Abstract
It has been widely assumed that elevated CDK2 kinase activity plays a contributory role in tumorigenesis. We have previously shown that mice overexpressing CDK4 under control of the keratin 5 promoter (K5CDK4 mice) develop epidermal hyperplasia and increased susceptibility to squamous cell carcinomas. In this model, CDK4 overexpression results in increased CDK2 activity associated with the noncatalytic function of CDK4, sequestration of p21Cip1 and p27Kip1. Furthermore, we have shown that ablation of Cdk2 reduces Ras-Cdk4 tumorigenesis, suggesting that increased CDK2 activity plays an important role in Ras-mediated tumorigenesis. To investigate this hypothesis, we generated two transgenic mouse models of elevated CDK2 kinase activity, K5Cdk2 and K5Cdk4D158N mice. The D158N mutation blocks CDK4 kinase activity without interfering with its binding capability. CDK2 activation via overexpression of CDK4D158N, but not of CDK2, resulted in epidermal hyperplasia. We observed elevated levels of p21Cip1 in K5Cdk2, but not in K5Cdk4D158N, epidermis, suggesting that CDK2 overexpression elicits a p21Cip1 response to maintain keratinocyte homeostasis. Surprisingly, we found that neither CDK2 overexpression nor the indirect activation of CDK2 enhanced skin tumor development. Thus, although the indirect activation of CDK2 is sufficient to induce keratinocyte hyperproliferation, activation of CDK2 alone does not induce malignant progression in Ras-mediated tumorigenesis.
Normal cell growth and differentiation requires precise control of the mechanisms that govern the entry into, passage through, and exit from the cell cycle. Progress through the G1 phase of the mammalian cell cycle is mediated by D-type cyclins (D1, D2, and D3), which associate and activate CDK4 and CDK6 kinases, resulting in their catalytic activation and substrate recognition.1,2 CDK2 is considered a unique kinase that binds to cyclin E regulating S phase entry. The pRb family of proteins, pRb, p107, and p130, are key substrates for cyclin Ds/CDK4,6 and cyclin E/CDK2 complexes, that negatively regulate the passage of cells from G1 to S phase.2 The Cip/Kip family of CDK inhibitors, p21Cip1, p27Kip1, and p57Kip2, form inactive complexes with CDK2-cyclin E and CDK2-cyclin A and also bind CDK4,6/cyclin D complexes but do not interfere with their kinase activities.3,4 Thus, cyclin D-CDK4 sequester p27Kip1, controlling the amount available for inhibition of CDK2 activity. We and others have demonstrated that indirect activation of CDK2 occurs by sequestration of p27Kip1 on forced expression of CDK4.5,6,7 The role of CDK2 in cell proliferation has been supported by several founder reports in this field. A dominant-negative form of CDK2 prevents growth of cells in culture,8 and microinjection of antibodies against CDK2, cyclin E, or cyclin A block initiation of DNA synthesis in mammalian cells.9,10,11 However, in the last few years the concept that CDK2 is crucial for controlling entry into S phase was challenged when two independent groups reported the generation of Cdk2-null mice.12,13 These mice are viable, develop normally, and show defects in meiosis, but not in mitosis. Also, CDK2 appears to be dispensable for cell-cycle inhibition and tumor suppression mediated by p27Kip1 and p21Cip1.14 Although CDK2 kinase activity is found elevated in human tumors, CDK2 is not directly affected by mutations and only a small subset of human tumors have Cdk2 gene amplification or elevated protein expression.15,16,17 However, CDK2 regulatory binding partner, cyclin E, is frequently amplified and overexpressed in human tumors.18,19,20 Likewise, loss of CDK2 inhibitor p27Kip1 is a common event in human and experimental tumors. For these reasons, it has been widely assumed that elevated CDK2 activity plays a causal role in human tumor development. Coincidently, mouse models for CDK2 regulatory proteins, cyclin E and p27Kip1, are susceptible to tumor development.21,22,23,24,25,26 Similarly, forced expression of cyclin D1-CDK2 fusion protein under the control of MMTV promoter results in the development of spontaneous mammary tumors.27 However these mice exhibit a phenotype similar to MMTV-cyclin D1 mice and the consequences of CDK2 activation alone have not been investigated.28 Therefore, it remains unclear whether elevated CDK2 kinase activity per se is a cause or consequence of tumorigenesis.
Our previous studies demonstrated that induction of keratinocyte proliferation by forced expression of CDK4 is followed by CDK2 activation.7,29,30 We also demonstrated that ablation of Cdk4 results in the reduction of CDK2 activity in mouse epidermis because of redistribution of p21Cip1 and p27Kip1.30 More recently, we reported that ablation of Cdk2 in a K5Cdk4 transgenic (Tg) mice reduces tumor development and CDK4-mediated malignant progression to squamous cell carcinoma (SCC).31 These findings suggest that CDK2 kinase activity contributes to CDK4-mediated keratinocyte hyperproliferation, as well as the development and progression of mouse skin tumors. Therefore, we hypothesized that elevated CDK2 kinase activity would enhance normal keratinocyte proliferation and mouse skin carcinogenesis. To investigate this hypothesis, we generated two Tg mouse models of elevated CDK2 kinase activity, K5Cdk2 and K5Cdk4D158N. The bovine keratin 5 (K5) promoter drives the expression of Cdk2 or mutant Cdk4D158N to the basal cell layer of epidermis and other epithelial tissues. The D158N mutation blocks the kinase activity of CDK4 resulting in a kinase dead protein, although it retains the ability to bind D-type cyclins and p27/p21 allowing it to sequester these inhibitors and indirectly activate CDK2 (noncatalytic function of CDK4). Herein, we demonstrate that indirect activation of CDK2 through overexpression of CDK4D158N results in epidermal hyperplasia associated with increased keratinocyte proliferation. In contrast, the direct overexpression of CDK2 does not affect keratinocyte proliferation. Interestingly, immunoblot analysis revealed elevated levels of p21Cip1 in K5Cdk2 epidermis compared to wild-type (Wt) littermates, but not in K5Cdk4D158N epidermis. The latter suggests that overexpression of CDK2, but not its indirect activation, elicits a p21Cip1 response to maintain keratinocyte homeostasis. More importantly, we report that neither the direct overexpression nor the indirect activation of CDK2 increases the susceptibility to mouse skin tumor development or progression to SCC under a two-stage carcinogenesis protocol. Thus, we conclude that although the CDK4 sequestering activity is enough to induce keratinocyte hyperproliferation, concomitant activation of both CDK4 and CDK2 kinase activities are required to induce malignant phenotype. Furthermore, this work shows that Cdk2 does not behave as an oncogene in ras-mediated tumorigenesis.
