Abstract
Endometrial cancer has been generally categorized into two broad groups of tumors, type I (TI) and type II (TII), with distinct epidemiological/clinical features and genetic alterations. Because telomere attrition appears to trigger genomic instability in certain cancers, we explored the role of telomere dysfunction in endometrial cancer by analyzing telomeres and other markers of telomere status in both tumor types. We describe a new method, telomere chromogenic in situ hybridization, which permitted us to detect cells with short telomeres relative to control (stromal) cells within the same tissue section. Using this method, we found that both types of tumor cells had short telomeres. However, only TII tumors were significantly associated with critical telomere shortening in adjacent, morphologically normal epithelium, suggesting that telomere shortening contributes to the initiation of TII but not TI tumors. To explore this hypothesis, we analyzed mice with critically short telomeres and documented distinctive endometrial lesions that histologically resembled the in situ precursor of TII serous carcinomas; these lesions have not been observed previously in TI mouse models of endometrial cancer. Based on this and previous studies, we propose a model in which telomere attrition contributes to the initiation of TII and progression of TI endometrial cancers.
Endometrial cancer is the most common cancer of the female reproductive tract.1 Although all endometrial cancers are believed to arise from a single cell type—the columnar epithelium that lines the inner surface of the uterus—endometrial cancers are subclassified into two classes of tumors with divergent epidemiological, clinical, histopathological, and molecular features.2,3 Type I (TI) endometrial cancers are strongly associated with estrogen-related risk factors such as obesity and unopposed estrogen therapy, and have a relatively good prognosis. In contrast, type II (TII) cancers have no association with estrogen-related risk factors, a much higher median age of onset, and a poor prognosis. These tumors are also histopathologically distinct, with TI being comprised of tumors with so-called “endometrioid” histology (and variants thereof such as endometrioid adenocarcinomas with squamous or mucinous differentiation), whereas TII tumors include uterine serous adenocarcinomas.4,5 TI and TII tumors also have distinct genetic and molecular profiles. Microsatellite instability and defects in DNA mismatch repair are common in TI, but rare in TII tumors.6,7,8 Furthermore, TI and TII cancers have reciprocal mutational spectra. Mutations in PTEN, KRAS, and the CTNNB1 locus that encodes β-catenin are frequent in TI, but rare in TII tumors, which in contrast have a very high rate of P53 mutations.3,9 Finally, TI tumors have relatively minor chromosomal abnormalities, whereas TII tumors exhibit abnormal, complex karyotypes characterized by hyperploidy and abnormal chromosomes with numerous end-to-end fusions.10,11,12,13
Genomic instability is one of the hallmarks of cancer,14 and there is growing evidence that telomere attrition triggers genomic instability in epithelial carcinogenesis.15 The telomerase holoenzyme that maintains telomeres consists of RNA catalytic and reverse transcriptase subunits (hTERC and hTERT, respectively), and because of the lack of hTERT expression in most adult somatic cells, telomeres progressively shorten with age, particularly in those tissue compartments or cell types that are highly proliferative and thus have an increased replicative history. Eventually, telomere attrition leads to naked chromosome ends repaired by nonhomologous end-joining, in turn leading to dicentric chromosomes that cannot be resolved at mitosis. This results in additional double-stranded DNA breaks (DSBs) similarly repaired by nonhomologous end-joining, thereby initiating a vicious cycle of chromosome bridging, fusion, and breakage.16 This model accounts for many heretofore unexplained aspects of cancer, such as the exponential increase in the incidence of most epithelial cancers with advancing age, and observations that carcinomas tend to be aneuploid with highly abnormal chromosomes that result from multiple nonreciprocal translocations.
The TP53 protein plays an essential and central role in sensing the genomic damage triggered by telomere attrition. By promoting apoptosis or senescence in the context of critically shortened telomeres, TP53 mediates many of the aging and cancer-prone phenotypes seen in short telomere hTERC-deficient mice bred for several generations.15 Inactivation of the p53 gene ameliorates the aging phenotypes seen in mice with short telomeres, but also greatly increases the incidence of spontaneous cancers, particularly carcinomas.15,17 Thus, telomere attrition and p53 inactivation (be it by direct mutation or other indirect mechanisms such as MDM2 amplification) appear to potently synergize to promote epithelial carcinogenesis.
These observations taken together suggest that telomere attrition might be an important molecular event driving endometrial carcinogenesis. In particular, this may help explain the striking association of TII tumors with advanced age and p53 inactivation. To explore this hypothesis, we have evaluated telomere lengths in situ using a novel chromogenic method (Telo-CISH), and also analyzed another molecular marker (pH2AX) associated with double-stranded breaks (DSBs) and telomere attrition. Both TI and TII tumors exhibited telomere shortening; however, only TII tumors were associated with telomere shortening in adjacent normal epithelium, and they more frequently exhibited pH2AX foci, a marker of DSBs, consistent with the notion that telomere shortening drives the initiation of TII tumors. Furthermore, we demonstrate that telomere attrition in mice (which normally have longer telomeres than humans) promotes the formation of distinct TII-like precursor lesions unlike those seen in other mouse models of endometrial cancer, all of which to date result in TI-like cancers or precancers. Our findings suggest that telomere attrition contributes differentially to TI and TII endometrial cancers.
Materials and Methods
Telo-CISH and Telomere Length Southern Analysis
Use of archival human tumor samples was approved by the University of Texas Southwestern Medical Center Institutional Review Board. Cases with mixed histology (eg, mixed endometrioid/serous) or of subtypes whose assignation as TI versus TII is less established (eg, clear cell), were excluded. Standard histological criteria were used.4,5 Tissue blocks were selected that contained both malignant and morphologically normal epithelium.
