Abstract
Several lines of evidence indicate that the Th2 immune response is associated with endometriosis. Although an increased concentration of interleukin (IL)-4, a typical Th2 cytokine, has been reported in endometriotic tissues, the implication of this for endometriosis has not been determined. To investigate a possible role of IL-4 in the development of endometriosis, we examined the presence of IL-4-producing cells in endometriotic tissues and the effect of IL-4 on proliferation of endometriotic stromal cells. Endometriotic stromal cells were isolated from endometriotic tissues obtained from women undergoing surgery for endometrioma. Immunohistochemistry of endometriotic tissues revealed that IL-4-positive cells were abundant in the stroma. The effect of IL-4 on proliferation of endometriotic stromal cells was studied using cell counting and BrdU incorporation assays. IL-4 (0.1 to 10 ng/ml) significantly increased cell number and BrdU incorporation in a dose-dependent manner, and the proliferative effect of IL-4 was inhibited by anti-IL-4 receptor antibody. IL-4-induced activation of mitogen-activated protein kinases in endometriotic stromal cells was examined by Western blotting. IL-4 induced phosphorylation of p38 mitogen-activated protein kinase, stress-activated protein kinase/c-Jun kinase, and p42/44 mitogen-activated protein kinase and inhibitors of these kinases suppressed IL-4-induced proliferation of endometriotic stromal cells. These findings suggest that proliferation of endometriotic stromal cells induced by locally produced IL-4 is involved in the development of endometriosis.
Endometriosis is an enigmatic disease that deteriorates the health of women of reproductive age.1,2 A widely believed etiology is that endometrial debris in retrograde menstruation implants, survives and grows in the peritoneal cavity.3 However, it remains unknown why endometrial implants develop to substantial endometriotic lesions. Numerous lines of evidence suggest that aberrant immune responses and inflammatory reactions are involved in the pathogenesis of endometriosis.4,5,6
Women with endometriosis have characteristics of autoimmune disease, such as increased polyclonal B-cell activity, abnormalities in T- and B-cell function, and familial inheritance.5,6,7 High prevalence of autoimmune disease in endometriotic women supports an autoimmune aspect of endometriosis.8 Allergies and asthma are also reported at high rates in endometriotic women. In addition, a recent genome-wide transcriptional profiling study revealed that endometriosis exhibits a gene expression signature reminiscent of other autoimmune disorders.9
It is well known interleukin (IL)-4 is a distinguished molecule in autoimmunity and allergy.10,11 In view of the autoimmune and allergic background of endometriotic women, IL-4 is speculated to play a role in the pathogenesis of endometriosis. The notion is underpinned by the evidence that the levels of IL-4 mRNA and protein in peripheral blood monocytes and peritoneal fluid cells are elevated in women with endometriosis.12,13 However, localization of IL-4 and effects of IL-4 in endometriotic cells have been unknown.
IL-4 exerts its effect on immune cells.11 In addition, actions of IL-4 on several nonimmune cells have been reported.10 Interestingly, IL-4 stimulates or inhibits cell proliferation in different cells and settings.14,15,16,17,18,19 The biological function of IL-4 is mediated by a specific IL-4 receptor that is linked to several different intracellular signal cascades.11 To address the possible implication of IL-4 in endometriosis, we studied localization of IL-4 in endometriotic tissues and effects of IL-4 on the proliferation of endometriotic stromal cells (ESCs).
Materials and Methods
Reagents and Materials
Type I collagenase and antibiotics (mixture of penicillin, streptomycin, amphotericin B) were purchased from Sigma (St. Louis, MO). Dulbecco’s modified Eagle’s medium/Ham’s F12 medium (DMEM/F12) and 0.25% trypsin-ethylenediaminetetraacetic acid were from Life Technologies (Rockville, MD). Mitogen-activated protein kinase (MAPK) inhibitors SB202190, SP600125, and PD98059 [inhibitors for p38 MAPK, stress-activated protein kinase/c-Jun kinase (SAPK/JNK), and p42/44 MAPK, respectively], a PKA inhibitor H89, and a nuclear factor (NF)-κB inhibitor SN50 were from Calbiochem (La Jolla, CA). Rabbit antibodies of total p38 MAPK, phosphorylated (phospho-) p38 MAPK, total SAPK/JNK, phospho-SAPK/JNK, total p42/44 MAPK, and phospho-p42/44 MAPK were from New England BioLabs (Beverly, MA). Mouse anti-human IL-4 antibody (MAB304), mouse anti-human IL-4 receptor antibody (MAB230), and recombinant human IL-4 were from R&D Systems (Minneapolis, MN). Isotype mouse IgGs (IgG1 and IgG2a) were from Dako Cytomation (Glostrup, Denmark). Charcoal/dextran-treated fetal bovine serum was from Hyclone (Logan, UT). Deoxyribonuclease I was from Takara (Tokyo, Japan).
Collection of Tissues
Endometriotic tissues were obtained from patients (n = 32) with ovarian endometriomas undergoing laparoscopy or laparotomy after obtaining written informed consent under a study protocol approved by the institutional review board of the University of Tokyo. The mean age of the patients was 35.2 years (SD, 5.7). These patients had not received hormones or GnRH agonist for at least 3 months before surgery. The stages of endometriosis were III (n = 14) and IV (n = 18), and the mean rASRM score was 56.6 (SD, 34.9). Endometriotic tissues were obtained from the cyst wall of ovarian endometrioma. Samples were collected under sterile conditions and transported to the laboratory on ice in DMEM/F12.