Materials and Methods
Development of Tg Mice
K5Cdk2 and K5Cdk4D158N Tg mice were developed by cloning a cDNA containing the human-Cdk4 carrying the D158N mutation or human-Cdk2 into the vector pBK5, which contains the 5.2-kb bovine keratin 5 regulatory sequences, β-globin intron 2, and the 3′ SV40-polyadenylylation sequences. These constructs were designated as pK5Cdk4D158N and pK5Cdk2. The transgene was excised from the plasmid vector by digestion with BssHII and microinjected into the FVB strain, at Science Park Transgenic Mouse Facility, M.D. Anderson Cancer Center, Smithville, TX. Several founders for each Tg mouse (K5Cdk2 and K5Cdk4D158N) were obtained from the transgenic facility. Positive founders were genotyped by polymerase chain reaction (PCR) using specific primers for the human transgenes Cdk2 and Cdk4, although not annealed to the endogenous genes. Two Tg lines for each transgene were used in these studies.
Two-Stage Chemical Carcinogenesis
For the two-stage carcinogenesis, newborn mice were initiated at day 1 after birth with a single topical application of 50 μg of 7,12-dimethylbenz(a)anthracene (DMBA) in 50 μl of acetone on dorsal mouse skin. At day 21, mice received 2.5 μg of tumor-promoting agent in 200 μl of acetone twice a week for 25 weeks. Skin tumors were counted once a week until the end of the experiment at 40 weeks. Malignant progression to SCC was determined by macroscopic observation and further confirmed by histopathological analysis of paraffin-embedded hematoxylin and eosin (H&E)-stained cross sections. Twenty mice were used for each experimental group (K5Cdk4D158N, K5Cdk2, and the respective Wt mice littermates).
Western Blots and Kinase Assays
For immunoblots, protein lysates were collected from epidermal skin scrapes with RIPA lysis buffer, 150 mmol/L NaCl, 1.0% IGEPAL, 0.5% DOC, 0.1% sodium dodecyl sulfate, 50 mmol/L Tris (pH 8.0). For immunoblot analysis of skin tumors, papillomas were snap-frozen in liquid N2 and crushed with a pestle and mortar. Homogenates from epidermal scrapes or papillomas were sonicated and centrifuged at 14,000 rpm at 4°C. Supernatants were boiled in 2× Laemmli sample buffer for Western blot analysis or stored at −80°C. To assess CDK2 kinase activities, proteins were extracted and immunoprecipitated in Nonidet P-40 lysis buffer (Tris, pH 7.5, 150 mmol/L NaCl, 0.5% Nonidet P-40, 50 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L dithiothreitol, 1 mmol/L phenylmethyl sulfonyl fluoride). For CDK4 kinase activity proteins were extracted and immunoprecipitated with Tween 20 buffer (50 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L EDTA, 2.5 mmol/L EGTA, 10% glycerol, 0.1% Tween 20, 1 mmol/L NaF, 1 mmol/L Na3VO4, and 1 mmol/L dithiothreitol). Briefly, 250 μg of protein lysates were immunoprecipitated with 2.5 μg of antibodies against CDK2 (M-20) or CDK4 (C-22), (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 hours at 4°C, then incubated with 35 μl of protein A-agarose bead. Beads were washed twice each with IP buffer and once with kinase buffer (50 mmol/L HEPES, pH 7, 10 mmol/L MgCl2, 5 mmol/L MnCl2). Then, 30 μl of kinase buffer, 1 μg of pRb or histone H1 (Upstate Biotechnology Inc., Charlottesville, VA) substrate, 5 μCi of [γ-32P]ATP (6000 Ci/mmol), 1 mmol/L dithiothreitol, and 5 μmol/L ATP were added to the bead pellet and incubated for 30 minutes at 30°C. Sodium dodecyl sulfate sample buffer was added, and each sample was boiled for 3 minutes to stop reaction and electrophoresed through polyacrylamide gels. Western blot and kinase assay bands were quantified using UN-SCAN-IT, gel version 6.1 software for Windows (Silk Scientific, Inc., Orem, UT).
Immunostaining
Epithelial cell proliferation was measured by intraperitoneal injection of bromodeoxyuridine (BrdU) 30 minutes before the mice were sacrificed by CO2 asphyxiation. BrdU incorporation was detected by immunohistochemical staining of paraffin-embedded skin sections with mouse anti-BrdU (Ab-2) monoclonal antibody (Calbiochem, San Diego, CA), biotin-conjugated anti-mouse antibody (Vector Laboratories, Inc., Burlingame, CA) and an avidin-biotin-peroxidase kit (Vectastain Elite; Vector Laboratories, Inc.) with diaminobenzidine as chromogen. Apoptotic cells were determined by terminal deoxynucleotidyl transferase dUTP nick-end labeling assays as was previously described.32 The number of apoptosis-positive cells in the hair follicle was determined in sections of 200 μm2 with a reticule grid. In all cases, 10 to 12 fields were counted per section on a total of eight paraffin-embedded sections representing four mice per genotype.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 4 software (GraphPad Software, San Diego, CA).
Results
Generation of K5Cdk2 Tg Mice and Gross Analysis of Mouse Epidermis
Expression of human Cdk2 was targeted to the epidermis using the 5′ regulatory sequence of the keratin 5 (K5) gene, which drives the expression of transgenes to the basal cell layer of the stratified squamous epithelia.33 Two founder mice were used to establish lines K202 and K203 of K5Cdk2 Tg mice in an FVB/N background (Figure 1A). Immunohistochemical staining using antibodies against CDK2 of paraffin-embedded skin verified that exogenous CDK2 was expressed and restricted to the keratin 5-expressing basal cell layer of the epidermis (Figure 1B). The level of CDK2 overexpression was similar between both K202 and K203 Tg lines (data not shown). Thus, line K202 was used for all experiments described in this article. Western blot analysis verified that K5Cdk2 mice exhibit an 11.3-fold increase of CDK2 protein expression in epidermis (Figure 1C). Consistent with previous reports, K5 promoter also drives expression of transgene in thymus where CDK2 expression shows a fourfold increase compared to Wt littermates (Figure 1C).
