Abstract
Biogenic amines are important messenger substances in the central nervous system and in peripheral organs of vertebrates and of invertebrates. The honeybee, Apis mellifera, is excellently suited to uncover the functions of biogenic amines in behaviour, because it has an extensive behavioural repertoire, with a number of biogenic amine receptors characterised in this insect.
In the honeybee, the biogenic amines dopamine, octopamine, serotonin and tyramine modulate neuronal functions in various ways. Dopamine and serotonin are present in high concentrations in the bee brain, whereas octopamine and tyramine are less abundant. Octopamine is a key molecule for the control of honeybee behaviour. It generally has an arousing effect and leads to higher sensitivity for sensory inputs, better learning performance and increased foraging behaviour. Tyramine has been suggested to act antagonistically to octopamine, but only few experimental data are available for this amine. Dopamine and serotonin often have antagonistic or inhibitory effects as compared to octopamine.
Biogenic amines bind to membrane receptors that primarily belong to the large gene-family of GTP-binding (G) protein coupled receptors. Receptor activation leads to transient changes in concentrations of intracellular second messengers such as cAMP, IP3 and/or Ca2+. Although several biogenic amine receptors from the honeybee have been cloned and characterised more recently, many genes still remain to be identified. The availability of the completely sequenced genome of Apis mellifera will contribute substantially to closing this gap.
In this review, we will discuss the present knowledge on how biogenic amines and their receptor-mediated cellular responses modulate different behaviours of honeybees including learning processes and division of labour.
Key Words: Serotonin, dopamine, octopamine, tyramine, honeybee, behaviour, division of labour, amine receptors
1. INTRODUCTION
Biogenic amines play a pivotal role in the control and modulation of complex behaviours both in vertebrates and invertebrates. The group of biogenic amines consists of seven main members. Whereas dopamine (DA), serotonin (5-hydroxytryptamine, 5-HT), and histamine (HA) are present both in the vertebrate and invertebrate nervous systems, two pairs of biogenic amines are rather specific to either vertebrates or invertebrates. The catecholamines norepinephrine and epinephrine are important regulators of vertebrate physiology. In invertebrates, however, these amines appear to be substituted by the phenolamines tyramine (TA) and octopamine (OA), which control and modulate similar physiological functions [10,11,40,44,45,106,140].
DA and 5-HT are present in high amounts in the vertebrate central nervous system (CNS). A failure of the dopaminergic system can lead to motor or movement disorders, addiction, paranoia, and schizophrenia [91,162] (for reviews see [166,169]). Serotonin modulates emotion, mood, sleep and appetite. In humans, 5-HT has been implicated in the aetiology of neurological diseases including depression, anxiety and schizophrenia. In addition, 5-HT has an impact on migraine, hypertension, pulmonary hypertension, eating disorders and vomiting (for reviews see [72,75]). HA is considered an important mediator of allergy and inflammation in vertebrates [76,160]. In the vertebrate CNS, HA is synthesised from a small population of neurons located in the posterior hypothalamus. These neurons project to most cerebral areas and have been implicated in cardiovascular control, hormonal secretion, thermoregulation and memory functions [160].
When it comes to studying the function of aminergic systems, invertebrates have a number of advantages over vertebrates. Obviously, invertebrate nervous systems are less complex than those of vertebrates. In addition, the biogenic amines seem to have similar functional properties in vertebrates and invertebrates. Among the invertebrate species, honeybees (Apis mellifera) in particular are ideal subjects to experimentally examine the functional role of biogenic amines. These insects possess a remarkably rich behavioural repertoire. Bees, for example, learn sensory cues very fast and reliably. In addition, they display a complex division of labour (for reviews see [57,62,99]).
