Abstract
While overexpression of several aquaporins (AQPs) has been reported in different types of human cancer, the role of AQPs in carcinogenesis has not been clearly defined. Here, by immunochemistry, we have found expression of AQP5 protein in 62.8% (59/94) of resected colon cancer tissue samples as well as association of AQP5 with liver metastasis. We then demonstrated that overexpression of human AQP5 (hAQP5) induces cell proliferation in colon cancer cells. Overexpression of wild-type hAQP5 increased proliferation and phosphorylation of extracellular signal-regulated kinase-1/2 in HCT116 colon cancer cells whereas these phenomena in hAQP5 mutants (N185D and S156A) were diminished, indicating that both membrane association and serine/threonine phosphorylation of AQP5 are required for proper function. Interestingly, overexpression of AQP1 and AQP3 showed no differences in extracellular signal-regulated kinase-1/2 phosphorylation, suggesting that AQP5, unlike AQP1, may be involved in signal transduction. Moreover, hAQP5-overexpressing cells showed an increase in retinoblastoma protein phosphorylation through the formation of a nuclear complex with cyclin D1 and CDK4. Small interfering RNA analysis confirmed that hAQP5 activates the Ras signaling pathway. These data not only describe the induction of hAQP5 expression during colorectal carcinogenesis but also provide a molecular mechanism for colon cancer development through the interaction of hAQP5 with the Ras/extracellular signal-regulated kinase/retinoblastoma protein signaling pathway, identifying hAQP5 as a novel therapeutic target.
The aquaporins (AQP) represent a family of transmembrane water channel proteins widely distributed in various tissues throughout the body and play a major role in transcellular and transepithelial water movement.1,2 So far, since the initial discovery of AQP1,3,4 ten distinct AQPs have been characterized in humans and there has been an increasing understanding of their roles in human pathophysiology.2 However, only recently has the role of these various hAQPs in human carcinogenesis become an area of active research and, although some expression profile and preliminary functional evidences have been provided, confirmatory data on the role of hAQPs as one of the key elements directly involved in human carcinogenesis are still lacking.5
Expression of hAQP1 is frequently related with colon cancers, brain tumors, and microvessels of multiple myeloma paralleling angiogenesis.6,7,8 Likewise, expression of AQP5 was increased in pancreatic cancer, as was AQP3 in renal cell cancer.9,10 Also, we have previously reported the induction of AQP5 expression in the early colon cancer development.7
Saadoun et al reported that AQP1 deletion impairs tumor microvessel proliferation, which produces extensive tumor necrosis suggesting an indirect role of AQP1 as a key element involved in angiogenesis.11 However, in the functional level, AQP1 is shown to be involved in cell cycle control.7,12 Most recently, based on novel oncogenic properties of AQP1, increased levels of AQP1 were suggested to play a role in tumorigenesis.13 This study, reported from our group, did not examine the AQP1 signal transduction pathway leading to enhanced cell proliferation and, therefore, provided only preliminary evidences for the role of AQPs in human carcinogenesis.
Recently, our group found further evidence regarding the role of AQPs in human carcinogenesis, specifically AQP514; The ectopic expression of wild-type (WT) AQP5 induced many phenotypic changes characteristic of transformation both in vitro and in vivo. Cells of the mouse fibroblast cell line NIH3T3 stably expressing WT AQP5 were found to have an increased rate of cell proliferation and colony formation when compared to the controls. These results were confirmed in vivo using athymic mice models.13 Furthermore, the biochemical mechanism leading to transformation in AQP5-overexpressing NIH3T3 cells has been elucidated.15 The overexpression of AQP5 in NIH3T3 cells demonstrated a significant effect on Ras activity and, thus, cell proliferation. This influence was shown to be mediated by phosphorylation of the protein kinase A (PKA) consensus site in AQP5, which provided an association between AQP5 and the Ras signal transduction pathway.
Based on these observations in NIH3T3 cells, we have investigated the role of AQP5 in the development of colon cancer. We have initiated our study by examining the expression of AQP5 in colon cancer cell lines and multiple resected colon cancer tissue samples. Then we have studied the oncogenic property of AQP5 in colon cancer cell lines and further examined phosphorylation of extracellular signal-regulated kinase (ERK) and retinoblastoma protein (Rb).
Materials and Methods
Cell Culture
The human colon cancer cell line HCT116 was a gift from Dr. Bert Vogelstein (Johns Hopkins University, Baltimore, MD) and the other cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA). The human colon cancer cell lines: HCT116, DLD1 cell, RKO, and SW480 were cultured in McCoy’s medium. The human normal lung (BEAS2B) cells and the human lung cancer H838 cell line were cultured in F-12K (Ham’s F-12K Nutrient Mixture, Kaighn’s Mod.) and RPMI1640 medium, respectively. All medium (Gibco) were supplemented with 10% fetal bovine serum and antibiotics (Gibco) at 37°C in a humidified environment containing 5% CO2.
Tissue Microarray and Immunohistochemistry
Human rectal cancer tissue microarrays (TMAs) were constructed using paraffin-embedded tissues of 94 samples obtained from Asan Medical Center, Seoul Korea. The colon cancer samples were obtained from 1994 to 2002. Samples were randomly selected from colon cancer patients both with liver metastasis and without.
