Abstract
There is accumulating evidence that the nucleus accumbens (NAc) plays an important role in the pathophysiology of depression. Given that clinical depression is marked by anhedonia (diminished interest or pleasure), dysfunction of the brain reward pathway has been suggested as contributing to the pathophysiology of depression.
Since the NAc is the center of reward and learning, it is hypothesized that anhedonia might be produced by hampering the function of the NAc. Indeed, it has been reported that stress, drug exposure and drug withdrawal, all of which produce a depressive-phenotype, alter various functions within the NAc, leading to inhibited dopaminergic activity in the NAc.
In this review, we describe various factors as possible candidates within the NAc for the initiation of depressive symptoms. First, we discuss the roles of several neurotransmitters and neuropeptides in the functioning of the NAc, including dopamine, glutamate, γ-aminobutyric acid (GABA), acetylcholine, serotonin, dynorphin, enkephaline, brain-derived neurotrophic factor (BDNF), cAMP response element-binding protein (CREB), melanin-concentrating hormone (MCH) and cocaine- and amphetamine-regulated transcript (CART). Second, based on previous studies, we propose hypothetical relationships among these substances and the shell and core subregions of the NAc.
Key Words: Depression, nucleus accumbens, glutamate, dopamine, γ-aminobutyric acid (GABA), acetylcholine, dynorphin, cAMP response element-binding protein (CREB)
INTRODUCTION
The nucleus accumbens (NAc), a part of the ventral striatum, is involved in motivation, reward, motor function and learning. Indeed, drugs of abuse increase locomotor activity, and preferentially and markedly increase dopamine release in the NAc. Recent studies have demonstrated that the NAc may play an important role in the etiology and pathophysiology of depression, although direct findings have never been reported. Exposure to chronic stress or withdrawal from long-term ingestion of drugs of abuse causes anhedonia (diminished interest or pleasure), a core symptom of major depressive disorder, in both humans and rodents [5,8,170]. Development of anhedonia has been ascribed to dysfunction of the reward pathway, in which the NAc plays a pivotal role [37,173]. Chronic stress or psychostimulant withdrawal has been reported to induce dramatic neurochemical alterations in the NAc, leading to depressive phenotypes [8,37,106,123].
In this review, we focus on several neurotransmitters and neuromodulators, which appear to play crucial roles in reward and regulation of mood in the NAc and describe alterations of these molecules in depressive states.
CLINICAL STUDIES
The involvement of the NAc in depression has been noted in the clinical studies although a direct indication has never been found in the NAc of depressed patients. Related study is that increased apathy is associated with lower volume of the NAc in patients infected with human immunodeficiency virus [122].An fMRI study using cocaine-dependent subjects indicated that cocaine-induced activation in the NAc is correlated with behavioral measures of euphoria [20]. Neuropsychological study showed that recently depressed cases made faster decisions in the context of betting more of their available points in the decision-making test [85] and an fMRI study using healthy adults demonstrated that ventral striatum is activated in reward processes in the decisionmaking test [46]. Taken together, it is likely that the NAc of depressed patients is impaired in reward and decision- making. Furthermore, a recent study reported that immunolabeling of protein kinase A subunits was significantly decreased in the NAc of young suicide victims [117]. The previous studies indicated the involvement of NAc in cognition and response to aversive and rewarding stimulus in animal models of depression (shown in Table 1).
Table 1.
The Functions of NAc in Animal Models of Depression
| Function |
|---|
| Cognition |
| • Glutamate injected into the NAc decreased swimming in the forced swim test [134]. |
| • Blockade of κ-opioid receptor in the NAc shell or core of learned helplessness rats improved behavioral deficits in the conditioned avoidance test [147]. |
| • Blockade of CREB in the NAc of learned helplessness animals produced an antidepressant-like effect in the conditional avoidance test [111]. |
| Under steady state |
| • Flinders sensitive line rats showed the decreased 5-HT turnover (5-HIAA/5-HT) and the increased DA turnover (DOPAC/DA) in the NAc [178,179]. |
| • Chronic mild stress caused a decrease in dopamine D2 receptors in the NAc [118]. |
| • Chronic mild stress did not affect basal dialysate DA in the NAc [37]. |
| • Learned helplessness rats showed the decreased densities of dopamine D2 receptors in the NAc core [81]. |
| • Learned helplessness rats showed increased expression of dynorphin A and B in the NAc shell and core [147]. |
| • Cocaine withdrawal elevated basal levels of GABA, reduced basal levels of extracellular glutamate, and did not alter basal levels of DA in the NAc [172]. |
| Response to aversive stress |
| • Chronic mild stress reversed the inhibitory response of DA transmission by the tail-pinch trial in the NAc shell [37]. |
| • Forced swim stress failed to increase extracellular levels of DA in the NAc of Flinders sensitive line rats [173]. |
| • In response to forced swim stress, Wistar-Kyoto rats exhibited a reduction in DA turnover (DOPAC/DA) and failed to increase 5-HIAA levels in the NAc [35]. |
| Response to reward |
| • Chronic mild stress blunted the stimulatory response of DA transmission to food in the NAc shell [37]. |
| • The increases in dopamine output observed in chronic stressed animals after cocaine administration were significantly lower than those observed in control rats [53]. |
THE NAC SHELL AND CORE SUBREGIONS
A recent study reported that selective lesions in the NAc core profoundly impaired the acquisition of drug-seeking behavior that was maintained by drug-associated conditioned reinforcers, but had only a minor effect on the acquisition of cocaine response under a schedule of continuous reinforcement, indicating that the NAc core is involved in the control of goal-directed behavior by associative processes, whereas the NAc shell controls the enhancing effects of drugs [70]. Thus, Pavlovian conditioned stimuli influence appetitive behavior such as drug-seeking. This drug-seeking behavior is characteristic of impulsivity, the mechanism of which is thought to be controlled in the NAc centered neuronal networks [72]. Increased impulsivity is often observed in depression. Therefore, it is conceivable that the NAc is involved in problem-solving ability, as increased impulsivity must be assumed to have some effect on decision-making or suicide attempts. Another study demonstrated that habituation of dopamine responses to aversive stimuli was not observed in the NAc core, but was observed in the NAc shell, indicating that the NAc core handles generic motivational value whereas the NAc shell integrates the motivational valence and novelty [11]. In the field of psychiatry, resistant habituation reminds us of various symptoms such as recurrent luminescence, predicted anxiety or impulsive thinking. The recent study demonstrated that NAc core lesions retard instrumental learning with delayed reinforcement and increased sensitivity to differences in reinforcer magnitude, indicating that the NAc core is required for normal preference for a large, delayed reward over a small, immediate reward (self-controlled choice) [24].
CONNECTIVITY
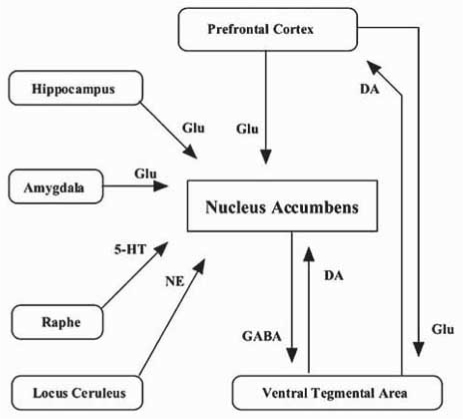
The NAc contains small populations of γ-aminobutylic acid (GABA)-containing and cholinergic interneurons, in addition to a large number of efferent GABAergic medium spiny projecting neurons [102]. The activity of projecting medium spiny neurons is regulated by glutamatergic afferents arising from the prefrontal cortex, hippocampus and amygdala, by dopaminergic afferents from the ventral tegmental area [124], by serotonergic afferents from the raphe nucleus, and by noradrenergic afferents from the locus ceruleus. A schematic diagram is shown in Fig. 1. These afferents converge primarily on GABA neurons, the main output cells of this region [102]. In addition, GABAergic projection neurons receive inhibitory input, primarily from a small population of GABAergic and cholinergic interneurons [75] but also through feedfoward inhibition [125] and axon collaterals of neighboring medium spiny neurons [29].
Fig. (1).
Connectivity of the NAc with other brain regions such as prefrontal cortex, ventral tegmental area (VTA). Activity of the NAc is mainly regulated by dopaminergic (from VTA) and glutamatergic afferents (from frontal cortex, amygdala and hippocampus). In addition, inputs of GABAergic, serotonergic (from raphe) and adrenergic afferents (from locus ceruleus) as well as cholinergic interneuons (within the NAc) also modified activity of the NAc.
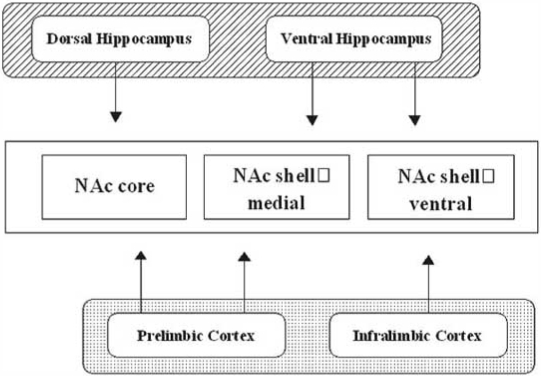
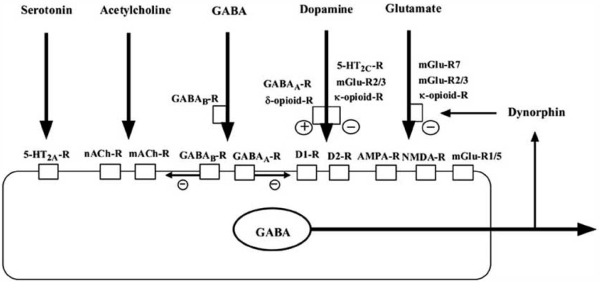
Medium spiny neurons project to a restricted part of the globus pallidus and the substantia nigra from the core, whereas from the shell, they project not only to the subcommissural part of the ventral pallidum and the ventral tegmental area but also to widespread areas in the hypothalamus and extended amygdala [102]. NAc output is regulated by numerous systems including glutamatergic afferent from the medial prefrontal cortex and hippocampus, both of which differentially regulate activities in the shell and core subregions (Fig. 2). Furthermore, various systems such as glutamate, dopamine, GABA, acetylcholine, serotonin and so on regulate each other through pre- and post- receptors (Fig. 3). A schematic diagram about the relationships among typical receptors is shown in Fig. 3. Recently, an emerging body of evidence suggests that the activities of medium spiny projecting neurons, as well as these afferents neurons, are regulated locally by several neuropeptides and transcriptional factors, which are expressed in the NAc, and which are up- or down-regulated by various stressful situations [110].
Fig. (2).
Schematic illustration of connections of the NAc shell and core regions with subregions of hippocampus and prefrontal cortex. Please note that three portions of the NAc could be affected efficiently by two regions of hippocampus and prefrontal cortex.
