Abstract
Growing evidence suggests that survivin, a member of the inhibitor of apoptosis gene family, is responsible for drug resistance in cancer cells, yet little is known about its role in the endothelial cells of the tumor vasculature. We have previously reported that tumor-associated endothelial cells derived from gliomas (TuBECs) are resistant to anticancer chemotherapy whereas normal brain endothelial cells (BECs) are sensitive. The focus of this study is to investigate the mechanism behind this chemoresistance. Here we show that survivin is constitutively overexpressed in the glioma vasculature but not in the blood vessels of normal brain. To determine whether survivin contributes to TuBEC chemoresistance, we used a lentiviral siRNA system or the drug roscovitine to down-regulate survivin expression. Reduced levels of survivin sensitized TuBECs to the chemotherapeutic agents VP-16, paclitaxel, thapsigargin, and temozolomide. This cell death was mediated through caspases 7 and 4. Conversely, forced expression of survivin in BECs was protective against drug cytotoxicity. These data suggest that overexpression of survivin in endothelial cells serves as a protective mechanism that defends the vasculature from drug cytotoxicity. Our studies demonstrate that targeting survivin may be an effective approach to chemosensitization and anti-vascular therapy for brain tumors.
The tumor vasculature is critical for cancer growth.1 This is particularly relevant in the highly vascular primary brain tumor, glioblastoma multiforme (glioma), a grade IV astrocytoma.2 The blood vessels within tumors have been shown to be abnormal in their structure and function as compared to the normal vasculature.3,4 Studies from this laboratory have shown that the tumor-associated endothelial cells derived from gliomas (TuBECs) have different properties than normal brain endothelial cells (BECs). TuBECs actively secrete pro-angiogenic factors,5 have increased migration,6 undergo G0/G1 cell-cycle arrest,7 and are resistant to drugs as compared to BECs.7 The mechanism of this chemoresistance in TuBECs is not known and is the focus of this study.
Studies have shown that chemoresistance in tumor cells can be correlated to an overexpression of the inhibitor of apoptosis survivin. This bifunctional protein inhibits apoptosis and promotes cell division.8 Survivin is also localized to various areas of the cell; mitochondrial and cytosolic survivin suppress apoptosis through blocking the activity of caspases 9, 3, and 7,9,10,11 whereas nuclear survivin is induced at G2/M of the cell cycle to ensure proper mitosis and cytokinesis.12 Survivin is typically found at low levels in normal cells but is elevated in many solid and hematologenous cancers.13 In various tumors, high survivin levels are correlated with poor prognosis, decreased apoptosis, increased angiogenesis, and chemoresistance in cancer cell lines.14,15 However, little is known of survivin’s function in the endothelial cells of the tumor vasculature.
In this study we present the novel findings that survivin is overexpressed in primary cultures of endothelial cells derived from human glioma tissues and that survivin protects these cells from chemotherapeutic agents. We decreased survivin through genetic and pharmacological approaches and found that survivin is responsible for the chemoresistance in TuBECs. Furthermore, forced expression of survivin protected BECs from cytotoxic drugs. Thus survivin is a prosurvival player in tumor-associated endothelial cells, and reducing survivin in the tumor vasculature can be an effective chemosensitizing mechanism and a valuable target for anti-angiogenic therapy.
Materials and Methods
Primary Endothelial Cell Isolation and Culture
Isolation of BECs and TuBECs from human normal brain and glioma tissues was previously described.5 Briefly, tissues were obtained and handled in agreement with the Keck School of Medicine, University of Southern California Institutional Review Board guidelines. The tissues were washed three times with RPMI 1640 medium (Life Technologies, Inc., Grand Island, NY) containing 2% fetal calf serum (Omega Scientific, Tarzana, CA), and 1% penicillin/streptomycin (Life Technologies, Inc.). The tissue was then minced into small pieces, and fresh medium was added. The mixture was transferred to a centrifuge tube, and an equal volume of a 30% dextran solution (Sigma-Aldrich, St. Louis, MO) was added, bringing the mixture to a final concentration of 15% dextran. The resulting mixture was then centrifuged for 10 minutes at 10,000 rpm to pull down the brain microvessels. The microvessel pellet was resuspended in 1 mg/ml of collagenase-dispase in RPMI 1640 medium supplemented with 2% fetal calf serum (FCS) (RPMI-2% FCS) and incubated in a shaking 37°C water bath for 1 hour. Subsequently, 10 ml of RPMI-2% FCS was added to the cells and centrifuged at 1200 rpm for 5 minutes. The pellet was resuspended in 20 ml of the RPMI-2% FCS and centrifuged again. The final pellet was resuspended in the endothelial cell culture medium [RPMI 1640 medium supplemented with 100 ng/ml endothelial cell growth supplement (Upstate Biotechnologies, Rochester, NY), 2 mmol/L l-glutamine (Life Technologies, Inc.), 10 mmol/L HEPES (Life Technologies, Inc.), 24 mmol/L sodium bicarbonate (Life Technologies, Inc.), 300 U heparin USP (Sigma-Aldrich), 1% penicillin/streptomycin, and 10% FCS]. Cells were plated on precoated gelatin flasks, and the medium was changed every 3 or 4 days until the cell cultures became 80% confluent. Endothelial cells were then purified from the cellular mixture by selecting cells that bind diacetylated low-density lipoprotein (di-LDL). Subconfluent cells were incubated with 10 ng/ml of fluorescent di-LDL for 4 hours at 37°C and then analyzed using fluorescence-activated cell sorting analysis (see Supplemental Figure S1 at http://ajp.amjpathol.org).
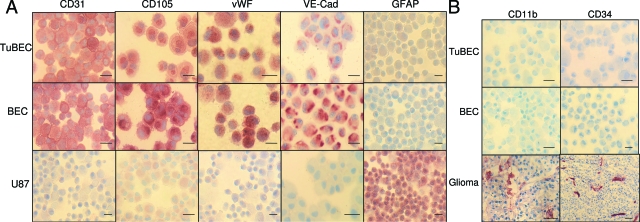
After the sorting procedure, the purity of BECs and TuBECs was confirmed by immunostaining for specific endothelial cell markers: CD31/PECAM-1 (Santa Cruz Biotechnology, Santa Cruz, CA), von Willebrand Factor (DAKO, Carpinteria, CA), VE-cadherin (R&D Systems, Minneapolis, MN), and CD105/endoglin (Santa Cruz Biotechnology) and was found to be 100% positive (see Figure 1). BECs and TuBECs were negative for astrocyte cell marker glial fibrillary acidic protein (DAKO), progenitor endothelial cell marker CD34 (DAKO), and macrophage/microglia marker CD11b (DAKO). BEC and TuBEC cultures were grown onto 1% gelatin-coated surfaces and were used up to passage 6. Digital images were taken of the cultured cells using a Sony (Park Ridge, NJ) DSC-P9 camera and Nikon (Tokyo, Japan) TMS microscope (objective lens: × 10 magnification, 0.25 numerical aperture) at room temperature. BECs and TuBECs were propagated in medium containing endothelial cell growth supplement, as described above. In performing experiments, however, endothelial cell growth supplement was removed from the culture medium.