Telo-CISH was performed using a custom-synthesized digoxigenin conjugated telomere probe Dig-OO-(C3TA2)3 (Biosynthesis, Lewisville, TX), where OO represents a Fmoc-AEEA-OH (8-amino-3,6-dioxaoctonic acid) linker. Probe was solubilized in dH20, aliquoted, and stored at −20°C. Five-μm tissue sections were deparaffinized in xylene and hydrated in an ethanol series. Slides were boiled gently in 10 mmol/L sodium citrate for 10 minutes and treated with 0.5% pepsin (pH 2) for 10 minutes. The duration of pepsin treatment should be optimized for each tissue because overdigestion results in tissue loss, and underdigestion results in decreased signal. Denaturation was performed by covering the tissue section with hybridization buffer, placing coverslips, and incubating the slide at 95°C for 5 minutes. Hybridization was performed overnight at 37°C in hybridization buffer [70% formamide, 10 mmol/L Tris pH 7.5, and 8.5 mmol/L MgSO4 with 10% blocking agent (catalog no. 1096176; Roche, Nutley, NJ) prepared in 0.1 g/ml of maleic acid buffer (catalog no. 1585762, Roche)] with 4 μg of probe per ml of hybridization buffer. Denaturation and hybridization steps were performed in a digital, temperature-controlled hybridization unit (catalog no. 240000; Boekel Scientific, Feasterville, PA). Slides were washed in 0.1× standard saline citrate at 55°C for 30 minutes × 2, then blocked in phosphate-buffered saline (PBS) and 2% bovine serum albumin for 30 minutes at room temperature. Polyclonal rabbit anti-digoxigenin/horseradish peroxidase (1:1000; DAKO, Carpinteria, CA) was applied in PBS and 2% bovine serum albumin for 1 hour at room temperature. Slides were washed with Tris-buffered saline plus 0.1% Tween for 5 minutes × 4 and signal was detected with freshly prepared liquid diaminobenzidine (DAKO). Slides were incubated in diaminobenzidine at room temperature for ∼1 hour, lightly counterstained with hematoxylin, and mounted in Permount (Fisher Scientific, Pittsburgh, PA). Visualization of signals was performed with standard bright-field optics using a ×100 (oil immersion) objective. Telomere length Southern analysis was performed using the TeloTAGGG kit (Roche Applied Science) per the manufacturer’s instructions. Normal matched DNA samples were obtained from tissues not involved by tumor such as ovary, fallopian tube, or cervix.
Immunohistochemistry (IHC) and Chromosome 7 Chromogenic in Situ Hybridization (CISH)
For IHC, 5-μm sections were deparaffinized in xylene and hydrated in a graded ethanol series. Slides were then boiled in 10 mmol/L sodium citrate for antigen retrieval and allowed to cool slowly at room temperature for 20 minutes. Endogenous peroxidases were blocked with 3% H2O2 in dH2O for 30 minutes. Slides were incubated with primary antibodies against p53 (1:100, catalog no. RM-9105; LabVision, Fremont, CA) and pH2AX (phosphorylated at serine 319) (1:1000, catalog no. 613401; Biolegend, San Diego, CA). Detection was performed using horseradish peroxidase-conjugated secondary antibodies with the Impress detection system (Vector Laboratories, Burlingame, CA). Slides were counterstained with hematoxylin and mounted in Permount. Cases with <5% positive cells (dot-like nuclear pattern) were scored as pH2AX-negative. CISH was performed using a chromosome 7 centromeric probe (Invitrogen, Carlsbad, CA), with signal detection performed with the CISH detection kit (Invitrogen) per the manufacturer’s instructions. Slides were counterstained with hematoxylin and mounted in Permount. Cases were scored in a blinded manner as follows: no aneuploidy, no cells with >2 signals; mild aneuploidy, 3 or more signals in ≤5% of cells; moderate aneuploidy, 3 or more signals in 5 to 20% of cells; and severe aneuploidy, 3 or more signals in >20% of cells.
Mouse Strains and Husbandry
This study was approved by an institutional animal care and use committee. Mice were housed in a barrier facility. G0i and G5i mice of mixed genetic background (C57/B6, 129, FVB/n) were generated as described.18 In brief, G4 mTerc−/− mice were crossed to G0 mTerc +/+ or −/− mice to generate the G5i and G0i cohorts and sibling controls.
Results
Case Selection and Telomere Length Analyses of TI and TII Tumors by Telo-CISH
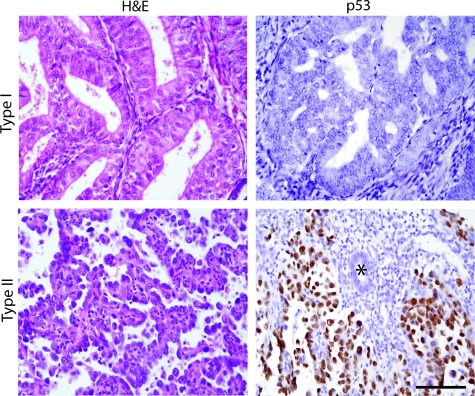
Archived blocks from age-matched TI (n = 14) and TII tumors (n = 15) were obtained; the median age was 65.1years for both groups. The selected TI tumors exhibited classic, well-differentiated endometrioid histology whereas TII cases were serous carcinomas (see Figure 1 for representative histologies). To validate these cases, p53 IHC was performed. Most TII endometrial cancers express high levels of p53 protein, presumably because of the functional inactivation of p53, which suppresses the Mdm2-dependent feedback loop that normally targets p53 for degradation.19,20 As expected, the majority of TII tumors showed strong p53 expression, whereas most TI tumors did not (13 of 14 versus 1 of 15; P = 2 × 10−6, Fisher’s exact test), confirming that our TI and TII tumor sets differ significantly with respect to p53 status and thus represent distinct biological entities (Figure 1).
Figure 1.
Case selection and p53 status of TI and TII endometrial cancers. H&E and p53 immunostaining of representative TI and TII tumors; asterisk = morphologically normal, p53-negative gland serving as internal negative control. TI case shows usual endometrioid histology including well-formed glands whereas TII case shows papillary serous histology with high-grade, atypical nuclei. Scale bar = 100 μm.
