Abstract
Formation of bile ducts in culture is important for reconstructing hepatic organoids with bile drainage systems. However, morphogenic factors of biliary epithelial cells (BECs) have been poorly understood because of the lack of experimental models. Here, we demonstrated that rat BECs formed bile ductular networks in dynamic culture, when culture conditions were sequentially controlled. BEC morphogenesis was achieved through two-dimensional culture on collagen gel, collagen gel sandwich configuration, and 1% dimethylsulfoxide stimulation. In this culture system, BECs developed into large bile duct structures (LBDs) that formed interconnected networks of continuous lumens. LBD luminal surfaces possessed well developed microvilli, consisted of 7 to 10 BECs, and their inner diameters measured 20 to 50 μm. Quantitative PCR analysis revealed that the cells in LBDs expressed apical and basal domain markers of BECs. Immunofluorescent staining identified apical domain markers such as Cl−/HCO3− anion exchanger 2 and cystic fibrosis transmembrane regulator on the luminal surface of LBDs, responding to secretin stimulation as well as laminin protein surrounding LBDs. Furthermore, the cells in LBDs transported metabolized fluorescein from the basal side to the luminal space, further demonstrating that the reconstructed LBDs were functionally and morphologically similar to the bile ducts in vivo. The culture model described here will be useful in reconstructing hepatic tissues as well as in understanding the mechanism of bile duct development and its disruption in disease.
Liver transplantation is the only treatment for end-stage liver disease, but donor organ shortages are a serious issue. Transplantation of tissue-engineered livers represents an alternative to donated liver transplantation.1 Although many studies have attempted to replace liver function using a hybrid bioartificial liver,2,3 it is still difficult to maintain differentiated hepatocytes in vitro. One problem in the bioreactor is the accumulation of bile, which is toxic to hepatocytes.4 To reconstruct hepatic tissues with a bile drainage system, it is important to control the morphogenesis of biliary epithelial cells (BECs) in vitro. In particular, reconstructing liver-like tissues, in which hepatocytes and BECs maintain the liver microarchitecture, is important.
In vivo, bile canaliculi, which are formed by hepatocytes, merge into bile ducts formed by BECs via canals of Hering. Therefore, bile ducts should be reconstructed from BECs to achieve hepatic organoids with bile drainage systems. Although some laboratories have tried to establish an in vitro BEC culture system, BEC morphogenesis, such as well developed duct formation, remains difficult. Yang et al5 established BEC lines from the primary culture of bile duct fragments cultured in collagen gel. The cells could transiently form duct-like structures, but the ducts soon disappeared in the culture. On the other hand, Sirica et al6,7 developed a BEC culture model in which cells isolated from a rat liver with massive bile ductular hyperplasia formed duct-like structures in collagen gel. However, they only formed disconnected tubular structures. Auth et al8,9,10 showed that human BECs could organize into luminal ducts in collagen gel when cocultured with hepatocytes. Although BECs shown in those studies formed duct-like structures, interconnected branching bile ductular networks have not been constructed in vitro. In particular, the critical factors of BEC morphogenesis in the process of ductular formation remain poorly characterized. BECs were cultured in the same conditions throughout the culture period. However, the critical factors of the BEC morphogenesis may change with time in culture because ductular formation can be achieved by a sequence of cellular events, such as migration, proliferation, rearrangement, and differentiation. Therefore, we hypothesized that BEC morphogenesis can be controlled by dynamic changes of culture conditions responding to the stage of BEC morphogenesis.
In the present study, we showed that BEC morphogenesis was promoted in a stepwise manner under the temporal control of culture conditions in response to the stage of BEC morphogenesis. First, BECs were cultured on collagen gel to proliferate and form colonies. The BEC colonies were then overlaid with collagen gel to make a collagen-gel sandwich configuration that provided the cells with a three-dimensional (3D) contact with extracellular matrix. The 3D environment induced the formation of small bile duct structures (SBDs). The SBDs finally developed into interconnected networks of large bile duct structures (LBDs) with continuous lumens following dimethylsulfoxide (DMSO) stimulation. DMSO was added to the culture medium to progress BEC morphogenesis from SBD to LBD formation because it stimulates differentiation of hepatocytes in culture.11 The morphology of LBDs was similar to that of bile ducts in vivo, and the cells in LBDs recovered polarity and acquired secretory function. This experimental approach will be useful to reconstruct functional bile ducts in vitro.
Materials and Methods
Isolation and Culture of BECs
BECs were isolated from Sprague-Dawley rats (250 to 350 g; Nippon Bio-Supp. Center, Tokyo, Japan). First, hepatocytes and hepatic nonparenchymal cells were removed from bile ductular tree by the two-step liver perfusion method of Seglen.12 The remaining tissues were carefully cut with scissors into small fragments (<1 mm) in Leibovitz L-15 medium (Invitrogen, Carlsbad, CA) with 20 mmol/L HEPES (Dojindo, Kumamoto, Japan), 1.1 g/L galactose (Katayama Chemical, Osaka, Japan), 30 mg/L l-proline, 0.1 μmol/L insulin, 10−7 M dexamethasone (Sigma-Aldrich, St. Louis, MO), and antibiotics. This mixture was shaken for 50 minutes at 37°C in L-15 medium supplemented with 400 U/ml collagenase (Yakult Pharmaceutical, Tokyo, Japan), 700 U/ml hyaluronidase (type I-S; Sigma-Aldrich), 0.1 μmol/L insulin, 10−7 M dexamethasone, and antibiotics, and filtered by 20-μm mesh.7 The digested cells were centrifuged at 850 × g for 10 minutes at 4°C, and the pellet was resuspended in the medium and centrifuged twice at 850 × g for 5 minutes at 4°C. Thereafter, the pellet was suspended in culture medium.
The obtained cells were seeded on rat-tail collagen gel formed in culture dishes (35-mm dish, 24-well culture plate; Corning, Corning, NY). Collagen solution (4 mg/ml rat-tail collagen in the mixture of 10× Dulbecco’s modified Eagle’s medium and NaOH) was polymerized at 37°C for 10 to 15 minutes. The culture medium used was Dulbecco’s modified Eagle’s medium (Sigma-Aldrich) supplemented with 5 μg/ml transferrin (Wako Pure Chemical, Tokyo, Japan), 0.1 μmol/L insulin, 10−7 M dexamethasone, 10 mmol/L nicotinamide (Katayama Chemical), 1 mmol/L ascorbic acid 2-phosphate (Wako Pure Chemical), 10 ng/ml epidermal growth factor (BD biosciences, Bedford, MA), 10 ng/ml hepatocyte growth factor (Sigma-Aldrich), 10% fetal bovine serum, and antibiotics. The cells were placed in a humidified, 5% CO2 incubator at 37°C, and the medium was changed to remove dead cells 1 day after inoculation. The medium was subsequently changed every other day. BECs were overlaid with collagen gel 4 to 5 days after inoculation. DMSO (Sigma-Aldrich) was added to the culture medium at the final concentration of 1% from days 6 to 8 (144 to 192 hours after inoculation).
Phase-Contrast Microscopy
Cells were photographed on a phase-contrast microscope (Nikon, Tokyo, Japan) equipped with a charge-coupled device camera (Axio Cam MRc5; Carl Zeiss, Hallbergmoos, Germany) by using AxioVision software (Carl Zeiss). Time-lapse microscopy was performed with a phase-contrast microscope (Nikon) equipped with a charge-coupled device camera (Roper Scientific, Trenton, NJ) by using MetaMorph imaging system (Universal Imaging, Downingtown, PA). Cells were placed in a humidified 5% CO2 chamber (Sankei, Tokyo, Japan) at 37°C, and images of the cells were recorded at 10-minute intervals for 48 hours, starting at days 1 and 3 of culture.