Fig. (3).
Schema of regulation of GABAergic medium spiny projecting neurons and the relationships among dopamine, glutamate, GABA, acetylcholine, enkephaline, dynorphin and their receptors in the NAc. Activity of GABAergic medium spiny neurons in the NAc is heavily regulated by dopaminergic (from ventral tegmental area) and glutamatergic afferents (from frontal cortex, amygdala and hippocampus) as well as GABAergic and cholinergic interneuons. Dopaminergic and glutamatergic activity in the NAc is also regulated by neuropeptides such as dynorphin and enkephalin. Regulation by other neurotransmitters such as serotonin and neuropeptides such as MCH and CART peptides may be involved.
DOPAMINE
The dopamine system is distributed to restricted brain regions known as the mesocortical, mesolimbic, and nigrostriatal dopamine projections. The mesocortical dopamine system supplies projections to the medial prefrontal and cingulate cortex, whereas the mesolimbic dopamine system innervates the NAc as well as the amygdala and septum. The dopaminergic system has been implicated in depression, and accumbal dopamine has been of particular interest, as dopamine in the NAc has been shown to be associated with motivation, reward, and hedonia [79]. Dopaminergic abnormalities within the limbic areas of the brain were observed in several animal models of depression [42,81,178]. Disruption of dopaminergic function within the NAc causes anhedonia, a cardinal symptom of depression in human as well as in rodents [5,170].
It is well known that withdrawal from long-term use of drugs of abuse such as phencyclidine, amphetamine and nicotine precipitates withdrawal syndromes characterized by affective symptoms including anhedonia [8], and reduces extracellular dopamine concentrations within the NAc [123]. Similarly,subchronic treatment with cocaine induces changes in tyrosine hydroxylase activity in the NAc [13,164]. Moreover, the mesolimbic dopamine system appears to be highly susceptible to stress. Thus, it has been reported that acute exposure to different forms of stress increases dopa-mine release in the NAc [73,163]. In contrast, long-term exposure to various unavoidable stress factors decreases dopamine release in the NAc shell [37,38,143]. Furthermore, it has also been reported that mice exposed to escapable foot shock show an increase in dopamine release in the NAc, while those exposed to inescapable foot shock show a decrease in dopamine release in the NAc [22]. In addition, prolonged exposure to the forced swimming test, an animal model of depression, promotes a reduction of dopamine release in the NAc [138]. Both homovanillic acid (HVA) and 3,4-dihydroxyphenylacetic acid (DOPAC) contents were reported to decrease in the NAc after forced swimming stress, and this reduction lasts for a longer time in animal models of depression, using Flinders Sensitive Line rats [173] and Wistar-Kyoto rats [35]. Furthermore, chronic stress was reported to reduce the cocaine-induced increase in dopamine output in the NAc shell [53], indicating an impairment of responsiveness to both aversive and pleasurable stimulus. In contrast, it has been demonstrated that chronic administration of the antidepressant imipramine increases both basal extracellular dopamine levels and a dopamine agonist amphetamine-induced extracellular dopamine levels intheNAc[69].However, another study reported that chronic administration with antidepressants such as imipramine and fluoxetine prevented an NMDA receptor antagonist phencyclidine-increased dopamine turnover (DOPAC/dopamine ratio) in the NAc [36]. These reports support the proposed relationship between depressive behavior and dopaminergic activity in the NAc.
This hypothesis is supported by reports describing how dopaminergic agents such as dopamine reuptake inhibitors, presumably by increasing synaptic available dopamine concentration, have been successful in treating major depression [54,181]. Consistent with these findings, it has been demonstrated that pramipexole, a D2 dopamine receptor agonist, reverses anhedonia induced by chronic exposure to mild unpredictable stress [171], and a D2/D3 dopamine receptor agonist exhibits antidepressant-like effects in numerous depression models including learned helplessness paradigm and chronic mild stress model [104]. Moreover, it has been reported that stimulation of D2/3 dopamine receptors, but not of the D4 dopamine receptor, exhibits an antidepressant-like effect in the forced swimming test [12]. However, it should be noted that these dopamine agonists do not necessarily act within the NAc, and that other regions such as the prefrontal cortex, amygdala and ventral tegmetal area should be taken into account.
Both the shell and core of NAc receive a dense afferent dopaminergic innervation from the ventral mesencephalon [102]. The psychotomimetic drug phencyclidine, a noncompetitive NMDA receptor antagonist, elevated extracellular dopamine levels in the NAc shell but not in the core [95]. Phencyclidine, when injected directly into the shell, but not into the core, results in reward activity [26]. These results suggest that dopaminergic (and glutamateregic) input to the shell region of NAc may be more involved in the reward effect than similar input to the core region.
Dopaminergic activity in the NAc is regulated by numerous substances including glutamate, serotonin, GABA, acetylcholine, and neuropeptides. Terminal dopamine release in the NAc is under tonic GABAergic inhibition mediated mainly by GABAA (and possibly by GABAB) receptors (see GABA section; Fig. 3). Serotonin regulates dopamine release in this region both positively and negatively (see serotonin section; Fig. 3). Endgenous opioid peptides, dynorphin and enkephalin, negatively and positively regulate dopamine release, respectively (see dynorphin and enkephalin sections; Fig. 3).
It has been reported that dopamine directly modulates a number of postsynaptic conductances in the NAc [113], as did reward-predictive cues [114]. Indeed, inactivating dopamine neurons reduces excitation in the NAc induced by reward-predictive cues [176]. A recent study reported that D1 receptors in the NAc may act as a determinant in reward and participate in reward prediction and spatial learning [165]. It has been reported that dopamine inhibits both glutamate and GABA release in the region [124]. It has been suggested that dopamine preferentially inhibits GABA release in the NAc, producing a net excitatory effect on projecting medium spiny neurons [64], and this may explain how dopamine can excite medium spiny neurons, while dopamine inhibits glutamate release.
Another recent study reported that chronically stressed rats showed a decrease in the number of dopamine transporter (DAT) binding sites, and an increase in the number of D1 binding sites in the NAc shell [143]. In contrast, chronic treatment with antidepressants increased expression of do-pamine receptors, including the D2 and D3 types, in the NAc shell [2,86]. Likewise, electroconvulsive shock treatment increased D3 dopamine receptor mRNA and binding in the NAc shell [86]. In contrast, it has been reported that chronic mild stress decreased the number of D2 dopamine receptors binding in the NAc, which are reversed by chronic treatment with imipramine [118]. Interestingly, reduced D2 dopamine receptor expression due to chronic mild stress was observed in both the NAc shell and core, although antidepressant treatment (imipramine and fluoxetine) countered the decreased D2 dopamine receptor mRNA in the shell, but not in the core [44], suggesting that a stress-induced change in the number of D2 dopamine receptor in the NAc shell is more reversible than similar changes in the NAc core.
GLUTAMATE
Glutamate, a major excitatory neurotransmitter in the brain, is involved in several physiological and pathological conditions. Dysfunction of excitatory amino acid transmission may play a role in the etiology and pathophysiology of depression [121]. In clinical studies, it has been reported that elevated glutamate levels were observed in plasma and cerebrospinal fluid of depressed patients [4,87,98], and the administration of antidepressants has been shown to decrease plasma glutamate levels significantly in patients diagnosed with depression [89]. Furthermore, it has been reported that a single intravenous dose of ketamine (a non-competitive NMDA receptor antagonist) had antidepressant effects in depressed patients [14]. Several lines of preclinical evidence indicate that manipulation of glutamatergic transmission is effective in treating depressive behavior. For example, it has been reported that various types of compounds such as NMDA receptor antagonists [119,150], mGluR5 antagonists [160], AMPA potentiators [88], and mGluR2/3 antagonists [28] show antidepressant-like activity in a variety of animal models.
A previous study reported that withdrawal from repeated cocaine use reduced the extracellular content of glutamate in the NAc core [130], and that microinjection of AMPA into the NAc increased motor activity in cocaine sensitized rats, which was blocked by the microinjection of non-NMDA antagonists into the NAc core [130]. Moreover, a recent study has reported that the injection of NMDA receptor antagonists MK-801 or AP-5 into the NAc results in antidepressant-like behavior in forced swimming tests [134]. Since non-competitive NMDA receptor antagonists have been shown to increase glutamate release in the NAc [1], some antidepressant effects of NMDA antagonists could be mediated through increased glutamate release [142]. The possible mechanism may be that the blockade of NMDA receptor on GABA neurons reduces GABA release, which in turn reduces the inhibition of glutamate neurons, with released glutamate acting on the AMPA receptor. Although this hypothesis remains to be proven, it may help to explain the seemingly paradoxical antidepressant effects of AMPA receptor potentiators and mGluR2/3 antagonists. Given that blockade of mGluR2/3 (presynaptic autoreceptor) increases glutamate release and that stimulation of postsynaptic AMPA receptor is involved in the anti-anhedonic action of mGluR2 antagonists [77], increased glutamate transmission in the NAc (possibly mediated through postsynaptic AMPA receptor) might be involved in this antidepressant action. It was also reported that injection of mGluR2/3 antagonists into the NAc increased dopamine release [68]. Of note, recent studies with KO mice lacking mGluR2 showed enhanced place preference associated with cocaine and increased dopamine release in the NAc, and showed reduced immobility time in the forced swimming test [107]. Likewise, it has been reported that KO mice lacking mGluR7, which also functions as a presynaptic autoreceptor, exhibit antidepressant-like activity [33]. These results indicate the respective roles of dopamine and glutamate in the NAc (Fig. 3). In support of these findings, it has been reported that transcranial magnetic stimulation, an effective treatment for major depression, increases the release of dopamine and glutamate in the NAc [177].
In contrast, it was reported that injection of glutamate into the NAc produces depressive behavior [134]. These reports also indicated that enhanced glutamate release by forced swimming on the second day coincides with decreased swimming [134]. Therefore, these data suggest the implication of increased glutamate activity in the NAc in depressive behavior. In the context of the hypothesis discussed above, increased glutamate appears to act on NMDA receptors of GABAergic neurons, which results in an increase of inhibitory tone.
Of note, local NMDA receptor blockade in the NAc core impaired spatial learning, while similar manipulation in the NAc shell had no effect [156]. AMPA receptor blockade in the NAc core impaired working memory [92]. A recent study reported that spatial memory consolidation required co-activation of glutamate and dopamine receptors within the NAc [49]. Furthermore, a previous study showed that NMDA receptors in the NAc are are involved in consolidation of spatial information, whereas AMPA receptors in the NAc are important for spatial discrimination [139]. Another recent study showed that NMDA and AMPA receptors in the NAc core play a critical role in invigorating response during instrumental learning, but are less important in guiding response according to reward-predictive cues [57]. NMDA receptor antagonism in the NAc impairs response-reinforcement learning to obtain food [76]. This appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the NAc core [157]. It is now believed that these types of information on learning and memory are weakly related with depression. However, given that reward sometimes requires learning and that the loss of pleasure observed in depression may reflect an inability to obtain reward, it is possible that enhancement of the learning and memory of new experiences could interfere with the memory of the stressful experience and thereby result in an antidepressant effect [146].