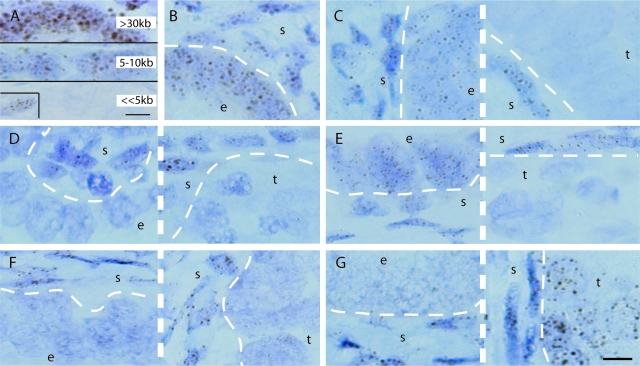
To explore the hypothesis that telomere shortening contributes to endometrial carcinogenesis, we sought to assess telomere lengths in situ. Peptide nucleic acid (PNA)-fluorescence in situ hybridization (FISH), which uses fluorescently-labeled PNA probes, has been used to analyze telomere lengths in human tissue sections.21,22,23,24 However, one limitation of this technique is that tissue architecture is not well visualized in dark-field microscopy, making it difficult to reliably distinguish between normal and malignant endometrial epithelium. To overcome this limitation, we adapted the PNA-FISH technique and developed a chromogenic method, which we have termed Telo-CISH (telomere chromogenic in situ hybridization). A telomeric PNA probe conjugated to digoxigenin was synthesized and hybridized to tissue sections, followed by incubation with an anti-digoxigenin antibody/horseradish peroxidase conjugate. Detection with the chromogen diaminobenzidine resulted in telomeric signals that were permanent and could be visualized via conventional bright-field microscopy, greatly facilitating observation of telomere signals in the context of the underlying tissue architecture. The signals were nuclear and dot-like, consistent with specific telomere detection (Figure 2). To determine whether Telo-CISH could be used as a qualitative assay for telomere length, we compared signals in three different samples processed identically. Laboratory mouse strains have average telomere lengths >30 kb, whereas human telomeres are much shorter, in the range of 5 to 10 kb.25,26 Normal human tissues gave rise to readily detectable telomeric signals, but, as expected, mouse tissues produced much more intense signals (Figure 2A). Intraluminal cells in prostatic intraepithelial neoplasia showed virtually absent telomeric signals, consistent with previous PNA-FISH studies documenting severe telomere attrition in prostatic intraepithelial neoplasia lesions (Figure 2A).22,23 Thus, Telo-CISH can be used for qualitative telomere length determinations in tissue sections and should be able to differentiate between cells with large differences in average telomere length within tissue sections. Epithelial cells in other neoplastic and preneoplastic lesions, including ductal carcinoma in situ of the breast, as well as invasive breast and prostate cancers, also exhibited decreased telomere signals by Telo-CISH (unpublished data).
Figure 2.
Telo-CISH analysis of TI and TII endometrial cancers, sections counterstained with hematoxylin. e, Morphologically normal epithelium; s, stroma; t, malignant epithelium. Thick dashed lines separate different fields from same slide/case; thin dashed lines show epithelial/stromal interfaces. A: Telo-CISH signals correlate with telomere length. Top: Mouse colon; middle: normal human endometrium from adult female; bottom: luminal cells from prostatic intraepithelial lesion (inset: stromal cells with strong telomere signals serving as internal positive control). B: Normal endometrium showing strong signals in both epithelium and subjacent stromal cells. C: TI, 73 years old. D: TI, 56 years old. E: TII, 76 years old. F: TII, 56 years old. G: TII, 66 years old. Scale bars = 5 μm.
Telo-CISH was performed on tissue sections of all 29 TI and TII cases. A control normal endometrial sample showed approximately equivalent telomere signals in stroma and epithelium (Figure 2B), demonstrating that the digoxigenin-labeled telomere probe hybridized well to both epithelial and stromal cell nuclei. Telo-CISH revealed diverse patterns of telomere lengths in TI and TII tumors. In the majority of TI tumors, nonmalignant nonhyperplastic (morphologically normal) epithelium showed strong signals, whereas adjacent tumor epithelium showed dramatically decreased signals (Figure 2C). A small minority of TI cases showed decreased telomere signals in the tumor as well as adjacent nonmalignant epithelium (Figure 2D, Table 1). This argues that in most TI tumors, telomere shortening is not an initiating tumorigenic event but rather occurs secondarily as a consequence of hyperproliferation and the increased replicative history of the tumor cells. With TII tumors, these patterns were reversed, with a minority of cases showing decreased signals in malignant epithelium only (Figure 2E), but the majority showed significant telomere attrition even in epithelium that was morphologically entirely normal (Figure 2F) (TII neoplasms do not progress via intermediate hyperplasias, and the malignant and normal epithelium are readily distinguishable at lower magnification). In one TII tumor, morphologically normal cells did show short telomeres relative to stromal cells, but the tumor cells showed very strong telomere signals, even stronger than the stromal cells (Figure 2G). In this case, telomere attrition was likely followed by the reactivation of hTERT or another mechanism such as ALT that stabilizes telomeres.27
Table 1.
Telo-CISH Study of Endometrial Cancer Cases
| Telomere signals in normal versus tumor epithelium | Type I | Type II |
|---|---|---|
| Decreased in normal and tumor | 3/14 | 11/15 |
| Decreased in tumor only | 11/14 | 3/15 |
| Decreased in normal, increased in tumor | 0/14 | 1/15 |
Qualitative comparison of signals in morphologically normal epithelium and tumor relative to adjacent stroma were determined. Cases fell into three general categories as summarized.
As summarized in Table 1, only 3 of 14 TI tumors showed decreased telomere signals in morphologically normal epithelium, versus 11 of 15 TII tumors (P = 0.007, Fisher’s exact test) These data demonstrate that telomere shortening is a general feature of TI and TII carcinogenesis, but more specifically, suggest that such telomere shortening is an important mechanism driving the initiation of TII tumors (see model presented in the Discussion).
