Abstract
The lymphatic system plays an important role in inflammation and cancer progression, although the molecular mechanisms involved are poorly understood. As determined using comparative transcriptional profiling studies of cultured lymphatic endothelial cells versus blood vascular endothelial cells, growth hormone receptor was expressed at much higher levels in lymphatic endothelial cells than in blood vascular endothelial cells. These findings were confirmed by quantitative real-time reverse transcriptase-polymerase chain reaction and Western blot analyses. Growth hormone induced in vitro proliferation, sprouting, tube formation, and migration of lymphatic endothelial cells, and the mitogenic effect was independent of vascular endothelial growth factor receptor-2 or -3 activation. Growth hormone also inhibited serum starvation-induced lymphatic endothelial cell apoptosis. No major alterations of lymphatic vessels were detected in the normal skin of bovine growth hormone-transgenic mice. However, transgenic delivery of growth hormone accelerated lymphatic vessel ingrowth into the granulation tissue of full-thickness skin wounds, and intradermal delivery of growth hormone resulted in enlargement and enhanced proliferation of cutaneous lymphatic vessels in wild-type mice. These results identify growth hormone as a novel lymphangiogenic factor.
Lymphatic vessels play an important role in tissue fluid homeostasis and lipid uptake as well as in pathological conditions such as inflammation and cancer dissemination.1,2 Recent studies have highlighted a direct correlation between the occurrence of lymph node metastases and lymphatic vessel density in several types of human cancer, including cutaneous malignant melanoma3 and head and neck cancer.4 Moreover, in mouse models of cancer, tumor-induced lymphangiogenesis potently promotes tumor metastasis to lymph nodes and other sites.5,6 Lymphangiogenesis also occurs in tumor-draining lymph nodes, and lymph node lymphangiogenesis is associated with enhanced metastasis to distant organs in mouse tumor models and also in some types of human cancer.7,8,9,10 Thus, there has been a quest for identifying the molecular mediators of lymphangiogenesis.
The first specific lymphangiogenic factors identified were vascular endothelial growth factor-C (VEGF-C) and VEGF-D, which activate VEGF-receptor-3 (VEGFR-3)11 expressed on lymphatic endothelial cells (LECs) but not on blood vascular endothelial cells (BVECs) under normal conditions.12 During embryogenesis, VEGF-C is required for the development of the lymphatic vascular system,13 and overexpression of VEGF-C or -D in experimental tumors promotes tumor lymphangiogenesis and metastasis. Furthermore, VEGF-C and -D expression levels have been shown to correlate with the incidence of metastases in a large number of human tumor types.14 More recently, additional lymphangiogenic factors have been identified, including VEGF-A,7,15,16,17 hepatocyte growth factor,18 angiopoietin-1,19 insulin-like growth factors I and II (IGF-I and -II),20 and platelet-derived growth factor-BB.21 However, the relative importance of these factors in physiological and pathological lymphangiogenesis is currently unclear, and additional factors are likely involved.
Growth hormone receptor (GHR) belongs to the cytokine receptor superfamily and is expressed by a number of different cell types including fetal mesenchymal tissue22 from which vessels develop. On binding of its ligand, the pituitary gland-derived growth hormone (GH, also known as somatotropin), the GHR forms homodimers leading to receptor autophosphorylation and activation of insulin receptor substrate 1 and 2, janus kinase 2, and phosphatidylinositol-3 kinase.23 In addition to production by the pituitary gland, ectopic GH expression has also been found in vivo in the developing rat lung,24 normal and neoplastic human lymphoid tissues and endothelial cells,25 in normal and cancerous breast tissue,26 as well as in pancreatic cancer.27 In vitro GH expression has been described in human dermal fibroblasts, in T-cell lymphoma and monocyte lymphoma cell lines,28 in peripheral blood mononuclear cells,29 and in murine granulocytes and macrophages.30
GH signaling has been implicated in promoting postnatal longitudinal growth, in carbohydrate metabolism, in adipocyte maturation, and in the maintenance and development of the immune system.31 GH also has been shown to enhance the proliferation of human retinal microvascular endothelial cells32 and of bovine brain capillary endothelial cells,33 and to promote the formation of capillary-like structures by human umbilical cord vein cells in vitro.34 In vivo, GH exerts an angiogenic effect in the late-stage chicken-chorioallantoic membrane assay,35 and transgenic mice expressing bovine GH under the rat phosphoenolpyruvate carboxykinase promoter (bGH tg) show enhanced vascularization of the wound granulation tissue.36 However, the potential activity of GH in lymphatic vessel formation and function is not known.
In previous gene expression profiling studies of cultured human LECs and BVECs, we detected that GHR expression levels were higher in LECs than in BVECs.37 In the present study, we investigated the in vitro and in vivo expression and function of GHR and GH, respectively, with regard to potential activity on the lymphatic vascular system. We found that GHR was expressed at much higher levels in LECs than in BVECs, as evaluated by quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR) and by Western blot analyses. GH induced in vitro proliferation, sprouting, tube formation, and migration of LECs, and the mitogenic effect was independent of VEGFR-2 or -3 activation. GH also inhibited serum starvation-induced LEC apoptosis. No major alterations of lymphatic vessels were detected in the normal skin of bGH tg mice. However, transgenic delivery of GH accelerated lymphatic vessel ingrowth into the granulation tissue of full-thickness skin wounds, and intradermal delivery of GH resulted in enlargement and enhanced proliferation of cutaneous lymphatic vessels in wild-type mice. Together, these findings identify GH as a novel lymphangiogenic factor and suggest a potential role in pathological conditions associated with lymphatic vessel activation.
Materials and Methods
Cells
Dermal BVECs and LECs were isolated from neonatal human foreskins by immunomagnetic purification as previously described.18 The lineage-specific differentiation was confirmed by real-time RT-PCR for the lymphatic vascular markers Prox1, LYVE-1, and podoplanin, and for the blood vascular endothelial markers VEGFR-1 and VEGF-C, as well as by immunostains for CD31, LYVE-1, and Prox1 as described.37 Cells were cultured in endothelial basal medium (Cambrex, Verviers, Belgium) supplemented with 20% fetal bovine serum (Gibco, Paisley, UK), antibiotic antimycotic solution (1×; Fluka, Buchs, Switzerland), l-glutamine (2 mmol/L, Fluka), hydrocortisone (10 μg/ml, Fluka), and N6,2′-O-dibutyryladenosine-3′,5′-cyclic monophosphate sodium salt (25 μg/ml, Fluka) for up to 11 passages. Cells were grown in a humidified atmosphere at 37°C and 5% CO2.