The immunostaining procedures were performed using the Benchmark automatic immunostaining device (Ventana Medical System, Tucson, AZ) with affinity-purified goat antibody raised against the 19-amino acid sequence (aa 251-269) of the COOH terminus of human AQP5 (α Diagnostic, San Antonio, Texas) at a 1:50 dilution. Tissue array sections (4 μm-thick) were deparaffinized in xylene, rehydrated in graded alcohols, and treated with 3% hydrogen peroxide in methanol at room temperature for blocking of endogenous peroxidase activity. The AQP5 antibody was visualized using the avidin-biotin-peroxidase technique (DAKO LSAB kit; DAKO Cytomation, Carpinteria, CA) and followed by chromogen detection with diaminobenzidine. Negative controls were performed by omitting the primary antibody incubation step.
Immunohistochemistry on the TMAs was graded semiquantitatively considering both staining intensity and percentage of positive tumor cells by study pathologists blinded to the clinicopathologic variables. The staining intensity was arbitrarily scored on a scale of four grades: 0, no staining of cancer cells; 1, weak staining; 2, moderate staining; 3, strong staining (Figure 1B, see below). The proportion of positive tumor cells was scored as following: 0, 0%; 0.1, 1% to 9%; 0.5, 10 to 49%; 1, 50% or more. AQP5 expression in the cancer tissue was defined as positive when the product of intensity score by percentage score is 2 or more. Both histological type and grade were confirmed on H&E-stained TMA slides.
Figure 1.
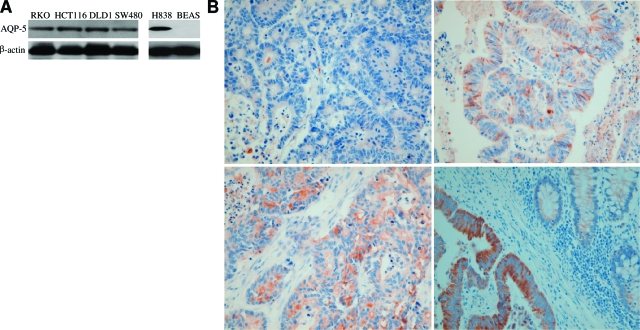
Expression profile of AQP5 in colon cancer cell lines and colon cancer tissues. A: Western blotting analysis using AQP5 antibody was performed in four colon cancer cell lines (RKO, HCR116 DLD1, and SW480). A non small cell lung cancer cell line (H838) and normal lung epithelial cell lines (BEAS) were loaded as positive and negative controls, respectively. All of the cell lines except BEAS expressed AQP5. The name of each cell line is marked on the top of each lane and β-actin was shown as the loading control. B: Immunohistochemistry was done on TMAs of colon cancer samples. Photomicrographs of AQP5 immunostaining are shown with the examples of score 0 (upper left), score 1 (upper right), score 2 (lower left), and score 3 (lower right). Note the absence of AQP5 expression in normal colonic mucosa (right part of lower right). Magnification = original ×400).
Small Interfering RNA Treatment, Drug Treatment, and Ras and ERK Activity Assays
Small interfering RNA (siRNA) against AQP5 was generated using the Silencer siRNA Construction Kit (Ambion) according to the manufacturer’s recommendations. For siRNA #1, the oligonucleotide sequences were 5′-AAAACTCTGCGAACACGGCCCCTGTCTC-3′ (sense) and 5′-AAGGCCGTGTTCGCAGAGTTCCTGTCTC-3′ (antisense). For siRNA #2, the oligonucleotide sequences were 5′-AAGAGCAGCCAGTGAAGTAGACCTGTCTC-3′ (sense) and 5′-AATCTACTTCACTGGCTGCTCCCTGTCTC-3′ (antisense). Control scrambled siRNAs were purchased from Santa Cruz. Cells were transfected with 75 nmol/L of siRNA using RNAi-Max transfection reagent (Invitrogen) in Opti-MEM I reduced serum medium (Invitrogen) and cultured at 37°C in a 5% CO2 atmosphere. The medium was removed and replaced with fresh culture medium supplemented with 10% fetal bovine serum. The growth of each cell line was measured by MTT assay in 2, 4, and 6 days after transfection (Invitrogen). Measurements were made in triplicate for each of the nonstable cell lines and the experiments were repeated three times.
Construction of hAQP5 cDNA Expression Construct and Mutants
Human AQP5 cDNA was amplified by PCR using primers and then inserted into the EcoR I and XhoI sites of pcDNA 3.1(+) (Invitrogen). Cloned genes were confirmed by restriction analysis and by DNA sequencing of both strands. Serine 156 or asparagine 185 are replaced with alanine or aspartic acid, respectively, by PCR-based site-directed mutagenesis (Invitrogen) to produce the S156A or N185D AQP5 mutant (Figure 2A, see below) based on the AQP5 pcDNA3.1 construct and verified by DNA sequencing of both strands of the mutants. Expression constructs were transfected into HCT116 cells with FuGENE 6 (Roche, Switzerland) according to manufacturer’s recommendations. Transfectants were selected with 2 mg/ml G418 (Cellgro, Herndon, VA) for HCT116, and selected clones were screened with Western blotting using AQP5 antibody and/or RT-PCR (Sense: 5′-AAGAAGGAGGTGTGTTCAGTTGCCTTCTTCA-3′ and antisense: 5′-GTGTGCCGTCAGCTCGATGGTCTTCTTCCG-3′). Two sets of stable cones and control clones were engineered to confirm the reproducibility of the experiments.