Transmission Electron Microscopy
For transmission electron microscopy, cells were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) overnight at 4°C, postfixed in 2% OsO4 for 2 hours, dehydrated through a graded series (70 to 100%) of ethanol, and embedded in Epon-812. Semithin (1 μm) and ultrathin (100 nm) sections were cut perpendicularly and parallel to the dish surface using an LKB ultramicrotome (LKB, Bromma, Sweden). The semithin sections were stained with 0.1% methylene blue and examined using a light microscope. The ultrathin sections were stained with uranyl acetate followed by lead citrate and examined at 80 kV using a JEM-100S transmission electron microscope (JEOL, Tokyo, Japan).
Immunocytochemistry and Cytochemical Staining
The primary antibodies used were mouse anti-cytokeratin (CK) 19; Novocastra, Newcastle, UK) for a BEC marker, rat anti-zonula occludens-1 (Chemicon International, Temecula, CA) for tight junctions, mouse anti-vimentin (DAKO, Copenhagen, Denmark) for fibroblastic cells, rabbit anti-laminin (Sigma-Aldrich) for the basement membrane matrix, and rabbit anti-cytokeratins (DAKO). Alexa Fluor 594-conjugated anti-rat/mouse/rabbit IgG antibodies (Invitrogen) and Alexa Fluor 488-conjugated anti-mouse/rabbit IgG antibodies (Invitrogen) were used as the secondary antibodies.
For frozen sections, samples were fixed in cold absolute ethanol and frozen with OCT compound in liquid nitrogen. Sections were cut at 7-μm thickness in a cryostat and air-dried. Immunofluorescent staining was performed for the cells and frozen sections. Samples were fixed in cold absolute ethanol or 3% paraformaldehyde. When cells were fixed in paraformaldehyde, cells were treated with 0.2% Triton-X to permeabilize cell membranes. After rinsing with PBS, the cells were incubated with BlockAce (Dainippon Pharm., Tokyo, Japan) at room temperature for 1 hour. Thereafter, the samples were incubated with primary antibodies at room temperature for 6 hours. After rinsing with PBS, the samples were incubated with secondary antibodies. The samples were mounted with glycerin containing 1 μg/ml 4′,6-diamino-2-phenylindole (Sigma-Aldrich) for counterstaining of cell nuclei. Digital images of the fluorescence distribution in the cells were obtained using a confocal laser-scanning microscope (PASCAL, Carl Zeiss). LSM Image Browser (Carl Zeiss) and Imaris (Bitplane, Zurich, Switzerland) were used for image processing.
The activity of γ-glutamyl transpeptidase was examined by the method of Rutenburg et al13 with some modification using γ-glutamyl-4-metoxy-2-naphthylamide (Sigma-Aldrich) as a substrate.
Paraffin-embedded samples of BECs that reconstructed ductular structures were used for periodic acid-Schiff (PAS) staining. Paraffin sections were cut perpendicular to the ductular structure at 10-μm thickness. Paraffin sections were deparaffinized and stained with PAS reagents (Merck, Darmstadt, Germany).
Secretin Treatment and Immunofluorescent Staining for BEC Apical Domain Markers
To stimulate the relocation of apical membrane proteins in BECs, the cells that formed LBDs were cultured in the DMSO-free medium supplemented with 10−5 M rat secretin (Backem AG, Bubendorf, Switzerland) for 30 minutes. The cells in the collagen sandwich configuration were then fixed in 2% paraformaldehyde for 15 minutes at room temperature. Next, the samples were incubated with Block Ace supplemented with 10% goat serum (Vector Laboratories, Burlingame, CA) for 30 minutes at room temperature. Thereafter, the upper layer of collagen gel was carefully peeled off and the samples were incubated for 6 hours with primary antibodies against apical membrane proteins, rabbit anti-cystic fibrosis transmembrane regulator (CFTR; Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-Cl−/HCO3− anion exchanger 2 (AE2; Chemicon). After rinsing with PBS, the samples were incubated for 3 hours with Alexa Fluor 488-conjugated anti-rabbit IgG (Invitrogen). The samples were then counterstained for CK19 as described above. Digital images of the fluorescence distribution in the cells were obtained using a confocal laser-scanning microscope (Carl Zeiss) and analyzed with LSM Image Browser (Carl Zeiss).
Uptake and Secretion of Fluorscein Diacetate
Fluorescein diacetate (FD; Sigma-Aldrich) is metabolized by intracellular esterase and becomes fluorescein. To investigate the secretory function of BECs that formed ductular structures, the cells were treated with 2.5 μg/ml FD for 20 minutes at 37°C. Thereafter, the cells were rinsed with warmed medium and photographed immediately using a confocal laser-scanning microscope (Carl Zeiss). Z-stacks of the fluorescent images were automatically collected at 0.89-μm intervals.
RNA Isolation and Quantitative PCR Analysis
Quantitative real-time polymerase chain reaction (QPCR) analysis was performed to identify mRNA transcription in the cells that formed ductular structures. Total RNA was isolated from LBD-forming cells and those cultured on collagen gel before overlaying collagen gel using the RNeasy RNA isolation kit (Qiagen, Hilden, Germany). As a positive control, the total RNA of isolated bile duct fragments was also prepared. Reverse transcription was performed on 1.0 μg of total RNA using the oligo(dT)20 primer and the SuperScript III reverse transcriptase (Invitrogen). The gene-specific primers used were designed using Primer3 software14; most primers were designed to amplify a region spanning the splice site of exon (Table 1). The analysis was performed on apical domain-associated markers such as Aquaporin (AQP) 1, multidrug resistance-associated protein (MRP) 2, and CFTR15; BEC basolateral domain markers such as AQP4 and MRP316,17; BEC markers such as CK19 and hepatocyte nuclear factor (HNF) 6; and hepatocyte markers such as albumin and HNF4α. The mRNA expression of glyceraldehide-3-phosphate-dehydrogenase was used for normalization. QPCR mixtures were composed of SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA), 10 μmol/L each primer, 10 nmol/L reference dye, and 1 μl cDNA in a total volume of 20 μl. The samples were incubated at 95°C for 5 minutes, followed by 45 cycles of 95°C for 5 seconds, and 65°C for 30 seconds using the Mx3000P real-time PCR system (Stratagene). Amplification plots were analyzed using MxPro software (Stratagene). A standard curve was generated using amplification plots of serial dilutions of mixed sample cDNAs. Plotting Ct values obtained for each cDNA on the standard curve enabled us to calculate the fold-difference between each experimental sample cDNA, normalized by the amount of glyceraldehide-3-phosphate-dehydrogenase.
Table 1.