Figure 1.
Characterization of TuBECs and BECs as endothelial cells. Cytospin preparations of TuBECs, BECs, and U87MG and frozen glioma tissues were fixed in acetone, blocked in 5% goat or horse serum, and stained with antibodies to the following: CD31, CD105, von Willebrand Factor (vWF), VE-cadherin (VE-Cad), glial fibrillary acidic protein (GFAP), CD11b, and CD34. The red precipitate denotes positive staining. Scale bars = 10 μm.
Cell Death Detection Enzyme-Linked Immunosorbent Assay (ELISA)
Cells in RPMI with 10% FCS were plated onto 96-well plates at 5 × 103 cells/well. Cells were treated with VP-16 (Calbiochem, La Jolla, CA), paclitaxel (Sigma-Aldrich), temozolomide (Schering-Plough, Kenilworth, NJ), or thapsigargin (Calbiochem), alone or in combination with roscovitine (Sigma-Aldrich). After treatment, cells were lysed and immediately analyzed using the Cell Death Detection ELISAPlus kit (Roche Diagnostics, Indianapolis, IN). Absorbance was measured at 405 nm, and percent cell death was calculated based on a 100% positive cell death control. The groups were treated in duplicate and the experiments were repeated at least twice.
Immunostaining
Cytocentrifuge cell preparations and cells grown onto chambered slides were fixed with acetone, blocked with 5% goat serum, and incubated overnight with anti-survivin polyclonal antibody (1:100) (Santa Cruz Biotechnology). Samples were incubated with biotinylated goat anti-rabbit antibody (1:400) (Vector Laboratories, Burlingame, CA) for 45 minutes, treated with the avidin-biotin-peroxidase complex (Vector Laboratories) for 30 minutes, and then treated with aminoethyl carbazol substrate for 15 minutes (Vector Laboratories). Samples were counterstained with hematoxylin for 1.5 minutes. A red precipitate denotes positive staining. Specificity of the anti-survivin polyclonal antibody was tested and confirmed through the use of monoclonal survivin antibodies and survivin blocking peptides (see Supplemental Figure S2, A and B, at http://ajp.amjpathol.org).
Confocal Microscopy
Double staining was performed on glioma and normal brain tissues fixed with acetone. Tissues were co-incubated with anti-survivin polyclonal (1:100) and anti-CD105 monoclonal (1:100) antibodies, and subsequently treated with Texas Red anti-rabbit (1:400) and fluorescein anti-mouse secondary antibodies (1:200) (Vector Laboratories). Mounting medium containing the fluorescent blue 4,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories) was used to identify nuclear staining. Rabbit and mouse IgG isotype-matched controls and the omission of primary antibody were used as negative controls. The staining was analyzed using a Zeiss LSM510 confocal microscope (Zeiss, Thornwood, NY); red color denotes survivin positivity, green color represents CD105 positivity, and yellow color signifies positive staining for both survivin and CD105.
Western Blot
Western blots were performed as previously described.7 Membranes were incubated overnight with antibodies to survivin (1:250) (Santa Cruz Biotechnology), caspase 7 (1:1000) (BD Pharmingen, Franklin Lakes, NJ), caspase 4 (1:500) (BD Pharmingen), CHOP (1:500) (Santa Cruz Biotechnology), or GAPDH (1:5000) (Santa Cruz Biotechnology) and then incubated with horseradish peroxidase-conjugated (Santa Cruz Biotechnology) or fluorescent-conjugated (Pierce, Rockford, IL) secondary antibodies (1:5000 to 1:15,000) for 45 minutes. Protein bands were detected either by chemiluminescence using the SuperSignal substrates (Pierce) or by Odyssey infrared imaging (LI-COR Biosciences, Lincoln, NE). Specificity of the anti-survivin polyclonal antibody was confirmed in comparison to a monoclonal survivin antibody (see Supplemental Figure S2C at http://ajp.amjpathol.org).
Generation of Lentiviral siRNA and Expression Vectors and Viral Infection
The following siRNA sequences (sense) were used: 5′-GGCTGGCTTCATCCACTGC-3′ (survivin) and 5′-GTGACCAGCGAATACCTGT-3′ (LacZ). The siRNA sequences were subcloned into the lentiviral siRNA delivery vector FG-12 at XbaI and XhoI sites, as previously described.16 The human wild-type survivin gene (pEYFP-N1-survivin) was kindly provided by Dr. Jeroen Pouwels and Dr. Anu Kukkonen (VTT Medical Biotechnology, Turku, Finland). Survivin was amplified using the primers 5′-CTAGTCTAGAGCCACCATGGGTGCCCCGACGTT-3′ and 5′-CGGGAATTCTCAATCCATGGCAGCCAGCTGCT-3′, and subcloned into the lentiviral expression vector pRRLsinCMV at XbaI and EcoR1 sites. Green fluorescent protein (GFP) was amplified from pEGFP with the primers 5′-CTGTCGGATCCGGAACCGTCAGATCCGCTA-3′ and 5′-CTGCAGAATTCGAAGCTTGAGCTCGAG-3′, and subcloned into pRRLsinCMV at BamH1 and EcoR1 sites. Correct orientation and sequence for both FG-12 and pRRLsinCMV cloning was confirmed through restriction enzyme digestion and DNA sequencing. The FG-12 or pRRLsinCMV constructs were used to transfect 293T cells along with packaging vectors pMDG and pCMVΔR8.2.17 The viral supernatant was collected and used to infect primary endothelial cell cultures. For the FG-12 system, infection efficiency was monitored through GFP labeling.18 The cells were then evaluated through Western blot analysis.
Lactate Dehydrogenase (LDH) Release Assay
Cells were plated onto gelatin-coated six-well plates at 5 × 104 cells/well. Cells were treated with VP-16, paclitaxel, or thapsigargin for 96 hours. The culture supernatants were collected, centrifuged at 1000 × g for 10 minutes, and the LDH release assay was performed according to manufacturer’s protocol (Sigma-Aldrich). Absorbance was read at 490 nm. The fold increase of LDH release was based on the OD readings from untreated, uninfected cells. Groups were treated in triplicate and experiments were repeated at least twice.
MTT Cell Viability Assay
Cells were seeded in triplicates onto 96-well plates in RPMI/10%FCS at 3 × 103 cells/well. Cells were treated with the designated drugs: VP-16, paclitaxel, thapsigargin, and/or roscovitine. After treatment, cells were incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent (Sigma-Aldrich) for 4 hours. Medium was removed and dimethyl sulfoxide (DMSO) was added. Absorbance was measured at 490 nm, and percentage cell viability was calculated relative to untreated controls. Experiments were repeated three times.
Statistical Analysis
Values are presented as the mean ± SEM. Statistical significance was evaluated using the Student’s two-tailed t-test. P < 0.05 was considered statistically significant.