Immunohistochemistry
Endometriotic tissue samples were washed in phosphate-buffered saline (PBS), embedded in OCT compound (Sakura, Tokyo, Japan), and snap-frozen in liquid nitrogen. Cryosections were cut at an 8-μm thickness and mounted on poly-l-lysine-treated slides. Sections were fixed in acetone for 30 minutes on ice and washed in PBS for 5 minutes twice. Sections were treated with 3% H2O2 for 15 minutes to eliminate endogenous peroxidase. After blocking with nonspecific staining blocking reagent, the sections were incubated with 100 μg/ml of anti-human IL-4 antibody or 100 μg/ml of mouse IgG1 isotype control for 60 minutes at room temperature and incubated with peroxidase-conjugated goat anti-mouse secondary antibody (labeled polymer-horseradish peroxidase anti-mouse, Dako Cytomation) for 30 minutes. Staining was detected with the vector novaRED substrate kit (Funakoshi, Tokyo). All sections were counterstained with hematoxylin and evaluated under a light microscope. As a positive control, we stained amniochorionic membranes.20
Isolation, Purification, and Culture of ESCs
The procedure was performed as described previously.21,22,23,24 Briefly, endometriotic tissue was minced into small pieces, incubated in DMEM/F12 with type I collagenase (2.5 mg/ml) and deoxyribonuclease I (15 U/ml) for 1 to 2 hours at 37°C, and filtered through nylon cell strainers with apertures of 100 μm, and then 70 μm. Stromal cells remaining in the filtrate were centrifuged at 200 × g for 5 minutes, washed with PBS, resuspended in DMEM/F12, and plated onto 100-mm dishes and allowed to adhere at 37°C for 30 minutes, after which nonadhering epithelial cells and blood cells were removed with PBS rinses. ESCs were cultured in DMEM/F12 containing 5% fetal bovine serum, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 0.25 μg/ml amphotericin B. When the cells became confluent in 2 or 3 days, they were dissociated with 0.25% trypsin-ethylenediaminetetraacetic acid, harvested by centrifugation at 200 × g for 5 minutes, replated in six-well plates at 2 × 105 cells/well for reverse transcription and polymerase chain reaction (RT-PCR) and Western blotting, or 96-well plates at 0.5 × 104 cells/well for cell proliferation assay, and incubated at 37°C in a humidified 5% CO2/95% air environment for 24 hours. The complete media were then removed and replaced with fresh serum-free media containing antibiotics, and the cells were cultured for an additional 24 hours. Purification of the stromal cell population was determined by immunocytochemical staining before confluency for the following antibodies: vimentin (stromal cells), cytokeratin (epithelial cells), CD45 and CD68 (monocytes and other leukocytes), and von Willebrand factor (endothelial cells). The purity of the stromal cell was more than 98%, as judged by positive cellular staining for vimentin and negative cellular staining for cytokeratin, CD45, CD68, and von Willebrand factor.
Treatment of ESCs
To evaluate dose effects of IL-4 on cell proliferation of ESCs, the wells were replenished with serum-free media with different concentrations of IL-4. To evaluate the effect of anti-IL-4 receptor neutralizing antibody on IL-4-induced proliferation of ESCs, the cells were preincubated with the antibody or isotype IgG2a for 30 minutes before IL-4 treatment (1 ng/ml). To evaluate effects of inhibitors of MAPK, PKA, and NF-κB on IL-4-induced proliferation of ESCs, the cells were preincubated with SB202190 (10 μmol/L), SP600125 (10 μmol/L), PD98059 (25 μmol/L), mixture of all of the MAPK inhibitors, H89 (5 μmol/L), or SN50 (50 μmol/L) for 1 hour before IL-4 treatment. To evaluate proliferative effect of tumor necrosis factor (TNF)-α and IL-4 on ESCs, the cells were treated with 1 ng/ml of IL-4 and/or 0.1 ng/ml of TNF-α. The conditions of the treatment were determined with reference to our previous studies.21,25,26,27
Cell Proliferation Assay
To measure the proliferative activity of ESCs, we measured the cell number of ESCs and BrdU incorporation. The number of ESCs was measured using cell counting kit-8 (CCK-8; Dojindo, Kumamoto, Japan) according to the manufacturer’s instructions. Briefly, ESCs were treated with IL-4 for 72 hours, and 10-ml CCK-8 solutions with tetrazolium salt WST-8 were added and incubated at 37°C for an additional 2 hours. WST-8 is bioreduced by cellular dehydrogenases to an orange formazan product in culture medium. The amount of formazan, which is directly proportional to the number of living cells, was evaluated by measurement of the optical density at 450 nm in the DigiScan microplate reader (ASYS Hitech GmbH, Eugendorf, Austria). The BrdU proliferation assay was performed as reported previously23,24,28 using the Biotrak cell proliferation enzyme-linked immunosorbent assay system (Amersham Biosciences, Little Chalfont, UK) according to the manufacturer’s instructions. Briefly, ESCs were treated with serum-free medium with different concentrations of IL-4 (0.1 to 10 ng/ml) for 48 hours, and 100-μl BrdU solutions were added and incubated at 37°C for an additional 2 hours. After removing the culture medium, the cells were fixed and the DNA denatured by the addition of 200 μl/well fixative. The peroxidase-labeled anti-BrdU bound to the BrdU incorporated in the newly synthesized, cellular DNA. The immune complexes were detected by the subsequent substrate reaction, and the resultant color was read at 450 nm in the DigiScan microplate reader.