Telomere Length Determinations by Southern Analysis
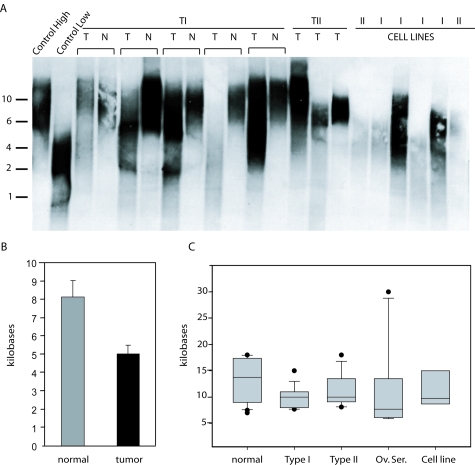
Telo-CISH permits direct comparisons of small numbers of cells visualized in situ, but does not readily provide a global view of telomere length in all cells in a biological sample. To confirm the somewhat unanticipated finding that a significant proportion of early TI tumors already have significant telomere shortening (given our initial hypothesis that telomere shortening drives TII tumorigenesis), we analyzed 20 TI tumors (predominantly grade I) where matched normal DNA was available, permitting the most accurate assessment of telomere shortening in individual endometrial tumors. Tumor DNA was prepared from frozen tumors and analyzed by telomere length Southern analysis (Figure 3A), with the median telomere lengths determined by standard methods (Figure 3B). This confirmed significant telomere attrition in TI tumors (P = 0.0068, Fisher’s exact test). Analysis of additional TI, TII, and endometrial cancer cell line DNA samples for which matched samples were not available (n = 9, 10, and 6, respectively) also demonstrated similar degrees of telomere attrition, as did, for comparison, ovarian papillary serous adenocarcinomas (n = 10) (Figure 3C). These findings confirm that telomere shortening is a general feature of both TI and TII endometrial cancers.
Figure 3.
Telomere length quantitation by Southern blot analysis. A: Representative Southern blot showing matched tumor and control DNAs from the same patient, unmatched TII tumors, and TI and TII cell lines. Size markers (kb) are shown on the left. Control high and low DNA samples correspond to early and late passage cultured cells. T, Tumor; N, normal. B: Mean telomere length in matched TI tumor versus normal (n = 20) as determined by Southern analysis; error bars represent SEM. C: Box plots showing absolute telomere lengths in unmatched normal endometrium (n = 20), TI (n = 9), and TII (n = 10) endometrial cancers, ovarian serous carcinomas (n = 10), and endometrial cancer cell lines (n = 6) as determined by Southern analysis. The boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Error bars above and below the box indicate the 90th and 10th percentiles, with outliers shown as black circles.
Aneuploidy and DNA Damage in TI versus TII Tumors
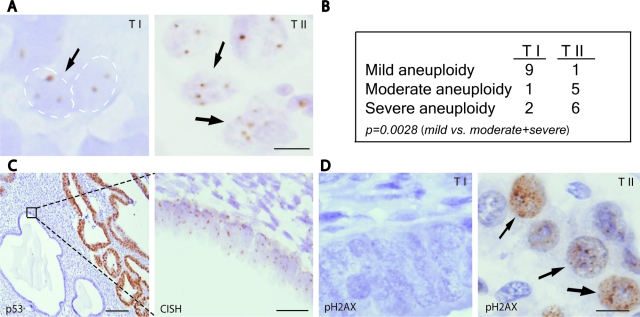
The finding that significant telomere shortening is present in the morphologically normal epithelium adjacent to TII but not TI cancers suggests that telomere shortening can drive endometrial cancer initiation, serving as a mutagenic mechanism that actively promotes cancer via breakage-fusion-bridge cycles.16 If so, then aneuploidy and DNA DSBs may be detectable in this premalignant adjacent epithelium. To explore this possibility, we performed ploidy analyses by CISH, which permits direct visualization and assessment of aneuploidy in the context of tissue morphology in routine sections. We used a centromeric probe for chromosome 7 because it is among the most common chromosomes showing aneuploidy in ovarian/uterine tumors,28 but other chromosomes could also in principle be used as general markers of aneuploidy. Although the use of a single centromeric probe likely underestimates the degree of aneuploidy, analysis of TI and TII tumors revealed significant differences. Most TI tumors showed very mild aneuploidy that was difficult to detect; in contrast, TII tumors showed moderate to severe aneuploidy that was readily apparent in tumor cells (see Materials and Methods for criteria), and this difference was statistically significant (P = 0.0028, Fisher’s exact test) (Figure 4, A and B). Notably, in at least three TII cases, we observed clear evidence of aneuploidy in the adjacent, morphologically normal epithelium that was p53-negative (Figure 4C). This indicates that significant aneuploidy is present, at least in some cases, even in the morphologically normal epithelium that presumably represents an early precursor to the adjacent TII tumor, and further suggests that normal p53 function is the key tumor suppressive mechanism restricting the growth of such aneuploid cells.
Figure 4.
Ploidy and DNA damage analyses. A: CISH using chromosome 7 centromeric probe. Normal nuclei show either one or two signals (attributable to sectioning of nuclei), but three or more signals indicate aneuploidy. Arrows indicate aneuploid nuclei with three or more signals. B: Table summarizing CISH analysis of 24 TI and TII tumors. C: Immunohistochemical and CISH analyses of representative TII case. p53 IHC (left) highlights malignant (strongly positive) versus normal epithelium (negative). CISH (right) performed on adjacent serial section shows significant aneuploidy in p53-negative morphologically normal endometrial epithelium. D: Representative pH2AX-positive and -negative TI and TII tumors. All slides counterstained with hematoxylin. Scale bars: 10 μm (A, D); 100 μm (C, left); 25 μm (C, right).