Results
Endothelial Cells Are Isolated from Human Glioma and Normal Brain Tissues
Fresh human glioma and normal brain specimens were processed for the isolation and culture of endothelial cells, as extensively described in the Materials and Methods. GBM tissues were received after surgery from glioma patients, whereas normal brain specimens were obtained from trauma or epileptic patients. The characterization of TuBECs and BECs was validated through 100% positive immunostaining for the following endothelial cell markers: CD31, von Willebrand Factor, CD105, and VE-cadherin (Figure 1). TuBECs and BECs, however, stained negative for the astrocyte/glial cell marker GFAP and for the microglia/macrophage marker CD11b (Figure 1). The glioma cell line U87MG was 100% positive for GFAP expression, whereas glioma tissues stained positive for CD11b and CD34. Interestingly, TuBECs and BECs were also negative for CD34, a marker of precursor endothelial cells, which suggests that TuBECs and BECs are at their end stage of differentiation (Figure 1).
Tumor-Associated Endothelial Cells Derived from Gliomas Are Chemoresistant to Different Classes of Cytotoxic Drugs
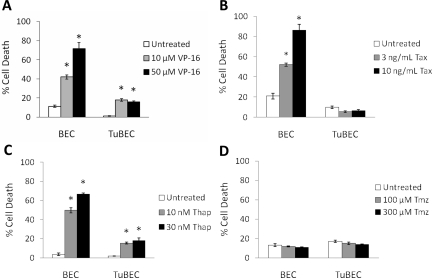
TuBECs and BECs were treated with drugs that induce cytotoxicity via different mechanisms, particularly VP-16 (10, 50 μmol/L), paclitaxel (3, 10 ng/ml), thapsigargin (10, 30 nmol/L), and temozolomide (100, 300 μmol/L). VP-16 inhibits topoisomerase II, whereas paclitaxel affects microtubule stability.19,20 Thapsigargin targets calcium pumps in the endoplasmic reticulum (ER),21 and temozolomide is a DNA alkylating agent.22 After drug treatment, cell death was analyzed using the Cell Death Detection ELISA assay. TuBECs were relatively resistant to VP-16, paclitaxel, and thapsigargin, whereas the BECs were sensitive. After 72 hours TuBECs exhibited much lesser cell death than BECs; BECs underwent 72% cell death with 50 μmol/L VP-16 (Figure 2A). TuBECs and BECs were also treated with paclitaxel, and a similar trend was observed. Paclitaxel at both doses had no significant effect on TuBEC viability as compared to untreated cells (P = 0.116 and P = 0.189), but BECs exhibited four times more cell death at the higher concentration (Figure 2B). This chemoresistance in TuBECs was again observed with thapsigargin treatment. TuBECs treated with 30 nmol/L thapsigargin showed 18% cell death whereas treatment of BECs showed 65% cell death, respectively (Figure 2C). Similar findings for VP-16, paclitaxel, and thapsigargin treatments were also detected in the MTT cell viability assay (data not shown). Interestingly, both TuBECs and BECs were not affected by 7-day temozolomide treatment as compared to untreated cells, even at 300 μmol/L (P = 0.136, TuBECs; and P = 0.227, BECs) (Figure 2D). These studies indicate that TuBECs are resistant to different classes of cytotoxic drugs.
Figure 2.
TuBECs and BECs respond differently to various cytotoxic agents. TuBECs and BECs were treated with VP-16 (A; 10, 50 μmol/L), paclitaxel (Tax) (B; 3, 10 ng/ml), thapsigargin (Thap) (C; 10, 30 nmol/L), or temozolomide (Tmz) (D; 100, 300 μmol/L). Untreated cells were incubated in medium containing vehicle (0.1% DMSO). After 72 hours of VP-16, paclitaxel, and thapsigargin treatment and after 7 days of temozolomide treatment, cell lysates were analyzed using the Cell Death Detection ELISA assay. Percent cell death was calculated based on 100% cell death positive control. *P < 0.05, comparisons are made between the untreated controls and the drug-treated cells.
TuBECs Express High Levels of Survivin in Vitro and in Situ in Glioma Tissue
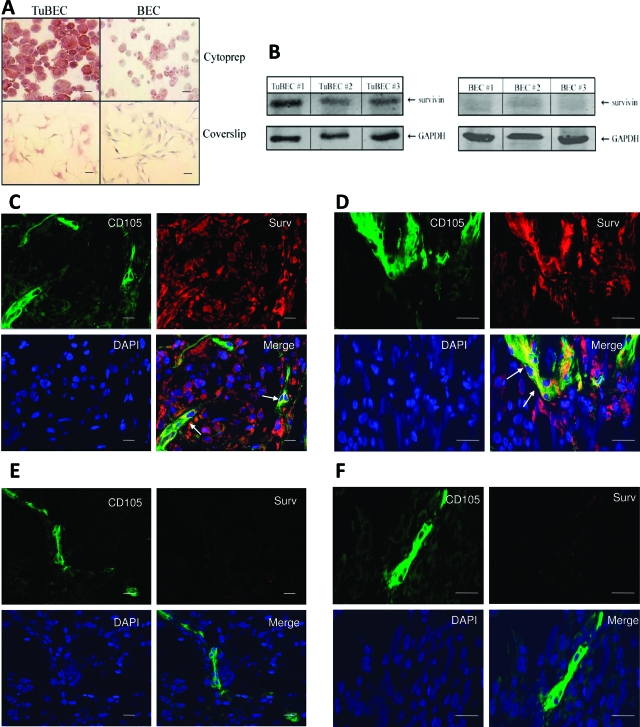
Because survivin is overexpressed in a variety of tumors including gliomas,15 we wanted to explore whether survivin is also overexpressed in the tumor vasculature. To accomplish this, we immunostained cell preparations of TuBECs and BECs with an antibody to survivin. The results showed that TuBECs stained intensely positive for survivin compared to BECs (Figure 3A). Survivin staining was also performed on cells grown on glass chambered slides; again, survivin expression was greater in TuBECs compared to BECs (Figure 3A). To confirm the specificity of survivin staining, a survivin blocking peptide was added along with the antibody and shown to have reduced the survivin staining (see Supplemental Figure S2B at http://ajp.amjpathol.org). Western blot analysis was also performed on TuBECs and BECs (Figure 3B). This is representative of 10 TuBEC and 11 BEC specimens from different patients. The data demonstrate that survivin is highly expressed in TuBECs and minimally expressed in BECs.
Figure 3.
TuBECs and the glioma vasculature overexpress survivin. A: Cytocentrifuge preparations (cytoprep) or glass chambered slides (coverslip) of TuBECs and BECs were immunostained with anti-survivin antibody. The red precipitate represents positive staining; hematoxylin (blue) staining denotes nuclei. B: Lysates of TuBECs and BECs from three different glioma patient specimens and three different normal brain tissues were analyzed for survivin expression using Western blot analysis. C and D: Glioma tissues were immunostained with anti-survivin antibody (red) and anti-CD105 antibody (green), and mounted in DAPI-containing medium (blue) to identify nuclei. Confocal microscopy was used to analyze the staining. In the merged image, survivin was localized to the CD105-positive blood vessels of the glioma tissue (arrow). Lower (C) and higher (D) magnification confocal images are presented. E and F: Normal human brain tissues were stained with antibodies to survivin (red) and CD105 (green), and mounted in DAPI-containing medium (blue). Confocal analysis at low (E) and high (F) magnification show that the merged image exhibited CD105-positive cells only; survivin was not visible in normal brain tissues. Scale bars: 10 μm (A); 100 μm (C–F).
