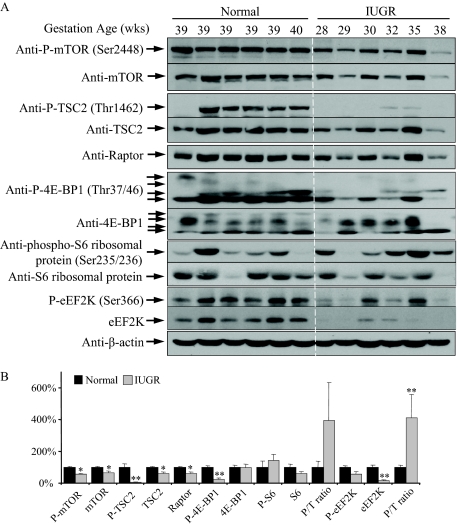
Besides influencing the AKT-GSK3-eIF2B pathway, AKT also tunes the protein synthesis rate through regulation of mTOR signaling. Both mTOR Ser2448 and TSC2 Thr1462 phosphorylation sites are direct substrates of the kinase AKT.29,31 Activity of mTORC1 depends not only on the activity of mTOR kinase and raptor protein level, but also on removal of an inhibitory complex TSC2/TSC1. In IUGR placentas, mTOR and P-mTOR at Ser2448, TSC2 and P-TSC2 at Thr1462, and raptor protein were all decreased as compared with controls (Figure 3, A and B). Dephosphorylation of TSC2 at Thr 1462 promotes binding of TSC2 to mTORC1, and the combined result was that activation of the downstream effector, P-4E-BP1 at Thr37/46, was reduced by approximately 75% in IUGR placentas, while 4E-BP1 remained constant (Figure 3, A and B). Multiple bands indicative of additional phosphorylation sites were observed for both P-4E-BP1 and 4E-BP1, as shown in antibody datasheets, and all were included in the quantification.
Figure 3.
Reduction of mTOR signaling in IUGR placentas. A: Western blotting demonstrated reduced phosphorylation of mTOR, TSC2 and 4E-BP1, but not of S6 and eEF2K. Levels of total mTOR, TSC2, raptor, eEF2K but not 4E-BP1 and S6, were lower in IUGR placentas. B: Densitometry of band intensity in IUGR expressed relative to normal controls (100%). Phosphorylation status is presented as the ratio between phosphorylated and total protein (P/T), both normalized to β-actin. Data are mean ± SEM for six placentas per group. *P < 0.05 and **P < 0.01.
mTORC1 can also regulate the activity of the S6K1, which in turn provides an addition mechanism to control protein translation. Both ribosomal protein S6 and eEF2K are substrates of the S6Ks.35 In IUGR, although the levels of P-S6 and S6 protein were not significantly changed compared to controls, there was a trend toward an increase, rather than the expected decrease, in the P-S6/S6 ratio (Figure 3, A and B). This suggested possible activation of the S6Ks, which was confirmed by the significant increase in ratio of P-eEF2K/eEF2K (Figure 3B). However, total eEF2K protein was significantly decreased, indicating a reduction in overall eEF2K activity in IUGR placentas (Figure 3, A and B). This implies an increase of eEF2 activity, which may facilitate translation elongation in those placentas.
Decreased Akt Protein Reduces mTOR Signaling and Impairs Murine Placental Growth
Akt1 null, but not Akt2 or Akt3 null, mice exhibit a late-onset IUGR phenotype, confirming the important role of Akt signaling in both placental and fetal growth.28 Although it has been reported that the IUGR arises primarily due to a compromise of placental vascularization,28 we investigated whether knock-out of Akt1 protein in mice may also result in impairment of mTORC1 signaling and 4E-BP1 phosphorylation, thereby slowing placental growth via translation inhibition.
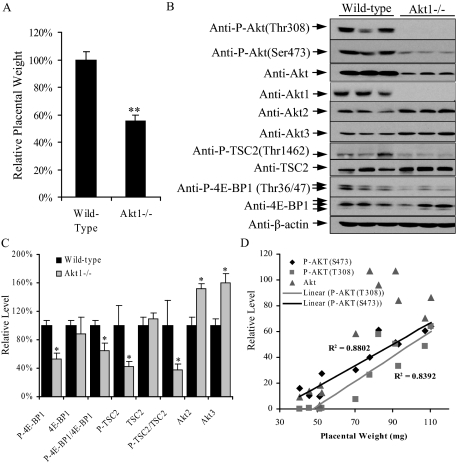
In our sample of E18.5 placentas from Akt1 null (Akt1−/−) mice placental mass was reduced by 45% compared to wild-type (WT) (Figure 4A). Akt1 appears to be the major Akt isoform in the mouse placenta as total Akt protein was reduced by over 90% in Akt1−/− placentas (Figure 4B). In addition, P-Akt at both Ser473 and Thr308 fell by more than 80% (Figure 4B). Interestingly, the protein levels of Akt2 and Akt3 increased by 50% and 60% respectively in the Akt1−/− placentas, suggesting a compensatory mechanism (Figure 4, B and C). This decrease of Akt signaling was associated with reductions in phosphorylation of TSC2 at Thr1462, and 4E-BP1 at Thr37/46, of 50% and 60% respectively, equivalent to our observations in the human IUGR placentas (Figure 3B).