The histone H2AX, a member of the histone H2A family, is rapidly phosphorylated at serine 139 at sites of DSBs, where such phosphorylated H2AX (pH2AX, also known as γ-H2AX) is believed to recruit additional factors to effect DSB repair.29 We thus sought to define patterns of pH2AX expression in endometrial cancers. In some control specimens (proliferative, secretory, and atrophic endometrium, and in other normal tissues such as colon) nonspecific diffuse nuclear staining was observed (data not shown). The basis of this nonspecific staining has not been explored, but may relate to cross-reactivity with the unphosphorylated form of H2AX, or some other nuclear antigen. In any case, this nonspecific staining was readily distinguished from the dot-like pattern that signifies bona fide double DNA stranded-breaks, and only dot-like pH2AX expression was scored as positive. All TII cases (12 of 12, 100%) were pH2AX-positive, whereas only a minority (5 of 12, 42%) of TI tumors were pH2AX-positive (Figure 4D), a difference that is statistically significant (P = 0.0045, Fisher’s exact test). That pH2AX expression significantly differs among TI and TII tumors prompted us to consider the possibility that pH2AX might serve as a diagnostic marker to, eg, identify TII tumors. For example, p53 IHC is sometimes used to confirm the diagnosis of uterine serous carcinoma, but this test is associated with false-negatives (likely because of the biallelic mutations that eliminate p53 protein). However, pH2AX status by IHC correlated less well than p53 expression with respect to TI versus TII status. Furthermore, we found that pH2AX positivity tended to be much more variegated and regional, and hence more difficult to interpret than p53 IHC (data not shown). Nonetheless, this is the first demonstration, as far as we are aware, that pH2AX correlates with telomere dysfunction in human tissue samples.
Analysis of Telomere Attrition in Relation to TII Endometrial Neoplasia in Vivo
Although analysis of telomere status and other markers in banked human tumor samples can provide important insights into telomere biology, the inability to follow the progression of lesions serially or perform other experimental manipulations represent important technical limitations. As a first step toward the generation of mouse models that would overcome these limitations and to further explore the role that telomere attrition might have on endometrial carcinogenesis, we studied mice bearing null mutations in mTerc, the RNA component required for telomerase holoenzyme activity.30,31 To generate animals with shortened telomeres, we used a validated mating strategy to generate G5i (generation 5 intermediate) animals that are prone to genomic instability because of the critically shortened telomeres (in half of their chromosomes) and control G0i animals that have capped, stabilized telomeres.18 We analyzed 14 experimental and control female mice for these studies. Experimental G5i animals are minute, exhibit accelerated aging, are in very poor health, and also exhibit increased incidence of spontaneous neoplasia.
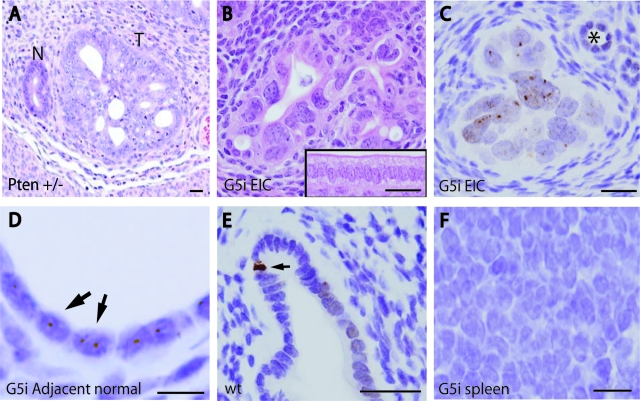
The 14 mice (five G5i, nine G0i) were 13 to 16 months of age before necropsy, which revealed in several mice the presence of diverse tumors, predominantly sarcomas, as has been previously described. None of the mice developed frankly invasive endometrial adenocarcinomas, but four of five G5i mice developed striking preneoplastic endometrial precancers (P = 0.005, Fisher’s exact test) closely resembling endometrial intraepithelial carcinoma (EIC), a lesion considered to be the histological in situ precursor to TII papillary serous adenocarcinomas.4,5,32 These EIC-like lesions differed from TI hyperplasias seen in women or Pten−/+ mice in that architectural irregularities (crowding, and so forth), were mild and focal, whereas nuclear atypia and anisometry were extremely severe (Figure 5, A and B). Although we cannot exclude early, microscopic invasion, these lesions were confined to the endometrium and not associated with obvious invasive tumor growth, gross tumor formation, or metastases. These EIC-like lesions were not observed in G0i or control wild-type animals. These findings are notable in that such TII-like lesions have not been previously documented in the mouse, and represent the first evidence that mouse models of TII endometrial carcinogenesis can be developed (see Discussion).
Figure 5.
Mouse model of TII precancers. A and B: H&E stains; C–F, pH2AX IHC counterstained with hematoxylin. A: Endometrium from Pten+/− female (6 weeks) showing spontaneous endometrial lesion illustrating TI histology. T, Neoplastic focus; N, adjacent morphologically normal gland. B: Endometrium from fifth generation mTerc−/− (G5i) mouse (14 months). Inset shows morphologically normal surface epithelium from control G0i mouse (15 months). Note that EIC lesion is characterized by extreme nuclear anisometry and mild architectural abnormalities, whereas normal epithelium consists of well-organized, polarized columnar epithelium. C: pH2AX IHC from same G5i uterus in B shows many nuclear foci consistent with DSBs; asterisk indicates morphologically normal gland with small, nonatypical and isometric nuclei. D: Numerous pH2AX foci in adjacent normal epithelium. E: pH2AX in control G0i mouse shows occasional apoptotic cell (arrow) and rare pH2AX foci that are difficult to discern and far rarer than in G5i uteri. F: Spleen from G5i animal showing absence of pH2AX foci. Scale bars: 25 μm (A–C, E); 10 μm (D, F).
To confirm the prediction that genomic instability and DSBs participate in the genesis of these EIC-like lesions, pH2AX IHC was performed (Figure 5C). This revealed the presence of multiple widespread discrete pH2AX-positive foci, indicating that these murine EIC-like lesions harbor DSBs and significant genomic instability because of the critical telomere shortening and chromosome bridging, fusion, and breakage cycles. Of note, similar pH2AX-positive foci were also observed in adjacent, morphologically normal epithelium, indicating that DSBs predate the formation of the EIC-like lesions and are thus likely to be critical in their genesis. Such pH2AX-positive foci are not common in endometria from control G0i or wild-type mice (Figure 5D), other than rare apoptotic cells that are strongly and diffusely pH2AX-positive, a pattern distinguishable from the discrete foci seen with DBSs (pH2AX is a known marker of apoptotic cells, because of their widespread DNA breakage) (Figure 5E). Also, pH2AX foci were not observed in all G5i tissues, including some tissues with high proliferation rates such as spleen (Figure 5F), arguing that tissue-specific factors contribute to the development of DSBs and genomic instability, and that the endometrium may be relatively sensitive to these insults. Thus in summary, analyses of mice with short telomeres combined with Telo-CISH studies together support the notion that telomere attrition contributes to the initiation of TII cancers.