RT-PCR
Total RNA was extracted from ESCs randomly selected out of the study population, using the RNAeasy mini kit (Qiagen, Hilden, Germany). RT was performed using Rever Tra Ace-a (Toyobo, Tokyo, Japan). One μg of total RNA was reverse-transcribed in a 20-μl total volume and cDNA was amplified using oligonucleotide primers. IL-4 receptor primers (sense, 5′-CAAGCTCTTGCCCTGTTTTC-3′; antisense, 5′-TGCACAGAAGCTCCCTTTTT-3′) were chosen to amplify a 238-bp fragment. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers (Toyobo) were used to ensure RNA amounts. The PCR condition of IL-4 receptor was 30 cycles at 98°C for 10 seconds, 60°C for 4 seconds, 74°C for 15 seconds. Each PCR product was purified with a Qiaex II gel extraction kit (Qiagen) and their identities were confirmed using an ABI Prism 310 genetic analyzer (Applied Biosystems, Foster City, CA).
Western Blotting
Western blotting was performed as reported previously.21,29 Cultured cells were homogenized in a lysis buffer containing 50 mmol/L Tris/HCl (pH 6.8), 2% sodium dodecyl sulfate, 10% glycerol, 50 mmol/L dithiothreitol, and 0.1% bromophenol blue, and diluted to 1 mg of total protein/ml. Concentrations of total protein in the homogenized cells were measured by a protein assay kit (Bio-Rad Laboratories, Hercules, CA). Samples were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Proteins were blotted onto a nitrocellulose membrane and incubated with rabbit antibodies to total p38 MAPK (1:1000), to phospho-p38 MAPK (1:1000), to total SAPK/JNK (1:1000), to phospho-SAPK/JNK (1:1000), to total p42/44 MAPK (1:1000), or to phospho-p42/44 MAPK (1:1000), as primary antibodies, and anti-rabbit horseradish peroxidase antibody (1:1000, Amersham Biosciences) as a secondary antibody. Immune complexes were visualized by the ECL Western blotting system (Amersham Biosciences).
Statistical Analysis
Data were evaluated using analysis of variance with post hoc analysis (Fisher’s protected least significance). A P value less than 0.05 was accepted as significant.
Results
Immunoreactive Cells for IL-4 Were Present in the Stroma of Endometriotic Tissue
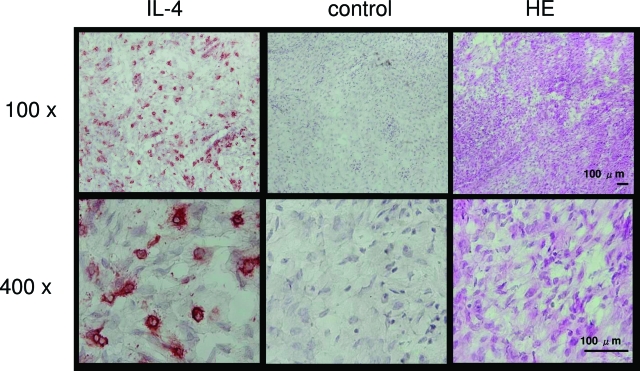
Figure 1 shows the cells stained for IL-4 in the stroma of endometriotic tissue. The number of IL-4-positive cells was 14% of total cells. No staining was seen when mouse IgG1 was used as a primary antibody.
Figure 1.
Immunohistochemistry of IL-4 in the human endometriotic tissue. Sections were immunostained with anti-human IL-4 antibody (IL-4), mouse IgG1 (control), and H&E. The result is representative of four separate experiments using samples from four different patients. Original magnifications: ×100 (top); ×400 (bottom).
Expression of IL-4 Receptor mRNA and Proliferative Effect of IL-4 on ESCs
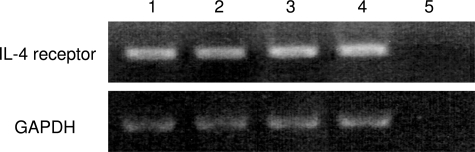
We first examined gene expression of the IL-4 receptor in ESCs. As shown in Figure 2, RT-PCR analysis demonstrated that IL-4 receptor mRNA was expressed in ESCs. Next, we studied the proliferative effect of IL-4 on ESCs. As depicted in Figure 3A, IL-4 (0.1 to 10 ng/ml) increased the cell number of ESCs in a dose-dependent manner. The increase at a dose of 10 ng/ml was 1.4-fold of the control. IL-4 (0.1 to 10 ng/ml) also increased BrdU incorporation in ESCs in a dose-dependent manner (Figure 3B). To determine whether the proliferative effect is mediated by IL-4 receptor on ESCs, we used anti-IL-4 receptor neutralizing antibody in addition to IL-4. In consequence, anti-IL-4 receptor neutralizing antibody significantly inhibited IL-4-induced proliferation of ESCs approximately to the control level (Figure 3C).