Quantitative Real-Time RT-PCR
Total cellular RNA was extracted from confluent LECs or BVECs using the Trizol reagent (Invitrogen, Paisley, UK) and treated with RQ RNase-free DNase (Catalys AG, Wallisellen, Switzerland) in the presence of RNase inhibitor (Applied Biosystems, Rotkreuz, Switzerland). The expression levels of vascular lineage-specific genes and of GHR were examined by real-time PCR using the 7900HT fast real-time PCR system and TaqMan one-step RT-PCR master mix reagents (Applied Biosystems). Primer and probe sequences for detection of Prox-1, LYVE-1, and VEGFR-1 have been described previously.37 For detection of GHR, the forward primer 5′-CATTGCCCTCAACTGGACTT-3′ and reverse primer 5′-GTGGTGCTTCCCATCTCACT-3′ were used in combination with SYBR Green PCR master mix (Applied Biosystems). IGF-I, VEGF-A, and VEGFR-2 mRNA expression levels were determined by TaqMan gene expression assays (Applied Biosystems) after treatment with 100 ng/ml of recombinant human GH (R&D Systems, Abingdon, UK) for 30 minutes, 4 hours, or 24 hours. All RT-PCR results were normalized by the expression levels of β-actin.18
Immunoblotting and Enzyme-Linked Immunosorbent Assay
LECs and BVECs were grown to 80% confluence. Total protein was extracted in lysis buffer (20 mmol/L Tris, 150 mmol/L sodium chloride, 5 mmol/L ethylenediaminetetraacetic acid, 1% Triton X, 25 mmol/L sodium fluoride, 1 mmol/L phenylmethyl sulfonyl fluoride, 1 mmol/L sodium metavanadate, 10% glycerol, one tablet of ethylenediaminetetraacetic acid-free protease inhibitor cocktail per 10 ml extraction buffer (Roche, Basel, Switzerland)) and the protein concentration was determined using the NanoOrange protein quantitation kit (Invitrogen). Eighty μg of total protein were immunoprecipitated with a mouse anti-GHR antibody (0.25 μg; Abcam, Cambridge, UK), separated in an 8% acrylamide separating gel and immunoblotted with the same GHR antibody (2 μg/ml) and an anti-mouse horseradish peroxidase antibody (1:2000; Amersham, Duebendorf, Switzerland). In an additional study, 2 ml of supernatant were obtained from LECs treated with 100 ng/ml of GH (R&D Systems) for 6 hours or 24 hours. After immunoprecipitation of IGF-I using a goat anti-IGF-I antibody (5 μg, R&D Systems), IGF-I was detected by incubation with a goat anti-IGF-I antibody (0.2 μg/ml, R&D Systems) and anti-goat horseradish peroxidase antibody (1:2000, Invitrogen). Recombinant human IGF-I (R&D Systems) was used as a positive control. Protein bands were visualized by the ECL plus Western blotting detection system (Amersham). For detection of IGF-I by enzyme-linked immunosorbent assay, 200 μl of immunoprecipitated tissue culture supernatant, and a dilution series of recombinant IGF-I were mixed with carbonate buffer (sodium hydrogen carbonate and sodium carbonate, 0.1 mol/L, pH 8.8) and incubated overnight in Nunc-Immuno 96-microwell plates (VWR, Dietikon, Switzerland) at 4°C. IGF-I was detected by incubation with 0.75 μg/ml goat anti-IGF I antibody (R&D Systems) and anti-goat horseradish peroxidase antibody (1:1000, Invitrogen). Detection was performed using BM Blue POD substrate (Roche) and stopped by applying sulfuric acid. Absorbance was measured by using a VersaMax microplate reader (Bucher Biotec AG, Basel, Switzerland).
Immunofluorescence Staining and Morphometric Analyses
Immunofluorescence analyses were performed on 6-μm frozen sections of human neonatal foreskins and of mouse skin samples after implantation of Matrigel containing recombinant human GH or phosphate-buffered saline (PBS), as well as of 6-μm paraffin sections of skin samples obtained from bGH transgenic and age-matched wild-type mice (kindly provided by Dr. Sabine Werner, ETH Zurich, Switzerland).36 The antibodies used were reactive against the lymphatic-specific markers podoplanin38 (D2-40, 1:100; Signet, Dedham, USA) and LYVE-138 (reactive against both human and mouse,1:1000; Upstate, Dundee, UK), against the panendothelial marker CD3139 (anti-human and anti-mouse, 1:50; Becton Dickinson, Allschwil, Switzerland) and against GH receptor (anti-human, 1:20; R&D Systems). Corresponding secondary antibodies were labeled with Alexa 488 or Alexa 594 (Invitrogen). Frozen sections were fixed in −20°C acetone for 2 minutes and 4°C 80% methanol for 5 minutes. Antigen retrieval was performed by boiling the sections for 10 minutes in citric acid buffer. For BrdU stains (Alexa 594-conjugated antibody, 1:60; Invitrogen), tissues were pretreated with 2 N HCl for 20 minutes at room temperature. Nuclei were counterstained with 20 μg/ml Hoechst trihydrochloride, trihydrate (nitrogen). Sections were examined by an Axioskop 2 plus microscope and pictures were taken using a AxioCam MRc (Carl Zeiss AG, Feldbach, Switzerland). The vessel density, vessel size, and average area occupied by vessels were determined in CD31/LYVE-1-stained sections obtained from wild-type and bGH transgenic mice as described,18 using the IPLab software (Scanalytics, Rockville, MD). Three pictures each were taken of hot-spot areas in normal tail skin (wild-type: n = 10 female and n = 6 male; transgenic: n = 9 female and n = 6 male), and four in areas surrounding or within the granulation tissue of 5-day-old skin wounds (wild-type: n = 5 female; transgenic: n = 6 female). For the Matrigel study, blood and lymphatic vessels as well as BrdU-positive LECs were analyzed in three areas in close proximity to the Matrigel. Morphometric analyses were performed by an investigator blinded to the identity of the samples. Statistical analyses were performed using the two-tailed unpaired Student’s t-test (Graph Pad Prism 4; GraphPad Software Inc., San Diego, CA).
Proliferation, Migration, Apoptosis, Tube Formation, and Sprouting Assays
Proliferation, migration, and tube formation assay were primarily performed as described.18 For proliferation assays, BVECs and LECs (1.25 to 1.5 × 103) were seeded into fibronectin- or collagen type I-coated 96-well plates and were treated with different concentrations of GH (0 to 1000 ng/ml, R&D Systems) or with an equal volume of PBS in endothelial basal medium containing 2% fetal bovine serum. LECs were also incubated with GH (100 ng/ml) together with goat anti-human GHR antibody (1 μg/ml, R&D Systems), human anti-human VEGFR-3 antibody (clone hF4-3C6, 1 μg/ml), human anti-human VEGFR-2 (clone 1121b, 10 μg/ml; kind gift of Dr. Bronek Pytowski, Imclone Systems Inc., New York, NY), goat anti-human IGF I antibody (10 μg/ml, R&D Systems), or control IgG (1 μg/ml or 10 μg/ml, respectively). After 72 hours, cells were incubated with 5-methylumbelliferylheptanoate as described.40 The intensity of fluorescence, proportional to the number of viable cells, was measured using a SpectraMax Gemini EM microplate reader (Bucher Biotec AG).
Haptotactic cell migration was performed in the presence or absence of GH (10 to 1000 ng/ml) as described.18 In additional studies, cells were pre-incubated with a blocking anti-GHR antibody (1 μg/ml, R&D Systems), a blocking anti-integrin α9β1 antibody (1 μg/ml; Chemicon, Temecula, CA), or control IgG for 10 minutes, and were then seeded into the upper chambers of transwell migration chambers and incubated for 3 hours in the presence or absence of GH (100 ng/ml). Migrated cells were stained with Calcein AM (Invitrogen) and fixed in 4% paraformaldehyde. The fluorescence intensity, proportional to the number of transmigrated cells, was measured using a SpectraMax Gemini EM reader.
For tube formation assays, confluent LEC monolayers were overlaid with collagen type I gels as described18 (1 mg/ml; Cohesion, Palo Alto, CA) containing GH (100 ng/ml) or an equal volume of PBS. Tubes were evaluated for up to 20 hours. Three pictures of hot-spots were taken per well and total tube length was analyzed using the IP Lab software as described.18
Spheroid sprouting assays were performed as described41 with the following variation. Spheroids were generated in hanging drops without methylcellulose in Nunclone plates (1000 cells in 20 μl medium; Nunc GmbH, Wiesbaden, Germany). After 24 hours of incubation, spheroids were collected and mixed with collagen type I solution with or without GH (100 ng/ml). Spheroid sprouting was analyzed after 16 hours, using an Axiovert 200M microscope (Carl Zeiss AG). Quantitative sprout length analysis was performed using the IP Lab software.