Figure 1.
Development and screening of K5CDK2 Tg mice. A: PCR amplification of DNA extracted from mouse tails. Two mice showing integration of K5CDK2 plasmid DNA were chosen as founders of the two independent Tg lines. Purified plasmid DNA from pK5CDK2 (+) and empty K5 plasmids (Blnk) were used as positive and negative PCR controls. B: Immunohistochemical analysis for CDK2 shows elevated levels of CDK2 in basal cell layer of the epidermis of K5CDK2 Tg mice compared to Wt littermates. E, Epidermis; D, dermis. C: Western blot analysis of epidermis lysates (lanes 1 and 3) and thymus (lanes 2 and 4) of second generation K5CDK2 Tg (+) and Wt littermates shows elevated levels of CDK2 protein in K5-expressing tissues. Actin was used as loading control.
To determine the in vivo consequences of forced CDK2 expression we examined formalin-fixed paraffin-embedded skin cross sections. Microscopic examination of H&E sections from K5Cdk2 mice shows that epidermal and dermal morphology was indistinguishable from that of Wt littermates (Figure 2A). Further characterization of K5Cdk2 epidermis was performed by quantifying the number of nucleated cells in the epidermis, assaying in vivo keratinocyte proliferation via BrdU incorporation and determining the number of apoptotic cells by tunnel assay. Mild epidermal hyperplasia was observed in K5Cdk2 epidermis as showed by the increased number of nucleated cells in the interfollicular epidermis (Figure 2B, P < 0.05). However, the level of proliferation (BrdU labeling index) was similar between K5Cdk2 and Wt littermates (Figure 2B). To verify the effect of CDK2 overexpression on the survival rate of keratinocytes, we assessed the level of apoptosis in follicular and interfollicular epidermis from K5Cdk2 Tg mice. An increased number of follicular apoptotic cells was found in K5Cdk2 mice in comparison to Wt mice (0.0176 and 0.0043, respectively; P < 0.05) (Figure 2B). On the other hand, no differences were observed in interfollicular apoptosis between K5Cdk2 and Wt littermates (data not shown). These results suggest that overexpression of CDK2 does not affect the rate of normal keratinocyte proliferation, although it may compromise the survival rate of follicular keratinocytes.
Figure 2.
Skin phenotype of K5CDK2 Tg mice. A: Representative paraffin sections of skin from K5CDK2 Tg mice and normal littermates (Wt) stained with H&E. Arrow denotes epidermis (E). D, Dermis. B: Quantification of the number of nucleated cells per 200 μm of interfollicular epidermis in K5CDK2 and Wt mice on H&E-stained cross sections. In vivo proliferation assay using BrdU incorporation (labeling index) in the basal cell layer of mouse epidermis and quantification of the number of apoptotic cells (apoptotic labeling index) in the hair follicle of K5CDK2 and Wt mice using terminal deoxynucleotidyl transferase dUTP nick-end labeling assay. Four mice for each genotype were used to determine the BrdU labeling index, number of nucleated cells, and apoptosis.
Biochemical Analysis of K5Cdk2 Mouse Epidermis
As aforementioned, epidermal lysates from K5Cdk2 mice show an 11.3-fold increase of CDK2 protein expression in mouse epidermis in comparison to Wt littermates (Figures 1C and 3A). To study whether forced expression of CDK2 affects related cell-cycle regulators we assessed the protein levels of CDKs, CKIs (cyclin-dependent kinase inhibitors), and cyclins. In general, no changes in protein expression were observed for CDK4 and CDK6 nor their cyclin binding partner, cyclin D1. Likewise, cyclins A and cyclin E, although they bind to CDK2, were unaltered (Figure 3A). Importantly, p21Cip1 protein expression increased fivefold in K5Cdk2 epidermis in comparison to Wt mice, whereas, p27Kip1 levels appear unchanged (Figure 3A). Several reports have shown that p53/p21 form an inducible barrier that protect cells against cyclin E deregulation34,35 and co-transfection of cyclin E and CDK2 in culture results in the transactivation of p53 signaling.36 Thus, it is possible that elevated CDK2 protein levels in mouse epidermis mimics the action of elevated cyclin E inducing a p53/p21 response and partially blocking CDK2 activity. Nevertheless, we did not observe increased p53 levels or p53 activation (phospho-p53 Ser 15) in K5Cdk2 compared with Wt epidermis (Figure 3A). In addition, we could not detect any accumulation of Mdm2, a p53 regulated gene, in K5Cdk2 epidermis (data not-shown). However, these data cannot rule out the possibility that p53 is involved in p21Cip1 induction. To determine the effect of elevated p21Cip1 levels on CDK2 kinase activity, we performed in vitro kinase assays using histone H1 as substrate. Consistent with the elevated expression of CDK2, CDK2-kinase activity increased 1.7-fold in Tg mice compared to Wt littermates (Figure 3B). To determine whether elevated p21Cip1 levels bind to transgenic CDK2, we immunoprecipitated CDK2 and immunoblotted for p21Cip1 (Figure 3C). We found that elevated levels of p21Cip1 bind to CDK2 and likely results in a partial attenuation of CDK2 activity in K5Cdk2 Tg mice. In addition, we immunoprecipitated cyclin E and immunoblotted for CDK2, which shows that transgenic CDK2 binds to endogenous cyclin E (Figure 3C). After prolonged exposure of the membrane we also observed complexes between endogenous CDK2 and cyclin E, p21Cip1 in Wt samples (data not shown). It is widely accepted that p27Kip1 and p21Cip1 act as assembly factors for CDK4/D-type cyclin complexes. Thus, we reasoned that the increase CDK2/p21 binding reduce the amount of p21Cip1 available to bind CDK4 complexes decreasing CDK4 kinase activity. However, CDK4 kinase activity was not altered in keratinocytes from K5Cdk2 Tg mice, showing comparable kinase activity against pRb substrate as in Wt keratinocytes (Figure 3B). Altogether these results suggest that elevated p21Cip1 levels may act as a compensatory mechanism in response to the forced expression of CDK2 to maintain keratinocyte homeostasis. However, p21Cip1 levels do not completely inhibit transgenic CDK2 activity, which remains 1.7-fold higher than Wt mice.
