Abstract
Inflammatory breast carcinoma (IBC) is a particularly lethal form of breast cancer characterized by exaggerated lymphovascular invasion, which is a phenotype recapitulated in our human xenograft MARY-X. MARY-X generated spheroids in vitro that resemble the embryonal blastocyst. Because of the resemblance of the spheroids to the embryonal blastocyst and their resistance to traditional chemotherapy/radiotherapy, we hypothesized that the spheroids expressed a stem cell-like phenotype. MARY-X spheroids expressed embryonal stem cell markers including stellar, rex-1, nestin, H19, and potent transcriptional factors, oct-4, nanog, and sox-2, which are associated with stem cell self-renewal and developmental potential. Most importantly, MARY-X spheroids expressed a cancer stem cell profile characterized by CD44+/CD24−/low, ALDH1, and most uniquely, CD133. A significant percentage of single cells of MARY-X exhibited distinct proliferative and morphogenic potencies in vitro. As few as 100 cells derived from single-cell clonogenic expansion were tumorigenic with recapitulation of the IBC phenotype. Prototype stem cell signaling pathways such as notch3 were active in MARY-X. The stem cell phenotype exhibited by MARY-X also was exhibited by the lymphovascular emboli of human IBC cases independent of their molecular subtype. This stem cell-like phenotype may contribute to the aggressive nature of IBC but also may lend itself to selective targeting.
Inflammatory breast cancer (IBC) is an aggressive form of human breast cancer characterized by florid lymphovascular invasion (LVI) and early metastasis.1,2 LVI is considered an important rate-limiting step in the metastatic process and is characterized by the formation of tumor emboli within lymphovascular channels.3,4 These emboli are relatively resistant to radiotherapy and chemotherapy because the tight aggregates of tumor cells exert autocrine and paracrine cytoprotective effects from these therapies by unknown mechanisms.5,6 These emboli are also efficient at escaping local organ confinement with subsequent distant implantation. They therefore exhibit a strong penchant for both distant metastasis as well as local recurrence and confound attempts at both local as well as systemic control. The tumor emboli bear a strong resemblance to the human embryonal blastocyst, a structure also efficient at escaping local organ confinement with subsequent distant implantation. The IBC phenotype has been successfully recapitulated in a human xenograft model of IBC termed MARY-X.3,4,5,6,7 This model exhibits florid LVI in vivo, which generates tight aggregates of tumor cells (spheroids) in vitro. These spheroids also bear a strong resemblance to the blastocyst. Because the blastocyst is the source of human embryonal stem cells (ESs), cells capable of self renewal, proliferation and differentiation along multiple lineages and because tumor stem cells have been implicated in the properties of local recurrence, distant metastasis, and drug resistance that human cancers exhibit, we hypothesized that the tumor lymphovascular emboli of MARY-X and its derived spheroids might, in fact, express a stem cell-like phenotype reflected by both stem cell markers and stem cell biology. In addition we could test this hypothesis in human cases of IBC and, if confirmed, might explain some of the aggressive behavior of IBC.
Materials and Methods
Institutional Approvals
Use of human tissues was approved by The Ohio State University Cancer Institutional Review Board under protocol 2006C0042. Specifically 25 cases of primary IBC with florid LVI and 25 non-IBC cancers and 5 normal breast tissues were selected from a patient database in CoPath and The Ohio State University’s Information Warehouse and anonymized. These tissues were available both as archival paraffin blocks as well as OCT-embedded frozen material. We chose IBC and non-IBC cases that comprised the major molecular subtypes of human breast cancer. All linkers to patient identity were removed before study. All animal and in vitro studies were approved by The Ohio State University’s Animal Care and Use Committee, protocol 2007A0218 and by The Ohio State University’s Institutional Biosafety Committee, protocol 2007R0057.
Cell Lines and Xenograft Studies
MARY-X was previously established from a patient with IBC and exhibited the phenotype of florid LVI with tumor emboli formation in nude/scid mice.3,4,5,6,7 MARY-X gave rise to tight aggregates of tumor cells termed spheroids in vitro, which could be propagated in suspension culture. These primary spheroids could be maintained in suspension culture for periods up to 3 months by growing them in either keratinocyte serum-free medium with supplements (Life Technologies, Inc., Gaithersburg, MD) or in minimal essential medium with 10% fetal calf serum (Life Technologies, Inc.). The individual tumor cells were held together within the spheroids by an overexpressed E-cadherin axis,3,4 which if neutralized with anti-E-cadherin or ethylenediaminetetraacetic acid, released individual single cells. As comparisons we chose non-IBC lines that included common estrogen receptor (ER)-positive (MCF-7) and ER-negative (MDA-MB-231 and MDA-MB-468) (American Type Culture Collection, Manassas, VA). As additional comparisons, we chose cell lines reflective of normal epithelial and myoepithelial cells of the breast or their surrogates: human mammary epithelial cells (Clonetics, San Diego, CA) and HMS-1, a human myoepithelial line previously established by us.8 As a positive control for known stem cell markers and stem cell signaling, we chose a human embryonal carcinoma cell line, HTB106 (American Type Culture Collection).
Immunocytochemical and Western Blot Studies
Primary murine monoclonal antibodies included anti-human CD133 (clones AC133, unconjugated; AC133, phycoerythrin (PE)-conjugated; AC141, PE-conjugated; 293C3, PE-conjugated; Miltenyi Biotec, Inc., Auburn, CA); anti-human CD44 (clone 515, PE-conjugated; BD Pharmingen, San Jose, CA); anti-human CD24 (clone eBioSN3, fluorescein isothiocyanate-conjugated; eBioscience, San Diego, CA); anti-human E-cadherin (clone HECD-1, unconjugated; Zymed Laboratories, Inc., South San Francisco, CA); anti-human CK7 (clone OV-TL 12/30, unconjugated; DAKO, Carpinteria, CA); anti-human SMA (clone 1A4, unconjugated; DAKO); anti-human ALDH1 (clone 44, unconjugated; BD Pharmingen); and anti-human ABCG2 (clone 5D3, fluorescein isothiocyanate-conjugated; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Control antibodies included mouse IgG1 (clone X40, unconjugated; BD Pharmingen); mouse IgG2b (clone MPC-11, unconjugated; BD Pharmingen). Secondary antibodies included goat anti-mouse IgG, fluorescein isothiocyanate-conjugated (BD Pharmingen); goat anti-mouse IgG, Texas Red-conjugated (Rockland Immunochemicals, Gilbertsville, PA); rabbit anti-mouse IgG, horseradish peroxidase-conjugated (Cell Signaling Technology, Inc., Danvers, MA).
Primary rabbit polyclonal antibodies included anti-human CD133, unconjugated (Abcam, Cambridge, MA), anti-human notch1, unconjugated; anti-human notch2, unconjugated; anti-human notch3, unconjugated; anti-human notch4, unconjugated; anti-human jagged1, unconjugated; and anti-human DLL1, unconjugated (Santa Cruz Biotechnology, Inc.). The notch antibodies recognized a notch intracellular domain (Nicd) of their respective receptor that was cleaved and translocated to the nucleus when notch signaling occurred. Control antibodies included normal rabbit IgG, unconjugated (Sigma-Aldrich, St. Louis, MO). Secondary antibodies included goat anti-rabbit IgG, horseradish peroxidase-conjugated (Cell Signaling Technology, Inc.), and goat anti-rabbit IgG, fluorescein isothiocyanate-conjugated (Sigma-Aldrich).
For immunocytochemical studies, MARY-X spheroids were fixed in cold methanol:acetone (1:1) for 20 minutes. Cells were blocked with phosphate-buffered saline (PBS) plus 1% normal donkey serum for 30 minutes and followed by incubation with the respective antibodies for 45 minutes. For the unconjugated antibodies, incubation with secondary antibody occurred for 30 minutes. The nuclei were counterstained with Hoechst 33342 dye (5 μg/ml) (Sigma-Aldrich). The stained cells were imaged on a Nikon fluorescent microscope using the Roper camera and MetaVue software (Universal Imaging Corporation, Downington, PA).