For apoptosis assays, LECs (one 60-mm plate each) were treated with 100 ng/ml GH or with PBS for 60 hours in endothelial basal medium containing 0.2% bovine serum albumin. As a positive control, 200 μmol/L hydrogen peroxide was applied for 24 hours. Cells were stained using the In Situ Cell Death Detection kit (Roche), and fluorescence was detected on a FACS DIVA flow cytometer (Becton Dickinson). Events (n = 30,000) were collected for each sample and the data were analyzed using Flowjo Version 6.3.4 (Tree Star Inc., Ashland, OR).
For all in vitro studies, three independent experiments were performed, except for the migration assay using anti-integrin α9β1 antibody and the TUNEL assay, which were performed twice. Migration, tube formation, sprouting, and proliferation assays using blocking antibodies were performed in triplicates each. For proliferation assays with different GH concentrations, eight wells per condition were used. The apoptosis assays were performed in single plates. Treatment with VEGF-A (20 ng/ml) was used as a positive control. Figures illustrate results of one representative experiment. Statistical analyses were performed using the two-tailed unpaired Student’s t-test (Graph Pad Prism 4).
In Vivo Matrigel Lymphangiogenesis Assay
FVB wild-type mice (female, 9 weeks old, n = 8 per group; Charles River, Sulzbach, Germany) were intradermally injected with 50 μl of growth factor reduced Matrigel (Becton Dickinson) containing 1 μg/ml of GH (n = 8) or an equal volume of PBS (n = 8). After 7 days, 3 hours before tissue harvest, 300 μl of BrdU (40 mmol/L; Sigma, Buchs, Switzerland) were injected intraperitoneally. Tissues were embedded and frozen in optimal cutting temperature (OCT) compound (Sakura Finetek, Zoeterwoude, The Netherlands). Immunohistochemistry and morphometric analyses for mouse LYVE-1 and CD31 were performed as described above by an investigator blinded to the identity of the samples. For statistical analyses the two-tailed unpaired Student’s t-test was used (Graph Pad Prism 4). The animal study was approved by the Veterinaeramt des Kantons Zuerich, Zuerich, Switzerland, permission number 123/2005.
Results
Enhanced Expression of GHR by LECs as Compared to BVECs
To identify genes that are more highly expressed in LECs than in BVECs, we reanalyzed our previously reported gene expression profiles comparing LECs and BVECs.18,42 These results showed that GH receptor mRNA was more strongly expressed by LECs than by BVECs (5.72-fold ± 0.99; n = 3). The difference in GHR gene expression levels was confirmed by real-time RT-PCR in two matched pairs of LECs and BVECs that were obtained from the same donor each, with an up to 30-fold increase of GHR mRNA levels in LECs (Figure 1A). Immunoprecipitation and Western blot analyses of cell lysates confirmed that these differences in GHR mRNA expression also correlated with the protein levels (Figure 1B).
Figure 1.
GHR is expressed at higher levels in human LECs as compared to BVECs in vitro. A: Real-time RT-PCR of two matched pairs of LECs (L) and BVECs (B) that were obtained from the same donor each, revealed an up to 30-fold increase in GHR mRNA levels in LECs. Bars represent mean + SD. B: Immunoprecipitation and Western blot analyses of cell lysates confirmed that these differences in GHR mRNA expression also correlated with the protein levels.
Lymphatic Vessels in Normal Skin Express GHR
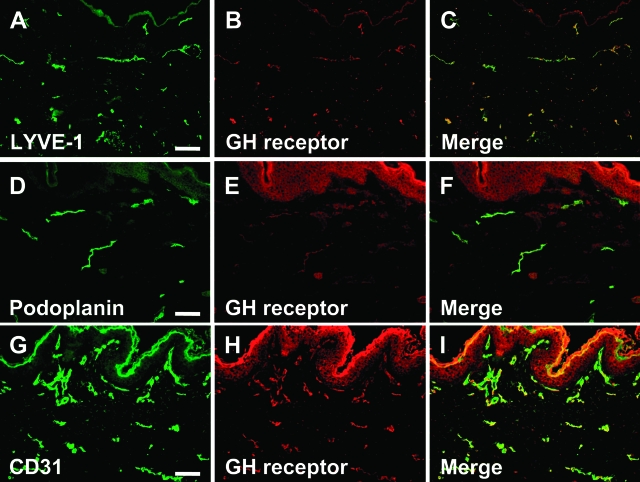
To investigate whether GHR is also expressed by lymphatic vessels in situ, we performed double-immunofluorescence analyses of normal human foreskin for GHR and for the lymphatic markers LYVE-1 or podoplanin and the panvascular marker CD31. LYVE-1-positive and podoplanin-positive lymphatic vessels also expressed GHR (Figure 2, A–F). The majority of CD31-positive vessels were labeled for GHR (Figure 2, G–I). In agreement with previous results, GHR expression was detected on epidermal keratinocytes and dermal fibroblasts.43 Double-immunofluorescence staining of murine skin revealed expression of GHR by LYVE-1-positive lymphatic vessels (data not shown).
Figure 2.
GHR is expressed by lymphatic vessels in normal human skin. A–F: Double-immunofluorescence analyses of normal human foreskin revealed a co-expression of the lymphatic-specific markers LYVE-1 (A, green) and podoplanin (D, green) with GH receptor (B and E, red; C and F, merged pictures). G–I: All CD31-positive vessels (G, green) were labeled for GH receptor (H, red; I, merged picture), confirming GH receptor expression by lymphatic as well as blood vessels in vivo. Scale bars = 100 μm.