We then performed double staining on human glioma tissues to determine whether survivin expression can be detected in the glioma vasculature in situ (Figure 3, C and D). Tissues were co-stained for CD105/endoglin, a marker for endothelial cells, and survivin; DAPI blue staining identified nuclei. Normal brain tissues were stained using the same procedure (Figure 3, E and F). The staining of the tissues was analyzed through confocal microscopy. Merging CD105 and survivin staining revealed that the tumor-associated vasculature within the glioma tissue exhibited positive survivin expression, as denoted by the yellow color (Figure 3, C and D; arrows). Normal brain tissues were negative for survivin (Figure 3D). Two representative fields are shown for both the glioma and normal brain tissues, one at a lower magnification (Figure 3, C and E) and another at a higher magnification (Figure 3, D and F). These studies demonstrate that blood vessels of glioma tissues express elevated levels of the survivin protein.
Reduction of Survivin Levels Using siRNA Chemosensitizes TuBECs
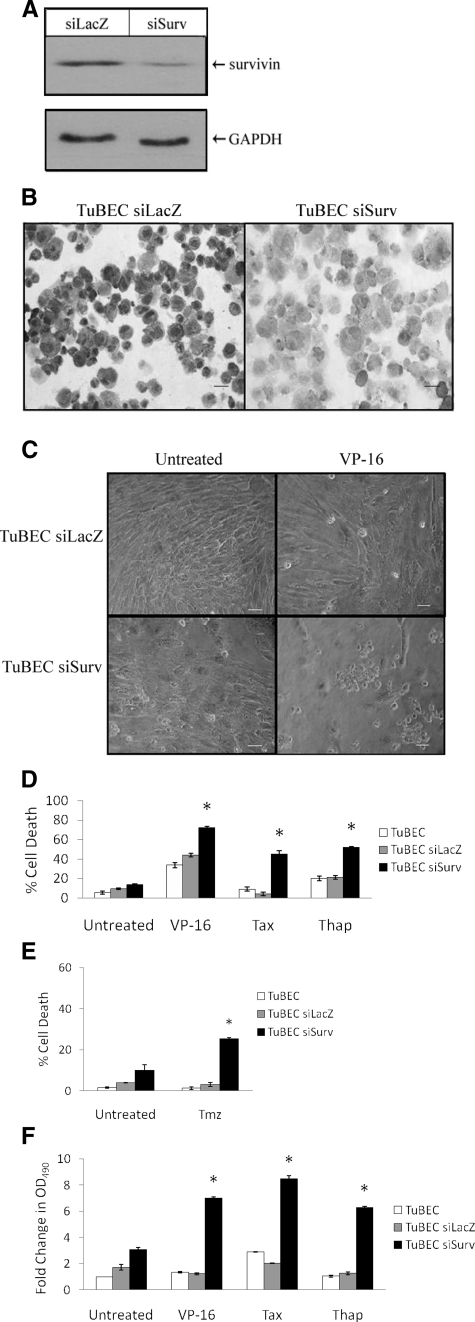
To test whether survivin protects TuBECs from chemotherapeutic agents, a lentiviral vector carrying the siRNA targeted against survivin (siSurv) was constructed. A siRNA against the nonmammalian gene LacZ was also used (siLacZ) as a control. Five days after infection, TuBECs with siSurv or siLacZ were evaluated for survivin expression using Western blot analysis (Figure 4A). Survivin protein was reduced in TuBECs infected with siSurv. This down-regulation of survivin was sustained for at least 3 weeks, as indicated by immunostaining of TuBEC siSurv and TuBEC siLacZ performed 21 days after infection (Figure 4B). These data also emphasize the specificity of the reagents used in these studies.
Figure 4.
Survivin knockdown with siRNA sensitizes TuBECs to chemotherapeutic agents. TuBECs were infected with either lentivirus expressing siRNA to survivin (siSurv) or LacZ (siLacZ). A: Cell lysates of lentiviral-infected cells, prepared 5 days after infection, were analyzed using Western blot analysis. B: The stable knockdown of survivin in TuBECs was confirmed with immunostaining for survivin of cells 21 days after infection. C: TuBEC siSurv and TuBEC siLacZ cultures were incubated with VP-16 (50 μmol/L) or vehicle (0.1% DMSO) for 96 hours and examined with an inverted microscope. D: TuBEC siSurv or control cells, uninfected or infected with lenti-siLacZ, were incubated with VP-16 (50 μmol/L), paclitaxel (Tax) (10 ng/ml), or thapsigargin (Thap) (30 nmol/L); untreated cells were exposed to vehicle (0.1% DMSO). After 96 hours, cell death was quantified using the Cell Death Detection ELISA. E: TuBEC siSurv and control cells were treated with temozolomide (Tmz) (300 μmol/L), and after 7 days, cytotoxicity was measured using the Cell Death Detection ELISA. F: TuBEC siSurv, TuBEC siLacZ, and uninfected TuBECs were treated with VP-16, paclitaxel, thapsigargin, or vehicle for 96 hours. Supernatants were then analyzed using the LDH release assay; optical density (OD) was read at 490 nm. Data are expressed as fold change in LDH release using uninfected, untreated TuBECs as the baseline value (*P < 0.05, comparisons were made for drug-treated TuBEC siSurv to drug-treated TuBEC siLacZ). Scale bars = 10 μm.
We then examined whether reduced survivin levels would affect TuBEC sensitivity to drugs. TuBECs, TuBEC siSurv, and TuBEC siLacZ were treated with VP-16 for 96 hours. In observing the cells in culture, it was apparent that VP-16 was effective in killing the cells infected with siSurv (Figure 4C). These cultures exhibited floating and rounded cells, typical of cell death. The TuBECs infected with siLacZ, however, had little response to VP-16 and exhibited morphology of healthy cells. To quantify the results observed in culture, the Cell Death Detection ELISA was used. Results show that the drugs had relatively little effect on uninfected TuBECs and TuBECs infected with siLacZ; in contrast, TuBECs infected with siSurv demonstrated significantly increased sensitivity to these agents (Figure 4D). TuBECs infected with siSurv and treated with VP-16 or thapsigargin for 96 hours demonstrated nearly a twofold increase in cell death (72%) (P = 0.007) and (55%) (P = 0.005), respectively. TuBECs infected with siSurv and treated with paclitaxel for 96 hours, exhibited a ninefold increase in cell death to 45% (P = 0.009). Incubation of TuBECs for 4 days with temozolomide, the principle drug used for glioma therapy, had no effect on cell death, in the absence or presence of siSurv (data not shown). However, after 7 days of temozolomide treatment, siSurv-infected TuBECs exhibited increased cell death (25%) compared to untreated or control infected TuBECs (P = 0.001) (Figure 4E). Similar results for VP-16, paclitaxel, and thapsigargin treatments were obtained using the LDH release assay (Figure 4F). These data indicate that decreasing survivin protein levels in TuBECs will chemosensitize these vascular cells to anti-tumor agents.