Sequences of QPCR primers
| Primer | Sequence | Size (bp) |
|---|---|---|
| AQP1 | 5′-CTGTGCGTTCTGGCTACCAC-3′ | 154 |
| 5′-GCACAGCAGAGCCAAATGAC-3′ | ||
| MRP2 | 5′-GAGGGTGGTGACAACCTGAG-3′ | 200 |
| 5′-ATGGTGTGCAGCCTGTGAG-3′ | ||
| CFTR | 5′-GGTCATAGAGCAGGGCAATG-3′ | 186 |
| 5′-TGCACTTCTTCCTCCGTCTC-3′ | ||
| AQP4 | 5′-CGGTTCATGGAAACCTCACT-3′ | 191 |
| 5′-CATGCTGGCTCCGGTATAAT-3′ | ||
| MRP3 | 5′-GCTGGAAGGTGCAGTGTCTG-3′ | 206 |
| 5′-TGCCCTTCTCTCCAATCTCTG-3′ | ||
| CK19 | 5′-TTCCGGACCAAGTTTGAGAC-3′ | 166 |
| 5′-CCTCGTGGTTCTTCTTCAGG-3′ | ||
| HNF6 | 5′-GACCATGGCCTGTGAAACTC-3′ | 188 |
| 5′-TGAAACTACCGCTCACGTTG-3′ | ||
| ALB | 5′-TCAACAAGGAGTGCTGTCACG-3′ | 198 |
| 5′-CAGCTATTGAGGGCAGATCG-3′ | ||
| HNF4α | 5′-CTGCAGGCTCAAGAAGTGC-3′ | 161 |
| 5′-GGGAGGTGATCTGCTGAGAC-3′ | ||
| GAPDH | 5′-ATCAACGGGAAACCCATCAC-3′ | 211 |
| 5′-TCTCGTGGTTCACACCCATC-3′ |
Results
Ductular Formation Process
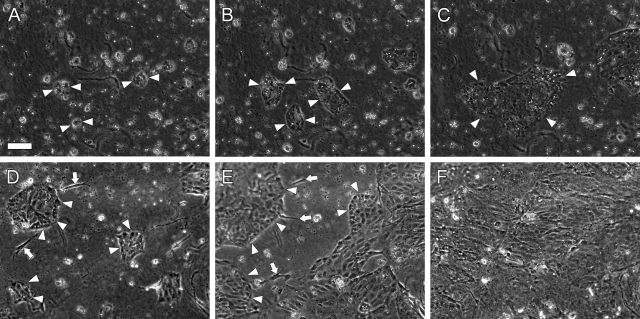
The isolated BEC fraction contained BECs as a single cell and as aggregates. The cells attached to the surface of the collagen gel within 1 day after inoculation, and some cells formed small colonies (arrowheads, Figure 1A). Time-lapse images revealed that BECs cultured on collagen gel migrated to assemble into colonies (Figure 1, B–C). The cells in the colony proliferated, and the colonies expanded with time in culture (Figure 1, D–F). Time-lapse video revealed that the proliferating cells were observed regardless of the location within the colonies (data not shown). The isolated BEC fraction also contained small amount of fibroblastic cells, which were observed surrounding BEC colonies in the early stage of the culture (arrows, Figure 1, D–E).
Figure 1.
Time-lapse images of BECs cultured on collagen gel before collagen-gel overlay. A–C: Corresponding phase-contrast images of BEC colonies were taken at 10 minutes intervals for 48 hours, starting from day 1 of the culture. A: Day 1. Single and aggregates of BECs attached to the surface of collagen gel and some cells formed small colonies (arrowheads). B: Day 2. BECs migrated to assemble into colonies, while some cells proliferated in the colonies. Arrowheads indicate corresponding colonies. C: Day 3. BEC colonies merged into a colony in which the cells actively proliferate (arrowheads). D–F: Corresponding images taken at 10 minutes intervals for 48 hours, starting from day 3 of culture. Images were taken at day 3 (D), day 4 (E), and day 5 (F). BEC colonies were enlarged by proliferating cells. Some fibroblastic cells located around BEC colonies (arrows; D, E). Scale bar, 50 μm.
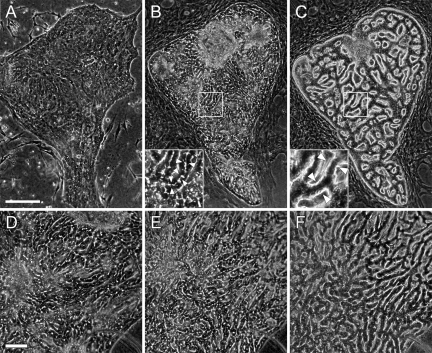
BECs formed large colonies about 4 days after inoculation, by intensive proliferation and migration on collagen gel until additional collagen gel was overlaid on the colonies (Figure 2A). After overlaying collagen gel, white lines became detectable in colonies under phase-contrast microscopy (arrowheads, Figure 2B inset). The white lines were then connected to each other and developed into anastomosing networks. These structures, called SBDs, were often observed between the cells 3 to 5 days after collagen-gel overlay (Figure 2B). After SBDs were formed, DMSO was added to culture medium. Several days after the DMSO addition, cells began to rearrange and form 3-D structures, called LBDs. Although BECs formed monolayers throughout the colony on collagen gel, the intercellular space in which no cells spread gradually increased in the colony when the cells were overlaid with collagen gel and DMSO was added to the medium. From 10 to 14 days after DMSO addition, LBDs were formed in most parts of a colony (Figure 2C). On the other hand, when BECs were cultured without DMSO, no LBD was observed; the cells maintained SBDs even at day 31 (Figure 2, D–E). However, SBDs could construct LBDs whenever DMSO was added to the cells, even after SBDs were cultured for more than 30 days without DMSO (Figure 2F). In addition, fibroblastic cells proliferated and covered BEC colonies when the cells were cultured without DMSO.
Figure 2.
Morphological changes of BECs in the process of ductular formation. A–C: Corresponding phase-contrast images of a BEC colony were taken at day 5 (A), day 9 (B), and day 18 (C) of culture. A: BECs maintained a flat colony one day after starting collagen-gel sandwich culture. B: BECs formed SBDs within 3 to 5 days in collagen-gel sandwich culture. Insets in B and C are enlarged images of rectangles in B and C, respectively. Intercellular white lines indicate SBD (arrowheads, B). C: BECs reconstructed LBDs when cultured with the medium containing 1% DMSO. The cells arranged into 3D structures that show clear outlines with white edges (arrowheads, C). Note that the size of the BEC colony is constant during bile duct formation. D–E: Phase-contrast images of a BEC colony before and after DMSO addition. D: SBDs formed by BECs at day 8 of culture. E: BECs maintained SBDs even at day 31 when they were cultured without DMSO addition. F: DMSO was supplemented to the culture medium at day 31, and the cells were photographed at day 44. Note that LBDs were formed after the DMSO addtion. The images of the same fields are shown (A–C, D–F). Scale bars: 200 μm (A); 100 μm (D).
Branching Interconnected Networks Formed by LBDs
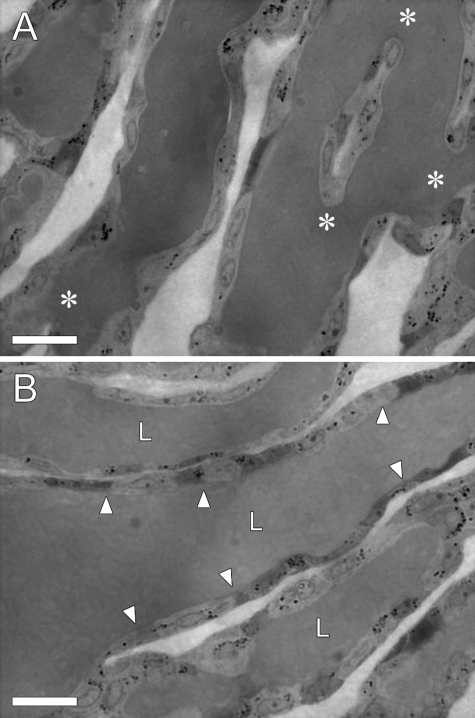
Horizontal sections of LBDs were stained with methylene blue and examined by light microscopy. Figure 3 shows that LBDs were continuously connected to form ductular networks, which were different from intercellular hollows. LBDs formed complicated networks with branching and merging points (asterisks, Figure 3A). Some LBDs aligned parallel to each other without intersection (Figure 3B).
Figure 3.
Bright-field images of horizontal sections of LBDs stained with methylene blue. A: LBDs formed interconnected networks. Asterisks indicate branching points of the networks. B: LBDs formed continuous luminal structures (L), which are lined by BEC monolayer (arrowheads). Scale bars: 20 μm.