Discussion
Recent studies using telomere PNA-FISH have demonstrated that telomere attrition is associated with and likely drives the progression of many epithelial malignancies, including breast, prostate, and pancreatic cancer.22,24,33 Here, we demonstrate that endometrial cancer is another epithelial malignancy in which telomere shortening is associated with and likely contributes to tumorigenesis. Furthermore, we describe a new approach for the chromogenic detection of telomeres that may be useful in either the research or clinical setting. For example, because telomeres serve as a biological clock and correlate with replicative history, Telo-CISH could serve as a marker of neoplasia (ie, to distinguish dysplasia versus reactive changes) or chronic disease; ie, inflammatory bowel disease, in which telomere shortening promotes genomic instability.34 However, one limitation of Telo-CISH compared to PNA-FISH is that it may not be as readily quantifiable, lending itself to more qualitative analyses.
It is well documented that TII endometrial cancers are highly aneuploid, whereas TI endometrial cancers are nearly diploid. Indeed, several TI endometrial cancer cell lines are euploid (46, XX) or have only one or two abnormal or extra chromosomes.10,11,12,13,35,36 At the same time, defects in mismatch repair are strongly associated with TI but not TII tumors. Indeed, the wide variety of mechanisms resulting in TI mismatch repair defects (acquired somatic mutation in mismatch repair genes, promoter hypermethylation, or germline mutations as in hereditary nonpolyposis colorectal cancer/Lynch syndrome), argues for the importance of a mismatch repair-driven mode of genomic instability in TI carcinogenesis.37 It is not surprising that such mismatch repair-driven tumors would also undergo telomere attrition. Because most TI tumors progress through a series of intermediates (simple hyperplasia, complex hyperplasia, and so forth) that likely require many years to develop, these lesions would be expected to have a history of increased mitotic divisions driving telomere shortening. However, it is also possible that in some tumors, mutations attributable to mismatch repair defects and telomere attrition occur more or less simultaneously, acting as synergistic mechanisms promoting genomic instability.
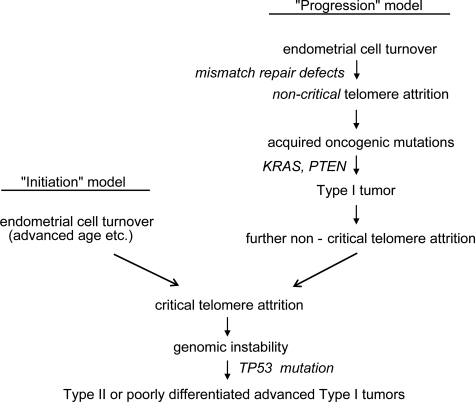
We propose that such telomere shortening in the setting of mismatch repair defects is likely an epiphenomenon that, at least initially, is not a major mechanism driving tumor progression. By the time a tumor is fully established, however, the continual acceleration of cell turnover would eventually lead to telomere crisis. Consistent with this hypothesis we (unpublished data) and others have documented telomerase reactivation in the vast majority of endometrial cancers.38,39,40 A prediction of this model, illustrated in Figure 6, is that advanced TI tumors should exhibit an increased incidence of TP53 mutations and have abnormal karyotypes. Poorly differentiated and advanced stage TI tumors indeed have a much higher incidence of TP53 mutations and (mutant) p53 overexpression by IHC, and are also well-documented in previous studies to be more aneuploid and have abnormal karyotypes.41,42,43,44 Although some aspects of this model need to be refined, we propose that both TII and advanced TI tumors converge to some extent through the common mechanism of telomere attrition and the inactivation of the telomere length checkpoint controlled by p53. Consistent with this view, poorly differentiated/undifferentiated TI tumors behave clinically more like TII tumors, being associated with a much worse prognosis than well-differentiated TI tumors. Such advanced TI tumors do retain distinctive molecular signatures (such as MSI), and differ in some respects from TII tumors (such as in their patterns of spread).42,45,46 The elucidation of environmental or genetic factors influencing telomere length in endometrium as a function of aging remains an important issue for future investigations. The TERT promoter contains multiple estrogen receptor binding sites and estrogen induces TERT expression and telomerase activity. Excess estrogen exposure associated with TI tumorigenesis may serve to protect telomeres and thus partially explain the different roles of telomere attrition in TI and TII tumorigenesis.38,39,47,48,49
Figure 6.
Proposed model of the role of telomere attrition in the initiation and progression of endometrial cancer.
An oncogene-induced DNA damage model of carcinogenesis has recently been proposed. Activated oncogenes such as ras, myc, and E2F1 appear to induce DNA DSBs in various cell types and animal models, reportedly in the absence of telomere erosion. Oncogenes may induce such DNA DSBs through so-called DNA replication stress, in which deregulation of DNA replication proteins leads to an increase of stalled replication forks. DSBs and genome instability have been documented in precancers.50 Because telomere attrition also characterizes precancers including endometrial hyperplasias, it may prove difficult to reliably ascertain the relative contribution of oncogene-induced DNA damage, but it remains a potential mechanism that could further contribute to genomic instability in endometrial cancer, particularly because activating mutations of some oncogenes (ie, KRAS) are present in endometrial hyperplasias.