These reports indicate that glutamate in the NAc is involved in the production of behavioral depression. It is conceivable that the antidepressant role of glutamate might be ascribed to the blockade of NMDA receptors and activation of AMPA receptors, although the precise mechanism remains to be proven.
GABA
γ-Aminobutyric acid (GABA) is the most abundant inhibitory neurotransmitter in the mammalian brain, where it is widely distributed. It has recently been suggested that major depressive disorder is associated with dysfunction of GABAergic transmission [19,83]. Reports have indicated that plasma and cerebrospinal fluid GABA levels were reduced in depressed patients [127,128]. In contrast, antidepressant treatments with selective serotonin reuptake inhibitors (SSRIs), mood stabilizers or electroconvulsive therapy appear to eliminate the deficit in GABA levels associated withmajor depressive disorder [83].Likewise, reducedGABA transmission has been reported in animal models of depression such as the forced swimming test [18] and learned helplessness model [129]. A recent study reported that pretreatment GABAB receptor agonist baclofen antagonizes cocaine- and morphine-induced dopamine release in the NAc, indicating the involvement of GABAB receptros in the NAc in reward processes [47].
In the NAc, GABA has been recognized as playing a paramount role in fast transmission. GABA is utilized by common, spiny projection neurons and by one category of aspiny interneurons [102]. The flow of information through the NAc is dependent upon the activity of the neuron: a GABAergic, medium-sized spiny projection cell, which contributes to the excitability of the nucleus and forms an important substrate for neuromodulation. There are two major receptor subtypes for GABA: ionotropic GABAA receptors and metabotropic GABAB receptors, both of which are expressed in the NAc [97]. Of these, GABAA receptors consist mostly of postsynaptic receptors, whereas GABAB receptors are localized presynaptically and postsynaptically (Fig. 3). Functional differences of GABAA and GABAB receptors in the NAc are suggested. It has been reported that GABAA receptors exert inhibitory control on dopamine release within the NAc, although the role of GABAB receptors is less prominent [136].
Psychostimulant withdrawal is thought to be as a model of depression [8]. Withdrawal from repeated cocaine administration increased the extracellular content of GABA in the NAc core, which might be a result of the functional desensitization of GABAB presynatic autoreceptor [172]. The extracellular GABA provides inhibitory tone on GABAB presynaptic autoreceptors to regulate GABA release [172] (Fig. 3). Within the NAc, activation of either GABAA or GABAB receptors inhibits acetylcholine release, although tonic GABA inhibition appears mainly under the influence of GABAA receptors [133]. A recent study using the unilateral injection technique has indicated that GABAA receptors in the NAc shell exert inhibitory control on dopamine-mediated turning behavior, whereas GABAB receptors in the NAc shell exert inhibitory control over acetylcholine-mediated turning behavior [3] (Fig. 3). Because cholinergic interneurons perform excitatory regulation of dopamine release in the NAc (presumably through nicotinic receptor), the inhibitory effects of GABA on acetylcholine release inhibit dopamine release. It has been suggested that reduced dopamine release in the NAc may cause anhedonia. Therefore, direct or indirect regulation of dopaminergic activity by GABAergic neurons within the NAc may be responsible for the etiology of depression, although the significance of each receptor subtype (GABAA and GABAB) within the NAc has yet to be fully elucidated.
A recent study showed that infusion of allopregnanolone into the NAc produced antidepressant-like effects in ovariectomized rats [105]. Allopregnanolone is a neurosteroid, which is known to be an agonist for GABAA receptors. In human beings, cerebrospinal fluid concentrations of allopregnanolone were reduced in depressed patients and recovered after antidepressant treatments [137,166]. However, allopregnanolone works in other regions such as the prefrontal cortex and hippocampus where GABAA receptors are widely distributed. Further study will be required to gain an understanding of the role of allopregnanolone in the NAc.
ACETYLCHOLINE
The central cholinergic system may play a role in depressive disorders. Stimulation of the central cholinergic system with cholinomimetics or cholinesterase inhibitors causes depressive phenotypes such as depressed mood, dysphoria and anhedonia [39]. Depressed human subjects are supersensitive to cholinergic challenge relative to controls [39]. Moreover, anti-muscarinic agents have been reported to have antidepressant-like activity in rodents [30]. The implication of hypercholinergic activity in depressive behavior was further supported by the fact that the Flinders Sensitive Line rats, which display depressive phenotypes, were developed by selective breeding for increased sensitivity to the cholinesterase inhibitor [115]. This genetic animal model of depression showed depressive behavior such as enhanced immobility in the forced swim test [116].
The NAc contains aspiny cholinergic interneurons, which interact with dopaminergic input, glutamatergic input as well as with the GABAergic medium spiny projection neurons [102]. Acetylcholine and dopamine have the opposite effects on the NAc. It should be noted that acetylcholine enhancement in the NAc is reported to prevent addictive behavior with cocaine and morphine [63]. The cholinergic neurons are more densely distributed in the shell than in the core [102]. The cholinergic interneurons control the excitability and membrane properties of these output neurons mainly by stimulating post-synaptic muscarinic receptors [102]. Thus, the cholinergic interneurons appear to be well suited for controlling the transmission of limbic information to the accumbens target nuclei. It has been reported that acetylcholine release in the NAc increases response to an aversively conditioned taste stimulus [96] or during withdrawal from drugs of abuse [135], indicating the implication of the accumbal cholinergic system in reward, emotion or depression.
It has been reported that injection of the M1-muscarinic receptor antagonist pirenzepine into the NAc exhibits an antidepressant effect in the forced swimming test without changing locomotor activity [30]. In contrast, local injection of the M2-muscarinic receptor antagonist gallamine results in depressive-like behaviors in the same paradigm and an increase in acetylcholine release in the NAc [30]. Increased acetylcholine may stimulate post-synaptic M1-muscarinic receptor to cause depressive behavior.
Injection of nicotine into the NAc increases dopamine release in the NAc [103]. In this context, the mechanism of nicotine is similar to that of drugs of substance abuse. Withdrawal symptoms from either nicotine or substances of abuse can be considered from the point of view of dopamine release in the NAc. Therefore, it is unsurprising that nicotine and nicotine receptor (α4β2) agonist showed antidepressant-like activity in rodents [48,144]. However, some contradictory reports indicate that nicotine receptor antagonism may mediate antidepressant-like activity [148,152]. Indeed, it has also been reported that antidepressants inhibit nicotine receptors [51,60] and that nicotine receptor antagonist enhances the antidepressant effect of imipramine and citalopram [132]. This discrepancy may stem from the differential expression of nicotine receptor subunits or differential selectivity of the compound for the subunits. The role of nicotine receptors in the NAc in depression remains to be verified.
SEROTONIN
Serotonin (5-HT) is known to be involved in depression, aggression, and suicidality. As it has been demonstrated that SSRIs serve as effective medication for major depressive disorder, much attention has been focused on the serotonergic system of the brain. Serotonergic responsivity was reported to be dulled in a subgroup of young depressed patients [94].
It has been postulated that serotonergic neurons in the NAc play an important role in motivation, reward and certain forms of stress [100,108], and short duration immobilization stress has been shown to alter 5-HT levels in the shell of the NAc [100]. Finders Sensitive Line rats, a genetic model of depression, showed a decrease in 5-HT turnover (5-HIAA/5-HT ratio) in the NAc [180]. Wistar-Kyoto rats, which are prone to develop stress-induced depression, exhibited significantly lower tissue levels in 5-HT and 5-HIAA in the NAc in stress conditions in spite of the fact that normal control rats exhibit a significant increase of 5-HIAA in response to stress [35], indicating the dulled serotonergic responsivity in the NAc in depression. Similarly, another model of depression, the olfactory bulbectomized rat displayed blunted serotonergic response to a challenge with a metabolic stressor lipopolysaccharide [32]. Other model of depression, chronically stressed rats, also showed a reduced 5-HT response, but not the basal levels, in the NAc shell after cocaine administration [93], indicating the dulled pleasure stimulation.
It has been reported that serotonergic transmission regulates dopamine release within the NAc, both positively and negatively, while local application of serotonin into the NAc has been reported to stimulate dopamine release [120]. It is considered that the mal-regulation of dopaminergic activity in the NAc by 5-HT may be involved in a depressive phenotype [40]. In support of this hypothesis, an increased inhibitory effect of 5-HT2C receptors on accumbal dopamine release was observed in Flinders Sensitive Line rats, an animal model of depression [42], and chronic antidepressant treatment normalized the serotonin-dopamine interaction as well as depressive behavior in the forced swimming test [178]. Serotonin-induced alterations in dopamine release involve the interaction of other types of serotonin receptors. Activation of 5-HT1A, 5-HT2A and 5-HT3 receptors induces dopamine release in the NAc [23,120,174] while activation of 5-HT2C receptors reduces dopamine release in the NAc [42].
It has been reported that local injection of serotonin into the NAc increased the release of β-endorphin in the region, which is enhanced by co-infusion of fluoxetine [179]. Since β-endorphin in the NAc has rewarding and reinforcing effects [78], this interaction has been postulated to be involved in depression.
Both core and shell subregions of the NAc received a dense serotonergic innervation from the raphe nucleus [167], while 5-HT-labeled axon terminals in the shell are more numerous and more frequently formed synaptic contacts of the symmetric variety relative to the 5-HT-labeled axon terminals in the core region [168]. In the NAc shell, 5-HT-containing axon terminals form mainly symmetric, inhibitorytype, synapses with GABAergic neurons and their targets, indicating a neuromodulatory role (largely inhibition) for 5-HT on GABAergic neurons in the NAc shell [167]. The authors demonstrated that 5-HT- and GABA-labeled axons were also frequently apposed, suggesting other presynaptic sites for modulatory interactions in the NAc shell. Moreover, a relatively high incidence of convergence between serotonergic and GABAergic terminals on common dendrites, presumably of cholinergic neurons, has been observed [167], suggesting that 5-HT afferents may have direct effects on cholinergic neurons (inhibition) and indirect effects of excitation involving inhibition of inhibitory GABAergic neurons.
DYNORPHIN/κ OPIOID RECEPTOR
It has been reported that κ opioid receptor agonists increases immobility time in the rat forced swimming test without effects on locomotor activity [90], suggesting that activation of κ opioid receptor produces depressive phenotypes. To support this hypothesis, it has been reported that a κ opioid receptor antagonist as well as gene disruption of prodynorphin attenuate increased immobility following exposure to repeated forced swim stress [101].