Figure 3.
Cell-cycle regulatory protein expression, CDK kinase activities, and complex formation in K5CDK2 epidermis. A: Epidermal lysates from Wt and K5CDK2 mice were probed with antibodies for CDKs, cyclins, CKIs, total and ser15-phopho-p53, pRb, and actin as loading control. B: CDK in vitro kinase assays for CDK2 and CDK4 using H1 and pRb peptides as substrates. Epidermal lysate from K5CDK2 (Tg) and Wt littermates were immunoprecipitated with antibodies against CDK2 (IP CDK2), CDK4 (IP CDK4), or normal rabbit IgG (Cont.) and incubated with respective substrates, H1 or pRb. C: Fresh epidermal lysates from K5CDK2 Tg mice and Wt littermates were immunoprecipitated with polyclonal antibodies against cyclin E (IP Cyc E) and CDK2 (IP-CDK2) and immunoblotted with polyclonal antibody for CDK2 (IB-CDK2) and p21Cip1 (IB-p21).
Generation of K5Cdk4D158N Tg Mice and Gross Analysis of Mouse Epidermis
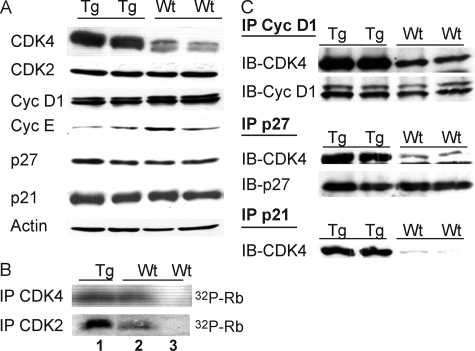
Our previous studies showed that CDK4 overexpression leads to increased epidermal proliferation and tumorigenesis attributable in part to the increased kinase activity of CDK2.7,29,30 However, CDK2 levels have not been found elevated in human tumors. To gain a better understanding of the role of elevated CDK2 activity mediated by overexpression of CDK4 in epidermal proliferation and tumorigenesis, we generated K5Cdk4D158N Tg mice. CDK4D158N is a kinase inactive protein that does not phosphorylate pRb, but retains the ability to bind and sequester p21/p27 leading to indirect activation of CDK2. Biochemical analysis of mouse epidermis confirmed overexpression of CDK4D158N in Tg mouse epidermis (Figure 4A). We examined the protein levels of CDK2, cyclin D1, p21Cip1, and p27Kip1, which showed similar levels of expression compared to Wt littermates (Figure 4A). To determine the functional consequences of CDK4D158N expression we conducted in vitro kinase assays for CDK4 and CDK2 in K5Cdk4D158N and Wt epidermal lysates. CDK4 kinase assay confirmed that CDK4D158N is kinase-inactive because K5Cdk4D158N keratinocytes have similar activity toward pRb as Wt keratinocytes (Figure 4B). In contrast, overexpression of CDK4D158N resulted in a fivefold induction of CDK2 kinase activity (Figure 4B). The latter is consistent with previous data in which overexpression of Wt CDK4 results in an induction of CDK2 kinase activity.7 Interestingly, cyclin E protein levels decreased 2.2-fold in K5Cdk4D158N compared to Wt mice (Figure 4A). Although the mechanism involved in cyclin E reduction is unknown, it is clear that the CDK2 kinase activity was not affected for this mild reduction (Figure 4B). To determine the mechanism of increased CDK2 activity in K5Cdk4D158N mice we pulled down CDK4 and assessed the amount of complex formation with p21Cip1, p27Kip1, and cyclin D1. We found increased complex formation between CDK4D158N/cyclin D1, CDK4D158N/p27Kip1, and CDK4D158N/p21Cip1 in Tg keratinocytes compared to Wt keratinocytes (Figure 4C).
Figure 4.
Cell-cycle regulatory protein expression, CDK kinase activities, and complex formation in K5CDK4D158N epidermis. A: Epidermal lysates from Wt and K5CDK4D158N (Tg) mice were probed with antibodies for CDK4, CDK2, cyclin D1, cyclin E, p27Kip1, p21Cip1, and actin as loading control. B: CDK2 and CDK4 in vitro kinase assays using pRb peptide as substrates. Epidermal lysates from K5CDK4D158N (Tg) and Wt littermates were immunoprecipitated with antibodies against CDK4, CDK2, or normal rabbit IgG (lane 3) and incubated with pRb peptides. C: Fresh epidermal lysates from K5CDK4D158N Tg mice and Wt littermates were immunoprecipitated with polyclonal antibodies against cyclin D1(IP Cyc D1), p27Kip1 (IP p27), and p21Cip1 (IP p21) and immunoblotted with polyclonal antibody for CDK4 (IB-CDK4), cyclin D1 (IB-Cyc D1), and p27Kip1 (IB-p27).
Histopathological analysis of paraffin-embedded skin cross-sections showed hyperplastic epidermis in K5Cdk4D158N in comparison to epidermis of Wt mice (Figure 5A). In fact, quantification of the number of nucleated cells per 200 μm of interfollicular epidermis revealed an elevated number of nucleated cells in K5Cdk4D158N compared to normal littermates (87.5 and 74.6 cells/200 μm, respectively; P < 0.05) (Figure 5B). These results evidence a clear difference between CDK2 overexpression (K5Cdk2 mice) and the indirect activation of CDK2 (K5Cdk4D158N). Consistent with the observed hyperplastic phenotype, in vivo proliferation assays demonstrated that K5Cdk4D158N have a fourfold increase in the number of proliferative keratinocytes (BrdU-positive cells) in comparison to Wt littermates (P < 0.05) (Figure 5B). These data highlights that the indirect activation of CDK2 kinase activity, but not CDK2 overexpression, induces keratinocyte proliferation.7 As mentioned, K5Cdk4D158N epidermal lysates do not display increased p21Cip1 protein levels (Figure 4A), as observed in K5Cdk2 epidermis, suggesting that elevated CDK2 protein overexpression, but not the indirect activation of CDK2 kinase activity, elicits the induction of p21Cip1.
Figure 5.