Although we detected telomere shortening in TII tumors and note that there is no other well-documented pathway of genomic instability in these tumors, this correlation does not firmly establish a causal relationship between telomere shortening and the initiation of TII tumors. Telomere length analyses of human samples may be obscured by a number of confounding variables, such as telomerase reactivation or ALT. To begin to establish this link in an in vivo experimental model system, we analyzed the endometria of mice with critical telomere shortening, and found that by 1 year of age, most contained focal, highly atypical preneoplastic lesions that histologically closely resemble EIC, the in situ precursor to serous TII cancers.5 It is likely that additional mutations are needed to drive the formation of fully invasive TII cancers in the context of short telomeres, particularly p53, but also perhaps additional oncogenic mutations that promote cell-cycle progression or bypass other cellular senescence checkpoints. Although our findings represent a first step toward the development of mouse models of TII endometrial carcinogenesis, the generation of practicable models will benefit from alternative approaches that do not require serial breeding to generate experimental cohorts. Functional inactivation of components of the Shelterin complex that bind to and protect telomeric DNA offers one promising avenue for such investigations.51
Acknowledgments
We thank Jesper Graakjaer, Georgene Hockey, Lane Shirley, Leni Jacobs, and the MD Anderson Uterine Cancer Spore Pathology Core Facility for technical assistance.
Footnotes
Address reprint requests to Diego H. Castrillon, UT Southwestern Medical Center, Department of Pathology, 6000 Harry Hines Blvd., Dallas, TX 75390-9072. E-mail: diego.castrillon@utsouthwestern.edu.
Supported by the MD Anderson P50 Uterine Cancer Spore Pilot Project Award (to D.H.C.) and the Sidney Kimmel Translational Science Award (to D.H.C.).
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- Ellenson LH, Wu TC. Focus on endometrial and cervical cancer. Cancer Cell. 2004;5:533–538. doi: 10.1016/j.ccr.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Clement PB, Young RH. Endometrioid carcinoma of the uterine corpus: a review of its pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol. 2002;9:145–184. doi: 10.1097/00125480-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Clement PB, Young RH. Non-endometrioid carcinomas of the uterine corpus: a review of their pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol. 2004;11:117–142. doi: 10.1097/00125480-200405000-00001. [DOI] [PubMed] [Google Scholar]
- Goodfellow PJ, Buttin BM, Herzog TJ, Rader JS, Gibb RK, Swisher E, Look K, Walls KC, Fan MY, Mutch DG. Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselected series of endometrial cancers. Proc Natl Acad Sci USA. 2003;100:5908–5913. doi: 10.1073/pnas.1030231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Douglas W, Lia M, Edelmann W, Kucherlapati R, Podsypanina K, Parsons R, Ellenson LH. DNA mismatch repair deficiency accelerates endometrial tumorigenesis in Pten heterozygous mice. Am J Pathol. 2002;160:1481–1486. doi: 10.1016/S0002-9440(10)62573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D, Soslow RA, Levine DA, Tornos C, Chen SC, Hummer AJ, Bogomolniy F, Olvera N, Barakat RR, Boyd J. Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J Clin Oncol. 2006;24:1745–1753. doi: 10.1200/JCO.2005.04.1574. [DOI] [PubMed] [Google Scholar]
- Schlosshauer PW, Pirog EC, Levine RL, Ellenson LH. Mutational analysis of the CTNNB1 and APC genes in uterine endometrioid carcinoma. Mod Pathol. 2000;13:1066–1071. doi: 10.1038/modpathol.3880196. [DOI] [PubMed] [Google Scholar]
- Ellenson LH. The molecular biology of endometrial tumorigenesis: does it have a message? Int J Gynecol Pathol. 2000;19:310–313. doi: 10.1097/00004347-200010000-00003. [DOI] [PubMed] [Google Scholar]
- Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol. 2006;24:4783–4791. doi: 10.1200/JCO.2006.06.7173. [DOI] [PubMed] [Google Scholar]
- Bardi G, Pandis N, Schousboe K, Holund B, Heim S. Near-diploid karyotypes with recurrent chromosome abnormalities characterize early-stage endometrial cancer. Cancer Genet Cytogenet. 1995;80:110–114. doi: 10.1016/0165-4608(94)00171-7. [DOI] [PubMed] [Google Scholar]
- Faruqi SA, Satyaswaroop PG, LiVolsi VA, Deger RB, Noumoff JS. Establishment and characterization of a poorly differentiated lethal human endometrial carcinoma cell line (NOU-1) with karyotype 46, XX. Cancer Genet Cytogenet. 2002;138:44–49. doi: 10.1016/s0165-4608(01)00658-6. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Artandi SE, DePinho RA. A critical role for telomeres in suppressing and facilitating carcinogenesis. Curr Opin Genet Dev. 2000;10:39–46. doi: 10.1016/s0959-437x(99)00047-7. [DOI] [PubMed] [Google Scholar]
- DePinho RA, Wong KK. The age of cancer: telomeres, checkpoints, and longevity. J Clin Invest. 2003;111:S9–S14. [PubMed] [Google Scholar]
- Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- Lax SF, Kurman RJ. A dualistic model for endometrial carcinogenesis based on immunohistochemical and molecular genetic analyses. Verh Dtsch Ges Pathol. 1997;81:228–232. [PubMed] [Google Scholar]
- Wu X, Bayle JH, Olson D, Levine AJ. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- van Heek NT, Meeker AK, Kern SE, Yeo CJ, Lillemoe KD, Cameron JL, Offerhaus GJ, Hicks JL, Wilentz RE, Goggins MG, De Marzo AM, Hruban RH, Maitra A. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol. 2002;161:1541–1547. doi: 10.1016/S0002-9440(10)64432-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker AK, Hicks JL, Platz EA, March GE, Bennett CJ, Delannoy MJ, De Marzo AM. Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res. 2002;62:6405–6409. [PubMed] [Google Scholar]