Figure 4.
mTOR signaling is reduced in Akt1−/− placentas. A: Placental weight of Akt1−/− mice. B: Western blot showing reduced phosphorylation of Akt, Tsc2 and 4E-BP1 and increased Akt2 and Akt3 total protein. C: Densitometry of band intensity in Akt1−/− relative to WT (100%). Phosphorylation status is presented as the ratio between phosphorylated and total protein, both normalized to β-actin. Data are mean ± SEM for three placentas per group within a single litter. *P < 0.05. D: A positive correlation exists between placental mass and the phosphorylation status of Akt (normalized with β-actin), but not with total Akt. n = 12 placentas with different Akt1 genotypes.
We observed a large variation in the mass of both wild-type (WT) and Akt1−/− placentas, both within and between litters. Variation within a litter reached 20%, (78 to 91.8 mg in WT and 40.8 to 51.7 mg in Akt1−/−) and between litters was up to 40% (78 to 110.4 mg in WT). To determine whether Akt activity relates to this variation in placental mass we measured the Akt phosphorylation level at both Ser473 and Thr308 from 12 mouse placentas with different Akt1 genotypes. A strong positive correlation was found between placental weight and both P-Akt(S473) and P-Akt(T308) (R2 = 0.88 and 0.84 respectively), but not with total Akt protein (Figure 4D). These data demonstrate that AKT activity plays a central role in regulating placental development via mTOR signaling.
The question remained as to how P-eIF2α is activated in the IUGR placentas, initiating this cascade of downstream consequences.
A Gradation of ER Stress in IUGR Alone and Preeclampsia with IUGR Placentas
Before investigating the placental tissues for evidence of ER stress, we verified how cellular biomarkers relate to its severity. An increase of ER chaperone proteins GRP78 and GRP94, expression of CHOP protein, and splicing of Xbp-1 mRNA are all biomarkers of ER stress.23 Anti-KDEL antibody is widely used to detect both GRP78 and GRP94 by recognizing the KDEL domain at the end of C termini of the chaperones.
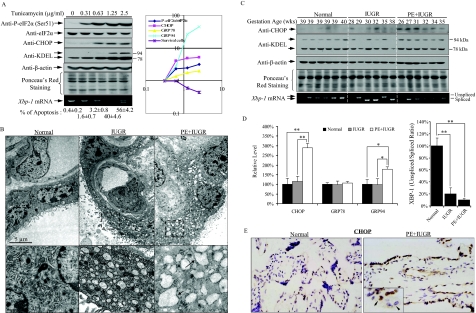
Tunicamycin induces ER stress by inhibition of N-linked glycosylation, and we performed a dose-response study incubating JEG-3 cells in the absence or presence of tunicamycin for 24 hours. Increased concentrations were closely associated with increased levels of CHOP, GRP78 and GRP94 proteins, and splicing of Xbp-1 mRNA (Figure 5A, left panel). As expected, there was a gradual increase of apoptotic cell death in JEG-3 cells (Figure 5A, left panel), mediated possibly through CHOP.
Figure 5.
Increased ER stress in IUGR placentas. A: Left panel, tunicamycin induced a dose-dependent increase of P-eIF2α (Ser51), CHOP, GRP78, GRP 94, and apoptotic cell death. RT-PCR also revealed increased splicing of Xbp-1 mRNA. Right graph, densitometric quantification of all bands plotted on a log scale. B: Electron micrographs showing regular cis-ternae of RER (arrowed) in control placenta and dilated cis ternae in IUGR and PE+IUGR placentas. Magnification = 14K. C: Increased ER stress biomarkers, CHOP and GRP94 in PE+IUGR placentas while increased spliced variant of Xbp-1 mRNA in both IUGR and PE+IUGR placentas. D: Densitometry of band intensity in those placentas expressed relative to normal controls (100%). E: IHC showed the nuclear localization of CHOP in both syncytiotrophoblast and endothelial cells in PE+IUGR placentas.
There was a progressive elevation of P-eIF2α at Ser51. When the data were plotted on a Log scale, there were strong positive correlations between the levels of P-eIF2α, CHOP, GRP78, and GRP94 proteins, but negative correlations with cell survival (Figure 5A, right graph). These data strongly suggest that the greater the severity of ER stress, the higher the level of P-eIF2α, and that when the stress reaches a certain level it induces apoptotic cell death. Therefore, we questioned whether the higher level of P-eIF2α observed in the PE+IUGR placentas in Figure 1, A and B was the result of greater ER stress.