Forced Overexpression of Survivin in BECs Protects These Cells from Cytotoxic Drugs
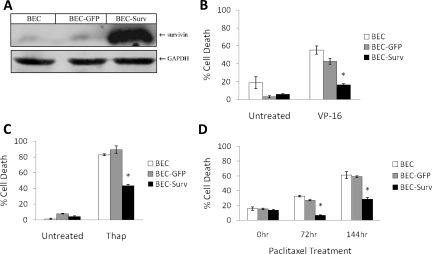
To determine whether survivin overexpression will be sufficient to confer chemoresistance on normal BECs, we infected BECs with a lentiviral expression vector containing the human wild-type survivin gene (BEC-Surv); to serve as a control, BECs were infected with a lentivirus containing GFP (BEC-GFP). Western blot analysis detected the increased survivin expression in BEC-Surv (Figure 5A). Once again the specificity of the antibody to survivin was confirmed. Survivin levels of BEC-GFP remained similar to those of uninfected BECs, suggesting the lentivirus had no secondary effects on survivin expression.
Figure 5.
Forced overexpression of survivin protects BECs from cytotoxic drugs. A: BECs were infected with lentivirus-expressing green fluorescent protein (GFP) or wild-type survivin (Surv) and examined using Western blot analysis. Uninfected BECs, BEC-GFP, and BEC-Surv were treated with VP-16 (B; 50 μmol/L) and thapsigargin (Thap) (C; 30 nmol/L) for 72 hours, or paclitaxel (Tax) (D; 3 ng/ml) for 72 or 144 hours; untreated cells were incubated with vehicle (0.1% DMSO). Cell death was quantified with the Cell Death Detection ELISA; percent cell death was determined based on 100% positive cell death control (*P < 0.05, comparisons were made between drug-treated BEC-Surv and drug-treated uninfected BECs).
Uninfected BECs, BEC-Surv, and BEC-GFP were then treated with drugs (VP-16, thapsigargin, and paclitaxel), and cytotoxicity was measured. The results show that overexpression of survivin resulted in reduced cytotoxicity. In Figure 5B, BEC-Surv were more resistant to VP-16 after 72 hours of treatment as compared to BECs (P = 0.019). A similar trend was also seen with thapsigargin, with significantly less death observed with BEC-Surv (43%) than with BECs (82%) (P = 0.010) (Figure 5C). This protective effect of survivin was also exhibited with paclitaxel treatment after 72 and 144 hours (P = 0.002 and P = 0.008, respectively) (Figure 5D). These data demonstrate that the overexpression of survivin in normal endothelial cells is protective and causes these sensitive cells to become resistant to cytotoxic drugs.
Reduced Survivin Levels Potentiate Caspase Activation in TuBECs
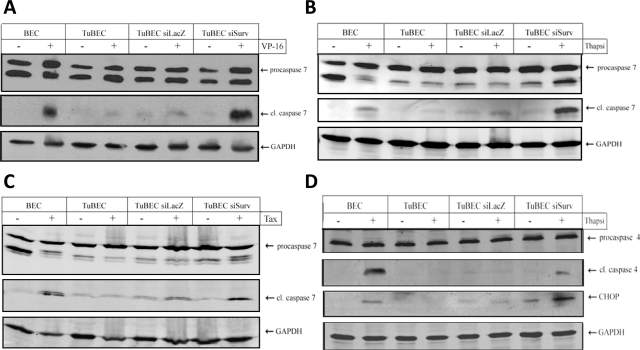
We next examined the activation of different caspases involved in apoptosis to better understand the mechanism by which reduced expression of survivin enhances cell death in TuBECs. Cells were treated with VP-16 and tested for caspase 7 cleavage, an effector caspase shown to interact with survivin.9,11 Treatment of BECs with VP-16 caused cleavage of caspase 7 (Figure 6A), whereas VP-16 had no effect on treated TuBECs. However, TuBECs with reduced survivin demonstrated activation of procaspase 7 when treated with the drug. Similar results were obtained with thapsigargin (Figure 6B) and paclitaxel treatments (Figure 6C). We also detected an intermediate product of procaspase 7 (32 kDa); this intermediate product naturally occurs in cells and was not indicative of caspase 7 activity. We then investigated the initiator caspase 4, a marker of ER stress-induced apoptosis (caspase 12 in mice).23 The results show that TuBEC siSurv treated with thapsigargin exhibited caspase 4 cleavage (Figure 6D), whereas untreated or control-infected TuBECs demonstrated negligible reactivity. We then analyzed thapsigargin-treated TuBECs and BECs for the induction of CHOP, a pro-apoptotic mediator of the ER stress pathway.23 The results were similar to those observed with caspase 4 cleavage; thapsigargin-treated TuBEC siSurv demonstrated remarkable CHOP induction (Figure 6D). These data indicate that survivin is actively blocking the apoptotic pathway in TuBECs. However, the primary site or sites of this inhibition in the apoptotic cascade are not known.
Figure 6.
Reduced survivin expression in TuBECs permits caspase activation. Uninfected BECs, uninfected TuBECs, TuBECs infected with a lentivirus-expressing siRNA to LacZ (TuBEC siLacZ), or TuBECs infected with lentivirus-expressing siRNA to survivin (TuBEC siSurv) were treated with VP-16 (A; 50 μmol/L), thapsigargin (Thap) (B; 30 nmol/L), or paclitaxel (Tax) (C; 10 ng/ml) for 72 hours. Untreated cells were exposed to vehicle (0.1% DMSO). Western blot analysis was used to determine the presence of procaspase 7 (35 kDa) and cleaved caspase 7 (20 kDa). D: BECs, TuBECs, TuBEC siLacZ, and TuBEC siSurv were treated with thapsigargin for 48 hours and analyzed for procaspase 4 (45 kDa), cleaved caspase 4 (30 kDa), and CHOP (30 kDa).
Roscovitine Reduces Survivin Expression and Enhances TuBEC Chemosensitivity
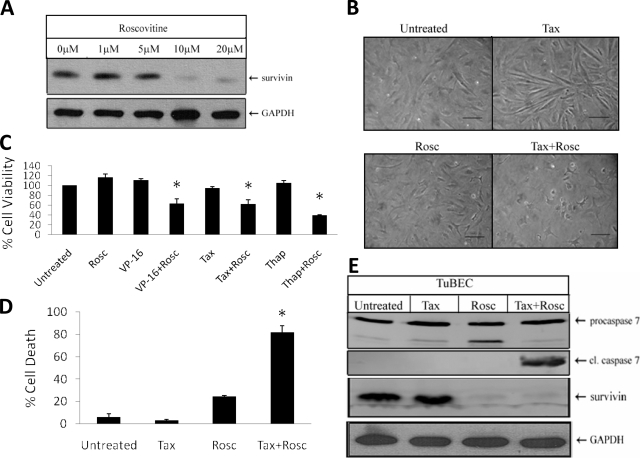
Targeting survivin expression has clinical relevance because it enhances the response of tumor-associated endothelial cells to different classes of drugs. However, lentiviral vectors are not currently available for use in the clinic. Therefore, we examined the effects of pharmacological inhibitors of survivin. The drug roscovitine, a Cdk1 inhibitor that is used in vitro and in vivo, was shown to reduce survivin levels24 by decreasing the stability of the survivin protein in tumor cell lines. To determine whether this agent could alter survivin expression in TuBECs, the cells were treated with roscovitine for 24 hours and analyzed for survivin protein using Western blot analysis (Figure 7A). Maximal down-regulation of survivin was observed with 10 μmol/L treatment, and therefore this dose was used for all subsequent experiments.