GH Induces LEC Proliferation Independently of VEGFR-2 or VEGFR-3 Activation in Vitro
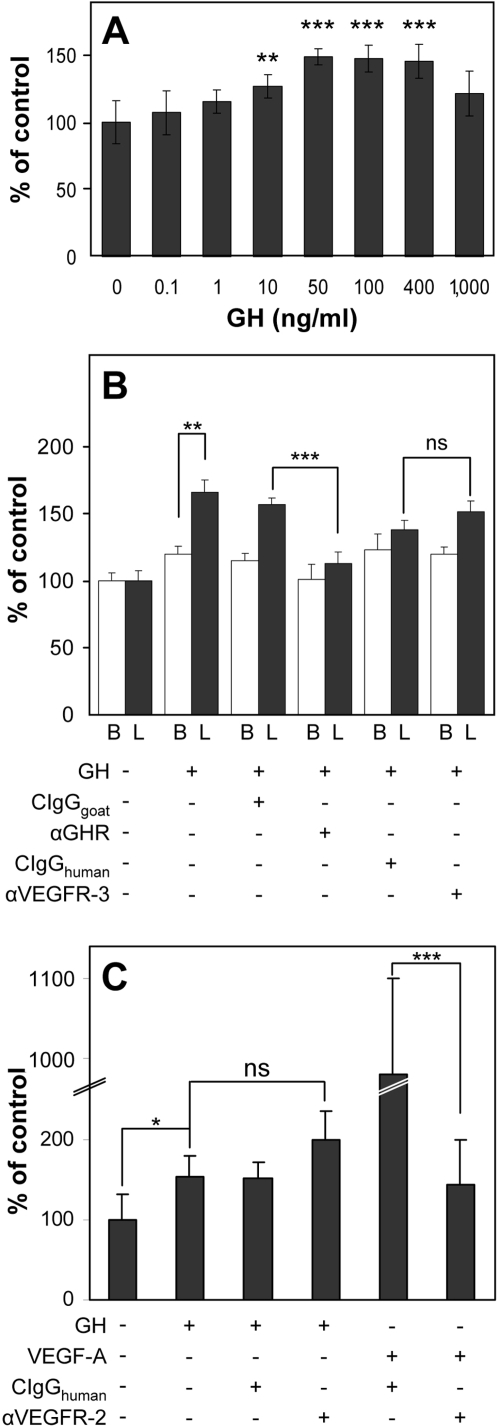
We next investigated whether GH might promote proliferation of LECs and whether the different receptor expression levels in LECs and BVECs in vitro might also lead to different response rates. GH enhanced LEC proliferation with a minimal effective concentration of 10 ng/ml (P = 0.0073, Figure 3A). At this concentration no effect on BVECs could be observed (data not shown). Treatment with 100 ng/ml of GH led to a more than 1.65-fold induction of proliferation in LECs (P = 0.0002, Figure 3B) but only to a 1.2-fold induction in BVECs (P = 0.0048, Figure 3B) as compared with untreated controls. Thus, LECs showed a significantly increased sensitivity toward induction of proliferation by GH, as compared with BVECs. Addition of an anti-GHR antibody completely abolished the GH induced proliferation in both cell types, whereas addition of an isotype control antibody had no effect (Figure 3B). To investigate whether GH induces endothelial cell proliferation directly or indirectly via activation of VEGFRs, as has been described for FGF-2,44,45 cells were treated with GH in the absence or presence of blocking anti-VEGFR-3 or anti-VEGFR-2 antibodies. Blockade of VEGFRs had no inhibitory effect on GH-induced endothelial cell proliferation (Figure 3, B and C), indicating that the mitogenic effects are independent of VEGFR signaling. Blockade of VEGFR-2 inhibited VEGF-A-induced LEC proliferation, confirming the blocking activity of the antibody (Figure 3C). Qualitative real-time RT-PCR analyses of LECs after GH treatment for 30 minutes, 4 hours, or 24 hours revealed similar levels of VEGFR-2 mRNA expression as compared with control LECs, whereas VEGF-A mRNA levels were below the detection limit in all samples (data not shown), further confirming that the GH effects were independent of VEGFR signaling.
Figure 3.
GH induces LEC proliferation independent of VEGFR-2 or VEGFR-3 activation in vitro. A: Dose-dependent effect of GH on LEC proliferation, as compared to untreated cells, with a minimal effective concentration of 10 ng/ml (P = 0.0073). B: Treatment with 100 ng/ml of GH led to a more than 1.65-fold induction of proliferation in LECs (L, black bars) but only to a 1.2-fold induction in BVECs (B, white bars), as compared with untreated controls. Addition of an anti-GH receptor (aGHR) antibody completely abolished the GH-induced proliferation in both cell types whereas addition of an anti-VEGFR-3 antibody (aVEGFR3) or isotype control antibodies (CIgGgoat and CIgGhuman) had no inhibitory effect. C: Addition of an anti-VEGFR-2 antibody (aVEGFR2) or isotype control antibody (CIgGhuman) did not inhibit the stimulation of LEC proliferation by GH (100 ng/ml). Addition of the anti-VEGFR-2 antibody completely blocked VEGF-A-induced proliferation (P < 0.001), confirming its blocking activity. Bars represent mean + SD. ns, Not significant. *P < 0.05, **P < 0.01, ***P < 0.001.
GH Induces Tube Formation, Sprouting, and Migration of LECs in Vitro
We investigated whether GH might also exert stimulatory effects on other processes implicated in lymphangiogenesis, namely tube formation, sprouting, and migration. For tube formation assays, confluent LEC monolayers were overlaid with a collagen type I gel containing or not 100 ng/ml of GH. GH treatment significantly induced formation of tube-like structures as compared with untreated controls (P < 0.001; Figure 4, A and B). Preincubation of the cells with a blocking antibody against GHR, but not with an isotype control antibody, inhibited the GH effects. Blockade of the GHR in the absence of GH stimulation did not reduce baseline LEC tube formation (Figure 4B).
Figure 4.
Treatment of LECs with GH induces tube formation, sprouting, and migration, and inhibits apoptosis in vitro. A: Confluent LEC monolayers were overlaid with a collagen type I gel containing or not 100 ng/ml of GH. GH treatment significantly induced formation of tube-like structures as compared with untreated controls (P < 0.001). A and B: Preincubation of the cells with a blocking antibody against GHR (aGHR), but not with an isotype control antibody (CIgG), inhibited the GH effects. C: Addition of GH promoted chemotactic LEC migration with a minimal effective concentration of 100 ng/ml (P = 0.0091). D: Preincubation with an anti-GHR antibody, but not with a blocking anti-integrin a9b1 antibody, significantly inhibited the migratory response toward GH, as compared to isotype controls (P = 0.0017). Bars represent mean + SD. ns, Not significant. **P < 0.01, ***P < 0.001. E: Spheroids produced by LECs, cultured in collagen type I gels, formed significantly more sprouts on incubation with 100 ng/ml of GH as compared to controls, but less than the treatment with 20 ng/ml of VEGF-A. F: Treatment of LEC monolayer cultures with 100 ng/ml of GH reduced serum starvation-induced apoptosis (gray line) as compared to vehicle-treated cells (black line). Hydrogen peroxide treatment was used as a positive control (dotted line). Scale bars = 100 μm (B).
The chemoattractive activity of GH in LECs was tested using a transwell migration assay. Addition of GH promoted chemotactic LEC migration with a minimal effective concentration of 100 ng/ml (P = 0.0091, Figure 4C). Preincubation with an anti-GHR antibody significantly inhibited the migratory response toward GH, as compared to isotype controls (P = 0.0017, Figure 4D). Integrin α9β1 has been recently described to partially mediate the effects of HGF on LEC migration.18 Therefore, we next investigated whether the integrin α9β1 might also play a role in mediating GH’s effects on LEC migration. However, incubation with a blocking anti-integrin α9β1 antibody did not reduce the GH-induced stimulation of LEC chemotactic migration (Figure 4D).
To test the ability of GH to induce sprouting, we embedded spheroids produced by LECs in collagen type I gels containing 100 ng/ml of GH or vehicle alone. Spheroids formed more sprouts on incubation with GH as compared to the controls (Figure 4E). When compared to VEGF-A-treated spheroids, GH stimulation was less potent and led to shorter sprouts. Treatment of LEC monolayer cultures with GH also reduced serum starvation-induced apoptosis (Figure 4F).