In the honeybee and in other invertebrate species, biogenic amines act as neurotransmitters, neuromodulators or neurohormones (for reviews see [10,37,40,135,136]). In this review, we summarise the present knowledge on how DA, TA, OA and 5-HT contribute to neuronal functions and behaviour of bees. Insights from molecular cloning approaches of biogenic-amine receptors will be provided. The role of HA in honeybees will not be discussed in detail, because only limited information is available on this amine. Generally, HA has been established as the neurotransmitter that is released from insect photoreceptor cells in response to illumination (for reviews see: [118,119,172]). Once released, it activates chloride currents in postsynaptic monopolar cells [66,67,164]. These HA-gated chloride channels have been cloned from Drosophila melanogaster [55,188]. Apart from photoreceptors, HA modulates mechanosensory transduction in Drosophila [97]. Evidence for HA acting as an inhibitory transmitter in the honeybee antennal lobe was provided by a Ca2+ imaging study [142]. The spontaneous activity and the odour responses of antennal lobe projection neurons were strongly reduced during bath application of HA [142].
We will start this overview with a brief introduction to the bee-brain anatomy, before we discuss the amines, their receptors and their behavioural functions in the honeybee.
2. THE HONEYBEE BRAIN
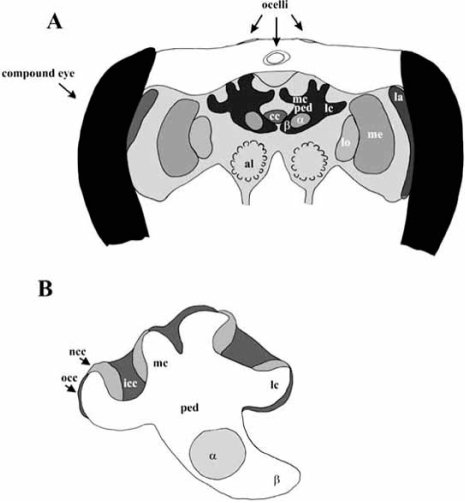
The brain of the honeybee consists of three major regions: protocerebrum, deutocerebrum and tritocerebrum. The protocerebrum contains the optic lobes (lamina, medulla and lobula), a pair of mushroom bodies (MBs) and the central complex (Fig. (1A)). The optic lobes receive and process sensory information from the compound eyes. Each MB consists of a lateral and a median calyx, which are connected to the α- and β-lobes via the pedunculus (Fig. (1B)). The MBs comprise a large number of densely packed intrinsic neurons, called Kenyon cells (after Kenyon, [80]), whose dendritic extensions form the cup-shaped calyces of the MBs. Three subpopulations of Kenyon cells have been distinguished based on their highly distinctive morphological profiles: outer compact cells, which are later in development pushed outward by non-compact cells, and inner compact cells, which, in turn, push the non-compact cells outward and which reside at the centre of each calycal cup (Fig. (1B), [49]]). The calyces are the main input regions of the MBs for visual, olfactory and mechanosensory information, whereas the α- and β-lobes are the output regions [32,100,109,141, 151,157]. Insect MBs are generally believed to be higher-order sensory integration centres necessary for insect learning and memory [38,62,69,98,100,170]. The central complex comprises a group of neuropils in the centre of the insect brain. It consists of the protocerebral bridge and the central body. The latter is subdivided into an upper division, a lower division and a pair of noduli. The central complex connects both brain hemispheres, coordinates information from both brain sides, and is presumably involved in motor control [70,171].
Fig. (1).
A. Schematic drawing of major compartments of the honeybee brain. The protocerebrum contains the optic lobes, the lamina (la), medulla (me) and lobula (lo), a pair of mushroom bodies (mb) and the central complex (cc). Each mushroom body consists of a lateral calyx (lc) and a median calyx (mc), which are connected via the pedunculus (ped) to the α-lobe (α) and the β-lobe (β). The antennal lobe (al) is part of the deutocerebrum, which also contains the dorsal lobe (not shown). The tritocerebrum (not shown) is composed of two small bilateral lobes at the base of the brain.