For Western blots, normal and tumor cell lines were harvested and then frozen immediately. The frozen cell pellets were then extracted with buffer (1% Triton X-100, 150 mmol/L NaCl, 10 mmol/L sodium phosphate, and 10 mmol/L ethylenediaminetetraacetic acid) containing an enzyme inhibitor cocktail (Pierce Biotechnology, Inc., Rockford, IL) for 4 hours at 4°C with gentle agitation. The samples were then centrifuged at 13,000 × g at 4°C for 15 minutes. Protein concentrations were determined using the BCA protein assay (Pierce Biotechnology, Inc.) according to the manufacturer’s instructions. Samples containing equal protein were boiled in Laemmli buffer under reducing conditions, run on a 4 to 15% sodium dodecyl sulfate-polyacrylamide gel, and transferred to a polyvinylidene difluoride membrane that was then incubated with the respective rabbit primary and horseradish peroxidase-labeled goat anti-rabbit secondary antibodies and the signal was detected with the Supersignal West Dura extended duration substrate (Pierce Biotechnology, Inc.). A monoclonal antibody to β-actin (Cell Signaling Technology, Inc.) was used to normalize for protein loading followed by rabbit anti-mouse IgG, horseradish peroxidase-conjugated (Cell Signaling Technology, Inc.).
RNA Isolation and Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Studies
Total RNA was isolated using RNeasy and treated with DNase I to remove contaminating DNA according to the manufacturer’s instructions (Qiagen, Valencia, CA). RNA quality and quantity were determined by measuring absorbance at 260 and 280 nm. Oligo(dT) primers (Integrated DNA Technologies, Coralville, IA) were used with Superscript II reverse transcriptase (Invitrogen Corporation, Carlsbad, CA) for cDNA synthesis from 1 μg of total RNA following the guidelines provided by the manufacturer. PCR was then conducted with Taq polymerase (Invitrogen Corporation). The forward and reverse primer pairs were as follows: oct-4: 5′-CTGCCTGCCCTTCTAGGAAT-3′ and 5′-TCTACTGTGTCCCAAGCTTCTT-3′; nanog: 5′-GATCGGGCCCGCCACCATGAGTGTGGATCCAGCTTG-3′ and 5′-GATCGAGCTCCATCTTCACACGTCTTCAGGTTG-3′; sox-2: 5′-CCTCCGGGACATGATCAG-3′ and 5′-TTCTCCCCCCTCCAGTTC-3′; stellar: 5′-GGACCCATCACAGTTTAATCC-3′ and 5′-GAAACTGCAGGGACATTTGA-3′; H19: 5′-TTCCAGGCAGAAAGAGCAAGAGGGC-3′ and 5′-AGACGTCCTGCTGCAACTCCCCGAG-3′; rex-1: 5′-CAACTGAAGAAACGGGCAAA-3′ and 5′-CTCAAGCGAATTCTCTCCAA-3′; nestin: 5′-AGAGGGGAATTCCTGGAG-3′ and 5′-CTGAGGACCAGGACTCTCTA-3′; cd133: 5′-ATCAGAACTGCAATCTGCACA-3′ and 5′-AGAAGATCCCTGTCACAATTCC- 3′; hes-1: 5′-ATGGAGAAAAATTCCTCGTCCC-3′ and 5′-TTCAGAGCATCCAAAATCAGTGT-3′; hes-5: 5′-CTCAGCCCCAAAGAGAAAAA-3′ and 5′-GACAGCCATCTCCAGGATGT-3′; hey-1: 5′-GAAACTTGAGTTCGGCTCTAGG-3′ and 5′-GCTTAGCAGATCCTTGCTCCAT-3′; hey-2: 5′-AGGGGGTAAAGGCTACTTTGA-3′ and 5′-TGGCGCAAGTGCTGAGATG-3′. β-Actin was used as control: 5′-CATGTACGTTGCTATCCAGGC-3′ and 5′-CTCCTTAATGTCACGCACGAT-3′. The mixture was first heated at 94°C for 2 minutes in a PTC-200 DNA engine thermal cycler (Bio-Rad, Hercules, CA). Amplification was performed for 33 cycles at 94 degrees for 30 seconds, 55°C for 30 seconds, and 68°C for 60 seconds, followed by 72°C for 10 minutes. The PCR products were separated on 1.5% agarose gels by electrophoresis. Digital images were captured on a Fotodyne (Hartland, WI) gel documentation system.
In Vitro and in Vivo Manipulations of MARY-X Spheroids
The primary spheroids derived from MARY-X were collected by gentle centrifugation (800 rpm) and dissociated either enzymatically (10 minutes in 0.05% trypsin), by ethylenediaminetetraacetic acid chelation or with anti-E-cadherin antibodies according to previous methods3,4,5,6,7 with subsequent physical manipulations using a fire-polished Pasteur pipette. The cells obtained from dissociation were neutralized with Hanks’ balanced salt solution and then sieved through a 40-μ cell strainer and analyzed for cellularity with a phase contrast microscope and determined to be singular.
For the side population (SP) experiments, 1 × 106 cells/ml were incubated in prewarmed Dulbecco’s modified Eagle’s medium with 5% fetal calf serum containing freshly added Hoechst 33342 (5 μg/ml, final concentration) for 60 minutes at 37°C. After this time, the cells were spun down and resuspended in cold PBS with 2% bovine serum albumin. The cells were then exposed to UV light at 350 nm and viewed under a fluorescent microscope. The SP was defined as those cells that were capable of excluding the dye.
In another set of experiments, the single cell suspension was labeled with PE-conjugated anti-CD133 (clone 293C3) and sorted. Before sorting, cells were mixed with 7-amino-actinomycin (7-AAD) (BD Pharmingen) for 10 minutes to identify the nonviable cells. Cells (1.2 × 107) were sorted using a BD FACSAria cell-sorting system (BD Biosciences, San Jose, CA) and 8.5 × 106 cells were recovered and verified to be CD133+ and 7-AAD−. A minor population of CD133− was also recovered. After sorting, each population was studied in vitro and in vivo. To test the self-renewal and proliferation capacity of the MARY-X cells, 102 single cells were plated in 96-well plates using limiting clonal dilution and monitored with phase contrast microscopy. In this clonogenic assay, single cells were grown in serum-free mammary epithelial growth medium, (BioWhittaker, Walkersville, MD), supplemented with B27 (Invitrogen Corporation), 20 ng/ml epidermal growth factor, 20 ng/ml basic fibroblast growth factor (BD Biosciences), and 4 μg/ml heparin (Sigma-Aldrich). Bovine pituitary extract was excluded. The ability of single cells to proliferate into secondary spheroids was recorded. Cells exhibiting this clonogenic potential were injected into groups of 10 immunodeficient mice (nude or scid) and tumorigenicity and the IBC phenotype was assessed. In these experiments, secondary spheroids of varying sizes containing different numbers of cells were injected to gauge the tumorigenicity threshold.
In a third set of experiments, to test the morphogenic capacity of the MARY-X cells, sorted single cells were also suspended in three-dimensional growth factor-reduced Matrigel (BD Biosciences):Dulbecco’s modified Eagle’s medium/F-12 medium mixture (1:1) with 5% fetal calf serum, 5 μg/ml insulin (Sigma-Aldrich), 1 μg/ml hydrocortisone (Sigma-Aldrich), 10 μg/ml cholera toxin (Sigma-Aldrich), and 10 ng/ml epidermal growth factor (BD Biosciences) in a 24-well plate at a density of 200 cells per well. The cells were observed for several weeks for morphogenic changes. Representative foci were fixed, sectioned, and subjected to immunohistochemistry (IHC) studies.
Because MARY-X spontaneously forms spheroids in vitro, whereas the non-IBC carcinoma and normal cell lines that were used in the comparative studies normally grow as monolayers, it was necessary to induce spheroid growth in these non-IBC lines so that they would provide a more valid comparison. For the human mammary epithelial cells, single cells were dissociated by TrypLE express (Invitrogen Corporation) and seeded in ultralow attachment plates (Corning, Inc., Corning, NY) at a density of 20,000 viable cells/ml in primary culture. Cells were grown in a serum-free mammary epithelial growth medium, supplemented with B27, 20 ng/ml epidermal growth factor, 20 ng/ml basic fibroblast growth factor, and 4 μg/ml heparin. After 7 to 10 days, human mammary epithelial cell spheroids (mammospheres) were collected by centrifugation (800 rpm) using previously established methods.9 For HMS-1 cells it was discovered that at superconfluent densities, HMS-1 cells form spheroids that loosely adhere to the underlying monolayer and can be harvested with gentle agitation.8 For MCF-7, MDA-MB-231, and MDA-MB-468 cell lines, induction of spheroids could be achieved by first coating the plates with a layer of 1% agar to prevent cell attachment. Then the cells were seeded at a density of 20,000 cells/ml in Dulbecco’s modified Eagle’s medium with 10% fetal calf serum. The induced spheroids were harvested at 4 to 5 days. The spheroids derived from normal and non-IBC lines formed loosely adherent aggregates in suspension culture in contrast to the high-density tightly bound superadherent aggregates exhibited by the MARY-X spheroids.