Using transmission EM, we found the ER cis ternae within the syncytiotrophoblast to be markedly dilated in both IUGR and PE+IUGR placentas, similar to Lister’s findings50 (Figure 5B). We did not observe equivalent dilation within the cytotrophoblast cells, or the cells of the villous core. By contrast, mitochondria within the syncytiotrophoblast displayed normal appearances, with regularly arranged cis ternae and narrow intracistal spaces. Thus, the dilation of the ER appeared to be cell-type specific, and could not be accounted for by a delay in tissue fixation. Visual comparison indicated that the size of the dilated cis ternae was greater in the PE+IUGR placentas than in those from IUGR alone, suggesting greater loss of ionic homeostasis within the former.
This view was supported by the molecular biomarkers. GRP94 was elevated in the PE+IUGR placentas compared to both normal and IUGR placentas (Figure 5, C and D, left graph), but no change in GRP78 protein (Figure 5, C and D, left graph). We also observed a 2.9-fold elevation of CHOP protein in PE+IUGR placentas, but not in the IUGR placentas (Figure 5, C and D, left graph). Apoptosis is significantly higher in PE+IUGR than in IUGR placentas, although estimates vary depending on the criteria used.51 CHOP requires translocation to the nucleus to regulate gene expression. Immunohistochemistry revealed an increase in CHOP staining in the nuclei of both the syncytiotrophoblast and the fetal endothelial cells in the PE+IUGR placentas (Figure 5E). There was also a significant increase in splicing of Xbp-1 mRNA in IUGR and PE+IUGR placentas compared to the controls (Figure 5, C and D, right graph), with a trend to even higher splicing in the latter although this difference was not statistically significant.
To conclude, the ultrastructural findings and increased expression of CHOP and GRP94 proteins suggest a greater degree of ER stress in the PE+IUGR placentas. Syncytiotrophoblast is not the only cell type that suffers ER stress, for we also observed an increase of immunoreactivity of CHOP in the fetal endothelial cells. ER stress might thus contribute to the increased trophoblastic apoptosis and fetal vascular remodeling observed in PE+IUGR placentas through induction of CHOP.
Low-Grade ER Stress Reduces Cell Proliferation
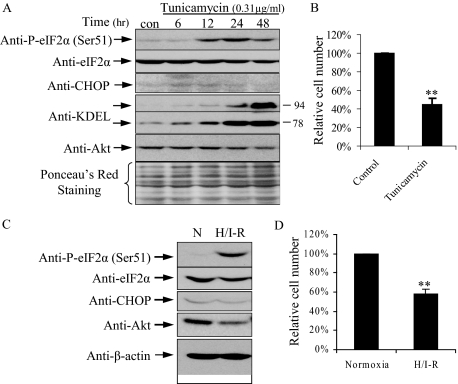
Although an increased rate of apoptosis could account for the smaller placental size in PE+IUGR (Table 1), the question arises as to how the lower levels of ER stress that do not induce apoptosis cause equivalent smaller sized placentas in IUGR pregnancies. In the tunicamycin dose-response study, at a dose of 0.31 μg/ml, there were increases in P-eIF2α, GRP78 and GRP94 proteins, and splicing of Xbp-1 mRNA without induction of CHOP and apoptosis (Figure 5A). Therefore, we investigated the effect of this sublethal dosage of tunicamycin (referred to as low-grade ER stress) on cell proliferation. With increasing length of incubation up to 48 hours there was a gradually elevation of GRP 78 and GRP 94 proteins, while CHOP was not detected throughout (Figure 6A). P-eIF2α peaked at 24 hours and declined slowly at 48 hours (Figure 6A). Strikingly, relative cell number was reduced by more than 50% following 48 hours of tunicamycin treatment compared to the untreated cells (Figure 6B). To eliminate a drug and cell-type specific effect, experiments were also performed using dithiothreitol, which prevents disulfide bond formation, and were repeated in JAR cells. Similar results were obtained (data not shown). Interestingly, JAR cells tolerated a threefold higher concentration of tunicamycin than JEG-3 cells, but application of a sublethal dose (1 μg/ml) for 48 hours reduced proliferation by 44% ± 5 (average ± range, n = 2). These data show that low-grade ER stress slows placental cell growth without induction of cell death. The different tolerance of ER stress in different cell types might explain why only certain placental cell types suffer ER stress. Importantly, the data highlighted that the effects of ER stress persist even after the activating signals to eIF2α have eased.
Figure 6.
Low-grade ER stress induced by either sublethal dosage of tunicamycin or repetitive H/I-R reduces cell proliferation in JEG-3 cells. A and B: Sublethal dosage (0.3125 μg/ml) with tunicamycin for 48 hours. C and D: Repetitive H/I-R in a 3-hour cyclical pattern at 0.5% and 21% O2 in the presence of 1.1 mmol/L glucose for 48 hours. A and B: Western blot showing increased eIF2α phosphorylation, decreased Akt protein, and no induction in CHOP. B and D: Cell numbers were compared to the untreated control (100%). Data are mean ± SEM for three independent experiments. **P < 0.01.