Figure 2.
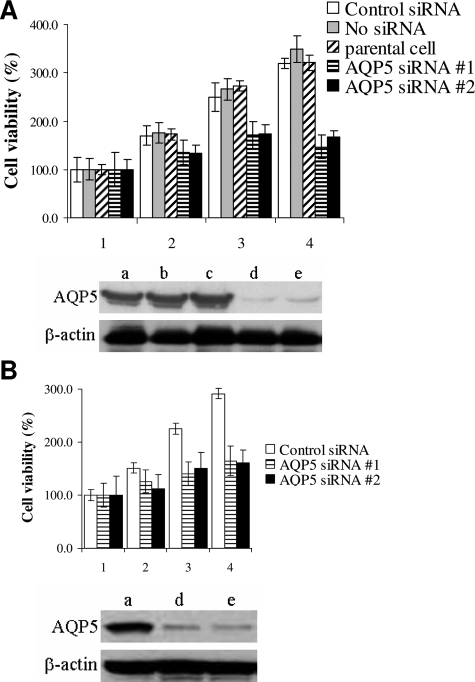
Loss of AQP5 is associated with decreased cell proliferation. The same cell numbers (5 × 105 cells) were plated onto 6-well plate and were transfected with AQP5 siRNA at 75 nmol/L (siRNA #1 and siRNA #2) with various controls. The MTT assay measured every 2 days after transfection (1: Before transfection, 2: 2 days, 3: 4 days, 4: 6 days after transfection). Western blotting analysis using AQP5 antibody was performed after 2 days transfection: (a) control siRNA, (b) no siRNA (reagent only), (c) parental cell, (d) AQP5 siRNA #1, and (e) AQP5 siRNA #2. A: Analysis of cell proliferation and AQP5 expression in HCT116 cells. B: Analysis of cell proliferation and AQP5 expression in DLD1 cells.
Nuclear and Cytosolic Protein Extraction and Immunoblotting
Nuclear proteins were extracted using Nuclear Extraction Kit as instructed by manufacturer (Sigma). For immunoblotting, lysates from cultured cells and tissues were prepared in ice-cold NP-40 lysis buffer (10 mmol/L Tris-Cl [pH 7.4], 137 mmol/L NaCl, 10% glycerol and 0.1% Nonidet P-40) containing an inhibitor cocktail of 10 mmol/L β-glycerol phosphate, 1 mmol/L phenylmethylsulfonyl fluoride, 10 mmol/L NaF, 10 mmol/L Na orthovanadate, 4.5 U/ml aprotinin (Sigma), and 1 μg/ml leupeptin (Sigma). Crude protein lysates (25 μg) were separated by 4 to 12% SDS-polyacrylamide gel electrophoresis (Invitrogen), transferred to nitrocellulose membranes (BIORAD), and blocked for 1 hour with 5% nonfat dry milk in Tris-buffered saline with 0.05% Tween 20. The following commercial antibodies were used for Western blot analysis: anti-AQP5 (1:200; α Diagnostic), anti-phospho-Rb (1:2500; Cell Signaling), and anti-Rb antibody (1:2500; Neomarkers), anti-phospho-ERK, and ERK antibody, anti-cyclin D1, and anti-CDK4 (1:2500; Santa Cruz Biotech), and mouse anti-β actin antibody (1:5000; Sigma). In immunoblotting, phospho-antibodies were applied first, followed by non-phospho-antibodies. Appropriate anti-rabbit and anti-mouse horseradish peroxidase-conjugated secondary antibodies (1:5000; Amersham) were used. Immunoreactive bands were detected by enhanced chemiluminescence (Pierce).
Immunoprecipitation
Samples were precleared with 50 μl of protein A-agarose beads at 4°C for 1 hour and clarified by centrifugation at 10, 000 rpm for 10 minutes. The precleared lysate (0.5 mg of total protein) was incubated with an antibody for 1 hour, then 50 μl of protein A-agarose beads was added, and the mixture was further incubated for 1 hour. After extensive washing with RIPA buffer, the immunoprecipitated proteins eluted from beads were subjected to 10% SDS-polyacrylamide gel electrophoresis under reducing condition.
Cell Proliferation Assay
For the proliferation assay, cell lines were grown in 6-well plates at a density of 1 × 104 cells per well. Measurements were made in triplicate for each of the cell lines and the experiments were repeated three times using MTT measurements (2, 4, and 6 days after transfection) and an additional time by counting cells with a hemacytometer (2 days after transfection).
Statistical Analysis
χ2 test was used to assess the association between AQP5 expression and demographic and clinicopathological variables. In addition, univariate and multivariate unconditional logistic regression analyses were performed to detect variables that affect liver metastasis status. All P values were derived from two-sided test and were considered to be statistically significant if less than 0.05. All statistical analyses were performed using STATA Statistical Software, 9.0 (Stata Corporation, College Station, TX).