Ductular Ultrastructures
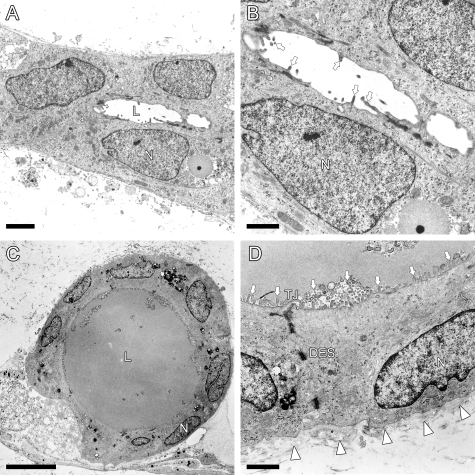
To investigate the ultrastructure of the cells consisting of SBDs and LBDs, vertical sections of BEC colonies were examined by transmission electron microscopy. SBDs were composed of 2 to 3 BECs and had small lumens of 2 to 5 μm in diameter (Figure 4A). Sparse microvilli were formed on the luminal surfaces (arrows, Figure 4B), and LBD luminal structures were lined by a single layer of 7 to 10 BECs, with an inner diameter of 20 to 50 μm (Figure 4C). As compared with SBDs, the cells of LBDs possessed large cytoplasms with many organelles. Well developed microvilli continuously covered the luminal surface of LBDs (arrows, Figure 4D). In addition, intercellular desmosomes (Figure 4D) and tight junctions (Figure 4D) developed at the apical poles of their lateral membranes. Furthermore, the polarity of typical BECs was shown by the existence of basement membrane-like structures beside basal membranes and nuclei localized in the bottom of the cells (Figure 4D). These characteristics of the cells in LBDs indicated the progression of maturation.
Figure 4.
Transmission electron micrographs of vertical sections of ductular structures. A: SBDs were formed between the cells cultured for 11 days. B: An enlarged image of an SBD in A. Sparse microvilli (arrows) were observed on the luminal surface. C: LBDs were reconstructed by the cells cultured for 50 days with the medium supplemented with 1% DMSO. Basolateral nuclei and a clear lumen are indicated. D: An enlarged image of the cells in (C). Numerous microvilli continuously covered the luminal surface (arrows). Adjacent cells were joined at the apical poles of their lateral membranes by tight junctions (TJ). A basement membrane (arrowheads) and desmosomes (DES) were also observed. N, nuclei; L, lumen. Scale bars: 2 μm (A); 1 μm (B); 10 μm (C); 1 μm (D).
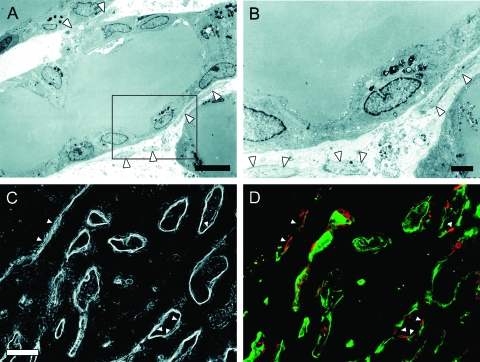
Fibroblastic Cells Surrounding LBDs
In this culture system, some fibroblastic cells were contaminated in the BEC fraction. Fibroblastic cells showing a spindle shape were mainly located around BEC colonies in the early stage of the culture (arrows, Figure 1, D–E). However, the cells were occasionally arranged along LBDs. In the transmission electron micrographs of a horizontal cross section of LBDs, cells with thin cytoplasm were observed along LBDs (arrowheads, Figure 5, A–B). To examine the phenotype of the cells, immunofluorescent staining for vimentin was performed. Most fibroblastic cells were vimentin-positive; the cells were arranged along LBDs (arrowheads, Figure 5, C–D), and no cluster formation of the cells was observed.
Figure 5.
Localization of fibroblastic cells around LBDs. A–B: Transmission electron micrographs of horizontal sections of LBDs. Cells were cultured for 50 days. An enlarged image of the region indicated by the box in A is shown in B. Arrowheads indicate fibroblastic cells that were located around LBDs. C–D: Corresponding phase-contrast (C) and immunofluorescence (D) images. Double immunostaining was performed for vimentin (red, a fibroblastic cell marker) and cytokeratins (green, a BEC marker). BECs forming LBDs were positive for cytokeratins. Arrowheads indicate vimentin-positive fibroblastic cells that were located around LBDs. Scale bars: 10 μm (A); 2 μm (B); and 50 μm (C).
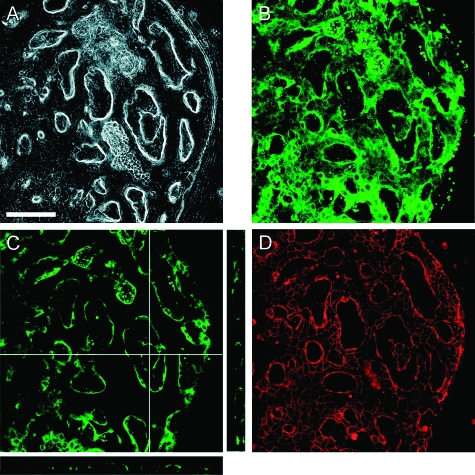
Phenotypes of Cultured BECs
The phenotypic property of the cells forming LBDs was examined by immunofluorescent staining for BEC markers. All LBD-forming cells were positive for CK19 (Figure 6, A–B). The cross section images of z-stacks also showed that BECs formed continuous luminal structures (Figure 6C). Zonula occludens-1, the tight junction-associated protein, was intensively detected at cell–cell borders (Figure 6D).
Figure 6.
Corresponding phase-contrast (A) and fluorescent images (B–D) of LBDs. Double immunostaining was performed for CK19 (green, a BEC marker) and zonula occludens-1 (red, tight junction-associated protein). A: The cells that reconstructed LBDs were fixed at day 31. B: The image for staining with CK19 is projected by 43 planes taken at 0.89-μm intervals in z-axis. C: The 15th image for staining with CK19. The cross-section images are three-dimensionally reconstructed by calculating 43 planes taken at 0.89-μm intervals in z-axis. The right image in C is the y-z axis section along the vertical line in the image. The lower image in C is the x-z axis section along the horizontal line in the image. D: The image for staining with ZO-1 is projected by 43 planes taken at 0.89-μm intervals in z-axis. Scale bar: 100 μm.
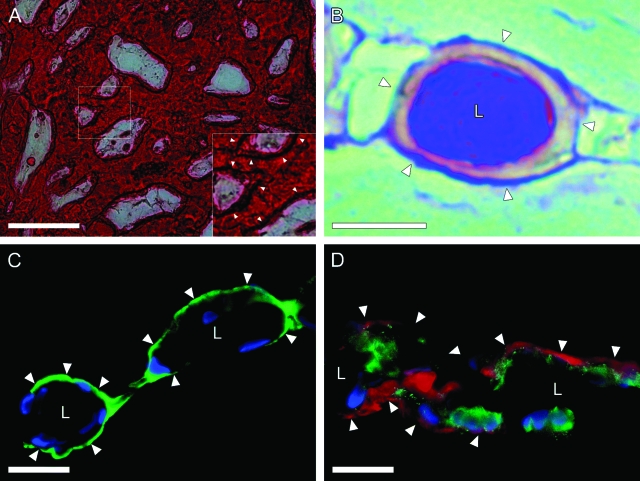
Polarity of LBDs was also examined by cytochemical staining. The LBD-forming cells exhibited strong γ-glutamyl transpeptidase activity, especially at their apical membranes (arrowheads, Figure 7A inset). PAS staining was performed to confirm the formation of basement membranes surrounding LBDs and the secretion of mucins into the ducts. PAS-positive materials existed surrounding LBDs, possibly representing a basement membrane (arrowheads, Figure 7B), and accumulated in the luminal space, which might be secretions (Figure 7B). The formation of basement membranes was also confirmed by immunofluorescent staining for laminin, one of the basement membrane matrices. A thin layer of laminin protein surrounded the LBDs (Figure 7, C–D). Type IV collagen was also observed around the LBDs (data not shown).
Figure 7.