Skin phenotype of K5CDK4D158N Tg mice. A: Representative paraffin sections of skin from K5CDK4D158N Tg mice and normal littermates (Wt) stained with H&E. B: Quantification of the number of nucleated cells per 200 μm of interfollicular epidermis in K5CDK4D158N and Wt mice on H&E-stained cross sections. In vivo proliferation assay using BrdU incorporation (labeling index) in the basal cell layer of mouse epidermis. Four mice for each genotype were used to determine the labeling index and the number of nucleated cells in the basal cell layer of the epidermis.
Elevated CDK2 Kinase Activity Does Not Enhance Mouse Skin Carcinogenesis
We have previously observed that forced expression of CDK4 (K5Cdk4 mice) results in increased CDK4/p21 and CDK4/p27 complexes and increased CDK2 kinase activity.7,29,30 Supporting a role for CDK4 in malignant progression, K5Cdk4 mice showed increased progression to SCC in a two-stage carcinogenesis protocol.29 In addition, we recently demonstrated that ablation of Cdk2 in K5Cdk4 Tg mice reduces the total number of chemically induced skin tumors.31 These results indicate that indirect activation of CDK2 plays an important role in skin tumor development and malignant progression. Therefore, we hypothesize that an increase in CDK2 kinase activity, whether induced directly or indirectly, can elicit a malignant skin tumor phenotype. To test this hypothesis, K5Cdk2 and K5Cdk4D158N Tg mice were subjected to a two-stage chemical carcinogenesis protocol. This protocol consists of a single application of a subcarcinogenic dose of DMBA followed with bi-weekly applications of a tumor-promoting agent, phorbol-12-myristate-13-acetate. DMBA initiation induces Ha-ras mutations, most commonly at codon 61. Tumor-promoting agent application then causes the clonal expansion of initiated cells, which results in skin papilloma development.37 Tumors were counted once a week to determine the kinetics of papilloma formation and were monitored for progression to SCC. Papilloma formation in K5Cdk2 and Wt mice began at 8 weeks of tumor-promoting agent promotion and reached a plateau at 28 weeks (Figure 6A). However, contrary to our hypothesis, increased levels of CDK2 did not enhance tumor formation. In fact, K5Cdk2 Tg mice developed a lower number of papillomas per mouse (multiplicity) in comparison to Wt mice (7 and 10.5, respectively, at 30 weeks) (Figure 6A). The incidence of papilloma formation was similar for both K5Cdk2 and Wt mice (100% and 95%, respectively) (Figure 6B). In addition, K5Cdk2 and Wt papillomas progressed to SCC at a similar frequency, 25% and 28% at 40 weeks, respectively (data not shown). Histopathological analysis also shows no significant differences in the degree of differentiation or tumor grade between K5Cdk2 and Wt papillomas (data not shown). Biochemical analysis of K5Cdk2 papillomas shows that CDK2 protein levels remain elevated, on average 8.4-fold, compared to Wt papillomas (Figure 6C). In contrast to epidermal lysates from normal K5Cdk2 untreated skin, immunoblot analysis of papilloma extracts did not reveal any persistent increase in p21Cip1 protein levels (Figure 6C). CDK2 in vitro kinase assays showed a 3.4-fold increase in kinase activity in papillomas from Tg mice compared to Wt papillomas whereas CDK4 kinase activity was similar in Wt and K5Cdk2 papillomas (Figure 6D). Analysis of other cell-cycle regulators such as CDK4, cyclin D1, cyclin A, and p15Ink4b does not show differential expression between K5Cdk2 and Wt tumors (Figure 6C). Thus, even with increased CDK2 protein levels and kinase activity, K5Cdk2 Tg mice do not exhibit an increased susceptibility to ras-mediated tumorigenesis.
Figure 6.
Kinetics of papilloma development and biochemical analysis of K5CDK2 tumors. A: Average number of papillomas per mouse (multiplicity) as a function of weeks of study. B: Percentage of mice with at least one papilloma as a function of weeks of study (incidence). K5CDK2 (▴) and Wt (▪). Twenty mice were used for each experimental group. C: Immunoblot analysis of Wt (−) and K5CDK2 (+) papilloma lysates developed with antibodies against CDK2, CDK4, cyclin D1, cyclin A, p21Cip1, and p15Ink4b. D: In vitro kinase assays for CDK2 and CDK4 in mouse skin papillomas. Papilloma lysates from K5CDK2 (+) and Wt littermates (−) were immunoprecipitated with antibodies against CDK2 (IP CDK2), CDK4 (IP CDK4), or normal rabbit IgG (control) and incubated with histone H1 (H1) or pRb peptides (Rb) as substrates.
To determine whether activation of CDK2, independent of increased CDK2 protein expression, alters the susceptibility to tumor development or malignant progression, we also subjected K5Cdk4D158N mice to a two-stage chemical carcinogenesis protocol. Similar to our observations in K5Cdk2 Tg mice, expression of CDK4D158N results in decreased number of papillomas per mouse compared to Wt littermates (11.5 and 16.5, respectively, at 30 weeks of promotion) (Figure 7A). The incidence of papilloma formation was the same for both K5Cdk4D158N and Wt mice (100%) (Figure 7B). Moreover, papillomas from K5Cdk4D158N progressed to SCCs at a similar rate in comparison to those derived from Wt littermates (data not shown).
Figure 7.
Kinetics of K5CDK4D158N papilloma development. A: Average number of papillomas per mouse (multiplicity) as a function of weeks of study. B: Percentage of mice with at least one papilloma as a function of weeks of study (incidence). K5CDK4D158N (▴) and Wt (▪). Twenty mice were used for each experimental group.