Results
AQP5 Is Overexpressed in Colon Cancer Cell Lines and Colon Cancer Tissues
To confirm the previous finding that AQP5 mRNA is expressed in colon cancer,7 we examined the expression of AQP5 at its protein level by performing western blotting analysis in four colon cancer cell lines: RKO, HCT 116, DLD1, and SW480 (Figure 1A). In addition, while BEAS human normal bronchial epithelial cell line showed no expression of AQP5 (negative control), H838, one of the non small cell lung cancer cell lines, exhibited the expression of AQP5 (positive control). We also did immunohistochemistry on 94 human colon cancer TMAs (Figure 1B). The percentages of samples with immunostaining score of 0, 1, 2, and 3 were 37.2% (35/94), 31.9% (30/94), 14.9% (14/94), and 16.0% (15/94), respectively. Thus, 62.8% (59/94) of the tissue samples as well as four colon cancer cell lines expressed AQP5 protein. Among tissue samples with AQP5 expression, 49.2% (29/59) showed moderate to strong expression of AQP5 (score of 2 and 3).
siRNA Targeting AQP5 Inhibits Cell Proliferation in Colon Cancer Cell Lines
To explore the role of AQP5 in colon cancer, we first knocked down AQP5 expression in colon cancer cell lines. HCT116 and DLD1 cells, which clearly express AQP5 (Figure 1A), was transfected with AQP5 siRNAs (75 nmol/L siRNAs) with various controls. The MTT assay measured every two days and Western blotting analysis demonstrated that HCT116 cells transfected with AQP5 siRNA exhibit decreased cell proliferation compared to the controls as time dependent manners (Figure 3A). DLD1 cell lines also showed similar results (Figure 3B).
Figure 3.
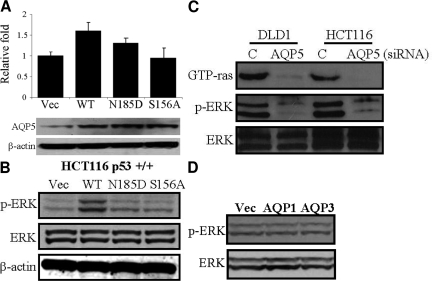
AQP5 induces cell proliferation by activating ERK phosphorylation. The HCT116 colon cancer cells were transfected with pCDNA vector and AQP5 WT, N185D, and S156A cDNAs as described in the materials and methods. A: Cell proliferation assay and AQP5 expression. The same cell numbers (5 × 105 cells) were plated onto a 6-well plate and incubated with complete media for 2 days, and the cell number was counted on the hemacytometer. Total proteins (25 μg) were performed by SDS-polyacrylamide gel electrophoresis (4 to 12% gradient gel) and transferred into nitrocellulose membrane. AQP5 expression level was confirmed by Western blotting. β-actin was loaded as controls. B: Activation of ERK by AQP5 overexpression. AQP5 WT showed the highest levels of cell proliferation and ERK phosphorylation. C: Inhibition of GTP-ras and ERK phosphorylation by AQP5 siRNA treatment. AQP5 siRNA #1 was treated into DLD1 and HCT116 colon cancer cell lines. D: Overexpression of AQP1 and AQP3 does not activate ERK1/2 phosphorylation.
hAQP5 Affects Cell Proliferation through Activating ERK Phsophorylation
To investigate how AQP5 overexpression affects human colon cancer cell line, AQP5 WT and its mutants pcDNAs (the site of second NPA motif [N185D] in the Loop E, and the other at the site of phosphorylation consensus sequences [S156A] in the Loop D) were transfected into HCT116 colon cancer cell line base on previous reports.1,3,15,16 WT and N185D transfected cells showed 1.6- and 1.3-fold increase in cell proliferation compared with vector transfected cells, respectively (Figure 2A). However, no increase in cell proliferation was observed in S156A transfected cells. A moderate (1.6-fold) difference in proliferation can be partly explained by the fact that HCT116 cell line, a well characterized human colon cancer cell line, already has a significant baseline expression of AQP5. However, the overexpression study results combined with the inhibition assay with siRNA altogether clearly demonstrate that the expression of AQP5 promotes cell proliferation. To examine whether AQP5 mediated cell proliferation might be involved in ERK phosphorylation, which is a central role of cell proliferation, ERK phosphorylation level was measured by western blotting using specific phosphor-ERK antibody. Interestingly, only WT transfected HCT116 cells showed an increase in ERK phosphorylation (Figure 2B). These findings suggest that cell proliferation and ERK phosphorylation by AQP5 may require both cAMP-protein kinase substrate phosphorylation (S156A) as well as membrane translocation of AQP5 (N185D). All of the above results with stable transfection were confirmed with another set of stable and control clones.
To verify these findings with siRNA experiment, we further knocked down AQP5 expression to see whether it affects ERK pathway. When AQP5 siRNA #1 was treated into both DLD1 and HCT116 colon cancer cell lines, siRNA treated cells demonstrated inhibition in both GTP-ras and ERK phosphorylation compared with mock treated cells, supporting our findings that AQP5 triggers cell proliferation through ERK pathway (Figure 2C). Cells treated with AQP5 siRNA #2 showed similar results (data not shown).