Polarity of the LBDs. A: Positive histochemical reaction for γ-glutamyl transpeptidase activity in BECs cultured for 26 days. The inset is an enlarged image of the box in A. The luminal surface is intensively positive for γ-glutamyl transpeptidase activity (arrowheads, inset). B: PAS staining image of paraffin section of LBDs (red-violet color; arrowheads). Note that LBDs were surrounded by basement membranes. C: Localization of laminin in frozen section of LBDs. Double immunofluorescent staining was performed for laminin (green) and 4′,6-diamino-2-phenylindole (blue). D: localization of laminin Triple immunofluorescent staining was performed for CK19 (green), laminin (red), and 4′,6-diamino-2-phenylindole (blue). Note that laminin protein surrounds LBDs. L, lumen. Scale bars: 100 μm (A); 20 μm (B–D).
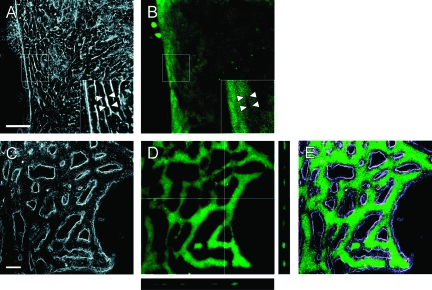
Secretory Function of SBDs and LBDs
When the BECs forming SBDs were treated with FD, the metabolized fluorescein was not secreted into SBDs but was detected only in the cytoplasm of the cells (Figure 8, A–B). The fluorescein was excreted into culture medium, and fluorescence in the cytoplasm gradually diminished. In contrast, when the cells forming LBDs were treated with FD, the metabolized fluorescein was secreted into the luminal space of LBDs and retained for at least 1 hour without leakage (Figure 8, C–E). The cross-section image of z-stacks revealed that LBDs were filled with the secreted fluorescein (Figure 8D). The merged fluorescent and phase-contrast images clearly showed that the fluorescein was excreted into LBDs (Figure 8E).
Figure 8.
Uptake and metabolization of FD by BECs forming SBDs and LBDs. A–B: Corresponding phase-contrast (A) and fluorescence (B) images of SBD-forming BECs. The insets are enlarged images of the box in A and B. The fluorescein was observed within the cytoplasms of BECs. No fluorescein was detected in intercellular spaces, which are shown as white areas under the phase-contrast image (arrowheads). C–E: Corresponding phase-contrast (C), fluorescence (D), and merged (E) images of LBD-forming BECs. The fluorescence image is projected by 44 planes taken at 0.89-μm intervals in z-axis. The right and lower images in D are the y-z axis and x-z axis sections along the lines, respectively. The merged image indicates that the fluorescein was secreted into LBDs, and accumulated without leakage. Scale bars: 50 μm (A and C).
QPCR Analysis
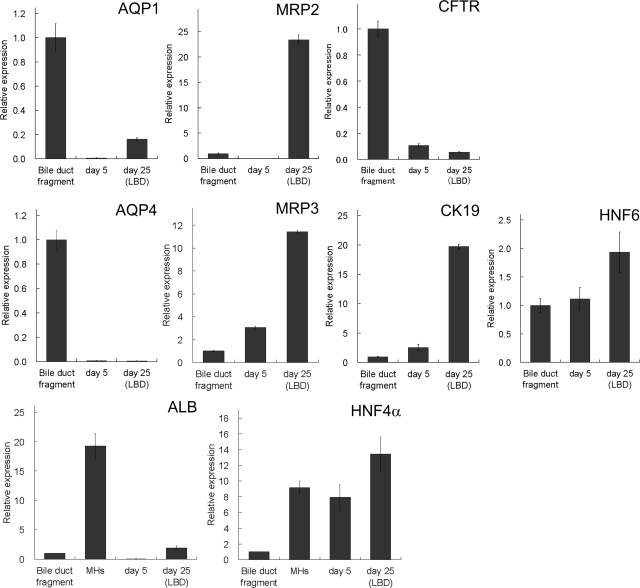
QPCR analysis was performed to examine mRNA expressions of LBD-forming BECs and the cells in monolayer before overlaying collagen gel (Figure 9). The LBD-forming BECs expressed higher levels of AQP1 and MRP2 mRNA than the cells in monolayer (day 5), whereas CFTR expression was low both in LBD-forming cells and in the monolayer cells, as compared with the expression in freshly isolated bile duct fragments. The basal domain marker MRP3 was also expressed higher in LBD-forming BECs than in the monolayer cells, while AQP4 expression decreased both in LBD-forming cells and the monolayer cells compared with the expression in freshly isolated bile duct fragments. Although both LBD-forming BECs and the monolayer cells expressed BEC markers such as CK19 and HNF6, they also expressed hepatocyte markers such as albumin and HNF4α.
Figure 9.
QPCR analysis for mRNA expressions of BEC and hepatocyte markers. The analysis was performed for apical (AQP1, MRP2, CFTR) and basal (AQP4, MRP3) domain markers of BECs, BEC markers (CK19, HNF6), and hepatocyte markers (albumin, HNF4α). The amount of mRNA expression was normalized with those of freshly isolated bile duct fragments. The amount of mRNA expressions of AQP1, MRP2, MRP3, CK19, and HNF6 increased in LBD-forming BECs [day 25 (LBD)] compared with that of the cells in monolayer (day 5), while that of CFTR and AQP4 diminished in culture. The LBD-forming BECs also expressed hepatocyte markers such as ALB and HNF4α. The mRNA expressions were also examined in freshly isolated mature hepatocytes (MHs) for ALB and HNF4α.
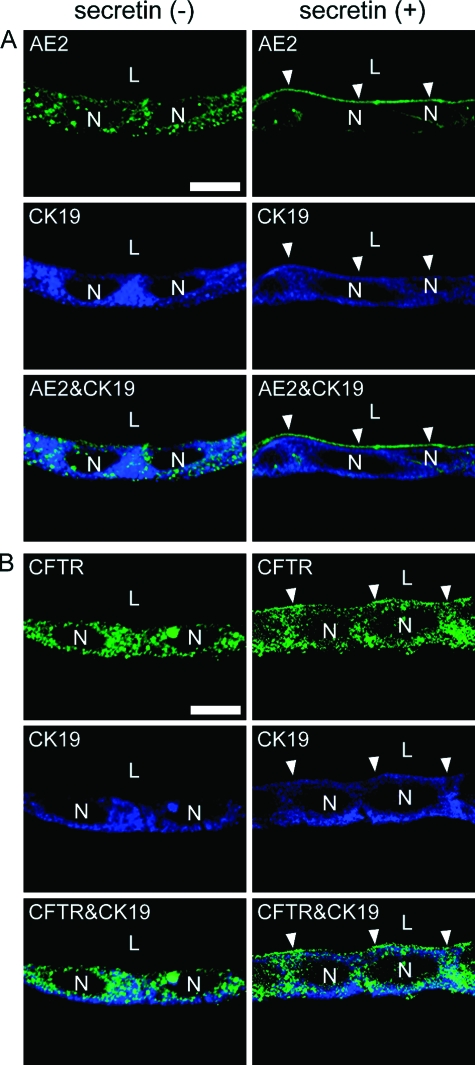
Secretin-Responsive Relocation of Apical Membrane Proteins, CFTR and AE2
Since BECs modify ductal bile flow, the cells have specialized transport systems located in the basolateral and apical membranes.18 We investigated such transport systems, focusing on apical membrane proteins. Immunofluorescent staining was performed to detect the localization of CFTR and AE2 proteins, which are a Cl− channel and a Cl−/HCO3− exchanger, respectively, localized to the apical membranes of BECs. Both CFTR and AE2 proteins were evenly distributed in the cytoplasm of BECs in the unstimulated state [secretin (−), Figure 10]. However, when BECs were treated with 10−5 M secretin for 30 minutes, AE2 intensively localized on the apical membranes of BECs [secretin (+), Figure 10A]. Although CFTR still localized in the cytoplasm of BECs after secretin treatment, some proteins also moved to the apical membranes [secretin (+), Figure 10B].
Figure 10.