Discussion
For more than 2 decades, the pRb/p16/CDK/cyclin pathway has been implicated in tumorigenesis. Specifically, CDK4 is frequently found amplified and/or overexpressed in human tumors.38,39,40,41,42 In addition, a germline mutation in the p16Ink4a binding domain of CDK4 (R24C) is associated with familial melanoma.43 In contrast, much less is known about the role of CDK2 in tumor development. Although CDK2 activity is frequently found elevated in human tumors, it has not been found directly mutated and is rarely overexpressed or genetically amplified in human tumors. However, amplification and altered expression of CDK2 binding partner, cyclin E, is encountered in human tumors and its expression serves as good prognostic indicator in some tumor types.26 Additionally, proteolytic processing of cyclin E into low molecular weight isoforms is a frequent event in breast tumors.25 In addition, these low molecular weight isoforms of cyclin E have been found to be refractory to Cip/Kip inhibitors resulting in the activation of CDK2 kinase activity.44 It has also been suggested that overexpression of CDK4 may contribute to tumor formation via the sequestration of p27/p21 from CDK2-cyclin E.45,46 Thus, it was widely accepted that elevated CDK2 kinase activity is a contributing factor in human tumors. Contrary to this idea, it has recently been shown that CDK2 activity is dispensable for cell-cycle progression in some cancer cell lines.47 Furthermore, CDK2-null MEFs and mouse skin remain susceptible to retroviral oncogenic transformation and chemical carcinogenesis, respectively, albeit with reduced efficiency.13,31 Here, we studied the role of CDK2 in normal and neoplastic proliferation by using two newly engineered Tg mouse models, K5Cdk2 and K5Cdk4D158N, in which transgenic expression was driven to epidermis.
CDK2 in Tumor Development
Our initial hypothesis was that elevated CDK2 kinase activity in mouse epidermis would enhance Ras-mediated tumorigenesis. Surprisingly, we found that elevated CDK2 kinase activity did not lead to an increased number of skin tumors, nor did it increase malignant progression of papillomas to SCC. Moreover, K5Cdk2 and K5Cdk4D158N Tg mice developed a lower number of papillomas in comparison to Wt littermates. Supporting these results, Lazarov and colleagues48 have shown that primary human keratinocytes co-transfected with Ras and CDK2 or CDK4D158N do not form invasive neoplasias when grafted into nude mice. In contrast, the same group showed that co-expression of Ras and CDK4 in human keratinocytes formed invasive neoplasias. Consistently, we have previously demonstrated that overexpression of CDK4 (K5Cdk4 Tg mice) collaborates with Ha-ras mutation to enhance malignant progression to SCC.29 Altogether, these results show that increased CDK4 activity, but not CDK2 activation, synergizes with Ras activation in mouse and human keratinocytes. In both, K5Cdk2 and K5Cdk4D158N models, CDK4 levels and kinase activity was unaltered, suggesting that increased CDK2 levels and/or activity is insufficient for tumor progression. The malignant phenotype observed in K5Cdk4 mice7,29 and the data presented here strongly suggest that CDK4 kinase activity, but not its noncatalytic function, is responsible for increased malignant progression.
We have also shown that increased CDK2 activity results in an elevated rate of apoptosis in the hair follicle, although no evident phenotype was observed in the mice hair. Carcinogen label-retaining experiments have shown that DMBA-initiated cells in the mouse skin localize in the hair follicle infundibulum and external root sheath.49 Thus, it is possible that the elevated apoptotic response triggered by elevated CDK2 activity and/or CDK2 protein levels limits skin tumor development observed in both K5Cdk2 and K5Cdk4D158N. We have recently showed that ablation of Cdk2 in K5Cdk4 mice (K5Cdk4/Cdk2−/− mice) results in a strong reduction of the number of papillomas and malignant progression to SCC compared to K5Cdk4 mice.31 Thus, CDK2 appears to be necessary for CDK4-induced tumor development, but does not behave as an independent oncogene. In this regard, absence of CDK2 activity can affect the rate of CDK4-induced proliferation reducing the number of papillomas, but elevated CDK2 kinase activity can trigger an apoptotic response, which limit tumor development.
CDK2 in Keratinocyte Proliferation
Several groundbreaking works in cell culture have shown that activation of CDK2 accelerates cell-cycle progression. Herein, we have obtained conflicting results regarding the effects of CDK2 activation on normal keratinocyte proliferation. On one hand, we have shown that increased CDK2 levels and kinase activity does not alter normal keratinocyte proliferation or the overall skin morphology of K5Cdk2 Tg mice. In contrast, K5Cdk4D158N mice have an increased rate of keratinocyte proliferation and exhibit epidermal hyperplasia. These data suggest that elevated protein levels of CDK2 induce a compensatory mechanism, which may keep CDK2 activity in check. To this effect, we observed elevated p21Cip1 protein expression in K5Cdk2, but not K5Cdk4D158N epidermal lysates. It has been suggested that p21Cip1 and p53 act as an inducible barrier for deregulated cyclin E.34 Likewise, it has been shown that co-transfection of cyclin E and CDK2 in cell culture results in the transactivation of p53 signaling.36 In addition, p53 has been shown to restrain cyclin E-associated genomic instability and cyclin ET393A knockin mice, which express a stable cyclin E mutant, accelerates tumorigenesis in the absence of p53.50,51 Therefore, it is possible that overexpression of CDK2 may also induce a p53 response that up-regulates p21Cip1 levels. Interestingly, elevated p21Cip1 levels result in increase CDK2/p21Cip1 complexes, suggesting that p21Cip1 is part of a putative compensatory mechanism triggered by overexpression of CDK2, partially attenuating its kinase activity. To this end, CDK2-kinase activity increased 1.7-fold in normal skin from K5Cdk2 Tg mice, whereas it was elevated fivefold in K5Cdk4D158N skin, suggesting that the induction of p21Cip1 elicited in K5Cdk2 keratinocytes partially blocks CDK2 activity to Wt or basal levels. The mechanism by which p21Cip1 is induced in K5Cdk2 epidermis remains unclear and is beyond the scope of this study. In any case, this compensatory mechanism was only elicited in nontransformed keratinocytes because papillomas from K5Cdk2 mice did not reveal increased p21Cip1 protein levels and CDK2 activity was augmented 3.4-fold compared to only 1.7-fold in K5Cdk2 normal keratinocytes.
Overall, we conclude that CDK4 sequestering activity is sufficient to produce keratinocyte hyperproliferation, presumably through CDK2 activation, however neither CDK2 overexpression nor the indirect activation of CDK2 enhances Ras-mediated skin tumorigenesis. In conjunction with our previous studies, the work presented here suggests that the concomitant activation of both CDK4 and CDK2 are required to elicit a malignant skin tumor phenotype.29,31 In summary, using two independent Tg mouse models we have demonstrated that the activation of CDK2 does not provide a significant selective advantage to neoplastic keratinocytes and to some extent this may explain why CDK2 amplification, mutation, or overexpression is infrequent in human malignancies.