The NAc is a brain region critically involved in aversive behaviors associated with dynorphin and its receptor (κ-opioid receptor) agonists. Indeed, microinjection of κ-opioid receptor agonists into the NAc causes conditioned place aversions that likely reflect dysphoric states [6]. It has also been reported that exposure to various stressors such as immobilization, forced swimming, and inescapable shock markedly increase dynorphin immunoreactivity in both the shell and core of NAc [147]. Given that depression in humans is often precipitated or worsened by physical or psychological stress, and stress is reported to produce depression [106], increased dynorphin expression in the NAc by stress could play an important role in formation of depression. Furthermore, there are several lines of evidence showing that blockade of κ opioid receptor produces antidepressant-like activity in animal models. Thus, intracerebroventricular or intraaccumbal injection of norBNI, a κ opioid receptor antagonist, decreases immobility in the forced swimming test [90] or reduces escape failure in the learned helplessness test [111,147].
Kappa opioid receptors have been reported to mediate aversion by regulating mesolimbic dopaminergic activity in the NAc [62]. Indeed, both systemic and intraaccumbal injection of the κ opioid receptor agonist reduce dopamine release in the NAc, while both systemic and intraaccumbal injection of the κ opioid receptor antagonist norBNI increase dopamine release [91,159]. Furthermore, injection of D1 receptor antagonists into the NAc attenuated the conditioned place aversion by the κ opioid receptor agonist [145], consistent with the supposition that κ opioid-mediated aversion is due to the inhibition of dopamine release in the NAc. It was reported that chronic cocaine administration increased κopioid receptors in the NAc [31]. Thus, given that the dopamine system in the NAc plays a major role in motivation and reward and could contribute to the anhedonia observed in depressed patients, it is suggested that a decrease in dopaminergic activity in the NAc by increased dynorphin expression due to exposure to stress might be responsible for the formation of depression.
The GABAergic spiny neurons in the NAc shell receive glutamatergic input from the prefrontal cortex, amygdala, and hippocampus, in addition to dopaminergic input from the ventral tegmental area. Interestingly, it was demonstrated that κ opioid receptor is present on the presynaptic terminals of the presumed excitatory synapses as well as on the dendrites of the medium spiny neurons [161], which raises the possibility that some of the effects mediated by κ opioid receptor may be attributable to the regulation of glutamatergic excitatory transmission. Indeed, it has been reported that the κ opioid receptor agonist decreases glutamate release in the NAc shell [65] and it is hypothesized that the behaviors induced by a κ opioid receptor agonists in the NAc shell are partially dependent on modulation of glutamatergic input to medium spiny neurons [65].
ENKEPHALIN/δ OPIOID RECEPTOR
A recent study reported that endogenous enkephalin modulates the basal hedonic state in pro-enkephalin knockout mice [151]. It has been reported that the enkephalin catabolism inhibitor exhibited antidepressant-like activity in learned helplessness and that this effect was reversed by the δ opioid receptor antagonist naltrindole [162]. Consistent with this hypothesis, δ opioid receptor agonists have been reported to display antidepressant-like activity in the forced swimming test [21,141], while KO mice lacking δ opioid receptor display a depressive-like phenotype in the forced swimming test [50].
It has been reported that chronic treatment with antidepressants increases [Met5]enkephalin-like immunoreactivity in the NAc [34]. Likewise, repeated electroconvulsive shocks cause an increase in [Met5]enkephalin content in the NAc [66]. In contrast, in another study chronic treatment with antidepressants was reported to decrease [Met]enkephaline content in the NAc [84]. Antidepressants seem to alter the peptide in the NAc, in spite of some conflicting results in previous reports.
The NAc contains δ opioid receptors, which are supposed to be located in the presynapse of dopaminergic terminals because local administration of δ opioid receptor agonists increase dopamine release in the NAc [158] (Fig. 3). Several lines of evidence have suggested the implication of enkephalin/δ opioid receptor in the NAc in depression. An increase in [Met5]enkephalin release in the NAc by exposure to a congener was curbed after chronic mild stress procedure [15], and the level of [Met5]enkephalin was reduced in the NAc in chronic mild stress model [43], suggesting that the activity of the enkephalinergic system in the NAc could be reduced in a stress-induced model of anhedonia. Indeed, δopioid receptor agonists, when infused into the NAc, increase dopamine release in the NAc [52], which may explain the antidepressant-like action of δ opioid receptor agonists. Although the precise mechanism mediated by antidepressant effects of δ opioid receptor remains to be determined, the interaction between δ opioid receptor and dopaminergic system in the NAc has been suggested as a possibility.
BDNF
BDNF is now recognized not only for its impact on neurons, but also for its implications in learning, motivation and regulation of mood. A recent study reported that serum BDNF levels were significantly reduced in antidepressant-free patients with depression, and were negatively correlated with the severity of depression [74]. Another study demonstrated that decreased serum BDNF levels in antidepressant-naïve patients recovered to normal levels associated with lower severity of depression after antidepressant medication [149].
Repeated stress decreases BDNF expression in the hippocampus [153], while antidepressant treatment as well as electroconvulsive seizure increases BDNF levels in the hippocampus [112]. Moreover, it has been reported that administration of BDNF into the hippocampus causes antidepressant-like effects in the forced swimming test and learned helplessness test [146]. These findings are in good agreement with the decreased serum BDNF levels in patients with depression, and strongly suggest that a decreased BDNF level in the hippocampus causes a depressive phenotype.
However, in contrast to the hippocampus, it has been reported that BDNF in the NAc is involved in the development of a depression-like phenotype. Thus, intra-NAc injection of adenoviral vector encoding truncated tyrosine kinase receptor B (TrkB) (which can act as a dominant-negative receptor) exhibits an antidepressant-like phenotype [45]. Moreover, it has been reported that chronic exposure to or withdrawal from drugs of abuse, conditions in which an anhedonic state can be produced, induces an increase in BDNF levels in the NAc [58]. In contrast, it has been reported that increased BDNF or stimulation of TrkB in the NAc enhance dopamine release and dopamine-related behaviors [109], and intra-NAc BDNF infusions enhances drug reward mechanisms [67]. These events contradict the finding that increased dopamine function and reward lead to antidepressant-like activity. The mechanism underlying the action of BDNF in the NAc remains to be elucidated.
CREB
CREB is a constitutively expressed transcription factor activated by phosphorylation through cAMP pathway and other intracellular signaling cascades [99]. Transgenic mice overexpressing CREB display depressive behavior in the learned helplessness test (increased escape failure), whereas mice overexpressing dominant-negative mutant CREB (mCREB) or rats with viral-expressed mCREB in the NAc showed a decrease in escape failure, an antidepressant-like effect [111]. Furthermore, mCREB expression decreased the expression of prodynorphin in NAc medium spiny neurons [111]. Thus, increased CREB (perphaps taken together with increased dynorphin) in the NAc could produce a depressive phenotype in animal models of depression.
It has been reported that elevated transcription of CREB in the NAc is associated with increased immobility in the forced swim test, while decreased CREB expression in the NAc exhibits the opposite effect, i.e., an antidepressant-like effect [131]. Acute and chronic stresses as well as chronic exposure to drugs of abuse have shown to activate CREB in the NAc [10,131]. It has been reported that increased CREB function in the NAc decreases reward responses to drugs of abuse, whereas decreased CREB function has the opposite effect [25,131]. Thus, rats expressing dominant-negative CREB spent more time in an environment associated with cocaine in a place conditioning study, while rats overexpressing CREB exhibited increased cocaine aversion [131].
Moreover, CREB activity in the NAc shell appears to control gating of behavioral responses to emotional stimuli [10], such that the increase in CREB function in the NAc shell following stress or drug exposure may contribute to symptoms of emotional numbing or anhedonia. This is interesting because cessation of drugs of abuse induces a withdrawal syndrome that is manifestly similar to the symptoms of major depressive disorder in humans [8]. Likewise, in rodents, withdrawal from drugs of abuse elevated the brain stimulation reward threshold in intracranial self-stimulation [59] and reduced motivation to obtain a sucrose solution under a progressive ratio schedule of reinforcement [9], both of which reflect anhedonia. Moreover, it has been shown that extracelluar dopamine level in the NAc is reduced during cocaine withdrawal [169]; thus it is presumed that increased CREB function in the NAc may inhibit dopaminergic activity.
In the NAc, prodynorphin has been identified as a target of CREB, and is induced by activation of cAMP-CREB cascade [110]. It has been reported that viral-mediated elevations of CREB in the NAc shell elevate local dynorphin mRNA, while overexpression of dominant-negative CREB in the NAc shell decreases dynorphin mRNA [25]. Consistent with this finding, it has been reported that injection of a κ opioid receptor antagonist into the NAc leads to the display of antidepressant effects in rodent models of depression [111,147]. Therefore, one plausible explanation of dysphoric and depressive phenotype by increased CREB in the NAc is that CREB-mediated increase in dynorphin within the NAc decreases local dopamine tone via actions at the κ opioid receptor on the terminals of mesolimbic dopamine neurons.
MCH
Melanin-concentrating hormone (MCH) is produced predominantly by neurons in the lateral hypothalamus and the zona incerta with extensive projections throughout the brain [16]. MCH has two receptor subtypes, designated MCH1R and MCH2R, both of which are G protein-coupled receptors [56]. An emerging body of evidence suggests that MCH1R plays an important role in the regulation of mood and stress.
MCH1R is highly expressed in the NAc shell [16,17,61, 140]. Moreover, it has recently been reported that MCH1R is co-expressed in dynorphin-positive medium spiny neurons in this region [55], and that injection of MCH into the NAc shell causes depressive behavior in the forced swimming test [55], suggesting that MCH may exert its depressive-like effects through interaction with MCH1R in the NAc shell.
Recently, it has been reported that MCH1R null mice exhibit upregulation of mesolimbic dopamine receptors with increased expression of dopamine D1- and D2-like receptors in the NAc shell, and that they show supersensitivity to D-amphetamine and dopamine D1 receptor agonist-induced locomotor hyperactivity [155]. Moreover, intracerebroventricular injection of MCH decreases DOPAC levels in the NAc [175]. These results indicate that MCH1R negatively modulates dopaminergic function in the NAc. Considering the co-expression of MCH1R with dynorphin, it is presumed that MCH inhibits dopaminergic activity in the NAc by positively regulating dynorphin expression or release, which may in turn inhibit dopamine release in the region; this mechanism may be involved, at least in part, in the depressive phenotype caused by MCH. Moreover, MCH has been reported to inhibit dopamine D1 receptor agonist-induced phosphorylation of Ser845 of the AMPA receptor subunit GluR1 in the NAc slices, the event, which leads to reduced AMPA transmission [55]. Thus, MCH1R may possibly negatively regulate AMPA transmission via D1 receptor in the NAc. This effect is of interest because it has been reported that AMPA potentiators have antidepressant effects in behavioral despair models such as the forced swim and tail suspension test [88], and this mechanism might also be involved in the depressive phenotype produced by MCH.
In accordance with the above findings, it has recently been reported that nonpeptidic MCH1R antagonists, when administered systemically, exhibit antidepressant effects in forced swimming test [17,27]. Moreover, injection of MCH1R antagonists to the NAc shell resulted in antidepressant effects in the forced swimming test [55]. Future studies remain to be investigated whether MCH or MCH1R expression in the NAc is increased following stress exposure or withdrawal from drugs of abuse.