Figure 7.
Down-regulation of survivin with roscovitine chemosensitizes TuBECs. A: TuBECs were treated with 1, 5, 10, and 20 μmol/L roscovitine or vehicle (0.1% DMSO) for 24 hours and analyzed for survivin expression using Western blot analysis. B: TuBEC cultures were treated for 72 hours with roscovitine (Rosc) (10 μmol/L) or paclitaxel (Tax) (10 ng/ml) alone or in combination. C: TuBECs were treated for 72 hours with roscovitine alone or in combination with VP-16 (50 μmol/L), paclitaxel, or thapsigargin (Thap) (30 nmol/L); untreated cells were exposed to vehicle. The data represent the MTT cell viability assay, and untreated TuBECs represented 100% cell viability (*P < 0.05, comparisons were made between the combination treatments and the drug alone treatments). D: TuBECs were treated for 72 hours with roscovitine or paclitaxel alone or in combination. The Cell Death Detection ELISA was performed, and percentage cell death was based on 100% positive cell death control (*P < 0.05, comparisons were made for Tax + Rosc treatment to Tax treatment alone and also for Tax + Rosc treatment to Rosc treatment alone). TuBECs were also treated with roscovitine or paclitaxel alone, or in combination for 48 hours. E: Western blot was performed to detect procaspase 7, cleaved caspase 7, and survivin expression. Scale bars = 10 μm.
We then tested whether roscovitine would alter the sensitivity of TuBECs to cytotoxic drugs. TuBECs were treated with roscovitine alone or in combination with VP-16, paclitaxel, or thapsigargin. TuBEC cultures incubated with paclitaxel or roscovitine alone for 72 hours appeared to be morphologically normal (Figure 7B). However, cells treated with both agents caused cell detachment and death. The MTT cell viability assay (Figure 7C) was used to quantitate these culture results and showed that roscovitine alone had negligible effects on TuBECs, whereas the combination of roscovitine with VP-16 (P = 0.011), paclitaxel (P = 0.001), or thapsigargin (P = 0.003) produced a significant decrease in endothelial cell viability as compared to the individual drugs alone. Similar results were obtained with the Cell Death Detection ELISA assay (Figure 7D). TuBECs treated with paclitaxel or roscovitine alone exhibited little cell death, but the combination treatment resulted in 82% cell death (P = 0.011 to roscovitine, P = 0.005 to paclitaxel). The cells treated with this drug combination underwent apoptosis, as determined by the TUNEL assay (see Supplemental Figure S3 at http://ajp.amjpathol.org). We also examined the effects of this combined treatment on caspase 7 cleavage and survivin expression. The results show that treatment with paclitaxel and roscovitine was effective in inducing caspase 7 activation, and that roscovitine decreased survivin alone and in combination with paclitaxel (Figure 7E). Thus, roscovitine sensitizes TuBECs to chemotherapeutic drugs in a similar manner to that seen with the lenti-siSurv approach.
Discussion
The present study provides compelling evidence that survivin is responsible for the chemoresistance observed in tumor-associated brain endothelial cells. We previously reported that TuBECs do not respond to celecoxib or CPT-11 treatments.7 Here, we extend these observations and show that TuBECs are resistant to different classes of drugs, particularly VP-16 (topoisomerase II inhibitor),19 paclitaxel (microtubule stabilizer),20 thapsigargin (Ca2+ pump inhibitor),21 and temozolomide (DNA alkylator).22 The mechanism of resistance in TuBECs was not known. In this study, we established that survivin, which is overexpressed in TuBECs, is responsible, at least in part, for the chemoresistance observed in these cells.
The protective function of survivin in endothelial cells, particularly those of the tumor vasculature, remains to be fully understood. Correlative studies have shown that elevated survivin expression coincides with increased microvessel density in brain,15 colorectal,25 and gastric26 cancers. To our knowledge, however, the role of survivin in endothelial cells isolated from tumors has not been established. Previous reports demonstrate that survivin protects endothelial cells from serum starvation,27 radiation,28 and chemotherapy.29 These studies, however, were performed on normal endothelial cells (eg, human umbilical vein endothelial cells or human dermal microvascular endothelial cells/dermal microvascular endothelial cells). Human umbilical vein endothelial cells and human dermal microvascular endothelial cells/dermal microvascular endothelial cells intrinsically have low levels of survivin and have been manipulated through cytokine stimulation or viral transduction to express survivin.27,30,31,32,33 Furthermore, in contrast to survivin in TuBECs, growth factor-induced survivin expression in normal endothelial cells is transitory, peaking at 12 hours and decreasing by 24 hours.33 Our studies use low passage, primary cultures of endothelial cells isolated from human brain tumor specimens of patients,5 not cell lines. In TuBECs, survivin levels remain constitutively high without treatment with any exogenous growth factor or genetic manipulation. The mechanism of this overexpression is currently under investigation and may likely be a response to the tumor microenvironment. Blanc-Brude and colleagues27 demonstrated that targeting survivin reduced tumor growth and angiogenesis in a breast cancer xenograft model; they did not, however, directly demonstrate that the tumor vasculature was a target of survivin modulation. Our present study extends their work, by showing conclusively that the tumor vasculature is the direct target for anti-survivin activity, and TuBECs do indeed express survivin. To support this, we analyzed the tumor vasculature in primary cultures and in vivo and showed that tumor-associated endothelial cells constitutively overexpress survivin and that survivin reduction sensitizes the tumor vasculature to drugs. Furthermore, our data agree with their concept that survivin provides a protective mechanism for the tumor microenvironment.
The function and mechanism of survivin has been widely studied in various types of tumors.14,34,35,36,37,38 Elevated survivin levels correlate with poor patient prognosis and likelihood of recurrence in many cancers, such as hepatocellular,39 non-small cell lung,40 and breast41 carcinomas. Survivin has bi-functional roles; it drives cell division and inhibits apoptosis, as reported in renal cancer carcinoma42 and lung cancer cells.35 Furthermore, in vivo studies have suggested the role of survivin in the tumorigenesis of melanomas.34 Survivin has been reported to inhibit mitochondrial apoptosis, prevent the incorporation of caspase-9 into the apoptosome, block cytochrome c release,27 and inhibit the effector caspases 3 and 7.11 How survivin functions to protect endothelial cells is still unclear. We show here that survivin protects against cytotoxicity induced by VP-16, paclitaxel, and thapsigargin by blocking the activation of caspase 7. Caspase 4 cleavage and CHOP induction were also clearly detected in TuBEC siSurv treated with thapsigargin, a drug that directly triggers ER stress.21 Caspase 4 and CHOP are specific mediators of ER stress-induced apoptosis.23 This suggests that survivin may also protect endothelial cells from the apoptotic pathway mediated through the ER stress mechanism. Survivin protection may therefore be dependent on the drug used to induce cytotoxicity, because caspase 4 cleavage was detected only with thapsigargin, and not with VP-16 or paclitaxel. In contrast to the other drugs tested, reduction of survivin protein slightly sensitized TuBECs to temozolomide. The effect of temozolomide on the tumor vasculature is of critical importance because this is the standard of care for glioma therapy. These data suggest that optimal glioma treatment using temozolomide alone may not be sufficient, and may require the combination with an anti-vascular agent. Thus, the function of survivin as a protective protein may differ depending on cell type and drug action.