Acknowledgments
We thank the Laboratory Animal Resources and the College of Veterinary Medicine histology core personnel at North Carolina State University, Christopher Sistrunk for helping edit this manuscript, and Kimberley Murphy for technical support.
Footnotes
Address reprint requests to M.L. Rodriguez-Puebla, North Carolina State University, CVM-MBS, 4700 Hillsborough St., Raleigh, NC 27606. E-mail: marcelo_rodriguez-puebla@ncsu.edu.
Supported by the National Institutes of Health (grants CA90864 and CA116328).
E.M. and P.L.M.d.M. contributed equally to this work.
Present address of E.M.: Lineberger Comprehensive Cancer Center, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC.
References
- Sherr CJ. D-type cyclins. Trends Biochem Sci. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Blain S, Montalvo E, Massague J. Differential interaction of the cyclin-dependent kinase (cdk) inhibitor p27Kip1 with cyclin A-cdk2 and cyclin D2-cdk4. J Biol Chem. 1997;272:25863–25872. doi: 10.1074/jbc.272.41.25863. [DOI] [PubMed] [Google Scholar]
- Labaer J, Garret MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Thieke K, Maier A, Saffrich R, Hanley-Hyde J, Ansorge W, Reed S, Sicinski P, Bartek J, Eilers M. Direct induction of cyclin D2 by Myc contributes to cell cycle progression and sequestration of p27. EMBO J. 1999;18:5321–5333. doi: 10.1093/emboj/18.19.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Roger I, Kim SH, Griffiths B, Sewing A, Land H. Cyclins D1 and D2 mediate myc-induced proliferation via sequestration of p27(Kip1) and p21(Cip1). EMBO J. 1999;18:5310–5320. doi: 10.1093/emboj/18.19.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliani de Marval P, Gimenez-Conti I, LaCava M, Martinez L, Conti C, Rodriguez-Puebla M. Transgenic expression of CDK4 results in epidermal hyperplasia and severe dermal fibrosis. Am J Pathol. 2001;159:369–379. doi: 10.1016/S0002-9440(10)61703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M, Theodoras A, Schumacher J, Roberts J, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:1559–1571. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LH, Lees E, Faha B, Harlow E, Riabowol K. The cdk2 kinase is required for the G1-to-S transition in mammalian cells. Oncogene. 1993;8:1593–1602. [PubMed] [Google Scholar]
- Berthet C, Aleem E, Coppola V, Tassarollo L, Kaldis P. Cdk2 knockout mice are viable. Curr Biol. 2003;13:1775–1785. doi: 10.1016/j.cub.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, Barbero JL, Malumbres M, Barbacid M. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet. 2003;35:25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- Martín A, Odajima J, Hunt SL, Dubus P, Ortega S, Malumbres M, Barbacid M. Cdk2 is dispensable for cell cycle inhibition and tumor suppression mediated by p27(Kip1) and p21(Cip1). Cancer Cell. 2005;7:591–598. doi: 10.1016/j.ccr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Marone M, Scambia G, Giannitelli C, Ferrandina G, Masciullo V, Bellacosa A, Benedetti-Panici P, Mancuso S. Analysis of cyclin E and CDK2 in ovarian cancer: gene amplification and RNA overexpression. Int J Cancer. 1998;75:34–39. doi: 10.1002/(sici)1097-0215(19980105)75:1<34::aid-ijc6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Kitahara K, Yasui W, Kuniyasu H, Yokozaki H, Akama Y, Yunotani S, Hisatsugu T, Tahara E. Concurrent amplification of cyclin E and CDK2 genes in colorectal carcinomas. Int J Cancer. 1995;62:25–28. doi: 10.1002/ijc.2910620107. [DOI] [PubMed] [Google Scholar]
- Shintani S, Mihara M, Nakahara Y, Kiyota A, Ueyama Y, Matsumura T, Wong DT. Expression of cell cycle control proteins in normal epithelium, premalignant and malignant lesions of oral cavity. Oral Oncol. 2002;38:235–243. doi: 10.1016/s1368-8375(01)00048-3. [DOI] [PubMed] [Google Scholar]
- Akama Y, Yasui W, Yokozaki H, Kuniyasu H, Kitahara K, Ishikawa T, Tahara E. Frequent amplification of the cyclin E gene in human gastric carcinomas. Jpn J Cancer Res. 1995;86:617–621. doi: 10.1111/j.1349-7006.1995.tb02442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyomarsi K, Herliczek T. The role of cyclin E in cell proliferation, development and cancer. Prog Cell Cycle Res. 1997;3:171–191. doi: 10.1007/978-1-4615-5371-7_14. [DOI] [PubMed] [Google Scholar]
- Eguchi N, Fujii K, Tsuchida A, Yamamoto S, Sasaki T, Kajiyama G. Cyclin E overexpression in human gallbladder carcinomas. Oncol Rep. 1999;6:93–96. doi: 10.3892/or.6.1.93. [DOI] [PubMed] [Google Scholar]
- Kiyokawa H, Kineman R, Manova-Todorova K, Soares V, Hoffman E, Ono M, Khanam D, Hayday A, Frohman L, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai LH, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- Gray-Bablin J, Zalvide J, Fox M, Knickerbocker C, DeCaprio J, Keyomarsi K. Cyclin E, a redundant cyclin in breast cancer. Proc Natl Acad Sci USA. 1996;93:15215–15220. doi: 10.1073/pnas.93.26.15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwell RM, Porter DC, Danes C, Keyomarsi K. Processing of cyclin E differs between normal and tumor breast cells. Cancer Res. 2000;60:481–489. [PubMed] [Google Scholar]
- Keyomarsi K, Conte DJ, Toyofuku W, Fox M. Deregulation of cyclin E in breast cancer. Oncogene. 1995;11:941–950. [PubMed] [Google Scholar]
- Corsino P, Davis B, Law M, Chytil A, Forrester E, Norgaard P, Teoh N, Law B. Tumors initiated by constitutive Cdk2 activation exhibit transforming growth factor beta resistance and acquire paracrine mitogenic stimulation during progression. Cancer Res. 2007;67:3135–3144. doi: 10.1158/0008-5472.CAN-06-3815. [DOI] [PubMed] [Google Scholar]
- Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- Miliani de Marval PL, Macias E, Conti CJ, Rodriguez-Puebla ML. Enhanced malignant tumorigenesis in Cdk4 transgenic mice. Oncogene. 2004;23:1863–1873. doi: 10.1038/sj.onc.1207309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliani de Marval PL, Macias E, Rounbehler R, Sicinski P, Kiyokawa H, Johnson DG, Conti CJ, Rodriguez-Puebla ML. Lack of cyclin-dependent kinase 4 inhibits c-myc tumorigenic activities in epithelial tissues. Mol Cell Biol. 2004;24:7538–7547. doi: 10.1128/MCB.24.17.7538-7547.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias E, Kim Y, Miliani de Marval PL, Klein-Szanto A, Rodriguez-Puebla ML. Cdk2 deficiency decreases ras/CDK4-dependent malignant progression, but not myc-induced tumorigenesis. Cancer Res. 2007;67:9713–9720. doi: 10.1158/0008-5472.CAN-07-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce AM, Gimenez-Conti IB, Schneider-Broussard R, Martinez LA, Conti CJ, Johnson DG. Increased E2F1 activity induces skin tumors in mice heterozygous and nullizygous for p53. Proc Natl Acad Sci USA. 1998;95:8858–8863. doi: 10.1073/pnas.95.15.8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez A, Bravo A, Jorcano J, Vidal M. Sequences 5′ of the bovine keratin 5 gene direct tissue- and cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation. 1994;58:53–64. doi: 10.1046/j.1432-0436.1994.5810053.x. [DOI] [PubMed] [Google Scholar]
- Minella AC, Swanger J, Bryant E, Welcker M, Hwang H, Clurman BE. p53 and p21 form an inducible barrier that protects cells against cyclin E-cdk2 deregulation. Curr Biol. 2002;12:1817–1827. doi: 10.1016/s0960-9822(02)01225-3. [DOI] [PubMed] [Google Scholar]
- He G, Siddik ZH, Huang Z, Wang R, Koomen J, Kobayashi R, Khokhar AR, Kuang J. Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene. 2005;24:2929–2943. doi: 10.1038/sj.onc.1208474. [DOI] [PubMed] [Google Scholar]
- Segawa K, Hokuto I, Minowa A, Ohyama K, Takano T. Cyclin E enhances P53-mediated transactivation. FEBS Lett. 1993;329:283–286. doi: 10.1016/0014-5793(93)80238-p. [DOI] [PubMed] [Google Scholar]
- Quintanilla M, Brown K, Ramsden M, Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Wunder JS, Eppert K, Burrow SR, Gokgoz N, Bell RS, Andrulis IL. Co-amplification and overexpression of CDK4. SAS and MDM2 occurs frequently in human parosteal osteosarcomas. Oncogene. 1999;18:783–788. doi: 10.1038/sj.onc.1202346. [DOI] [PubMed] [Google Scholar]
- Wei G, Lonardo F, Ueda T, Kim T, Huvos AG, Healey JH, Ladanyi M. CDK4 gene amplification in osteosarcoma: reciprocal relationship with INK4A gene alterations and mapping of 12q13 amplicons. Int J Cancer. 1999;80:199–204. doi: 10.1002/(sici)1097-0215(19990118)80:2<199::aid-ijc7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Nikitakis N, Drachenberg C, Papadimitriou J. MDM2 and CDK4 expression in carcinosarcoma of the esophagus: comparison with squamous cell carcinoma. Exp Mol Pathol. 2002;73:198–208. doi: 10.1006/exmp.2002.2465. [DOI] [PubMed] [Google Scholar]
- Khatib Z, Matsushime H, Valentine M. Coamplification of the Cdk4 gene with MDM2 and GL1 in human sarcomas. Cancer Res. 1993;53:5535–5541. [PubMed] [Google Scholar]
- Dei Tos A, Doglioni C, Piccini S, Sciot R, Furlanetto A, Boiocchi M, Dal Cin P, Maestro R, Fletcher C, Tallini G. Coordinate expression and amplification of the MDM2. CDK4, and HMGI-C genes in atypical lipomatous tumours. J Pathol. 2000;190:531–536. doi: 10.1002/(SICI)1096-9896(200004)190:5<531::AID-PATH579>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Zuo L. Germline mutation in the p16Ink4a binding domain of cdk4 in familial melanoma. Nat Genet. 1996;12:97–99. doi: 10.1038/ng0196-97. [DOI] [PubMed] [Google Scholar]
- Harwell RM, Mull BB, Porter DC, Keyomarsi K. Activation of cyclin-dependent kinase 2 by full length and low molecular weight forms of cyclin E in breast cancer cells. J Biol Chem. 2004;279:12695–12705. doi: 10.1074/jbc.M313407200. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- McConnell B, Gregory F, Stott F, Hara E, Peters G. Induced expression of p16Ink4a inhibits both CDK4- and CDK2-associated kinase activity by reassortment of cyclin-CDK-inhibitor complexes. Mol Cell Biol. 1999;19:1981–1989. doi: 10.1128/mcb.19.3.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell. 2003;3:233–245. doi: 10.1016/s1535-6108(03)00053-9. [DOI] [PubMed] [Google Scholar]
- Lazarov M, Kubo Y, Cai T, Dajee M, Tarutani M, Lin Q, Fang M, Tao S, Green C, Kharavi P. CDK4 coexpression with Ras generates malignant human epidermal tumorigenesis. Nat Med. 2002;8:1105–1114. doi: 10.1038/nm779. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Fischer SM, Slaga TJ. Evidence that a slowly cycling subpopulation of adult murine epidermal cells retains carcinogen. Cancer Res. 1986;46:3061–3066. [PubMed] [Google Scholar]
- Loeb KR, Kostner H, Firpo E, Norwood T, Tsuchiya D, Clurman BE, Roberts JM. A mouse model for cyclin E-dependent genetic instability and tumorigenesis. Cancer Cell. 2005;8:35–47. doi: 10.1016/j.ccr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Minella AC, Grim JE, Welcker M, Clurman BE. p53 and SCFFbw7 cooperatively restrain cyclin E-associated genome instability. Oncogene. 2007;26:6948–6953. doi: 10.1038/sj.onc.1210518. [DOI] [PubMed] [Google Scholar]