CART
Cocaine- and amphetamine-regulated transcript (CART) was identified as a mRNA acutely upregulated in the NAc after administration of cocaine or amphetamine in rats [41]. To date, several CART peptide fragments have been identified, and among them, two CART peptide fragments, CART-(55-102) and CART-(62-102), were found to be biologically active [7,82].
High levels of CART peptide immunoreactivity were found in both the shell and core of the NAc in rats [80] and monkeys [154] with more abundant expression in the shell region. In the shell, CART was found to be highly concentrated in medium spiny projection neurons that contain GABA [154], suggesting that CART peptide may be a co-transmitter with GABA in a subpopulation of projection neurons in the shell.
Moreover, it has been reported that CART peptideimmunoreactive dendrites were found to be in contact with tyrosine hydroxylase-positive terminals that displayed the ultrastructural features of dopamine containing boutons, indicating that CART peptide-immunoreactive neurons receive direct synaptic input from dopaminergic afferents [154]. In contrast, CART mRNA-containing cell bodies or CARTimmunoreactive cell bodies were not found in the VTA, whereas CART-containing axons and nerve terminals were found. Thus, CART might well be a region-specific chemical in the NAc and striatum.
It has been reported that injection of CART peptide into the NAc inhibits cocaine-induced hyperlocomotion [71], suggesting the implication of CART peptide in the reward pathway. CART is likely to act at least partly on GABAcontaining neurons. In the NAc, CART may possibly inhibit GABAoutputvia receptors (although unidentified) on GABA ergic neurons. Although the relationship between CART and depression has yet to be demonstrated, based on the implication of CART in the reward pathway through modulation of the GABA-dopamine interaction, it is highly likely that CART peptide in the NAc plays an important role in the pathophysiology of depression.
CONCLUDING REMARKS
Dysfunction of dopaminergic activity in the NAc may be deeply involved in the pathophysiology of depression. Reduced dopaminergic activity in the NAc was also observed in animal models of depression as well as after chronic mild stress. In this context, dopamine receptor agonists should provide beneficial effects in treating impaired NAc-related functions such as reward, motivation, and learning. Indeed, dopaminergic agents such as dopamine reuptake inhibitors have been reported to be effective in the treatment of patients with major depressive disorder [54,181]. Further studies are required to elucidate the importance of dopamine in the NAc in depression and the effectiveness of dopamine-related agents for the treatment of depression.
Dopaminergic activity in the NAc is regulated by numerous substances. These include dynorphin, MCH, and CART, which are postulated to negatively modulate dopamine release in the NAc. Interestingly, it has been reported that expression of dynorphin in the NAc is increased in animal models of depression, learned helplessness and psychostimulant withdrawal. Thus, the reduced dopaminergic activity seen in depression could be ascribed to increased expression of negative modulators of the dopamine system, including dynorphin, in the NAc under stressful conditions. Here, it should be noted that dopaminergic agents, by acting outside the NAc, generate not only beneficial effects in the treatment of depressive mood but also a number of adverse side effects. In this case, neuropeptides such as dynorphin, MCH, and CART might be ideal targets for therapeutic agents with fewer unwanted effects.
It is well recognized that GABAergic neurons function as the main neurons in the NAc, and that the flow of information through the NAc is dependent on the activity of GABAergic spiny projecting neurons. Moreover, it has also been recognized that the glutamatergic corticolimbic pathway plays an important role in the balance of activity between the cortical and subcortical regions. However, in spite of the crucial roles of these amino acids in the NAc, studies on the involvement of these amino acids in the NAc in depression have been hampered by the fact that they are abundantly and ubiquitously distributed throughout the brain; moreover, findings on local injection of agonists/antagonists for these amino acid receptors into the NAc are currently limited. Likewise, the role of cholinergic systems in the NAc in depression must be clarified, although here it is interesting to note that cholinergic neurons in the NAc are interneurons, but not projecting neurons. Although currently prescribed antidepressants exert their effects through the serotonin or norepinephrine systems, these neurotransmitters do not appear to be involved in the main functions within the NAc.
Depression may affect various brain regions, and different symptoms seen in subjects with depression may be attributed to different magnitudes of impairments among the affected brain regions. Accumulating evidence has clearly indicated the importance of the NAc in pathophysiology of depression, and the roles of several neurotransmitters, neuropeptides, and transcriptional factors in this region in depression have been investigated. Further studies are required to delineate the interaction among these molecules in the NAc, and to elucidate their implications in depressive disorder.
REFERENCES
- 1.Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ainsworth K, Smith SE, Zetterstrom TS, Pei Q, Franklin M, Sharp T. Effect of antidepressant drugs on dopamine D1 and D2 receptor expression and dopamine release in the nucleus accumbens of the rat. Psychopharmacology. 1998;140:470–477. doi: 10.1007/s002130050791. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama G, Ikeda H, Matsuzaki S, Sato M, Moibe S, Koshikawa N, Cools AR. GABAA and GABAB receptors in the nucleus accumbens shell differentially modulate dopamine and acetylcholine receptor-mediated turning behaviour. Neuropharmacology. 2004;46:1082–1088. doi: 10.1016/j.neuropharm.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Altamura CA , Mauri M C, Ferrara A, Moro A R, D’Andrea G, Zamberlan F. Plasma and platelet excitatory amino acids in psychiatry disorders. Am J Psychiatry. 1993;150:1731–1733. doi: 10.1176/ajp.150.11.1731. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. 4th Edition. Washington DC: American Psychiatric Press; 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 6.Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- 7.Bannon A W, Seda J, Carmouche M, Francis J M, Jarosinski MA, Douglass J. Multiple behavioral effects of cocaine- and amphetamine-regulated transcript (CART) peptides in mice: CART 42-89 and CART 49-89 differ in potency and activity. J Pharmacol Exp Ther. 2001;299:1021–1026. [PubMed] [Google Scholar]
- 8.Barr AM, Markou A, Phillips AG. A ‘crash’ course on psychostimulant withdrawal as a model of depression. Trends Pharmacol Sci. 2002;23:475–482. doi: 10.1016/s0165-6147(02)02086-2. [DOI] [PubMed] [Google Scholar]
- 9.Barr AM, Phillips AG. Withdrawal following repeated exposure to d-amphetamine decreases responding for a sucrose solution as measured by a progressive ratio schedule of reinforcement. Psychopharmacology. 1999;141:99–106. doi: 10.1007/s002130050812. [DOI] [PubMed] [Google Scholar]
- 10.Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci USA. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassareo V, De Luca MA, Di Chiara G. Differential expression of motivational stimulus properties by dopamine in nucleus accumbens shell versus core and prefrontal cortex. Neurosci. 2002;22:4709–4719. doi: 10.1523/JNEUROSCI.22-11-04709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basso AM, Gallagher KB, Bratcher NA, Brioni JD, Moreland RB, Hsieh GC, Drescher K, Fox GB, Decker MW, Rueter LE. Antidepressant-Like Effect of D(2/3) Receptor-, but not D(4) Receptor-Activation in the Rat Forced Swim Test. Neuropsychopharmacology. 2005;30:1257–1268. doi: 10.1038/sj.npp.1300677. [DOI] [PubMed] [Google Scholar]
- 13.Beitner-Johnson D, Nestler EJ. Morphine and cocaine exert common chronic actions on tyrosine hydroxylase in dopaminergic brain reward regions. J Neurochem. 1991;57:344–347. doi: 10.1111/j.1471-4159.1991.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 14.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 15.Bertrand E, Smadja C, Mauborgne A, Roques BP, Dauge V. Social interaction increases the extracellular levels of [Met]enkephalin in the nucleus accumbens of control but not of chronic mild stressed rats. Neuroscience. 1997;80:17–20. doi: 10.1016/s0306-4522(97)00136-x. [DOI] [PubMed] [Google Scholar]
- 16.Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 17.Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, Heurich R, Lichtblau H, Shaposhnik Z, Daniewska I, Blackburn TP, Branchek TA, Gerald C, Vaysse PJ, Forray C. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med. 2002;8:825–830. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- 18.Borsini F, Mancinelli A, D’Aranno V, Evangelista S, Meli A. On the role of endogenous GABA in the forced swimming test in rats. Pharmacol Biochem Behav. 1988;29:275–279. doi: 10.1016/0091-3057(88)90156-6. [DOI] [PubMed] [Google Scholar]
- 19.Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721– 737. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- 20.Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 21.Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002;26:744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- 22.Cabib S, Puglisi-Allegra S. Opposite responses of mesolimbic dopamine system to controllable and uncontrollable aversive experiences. J Neurosci. 1994;14:3333–3340. doi: 10.1523/JNEUROSCI.14-05-03333.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell AD, McBride WJ. Serotonin-3 receptor and ethanol-stimulated dopamine release in the nucleus accumbens. Pharmacol Biochem Behav. 1995;51:835–842. doi: 10.1016/0091-3057(95)00050-7. [DOI] [PubMed] [Google Scholar]
- 24.Cardinal RN, Cheung THC. Nucleus accumbens core lesions retard instrumental learning and performance with delayed reinforcement in the rat. BMC Neurosci. 2005;6:9. doi: 10.1186/1471-2202-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 26.Carlezon WA, Jr, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16:3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaki S, Funakoshi T, Hirota-Okuno S, Nishiguchi M, Shimazaki T, Iijima M, Grottick AJ, Kanuma K, Omodera K, Sekiguchi Y, Okuyama S, Tran TA, Semple G, Thomsen W. Anxiolytic- and antidepressant-like profile of ATC0065 and ATC0175: Nonpeptidic and orally active melanin-concentrating hormone receptor 1 antagonists. J Pharmacol Exp Ther. 2005;313:831–839. doi: 10.1124/jpet.104.081711. [DOI] [PubMed] [Google Scholar]