ALDEFLUOR Assay
The ALDEFLUOR kit (StemCell Technologies, Durham, NC) was applied to identify a cell population with high aldehyde dehydrogenase (ALDH) enzymatic activity. Single cells dissociated from MARY-X spheroids enzymatically (10 minutes in 0.05% trypsin) were first gated for viability by the absence of PI staining. The sorted viable cells were suspended at a density of 106 cells/ml in ALDEFLUOR assay buffer containing 1 μmol/L ALDH substrate bodipyaminoacetaldehyde (BAAA). The cells were incubated for 40 minutes at 37°C. At the same time, a sample was treated with 50 mmol/L diethylaminobenzaldehyde (DEAB), a specific ALDH inhibitor and studied in parallel. The cells were analyzed using a BD FACSAria cell-sorting and flow cytometric system (BD Biosciences).
Studies of Human IBC Cases
Twenty-five cases of IBC were studied immunocytochemically for CD133 expression and notch signaling. Twenty-five non-IBC breast cancers and five normal breast tissues were used as controls. Both the IBC and the non-IBC cases were selected from the molecular subtypes of human breast cancer, including ER-positive, Her-2/neu-positive, and triple-negative determined by previous IHC studies. For CD133, the primary antibody was a mouse anti-human CD133 (clone AC133, unconjugated; Miltenyi Biotec, Inc.), diluted 1:25 and incubated for 1 hour at room temperature. The detection system was a universal link/label system (catalog no. K0690, DAKO). For notch3, the primary antibody was a rabbit polyclonal anti-human notch3, unconjugated (Santa Cruz Biotechnology, Inc.), diluted 1:75, and incubated for 1 hour at room temperature. This notch antibody recognized the notch intracellular domain (Nicd) of the notch receptor that was cleaved and nuclear translocated during notch signaling. The secondary antibody was a goat anti-rabbit (catalog no. BA-1000; Vector Laboratories, Burlingame, CA), diluted 1:200 and incubated for 30 minutes. The detection system was Vectastain Elite (catalog no. PK-6100, Vector Laboratories). For both CD133 and notch3, the substrate chromogen was DAB+ (catalog no. K3468, DAKO). Both antibodies worked much better in the frozen material than in paraffin and so these IHC studies were performed on frozen sections. These cases were also studied for ALDH1 immunoreactivity using mouse anti- human ALDH1 (clone 44, unconjugated; BD Pharmingen) diluted 1/100 and incubated for 1 hour at room temperature. The detection system was a universal link/label system (catalog no. K0690, DAKO). The antibody worked well in both the frozen as well as the paraffin material, when the paraffin sections were processed by antigen retrieval. We elected to use the latter source for our studies.
Statistical Analysis
Differences in stem cell marker immunoreactivity between subgroups of human IBC and non-IBC were analyzed with the two-tailed Student’s t-test as well as analysis of variance. Weighted κ statistics were calculated to assess agreement between certain immunocytochemical measurements in the subgroups of human IBC and non-IBC.
Results
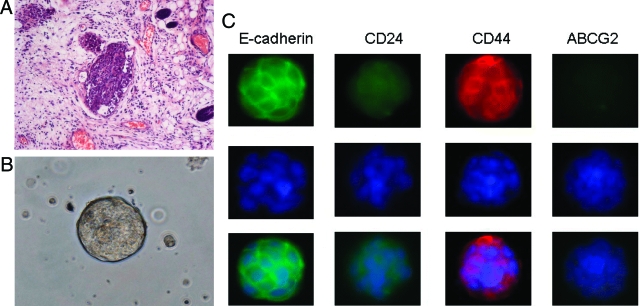
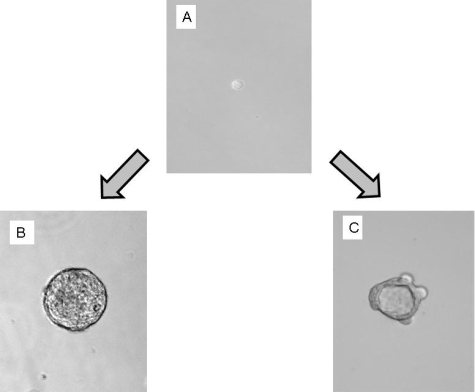
MARY-X manifested the phenotype of florid LVI in scid/nude mice (Figure 1A). The xenograft gave rise to tight aggregates of tumor cells termed spheroids in suspension culture (Figure 1B) mediated by an overexpressed E-cadherin axis (Figure 1C) and, as such, bore a strong resemblance to the human embryonal blastocyst, the source of ESs. The Mary-X spheroids, as three-dimensional aggregates, expressed a stem cell marker phenotype of CD44+/CD24−/low in immunofluorescence studies (Figure 1C). This phenotype of E-cadherin+/CD44+/CD24−/low was expressed in virtually 100% of the cells. We injected the MARY-X spheroids into a number of different locations in the mouse including the mammary fat pads, the flank, and back and the phenotype was the same: tumor nodules exhibiting florid LVI in vivo and generating numerous spheroids in vitro. MARY-X cells gained access to the lymphatics very early irrespective of their site of injection and metastasized to the regional lymph nodes as well as lungs. Their tumorigenicity and biology were independent of their site of injection.
Figure 1.
A: MARY-X manifested the phenotype of IBC in vivo, namely florid LVI. B: In vitro, MARY-X grew as spheroids, tight balls of tumor cells that resemble the embryonal blastocyst. Immunofluorescence studies of the spheroids revealed strong expression of membrane E-cadherin and membrane CD44 but weak to absent expression of CD24 and ABCG2. C: Top row is fluorescently labeled primary/secondary antibody, middle row is Hoechst 33342 nuclear counterstain, and bottom row is the composite image.
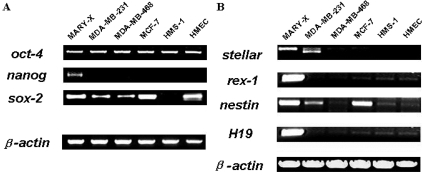
In addition, the MARY-X spheroids contained a large percentage of cells that expressed other stem cell markers known to be associated with normal ESs or pluripotent stem cells (Table 1 and Figure 2, A and B). Specifically the spheroids, by RT-PCR, expressed stellar, rex-1, nestin, H19, and other markers usually restricted to normal human embryonal or tissue progenitor cells (Table 1 and Figure 2B). Although some of these markers were expressed by non-IBC carcinoma and normal cell lines, only MARY-X expressed all of these stem cell markers (Figure 2B). Not only did the MARY-X spheroids express these general stem cell markers but, more specific transcription factors, including oct-4, nanog, sox-2, and others (Table 1 and Figure 2A), thought to have essential roles in pluripotency and self-renewal of embryonic stem cells10,11 and just recently shown to be capable of inducing reversion to an embryonic stem cell state when transfected into fetal, newborn, or adult fibroblasts.12,13 Although some of these transcription factors were detected in non-IBC carcinoma and normal cell lines, only MARY-X expressed all of them (Figure 2A). A complete list of stem cell markers and transcription factors demonstrated to be present in MARY-X is listed (Table 1).
Table 1.
Summary of MARY-X’s Expression of Known Human Stem Cell Markers in Normal Cells
| Stem cell type | Molecular marker | Significance | Expression in MARY-X spheroids |
|---|---|---|---|
| Embryonic stem cells (ES) or pluripotent stem cells (PS) | Oct-4 | Transcription factor essential for establishment and maintenance of undifferentiated PS | Yes |
| Pax-6 | Transcription factor expressed as ES differentiates into neuroepithelium | Yes | |
| Stellar | Specific marker of undifferentiated ES | Yes | |
| Alpha-fetoprotein (AFP) | Reflects endodermal differentiation of PS | Yes | |
| Rex-1 | Specific marker of undifferentiated ES | Yes | |
| Germ cell nuclear factor (GCNF) | Transcription factor expressed by PS | Yes | |
| Sox-2 | Transcription factor essential for establishment and maintenance of undifferentiated PS | Yes | |
| H19 | Marker developmentally regulated in skeletal muscle, smooth muscle, and fetal liver | Yes | |
| Nanog | Transcription factor unique to PS; essential for establishment/maintenance of undifferentiated PS | Yes | |
| Hematopoietic stem cells (HS) | CD34 | Indicative of HS and EP | No |
| c-kit | Cell-surface receptor on bone marrow cell types that identifies HS and MS | Yes | |
| Mesenchymal stem and progenitor cells (MS) | Bone morphogenetic protein receptor (BMPR) | BMPR identifies early mesenchymal lineages (MS) | Not determined |
| Stro-1 antigen | Cell-surface glycoprotein on subsets of bone marrow MS | Not determined | |
| Neural stem cells (NS) | CD133 | Identifies NS and HS | Yes |
| Nestin | Identifies NS | Yes | |
| Endothelial progenitor cells (EP) | Fetal liver kinase-1 (Flk-1) | Cell-surface receptor protein that identifies EP | Yes |
| Breast stem cells (BS) | ALDH1 | Enzyme present in normal mammary stem cells | Yes |
This table is meant as a general guide to stem cell marker expression in MARY-X. Many of these markers are also expressed by other types of normal stem cells in addition to the ones indicated. A more extensive listing is available from the National Institutes of Health at: http://stemcells. nih.gov/info/scireport/appendixe.asp#eii.