- Meeker AK, Gage WR, Hicks JL, Simon I, Coffman JR, Platz EA, March GE, De Marzo AM. Telomere length assessment in human archival tissues: combined telomere fluorescence in situ hybridization and immunostaining. Am J Pathol. 2002;160:1259–1268. doi: 10.1016/S0002-9440(10)62553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker AK, Hicks JL, Gabrielson E, Strauss WM, De Marzo AM, Argani P. Telomere shortening occurs in subsets of normal breast epithelium as well as in situ and invasive carcinoma. Am J Pathol. 2004;164:925–935. doi: 10.1016/S0002-9440(10)63180-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich U, Griese E, Schwab M, Fritz P, Thon K, Klotz U. Telomere length in different tissues of elderly patients. Mech Ageing Dev. 2000;119:89–99. doi: 10.1016/s0047-6374(00)00173-1. [DOI] [PubMed] [Google Scholar]
- Hemann MT, Greider CW. Wild-derived inbred mouse strains have short telomeres. Nucleic Acids Res. 2000;28:4474–4478. doi: 10.1093/nar/28.22.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn WC. Telomerase and cancer: where and when? Clin Cancer Res. 2001;7:2953–2954. [PubMed] [Google Scholar]
- Taetle R, Aickin M, Yang JM, Panda L, Emerson J, Roe D, Adair L, Thompson F, Liu Y, Wisner L, Davis JR, Trent J, Alberts DS. Chromosome abnormalities in ovarian adenocarcinoma: I. Nonrandom chromosome abnormalities from 244 cases. Genes Chromosom Cancer. 1999;25:290–300. [PubMed] [Google Scholar]
- Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci USA. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- Lax SF. Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular-based classification. Virchows Arch. 2004;444:213–223. doi: 10.1007/s00428-003-0947-3. [DOI] [PubMed] [Google Scholar]
- Meeker AK, Hicks JL, Iacobuzio-Donahue CA, Montgomery EA, Westra WH, Chan TY, Ronnett BM, De Marzo AM. Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin Cancer Res. 2004;10:3317–3326. doi: 10.1158/1078-0432.CCR-0984-03. [DOI] [PubMed] [Google Scholar]
- O'Sullivan JN, Bronner MP, Brentnall TA, Finley JC, Shen WT, Emerson S, Emond MJ, Gollahon KA, Moskovitz AH, Crispin DA, Potter JD, Rabinovitch PS. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat Genet. 2002;32:280–284. doi: 10.1038/ng989. [DOI] [PubMed] [Google Scholar]
- Kuramoto H, Tamura S, Notake Y. Establishment of a cell line of human endometrial adenocarcinoma in vitro. Am J Obstet Gynecol. 1972;114:1012–1019. doi: 10.1016/0002-9378(72)90861-7. [DOI] [PubMed] [Google Scholar]
- Yudate T, Isaka K, Okabe K, Takayama M. Establishment and characterization of the new cell line (EI) from a human endometrial adenocarcinoma. Hum Cell. 1995;8:43–48. [PubMed] [Google Scholar]
- Broaddus RR, Lynch HT, Chen LM, Daniels MS, Conrad P, Munsell MF, White KG, Luthra R, Lu KH. Pathologic features of endometrial carcinoma associated with HNPCC: a comparison with sporadic endometrial carcinoma. Cancer. 2006;106:87–94. doi: 10.1002/cncr.21560. [DOI] [PubMed] [Google Scholar]
- Lehner R, Enomoto T, McGregor JA, Shroyer AL, Haugen BR, Pugazhenthi U, Shroyer KR. Quantitative analysis of telomerase hTERT mRNA and telomerase activity in endometrioid adenocarcinoma and in normal endometrium. Gynecol Oncol. 2002;84:120–125. doi: 10.1006/gyno.2001.6474. [DOI] [PubMed] [Google Scholar]
- Kyo S, Kanaya T, Takakura M, Tanaka M, Inoue M. Human telomerase reverse transcriptase as a critical determinant of telomerase activity in normal and malignant endometrial tissues. Int J Cancer. 1999;80:60–63. doi: 10.1002/(sici)1097-0215(19990105)80:1<60::aid-ijc12>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Maida Y, Kyo S, Kanaya T, Wang Z, Tanaka M, Yatabe N, Nakamura M, Inoue M. Is the telomerase assay useful for screening of endometrial lesions? Int J Cancer. 2002;100:714–718. doi: 10.1002/ijc.10543. [DOI] [PubMed] [Google Scholar]
- Erkanli S, Eren F, Pekin S, Bagis T. BCL-2 and P53 expression in endometrial carcinoma. J Exp Clin Cancer Res. 2004;23:97–103. [PubMed] [Google Scholar]
- Risinger JI, Dent GA, Ignar-Trowbridge D, McLachlan JA, Tsao MS, Senterman M, Boyd J. p53 gene mutations in human endometrial carcinoma. Mol Carcinog. 1992;5:250–253. doi: 10.1002/mc.2940050403. [DOI] [PubMed] [Google Scholar]
- Micci F, Teixeira MR, Haugom L, Kristensen G, Abeler VM, Heim S. Genomic aberrations in carcinomas of the uterine corpus. Genes Chromosom Cancer. 2004;40:229–246. doi: 10.1002/gcc.20038. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Watanabe Y, Nanjoh T, Noda K. Evaluation of DNA ploidy in endometrial cancer. Gynecol Oncol. 1993;50:25–29. doi: 10.1006/gyno.1993.1158. [DOI] [PubMed] [Google Scholar]
- Lax SF, Kendall B, Tashiro H, Slebos RJ, Hedrick L. The frequency of p53. K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer. 2000;88:814–824. [PubMed] [Google Scholar]
- Riethdorf L, Begemann C, Riethdorf S, Milde-Langosch K, Loning T. Comparison of benign and malignant endometrial lesions for their p53 state, using immunohistochemistry and temperature-gradient gel electrophoresis. Virchows Arch. 1996;428:47–51. doi: 10.1007/BF00192926. [DOI] [PubMed] [Google Scholar]
- Boggess JF, Zhou C, Bae-Jump VL, Gehrig PA, Whang YE. Estrogen-receptor-dependent regulation of telomerase activity in human endometrial cancer cell lines. Gynecol Oncol. 2006;103:417–424. doi: 10.1016/j.ygyno.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Williams CD, Boggess JF, LaMarque LR, Meyer WR, Murray MJ, Fritz MA, Lessey BA. A prospective, randomized study of endometrial telomerase during the menstrual cycle. J Clin Endocrinol Metab. 2001;86:3912–3917. doi: 10.1210/jcem.86.8.7729. [DOI] [PubMed] [Google Scholar]
- Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, Orimo A, Inoue M. Estrogen activates telomerase. Cancer Res. 1999;59:5917–5921. [PubMed] [Google Scholar]
- Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319:1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]