Figure 2.
Gene expression of IL-4 receptor in ESCs. The samples were randomly selected out of the study population. Total RNA isolated from ESCs of four women with endometriosis was reverse-transcribed and amplified by PCR using primers for IL-4 receptor. Amplification of GAPDH was used to ensure RNA amounts. Lane 5 is negative control with water.
Figure 3.
A: Proliferative effect of IL-4 on ESCs. ESCs were treated with IL-4 at different concentrations for 72 hours. The proliferation of ESCs was evaluated by cell counting kit-8 (CCK-8). Values are the mean ± SEM of combined data from five independent experiments using different ESC preparations from different women. *P < 0.01; **P < 0.0001 (each versus control). B: Effect of IL-4 on BrdU incorporation in ESCs. ESCs were treated with IL-4 at different concentrations for 48 hours, and then BrdU incorporation into DNA was measured. Values are the mean ± SEM of combined data from four independent experiments using different ESC preparations from different women. *P < 0.01; **P < 0.0001 (each versus control). C: Effect of anti-IL-4 receptor neutralizing antibody on IL-4-induced proliferation of ESCs. After the preincubation with either anti-IL-4 receptor neutralizing antibody or isotype IgG2a for 30 minutes, the cells were treated with or without IL-4 for 72 hours. Proliferation of ESCs was evaluated by CCK-8. Values are the mean ± SEM of the combined data from five independent experiments using different ESC preparations. *P < 0.0001 (versus isotype IgG); **P < 0.0001 (versus IL-4 plus isotype IgG).
IL-4-Induced Phosphorylation of p38 MAPK, SAPK/JNK, and p42/44 MAPK in ESCs, and Effect of MAP Kinase Inhibitors on IL-4-Induced Proliferation of ESCs
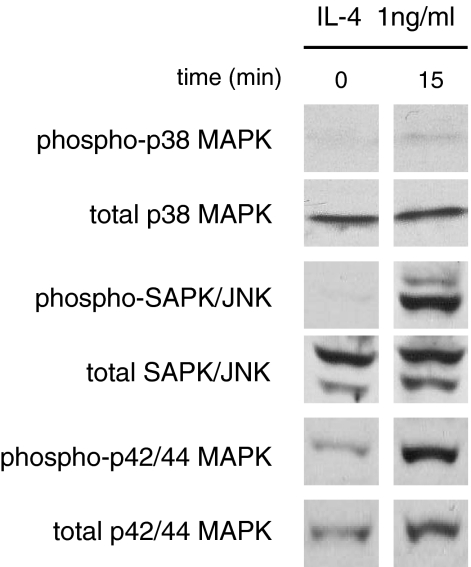
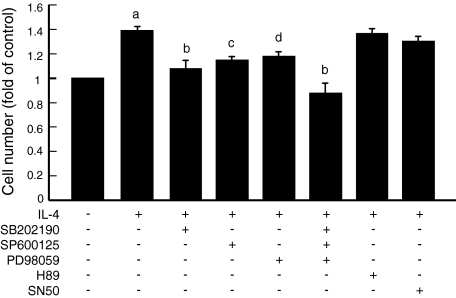
With the aim to study intracellular signaling involved in IL-4-induced proliferation of ESCs, we first examined phosphorylation of p38 MAPK, SAPK/JNK, and p42/44 MAPK. As shown in Figure 4, IL-4 stimulated the phosphorylation of p38 MAPK, SAPK/JNK, and p42/44 MAPK. We then tested inhibitors of these kinases for the inhibitory effect on IL-4-induced proliferation of ESCs. As shown in Figure 5, inhibitors of p38 MAPK (SB202190), SAPK/JNK (SP600125), and p42/44 MAPK (PD98059) significantly suppressed the IL-4-induced proliferation of ESCs. A mixture of these inhibitors suppressed the IL-4-induced proliferation more markedly. In contrast, inhibitors of PKA and NF-κB did not affect the proliferative effect of IL-4.
Figure 4.
IL-4 induced MAPK activation. ESCs were incubated with 1 ng/ml of IL-4 for 15 minutes. Cell extracts were prepared and assayed for phospho-/total p38 MAPK, phospho-/total SAPK/JNK and phospho-/total p42/44 MAPK by Western blotting. The result is representative of three separate experiments.
Figure 5.
After the preincubation with SB202190 (p38 MAPK inhibitor, 10 μmol/L), SP600125 (SAPK/JNK inhibitor, 10 μmol/L), PD98059 (p42/44 MAPK inhibitor, 25 μmol/L), mixture of all of the MAPK inhibitors, H89 (PKA inhibitor, 5 μmol/L), or SN50 (NF-κB inhibitor, 50 μmol/L) for 1 hour, the cells were treated with or without IL-4 for 72 hours. The proliferation of ESCs was evaluated by CCK-8. Values are the mean ± SEM of the combined data from eight independent experiments using different ESC preparations from different women. a, P < 0.0001 (versus control); b, P < 0.0001 (versus IL-4); c, P < 0.001 (versus IL-4); d, P < 0.005 (versus IL-4).