Figure 2.
A: The MARY-X spheroids, assessed by RT-PCR, expressed transcriptional factors associated with the maintenance of the stem cell state in ESs. These factors included: oct-4, nanog, and sox-2. B: In addition MARY-X expressed stem cell markers primarily restricted to normal ESs. These included stellar, H19, rex-1, and nestin.
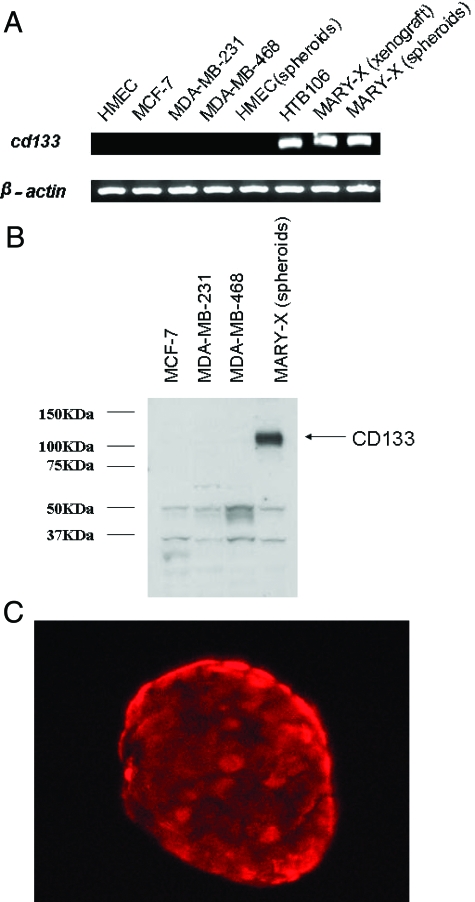
Most importantly, MARY-X spheroids also expressed a human cancer stem cell (CSC) marker profile (Table 2) characterized by strong expression of CD133 by both RT-PCR as well as Western blot (Figure 3, A and B). This CD133 expression could be detected by three different monoclonal antibodies (AC133, AC141, and 293C3) directed against distinct epitopes of CD133. CD133 was not at all expressed on non-IBC breast carcinoma lines nor on normal epithelial or myoepithelial cell surrogates (Figure 3, A and B). Strong CD133 expression was present, however, in the human embryonal carcinoma cell line, HTB106, which was used as a positive control. Immunofluorescence studies of the MARY-X spheroids, revealed strong surface expression of CD133 on >90% of the cells (Figure 3C), a finding confirmed by flow cytometry and cell sorting. Even when the non-IBC carcinoma and normal cells were grown as spheroids, CD133 was not expressed.
Table 2.
Summary of MARY-X’s Expression of Known Human Cancer Stem Cell Markers
| Human cancer type | Phenotypic marker | Side population | Studies* |
|---|---|---|---|
| Leukemia | CD34+/CD38−, CD44+ | Yes | 14 |
| Breast cancer | CD44+/CD24−/low, spheroid formation, ALDH1+, notch3 | Yes | 16, 28, 31 |
| Prostate cancer | CD44+/α2β1hi/CD133+ | Not determined | 20 |
| Melanoma | CD20+, spheroid formation | Yes | 30 |
| Brain cancer | CD133+, neurospheroid formation | Not determined | 15 |
| Retinoblastoma | ABCG2+, ALDH1+ | Yes | 44 |
| Colon cancer | CD133+/CD44+/EpCAM+/CD166+ | Not determined | 21, 39 |
| Pancreatic cancer | CD44+/CD24+/ESA+ | Not determined | 40 |
| Head and neck cancer | CD44+ | Not determined | 41 |
| Liver cancer | CD90+ | Side population | 42, 43 |
| MARY-X (IBC) | CD44+/CD133+/CD24−/low spheroid formation, ALDH1+, notch3 | No | Present study |
Cited studies listed in References.
Figure 3.
The MARY-X (xenograft and spheroids) expressed CD133 by both RT-PCR (A) and Western blot (B). C: Immunofluorescence studies using anti-CD133 (PE-conjugated clone AC133) depicted strong surface immunoreactivity. Non-IBC lines were uniformly negative for CD133 except for the control human embryonal carcinoma cell line, HTB 106.
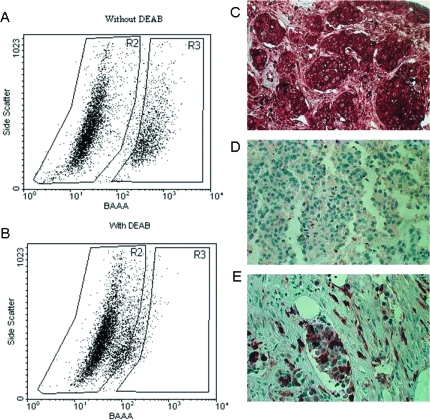
A high activity of aldehyde dehydrogenase (ALDH), the enzyme responsible for the oxidation of intracellular aldehydes, considered as a possible marker for both normal and CSCs was observed in the cells comprising the MARY-X spheroids. When the spheroids were disadhered into single cells, ∼23% of the cells exhibited ALDH activity by the ALDEFLUOR flow cytometric assay (Figure 4, A and B). The presence of ALDH activity was corroborated by the presence of similar levels of ALDH1 immunoreactivity within the lymphovascular emboli of MARY-X (data not shown).
Figure 4.
The ALDEFLUOR assay was applied to identify the ALDEFLUOR-positive population exhibiting high aldehyde dehydrogenase (ALDH) enzymatic activity within the MARY-X spheroids. A single cell suspension of disadhered cells was incubated with ALDEFLUOR substrate (BAAA) without (A) and with (B) the specific inhibitor of ALDH, DEAB, and used to establish the baseline fluorescence of these cells (R2) and to define the ALDEFLUOR-positive region (R3). In all experiments, cells were first gated on PI-negative cells (viable cells). The ALDEFLUOR-positive population (R3) is apparent (A) but abolished with the inhibitor (B). Approximately 23% of the cells of the MARY-X spheroids were ALDEFLUOR-positive. Immunocytochemical studies using anti-human ALDH1 in cases of IBC similarly revealed high percentages of ALDH1-positive cells. C: Some cases exhibited 100% ALDH1 immunoreactivity within their lymphovascular emboli. D: Many non-IBC cases were completely negative for ALDH1. E: In some non-IBC cases in which overall ALDH1 immunoreactivity was absent or low, their lymphovascular emboli contained enriched ALDH1 immunoreactivity.
MARY-X did not, however, express every known stem cell marker. In particular stem cell markers associated with hematopoietic stem cells such as CD34 were absent in MARY-X (Table 1). Another marker, ABCG2, a determinant of the Hoechst-negative phenotype of the SP found in a variety of stem cells (Table 2), was absent in the MARY-X spheroids (Figure 1C). A SP predictably was also absent in MARY-X (Table 2).
The dual capabilities of self-renewal/proliferation and differentiation/morphogenesis, two essential properties of stem cells were tested for in the MARY-X spheroids (Table 3) (Figures 5 and 6). Single cells dissociated from MARY-X spheroids and were sorted on the basis of CD133+ were subsequently seeded by limiting clonal dilution and cultured in the clonogenic assay. Approximately 10 to 30% of these single CD133+ cells were able to form secondary spheroids (Figure 5, A and B). CD133− cells lacked the ability to form spheroids. This clonogenic efficiency was similar to that found when single CD133+ cells of MARY-X were seeded together at low densities (1000 cells/ml). These observations demonstrated that secondary spheroids of MARY-X could be generated without the obligate requirement of cell aggregation/cell-cell contact. Both primary and secondary spheroids consisting of as few as 100 CD133+ cells were 90% tumorigenic, forming tumors with the ability to completely recapitulate the IBC phenotype of florid LVI, which could be transferred with serial passage. Therefore single CD133+ cells of MARY-X demonstrated a capacity for both proliferation as well as the ability to recapitulate the unique tumoral phenotype of LVI in perpetuity. This implied that single CD133+ cells of MARY-X exhibited the capacity for self-renewal. The CD133− cells, in contrast, were nontumorigenic. Disadhering the spheroids caused a profound degree of apoptosis after 4 to 6 hours in the majority of cells. The majority of the CD133+ and CD133−, when disadhered into single cells, then could not survive for long in culture. We were able to generate spheroids from the isolated CD133+ cells but not the CD133− cells. We were not able to keep the CD133− cells alive long enough to determine whether they could change into CD133+ cells.