Accelerated Lymphatic Vessel Ingrowth into the Wound Granulation Tissue of GH Transgenic Mice
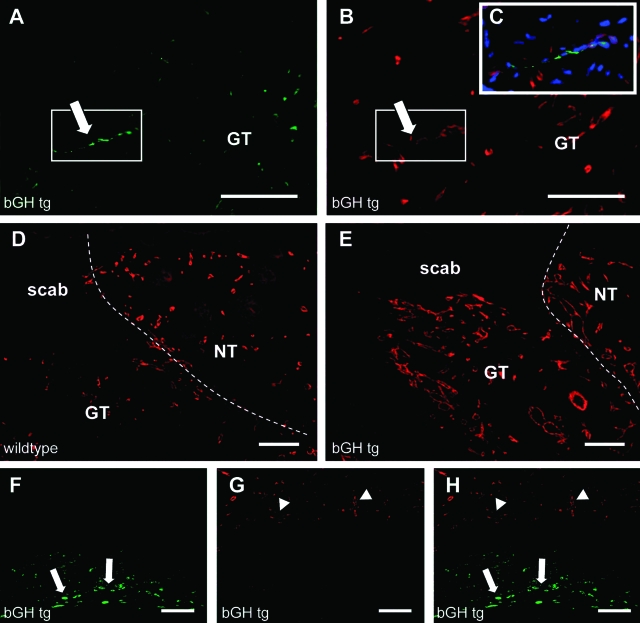
To investigate whether GH might also induce lymphangiogenesis in vivo, we analyzed transgenic mice that express bovine GH under the rat phosphoenolpyruvate carboxykinase-promoter (bGH tg mice).36 Double-immunofluorescence analyses of normal skin for the lymphatic vessel marker LYVE-1 and the panvascular marker CD31 revealed a slightly increased average size of lymphatic vessels in both female bGH tg (1937.939 μm2 ± 724.94 versus wild-type: 1539.45 μm2 ± 703.58; P = 0.24) and male bGH tg (2455.567 μm2 ± 765.58 versus wild-type: 2222.228 μm2 ± 433.014; P = 0.53) mice. It is of interest that the average size of lymphatic vessels was higher in male than in female wild-type mice. To study whether transgenic expression of bGH might enhance lymphatic vessel growth under conditions of lymphangiogenesis, we quantified lymphangiogenesis and angiogenesis during wound healing in bGH tg and control mice. Differential immunostaining of skin samples, obtained at day 5 after induction of full-thickness wounds,36 for LYVE-1 and CD31 revealed accelerated ingrowth of LYVE-1-positive/CD31-positive lymphatic vessels into the wound granulation tissue in bGH tg mice (three of six mice), as compared to wild-type controls (zero of five) (Figure 5, A–C). In both genotypes, massive accumulations of LYVE-1-positive macrophages were observed at the lower border of the granulation tissue. In contrast to lymphatic vessels, these macrophages did not express CD31 (Figure 5, F–H). There were no obvious differences in macrophage numbers between bGH tg and wild-type mice (data not shown). In agreement with previous results,36 we also found an increased density of blood vessels within and in close proximity to the granulation tissue of bGH tg mice (Figure 5, D and E).
Figure 5.
Accelerated lymphatic vessel ingrowth into the granulation tissue of bGH transgenic mice. A and B: Differential immunostains of skin samples for LYVE-1 (green) and CD31 (red) revealed accelerated ingrowth of LYVE-1-positive/CD31-positive lymphatic vessels into the wound granulation tissue in bGH tg mice (arrows). C: Merged picture of a LYVE-1/CD31-positive lymphatic vessel in the wound granulation tissue (blue, nuclei). D and E: Increased density of CD31-positive vessels within and in close proximity to the granulation tissue of bGH tg mice (E) compared to wild-type littermates (D: GT, granulation tissue; NT, normal tissue). F–H: Massive accumulation of LYVE-1-positive macrophages (arrows) were observed at the lower border of the granulation tissue in both genotypes. In contrast to lymphatic vessels, these macrophages did not express CD31. CD31-positive vessels are indicated (arrowhead). Scale bars = 100 μm.
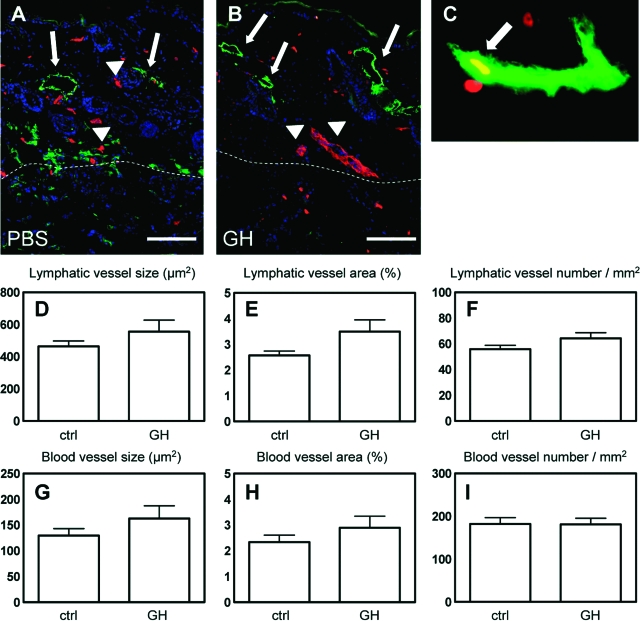
Intradermal Implantation of GH-Containing Matrigels Promotes Lymphatic Vessel Enlargement in Mice
We investigated whether intradermal delivery of GH to normal skin might promote lymphatic vessel activation. To this end, we implanted Matrigels containing 1 μg/ml of GH or an equal volume of PBS into the back skin of female FVB mice. After 7 days, differential immunostaining for LYVE-1 and CD31 revealed enlargement of lymphatic vessels in the skin surrounding GH-containing Matrigels but not in the skin surrounding control Matrigel implants (Figure 6, A–C). Double immunostaining for LYVE-1 and BrdU revealed the presence of several proliferating LECs in lymphatic vessels surrounding GH-containing implants (Figure 6C). This was only rarely seen in control implanted mice. We also found several enlarged LYVE-1-negative, CD31-positive blood vessels surrounding GH-containing Matrigel implants (Figure 6B). Quantitative image analyses of LYVE-1/CD31-stained sections showed a slight increase in the size of lymphatic (555.62 μm2 ± 188.21 versus 463 μm2 ± 82.94; P = 0.29) and blood vessels (162.87 μm2 ± 64.92 versus 129.76 μm2 ± 32.96; P = 0.28) surrounding GH-containing Matrigels (Figure 6, D and G). In contrast, the number of lymphatic and blood vessels was unchanged (Figure 6, F and I).
Figure 6.
Intradermal implantation of GH-containing Matrigels promotes lymphatic vessel enlargement in mice. A and B: Differential immunostains for LYVE-1 (green) and CD31 (red) revealed enlargement of lymphatic vessels (arrows) and blood vessels (arrowheads) in the skin surrounding GH-containing Matrigels and to a lesser extent in the skin surrounding control Matrigel implants. C: Double immunostains for LYVE-1 (green) and BrdU (red) revealed the presence of proliferating LECs in lymphatic vessels surrounding GH-containing implants (arrow). D–I: Quantitative image analyses of LYVE-1/CD31-stained sections showed an increase in the size (average size of individual vessels in μm2) and the average vascular area (percent of tissue area covered by vessels) of lymphatic (D and E) and blood vessels (G and H) surrounding GH-containing Matrigels, whereas, the number of lymphatic (F) and blood vessels (I) was unchanged.
Discussion
To date, several mediators of lymphangiogenesis have been identified. Among these, VEGF-C represents the most important lymphangiogenic factor in many human cancers.46 Moreover, there is increasing evidence that lymphatic vessel activation plays an important role in mediating acute and chronic inflammation.16,47 Therefore, identification of the molecular mediators of lymphatic vessel growth will likely provide novel insights into the pathomechanisms of disease. In a search for novel pathways involved in lymphangiogenesis, we have used transcriptional profiling of cultured human dermal BVECs and LECs to identify enhanced expression of GHR in lymphatic endothelium in vitro. These results were confirmed by quantitative real-time RT-PCR and by Western blot analyses. We also found that GH promotes LEC proliferation, migration, sprouting, and tube formation, and inhibits starvation-induced apoptosis. Moreover, our studies in transgenic mice and intracutaneous application of GH indicate that GH also activates lymphatic vessels in vivo.