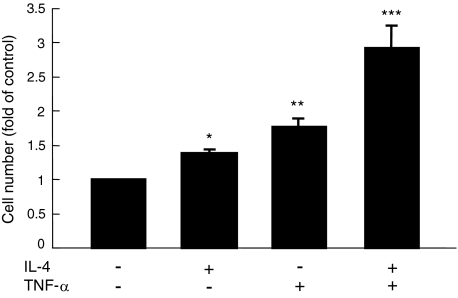
Effect of IL-4 on TNF-α-Induced Proliferation of ESCs
It has been previously reported that TNF-α has proliferative effect on ESCs.30 We examined whether IL-4 has an additional effect on TNF-α-induced proliferation of ESCs. As shown in Figure 6, addition of IL-4 to TNF-α showed a synergistic effect on ESC proliferation.
Figure 6.
Synergistic effect of IL-4 and TNF-α on ESC proliferation. The cells were treated with 1 ng/ml of IL-4 and/or 0.1 ng/ml of TNF-α for 72 hours. The proliferation of ESCs was evaluated by CCK-8. Values are the mean ± SEM of the combined data from five independent experiments using different ESC preparations from different women. *P < 0.05 (versus control); **P < 0.0005 (versus control); ***P < 0.0001 (versus all of the others).
Discussion
In the present study, we demonstrated the presence of IL-4-immunoreactive cells in the endometriotic tissue. Expression of IL-4 receptor was detected by RT-PCR in ESCs. We then showed that IL-4 stimulated the proliferation of ESCs, which was inhibited by the addition of anti-IL-4 receptor antibody. IL-4 stimulated the phosphorylation of p38MAPK, SAPK/JNK, and p42/44 MAPK in ESCs, and inhibitors of these MAPKs suppressed the IL-4-induced proliferation of ESCs. IL-4 also exerted a synergistic effect on TNF-α-induced proliferation of ESCs. These findings suggest important roles of IL-4 in the pathophysiology of endometriosis.
It has been shown that multiple immune cells, eg, macrophages, T lymphocyte, NK cells, mast cells, eosinophils, reside in endometriotic tissues.14,31,32 The immune cells in endometriotic tissues are suggested to be involved in the development of endometriosis by inducing various events such as inflammation, proliferation, invasion, angiogenesis, and fibrosis.4 In these events, cytokines from the immune cells play important roles, acting directly on ESCs or modulating other immune cell functions. In the present study, we detected many IL-4-immunoreactive cells in the endometriotic tissue. This finding is consistent with the report showing the increased expression of IL-4 mRNA and protein in lymphocytes in endometriotic tissues.12 In addition, IL-4 stimulated the proliferation of ESCs, suggesting that IL-4 plays as a local mediator to grow endometriotic lesion. These findings suggest that IL-4 is one of the cytokines in the immune network in endometriosis that promote the progress of the disease.
The present finding also has an implication in immunology of endometriosis. Multiple lines of evidence have shown that development of endometriosis is accompanied by the activation of a Th2 immune response.12,13,33 Because IL-4 is a typical Th2 cytokine, our findings extend the notion that a Th2 immune response may directly develop the disease through local IL-4 production. Interestingly, our recent study suggested that IL-17 stimulates the progress of endometriosis.25 IL-17 is a typical cytokine of Th17 cells, a novel member of helper T lymphocytes that has been believed to be only Th1 and Th2 for a long time. Therefore, both Th2 and Th17 immune response could contribute to the pathogenesis of the disease. Further study is warranted to elucidate the precise mechanism.
Anti-IL-4 receptor antibody abandoned the proliferation of ESCs induced by IL-4 in the present study. This finding clearly demonstrates that the proliferative effect of IL-4 on ESCs is exerted through its ligation to IL-4 receptor. It also implies that blocking IL-4 action on ESCs would be a possible treatment of endometriosis. Asthma is a disease in which IL-4 contributes to inflammation and airway obstruction. Aiming to develop a new drug for asthma therapy, drugs that prevent IL-4 binding to its receptor has been explored.34 A promising IL-4 receptor antagonist is under clinical trials, and the drug also might be useful in the treatment of endometriosis.
Downstream signal transduction of IL-4 receptor has been known to be diverse. In this study, we have shown that IL-4 activated p38 MAPK, SAPK/JNK, and p42/44 MAPK in ESCs. Moreover, inhibitors of p38 MAPK, SAPK/JNK, and p42/44 MAPK suppressed IL-4-induced proliferation of ESCs. We have observed similar findings in the previous study that activation of p38 MAPK, SAPK/JNK, and p42/44 MAPK were involved in the proliferation of ESCs by PAR2 activation.21 We have also reported that activation of p38 MAPK is higher in endometriotic tissues as compared to eutopic endometrium, and p38 MAPK inhibitor reduces endometriotic tissue in the experimental mouse model of endometriosis.35,36 Taken together, it is plausible that MAPKs are mediators used in common with various pro-endometriotic molecules and play a pivotal role in the development of endometriosis.