Table 3.
Summary of MARY-X’s Stem Cell Properties
| Stem cell properties exhibited by MARY-X spheroids | |
|---|---|
| Stem cell specific markers | Transcriptional determinants as well as specific markers (oct-4, sox-2, nanog, rex-1, and so forth) known to be restricted to normal embryonal or tissue stem cells were detected within the spheroids of MARY-X. In addition, CD44 and CD133, surface markers of cancer stem cells previously identified in a series of different human cancers, were strongly expressed on MARY-X spheroids |
| Proliferation | A single cell of MARY-X was able to generate secondary spheroids without initial cell aggregation/cell-cell contact; spheroids containing as few as 100 cells were fully tumorigenic |
| Morphogenesis | A single cell of MARY-X cultured in Matrigel generated acinar structures in vitro, composed of cells exhibiting separate epithelial and myoepithelial lineage markers |
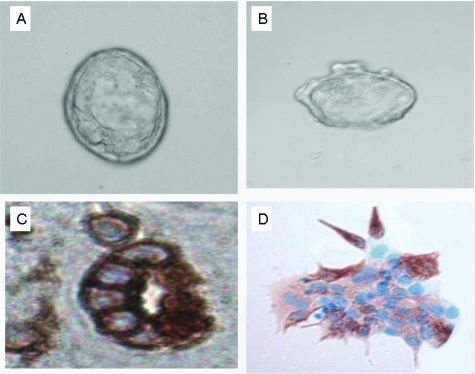
Figure 5.
A single stem cell of MARY-X (A) was capable of either proliferation into spheroids (B) or morphogenesis into acini (C) independent of any initial cell-cell contacts. Single cells of MARY-X could demonstrate either of these properties depending on the specific growth conditions. The secondary spheroids were fully tumorigenic and expressive of the IBC phenotype of florid LVI.
Figure 6.
More detailed examination of the acinar morphogenesis (A, B) revealed CK7-positive epithelial cells arranged centrally around a central lumen (A, C) and peripherally located spindle cells (B, D) exhibiting features of myoepithelial cells by strong SMA immunoreactivity.
To examine the morphogenic ability of MARY-X, single cells of the MARY-X spheroids were plated at very low densities (200 cells/1.5 ml of gel) in three-dimensional growth factor-reduced Matrigel, under conditions that promoted morphogenesis rather than proliferation. After 2 weeks of cultivation in Matrigel, acini were observed (Figure 5, A and C). We examined a number of different acinar structures (Figure 6, A and B) and showed by IHC studies with anti-CK7 and anti-SMA that these structures consisted of cells that had differentiated into either epithelial (Figure 6C) or myoepithelial cells (Figure 6D), recapitulating acinar development. Many of the acinar structures exhibited a spatial relationship between the myoepithelial and epithelial cells with the former arranged peripherally and the latter arranged more centrally around a lumen. This was reminiscent of the breast ductal-lobular unit. The acinar structures were therefore different from the single parental cell from which they were derived.
This manifestation of morphogenesis was likewise independent of cell-cell contact. CD133− cells, in contrast, lacked the ability to form acini. The percentage of single CD133+ cells capable of forming acini was ∼10 to 30% and similar to the percentage able to form spheroids in the clonogenic assay. The ability to form acini was considered evidence of morphogenic capacity. However single cells of MARY-X did not exhibit evidence of pluripotency, a trait exhibited by ESs. Pluripotency, at least in the context of ESs, is usually defined as exhibiting the ability to differentiate along the three germ lines: ectoderm, mesoderm, and endoderm. MARY-X, however, did not exhibit this property. However MARY-X’s ability to develop into acinar structures containing epithelial as well as myoepithelial cells could be interpreted as an example of multipotency.
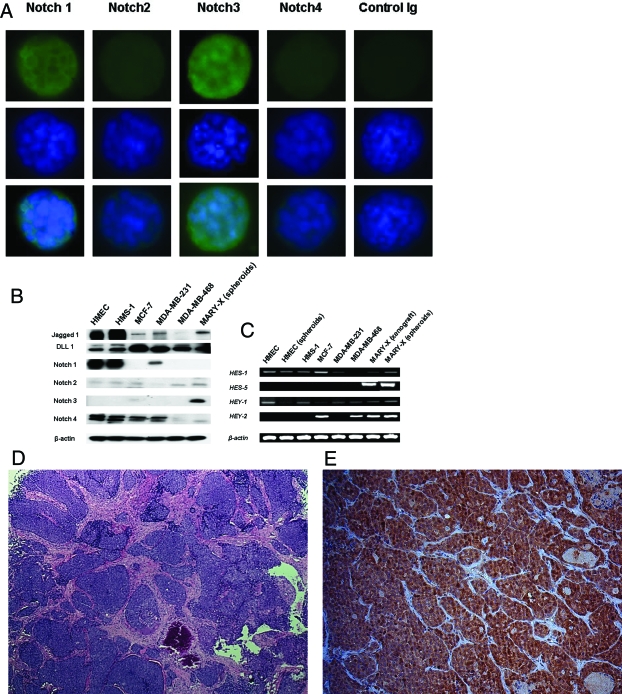
Prototype signaling pathways such as notch, which are thought to contribute to the maintenance of the developmental potential of the stem cell state, were studied in the MARY-X spheroids and compared to non-IBC carcinoma lines and normal epithelial/myoepithelial surrogates. Among notch receptors, only notch3 expression was increased in MARY-X spheroids by both immunocytochemistry and Western blot (Figure 7, A and B). More importantly, notch signaling through notch3 was dramatically increased in MARY-X (Figure 7, A and C) compared to non-IBC breast carcinomas and normal cell surrogates that essentially did not signal. Even when the non-IBC carcinoma and normal cells were grown as spheroids, notch activation and signaling did not occur. Notch 3 signaling was in evidence in the MARY-X spheroids by the detection of nuclear notch3 immunoreactivity, evidence that the Nicd had been cleaved and translocated to the nucleus. In addition expression of downstream genes: hes-1, hes-5, hey-1, and hey-2 were detected by RT-PCR. Notch ligands, jagged1, and DLL1 were also present in MARY-X but not at increased levels compared to non-IBC lines. The entire notch receptor was sequenced and found to be normal (data not shown). Specifically there were no activating mutations present. The reason for notch activation and signaling in MARY-X was not apparent.
Figure 7.
A: The MARY-X spheroids exhibited prominent notch signaling, particularly with notch3. Fluorescence studies with antibodies that recognized the Notch3 Nicd domain showed prominent nuclear immunoreactivity whereas similar intracellular domain antibodies to notch1 revealed only slight membrane immunoreactivity and no signaling. Top row is fluorescence-labeled primary/secondary antibody (notch 1 to 4 and control Ig), middle row is Hoechst 33342 nuclear counterstain, and bottom row is the composite image. B: Western blot confirmed strong notch3 expression in MARY-X compared to non-IBC lines. C: Although other notch family members were also expressed in non-IBC lines, there was no appreciable signaling except in MARY-X where all four of the notch-cleavage downstream genes (hes-1, hes-5, hey-1, and hey-2) were detected by RT-PCR. D: The MARY-X xenograft consisted of a confluence of central nodules evolving into peripheral lymphovascular emboli. E: All of these nodules uniformly exhibited notch3 signaling as evidenced by strong nuclear Nicd immunoreactivity.
Notch activation was homogeneously present throughout all of MARY-X. The MARY-X xenograft, in its central portion, consisted of a confluence of nodules in a mesenchymal stroma (Figure 7D). In its periphery these nodules transitioned to lymphovascular emboli. Both peripheral and central portions generated identical spheroids in vitro, which exhibited identical expression profiles. IHC studies of the xenograft also showed identical expression of notch3 nuclear immunoreactivity both in the central as well as in the peripheral nodules (Figure 7E). Therefore there was no difference between the main tumor mass and the lymphovascular emboli in terms of notch signaling. Furthermore there was also no geographical differences in CD133 or ALDH1 immunoreactivity.