GHR was one of the most potently up-regulated genes in LECs as compared to BVECs, indicating that strong GHR expression is characteristic for the lymphatic phenotype in vitro. It is of interest that GHR mRNA levels were also increased after infection of BVECs with the Kaposi’s sarcoma-associated herpesvirus (KSHV), which leads to a lymphatic reprogramming of blood vascular endothelium.42,48 The KSHV-induced lymphatic reprogramming is partially mediated by up-regulation of the transcription factor Prox1, and we have previously shown that siRNA-mediated Prox1 knockdown diminished the KSHV-mediated induction of GHR.42 Furthermore, our recent studies indicate that knockdown of Prox1 in primary human LECs results in inhibition of GHR expression (data not shown), indicating that GHR is indeed a Prox1 target gene.
In accordance with the enhanced GHR expression by LECs, its ligand GH more potently induced the proliferation of LECs as compared with BVECs in vitro. Previous studies have shown that GH stimulates the in vitro growth of human retinal microvascular endothelial cells32 and of bovine brain capillary endothelial cells33 but not of human umbilical cord vein endothelial cells.32 However, its comparative effects on LECs have not been previously characterized. Importantly, GH did not only stimulate LEC proliferation, but also promoted a number of other cellular functions involved in lymphangiogenesis, including tube formation and chemotactic migration. LEC treatment with GH inhibited LEC apoptosis, in accordance with previous results in other cell types including mammary carcinoma cells49 and in T cells.50
Previous studies have indicated that several lymphangiogenic factors might mediate their effects on LECs indirectly via paracrine or autocrine effects leading to activation of VEGFR-2 or VEGFR-3. In particular, the lymphangiogenic activity of basic fibroblast growth factor was inhibited by blockade of VEGFR-3 signaling.44 Importantly, we found that inhibition of VEGFR-2 or VEGFR-3 signaling by anti-VEGFR-2 or anti-VEGFR-3 blocking antibodies did not reduce the mitogenic activity of GH on LECs. These results indicate that GH, similar to hepatocyte growth factor18 and IGF-1,20 activates lymphatic endothelium independently of the VEGFR-signaling pathway in vitro.
Previously, IGF-I has been shown to mediate several of GH’s downstream effects in a number of cell types.31 However, after treatment of LECs with GH for up to 24 hours, we did not detect elevated IGF-1 mRNA expression levels, and IGF-1 protein was not detectable in LEC supernatants with and without GH treatment (data not shown), as studied by immunoprecipitation/Western blot analyses and enzyme-linked immunosorbent assays. Thus, autocrine IGF-1-mediated signaling does not appear to significantly contribute to the effects of GH on LECs in vitro. Similarly, previous studies in rat pancreatic β cells found that GH treatment did not result in up-regulation of IGF-1 expression, indicating that there are cell type-specific differences in the GH response.51
The integrin α9β1 is specifically expressed by LECs but not by BVECs52 in vitro and by lymphatic vessels in vivo.53 Integrin α9β1 acts as a receptor for VCAM-1, cytotactin, and osteopontin, and it plays an important role in lymphatic vessel formation. Integrin α9β1-deficient mice are characterized by chylothorax formation, a sign of impaired lymphatic fluid transport54, mediated by abnormal development of larger lymphatic vessels. Previous studies have revealed that the α9β1 integrin is important for VEGF-C and hepatocyte growth factor-mediated LEC migration.18,52 Our results indicate that GH-stimulated chemotactic LEC migration, in contrast, is independent of the α9β1 integrin.
The expression of GHR by LECs was confirmed in normal tissues in situ. We found that GHR was expressed by human dermal lymphatic vessels and also by other cell types, including fibroblasts and epidermal keratinocytes, in agreement with previous results.43 Whereas GHR was expressed at much higher mRNA and protein levels in cultured LECs than in BVECs, blood vessels were also found to express GHR in situ, as evaluated in immunofluorescence stains using a CD31 antibody. Although immunofluorescence stains are not suitable for an exact quantification of protein expression levels, these results indicate that factors in the tissue microenvironment play important roles in regulating endothelial cell expression of GHR. In agreement with the detected GHR expression on lymphatic vessels, chronic intradermal delivery of GH to the skin of mice induced lymphatic vessel enlargement surrounding the Matrigel implants, associated with increased LEC proliferation. GH also accelerated lymphatic vessel ingrowth into the granulation tissue of full-thickness wounds in bGH tg mice. In contrast, in VEGF-A transgenic mice—which show enhanced lymphangiogenesis—the first lymphatic vessels in the wound granulation tissue appeared at day 7 after wounding, whereas in wild-type mice, lymphatics are usually first seen in the granulation tissue after 14 days.15 Chronic transgenic delivery of bGH in transgenic mice did not lead to major alterations of lymphatic vessels in normal, unwounded skin. Together, these results indicate that the GH effects are dependent on the activation status of lymphatic vessels because they are seen in healing wounds and in the inflammatory tissue response to Matrigel implants. Activation in these conditions is likely mediated, at least in part, by accumulation of activated macrophages that are known to be potent sources of other lymphangiogenic factors such as VEGF-C and VEGF-A,55 which are also known to be elevated during wound healing.56,57 Similarly, it has been previously found that GH’s effects on blood vascular angiogenesis only occur at sites of blood vessel activation in mice.36 In accordance with these data, we found that intradermal GH delivery resulted in enlargement of cutaneous blood vessels, and that bGH tg mice showed enhanced blood vascular density in the wound granulation tissue. Thus, GH is both a lymphangiogenic and an angiogenic factor, similar to some other recently identified novel lymphangiogenic factors including hepatocyte growth factor,18 VEGF-A,16 IGF-I,20 and platelet-derived growth factor-BB.21 Further studies are needed to evaluate the relative contributions of each of these factors, as well as their possible synergistic activities, in different pathological conditions associated with lymphangiogenesis. In addition, tissue-specific and disease-specific characteristics of stromal cells might further modulate the lymphangiogenic response to these factors.
The clinical relevance of GH’s effects on lymphatic vessels remains to be established. Lymphatic vessel hyperplasia and activation has been reported in several chronic inflammatory diseases including psoriasis.16 Elevated circulating GH levels in psoriatic patients have been reported,58,59 and GH treatment correlated with relapses of psoriatic lesions.60 Somatostatin, an inhibitor of GH production by the pituitary gland, has been previously used to treat human psoriasis of the skin and psoriatic arthritis with some encouraging results.61 However, there are at present no published placebo-controlled, larger clinical studies using somatostatin or the more specific GH antagonist pegvisomant. GH expression has also been found in metastatic pancreatic cancer27 and in breast cancer,26 and up-regulated GHR expression levels have been implicated in colorectal cancer,62 in prostate cancer progression,63 and in breast cancer development.64 In mouse tumor xenotransplant models, GH receptor antagonists (pegvisomant, B2036-PEG) inhibited the growth of experimental breast cancer, colorectal cancer, and meningiomas.65,66,67 However, tumor (lymph)angiogenesis was not evaluated in these studies. Because tumor-induced lymphangiogenesis has been correlated with cancer metastases in several types of human tumors,68,69 it will be of interest to investigate whether GH expression in human cancers might also be associated with induction of lymphatic vessel growth and/or with enhanced frequency of metastases.
Footnotes
Address reprint requests to Michael Detmar, M.D., Institute of Pharmaceutical Sciences, Swiss Federal Institute of Technology, ETH Zurich, Wolfgang-Pauli-Str. 10, HCI H303, CH-8093 Zurich, Switzerland. E-mail: michael.detmar@pharma.ethz.ch.