Finally, to examine whether ERK phosphorylation is a universal phenomenon with AQPs, the same HCT116 cell line was transfected with pcDNA vectors containing either AQP1 or AQP3 cDNA. Intriguingly, unlike AQP5-, AQP1-, and AQP3-transfected cells showed no increase in ERK1/2 phosphorylation (Figure 2D), suggesting that AQP1 and AQP3 may not be involved in signal transduction.
hAQP5 Increases Phosphorylation of Retinoblastoma Protein through Cyclin D1/CDK4 Complex
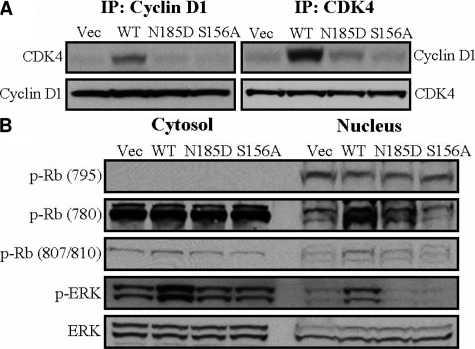
It is well known that extracellular growth signals are transduced through the Ras-MAPK pathway to the nucleus, where cyclin/CDK complexes phosphorylate retinoblastoma protein (Rb) and release E2F, causing transcription of genes that promote proliferation. Therefore, the complex formation between CDK4 and cyclin D1 is a crucial step for the Ras-MAPK signaling pathway.17,18 Accordingly, we performed immunoprecipitation and western blotting analysis in transfected cell lines using CDK4 and cyclin D1 antibodies. As expected, cells transfected with AQP5 WT showed an increase of the CDK4/cyclin D1 complex, whereas cells transfected with vector did not (Figure 4A, upper panel). However, overexpression of hAQP5 did not affect the expression levels of cyclin D1 and CDK4 (Figure 4A, lower panel).
Figure 4.
hAQP5 increases phosphorylation of retinoblastoma protein through cyclin D1/CDK4 complex. A: Overexpression of hAQP5 increases the complex of cyclin D1 and CDK4. Total proteins (0.5 mg) were immunoprecipitated and immunoblotted with each antibody as described in Materials and Methods. B: Overexpression of hAQP5 increases phosphorylation of retinoblastoma (Rb) protein. The transfected cells were fractionated into cytosol and nucleus and immunoblotted with phospo-Rb and Rb antibodies. ERK expression levels indicate equal amount of the protein samples loaded.
To further elucidate the downstream of Ras-MAPK pathway, the cells were fractionated into cytosolic and nuclear extracts using the Nuclear Extraction Kit (Sigma). AQP5 WT transfected cells revealed that phosphorylation of Rb at Serine 780 site was dramatically increased in the nuclear fraction. However, there was no difference in the phosphorylation of Rb in the cytosolic fraction (Figure 4B).
AQP5 Expression Is Associated with Lung Metastasis in Colon Cancer
Finally, we decide to find out whether AQP5 expression in colon cancer holds any clinical significance in our patient samples. Interestingly, with 94 TMAs randomly selected from colon cancer patients, we found that age and metastasis status were associated with AQP5 expression (χ2 test, P = 0.027, 0.006, respectively) (Table 1). No other demographic or clinopathologic variables including tumor size, differentiation, depth of invasion, and lymph node involvement were related with AQP5 expression (Table 1).
Table 1.
The Association between Clinicopathologic Characteristics of Colon Cancer Patients and AQP5 Expression
| Correlation | Aquaporin 5*
|
P value** | |
|---|---|---|---|
| − | + | ||
| Total (n = 94) | 65 (69.1%) | 29 (30.9%) | |
| Age (yr) | |||
| ≤30 | 15 (23.1%) | 1 (3.4%) | 0.027 |
| 31∼50 | 39 (60.0%) | 20 (69.0%) | |
| >51 | 11 (16.9%) | 8 (27.6%) | |
| Sex | |||
| Male | 38 (58.5%) | 17 (58.6%) | 0.988 |
| Female | 27 (41.5%) | 17 (41.4%) | |
| Size | |||
| <5 cm | 19 (29.2%) | 11 (37.9%) | 0.403 |
| ≥5 cm | 46 (70.8%) | 18 (62.1%) | |
| Differentiation | |||
| Well differentiated | 8 (12.3%) | 3 (10.3%) | 0.786 |
| Moderately differentiated | 54 (83.1%) | 24 (82.8%) | |
| Poorly differentiated | 3 (4.6%) | 2 (6.9%) | |
| Lymphovascular invasion | |||
| Absent | 41 (73.2%) | 19 (79.2%) | 0.573 |
| Present | 15 (26.8%) | 5 (20.8%) | |
| Lymph node metastasis | |||
| Absent | 32 (49.2%) | 10 (34.5%) | 0.184 |
| Present | 33 (50.8%) | 19 (65.5%) | |
| Liver metastasis | |||
| Absent | 48 (73.9%) | 13 (44.8%) | 0.006 |
| Present | 17 (26.2%) | 16 (55.2%) | |
AQP5 expression in the cancer tissue was defined as positive when the product of intensity score by percentage score is 2 or more.
P values were derived from chi-square test.
The proportion of positive AQP5 expression (defined as immunostaining intensity score of 2+ in Table 1) in colon cancer patients with liver metastasis (48.5%, 16/33) was 2.3 times higher than that in patients without metastasis (21.3%, 13/61). Likewise, the odds of having liver metastasis in patients with positive AQP5 expression was 3.46 times that in patients with negative AQP5 expression (P = 0.008, Table 2). Even when controlling for age and tumor size, the odds ratio of developing metastasis in patients with versus without positive AQP5 expression was as high as 3.21 (P = 0.017, Table 2).