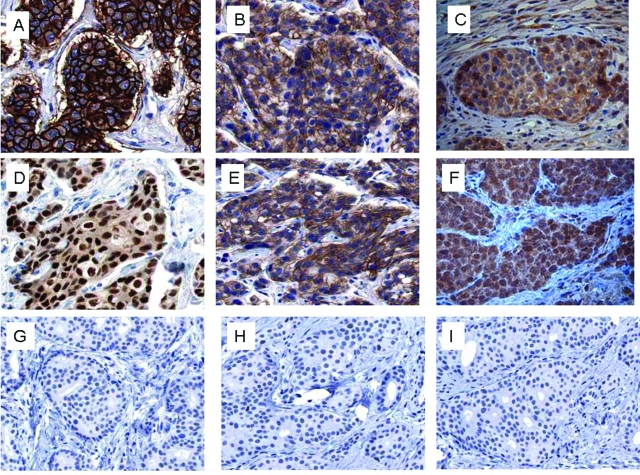
To test whether the observations made in MARY-X were applicable to actual human cases of IBC, we conducted CD133 and notch3 IHC studies of 25 cases of IBC, 25 cases of non-IBC, and 5 normal breast tissues. The IBC cases included 6 cases that were ER-positive, 11 cases that were Her-2/neu-positive, and 8 cases that were triple-negative. In selecting these cases, we chose cases that exhibited moderate to high-grade histology. The IBC cases by definition were all T4 stage and the non-IBC cases consisted of both T2 (20 cases) and T3 (5 cases). A significant portion of each IBC case manifested LVI with prominent tumor emboli. The non-IBC cases included 14 cases that were ER-positive, 6 cases that were Her-2/neu-positive, and 5 cases that were triple-negative. In these non-IBC cases, LVI with tumor emboli were uncommon. Of IBC cases, 22 of 25 (88%) expressed CD133 membrane immunoreactivity and 19 of 25 IBC cases (76%) expressed notch3 nuclear immunoreactivity and there was no significant differences among the subclasses of IBC for expression of either stem cell marker (Figure 8 and Table 4). The expression of CD133 membrane immunoreactivity was more homogeneous when present and the expression of nuclear notch3 immunoreactivity was more heterogeneous when present. In contrast the presence of either CD133 or notch3 immunoreactivity was low overall in the non-IBC cases (2 of 25 cases, 8%) (Figure 8). Interestingly two of five (40%) of the triple-negative non-IBC cases exhibited both CD133 and notch3 immunoreactivity (Table 4). The pattern of notch3 immunoreactivity in the two triple-negative non-IBC cases was similar to that in the IBC cases but significantly fewer cells were CD133-positive in the triple-negative non-IBC cases compared to the IBC cases in which the immunoreactivity was more homogeneous. CD133 and notch3 immunoreactivity were both completely absent in the normal breast tissues. The expression of CD133 and notch3 in the majority of the cases overall was highly concordant [weighted κ (95% confidence interval) = 0.86 (0.81, 0.91)]. Human IBC cases within their lymphovascular tumor emboli also exhibited a range of ALDH1 cytoplasmic immunoreactivity from 10 to 100% (Figure 4C). This immunoreactivity was heterogeneous in nature but was observed equally in all of the subtypes of IBC. In some of the non-IBC cases in which overall ALDH1 immunoreactivity was absent or low (Figure 4D), their lymphovascular emboli contained enriched ALDH1 immunoreactivity (Figure 4E).
Figure 8.
Immunocytochemical studies for stem cell markers, CD133, and activated notch3 in the different molecular subtypes of human IBC revealed the presence of CD133 and notch3 immunoreactivity independent of molecular subtype: IBC with Her-2/neu-positive lymphovascular tumor embolus (A) exhibited strong CD133 membrane immunoreactivity (B) and notch3 nuclear immunoreactivity (C). IBC with ER-positive lymphovascular tumor embolus (D) similarly exhibited strong CD133 membrane immunoreactivity (E) and notch3 nuclear immunoreactivity (F). In contrast a triple-negative non-IBC (G) was negative for CD133 (H) and notch3 nuclear immunoreactivity (I).
Table 4.
Stem Cell IHC Profile in the Major Molecular Subtypes of IBC and Non-IBC
| IBC
|
Non-IBC
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Triple negative (n = 8) | Her-2/neu+ (n = 11) | ER+ (n = 6) | Total (n = 25) | Triple negative (n = 5) | Her-2/neu+ (n = 6) | ER+ (n = 14) | Total (n = 25) | |
| CD133+ | 7 (88) | 10 (91) | 5 (83) | 22 (88) | 2 (40) | 0 (0) | 0 (0) | 2 (08) |
| Notch+ | 6 (75) | 9 (82) | 4 (67) | 19 (76) | 2 (40) | 0 (0) | 0 (0) | 2 (08) |
Absolute numbers of cases and (% positivity) are listed. Differences for both CD133 and notch in IBC versus non-IBC were highly significant (P < 0.001); differences among subclasses of IBC were not significant (P > 0.1). Strong agreement between CD133 and notch was present in the IBC cases [weighted κ (95% confidence interval) = 0.86 (0.81, 0.91)].
Discussion
MARY-X is a unique transplantable xenograft that faithfully maintains the phenotype of florid LVI with the formation of tumor emboli in vivo and spheroids in vitro, which strongly resemble the human embryonal blastocyst, the source of ESs. Recent experimental evidence in a variety of tumors and normal tissues has lent strong support to the stem cell hypothesis.10,11,12,13,14,15,16,17,18,19,20,21,22 It has been suggested that most solid cancers, for example, contain a cellular subpopulation termed a CSC subpopulation that retains key properties of ESs.15,16,17,20,21 Based on biological similarities shared by the lymphovascular embolus, the MARY-X spheroids, the human blastocyst, and stem cells, like anchorage independence and the ability to migrate and metastasize, we sought to investigate whether MARY-X directly exhibited stem cell-like characteristics. We found that MARY-X indeed exhibited a stem cell marker phenotype characterized by E-cadherin+/CD44+/CD24−/low. The human embryonal blastocyst also overexpresses E-cadherin.3,4,5,6,7 Furthermore, a population of cells from human breast cancers characterized by CD44+/CD24−/low has recently been identified that exhibit properties of CSCs.16 Other studies have suggested that human breast cancers with a high percentage of CD44+/CD24−/low cells exhibit a greater tendency of distant metastasis.23,24 MARY-X and IBC also exhibit a great tendency for distant metastasis.
In addition the spheroids of Mary-X contained a large percentage of cells that expressed other stem cell markers known to be associated with normal ESs or pluripotent stem cells (Table 1). These included stellar, rex-1, nestin, H19, and other markers primarily restricted to normal human ESs. Stellar, for example, also known as developmental pluripotency associated-3 (DPPA3) and rex-1, also known as zinc finger protein 42 homolog (ZFP42), are two specific markers of undifferentiated ESs. Although the functions of stellar and rex-1 are still not clear, the expressions of these two genes decrease with ES cell differentiation.10,11 Not only were the expression of these markers of ESs and pluripotent stem cells observed within MARY-X but expression of stem cell-related transcription factors (oct-4, nanog, sox-2, and others) were also observed (Table 1). These transcription factors have been demonstrated to play central roles in the self-renewal and pluripotency of human ESs. Just recently these transcription factors in combination have been shown to be capable of inducing reversion to an embryonic stem cell state when transfected into fetal, newborn, or adult fibroblasts.12,13 The expression of these same pleiotropic genes may also be important in the setting of IBC, in regulating and maintaining its stem cell-like state.
Not only did MARY-X express markers characteristic of normal human ESs but also markers characteristic of specific human CSC. Of these latter markers, the most impressive and the most specific marker was CD133, which was exhibited by >90% of the cells of the MARY-X spheroids. Human CD133 has been reported to be expressed in cancer-initiating cells from multiple tumor types, including leukemia, brain tumors, prostate cancers, and colon cancers (Table 2).15,20,21 In colon cancer CD133+ cells comprise 2.5% of the total tumor cell population.21 These studies highlight the potential importance of CD133 in CSC populations. However, the exact role of CD133 in CSC functions is still not clear. This present study is the first report of the strong expression of CD133 in an experimental model of human IBC.
MARY-X initially was derived from a patient with IBC. The xenograft was derived by placing explants of the patient’s tumor into nude/scid mice. Therefore MARY-X was not clonal initially. However when we obtained the spheroids from the xenograft, we disadhered the spheroids into single cells and regenerated the spheroids from single cells and reinjected them into the mouse. The single cell progeny also grew into the phenotype of MARY-X. So a clonal line was derived from the original MARY-X. A majority of the cells of MARY-X expressed stem cell markers, eg, CD133 and activated notch3. This was observed in both the original xenograft as well as the clonally-derived secondary xenograft. Even if a tumor is derived from a single cell, ie, clonal, the cancer oftentimes is very heterogeneous. Stem cell-like features can also be a manifestation of this heterogeneity.