Localization of AE2 (A) and CFTR (B) in LBDs in the absence (left) or presence (right) of secretin. BECs were treated with 10−5 M secretin at day 35 and double immunofluorescent staining was performed for AE2/CFTR (green) and CK19 (blue). The fluorescence distribution in the cells was obtained using a confocal laser-scanning microscope, and horizontal optical section images of LBDs are shown. When BECs were stimulated with secretin, both CFTR and AE2 were localized to the luminal surface of LBDs (arrowheads), whereas the proteins were evenly distributed in cytoplasm in the absence of secretin. L, lumen. N, nuclei. All images were taken at the same magnification. Scale bars: 10 μm.
Discussion
Stepwise Control for BEC Morphogenesis
In this study, we hypothesized that BEC morphogenesis could be achieved by the situational control of culture condition responding to the stage of BEC morphogenesis. Recent evidence suggests that temporal control of cellular microenvironments facilitates cellular morphogenesis. In our previous study, 3D hepatic tissues were successfully reconstructed by stacking two-dimensional tissues of small hepatocytes, hepatic progenitor cells,19 whereas the cells were unable to reconstruct well developed tissues when they were cultured directly in 3D configuration from the early stage of culture. On the other hand, Hui et al20 demonstrated that temporal control of cell–cell interaction followed by soluble signaling is important to maintain cellular functions. When hepatocytes had a contact-mediated signal in the early stage of culture, they could respond to paracrine signaling later, whereas the cells without the contact-mediated signal had no response to the paracrine signaling. These results suggest that sequential signaling in an organized manner is important for progression of cellular differentiation and morphogenesis.
In our culture system, BECs were cultured on collagen gel in the early stage of culture. This culture condition facilitated the proliferation of BECs. As shown in Figure 1, BECs actively migrated and proliferated to form large colonies; the major axis reached more than several hundred micrometers. The colony expansion was essential for constructing LBDs, because the size of the colony was related to LBD formation. The cells in small colonies took a longer time to form LBDs compared to those in the large colonies. LBDs developed well as the cells formed larger colonies before collagen-gel overlay. Growth factors such as epidermal growth factor and hepatocyte growth factor were important for colony expansion. The supplementation of both epidermal growth factor and hepatocyte growth factor dose-dependently enhanced the formation of large colonies (data not shown). In contrast, when cells were cultured without epidermal growth factor and hepatocyte growth factor, they did not spread on the collagen gel or form ductular structures. In addition, when the cells were overlaid with collagen gel from the beginning of the culture, no LBD could form (data not shown). Although BECs could also form colonies when they were cultured on a collagen-coated culture dish, the cells formed no SBD even if a collagen-gel was overlaid (data not shown). These results suggest that the initial expansion of BEC colonies on collagen gel is important for LBD formation.
The collagen-gel sandwich culture method is useful for making a 3D culture condition in which cells have a 3D contact with extracellular matrix. When hepatocytes were cultured between collagen-gel layers, the cells maintained a polygonal shape and differentiated function.21,22 An application of this 3D extracellular matrix condition to BEC culture was also effective. When BECs were cultured on collagen gel, the cells actively proliferated and their colonies rapidly expanded (Figure 1). However, once the cells were overlaid with collagen gel, the colony expansion was inhibited and the size of the BEC colonies was constant in the process of ductular formation (Figure 2). This suppression of colony expansion might initiate the SBD formation. When BECs were cultured without collagen-gel overlay, they formed no SBDs and diminished with time in culture (data not shown). The promotion of ductular morphogenesis by the collagen-gel sandwich configuration was also reported in the culture of various hepatic cells.9,23,24 The collagen-gel sandwich culture provides an environment that closely resembles the conditions normally experienced by the cells in vivo. Maintenance of 3D configuration seemed to facilitate BEC morphogenesis.
DMSO is known to stimulate the differentiation of various cells.11,25,26,27 However, DMSO had not been used for BEC cultures in previous studies. In the present experiment, DMSO clearly affected BEC morphogenesis in terms of LBD formation. Although the collagen-gel sandwich configuration induced SBD formation, BECs maintained the structure of SBDs and could not form LBDs without DMSO addition, even if they were cultured for more than a month (Figure 2E). However, the cells began forming LBDs after DMSO addition. These results suggest that the collagen-gel overlay progresses the BEC morphogenesis from monolayer to SBD formation, and that DMSO is important to progress the morphogenesis from SBD to LBD formation. Although the molecular basis of the effect of DMSO on cellular differentiation is still unclear, a recent molecular dynamics simulation suggested that DMSO increased fluidity of a cell membrane.28 The simulation showed that, as DMSO concentration increased, DMSO decreased the thickness of phospholipid membranes, increased fluidity of the membrane’s hydrophobic core, induced transient water pores, and finally destroyed the bilayer structure of membranes. The increase in membrane fluidity may facilitate migration and rearrangement of BECs to form LBDs, as well as localization of apical and basal membrane proteins to acquire polarity in our culture system. The formation of transient water pores suggested the permeation of solutes across cell membranes, which may enhance signals from the soluble factors in the culture medium. The various modes of action of DMSO may have important consequences for polarization and differentiation of cultured BECs through changing the localization and function of various transporters and channels at the apical surface of the cells.
DMSO also suppressed the proliferation of fibroblasts.29 In the present experiment, when the isolated cells were cultured without DMSO, fibroblastic cells intensively proliferated and finally disturbed the proliferation of BECs (data not shown). The interaction between BECs and fibroblastic cells is important in the stage of intrahepatic bile duct development.30 In our culture system, the fibroblastic cells seemed to stimulate the BEC morphogenesis. In fact, basement membrane formation was clearly observed between LBDs and the fibroblastic cells. Although the detailed mechanism is unknown, a certain signal from the cells in SBDs may stimulate the fibroblastic cells to produce extracellular matrix, resulting in the formation of basement membranes. This is consistent with the result of immunofluorescent staining for laminin: a layer of laminin protein surrounded LBDs (Figure 7, C–D). The PAS staining also allowed visualization of the basement membranes. Additional support for polarity of the cells forming LBDs was indicated by the localization of a strong γ-glutamyl transpeptidase activity at apical membranes of the cells. Therefore, LBDs are considered to be well differentiated ductular structures and are morphologically similar to bile ducts in vivo.
Reconstruction Process of Ductular Structures by BECs
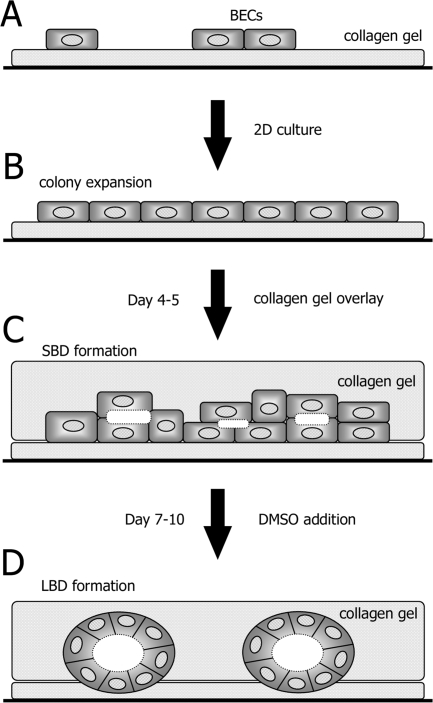
The process of ductular formation can be divided into two stages according to their structure and function. First, BECs formed SBDs between adjacent cells, which were induced by collagen-gel overlay. Second, BECs reconstructed LBDs, which were induced by DMSO addition. Changes in the corresponding phase-contrast images indicated that LBDs were constructed by the rearrangement of the cells in SBDs (Figure 2). BEC morphogenesis is summarized in Figure 11.
Figure 11.
Schematic diagram of BEC morphogenesis. A: BECs were seeded on collagen gel. B: Two-dimensional culture promoted expansion of BEC colonies. C: SBDs were constructed 4 to 5 days after collagen-gel overlay. D: LBDs were constructed 7 to 10 days after DMSO addition.