Supported by the National Institutes of Health (grant CA69184), the Swiss National Fund (grant 3100A0-108207), the Austrian Science Foundation (grant S9408-B11), the Cancer League Zurich, the Commission of the European Communities (grant LSHC-CT-2005-518178 to M.D.), and the Roche Research Foundation (to N.E.T.).
References
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- Cueni LN, Detmar M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol. 2006;126:2167–2177. doi: 10.1038/sj.jid.5700464. [DOI] [PubMed] [Google Scholar]
- Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa S, Mihm MC, Detmar M. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol. 2005;18:1232–1242. doi: 10.1038/modpathol.3800410. [DOI] [PubMed] [Google Scholar]
- Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, Cox G, Harris AL, Jackson DG. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62:1315–1320. [PubMed] [Google Scholar]
- Mattila MM, Ruohola JK, Karpanen T, Jackson DG, Alitalo K, Harkonen PL. VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int J Cancer. 2002;98:946–951. doi: 10.1002/ijc.10283. [DOI] [PubMed] [Google Scholar]
- Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa S, Brown LF, Kodama S, Paavonen K, Alitalo K, Detmar M. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood. 2007;109:1010–1017. doi: 10.1182/blood-2006-05-021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eynden GG, Vandenberghe MK, van Dam PJ, Colpaert CG, van Dam P, Dirix LY, Vermeulen PB, Van Marck EA. Increased sentinel lymph node lymphangiogenesis is associated with nonsentinel axillary lymph node involvement in breast cancer patients with a positive sentinel node. Clin Cancer Res. 2007;13:5391–5397. doi: 10.1158/1078-0432.CCR-07-1230. [DOI] [PubMed] [Google Scholar]
- Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol. 2007;170:774–786. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev. 2002;82:673–700. doi: 10.1152/physrev.00005.2002. [DOI] [PubMed] [Google Scholar]
- Partanen TA, Paavonen K. Lymphatic versus blood vascular endothelial growth factors and receptors in humans. Microsc Res Tech. 2001;55:108–121. doi: 10.1002/jemt.1162. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Williams RA, Achen MG. Lymphangiogenic growth factors as markers of tumor metastasis. APMIS. 2004;112:539–549. doi: 10.1111/j.1600-0463.2004.apm11207-0812.x. [DOI] [PubMed] [Google Scholar]
- Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, Bohlen P, Senger DR, Detmar M. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 2004;18:1111–1113. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, Lin C, Fiebiger E, Wei X, Wu Y, Hicklin D, Bohlen P, Detmar M. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–1057. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, Lawitts JA, Benjamin L, Tan X, Manseau EJ, Dvorak AM, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002;196:1497–1506. doi: 10.1084/jem.20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiya K, Hirakawa S, Ma B, Drinnenberg I, Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisada T, Oike Y, Yamada Y, Urano T, Akao M, Kubota Y, Maekawa H, Kimura Y, Ohmura M, Miyamoto T, Nozawa S, Koh GY, Alitalo K, Suda T. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- Bjorndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ, Cao Y. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:15593–15598. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Bjorndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, Ohhashi T, Jackson DG, Cao Y. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Edmondson SR, Thumiger SP, Werther GA, Wraight CJ. Epidermal homeostasis: the role of the growth hormone and insulin-like growth factor systems. Endocr Rev. 2003;24:737–764. doi: 10.1210/er.2002-0021. [DOI] [PubMed] [Google Scholar]
- Ridderstråle M. Signaling mechanism for the insulin-like effects of growth hormone—another example of a classical hormonal negative feedback loop. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:79–92. doi: 10.2174/1568008053174787. [DOI] [PubMed] [Google Scholar]
- Beyea JA, Olson DM, Harvey S. Growth hormone expression in the perinatal and postnatal rat lung. Dev Dyn. 2005;232:1037–1046. doi: 10.1002/dvdy.20255. [DOI] [PubMed] [Google Scholar]
- Wu H, Devi R, Malarkey WB. Localization of growth hormone messenger ribonucleic acid in the human immune system—a Clinical Research Center study. J Clin Endocrinol Metab. 1996;81:1278–1282. doi: 10.1210/jcem.81.3.8772612. [DOI] [PubMed] [Google Scholar]
- Stoll BA. Breast cancer: further metabolic-endocrine risk markers? Br J Cancer. 1997;76:1652–1654. doi: 10.1038/bjc.1997.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzat S, Ezrin C, Yamashita S, Melmed S. Recurrent acromegaly resulting from ectopic growth hormone gene expression by a metastatic pancreatic tumor. Cancer. 1993;71:66–70. doi: 10.1002/1097-0142(19930101)71:1<66::aid-cncr2820710112>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Palmetshofer A, Zechner D, Luger TA, Barta A. Splicing variants of the human growth hormone mRNA: detection in pituitary, mononuclear cells and dermal fibroblasts. Mol Cell Endocrinol. 1995;113:225–234. doi: 10.1016/0303-7207(95)03633-i. [DOI] [PubMed] [Google Scholar]
- Hattori N, Shimatsu A, Sugita M, Kumagai S, Imura H. Immunoreactive growth hormone (GH) secretion by human lymphocytes: augmented release by exogenous GH. Biochem Biophys Res Commun. 1990;168:396–401. doi: 10.1016/0006-291x(90)92334-v. [DOI] [PubMed] [Google Scholar]
- Kooijman R, Malur A, Van Buul-Offers SC, Hooghe-Peters EL. Growth hormone expression in murine bone marrow cells is independent of the pituitary transcription factor Pit-1. Endocrinology. 1997;138:3949–3955. doi: 10.1210/endo.138.9.5414. [DOI] [PubMed] [Google Scholar]
- Kopchick JJ, Parkinson C, Stevens EC, Trainer PJ. Growth hormone receptor antagonists: discovery, development, and use in patients with acromegaly. Endocr Rev. 2002;23:623–646. doi: 10.1210/er.2001-0022. [DOI] [PubMed] [Google Scholar]
- Rymaszewski Z, Cohen RM, Chomczynski P. Human growth hormone stimulates proliferation of human retinal microvascular endothelial cells in vitro. Proc Natl Acad Sci USA. 1991;88:617–621. doi: 10.1073/pnas.88.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struman I, Bentzien F, Lee H, Mainfroid V, D'Angelo G, Goffin V, Weiner RI, Martial JA. Opposing actions of intact and N-terminal fragments of the human prolactin/growth hormone family members on angiogenesis: an efficient mechanism for the regulation of angiogenesis. Proc Natl Acad Sci USA. 1999;96:1246–1251. doi: 10.1073/pnas.96.4.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerich B, Kurtz-Hoffmann J, Lindemann N. Influence of growth hormone on maintenance of capillary-like structures in an in vitro model of stromal vascular tissue—results from morphometric analysis. Artif Organs. 2005;29:338–341. doi: 10.1111/j.1525-1594.2005.29057.x. [DOI] [PubMed] [Google Scholar]
- Gould J, Aramburo C, Capdevielle M, Scanes CG. Angiogenic activity of anterior pituitary tissue and growth hormone on the chick embryo chorio-allantoic membrane: a novel action of GH. Life Sci. 1995;56:587–594. doi: 10.1016/0024-3205(94)00491-a. [DOI] [PubMed] [Google Scholar]