Survivin is an attractive target for cancer therapy because it is highly expressed in both cancer cells and the tumor vasculature. We used both a genetic and pharmacological approach to reduce survivin in TuBECs. However, because lentiviral agents are not currently used in the clinic, we tested a pharmacological agent, roscovitine, a Cdk1 inhibitor24 that prevents the phosphorylation of survivin and thereby decreases its stability. Co-treatment with roscovitine enhanced the response of TuBECs to VP-16, paclitaxel, and thapsigargin. VP-16 is used for treating gliomas,43 and so combined administration of this drug with roscovitine may be more efficient in treating brain tumors because of the additional anti-angiogenic effects. Our data are consistent with previous reports using other Cdk1 inhibitors, such as Purvalanol A and NU6140, which enhanced apoptosis in paclitaxel-treated HeLa cells.44,45 Similar findings were obtained showing that roscovitine enhanced cell death of glioma cells resistant to TRAIL by reducing survivin.46 We show here that decreased survivin can also enhance cytotoxicity of the tumor-associated vasculature, thereby making this an effective anti-vascular therapy.
Survivin had originally gained attention because it was widely and specifically overexpressed in tumor cells and promoted chemoresistance. Our studies reveal that survivin is also elevated in tumor-associated endothelial cells and is a powerful anti-apoptosis agent for the tumor vasculature. Thus anti-survivin therapy in combination with conventional chemotherapy would target the tumor vasculature as well as malignant cells, making this an appealing approach for cancer treatment.
Acknowledgments
We thank Dr. Jeroen Pouwels and Dr. Anu Kukkonen (VTT Medical Biotechnology, Turku, Finland) for kindly providing the human wild-type survivin gene, Mrs. Ligaya Pen and Mrs. Susan Su for their help in isolating human brain endothelial cells, and the Alzheimer’s Disease Research Center Neuropathology Core (University of Southern California) for some of the normal brain tissues used in this study.
Footnotes
Address reprint requests to Florence M. Hofman, Ph.D., University of Southern California, Keck School of Medicine, 2011 Zonal Ave., HMR 315A, Los Angeles, CA 90033. E-mail: hofman@usc.edu.
Supported by the California Breast Cancer Research Program (to F.M.H.) and the Wright Foundation (to F.M.H.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Louis DN, Pomeroy SL, Cairncross JG. Focus on central nervous system neoplasia. Cancer Cell. 2002;1:125–128. doi: 10.1016/s1535-6108(02)00040-5. [DOI] [PubMed] [Google Scholar]
- Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol. 2002;28:C947–C970. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Deambrosis I, Russo S, Deregibus MC, Camussi G. Altered angiogenesis and survival in human tumor-derived endothelial cells. FASEB J. 2003;17:1159–1161. doi: 10.1096/fj.02-0557fje. [DOI] [PubMed] [Google Scholar]
- Charalambous C, Hofman FH, Chen TC. Functional and phenotypic differences between glioblastoma multiforme-derived and normal human brain endothelial cells. J Neurosurg. 2005;102:699–705. doi: 10.3171/jns.2005.102.4.0699. [DOI] [PubMed] [Google Scholar]
- Charalambous C, Pen LB, Su YS, Milan J, Chen TC, Hofman FM. Interleukin-8 differentially regulates migration of tumor-associated and normal human brain endothelial cells. Cancer Res. 2005;65:10347–10354. doi: 10.1158/0008-5472.CAN-05-0949. [DOI] [PubMed] [Google Scholar]
- Charalambous C, Virrey JJ, Kardosh A, Jabbour MN, Qazi-Abdullah L, Pen L, Zidovetzki R, Schönthal AH, Chen TC, Hofman FM. Glioma-associated endothelial cells show evidence of replicative senescence. Exp Cell Res. 2007;313:1192–1202. doi: 10.1016/j.yexcr.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18:609–615. doi: 10.1016/j.ceb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest. 2004;114:1117–1127. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohi T, Okada K, Xia F, Wilford CE, Samuel T, Welsh K, Marusawa H, Zou H, Armstrong R, Matsuzawa S, Salvesen GS, Reed JC, Altieri DC. An IAP–IAP complex inhibits apoptosis. J Biol Chem. 2004;279:34087–34090. doi: 10.1074/jbc.C400236200. [DOI] [PubMed] [Google Scholar]
- Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, Chung CW, Jung YK, Oh BH. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. 2001;40:1117–1123. doi: 10.1021/bi001603q. [DOI] [PubMed] [Google Scholar]
- Vong QP, Cao K, Li HY, Iglesias PA, Zheng Y. Chromosome alignment and segregation regulated by ubiquitination of survivin. Science. 2005;310:1499–1504. doi: 10.1126/science.1120160. [DOI] [PubMed] [Google Scholar]
- Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Validating survivin as a cancer therapeutic agent. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- Zhen HN, Zhang X, Hu PZ. Survivin expression and its relation with proliferation, apoptosis, and angiogenesis in brain gliomas. Cancer. 2005;104:2775–2783. doi: 10.1002/cncr.21490. [DOI] [PubMed] [Google Scholar]
- Qin XF, An DS, Chen ISY, Baltimore D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci USA. 2003;100:183–188. doi: 10.1073/pnas.232688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S, Chen M, Woodley DT, Li W. Nckβ adapter controls neuritogenesis by maintaining cellular paxillin level. Mol Cell Biol. 2007;17:6001–6011. doi: 10.1128/MCB.01807-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay B, Fan J, Guan S, Li Y, Chen M, Woodley DT, Li W. A “traffic control” role for TGFbeta3: orchestrating dermal and epidermal cell motility during wound healing. J Cell Biol. 2006;172:1093–1105. doi: 10.1083/jcb.200507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meresse P, Dechaux E, Monneret C, Bertounesque E. Etoposide: discovery and medicinal chemistry. Curr Med Chem. 2004;11:2443–2466. doi: 10.2174/0929867043364531. [DOI] [PubMed] [Google Scholar]
- Marupudi NI, Han JE, Li KW, Renard VM, Tyler BM, Brem H. Paclitaxel: a review of adverse toxicities and novel delivery strategies. Expert Opin Drug Safety. 2007;6:609–621. doi: 10.1517/14740338.6.5.609. [DOI] [PubMed] [Google Scholar]