It is generally conceptualized that pelvic inflammation is a promoting factor for endometriosis. Increased activated macrophages in the peritoneal cavity of endometriotic women are suggested to produce proinflammatory cytokines and sustain self-perpetuating inflammation. TNF-α is a typical proinflammatory cytokine that plays multiple roles in the progression of endometriosis,30,37,38 and TNF-α-targeted suppression by specific drugs has been shown to inhibit the development of endometriosis in baboons.39,40 The present study demonstrated the synergistic effect of IL-4 and TNF-α to stimulate the proliferation of ESCs. The finding is interesting in that Th2 immune response may accelerate the progress of the disease in synergy with another inflammatory mediator.
In the present study, we used the tissues from ovarian endometrioma. It is suggested that endometriosis of ovarian, peritoneal, or deep pelvic lesions has different characteristics. In particular, a high steroid environment may affect ovarian lesions. It is well known that ovarian steroids have influence on various immune cells including T cells.41 Circulating IL-4 levels are demonstrated to be increased by estrogen.42,43 Therefore, a degree of contribution of IL-4 to the development of endometriosis might somehow be different in endometriotic lesions outside the ovary. Lastly, we would like to remark that the present study demonstrated the IL-4 effects on the proliferation and intracellular signaling in ESCs by the in vitro experiments. Further studies using in vivo animal models would be warranted to elucidate a definitive role of IL-4 in endometriosis.
In summary, the present study demonstrated that IL-4-immunoreactive cells are present in endometriotic tissues and that IL-4 stimulates the proliferation of ESCs. These findings suggest that locally produced IL-4 may be involved in the development of endometriosis.
Footnotes
Address reprint requests to Yutaka Osuga, M.D., Department of Obstetrics and Gynecology, Faculty of Medicine, University of Tokyo, 7-3-1, Hongo, Bunkyo-ku, Tokyo, 113-8655, Japan. E-mail: yutakaos-tky@umin.ac.jp.
This work was partially supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labour and Welfare.
Z.O. and Y.H. contributed equally to this study.
References
- Momoeda M, Taketani Y, Terakawa N, Hoshiai H, Tanaka K, Tsutsumi O, Osuga Y, Maruyama M, Harada T, Obata K, Hayashi K. Is endometriosis really associated with pain? Gynecol Obstet Invest. 2002;54(Suppl 1):18–21. doi: 10.1159/000066290. [DOI] [PubMed] [Google Scholar]
- Osuga Y, Koga K, Tsutsumi O, Yano T, Maruyama M, Kugu K, Momoeda M, Taketani Y. Role of laparoscopy in the treatment of endometriosis-associated infertility. Gynecol Obstet Invest. 2002;53(Suppl 1):33–39. doi: 10.1159/000049422. [DOI] [PubMed] [Google Scholar]
- Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151–154. [PubMed] [Google Scholar]
- Berkkanoglu M, Arici A. Immunology and endometriosis. Am J Reprod Immunol. 2003;50:48–59. doi: 10.1034/j.1600-0897.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75:1–10. doi: 10.1016/s0015-0282(00)01630-7. [DOI] [PubMed] [Google Scholar]
- Nothnick WB. Treating endometriosis as an autoimmune disease. Fertil Steril. 2001;76:223–231. doi: 10.1016/s0015-0282(01)01878-7. [DOI] [PubMed] [Google Scholar]
- Bancroft K, Vaughan Williams CA, Elstein M. Minimal/mild endometriosis and infertility. A review. Br J Obstet Gynaecol. 1989;96:454–460. doi: 10.1111/j.1471-0528.1989.tb02422.x. [DOI] [PubMed] [Google Scholar]
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715–2724. doi: 10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- Hever A, Roth RB, Hevezi P, Marin ME, Acosta JA, Acosta H, Rojas J, Herrera R, Grigoriadis D, White E, Conlon PJ, Maki RA, Zlotnik A. Human endometriosis is associated with plasma cells and overexpression of B lymphocyte stimulator. Proc Natl Acad Sci USA. 2007;104:12451–12456. doi: 10.1073/pnas.0703451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomarat P, Banchereau J. An update on interleukin-4 and its receptor. Eur Cytokine Netw. 1997;8:333–344. [PubMed] [Google Scholar]
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- Antsiferova YS, Sotnikova NY, Posiseeva LV, Shor AL. Changes in the T-helper cytokine profile and in lymphocyte activation at the systemic and local levels in women with endometriosis. Fertil Steril. 2005;84:1705–1711. doi: 10.1016/j.fertnstert.2005.05.066. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Yang BC, Wu MH, Huang KE. Enhanced interleukin-4 expression in patients with endometriosis. Fertil Steril. 1997;67:1059–1064. doi: 10.1016/s0015-0282(97)81439-2. [DOI] [PubMed] [Google Scholar]