Repetitive Hypoxia/Ischemia-Reperfusion Causes Sustainable Activation of eIF2α Phosphorylation and Reduces Cell Proliferation
Although we have demonstrated that ischemia is a potent activator of eIF2α phosphorylation in JEG-3 cells, the phosphorylation status falls to basal levels during the first 3 hours of re-oxygenation. Nonetheless, approximately 25% of the cells undergo apoptosis after 24 hour of re-oxygenation.23 As most cases of unexplained IUGR are associated with deficient conversion of the spiral arteries we speculate that repetitive hypoxia/ischemia-reperfusion (H/I-R) is the likely precipitating insult rather than a single episode.6 Therefore, we used media containing 10% of the normal glucose concentration with 0.5% and 21% O2 to perform H/I-R in a 3-hour cyclical pattern with JEG-3 cells for 48 hours to mimic the physiological environment in vivo. Repetitive H/I-R strongly activated eIF2α phosphorylation at serine 51 (Figure 6C). Consistently, it also down-regulated total AKT protein concentration (Figure 6C), but there was no induction of CHOP expression (Figure 6C). Crucially, unlike 48 hours of hypoxia, which induced approximately 20% cell death in JEG-3 cells, repetitive H/I-R did not trigger apoptosis or necrosis (data not shown). Instead, we observed a 40% reduction of cell proliferation (Figure 6D). These results confirm that repetitive H/I-R induces low-grade ER stress equivalent to that seen using sublethal dosage of tunicamycin and in human IUGR placentas, including increased P-eIF2α, decreased AKT protein, reduced cell proliferation but no induction of CHOP protein or cell death.
Discussion
This is the first study demonstrating evidence that PSI and the unfolded protein response play a major role in the pathophysiology of complications of human pregnancy, in particular IUGR. Increased P-eIF2α in the placenta induces an acute PSI that leads to suppression of AKT and proteins/kinases in the mTOR pathway. Reduced activity in this pathway leads ultimately to reduced phosphorylation of 4E-BP1, which in turn inhibits cap-dependent translation. This mechanism provides a positive feedback loop that enhances the PSI, and ensures no translation under conditions of stress even in nutrient enriched conditions. PSI is further reinforced by the reduction of eIF2Bε subunit protein level and increased GSK-3β activity, thereby preventing guanine nucleotide-exchange in the initiation stage of protein translation. Down-regulation of cyclin D1 facilitates cell cycle arrest, which in addition to the reduction of AKT activity will compromise cell proliferation.
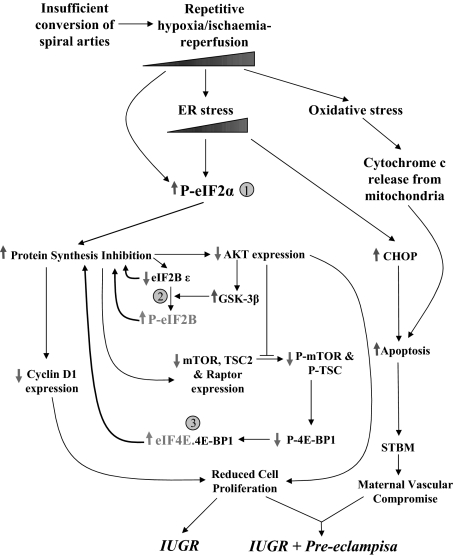
The end result will be a smaller placental size, the phenotype of IUGR. In addition, we have clearly demonstrated the existence of low-grade ER stress in IUGR placentas by both ultrastructural analysis and the results of ER stress biomarkers. Dilation of the ER lumen is seen during periods of ischemia in trophoblast and other cell types, where it is associated with activation of the unfolded protein response.23,52 This stress is more severe in PE+IUGR placentas. ER stress activates phosphorylation of eIF2α, and so is a strong candidate for mediation of the PSI following hypoxia/ischemia-reperfusion. In trophoblast-like cell lines induction of low-grade ER stress reduces cell proliferation, while more severe stress triggers apoptosis through expression of CHOP protein. Apoptotic syncytiotrophoblastic debris shed into the maternal circulation as microvillous fragments can impair maternal vascular endothelial function.53 The degree of ER stress may therefore be a critical factor that determines whether IUGR pregnancies are complicated by preeclampsia or not (Figure 7).
Figure 7.
Schematic diagram showing the three molecular mechanisms of protein synthesis inhibition and interactions with apoptotic pathways in the etiology of IUGR and PE+IUGR.
In this study, we chose term rather than preterm placentas as normal controls. Although preterm placentas would match the gestational age of the IUGR and PE+IUGR placentas, they are commonly associated with pathological processes54,55 and so there is considerable doubt as to whether they are truly normal. In addition, our previous results have shown that labor induces significant cellular stress within the placenta,56 and non- labored preterm healthy placentas delivered by elective caesarean section are almost impossible to obtain. On the other hand, we did not observe any correlation between gestational age and any of the data presented (Figure 1). Furthermore, the IUGR and PE+IUGR placentas where differences in the degree of ER stress were observed were age-matched, eliminating any gestational age bias.