- Denmeade SR, Isaacs JT. The SERCA pump as a therapeutic target: making a “smart bomb” for prostate cancer. Cancer Biol Ther. 2005;4:14–22. doi: 10.4161/cbt.4.1.1505. [DOI] [PubMed] [Google Scholar]
- Friedman HS, Kirby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–2597. [PubMed] [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirado OM, Mateo-Lozano S, Notario V. Roscovitine is an effective inducer of apoptosis of Ewing’s sarcoma family tumor cells in vitro and in vivo. Cancer Res. 2005;65:9320–9327. doi: 10.1158/0008-5472.CAN-05-1276. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Toyoda M, Shinohara H, Okuda J, Watanabe I, Yamamoto T, Tanaka K, Tenjo T, Tanigawa N. Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer. 2001;91:2026–2032. doi: 10.1002/1097-0142(20010601)91:11<2026::aid-cncr1228>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Lee GH, Joo YE, Koh YS, Chung IJ, Park YK, Lee JH, Kim HS, Choi SK, Rew JS, Park CS, Kim SJ. Expression of survivin in gastric cancer and its relationship with tumor angiogenesis. Eur J Gastroenterol Hepatol. 2006;18:957–963. doi: 10.1097/01.meg.0000230086.83792.56. [DOI] [PubMed] [Google Scholar]
- Blanc-Brude OP, Mesri M, Wall NR, Plescia J, Dohi T, Altieri DC. Therapeutic targeting of the survivin pathway in cancer: initiation of mitochondrial apoptosis and suppression of tumor-associated angiogenesis. Clin Cancer Res. 2003;9:2683–2692. [PubMed] [Google Scholar]
- Kim KW, Mutter RW, Cao C, Albert JM, Shinohara ET, Sekhar KR, Lu B. Inhibition of signal transducer and activator of transcription 3 activity results in down-regulation of survivin following irradiation. Mol Cancer Ther. 2006;5:2659–2665. doi: 10.1158/1535-7163.MCT-06-0261. [DOI] [PubMed] [Google Scholar]
- Tran J, Master Z, Yu JL, Rak J, Dumont DJ, Kerbel RS. A role for survivin in chemoresistance of endothelial cells mediated by VEGF. Proc Natl Acad Sci USA. 2002;99:4349–4354. doi: 10.1073/pnas.072586399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DS, Schechner JS, Adida C, Mesri M, Rothermel AL, Li F, Nath AK, Pober JS, Altieri DC. Control of apoptosis during angiogenesis by survivin expression in endothelial cells. Am J Pathol. 2000;156:393–398. doi: 10.1016/S0002-9440(10)64742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesri M, Morales-Ruiz M, Ackermann EJ, Bennett CF, Pober JS, Sessa WC, Altieri DC. Suppression of vascular endothelial growth factor-mediated endothelial cell protection by survivin targeting. Am J Pathol. 2001;158:1757–1765. doi: 10.1016/S0002-9440(10)64131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O'Connor DS, Li F, Altieri DC, Sessa WC. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- Grossman D, Kim PJ, Schechner J, Altieri DC. Inhibition of melanoma tumor growth in vivo by survivin targeting. Proc Natl Acad Sci USA. 2001;98:635–640. doi: 10.1073/pnas.230450097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olie RA, Simoes-Wust AP, Baumann B, Leech SH, Fabbro D, Stahel RA, Zangemeister-Wittke U. A novel antisense oligonucleotide targeting survivin expression induces apoptosis and sensitizes lung cancer cells to chemotherapy. Cancer Res. 2000;60:2805–2809. [PubMed] [Google Scholar]
- Ceballos-Cancino G, Espinosa M, Maldonado V, Melendez-Zajgla J. Regulation of mitochondrial Smac/DIABLO-selective release by survivin. Oncogene. 2007;26:7569–7575. doi: 10.1038/sj.onc.1210560. [DOI] [PubMed] [Google Scholar]
- Song Z, Yao X, Wu M. Direct interaction between survivin and Smac/DIABLO is essential for the anti-apoptotic activity of survivin during taxol-induced apoptosis. J Biol Chem. 2003;278:23130–23140. doi: 10.1074/jbc.M300957200. [DOI] [PubMed] [Google Scholar]
- Kumar P, Coltas IK, Kumar B, Chepeha DB, Bradford CR, Polverini PJ. Bcl-2 protects endothelial cells against γ-radiation via a raf-MEK-ERK-survivin signaling pathway that is independent of cytochrome c release. Cancer Res. 2007;67:1193–1202. doi: 10.1158/0008-5472.CAN-06-2265. [DOI] [PubMed] [Google Scholar]
- Ye CP, Qiu CZ, Huang ZX, Su QC, Zhuang W, Wu RL, Li XF. Relationship between survivin expression and recurrence, and prognosis in hepatocellular carcinoma. World J Gastroenterol. 2007;13:6264–6268. doi: 10.3748/wjg.v13.i46.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atikcan S, Unsal E, Demirag F, Köksal D, Yilmaz A. Correlation between survivin expression and prognosis in non-small cell lung cancer. Respir Med. 2006;100:2220–2226. doi: 10.1016/j.rmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Masuda Y, Kurizaki T, Haga Y, Murayama T, Ikei S, Kamei M, Takeno S, Kawahara K. Survivin expression predicts early recurrence in early-stage breast cancer. Anticancer Res. 2007;27:2803–2808. [PubMed] [Google Scholar]
- Sato A, Oya M, Ito K, Mizuno R, Horiguchi Y, Umezawa K, Hayakawa M, Murai M. Survivin associates with cell proliferation in renal cancer cells: regulation of survivin expression by insulin-like growth factor-1, interferon-gamma and a novel NF-kappaB inhibitor. Int J Oncol. 2006;28:841–846. [PubMed] [Google Scholar]
- Korones DN, Smith A, Foreman N, Bouffet E. Temozolomide and oral VP-16 for children and young adults with recurrent or treatment-induced malignant gliomas. Pediatr Blood Cancer. 2006;47:37–41. doi: 10.1002/pbc.20510. [DOI] [PubMed] [Google Scholar]
- O'Connor DS, Wall NR, Porter AC, Altieri DC. A p34(cdc2) survival checkpoint in cancer. Cancer Cell. 2002;2:43–54. doi: 10.1016/s1535-6108(02)00084-3. [DOI] [PubMed] [Google Scholar]
- Pennati M, Campbell AJ, Curto M, Binda M, Cheng Y, Wang LZ, Curtin N, Golding BT, Griffin RJ, Hardcastle IR, Henderson A, Zaffaroni N, Newell DR. Potentiation of paclitaxel-induced apoptosis by the novel cyclin-dependent kinase inhibitor NU6140: a possible role for survivin down-regulation. Mol Cancer Ther. 2005;4:1328–1337. doi: 10.1158/1535-7163.MCT-05-0022. [DOI] [PubMed] [Google Scholar]
- Kim EH, Kim SU, Shin DY, Choi KS. Roscovitine sensitizes glioma cells to TRAIL-mediated apoptosis by downregulation of survivin and XIAP. Oncogene. 2004;23:446–456. doi: 10.1038/sj.onc.1207025. [DOI] [PubMed] [Google Scholar]