- Blumenthal RD, Samoszuk M, Taylor AP, Brown G, Alisauskas R, Goldenberg DM. Degranulating eosinophils in human endometriosis. Am J Pathol. 2000;156:1581–1588. doi: 10.1016/S0002-9440(10)65030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechanet J, Briolay J, Rissoan MC, Chomarat P, Galizzi JP, Banchereau J, Miossec P. IL-4 inhibits growth factor-stimulated rheumatoid synoviocyte proliferation by blocking the early phases of the cell cycle. J Immunol. 1993;151:4908–4917. [PubMed] [Google Scholar]
- Prokopchuk O, Liu Y, Henne-Bruns D, Kornmann M. Interleukin-4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. Br J Cancer. 2005;92:921–928. doi: 10.1038/sj.bjc.6602416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atamas SP, Luzina IG, Dai H, Wilt SG, White B. Synergy between CD40 ligation and IL-4 on fibroblast proliferation involves IL-4 receptor signaling. J Immunol. 2002;168:1139–1145. doi: 10.4049/jimmunol.168.3.1139. [DOI] [PubMed] [Google Scholar]
- Falkensammer C, Johrer K, Gander H, Ramoner R, Putz T, Rahm A, Greil R, Bartsch G, Thurnher M. IL-4 inhibits the TNF-alpha induced proliferation of renal cell carcinoma (RCC) and cooperates with TNF-alpha to induce apoptotic and cytokine responses by RCC: implications for antitumor immune responses. Cancer Immunol Immunother. 2006;55:1228–1237. doi: 10.1007/s00262-006-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitsu Y, Fukuda K, Kumagai N, Nishida T. IL-4-induced cell proliferation and production of extracellular matrix proteins in human conjunctival fibroblasts. Exp Eye Res. 2003;76:107–114. doi: 10.1016/s0014-4835(02)00248-8. [DOI] [PubMed] [Google Scholar]
- Moraes-Pinto MI, Vince GS, Flanagan BF, Hart CA, Johnson PM. Localization of IL-4 and IL-4 receptors in the human term placenta, decidua and amniochorionic membranes. Immunology. 1997;90:87–94. doi: 10.1046/j.1365-2567.1997.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y, Osuga Y, Hirata T, Harada M, Morimoto C, Yoshino O, Koga K, Yano T, Tsutsumi O, Taketani Y. Activation of protease-activated receptor 2 stimulates proliferation and interleukin (IL)-6 and IL-8 secretion of endometriotic stromal cells. Hum Reprod. 2005;20:3547–3553. doi: 10.1093/humrep/dei255. [DOI] [PubMed] [Google Scholar]
- Hirota Y, Osuga Y, Koga K, Yoshino O, Hirata T, Harada M, Morimoto C, Yano T, Tsutsumi O, Sakuma S, Muramatsu T, Taketani Y. Possible implication of midkine in the development of endometriosis. Hum Reprod. 2005;20:1084–1089. doi: 10.1093/humrep/deh720. [DOI] [PubMed] [Google Scholar]
- Morimoto C, Osuga Y, Yano T, Takemura Y, Harada M, Hirata T, Hirota Y, Yoshino O, Koga K, Kugu K, Taketani Y. GnRH II as a possible cytostatic regulator in the development of endometriosis. Hum Reprod. 2005;20:3212–3218. doi: 10.1093/humrep/dei192. [DOI] [PubMed] [Google Scholar]
- Takemura Y, Osuga Y, Yoshino O, Hasegawa A, Hirata T, Hirota Y, Nose E, Morimoto C, Harada M, Koga K, Tajima T, Yano T, Taketani Y. Metformin suppresses interleukin (IL)-1beta-induced IL-8 production, aromatase activation, and proliferation of endometriotic stromal cells. J Clin Endocrinol Metab. 2007;92:3213–3218. doi: 10.1210/jc.2006-2486. [DOI] [PubMed] [Google Scholar]
- Hirata T, Osuga Y, Hamasaki K, Yoshino O, Ito M, Hasegawa A, Takemura Y, Hirota Y, Nose E, Morimoto C, Harada M, Koga K, Tajima T, Saito S, Yano T, Taketani Y. Interleukin (IL)-17A stimulates IL-8 secretion, cyclooxygensase-2 expression, and cell proliferation of endometriotic stromal cells. Endocrinology. 2008;149:1260–1267. doi: 10.1210/en.2007-0749. [DOI] [PubMed] [Google Scholar]
- Hirata T, Osuga Y, Hirota Y, Koga K, Yoshino O, Harada M, Morimoto C, Yano T, Nishii O, Tsutsumi O, Taketani Y. Evidence for the presence of toll-like receptor 4 system in the human endometrium. J Clin Endocrinol Metab. 2005;90:548–556. doi: 10.1210/jc.2004-0241. [DOI] [PubMed] [Google Scholar]
- Yoshino O, Osuga Y, Hirota Y, Koga K, Hirata T, Yano T, Ayabe T, Tsutsumi O, Taketani Y. Endometrial stromal cells undergoing decidualization down-regulate their properties to produce proinflammatory cytokines in response to interleukin-1 beta via reduced p38 mitogen-activated protein kinase phosphorylation. J Clin Endocrinol Metab. 2003;88:2236–2241. doi: 10.1210/jc.2002-021788. [DOI] [PubMed] [Google Scholar]