- 28.Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N, Yoshimizu T, Yasuhara A, Sakagami K, Okuyama S, Nakanishi S, Nakazato A. MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology. 2004;46:457–467. doi: 10.1016/j.neuropharm.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Chang HT, Kitai ST. Projection neurons of the nucleus accumbens: an intracellular labeling study. Brain Res. 1985;347:112–116. doi: 10.1016/0006-8993(85)90894-7. [DOI] [PubMed] [Google Scholar]
- 30.Chau DT, Rada P, Kosloff RA, Taylor JL, Hoebel BG. Nucleus accumbens muscarinic receptors in the control of behavioral depression: antidepressant-like effects of local M1 antagonist in the Porsolt swim test. Neuroscience. 2001;104:791–798. doi: 10.1016/s0306-4522(01)00133-6. [DOI] [PubMed] [Google Scholar]
- 31.Collins SL, Kunko PM, Ladenheim B, Cadet JL, Carroll FI, Izenwasser S. Chronic cocaine increases kappa-opioid receptor density: lack of effect by selective dopamine uptake inhibitors. Synapse. 2002;45:153–158. doi: 10.1002/syn.10091. [DOI] [PubMed] [Google Scholar]
- 32.Connor TJ, Song C, Leonard BE, Anisman H, Merali Z. Stressor-induced alterations in serotonergic activity in an animal model of depression. Neuroreport. 1999;10:523–528. doi: 10.1097/00001756-199902250-00015. [DOI] [PubMed] [Google Scholar]
- 33.Cryan JF, Kelly PH, Neijt HC, Sansig G, Flor PJ, van Der Putten H. Antidepressant and anxiolytic-like effects in mice lacking the group III metabotropic glutamate receptor mGluR7. Eur J Neurosci. 2003;17:2409–2417. doi: 10.1046/j.1460-9568.2003.02667.x. [DOI] [PubMed] [Google Scholar]
- 34.De Felipe MC, De Ceballos ML, Gil C, Fuentes JA. Chronic antidepressant treatment increases enkephalin levels in n. accumbens and striatum of the rat. Eur J Pharmacol. 1985;112:119– 122. doi: 10.1016/0014-2999(85)90247-x. [DOI] [PubMed] [Google Scholar]
- 35.De La Garza II R, Mahoney III JJ. A distinct neurochemical profile in WKY rats at baseline and in response to acute stress: Implications for animal models of anxiety and depression. Brain Res. 2004;1021:209–218. doi: 10.1016/j.brainres.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 36.De La Garza II R, Jentsch JD, Verrico CD, Roth RH. Adaptation of monoaminergic responses to phencyclidine in nucleus accumbens and prefrontal cortex following repeated treatment with fluoxetine or imipramine. Brain Res. 2002;958:20–27. doi: 10.1016/s0006-8993(02)03772-1. [DOI] [PubMed] [Google Scholar]
- 37.Di Chiara G, Loddo P, Tanda G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol Psychiatry. 1999;46:1624–1633. doi: 10.1016/s0006-3223(99)00236-x. [DOI] [PubMed] [Google Scholar]
- 38.Di Chiara G, Tanda G. Blunting of reactivity of dopamine transmission to palatable food: a biochemical marker of anhedonia in the CMS model? Psychopharmacology. 1997;134:351–353. doi: 10.1007/s002130050465. [DOI] [PubMed] [Google Scholar]
- 39.Dilsaver SC. Cholinergic mechanisms in depression. Brain Res. 1986;396:285–316. doi: 10.1016/0165-0173(86)90016-0. [DOI] [PubMed] [Google Scholar]
- 40.Di Matteo V, De Blasi A, Di Giulio C, Esposito E. Role of 5-HT(2C) receptors in the control of central dopamine function. Trends Pharmacol. Sci. 2001;22:229–232. doi: 10.1016/s0165-6147(00)01688-6. [DOI] [PubMed] [Google Scholar]
- 41.Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dremencov E, Newman ME, Kinor N, Blatman-Jan G, Schindler CJ, Overstreet DH, Yadid G. Hyperfunctionality of serotonin-2C receptor-mediated inhibition of accumbal dopamine release in an animal model of depression is reversed by antidepressant treatment. Neuropharmacology. 2005;48:34–42. doi: 10.1016/j.neuropharm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Dziedzicka-Wasylewska M, Papp M. Effect of chronic mild stress and prolonged treatment with imipramine on the levels of endogenous Met-enkephalin in the rat dopaminergic mesolimbic system. Pol J Pharmacol. 1996;48:53–6. [PubMed] [Google Scholar]
- 44.Dziedzicka-Wasylewska M, Willner P, Papp M. Changes in dopamine receptor mRNA expression following chronic mild stress and chronic antidepressant treatment. Behav Pharmacol. 1997;8:607–618. doi: 10.1097/00008877-199711000-00017. [DOI] [PubMed] [Google Scholar]
- 45.Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Zarahn E, Leibenluft E, Zametkin A, Towbin K, Blair J, Charney D, Pine DS. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 47.Fadda P, Scherma M, Fresu A, Collu M, Fratta W. Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine in the nucleus accumbens of rat. Synapse. 2003;50:1–6. doi: 10.1002/syn.10238. [DOI] [PubMed] [Google Scholar]
- 48.Ferguson SM, Brodkin JD, Lloyd GK, Menzaghi F. Antidepressant-like effects of the subtype-selective nicotinic acetylcholine receptor agonist, SIB-1508Y, in the learned helplessness rat model of depression. Psychopharmacology. 2000;152:295–303. doi: 10.1007/s002130000531. [DOI] [PubMed] [Google Scholar]
- 49.Ferretti V, Florian C, Costantini VJA, Roullet P, Rinaldi A, De Leonibus E, Oliverio A, Mele A. Co-activation of glutamate and dopamine receptors within the neucleus accumbens is required for spatial memory consolidation in mice. Psychopharmacology. 2005;179:108–116. doi: 10.1007/s00213-005-2144-3. [DOI] [PubMed] [Google Scholar]
- 50.Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nature Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- 51.Fryer JD, Lukas RL. Antidepressants noncompetitively inhibit nicotinic acetylcholine receptor function. J Neurochem. 1999;72:1117–1124. doi: 10.1046/j.1471-4159.1999.0721117.x. [DOI] [PubMed] [Google Scholar]
- 52.Fusa K, Takahashi I, Watanabe S, Aono Y, Ikeda H, Saigusa T, Nagase H, Suzuki T, Koshikawa N, Cools AR. The non-peptidic delta opioid receptor agonist TAN-67 enhances dopamine efflux in the nucleus accumbens of freely moving rats via a mechanism that involves both glutamate and free radicals. Neuroscience. 2005;130:745–755. doi: 10.1016/j.neuroscience.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 53.Gambarana C, Masi F, Tagliamonte A, Scheggi S, Ghiglieri O, De Montis MG. A chronic stress that impairs reactivity in rats also decreases dopaminergic transmission in the nucleus accumbens: a microdialysis study. J Neurochem. 1999;72:2039–2046. doi: 10.1046/j.1471-4159.1999.0722039.x. [DOI] [PubMed] [Google Scholar]
- 54.Garattini S. Pharmacology of amineptine, an antidepressant agent acting on the dopaminergic system: a review. Int Clin Psychopharmacol. 1997;12(Suppl 3):S15–S19. doi: 10.1097/00004850-199707003-00003. [DOI] [PubMed] [Google Scholar]
- 55.Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gibson WT, Pissios P, Trombly DJ, Luan J, Keogh J, Wareham NJ, Maratos-Flier E, O’Rahilly S, Farooqi IS. Melanin-concentrating hormone receptor mutations and human obesity: functional analysis. Obes Res. 2004;12:743–749. doi: 10.1038/oby.2004.89. [DOI] [PubMed] [Google Scholar]
- 57.Giertler C, Bohn I, Hauber W. Involvement of NMDA and AMPA/KA receptors in the nucleus accumbens core in instrumental learning guided by reward-predictive cues. Eur J Neurosci. 2005;21:1689–1702. doi: 10.1111/j.1460-9568.2005.03983.x. [DOI] [PubMed] [Google Scholar]
- 58.Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harrison AA, Liem YT, Markou A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology. 2001;25:55–71. doi: 10.1016/S0893-133X(00)00237-2. [DOI] [PubMed] [Google Scholar]
- 60.Hennings EC, Kiss JP, Vizi ES. Nicotinic acetylcholine receptor antagonist effect of fluoxetine in rat hippocampal slices. Brain Res. 1997;759:292–294. doi: 10.1016/s0006-8993(97)00343-0. [DOI] [PubMed] [Google Scholar]
- 61.Hervieu GJ, Cluderay JE, Harrison D, Meakin J, Maycox P, Nasir S, Leslie RA. The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, slc-1, in the central nervous system of the rat. Eur J Neurosci. 2000;12:1194–1216. doi: 10.1046/j.1460-9568.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- 62.Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology. 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- 63.Hikida T, Kitabatake Y, Pastan I, Nakanishi S. Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proc Natl Acad Sci USA. 2003;100:6169–6173. doi: 10.1073/pnas.0631749100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hjelmstad GO. Dopamine excites nucleus accumbens neurons through the differential modulation of glutamate and GABA release. J Neurosci. 2004;24:8621–8628. doi: 10.1523/JNEUROSCI.3280-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hjelmstad GO, Fields HL. Kappa opioid receptor inhibition of glutamatergic transmission in the nucleus accumbens shell. J Neurophysiol. 2001;85:1153–1158. doi: 10.1152/jn.2001.85.3.1153. [DOI] [PubMed] [Google Scholar]
- 66.Hong JS, Gillin JC, Yang HY, Costa E. Repeated electroconvulsive shocks and the brain content of endorphins. Brain Res. 1979;177:273–278. doi: 10.1016/0006-8993(79)90778-9. [DOI] [PubMed] [Google Scholar]
- 67.Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW. The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther. 1999;289:412–416. [PubMed] [Google Scholar]
- 69.Ichikawa J, Kuroki T, Meltzer HY. Differential effects of chronic imipramine and fluoxetine on basal and amphetamine-induced extracellular dopamine levels in rat nucleus accumbens. Eur J Pharmacol. 1998;350:159–164. doi: 10.1016/s0014-2999(98)00247-7. [DOI] [PubMed] [Google Scholar]
- 70.Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nature Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- 71.Jaworski JN, Kozel MA, Philpot KB, Kuhar MJ. Intra-accumbal injection of CART (cocaine-amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. J Pharmacol Exp Ther. 2003;307:1038–1044. doi: 10.1124/jpet.103.052332. [DOI] [PubMed] [Google Scholar]
- 72.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 73.Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- 74.Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 75.Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- 76.Kelley AE, Smith-Roe SL, Holahan MR. Responsereinforcement learning is dependent on N-methyl-D-aspartate receptor activation in the nucleus accumbens core. Proc Natl Acad Sci USA. 1997;94:12174–12179. doi: 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kenny PJ, Gasparini F, Markou A. Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther. 2003;306:1068–1076. doi: 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- 78.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 79.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 80.Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–132. [PubMed] [Google Scholar]