Protein synthesis is a complex process and can be divided into three main stages: initiation, elongation, and termination. Regulation can occur at either the initiation or elongation stages, and can be controlled via signal transduction pathways.38 We observed three mechanisms by which initiation may be suppressed in the IUGR placentas.
Firstly, eIF2 binds GTP and Met-tRNAi and transfers Met-tRNA to the 40S subunit to form the 43S pre-initiation complex. It is converted to eIF2.GDP in the process. eIF2B, a guanine nucleotide exchanger, promotes a new round of translation initiation by regenerating eIF2.GTP. Phosphorylation of the eIF2 subunit α at Ser51 acts as a competitive inhibitor of eIF2B, thus blocking recycling of eIF2 and attenuating translation initiation.57 Four kinases have been identified that directly phosphorylate eIF2α, including PRKR-like endoplasmic reticulum kinase, general control nonderepressible 2, heme-regulated eIF2α kinase, and double-stranded RNA-dependent protein kinase.14 These kinases are activated in response to different stresses: PRKR-like endoplasmic reticulum kinase by ER stress; general control nonderepressible 2 by amino acid deprivation; heme-regulated eIF2α kinase by hemin deficiency; and double-stranded RNA-dependent protein kinase by viral infection. Based on the molecular biomarkers we analyzed, the more severe ER stress in PE+IUGR placentas correlated with higher levels of P-eIF2α (Figure 1A), suggesting a causal link via activation of PRKR-like endoplasmic reticulum kinase.
A second mechanism for suppressing translation initiation involves GSK-3 that phosphorylates eIF2B, inhibiting its activity.39,48 In IUGR placentas, we observed a greater than 90% reduction in GSK-3β phosphorylation, with only a slight decrease in total protein (Figure 2), secondary to the reduction of AKT protein. Dephosphorylation of GSK-3β increases its activity, allowing phosphorylation of eIF2B.39 eIF2B is composed of five subunits, and only the ε subunit contains guanine nucleotide exchange catalytic activity. Therefore, the down-regulation of eIF2Bε subunit concentration observed in IUGR placentas indicates a further reduction of eIF2B activity.
The third block to initiation involves 4E-BP1. The eIF4 family of translation factors recruits mRNA to ribosomes by binding to the 5′cap structure of the mRNA and activating cap-dependent translation initiation.58 The activity of eIF4E can be regulated by a set of 4E binding proteins (4E-BPs) including 4E-BP1. 4E-BP1 binds to eIF4E, preventing its interaction with eIF4 family members.59 Phosphorylation of 4E-BP1 blocks this interaction. 4E-BP1 contains multiple phosphorylation sites, including Thr37/46, Thr70, Ser65, and Ser101.60 Although phosphorylation of Thr37/46 does not regulate the binding of 4E-BP1 to eIF4E directly, it is required for modification of Thr70, following which Ser65 undergoes phosphorylation.60,61,62,63 Therefore, the phosphorylation level of Thr37/46 can be used as a reporter for 4E-BP1 binding activity. Our observation of greatly decreased P-4E-BP1 at Thr37/46 in IUGR placentas without a change in total protein concentration thus indicates suppression of cap-dependent initiation of translation.
Our data confirm reduced mTOR signaling in IUGR. Indeed, the decreases in mTOR, TSC2, and raptor protein concentrations suggest that eIF2α operates upstream of the mTOR pathway, providing an overarching mechanism that ensures stress-induced attenuation of initiation of protein translation even in nutrient-rich conditions. Consistent with this, we observed a significant reduction of P-4E-BP1, as well as of P-TSC2 in Akt1 null mouse placentas (Figure 4).
The elongation stage of protein synthesis is controlled by the eukaryotic elongation factors (eEFs). eEF2 is phosphorylated at Thr56 by eEF2 kinase (eEF2K), impairing its interaction with the ribosome and thus inactivating elongation.64 The activity of eEF2K can be regulated by S6 kinases.35 Phosphorylation of eEF2K at Ser366 by S6Ks impairs its activity and hence favors activation of translation.35 In IUGR placentas we observed increased P-eEF2K, suggesting that S6Ks activity was maintained through other pathways, despite the reduction in mTOR signaling. This increased phosphorylation, coupled with the reduction in total eEF2K, suggests that the elongation process still functions in these placentas.
These results may explain the apparent paradox that proteins involved in stress responses, including GRP78, GRP94, CHOP and activating transcription factor 4, can bypass translation inhibition in the presence of P-eIF2α and increase in concentration.14 Other placental proteins, such as leptin and N-myc down-regulated gene 1, are also markedly increased in both IUGR and preeclampsia, and the associated rise in their mRNAs suggests a combination of both transcriptional and translational activation occurs.65,66 It has been reported that mRNAs containing small upstream open reading frames within their 5′-UTR regions or internal ribosome entry sites sequences are selectively translated independent of eIF2α regulation,67,68 although the latter is contentious.69 Genomic sequence analysis revealed that the genes mentioned above contain either upstream open reading frames or internal ribosome entry sites, or both68,70,71 (unpublished data).