- Thorey IS, Hinz B, Hoeflich A, Kaesler S, Bugnon P, Elmlinger M, Wanke R, Wolf E, Werner S. Transgenic mice reveal novel activities of growth hormone in wound repair, angiogenesis, and myofibroblast differentiation. J Biol Chem. 2004;279:26674–26684. doi: 10.1074/jbc.M311467200. [DOI] [PubMed] [Google Scholar]
- Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T, Detmar M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162:575–586. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16:773–783. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- Sauter B, Foedinger D, Sterniczky B, Wolff K, Rappersberger K. Immunoelectron microscopic characterization of human dermal lymphatic microvascular endothelial cells. Differential expression of CD31, CD34, and type IV collagen with lymphatic endothelial cells vs blood capillary endothelial cells in normal human skin, lymphangioma, and hemangioma in situ. J Histochem Cytochem. 1998;46:165–176. doi: 10.1177/002215549804600205. [DOI] [PubMed] [Google Scholar]
- Detmar M, Tenorio S, Hettmannsperger U, Ruszczak Z, Orfanos CE. Cytokine regulation of proliferation and ICAM-1 expression of human dermal microvascular endothelial cells in vitro. J Invest Dermatol. 1992;98:147–153. doi: 10.1111/1523-1747.ep12555746. [DOI] [PubMed] [Google Scholar]
- Korff T, Kimmina S, Martiny-Baron G, Augustin HG. Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. 2001;15:447–457. doi: 10.1096/fj.00-0139com. [DOI] [PubMed] [Google Scholar]
- Hong YK, Foreman K, Shin JW, Hirakawa S, Curry CL, Sage DR, Libermann T, Dezube BJ, Fingeroth JD, Detmar M. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat Genet. 2004;36:683–685. doi: 10.1038/ng1383. [DOI] [PubMed] [Google Scholar]
- Ginarte M, Garcia-Caballero T, Fernandez-Redondo V, Beiras A, Toribio J. Expression of growth hormone receptor in benign and malignant cutaneous proliferative entities. J Cutan Pathol. 2000;27:276–282. doi: 10.1034/j.1600-0560.2000.027006276.x. [DOI] [PubMed] [Google Scholar]
- Kubo H, Cao R, Brakenhielm E, Makinen T, Cao Y, Alitalo K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci USA. 2002;99:8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LK, Garcia-Cardena G, Farnebo F, Fannon M, Chen EJ, Butterfield C, Moses MA, Mulligan RC, Folkman J, Kaipainen A. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci USA. 2004;101:11658–11663. doi: 10.1073/pnas.0404272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS, Tille JC, Nisato R, Skobe M. Lymphangiogenesis and tumor metastasis. Cell Tissue Res. 2003;314:167–177. doi: 10.1007/s00441-003-0748-7. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D, Huttary N, Raab I, Regele H, Bojarski-Nagy K, Bartel G, Krober SM, Greinix H, Rosenmaier A, Karlhofer F, Wick N, Mazal PR. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12:230–234. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- Wang HW, Trotter MW, Lagos D, Bourboulia D, Henderson S, Makinen T, Elliman S, Flanagan AM, Alitalo K, Boshoff C. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat Genet. 2004;36:687–693. doi: 10.1038/ng1384. [DOI] [PubMed] [Google Scholar]
- Graichen R, Liu D, Sun Y, Lee KO, Lobie PE. Autocrine human growth hormone inhibits placental transforming growth factor-beta gene transcription to prevent apoptosis and allow cell cycle progression of human mammary carcinoma cells. J Biol Chem. 2002;277:26662–26672. doi: 10.1074/jbc.M109931200. [DOI] [PubMed] [Google Scholar]
- Dobashi H, Sato M, Tanaka T, Tokuda M, Ishida T. Growth hormone restores glucocorticoid-induced T cell suppression. FASEB J. 2001;15:1861–1863. doi: 10.1096/fj.00-0702fje. [DOI] [PubMed] [Google Scholar]
- De W, Breant B, Czernichow P, Asfari M. Growth hormone (GH) and prolactin (PRL) regulate IGFBP-3 gene expression in rat beta-cells. Mol Cell Endocrinol. 1995;114:43–50. doi: 10.1016/0303-7207(95)03640-s. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Alitalo K. Lymphatic endothelial regulation, lymphoedema, and lymph node metastasis. Semin Cell Dev Biol. 2002;13:9–18. doi: 10.1006/scdb.2001.0286. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr, Sheppard D. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van de Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D'Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170:1178–1191. doi: 10.2353/ajpath.2007.060018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G, Neidhardt M, Schmidt A, Geiger A. Correlation of growth hormone and aetiology of psoriasis. Arch Dermatol Res. 1981;270:129–140. doi: 10.1007/BF00408223. [DOI] [PubMed] [Google Scholar]
- Weber G, Pliess G, Heitz U. Growth hormone producing hyperplasia of pituitary gland in psoriasis. Arch Dermatol Res. 1985;277:345. doi: 10.1007/BF00509098. [DOI] [PubMed] [Google Scholar]
- Maghnie M, Borroni G, Larizza D, Lorini R, Girani MA, Rabbiosi G, Severi F. Relapsing eruptive psoriasis and immunological changes triggered by growth hormone therapy in a growth hormone-deficient girl. Dermatologica. 1990;181:139–141. doi: 10.1159/000247903. [DOI] [PubMed] [Google Scholar]
- Weber G, Klughardt G, Neidhardt M, Galle K, Frey H, Geiger A. Treatment of psoriasis with somatostatin. Arch Dermatol Res. 1982;272:31–36. doi: 10.1007/BF00510390. [DOI] [PubMed] [Google Scholar]
- Yang X, Liu F, Xu Z, Chen C, Li G, Wu X, Li J. Growth hormone receptor expression in human colorectal cancer. Dig Dis Sci. 2004;49:1493–1498. doi: 10.1023/b:ddas.0000042254.35986.57. [DOI] [PubMed] [Google Scholar]
- Weiss-Messer E, Merom O, Adi A, Karry R, Bidosee M, Ber R, Kaploun A, Stein A, Barkey RJ. Growth hormone (GH) receptors in prostate cancer: gene expression in human tissues and cell lines and characterization. GH signaling and androgen receptor regulation in LNCaP cells. Mol Cell Endocrinol. 2004;220:109–123. doi: 10.1016/j.mce.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Gebre-Medhin M, Kindblom LG, Wennbo H, Tornell J, Meis-Kindblom JM. Growth hormone receptor is expressed in human breast cancer. Am J Pathol. 2001;158:1217–1222. doi: 10.1016/S0002-9440(10)64071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagnaes-Hansen F, Duan H, Rasmussen LM, Friend KE, Flyvbjerg A. Growth hormone receptor antagonist administration inhibits growth of human colorectal carcinoma in nude mice. Anticancer Res. 2004;24:3735–3742. [PubMed] [Google Scholar]
- Divisova J, Kuiatse I, Lazard Z, Weiss H, Vreeland F, Hadsell DL, Schiff R, Osborne CK, Lee AV. The growth hormone receptor antagonist pegvisomant blocks both mammary gland development and MCF-7 breast cancer xenograft growth. Breast Cancer Res Treat. 2006;96:315–327. doi: 10.1007/s10549-006-9168-1. [DOI] [PubMed] [Google Scholar]
- McCutcheon IE, Flyvbjerg A, Hill H, Li J, Bennett WF, Scarlett JA, Friend KE. Antitumor activity of the growth hormone receptor antagonist pegvisomant against human meningiomas in nude mice. J Neurosurg. 2001;94:487–492. doi: 10.3171/jns.2001.94.3.0487. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Baldwin ME, Achen MG. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002;16:922–934. doi: 10.1096/fj.01-0945rev. [DOI] [PubMed] [Google Scholar]
- Tobler NE, Detmar M. Tumor and lymph node lymphangiogenesis—impact on cancer metastasis. J Leukoc Biol. 2006;80:691–696. doi: 10.1189/jlb.1105653. [DOI] [PubMed] [Google Scholar]