- 81.Kram ML, Kramer GL, Ronan PJ, Steciuk M, Petty F. Dopamine receptors and learned helplessness in the rat: an autoradiographic study. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:639–645. doi: 10.1016/s0278-5846(01)00222-6. [DOI] [PubMed] [Google Scholar]
- 82.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 83.Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, Epperson CN, Goddard A, Mason GF. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7:S71–S80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- 84.Kurumaji A, Mitsushio H, Takashima M. Chronic dietary treatment with antidepressants decrease brain Met-enkephalin-like immunoreactivity in the rat. Psychopharmacology. 1988;94:188–192. doi: 10.1007/BF00176843. [DOI] [PubMed] [Google Scholar]
- 85.Kyte ZA, Goodyer IM, Sahakian BJ. Selected executive skills in adolescents with recent first episode major depression. J Child Psychol Pschiat. 2005;46:995–1005. doi: 10.1111/j.1469-7610.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- 86.Lammers CH, Diaz J, Schwartz JC, Sokoloff P. Selective increase of dopamine D3 receptor gene expression as a common effect of chronic antidepressant treatments. Mol Psychiatry. 2000;5:378–388. doi: 10.1038/sj.mp.4000754. [DOI] [PubMed] [Google Scholar]
- 87.Levine J, Panchalingam K, Rapoport A, Gershon S, McClure RJ, Pettegrew JW. Increased cerebrospinal fluid glutamine levels in depressed patients. Biol Psychiatry. 2000;47:586–593. doi: 10.1016/s0006-3223(99)00284-x. [DOI] [PubMed] [Google Scholar]
- 88.Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P. Antidepressant-like actions of an AMPA receptor potentiator ( LY392098) Neuropharmacology. 2001;40:1028–1033. doi: 10.1016/s0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- 89.Maes M, Verkert R, Vandoolaeghe E, Lin A, Scharpe S. Serum levels of excitatory amino acids, serine, glycine, histidine, threonine, taurine, alanine and arginine in treatment-resistant depression: modulation by treatment with antidepressants and prediction of clinical responsivity. Acta Psychiat Scand. 1998;97:302–308. doi: 10.1111/j.1600-0447.1998.tb10004.x. [DOI] [PubMed] [Google Scholar]
- 90.Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- 91.Maisonneuve IM, Archer S, Glick SD. U50,488, a kappa opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett. 1994;181:57–60. doi: 10.1016/0304-3940(94)90559-2. [DOI] [PubMed] [Google Scholar]
- 92.Maldonado-Irizarry CS, Kelley AE. Excitatory amino acid receptors within nucleus accumbens subregions differentially mediate spatial learning in the rat. Behav Pharmacol. 1995;6:527–539. [PubMed] [Google Scholar]
- 93.Mangiavacchi S, Masi F, Scheggi S, Leggio B, De Montis MG, Gambarana B. Long-term behavioral and neurochemical effects of chronic stress exposure in rats. J Neurochem. 2001;79:1113–1121. doi: 10.1046/j.1471-4159.2001.00665.x. [DOI] [PubMed] [Google Scholar]
- 94.Mann JJ, McBride PA, Malone KM, DeMeo MD, Kelip JG. Blunted serotonergic responsivity in depressed patients. Neuropsychopharmacology. 1995;13:53–64. doi: 10.1016/0893-133X(95)00016-7. [DOI] [PubMed] [Google Scholar]
- 95.Marcus MM, Mathe JM, Nomikos GG Svensson TH. Effects of competitive and non-competitive NMDA receptor antagosnists on dopamine output in the shell and core subdivisions of the nucleus accumbens. Neuropharmacology. 2001;40:482–490. doi: 10.1016/s0028-3908(00)00199-4. [DOI] [PubMed] [Google Scholar]
- 96.Mark GP Weinberg, JB Rada, PV Hoebel BG. Extracellular acetylcholine is increased in the nucleus accumbens following the presentation of an aversively conditioned taste stimulus. Brain Res. 1995;688:184–188. doi: 10.1016/0006-8993(95)00401-b. [DOI] [PubMed] [Google Scholar]
- 97.Matsumoto RR. GABA receptors: are cellular differences reflected in function? Brain Res Brain Res Rev. 1989;14:203–225. doi: 10.1016/0165-0173(89)90001-5. [DOI] [PubMed] [Google Scholar]
- 98.Mauri MC, Ferrara A, Boscati L, Bravin S, Zamberlan F, Alecci M, Invernizzi G. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology. 1998;37:124–129. doi: 10.1159/000026491. [DOI] [PubMed] [Google Scholar]
- 99.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nature Rev. Mol. Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 100.McEwen BS, Angulo J, Gould E, Mendelson S, Watanabe Y. Antidepressant modulation of isolation and restraint stress effects on brain chemistry and morphology. Eur Psychiatry. 1993;8:(Suppl 2), 41S–48S. [Google Scholar]
- 101.McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meredith GE, Pennartz CM, Groenewegen HJ. The cellular framework for chemical signalling in the nucleus accumbens. Prog Brain Res. 1993;99:3–24. doi: 10.1016/s0079-6123(08)61335-7. [DOI] [PubMed] [Google Scholar]
- 103.Mifsud JC, Hernandez L, Hoebel BG. Nicotine infused into the nucleus accumbens increases synaptic dopamine as measured by in vivo microdialysis. Brain Res. 1989;478:365–367. doi: 10.1016/0006-8993(89)91518-7. [DOI] [PubMed] [Google Scholar]
- 104.Millan MJ, Brocco M, Papp M, Serres F, La Rochelle CD, Sharp T, Peglion JL, Dekeyne A. S32504, a novel naphtoxazine agonist at dopamine D3/D2 receptors: III. Actions in models of potential antidepressive and anxiolytic activity in comparison with ropinirole. J Pharmacol Exp Ther. 2004;309:936–950. doi: 10.1124/jpet.103.062463. [DOI] [PubMed] [Google Scholar]
- 105.Molina-Hernandez M, Tellez-Alcantara NP, Garcia JP, Lopez JIO, Jaramillo MT. Antidepressant-like actions of intra-accumbens infusions of allopregnanolone in ovariectomized Wistar rats. Pharmcol Biochem Behav. 2005;80:401–409. doi: 10.1016/j.pbb.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 106.Moreau JL, Scherschlicht R, Jenck F, Martin JR. Chronic mild stress-induced anhedonia model of depression; sleep abnormalities and curative effects of electroshock treatment. Behav Pharmacol. 1995;6:682–687. [PubMed] [Google Scholar]
- 107.Morishima Y, Miyakawa T, Furuyashiki T, Tanaka Y, Mizuma H, Nakanishi S. Enhanced cocaine responsiveness and impaired motor coordination in metabotropic glutamate receptor subtype 2 knockout mice. Proc Natl Acad Sci USA. 2005;102:4170–4175. doi: 10.1073/pnas.0500914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakahara D, Ozaki N, Miura Y, Miura H, Nagatsu T. Increased dopamine and serotonin metabolism in rat nucleus accumbens produced by intracranial self-stimulation of medial forebrain bundle as measured by in vivo microdialysis. Brain Res. 1989;495:178–181. doi: 10.1016/0006-8993(89)91234-1. [DOI] [PubMed] [Google Scholar]
- 109.Narita M, Aoki K, Takagi M, Yajima Y, Suzuki T. Implication of brain-derived neurotrophic factor in the release of dopamine and dopamine-related behaviors induced by methamphetamine. Neuroscience. 2003;119:767–775. doi: 10.1016/s0306-4522(03)00099-x. [DOI] [PubMed] [Google Scholar]
- 110.Nestler EJ, Barrot M, DiLeone RJ, Eisch A J, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 111.Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, Chen J, Neve R, Nestler EJ, Duman RS. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- 114.Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Cue-evoked firing of nucleus accumbens neurons encodes motivational significance during a discriminative stimulus task. J Neurophysiol. 2004;91:1840–1865. doi: 10.1152/jn.00657.2003. [DOI] [PubMed] [Google Scholar]
- 115.Overstreet DH. Selective breeding for increased cholinergic function: development of a new animal model of depression. Biol Psychiatry. 1986;21:49–58. doi: 10.1016/0006-3223(86)90007-7. [DOI] [PubMed] [Google Scholar]
- 116.Overstreet DH, Pucilowski O, Rezvani AH, Janowsky DS. Administration of antidepressants, diazepam and psychomotor stimulants further confirms the utility of Flinders Sensitive Line rats as an animal model of depression. Psychopharmacology. 1995;121:27–37. doi: 10.1007/BF02245589. [DOI] [PubMed] [Google Scholar]
- 117.Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Mondal AC, Shukla PK, Conley RR. Brain region specific alterations in the protein and mRNA levels of protein kinase A subunits in the post-mortem brain of teenage suicide victims. Neuropsychopharmacology. 2005;30:1548–1556. doi: 10.1038/sj.npp.1300765. [DOI] [PubMed] [Google Scholar]
- 118.Papp M, Klimek V, Willner P. Parallel changes in dopamine D2 receptor binding in limbic forebrain associated with chronic mild stress-induced anhedonia and its reversal by imipramine. Psychopharmacology. 1994;115:441–446. doi: 10.1007/BF02245566. [DOI] [PubMed] [Google Scholar]
- 119.Papp M, Moryl E. Antidepressant activity of non-competitive and competitive NMDA receptor antagonists in a chronic mild stress model of depression. Eur J Pharmacol. 1994;263:1–7. doi: 10.1016/0014-2999(94)90516-9. [DOI] [PubMed] [Google Scholar]
- 120.Parsons LH, Justice JB Jr. Perfusate serotonin increases extracellular dopamine in the nucleus accumbens as measured by in vivo microdialysis. Brain Res. 1993;606:195–199. doi: 10.1016/0006-8993(93)90984-u. [DOI] [PubMed] [Google Scholar]
- 121.Paul IA, and Skolnick P. Glutamate and depression. Ann NY Acad. Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- 122.Paul RH, Brickman AM, Navia B, Hinkin C, Malloy PF, Jefferson AL, Cohen RA, Tate DF, Flanigan TP. Apathy is associated with volume of the nucleus accumbens in patients infected with HIV. J Neuropsychiatry Clin. Neurosci. 2005;17:167–171. doi: 10.1176/appi.neuropsych.17.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology. 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pennartz CM, Dolleman-Van der Weel MJ, Kitai ST, Lopes da Silva FH. Presynaptic dopamine D1 receptors attenuate excitatory and inhibitory limbic inputs to the shell region of the rat nucleus accumbens studied in vitro. J Neurophysiol. 1992;67:1325–1334. doi: 10.1152/jn.1992.67.5.1325. [DOI] [PubMed] [Google Scholar]
- 125.Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 126.Pennartz CM, Kitai ST. Hippocampal inputs to identified neurons in an in vitro slice preparation of the rat nucleus accumbens: evidence for feed-forward inhibition. J Neurosci. 1991;11:2838–2847. doi: 10.1523/JNEUROSCI.11-09-02838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Petty F. GABA and mood disorders: a brief review and hypothesis. J Affect Disord. 1995;34:275–281. doi: 10.1016/0165-0327(95)00025-i. [DOI] [PubMed] [Google Scholar]
- 128.Petty F, Kramer GL, Gullion CM, Rush AJ. Low plasma √-aminobutyric acid levels in male patients with depression. Biol Psychiatry. 1992;32:354–363. doi: 10.1016/0006-3223(92)90039-3. [DOI] [PubMed] [Google Scholar]
- 129.Petty F, Sherman AD. GABAergic modulation of learned helplessness. Pharmacol Biochem Behav. 1981;15:567–570. doi: 10.1016/0091-3057(81)90210-0. [DOI] [PubMed] [Google Scholar]