The morphology of ductular structures was apparently different between SBDs and LBDs. SBDs had relatively small lumens, which were 2 to 5 μm in diameter. The ductules consisted of only 2 to 3 cells, which showed few organella in their cytoplasms and sparse microvilli formed on apical membranes. Therefore, SBDs seem to be poorly differentiated structures, which may transiently appear in the process of BEC morphogenesis. In contrast, LBDs had relatively large lumens, which were 20 to 50 μm in diameter. A single layer of BECs consisted of 7 to 10 cells lining luminal surfaces. The cells possessed many organelles in their cytoplasm and numerous microvilli in apical membranes. In addition, tight junctions existed in the apical portion of the lateral membranes, which might generate the polarity of the cells. In fact, the nuclei were located at the basal side of the cells, and basement membranes were developed beneath the cells.
LBDs had continuous lumens, which are apparently different from the ductular structures reported in previous studies,6,9,24 although SBDs were similar to the ductular structures reported by Yang et al.5 In their studies, the network of the ductular structures was not clearly shown. In our culture system, on the other hand, LBDs could form anastomosing networks.
Functional Studies of LBD-Forming Cells
FD treatment clearly revealed the functional difference between SBDs and LBDs. LBD-forming cells metabolized FD and secreted the metabolized fluorescein into the luminal space, whereas SBD-forming cells retained fluorescein within their cytoplasm, which means that the cells in SBDs can metabolize FD but not secrete the metabolites. These results suggest that BECs in LBDs may acquire the sorting and secreting functions of the metabolites. The result of QPCR analysis that the MRP2 mRNA expressed in LBD-forming cells supports the acquisition of the function, because MRP2, one of ATP-binding cassette transporters in the apical membrane, plays an important role in the secretion of the metabolized fluorescein into bile canaliculi.31,32 The MRP3 mRNA was more highly expressed in LBD-forming cells than in monolayer cells. In the liver, MRP3 is mainly expressed in basolateral membranes of BECs and is thought to be involved in the cholehepatic circulation of bile salts by transporting them from BECs into circulating blood.33 Although the LBD networks were well developed in this culture system, the ends of the networks were closed. Therefore, the inner pressure of the lumen may increase with time in culture, especially when the cells in LBDs differentiate into mature cells and the cellular functions become active, which may result in the up-regulation of excreting transporters such as MRP2 and MRP3.
AQP1 is a water-selective channel protein that mediates physiological function of bile ducts.34 AQP1 localization was regulated by secretin, the choleretic hormone that induces AQP1 redistribution from intracellular to apical membranes of BECs.35 LBD-forming cells expressed the AQP1 mRNA 16-fold stronger than that of the cells in monolayer. Since the AQP1 mRNA expression was detected in bile duct fragments, BECs appeared to lose the expression within 5 days of culture in two-dimensional conditions. However, the expression was partially recovered when they formed LBDs, although the amount of the expression was less than that of bile duct fragments. The partial up-regulation of the gene expression may show that the cells forming LBDs are still maturing. BECs express another member of the AQP family proteins, AQP4, which is constitutively expressed on the basolateral membranes.16 The AQP4 mRNA expression was very low in the cells in LBDs, as was the CFTR mRNA. These results also suggest the partial maturation of the cells forming LBDs. Although the expression and the localization of AQP1 protein were not examined in this experiment, we performed immunofluorescent staining of the other apical membrane proteins, CFTR and AE2. Tietz et al36 proposed that these proteins localized to an intracellular vesicular compartment in BECs in the unstimulated state, but that they redistributed to the apical membrane responding to secretin stimulation. Our results are consistent with the phenomenon that showed the localization of CFTR and AE2 changed to the luminal surface of LBDs responding to secretin stimulation.
Interestingly, LBD-forming cells expressed not only BEC markers but also hepatocyte markers, such as albumin and HNF4α. The appearance of the cells that are positive for both BEC and hepatocyte markers have been reported in the process of liver regeneration.37,38 When thermoreversible gelatin polymer was applied to a small defect of a normal rat liver, hepatic stem cells appeared in biliary ductules near the injured area and differentiated into mature hepatocytes.38 BECs were isolated from the liver tissues surrounding the injured lesion and cultured for a long time. Although the features of the cells were initially very similar to terminal BECs, the cells showing hepatocytic morphology appeared when the cells were covered with the thermorevesible gelatin polymer. These results suggest that the phenotype of BECs and hepatocytes can be switched to each other in response to their microenvironment. In the present experiment, although we examined the expression of highly differentiated hepatocyte-marker genes, such as tyrosine aminotransferase and tryptophan-2, 3-dioxygenase, they were not detected by QPCR analysis (data not shown). The origin of the cells is probably mature BECs in bile ducts, but may have a potential of hepatic progenitor cells that are committed to differentiate into BECs. Recently, it was reported that the cell– extracellular matrix interaction is important for differentiation of hepatic progenitor cells.39 They demonstrated that the basement membrane proteins, especially laminin, were important for the hepatic progenitor cells to express the phenotype of differentiated BECs. In our culture system, an accumulation of laminin observed around LBDs may stimulate the differentiation of BECs. As BECs forming LBDs are still on the way to maturation, other factors may be necessary for their full maturation. Further investigations should be performed to clarify the mechanism of BEC morphogenesis.
In conclusion, we found that collagen gel configuration and DMSO stimulation are key morphogenic factors of bile duct formation. We also demonstrated that the situational control of the cellular microenvironment is important for constructing well developed bile duct networks, which have similar morphology to that of bile ducts in vivo. This culture model is useful for reconstructing hepatic tissues with bile drainage system in vitro, as well as for understanding mechanism of bile duct development and its disruption in disease.
Acknowledgments
We thank Mr. Hiroshi Kohara for valuable discussion and Mr. Philip Bransford for help with the manuscript.
Footnotes
Address reprint requests to Ryo Sudo, Department of Biological Engineering, Massachusetts Institute of Technology, 500 Technology Square, Building NE47-323, Cambridge, Massachusetts 02139. E-mail: rsudo@2000.jukuin.keio.ac.jp.
Supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology, Japan; Grant-in-Aid for Scientific Research on Priority Areas, Young Scientists (B); and for the 21st Century Center of Excellence for “System Design: Paradigm Shift from Intelligence to Life”; and Keio University Special Grant-in-Aid for Innovative Collaborative Research Projects.