The syncytiotrophoblast layer of the human placenta is crucial for normal placental function. All secretory proteins require posttranslational modifications inside the ER lumen before passage to the Golgi apparatus for secretion. Any disturbance of the ER environment will induce unfolded protein response, influencing placental secretion of autocrine, paracrine and endocrine factors. Such perturbations can have devastating effects on placental development, and secondarily on the fetus. Thus, deletion of a trophoblast-specific Igf2 transcript, which encodes one of the major placental growth factors, insulin-like growth factor 2, in the mouse results in severe growth restriction of the placenta, and consequently of the pups.72 The phenotypes are similar to those seen in human IUGR.73,74 There is also increasing clinical evidence that during human pregnancy reduced placental growth precedes fetal growth restriction.2 Therefore, ER stress-induced placental growth restriction could have a major impact on fetal growth. Genetic manipulation of different molecules involved in the ER stress may elucidate the mechanisms for ER stress regulated placental growth. However, Grp78−/− mice fail to develop up to E3.575 while mice with knocked out PRKR-like endoplasmic reticulum kinase, Perk−/−, exhibit normal morphology at birth,76 suggesting that choosing the right molecule to study is crucial.
To conclude, our data provide the first molecular mechanistic explanation for the placental growth restriction seen in pathological pregnancies. We have identified protein synthesis inhibition as the key element in the pathophysiology. This also provides a rational explanation for the biochemical changes observed in IUGR pregnancies, such as the reduced concentration of circulating pregnancy-associated plasma protein-A, which may be useful as a diagnostic biomarker.77 Identification of protein synthesis inhibition provides a completely new insight into potential therapeutic interventions for IUGR.
Acknowledgments
We thank Dr CJP Jones, University of Manchester, for the loan of the EM blocks, and to Svitlana Korolchuk and Olivera Spasic-Boskovic for expert technical assistance.
Footnotes
Address reprint requests to Professor G. J. Burton, Centre for Trophoblast Research, Department of Physiology, Development and Neuroscience, Physiological Laboratory, Downing Street. Cambridge CB2 3EG, UK. E-mail: gjb2@cam.ac.uk.
See related Commentary on page 311
Supported by the Wellcome Trust (069027/Z/02/Z).
S. C.-J. and G. J. B. contributed equally to the work.
References
- Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol. 2000;182:198–206. doi: 10.1016/s0002-9378(00)70513-8. [DOI] [PubMed] [Google Scholar]
- Thame M, Osmond C, Bennett F, Wilks R, Forrester T. Fetal growth is directly related to maternal anthropometry and placental volume. Eur J Clin Nutr. 2004;58:894–900. doi: 10.1038/sj.ejcn.1601909. [DOI] [PubMed] [Google Scholar]
- Hafner E, Metzenbauer M, Hofinger D, Munkel M, Gassner R, Schuchter K, Dillinger-Paller B, Philipp K. Placental growth from the first to the second trimester of pregnancy in SGA-foetuses and pre-eclamptic pregnancies compared to normal foetuses. Placenta. 2003;24:336–342. doi: 10.1053/plac.2002.0918. [DOI] [PubMed] [Google Scholar]
- Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol. 1977;84:656–663. doi: 10.1111/j.1471-0528.1977.tb12676.x. [DOI] [PubMed] [Google Scholar]
- Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Nikaido T, Toki T, Kita N, Kanai M, Ashida T, Ohira S, Konishi I. Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch. 2004;444:49–55. doi: 10.1007/s00428-003-0903-2. [DOI] [PubMed] [Google Scholar]
- DeGracia DJ, Montie HL. Cerebral ischemia and the unfolded protein response. J Neurochem. 2004;91:1–8. doi: 10.1111/j.1471-4159.2004.02703.x. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Mori M. Nitric oxide and endoplasmic reticulum stress. Arterioscler Thromb Vasc Biol. 2006;26:1439–1446. doi: 10.1161/01.ATV.0000223900.67024.15. [DOI] [PubMed] [Google Scholar]
- Brostrom MA, Brostrom CO. Calcium dynamics and endoplasmic reticular function in the regulation of protein synthesis: implications for cell growth and adaptability. Cell Calcium. 2003;34:345–363. doi: 10.1016/s0143-4160(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- Harding HP, Ron D. Endoplasmic reticulum stress and the development of diabetes: a review. Diabetes. 2002;51 Suppl 3:S455–S461. doi: 10.2337/diabetes.51.2007.s455. [DOI] [PubMed] [Google Scholar]
- Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature. 2004;429:841–847. doi: 10.1038/nature02656. [DOI] [PubMed] [Google Scholar]
- Song BL, Sever N, DeBose-Boyd RA. Gp78, a membrane-anchored ubiquitin ligase, associates with Insig-1 and couples sterol-regulated ubiquitination to degradation of HMG CoA reductase. Mol Cell. 2005;19:829–840. doi: 10.1016/j.molcel.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa A, Nishitoh H, Tobiume K, Takeda K, Ichijo H. Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: advanced findings from ASK1 knockout mice. Antioxid Redox Signal. 2002;4:415–425. doi: 10.1089/15230860260196218. [DOI] [PubMed] [Google Scholar]
- Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, Tsujimoto Y, Tohyama M. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004;165:347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Yung HW, Korolchuk S, Tolkovsky AM, Charnock-Jones DS, Burton GJ. Endoplasmic reticulum stress exacerbates ischemia-reperfusion-induced apoptosis through attenuation of Akt protein synthesis in human choriocarcinoma cells. FASEB J. 2007;21:872–884. doi: 10.1096/fj.06-6054com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294:1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Skeen JE, Bhaskar PT, Chen CC, Chen WS, Peng XD, Nogueira V, Hahn-Windgassen A, Kiyokawa H, Hay N. Akt deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Cancer Cell. 2006;10:269–280. doi: 10.1016/j.ccr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- Nave BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344:427–431. [PMC free article] [PubMed] [Google Scholar]
- Sekulic A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, Abraham RT. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci. 2003;28:573–576. doi: 10.1016/j.tibs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. United at last: the tuberous sclerosis complex gene products connect the phosphoinositide 3-kinase/Akt pathway to mammalian target of rapamycin (mTOR) signalling. Biochem Soc Trans. 2003;31:573–578. doi: 10.1042/bst0310573. [DOI] [PubMed] [Google Scholar]
- Raught B, Gingras AC, Gygi SP, Imataka H, Morino S, Gradi A, Aebersold R, Sonenberg N. Serum-stimulated, rapamycin-sensitive phosphorylation sites in the eukaryotic translation initiation factor 4GI. EMBO J. 2000;19:434–444. doi: 10.1093/emboj/19.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- Shiota C, Woo JT, Lindner J, Shelton KD, Magnuson MA. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev Cell. 2006;11:583–589. doi: 10.1016/j.devcel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403:217–234. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- Welsh GI, Miller CM, Loughlin AJ, Price NT, Proud CG. Regulation of eukaryotic initiation factor eIF2B: glycogen synthase kinase-3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Lett. 1998;421:125–130. doi: 10.1016/s0014-5793(97)01548-2. [DOI] [PubMed] [Google Scholar]
- Leegwater PA, Vermeulen G, Konst AA, Naidu S, Mulders J, Visser A, Kersbergen P, Mobach D, Fonds D, van Berkel CG, Lemmers RJ, Frants RR, Oudejans CB, Schutgens RB, Pronk JC, van der Knaap MS. Subunits of the translation initiation factor eIF2B are mutant in leukoencephalopathy with vanishing white matter. Nat Genet. 2001;29:383–388. doi: 10.1038/ng764. [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, van Berkel CG, Herms J, van Coster R, Baethmann M, Naidu S, Boltshauser E, Willemsen MA, Plecko B, Hoffmann GF, Proud CG, Scheper GC, Pronk JC. eIF2B-related disorders: antenatal onset and involvement of multiple organs. Am J Hum Genet. 2003;73:1199–1207. doi: 10.1086/379524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todros T, Ferrazzi E, Groli C, Nicolini U, Parodi L, Pavoni M, Zorzoli A, Zucca S. Fitting growth curves to head and abdomen measurements of the fetus: a multicentric study. J Clin Ultrasound. 1987;15:95–105. doi: 10.1002/jcu.1870150203. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Cortinovis I, Bortolus R, Fedele L. [Standards of birth weight in Italy]. Ann Ostet Ginecol Med Perinat. 1991;112:203–246. [PubMed] [Google Scholar]
- Pardi G, Cetin I, Marconi AM, Lanfranchi A, Bozzetti P, Ferrazzi E, Buscaglia M, Battaglia FC. Diagnostic value of blood sampling in fetuses with growth retardation. N Engl J Med. 1993;328:692–696. doi: 10.1056/NEJM199303113281004. [DOI] [PubMed] [Google Scholar]
- Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol. 2002;22:7405–7416. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci USA. 2000;97:12625–12630. doi: 10.1073/pnas.220247197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- Wang X, Paulin FE, Campbell LE, Gomez E, O'Brien K, Morrice N, Proud CG. Eukaryotic initiation factor 2B: identification of multiple phosphorylation sites in the epsilon-subunit and their functions in vivo. EMBO J. 2001;20:4349–4359. doi: 10.1093/emboj/20.16.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass R, Sohn C. Increased oxidative stress in pre-eclamptic placenta is associated with altered proteasome activity and protein patterns. Placenta. 2003;24:979–984. doi: 10.1016/s0143-4004(03)00174-7. [DOI] [PubMed] [Google Scholar]