- 130.Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA. Jr. Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Popik P, Kozela E, Krawczyk M. Nicotine and nicotinic receptor antagonists potentiate the antidepressant-like effects of imipramine and citalopram. Br J Pharmacol. 2003;139:1196–1202. doi: 10.1038/sj.bjp.0705359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rada PV, Mark GP, Hoebel BG. In vivo modulation of acetylcholine in the nucleus accumbens of freely moving rats: II. Inhibition by gamma-aminobutyric acid. Brain Res. 1993;619:105–110. doi: 10.1016/0006-8993(93)91601-n. [DOI] [PubMed] [Google Scholar]
- 134.Rada P, Moreno SA, Tucci S, Gonzalez LE, Harrison T, Chau DT, Hoebel BG, Hernandez L. Glutamate release in the nucleus accumbens is involved in behavioral depression during the porsolt swim test. Neuroscience. 2003;119:557–565. doi: 10.1016/s0306-4522(03)00162-3. [DOI] [PubMed] [Google Scholar]
- 135.Rada P, Pothos E, Mark GP, Hoebel BG. Microdialysis evidence that acetylcholine in the nucleus accumbens is involved in morphine withdrawal and its treatment with clonidine. Brain Res. 1991;561:354–356. doi: 10.1016/0006-8993(91)91616-9. [DOI] [PubMed] [Google Scholar]
- 136.Rahman S, McBride WJ. Involvement of GABA and cholinergic receptors in the nucleus accumbens on feedback control of somatodendritic dopamine release in the ventral tegmental area. J Neurochem. 2002;80:646–654. doi: 10.1046/j.0022-3042.2001.00739.x. [DOI] [PubMed] [Google Scholar]
- 137.Romeo E, Strohle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- 138.Rossetti ZL, Lai M, Hmaidan Y Gessa GL. Depletion of mesolimbic dopamine during behavioral despair: partial reversal by chronic imipramine. Eur J Pharmacol. 1993;242:313–315. doi: 10.1016/0014-2999(93)90257-i. [DOI] [PubMed] [Google Scholar]
- 139.Roullet P, Sargolini F, Oliverio A, Mele A. NMDA and AMPA antagonist infusions into the ventral striatum impair differential steps of spatial information processing in a nonassociative task in mice. J Neurosci. 2001;21:2143–2149. doi: 10.1523/JNEUROSCI.21-06-02143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Saito Y Cheng M, Leslie FM Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001;435:26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- 141.Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmacol Sci. 2004;95:374–380. doi: 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- 142.Sanacora G, Rothman DL, Mason G, Krystal JH. Clinical studies implementing glutamate neurotransmission in mood disorders. Ann NY Acad Sci. 2003;1003:292–308. doi: 10.1196/annals.1300.018. [DOI] [PubMed] [Google Scholar]
- 143.Scheggi S, Leggio B, Masi F, Grappi S, Gambarana C, Nanni G, Rauggi R, De Montis MG. Selective modifications in the nucleus accumbens of dopamine synaptic transmission in rats exposed to chronic stress. J Neurochem. 2002;83:895–903. doi: 10.1046/j.1471-4159.2002.01193.x. [DOI] [PubMed] [Google Scholar]
- 144.Semba J, Mataki C, Yamada S, Nankai M, Toru M. Antidepressantlike effects of chronic nicotine on learned helplessness paradigm in rats. Biol Psychiatry. 1998;43:389–391. doi: 10.1016/s0006-3223(97)00477-0. [DOI] [PubMed] [Google Scholar]
- 145.Shippenberg TS, Bals-Kubik R, Herz A. Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs D-2 dopamine receptors. J Pharmacol Exp Ther. 1993;265:53–59. [PubMed] [Google Scholar]
- 146.Shirayama Y, Chen A C, Nakagawa S, Russell D S, Duman R S. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shirayama Y, Ishida H, Iwata M, Hazama G, Kawahara R, Duman R S. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- 148.Shytle RD, Silver AA, Lukas RJ, Newman MB, Sheehan DV, Sanberg PR. Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry. 2002;7:525–535. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- 149.Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu K, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, Iyo M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients without or with antidepressants. Biol Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 150.Skolnick P. Antidepressants for the new millenium. Eur J Pharmacol. 1999;375:31–40. doi: 10.1016/s0014-2999(99)00330-1. [DOI] [PubMed] [Google Scholar]
- 151.Skoublis PD, Lam HA, Shoblock J, Narayanant S, Maidment NT. Endogenous enkephalins, not endorphins, modulate basal hedonic state in mice. Eur J Neurosci. 2005;21:1379–1384. doi: 10.1111/j.1460-9568.2005.03956.x. [DOI] [PubMed] [Google Scholar]
- 152.Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharm Exp Ther. 2000;295:321–327. [PubMed] [Google Scholar]
- 153.Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smith Y, Kieval J, Couceyro PR, Kuhar MJ. CART peptide-immunoreactive neurones in the nucleus accumbens in monkeys: ultrastructural analysis, colocalization studies, and synaptic interactions with dopaminergic afferents. J Comp Neurol. 1999;407:491–511. doi: 10.1002/(sici)1096-9861(19990517)407:4<491::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 155.Smith DG, Tzavara ET, Shaw J, Luecke S, Wade M, Davis R, Salhoff C, Nomikos GG, Gehlert DR. Mesolimbic dopamine super-sensitivity in melanin-concentrating hormone-1 receptor-deficient mice. J Neurosci. 2005;25:914–922. doi: 10.1523/JNEUROSCI.4079-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Smith-Roe SL, Sadeghian K, Kelley AE. Spatial learning and performance in the radial maze is impaired after N-metyl-D-aspartate (NMDA) receptor blockade in striatal subregions. Behav Neurosci. 1999;113:703–717. doi: 10.1037//0735-7044.113.4.703. [DOI] [PubMed] [Google Scholar]
- 157.Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci. 2000;20:7737–7742. doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an In vivo microdialysis study. J Neurochem. 1990;55:1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- 159.Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Spooren WP, Gasparini F, Salt TE, Kuhn R. Novel allosteric antagonists shed light on mglu(5) receptors and CNS disorders. Trends Pharmacol. Sci. 2001;22:331–337. doi: 10.1016/s0165-6147(00)01694-1. [DOI] [PubMed] [Google Scholar]
- 161.Svingos AL, Colago EE, Pickel VM. Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. J Neurosci. 1999;19:1804–1813. doi: 10.1523/JNEUROSCI.19-05-01804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tejedor-Real P, Mico JA, Smadja C, Maldonado R, Roques BP, Gilbert-Rahola J. Involvement of delta-opioid receptors in the effects induced by endogenous enkephalins on learned helplessness model. Eur J Pharmacol. 1998;354:1–7. doi: 10.1016/s0014-2999(98)00423-3. [DOI] [PubMed] [Google Scholar]
- 163.Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an In vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- 164.Todtenkopf MS, De Leon KR Stellar JR. Repeated cocaine treatment alters tyrosine hydroxylase in the rat nucleus accumbens. Brain Res Bull. 2000;52:407–411. doi: 10.1016/s0361-9230(00)00277-x. [DOI] [PubMed] [Google Scholar]
- 165.Tran AH, Tamura R, Uwano T, Kobayashi T, Katsuki M, Ono T. Dopamine D1 receptors involved in locomotor activity and accumbens neural responses to prediction of reward associated with place. Proc Natl Acad Sci USA. 2005;102:2117–2122. doi: 10.1073/pnas.0409726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Axad Sci USA. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Van Bockstaele EJ, Chan J, Pickel VM. Pre- and postsynaptic sites for serotonin modulation of GABA-containing neurons in the shell region of the rat nucleus accumbens. J Comp Neurol. 1996;371:116–128. doi: 10.1002/(SICI)1096-9861(19960715)371:1<116::AID-CNE7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 168.Van Bockstaele EJ, Pickel VM. Ultrastructure of serotonin-immunoreactive terminals in the core and shell of the rat nucleus accumbens: cellular substrates for interactions with catecholamine afferents. J Comp Neurol. 1993;334:603–617. doi: 10.1002/cne.903340408. [DOI] [PubMed] [Google Scholar]
- 169.Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self- administration. Brain Res. 1992;593:314–318. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- 170.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 171.Willner P, Lappas S, Cheeta S, Muscat R. Reversal of stress-induced anhedonia by the dopamine receptor agonist, pramipexole. Psychopharmacology. 1994;115:454–462. doi: 10.1007/BF02245568. [DOI] [PubMed] [Google Scholar]
- 172.Xi Z-X, Ramamoorthy S, Shen H, Lake R, Samuvel DJ, Kalivas PW. GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. J Neurosci. 2003;23:3498–3505. doi: 10.1523/JNEUROSCI.23-08-03498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yadid G, Overstreet DH, Zangen A. Limbic dopaminergic adaptation to a stressful stimulus in a rat model of depression. Brain Res. 2001;896:43–47. doi: 10.1016/s0006-8993(00)03248-0. [DOI] [PubMed] [Google Scholar]
- 174.Yan QS. Activation of 5-HT2A/2C receptors within the nucleus accumbens increases local dopaminergic transmission. Brain Res Bull. 2000;51:75–81. doi: 10.1016/s0361-9230(99)00208-7. [DOI] [PubMed] [Google Scholar]
- 175.Yang SC, Shieh KR. Differential effects of melanin concentrating hormone on the central dopaminergic neurons induced by the cocaine- and amphetamine-regulated transcript peptide. J Neurochem. 2005;92:637–646. doi: 10.1111/j.1471-4159.2004.02896.x. [DOI] [PubMed] [Google Scholar]
- 176.Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci. 2004;24:2923–2933. doi: 10.1523/JNEUROSCI.5282-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zangen A, Hyodo K. Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. Neuroreport. 2002;13:2401–2405. doi: 10.1097/00001756-200212200-00005. [DOI] [PubMed] [Google Scholar]
- 178.Zangen A, Nakash R, Overstreet DH, Yadid G. Association between depressive behavior and absence of serotonin-dopamine interaction in the nucleus accumbens. Psychopharmacology. 2001;155:434–439. doi: 10.1007/s002130100746. [DOI] [PubMed] [Google Scholar]
- 179.Zangen A, Nakash R, Yadid G. Serotonin-mediated increases in the extracellular levels of beta-endorphin in the arcuate nucleus and nucleus accumbens: a microdialysis study. J Neurochem. 1999;73:2569–2574. doi: 10.1046/j.1471-4159.1999.0732569.x. [DOI] [PubMed] [Google Scholar]
- 180.Zangen A, Overstreet DH, Yadid G. High serotonin and 5-hydroxyindoleacetic acid levels in limbic brain regions in a rat model of depression: normalization by chronic antidepressant treatment. J Neurochem. 1997;69:2477–2483. doi: 10.1046/j.1471-4159.1997.69062477.x. [DOI] [PubMed] [Google Scholar]
- 181.Zung WW. Review of placebo-controlled trials with bupropion. J Clin Psychiatry. 1983;44:104–114. [PubMed] [Google Scholar]