MARY-X did not, however, express all of the known embryonal or tumoral stem cell markers. In particular stem cell markers associated with hematopoietic stem cells, ie, CD34 were absent in MARY-X. In addition a SP of cells able to exclude Hoechst 33342 was absent. The SP is a subset of cells with the capacity to efflux lipophilic dyes. Recent evidence suggested that the SP phenotype is associated with high-level expression of the ATP-binding cassette transporter protein ABCG2.22,25,26 ABCG2-positive and SP cells have been demonstrated to be enriched in normal stem cells as well as CSC. ABCG2 was also absent in MARY-X. However ABCG2 expression is not necessarily a sine qua non of either stemness or tumorigenicity.25,27
The cells derived from the MARY-X spheroids showed the properties of proliferation and morphogenesis (acinar development) in vitro (Table 3). However, although 10 to 30% of the cells exhibited clonogenic potential, their true cloning efficiency could not be accurately determined because of the compromised ability of single CD133+ cells to grow in clonogenic assays. When the primary spheroids were initially disadhered to generate a single cell population, many of the single cells either underwent frank apoptosis or had apoptosis signaling initiated.3,4,5,6,7 This compromised their innate clonogenic potential. Although 10 to 30% of the CD133+ cells of MARY-X were able to exhibit clonogenic potential, comparable clonogenic potential was exhibited by a number of the non-IBC lines such as MCF-7 and MDA-MB-231. However because disadherence-induced apoptosis occurred in MARY-X but not in the other non-IBC lines, the MARY-X spheroids likely possessed much greater innate clonogenic potential than these other non-IBC lines. The significant clonogenic potential of MARY-X was also illustrated by the fact that secondary spheroids consisting of only 100 cells were fully tumorigenic and capable of recapitulating the phenotype of florid LVI. This further reflected the proliferation capacity of MARY-X in vivo. Because the IBC phenotype of florid LVI, could be transferred in perpetuity with serial passage from single cells, single cells of MARY-X exhibited the capacity for self-renewal. We also observed the morphogenesis of single MARY-X cells grown in Matrigel into acini. Whereas many different breast cancer cell lines and, in fact, normal breast cells exhibit morphogenic changes in Matrigel, not all of the lines exhibit the same morphogenic changes and not all of the lines exhibit true acini that consists of an arrangement of epithelial cells exhibiting polarity around a central lumen. Unlike normal human mammary epithelial cells that are dependent on cell-cell contacts and a certain high density of plating to generate ductal/acinar structures, single MARY-X cells alone were capable of acinar morphogenesis. Of the MARY-X cells in Matrigel 10 to 30% exhibited evidence of this morphogenesis, but for the same reasons given for clonogenic potential, MARY-X cells likely possessed much greater morphogenic potential as well.
It is important to recognize and distinguish those stem cell-like properties of MARY-X that are an innate characteristic of this model from those that are related to the spheroid three-dimensional structures that the model exhibits. Other carcinoma cell lines and normal lines may exhibit or be induced to exhibit spheroid formation. There are obvious analogies between the MARY-X spheroids and other nonadhesive floating spheroids, such as neurospheroids15 and mammospheroids.9,28 Studies revealed that these non-IBC spheroids were composed of highly enriched stem-like cells. Actually, anchorage independence, which had been thought to be a hallmark of transformed cells, recently has been shown to be a property shared by normal tissue cells that exhibited stem cell properties.29 In addition, some tumor stem cells that were able to be propagated exhibited the phenotype of nonadherent spheroids.28,30 For example, a tumorigenic subpopulation exhibiting stem cell properties in melanoma was propagated as nonadherent spheroids in culture conditions suitable for human embryonic stem cells.30 Breast cancer-initiating cells have also displayed the capacity of extensive proliferation as clonal nonadherent spheroidal clusters.28 In our studies, the spheroid three-dimensional structures of MARY-X clearly allows for the expression of some of its stem cell-like properties. When the spheroids were disadhered by treatment with anti-E-cadherin, ethylenediaminetetraacetic acid, trypsin, or transfection with a dominant-negative E-cadherin mutant, for example, there was a dramatic loss of clonogenic potential and tumorigenicity, partly because of increased apoptosis.5,6,7 The microenvironment of the MARY-X spheroids, in particular, then created a spheroid niche that preserved the ability of its stem-like cells to survive, self-renew, and proliferate. However spheroid growth, alone, did not completely account for the stem cell marker phenotype exhibited. Even when the non-IBC carcinoma cell lines and normal cells were grown as spheroids, CD133 was not expressed, and conversely, even when the MARY-X spheroids, were disadhered, the single cells still expressed CD133. So the spheroid nature of MARY-X only partially accounted for its overall stem cell-like phenotype. Still this contribution is highly significant both in vitro and in vivo. Both the in vitro spheroids of MARY-X and the corresponding spheroid-like three-dimensional lymphovascular tumor emboli present in vivo are characterized by the close juxtaposition of tumor cells to each other, which promotes cytoprotective and other signaling pathways. We have recently observed, for example, decreased notch3 signaling when the spheroids of MARY-X were disadhered (unpublished observations).
Potential prototype signaling pathways such as notch, which are thought to contribute to the maintenance of the developmental potential of the stem cell phenotype, were observed in the MARY-X spheroids and found to be activated. Most specifically though, notch signaling through notch3 was dramatically increased in MARY-X compared to non-IBC breast carcinoma cell lines and normal cell surrogate lines that essentially did not signal. Even when the non-IBC carcinoma lines and normal cells were grown as spheroids, notch3 activation and signaling did not occur.
Notch, wnt, and hedgehog are important signaling pathways that play essential roles in ES renewal, maintenance of developmental potential, and proliferation.29 The observations that notch3 signaling occurred in intact MARY-X spheroids and the corresponding lymphovascular tumor emboli of the MARY-X xenograft but not in the non-IBC carcinoma lines suggest the possibility that IBC tumor emboli are dependent on this signaling.
Our MARY-X model also exhibited relatively high expression of ALDH enzymatically and specifically ALDH1 immunocytochemically. ALDH1 has been shown to be a marker for normal and malignant human mammary stem cells and a predictor of poor clinical outcome in human breast cancer.31 Whether the ALDH1+ population of MARY-X exhibits enhanced stem cell properties and/or increased metastasis remains to be determined.
It is important to verify that insights gained by studying the stem cell properties of MARY-X are applicable to human IBC. Our observations concerning CD133, notch3, and ALDH1 could be extended to human cases of IBC but it would be legitimate to question whether the results observed in MARY-X are generalizable to all of human IBC. After all, MARY-X is ER-negative, PR-negative, Her-2/neu-positive, and EGFR-positive3 and therefore expressive of a triple-negative or basal molecular subtype. It could be argued that the stem cell-like properties exhibited by MARY-X could be a manifestation of its basal origin rather than its IBC origin. Like non-IBC, IBC is a heterogeneous disease, notably in terms of survival and response to chemotherapy and it has been reported that the same molecular subtypes present in non-IBC are also present in IBC.32 The stem cell markers observed in MARY-X, namely CD133, notch3, and ALDH1 were not limited to triple-negative IBC, however, but were observed equally in ER-positive and Her-2/neu-positive IBC as well. Therefore the IBC stem cell-like phenotype transcends the conventional molecular subtypes. Although the stem cell-like phenotype may also occur in non-IBC triple-negative breast cancers,33,34,35,36 these observations really require additional study because the non-IBC triple-negative phenotype includes a potpourri of different cancers, which include medullary carcinoma, metaplastic carcinoma, and BRCA1-related carcinoma.
To gain additional insight into these stem cell-like signatures overall, we are planning a more extensive TMA analysis of CD133, notch3, and ALDH1 immunoreactivity in dermal versus breast emboli in IBC, emboli in IBC versus emboli in locally advanced breast cancer versus emboli in early stage breast cancer, and embolic versus nonembolic areas of IBC. For these studies we believe that comparative analyses of pooled laser-captured microdissected lymphovascular emboli from these different clinicopathological entities might be particularly revealing. We also plan to investigate the issue of whether lymphovascular tumor emboli in non-IBC cases express a different profile than the main tumor mass. This will tell us whether it is the lymphovascular embolus per se that contains the essential molecular signature of stemness independent of its IBC origin.