- Hirota Y, Osuga Y, Hirata T, Yoshino O, Koga K, Harada M, Morimoto C, Nose E, Yano T, Tsutsumi O, Taketani Y. Possible involvement of thrombin/protease-activated receptor 1 system in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2005;90:3673–3679. doi: 10.1210/jc.2004-0493. [DOI] [PubMed] [Google Scholar]
- Hirota Y, Osuga Y, Koga K, Yoshino O, Hirata T, Morimoto C, Harada M, Takemura Y, Nose E, Yano T, Tsutsumi O, Taketani Y. The expression and possible roles of chemokine CXCL11 and its receptor CXCR3 in the human endometrium. J Immunol. 2006;177:8813–8821. doi: 10.4049/jimmunol.177.12.8813. [DOI] [PubMed] [Google Scholar]
- Iwabe T, Harada T, Tsudo T, Nagano Y, Yoshida S, Tanikawa M, Terakawa N. Tumor necrosis factor-alpha promotes proliferation of endometriotic stromal cells by inducing interleukin-8 gene and protein expression. J Clin Endocrinol Metab. 2000;85:824–829. doi: 10.1210/jcem.85.2.6335. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Konno R, Netsu S, Sugamata M, Shibahara H, Ohwada M, Suzuki M. Localization of mast cells in endometrial cysts. Am J Reprod Immunol. 2004;51:341–344. doi: 10.1111/j.1600-0897.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- Oosterlynck DJ, Cornillie FJ, Waer M, Koninckx PR. Immunohistochemical characterization of leucocyte subpopulations in endometriotic lesions. Arch Gynecol Obstet. 1993;253:197–206. doi: 10.1007/BF02766646. [DOI] [PubMed] [Google Scholar]
- Podgaec S, Abrao MS, Dias JA, Jr, Rizzo LV, de Oliveira RM, Baracat EC. Endometriosis: an inflammatory disease with a Th2 immune response component. Hum Reprod. 2007;22:1373–1379. doi: 10.1093/humrep/del516. [DOI] [PubMed] [Google Scholar]
- Andrews AL, Holloway JW, Holgate ST, Davies DE. IL-4 receptor alpha is an important modulator of IL-4 and IL-13 receptor binding: implications for the development of therapeutic targets. J Immunol. 2006;176:7456–7461. doi: 10.4049/jimmunol.176.12.7456. [DOI] [PubMed] [Google Scholar]
- Yoshino O, Osuga Y, Hirota Y, Koga K, Hirata T, Harada M, Morimoto C, Yano T, Nishii O, Tsutsumi O, Taketani Y. Possible pathophysiological roles of mitogen-activated protein kinases (MAPKs) in endometriosis. Am J Reprod Immunol. 2004;52:306–311. doi: 10.1111/j.1600-0897.2004.00231.x. [DOI] [PubMed] [Google Scholar]
- Yoshino O, Osuga Y, Koga K, Hirota Y, Hirata T, Ruimeng X, Na L, Yano T, Tsutsumi O, Taketani Y. FR 167653, a p38 mitogen-activated protein kinase inhibitor, suppresses the development of endometriosis in a murine model. J Reprod Immunol. 2006;72:85–93. doi: 10.1016/j.jri.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Chen DB, Yang ZM, Hilsenrath R, Le SP, Harper MJ. Stimulation of prostaglandin (PG) F2 alpha and PGE2 release by tumour necrosis factor-alpha and interleukin-1 alpha in cultured human luteal phase endometrial cells. Hum Reprod. 1995;10:2773–2780. doi: 10.1093/oxfordjournals.humrep.a135790. [DOI] [PubMed] [Google Scholar]
- Zhang RJ, Wild RA, Ojago JM. Effect of tumor necrosis factor-alpha on adhesion of human endometrial stromal cells to peritoneal mesothelial cells: an in vitro system. Fertil Steril. 1993;59:1196–1201. doi: 10.1016/s0015-0282(16)55976-7. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Nugent NP, Cuneo S, Chai DC, Deer F, Debrock S, Kyama CM, Mihalyi A, Mwenda JM. Recombinant human TNFRSF1A (r-hTBP1) inhibits the development of endometriosis in baboons: a prospective, randomized, placebo- and drug-controlled study. Biol Reprod. 2006;74:131–136. doi: 10.1095/biolreprod.105.043349. [DOI] [PubMed] [Google Scholar]
- Falconer H, Mwenda JM, Chai DC, Wagner C, Song XY, Mihalyi A, Simsa P, Kyama C, Cornillie FJ, Bergqvist A, Fried G, D'Hooghe TM. Treatment with anti-TNF monoclonal antibody (c5N) reduces the extent of induced endometriosis in the baboon. Hum Reprod. 2006;21:1856–1862. doi: 10.1093/humrep/del044. [DOI] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Kamada M, Irahara M, Maegawa M, Ohmoto Y, Murata K, Yasui T, Yamano S, Aono T. Transient increase in the levels of T-helper 1 cytokines in postmenopausal women and the effects of hormone replacement therapy. Gynecol Obstet Invest. 2001;52:82–88. doi: 10.1159/000052948. [DOI] [PubMed] [Google Scholar]
- Kumru S, Godekmerdan A, Yilmaz B. Immune effects of surgical menopause and estrogen replacement therapy in peri-menopausal women. J Reprod Immunol. 2004;63:31–38. doi: 10.1016/j.jri.2004.02.001. [DOI] [PubMed] [Google Scholar]