References
- Griffith LG, Naughton G. Tissue engineering–current challenges and expanding opportunities. Science. 2002;295:1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- Allen JW, Hassanein T, Bhatia SN. Advances in bioartificial liver devices. Hepatology. 2001;34:447–455. doi: 10.1053/jhep.2001.26753. [DOI] [PubMed] [Google Scholar]
- Park JK, Lee DH. Bioartificial liver systems: current status and future perspective. J Biosci Bioeng. 2005;99:311–319. doi: 10.1263/jbb.99.311. [DOI] [PubMed] [Google Scholar]
- Strain AJ, Neuberger JM. A bioartificial liver—state of the art. Science. 2002;295:1005–1009. doi: 10.1126/science.1068660. [DOI] [PubMed] [Google Scholar]
- Yang L, Fabris RA, Hixson DC. Long-term culture and characteristics of normal rat liver bile duct epithelial cells. Gastroenterology. 1993;104:840–852. doi: 10.1016/0016-5085(93)91021-9. [DOI] [PubMed] [Google Scholar]
- Sirica AE, Gainey TW. A new rat bile ductular epithelial cell culture model characterized by the appearance of polarized bile ducts in vitro. Hepatology. 1997;26:537–549. doi: 10.1002/hep.510260302. [DOI] [PubMed] [Google Scholar]
- Sirica AE. Bile duct epithelial cell culture. Methods Mol Biol. 2002;188:37–52. doi: 10.1385/1-59259-185-X:37. [DOI] [PubMed] [Google Scholar]
- Auth MK, Okamoto M, Ishida Y, Keogh A, Auth SH, Gerlach J, Encke A, McMaster P, Strain AJ. Maintained function of primary human hepatocytes by cellular interactions in coculture: implications for liver support systems. Transpl Int. 1998;11:S439–S443. doi: 10.1007/s001470050516. [DOI] [PubMed] [Google Scholar]
- Auth MK, Joplin RE, Okamoto M, Ishida Y, McMaster P, Neuberger JM, Blaheta RA, Voit T, Strain AJ. Morphogenesis of primary human biliary epithelial cells: induction in high-density culture or by coculture with autologous human hepatocytes. Hepatology. 2001;33:519–529. doi: 10.1053/jhep.2001.22703. [DOI] [PubMed] [Google Scholar]
- Auth MK, Woitaschek D, Beste M, Schreiter T, Kim HS, Oppermann E, Joplin RE, Baumann U, Hilgard P, Nadalin S, Markus BH, Blaheta RA. Preservation of the synthetic and metabolic capacity of isolated human hepatocytes by coculture with human biliary epithelial cells. Liver Transpl. 2005;11:410–419. doi: 10.1002/lt.20367. [DOI] [PubMed] [Google Scholar]
- Isom HC, Secott T, Georgoff I, Woodworth C, Mummaw J. Maintenance of differentiated rat hepatocytes in primary culture. Proc Natl Acad Sci USA. 1985;82:3252–3256. doi: 10.1073/pnas.82.10.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Ruthenburg AM, Kim H, Fischbein JW, Hanker JS, Wasserkrug HL, Seligman AM. Histochemical and ultrastructural demonstration of γ-glutamyl transpeptidase activity. J Histochem Cytochem. 1969;17:517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Kirk KL. New paradigms of CFTR chloride channel regulation. Cell Mol Life Sci. 2000;57:623–634. doi: 10.1007/PL00000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli RA, Pham LD, Tietz PS, LaRusso NF. Expression of aquaporin-4 water channels in rat cholongiocytes. Hepatology. 2000;31:1313–1317. doi: 10.1053/jhep.2000.7986. [DOI] [PubMed] [Google Scholar]
- Kool M, van der Linden M, de Haas M, Scheffer GL, de Vree JM, Smith AJ, Jansen G, Peters GJ, Ponne N, Scheper RJ, Elferink RP, Baas F, Borst P. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc Natl Acad Sci USA. 1999;96:6914–6919. doi: 10.1073/pnas.96.12.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli RA, Tietz PS, Larusso NF. Regulated vesicle trafficking of membrane transporters in hepatic epithelia. J Hepatol. 2005;42:592–603. doi: 10.1016/j.jhep.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Sudo R, Mitaka T, Ikeda M, Tanishita K. Reconstruction of 3D stacked-up structures by rat small hepatocytes on microporous membranes. FASEB J. 2005;19:1695–1717. doi: 10.1096/fj.04-3269fje. [DOI] [PubMed] [Google Scholar]
- Hui EE, Bhatia SN. Micromechanical control of cell–cell interactions. Proc Natl Acad Sci USA. 2007;104:5722–5726. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989;3:174–177. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- LeCluyse EL, Audus KL, Hochman JH. Formation of extensive canalicular networks by rat hepatocytes cultured in collagen-sandwich configuration. Am J Physiol. 1994;266:C1764–C1774. doi: 10.1152/ajpcell.1994.266.6.C1764. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Tokusashi Y, Kadohama T, Nishimori H, Ogawa K. Hepatocytic cells form bile duct-like structures within a three-dimensional collagen gel matrix. Exp Cell Res. 1996;223:357–371. doi: 10.1006/excr.1996.0091. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Smith S, Wallace L, Sadamoto T, Okamoto M, Auth M, Strazzabosco M, Fabris L, Medina J, Prieto J, Strain AJ, Neuberger J, Joplin R. Ductular morphogenesis and functional polarization of normal human biliary epithelial cells in three-dimensional culture. J Hepatol. 2001;35:2–9. doi: 10.1016/s0168-8278(01)00078-2. [DOI] [PubMed] [Google Scholar]
- Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci USA. 1978;75:2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Tsao D, Siddiqui B, Whitehead JS, Arnstein P, Bennett J, Hicks J. Effects of sodium butyrate and dimethylsulfoxide on biochemical properties of human colon cancer cells. Cancer. 1980;45:1185–1192. doi: 10.1002/1097-0142(19800315)45:5+<1185::aid-cncr2820451324>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- McBurney MW, Jones–Villeneuve EM, Edwards MK, Anderson PJ. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature. 1982;299:165–167. doi: 10.1038/299165a0. [DOI] [PubMed] [Google Scholar]
- Gurtovenko AA, Anwar J. Modulating the structure and properties of cell membranes: the molecular mechanism of action of dimethyl sulfoxide. J Phys Chem B. 2007;111:10453–10460. doi: 10.1021/jp073113e. [DOI] [PubMed] [Google Scholar]
- Berliner DL, Rubmann AG. The influence of dimethyl sulfoxide on fibroblastic proliferation. Ann NY Acad Sci. 1967;141:159–164. doi: 10.1111/j.1749-6632.1967.tb34877.x. [DOI] [PubMed] [Google Scholar]
- Libbrecht L, Cassiman D, Desmet V, Roskams T. The correlation between portal myofibroblasts and development of intrahepatic bile ducts and arterial branches in human liver. Liver. 2002;22:252–258. doi: 10.1046/j.0106-9543.2002.01674.x. [DOI] [PubMed] [Google Scholar]
- Sudo R, Ikeda S, Sugimoto S, Harada K, Hirata K, Tanishita K, Mochizuki Y, Mitaka T. Bile canalicular formation in hepatic organoid reconstructed by rat small hepatocytes and nonparenchymal cells. J Cell Physiol. 2004;199:252–261. doi: 10.1002/jcp.10407. [DOI] [PubMed] [Google Scholar]
- Sudo R, Kohara H, Mitaka T, Ikeda M, Tanishita K. Coordinated movement of bile canalicular networks reconstructed by rat small hepatocytes. Ann Biomed Eng. 2005;33:696–708. doi: 10.1007/s10439-005-1690-5. [DOI] [PubMed] [Google Scholar]
- Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3). J Biol Chem. 2000;275:2905–2910. doi: 10.1074/jbc.275.4.2905. [DOI] [PubMed] [Google Scholar]
- Roberts SK, Yano M, Ueno Y, Pham L, Alpini G, Agre P, LaRusso NF. Cholangiocytes express the aquaporin CHIP and transport water via a channel-mediated mechanism. Proc Natl Acad Sci USA. 1994;91:13009–13013. doi: 10.1073/pnas.91.26.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli RA, Tietz PS, Pham LD, Rueckert L, Agre P, LaRusso NF. Secretin induces the apical insertion of aquaporin-1 water channels in rat cholangiocytes. Am J Physiol. 1999;276:G280–G286. doi: 10.1152/ajpgi.1999.276.1.G280. [DOI] [PubMed] [Google Scholar]
- Tietz PS, Marinelli RA, Chen XM, Huang B, Cohn J, Kole J, McNiven MA, Alper S, LaRusso NF. Agonist-induced coordinated trafficking of functionally related transport proteins for water and ions in cholangiocytes. J Biol Chem. 2003;278:20413–20419. doi: 10.1074/jbc.M302108200. [DOI] [PubMed] [Google Scholar]
- Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- Nagaya M, Kubota S, Suzuki N, Akashi K, Mitaka T. Thermoreversible gelation polymer induces the emergence of hepatic stem cells in the partially injured rat liver. Hepatology. 2006;43:1053–1062. doi: 10.1002/hep.21153. [DOI] [PubMed] [Google Scholar]
- Tanimizu N, Miyajima A, Mostov KE. Liver progenitor cells develop cholangiocyte-type epithelial polarity in three-dimensional culture. Mol Biol Cell. 2007;18:1472–1479. doi: 10.1091/mbc.E06-09-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]