The cancer stem hypothesis has revolutionized our understanding of carcinogenesis and tumor cell biology.37,38 It is believed that CSCs may account for many of the disease’s properties such as tumor latency, local recurrences, distant metastasis, and emerging drug resistance. Cancer stem cells are thought to exhibit increased resistance to the induction of apoptosis by cytotoxic agents and radiation therapy compared with proliferating and more differentiated cells. Some studies have suggested that CSCs may exhibit more angiogenic activity. We have not shown unequivocally that MARY-X contains either embryonal or tumoral stem cells because we have not directly demonstrated a specific subpopulation capable of both self-renewal as well as multipotency, although we have indirectly supported these claims. What we have shown is that MARY-X contains a significantly large population of cells that express stem cell-like properties. Recent studies have suggested that a high percentage of tumor cells exhibiting stem cell properties or expression of a stem cell gene signature is associated with advanced stage and poor prognosis for different types of human malignancies.39,40,41,42,43,44,45,46,47,48,49 All these observations can be made with MARY-X and IBC. The MARY-X spheroids, in particular, and IBC, in general, resist chemotherapy and radiation-induced apoptosis, display strong expression of many angiogenesis markers, including VEGF, flt1/VEGFR1, flk1/VEGFR2,44 and are associated with early distant metastasis and hence an advanced stage and poor prognosis. This stem cell-like phenotype may contribute to the aggressive nature of IBC but may also lend itself to selective targeting.
Footnotes
Address reprint requests to Sanford H. Barsky, M.D., Department of Pathology, The Ohio State University College of Medicine, 129 Hamilton Hall, 1645 Neil Ave., Columbus, OH 43210-1218. E-mail: sanford.barsky@osumc.edu.
Supported by the Department of Defense (U.S. Army Breast Cancer Research Program grant W81XWH-06-1-0631), the Strategic Initiative Grant Program at Ohio State, and The Donald A. Senhauser Endowment.
References
- Palangie T, Mosseri B, Mihura J, Campana F, Beuzeboc P, Dorval T, Garcia-Giralt E, Jouve M, Scholl S, Asselain B, Pouillart P. Prognostic factors in inflammatory breast cancer and therapeutic implications. Eur J Cancer. 1994;30A:921–927. doi: 10.1016/0959-8049(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Guérin M, Gabillot M, Mathieu MC, Travagle JP, Spielmann M, Andrieu N, Riou G. Structure and expression of c-erbB-2 and EGF receptor genes in inflammatory and non-inflammatory breast cancer: prognostic significance. Int J Cancer. 1989;43:201–208. doi: 10.1002/ijc.2910430205. [DOI] [PubMed] [Google Scholar]
- Alpaugh ML, Tomlinson JS, Shao ZM, Barsky SH. A novel human xenograft model of inflammatory breast cancer. Cancer Res. 1999;59:5079–5084. [PubMed] [Google Scholar]
- Tomlinson JS, Alpaugh ML, Barsky SH. An intact overexpressed E-cadherin/alpha, beta-catenin axis characterizes the lymphovascular emboli of inflammatory breast carcinoma. Cancer Res. 2001;61:5231–5241. [PubMed] [Google Scholar]
- Alpaugh ML, Tomlinson JS, Kasraeian S, Barsky SH. Cooperative role of E-cadherin and sialyl-Lewis X/A-deficient MUC1 in the passive dissemination of tumor emboli in inflammatory breast carcinoma. Oncogene. 2002;21:3631–3643. doi: 10.1038/sj.onc.1205389. [DOI] [PubMed] [Google Scholar]
- Alpaugh ML, Barsky SH. Reversible model of spheroid formation allows for high efficiency of gene delivery ex vivo and accurate gene assessment in vivo. Hum Gene Ther. 2002;13:1245–1258. doi: 10.1089/104303402320139023. [DOI] [PubMed] [Google Scholar]
- Alpaugh ML, Tomlinson JS, Ye Y, Barsky SH. Relationship of sialyl-Lewisx/a underexpression and E-cadherin overexpression in the lymphovascular embolus of inflammatory breast carcinoma. Am J Pathol. 2002;161:619–628. doi: 10.1016/S0002-9440(10)64217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht M, Safarians S, Calcaterra TC, Barsky SH. Establishment and characterization of a novel human myoepithelial cell line and matrix-producing xenograft from a parotid basal cell adenocarcinoma. In Vitro Cell Dev Biol. 1996;32:550–563. doi: 10.1007/BF02722982. [DOI] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Michael FC, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AT, Rodriguez RT, Bodnar MS. Human STELLAR, NANOG, and GDF3 genes are expressed in pluripotent cells and map to chromosome 12p13, a hotspot for teratocarcinoma. Stem Cells. 2004;22:169–179. doi: 10.1634/stemcells.22-2-169. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick J. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168–172. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris J, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino B. The ABC transporter Brcp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- Abraham BK, Fritz P, McClellan M, Hauptvoge P, Athelogou M, Brauch H. Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11:1154–1159. [PubMed] [Google Scholar]
- Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, Goulet R, Badve S, Nakshatri H. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrawala L, Calhoun T, Robin SB, Zhou JJ, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- Triel C, Vestergaard ME, Bolund L, Jensen TG, Jensen UB. Side population cells in human and mouse epidermis lack stem cell characteristics. Exp Cell Res. 2004;295:79–90. doi: 10.1016/j.yexcr.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster R. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci USA. 2003;100:6487–6492. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- Wicha MS, Liu SL, Dontu G. Cancer stem cells: an old idea—a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertucci F, Finetti P, Rougemont J, Charafe-Jauffret, Cervera N, Tarpin C, Nguyen C, Xerri L, Houlgatte R, Jacquemier J, Viens P, Birnbaum D. Gene expression profiling identifies molecular subtypes of inflammatory breast cancer. Cancer Res. 2005;65:2170–2178. doi: 10.1158/0008-5472.CAN-04-4115. [DOI] [PubMed] [Google Scholar]
- Bertucci F, Finetti P, Cervera N, Charafe-Jauffret, Mamessier E, Adelaide J, Debono S, Houvenaeghel G, Maraninchi D, Viens P, Charpin C, Jacquemier J, Birnbaum D. Gene expression profiling shows medullary breast cancer is a subgroup of basal breast cancers. Cancer Res. 2006;66:4636–4644. doi: 10.1158/0008-5472.CAN-06-0031. [DOI] [PubMed] [Google Scholar]
- Stylianou S, Clarke RB, Brennan K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 2006;66:1517–1525. doi: 10.1158/0008-5472.CAN-05-3054. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Oyama T, Ito E, Satoh H, Azuma S, Hayashi M, Shimizu K, Honma R, Yanagisawa Y, Nishikawa A, Kawamura M, Imai J, Ohwada S, Tatsuta K, Inoue J, Semba K, Watanabe S. Notch3 signaling pathway plays crucial roles in the proliferation of erbB2-negative human breast cancer cells. Cancer Res. 2008;68:1881–1888. doi: 10.1158/0008-5472.CAN-07-1597. [DOI] [PubMed] [Google Scholar]
- Sansone P, Storci G, Giovannini C, Pandolfi S, Pianetti S, Taffurelli M, Santini D, Ceccarelli C, Chieco P, Bonafe M. p66Shc/Notch-3 interplay controls self-renewal and hypoxia survival in human stem/progenitor cells of the mammary gland expanded in vitro as mammospheres. Stem Cells. 2007;25:807–815. doi: 10.1634/stemcells.2006-0442. [DOI] [PubMed] [Google Scholar]
- Kondo T. Stem cell-like cancer cells in cancer cell lines. Cancer Biomarkers. 2007;3:245–250. doi: 10.3233/cbm-2007-34-508. [DOI] [PubMed] [Google Scholar]
- Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey I, Gurney A, Huang EH, Simeone DM, Shelton A, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, Nakauchi H, Taniguchi H. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240–251. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- Ilse VA, Steven JV, Gert G, Eynden V, Benoy V, Dam P, Colpaert CG, Fox SB, Turley H, Harris AL, Marck VA, Vermeulen PB, Dirix LY. Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin Cancer Res. 2004;10:7965–7971. doi: 10.1158/1078-0432.CCR-04-0063. [DOI] [PubMed] [Google Scholar]
- Phillips HS, Kharbanda S, Chen RH, Forrest WF, Soriano RH, Wu TD, Misra A. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Glinsky GV, Berezovska O, Glinskii AB. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest. 2005;115:1503–1521. doi: 10.1172/JCI23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahad JP, Mills GB, Coombes KR. Stem cell-ness: a “magic marker” for cancer. J Clin Invest. 2005;115:1463–1467. doi: 10.1172/JCI25455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigel GM, Campbell LM, Narayan M, Federico GF. Cancer stem cell characteristics in retinoblastoma. Mol Vis. 2005;11:729–737. [PubMed] [Google Scholar]
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]