- Lister UM. Ultrastructure of the placenta in hypertensive states. The localization of enzmes hydrolysing adenosine triphosphate in placentae from normal and toxaemic pregnancies. J Obstet Gynaecol Br Commonw. 1966;73:439–451. doi: 10.1111/j.1471-0528.1966.tb05186.x. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- Schweikhart G, Kaufmann P. [Problems of distinction of normal, arteficial and pathological structures in mature human placental villi. I Ultrastructure of the syncytiotrophoblast (author’s transl)] Arch Gynakol. 1977;222:213–230. doi: 10.1007/BF00717599. [DOI] [PubMed] [Google Scholar]
- Smarason AK, Sargent IL, Starkey PM, Redman CW. The effect of placental syncytiotrophoblast microvillous membranes from normal and pre-eclamptic women on the growth of endothelial cells in vitro. Br J Obstet Gynaecol. 1993;100:943–949. doi: 10.1111/j.1471-0528.1993.tb15114.x. [DOI] [PubMed] [Google Scholar]
- Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann NY Acad Sci. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ, Kuczynski E. Markers of risk for preterm delivery. J Perinat Med. 1999;27:5–20. doi: 10.1515/JPM.1999.001. [DOI] [PubMed] [Google Scholar]
- Cindrova-Davies T, Yung HW, Johns J, Spasic-Boskovic O, Korolchuk S, Jauniaux E, Burton GJ, Charnock-Jones DS. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am J Pathol. 2007;171:1168–1179. doi: 10.2353/ajpath.2007.070528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haro C, Mendez R, Santoyo J. The eIF-2alpha kinases and the control of protein synthesis. FASEB J. 1996;10:1378–1387. doi: 10.1096/fasebj.10.12.8903508. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- Mader S, Lee H, Pause A, Sonenberg N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol Cell Biol. 1995;15:4990–4997. doi: 10.1128/mcb.15.9.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li W, Parra JL, Beugnet A, Proud CG. The C terminus of initiation factor 4E-binding protein 1 contains multiple regulatory features that influence its function and phosphorylation. Mol Cell Biol. 2003;23:1546–1557. doi: 10.1128/MCB.23.5.1546-1557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothe-Satney I, Brunn GJ, McMahon LP, Capaldo CT, Abraham RT, Lawrence JC., Jr Mammalian target of rapamycin-dependent phosphorylation of PHAS-I in four (S/T)P sites detected by phospho-specific antibodies. J Biol Chem. 2000;275:33836–33843. doi: 10.1074/jbc.M006005200. [DOI] [PubMed] [Google Scholar]
- Wang X, Beugnet A, Murakami M, Yamanaka S, Proud CG. Distinct signaling events downstream of mTOR cooperate to mediate the effects of amino acids and insulin on initiation factor 4E-binding proteins. Mol Cell Biol. 2005;25:2558–2572. doi: 10.1128/MCB.25.7.2558-2572.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price NT, Redpath NT, Severinov KV, Campbell DG, Russell JM, Proud CG. Identification of the phosphorylation sites in elongation factor-2 from rabbit reticulocytes. FEBS Lett. 1991;282:253–258. doi: 10.1016/0014-5793(91)80489-p. [DOI] [PubMed] [Google Scholar]
- Laivuori H, Gallaher MJ, Collura L, Crombleholme WR, Markovic N, Rajakumar A, Hubel CA, Roberts JM, Powers RW. Relationships between maternal plasma leptin, placental leptin mRNA and protein in normal pregnancy, pre-eclampsia and intrauterine growth restriction without pre-eclampsia. Mol Hum Reprod. 2006;12:551–556. doi: 10.1093/molehr/gal064. [DOI] [PubMed] [Google Scholar]
- Choi SJ, Oh SY, Kim JH, Sadovsky Y, Roh CR. Increased expression of N-myc downstream-regulated gene 1 (NDRG1) in placentas from pregnancies complicated by intrauterine growth restriction or preeclampsia. Am J Obstet Gynecol. 2007;196:45 e41–e47. doi: 10.1016/j.ajog.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Jan E, Thompson SR, Wilson JE, Pestova TV, Hellen CU, Sarnow P. Initiator Met-tRNA-independent translation mediated by an internal ribosome entry site element in cricket paralysis virus-like insect viruses. Cold Spring Harb Symp Quant Biol. 2001;66:285–292. doi: 10.1101/sqb.2001.66.285. [DOI] [PubMed] [Google Scholar]
- Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Some thoughts about translational regulation: forward and backward glances. J Cell Biochem. 2007;102:280–290. doi: 10.1002/jcb.21464. [DOI] [PubMed] [Google Scholar]
- Macejak DG, Sarnow P. Internal initiation of translation mediated by the 5′ leader of a cellular mRNA. Nature. 1991;353:90–94. doi: 10.1038/353090a0. [DOI] [PubMed] [Google Scholar]
- Kim YK, Jang SK. Continuous heat shock enhances translational initiation directed by internal ribosomal entry site. Biochem Biophys Res Commun. 2002;297:224–231. doi: 10.1016/s0006-291x(02)02154-x. [DOI] [PubMed] [Google Scholar]
- Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417:945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, Gannon M, Ma K, McNaughton K, Cavener DR. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22:3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GC, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM. Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab. 2002;87:1762–1767. doi: 10.1210/jcem.87.4.8430. [DOI] [PubMed] [Google Scholar]