Table 2.
The Association between Liver Metastasis and Clinicopathologic Factors in Colon Cancer Patients
| Odds ratio of developing liver metastasis (95% C.I.) | P value* | |
|---|---|---|
| Univariate analysis | ||
| Age (yr) | 1.05 (1.01–1.09) | 0.022 |
| Tumor size (cm) | 1.02 (0.78–1.33) | 0.897 |
| AQP5 expression (+ vs. −)** | 3.46 (1.39–8.70) | 0.008 |
| Multivariate analysis | ||
| Age (yr) | 1.04 (1.00–1.09) | 0.046 |
| Tumor size (cm) | 1.11 (0.83–1.49) | 0.463 |
| AQP5 expression (+ vs. −) | 3.21 (1.24–8.36) | 0.017 |
P values were derived from unconditional logistic regression analysis.
AQP5 expression in the cancer tissue was defined as positive when the product of intensity score by percentage score is 2 or more.
Discussion
We have previously demonstrated that overexpression of AQP5 in mouse fibroblasts, NIH3T3 cells, induces cell proliferation secondary to the activation of Ras, which is mediated by phosphorylation of AQP5 in its PKA consensus site (Ser 156).15 Although this study provided an association between AQP5 and the Ras signal transduction pathway, to define the role of AQP5 in human cancer, these results needed to be confirmed in human cancer cell lines. As we had previously reported an induced expression of AQP5 message in colon cancer tissue samples,7 we have decided to use colon cancer cells as a model to study the role of AQP5 with its related molecular pathways leading to enhanced proliferation. As a first step, we studied almost 100 cases of resected colorectal cancer tissue samples for the expression of AQP5 proteins. Then, we delineated the oncogenic properties of AQP5 in colon cancer cell line by overexpressing and blocking AQP5 message. Lastly, we have characterized some of its down stream molecular pathways.
From our initial report, by using in situ hybridization, all of 12 colon cancer tissue samples from five patients showed strong expression of AQP5 message in cancer cells with almost no staining in surrounding normal colonic mucosa. Consistent with these results, we have observed a major expression of AQP5 in cancer cells with almost no expression in normal colonic epithelium. Of note, in contrast to previous reports that showed 100% expression of AQP5 message, in our study, approximately 60% of tissues we had examined showed expression of AQP5 protein. At this point, although we do not have a clear idea about this discrepancy, we believe that the degree of AQP5 protein expression is still significant.
By using colon cancer cells carrying various AQP5 expression construct, we have identified that the oncogenic property of AQP5 is mediated by the activation of Ras, ERK, and Rb signaling pathway (Figure 2 and 4). This is the first report to demonstrate that human AQP5 affects Rb pathway secondary to the activation of Ras/ERK. The retinoblastoma (Rb) gene encodes a nuclear protein Rb, the phosphorylation of which plays a critical role in cell cycle regulation. On phosphorylation by CDKs, Rb releases E2F, which in turn transcribes genes required for DNA synthesis.18 Moreover, cAMP-induced proliferation involves both activation of PKA and phosphorylation of Rb.19 Our findings illustrate that overexpression of AQP5 induces extracellular growth signals that are transduced through the Ras-MAPK pathway to the nucleus, where cyclin/CDK complexes phosphorylate Rb and cause transcription of genes related with cell proliferation. As expected, the cell proliferation promoting property of AQP5 in colon cancer cells were further corroborated by siRNA experiments; Loss of AQP5 expression in colon cancer cells led to reduced cell proliferation and inhibition of GTP-ras and ERK phosphorylation (Figures 2D and 3). Of note, the overexpression of AQP1 and AQP3 in the colon cancer cells did not affect ERK phosphorylation (Figure 2E). It is interesting to note that AQP1 is not linked with ERK phosphorylation as this is consistent with our previous observation that the oncogenic property of AQP1 in NIH3T3 cells are not due to the activation of the ERK signal cascade, but because of its resistance to apoptosis.13 The role of AQP5 in apoptosis of cancer cells needs to be clarified.
Based on our findings from colon cancer cells, we have hypothesized that the expression level of AQP5 may provide a useful insight into the aggressiveness of colon cancer cells.7 Interestingly enough, our clinicopathologic correlation study showed a statistically significant association between the status of liver metastasis and level of AQP5 expression (Table 1 and 2). This can be partially explained by the fact that activated Ras/Raf/ERK pathway can induce epithelial-mesenchymal transition, which is one of the first steps in metastatic progression of cancer cells.20 We have recently reported that AQP5 triggers epithelial-mesenchymal transition in a normal human bronchial epithelial cell line (BEAS-2B).21
AQP5 expression is known to be regulated by cAMP through a PKA pathway.16,22,23 For example, cAMP treatment induced AQP5 expression in cultured mouse epithelial cells.24 It was also shown to have a biphasic effect on AQP5 expression.25 In contrast to these two reports, our group has recently demonstrated that membranous expression of AQP5 in human bronchial epithelial cells can occur irrespective of the PKA pathway26 and further suggested an existence of a novel, unidentified role of PKA pathways activated by AQP5.15 Our results with human colon cancer cells also indicate that both serine/threonine phosphorylation (S156A) and membrane translocation (N185D) of AQP5 play an important role in AQP5 mediated cell proliferation via Ras/ERK/Rb pathway (Figure 2 and 4) and clearly suggest a novel role of AQP5 in human pathophysiology.
Although it is still possible that activation of AQP5 may lead to an increase in its water permeability and thus increase the metabolic capacity of the cell, it appears to be unlikely as data in Xenopus oocytes suggest otherwise.27 Thus, activation of AQP5 via its PKA consensus site and subsequent activation of Ras/ERK pathway seems to be logical explanation for the oncogenic property of AQP5 in colon caner cells.
Presently, to explain how human AQP5 activates the Ras signaling pathway, while we have started to test the kinase activity of AQP5 itself, we recently identified several adaptor molecules including Grb2 that can bind to AQP5, thereby trigger Ras/ERK signal transduction pathway. For instance, AQP5 has a diproline peptide sequence (RTSPVGSP) in loop D,16,22,28 which has sequence similarity to the SH3 binding consensus site and it is suggested that AQP5 can directly interact with the SH3 domain of Grb228,29 (Lee et al, manuscript in prep). More importantly, as the interaction between AQP5 and its downstream pathways are dependent on phosphorylation of PKA consensus, clinical evidence in tissue samples from colon cancer patients demonstrating possible differences in the phosphorylation status of AQP5 as compared to normal tissue samples would demonstrate a unique opportunity in developing novel therapeutics targeting AQP5. In fact, a preliminary examination of the PKA consensus site in AQP5 in resected tissue samples from lung cancer patients has demonstrated that this site was phosphorylated in tumors but not in normal tissues.14 A more thorough examination of this phenomenon is warranted in colon cancer.
In humans, deficiencies in proper expression or localization of AQPs have also been associated with defects in water handling. However, the significance behind the aberrant expression of AQPs in human cancers has not been fully explored at its molecular level. Overall, our study, for the first time, not only describes an induced expression of AQP5 protein during colorectal carcinogenesis but also provides a molecular insight on how AQP5 protein expression can influence colon cancer development possibly through its interaction with the Ras/ERK/Rb signaling pathway. Currently, further animal studies are being designed to demonstrate the increased metastatic activity of colorectal cancer cells expressing WT AQP5 versus mock and other mutants. Although further detailed characterization of the molecular mechanism is warranted, our observations altogether suggest that hAQP5 may serve as a novel therapeutic target for the future treatment of colorectal cancer.
Footnotes
Address reprint requests to Chulso Moon, M.D., Ph.D., The Head and Neck Cancer Research Division, Department of Otolaryngology, The Johns Hopkins University, CRBII, 5M03, 1550 Orleans St. Baltimore MD 21231. E-mail: cmoon5@jhmi.edu; Se Jin Jang, M.D., Ph.D., Department of Thoracic Surgery, Asan Medical Center, College of Medicine, Ulsan University, Seoul, Korea. E-mail: jangsejin@amc.seoul.kr.
Supported in part by the SPORE grant P50 CA96784-01 (to C.M.), Fondation de France, AP-HP, and Lilly Fondation Grant (to J-C. S.), Cancer Research Grant from Pyung-Ya Foundation (to C.M.), and KOSEF research grant R01-2004-000-10670-0 and Korea Research Foundation grant KFR-2004-041-E00064 (to S.J.J).
S.K.K. and Y.K.C. contributed equally to this work.
References
- King LS, Agre P. Pathophysiology of the aquaporin water channels. Annu Rev Physiol. 1996;58:619. doi: 10.1146/annurev.ph.58.030196.003155. [DOI] [PubMed] [Google Scholar]
- Verkman AS, van Hoek AN, Ma T, Frigeri A, Skach WR, Mitra A, Tamarappoo BK, Farinas J. Water transport across mammalian cell membranes. Am J Physiol. 1996;270:C12–30. doi: 10.1152/ajpcell.1996.270.1.C12. [DOI] [PubMed] [Google Scholar]
- Preston GM, Smith BL, Zeidel ML, Moulds JJ, Agre P. Mutations in aquaporin-1 in phenotypically normal humans without functional CHIP water channels. Science. 1994;265:1585–1587. doi: 10.1126/science.7521540. [DOI] [PubMed] [Google Scholar]
- Moon C, Preston GM, Griffin CA, Jabs EW, Agre P. The human aquaporin-CHIP gene. Structure, organization, and chromosomal localization. J Biol Chem. 1993;268:15772–15778. [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Davies DC, Bell BA, Krishna S. Increased aquaporin 1 water channel expression in human brain tumours. Br J Cancer. 2002;87:621–623. doi: 10.1038/sj.bjc.6600512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C, Soria JC, Jang SJ, Lee J, Hoque MO, Sibony M, Trink B, Chang YS, Sidransky D, Mao L. Involvement of aquaporins in colorectal carcinogenesis. Oncogene. 2003;22:6699–6703. doi: 10.1038/sj.onc.1206762. [DOI] [PubMed] [Google Scholar]
- Vacca A, Frigeri A, Ribatti D, Nicchia GP, Nico B, Ria R, Svelto M, Dammacco F. Microvessel overexpression of aquaporin 1 parallels bone marrow angiogenesis in patients with active multiple myeloma. Br J Haematol. 2001;113:415–421. doi: 10.1046/j.1365-2141.2001.02738.x. [DOI] [PubMed] [Google Scholar]
- Kageyama Y, Sasaki S, Yamamura Y, Oshima H, Ikawa Y. Water channel protein subtype suggests the origin of renal cell carcinoma. J Urol. 1996;156:291–295. [PubMed] [Google Scholar]
- Burghardt B, Elkaer ML, Kwon TH, Racz GZ, Varga G, Steward MC, Nielsen S. Distribution of aquaporin water channels AQP1 and AQP5 in the ductal system of the human pancreas. Gut. 2003;52:1008–1016. doi: 10.1136/gut.52.7.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- Moon C, Williams JB, Preston GM, Copeland NG, Gilbert DJ, Nathans D, Jenkins NA, Agre P. The mouse aquaporin-1 gene. Genomics. 1995;30:354–357. doi: 10.1006/geno.1995.0029. [DOI] [PubMed] [Google Scholar]
- Hoque MO, Soria JC, Woo J, Lee T, Lee J, Jang SJ, Upadhyay S, Trink B, Monitto C, Desmaze C, Mao L, Sidransky D, Moon C. Aquaporin 1 is overexpressed in lung cancer and stimulates NIH-3T3 cell proliferation and anchorage-independent growth. Am J Pathol. 2001;168:1345–1353. doi: 10.2353/ajpath.2006.050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Lee J, Chae YK, Kim MS, Baek JH, Park JC, Park M, Smith IM, Trink B, Ratovitski E, Lee T, Park B, Jang SJ, Soria JC, Califano JA, Sidransky D, Moon C. Overexpression of AQP5, a putative oncogene, promotes cell growth and transformation. Cancer Lett. 2008;264:54–62. doi: 10.1016/j.canlet.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Lee J, Kim MS, Jang SL, Sidransky D, Moon C. The effect of aquaporin 5 overexpression on the Ras signaling pathway. Biochem Biophys Res Commun. 2008;367:291–298. doi: 10.1016/j.bbrc.2007.12.073. [DOI] [PubMed] [Google Scholar]
- Raina S, Preston GM, Guggino WB, Agre P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J Biol Chem. 1995;270:1908–1912. doi: 10.1074/jbc.270.4.1908. [DOI] [PubMed] [Google Scholar]
- Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- Mitra AP, Datar RH, Cote RJ. Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J Clin Oncol. 2006;24:5552–5564. doi: 10.1200/JCO.2006.08.2073. [DOI] [PubMed] [Google Scholar]
- Medina DL, Toro MJ, Santisteban P. Somatostatin interferes with thyrotropin-induced G1-S transition mediated by cAMP-dependent protein kinase and phosphatidylinositol 3-kinase. Involvement of RhoA and cyclin E x cyclin-dependent kinase 2 complexes. J Biol Chem. 2000;275:15549–15556. doi: 10.1074/jbc.275.20.15549. [DOI] [PubMed] [Google Scholar]
- Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39:305–318. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- Chae YK, Woo J, Kim M, Kang S, Kim MS, Lee J, Lee SK, Gong G, Kim YH, Soria JC, Jang SJ, Sidransky D, Moon C. Expression of aquaporin 5 (AQP5) promotes tumor invasion in human non small cell lung cancer. PLoS ONE. 2008;3:e2162. doi: 10.1371/journal.pone.0002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem. 1997;272:14800–14804. doi: 10.1074/jbc.272.23.14800. [DOI] [PubMed] [Google Scholar]
- Stork PJ, Schmitt JM. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2000;12:258–266. doi: 10.1016/s0962-8924(02)02294-8. [DOI] [PubMed] [Google Scholar]
- Yang F, Kawedia JD, Menon AG. Cyclic AMP regulates aquaporin 5 expression at both transcriptional and post-transcriptional levels through a protein kinase A pathway. J Biol Chem. 2005;278:32173–32180. doi: 10.1074/jbc.M305149200. [DOI] [PubMed] [Google Scholar]
- Sidhaye V, Hoffert JD, King LS. cAMP has distinct acute and chronic effects on aquaporin-5 in lung epithelial cells. J Biol Chem. 2004;280:3590–3596. doi: 10.1074/jbc.M411038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Chae YK, Jang SJ, Kim MS, Baek JH, Park JC, Trink B, Ratovitski E, Lee T, Park B, Park M, Kang JH, Soria JC, Lee J, Califano J, Sidransky D, Moon C. Membrane trafficking of AQP5 and cAMP dependent phosphorylation in bronchial epithelium. Biochem Biophys Res Commun. 2008;366:321–327. doi: 10.1016/j.bbrc.2007.11.078. [DOI] [PubMed] [Google Scholar]
- Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem. 1997;272:16140–6146. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]
- Mayer BJ. SH3 domains: complexity in moderation. J Cell Sci. 2001;114:1253–1263. doi: 10.1242/jcs.114.7.1253. [DOI] [PubMed] [Google Scholar]
- Kay BK, Williamson MP, Sudol M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000;14:231–241. [PubMed] [Google Scholar]