B. Schematic drawing of a mushroom body with the three subpopulations of Kenyon cells. The outer compact cells (occ) are born first and later pushed outward by the non-compact cells (ncc). These cells are later pushed outward by the inner compact cells (icc), which reside at the centre of each calycal cup (after [47]).
The deutocerebrum harbours the antennal lobes (ALs) and the dorsal lobes [165]. The ALs contain approximately 160 glomeruli and receive sensory inputs from olfactory receptor cells on the antennae, whereas antennal mechanosensory fibres mainly project to the dorsal lobes [174].
The tritocerebrum is composed of two relatively small bilateral lobes at the base of the brain. These are adjacent to the subesophageal ganglion (SOG). The compartments of the tritocerebrum are known to have sensory and motor connections to the mouth and the digestive tract. The SOG is a relay station between the brain and the ventral nerve cord. It processes sensory inputs from the proboscis and from the brain and projects to the motor neurons of the proboscis and mandibles [110]. Important modulatory neurons, such as ventral unpaired median neurons (VUM neurons), have their somata in the SOG [86].
3. DISTRIBUTION OF BIOGENIC AMINES IN THE BRAIN OF ADULT HONEYBEES
Immunocytochemical studies have shown that biogenic amines are only synthesised by a relatively small number of interneurons in the insect brain [70]. Antisera raised against DA, OA and 5-HT specifically stain these interneurons, which often possess widespread projections. When biogenic amines are released from these neurons, they modulate the activity of target neurons in various parts of the brain [8,60,70]. Biochemical and pharmacological studies [53,68, 106,143,152,75,181] have shown that DA and 5-HT are present in high concentrations in the bee brain (DA: 1240 pmol/brain; 5-HT: 6-21 pmol/brain), whereas the amount of OA is lower (2-10 pmol/brain). The amount of TA was recently determined in bees [144]. It varied between 0.3 and 2 pmol/brain and is considerably lower than that of OA. Only trace amounts of norepinephrine have been detected in the bee brain [53,106].
3.1. Dopamine
Approximately 330 DA-immunoreactive somata have been identified in each brain hemisphere and the SOG [147,159]. Most of these somata are clustered. One cluster is located below the lateral calyx of the MB. Two clusters are located in the anterior-ventral protocerebrum. Additional DA-immunoreactive somata are organised in small groups around the protocerebral bridge, below the optic tubercles, proximal to the ventral margin of the lobula, and in the lateral and ventral somatal rind of the SOG.
The MBs and the central body are surrounded by a dense network of DA-immunoreactive fibres. The processes project into the MB neuropil and into the somatal rind, where they synapse onto Kenyon cell bodies [17]. In the deutocerebrum, the ALs are innervated by fine projections of DA-immunoreactive interneurons [147,159]. With this innervation pattern and because single DA-immunoreactive neurons can have wide projection fields, it is assumed that DA participates in modulatory processes rather than in local neuronal interactions [104]].
3.2. Octopamine and Tyramine
The distribution of OA-immunoreactivity has been investigated in great detail [7,86]. Five cell clusters containing more than 100 OA-immunoreactive somata have been identified in the honeybee brain. These comprise a number of neurosecretory cells, a cell cluster located mediodorsal to the AL, a group of cells distributed on both sides of the protocerebral midline, another group between the lateral protocerebral lobes and the DLs, and single somata on either side of the central body [7,86]. The distribution pattern of OA in the bee brain is particularly interesting, because an identified octopaminergic neuron (VUMmx1, [60]) is important for olfactory conditioning (see below). The soma of this neuron is located in the SOG. VUMmx1 has an amazingly extensive axonal projection with ramifications in the ALs, the calycal region of the MBs, the lateral protocerebrum and the SOG.
Similar to dopaminergic fibres, OA-immunoreactive fibres innervate most neuropils of the brain [86,163]. The strongest labelling was observed in the central complex [36]. In the MBs, a striking compartmentalisation was described: the calyces are innervated by extrinsic varicose OA-immunoreactive fibres, whereas large parts of the α- and β-lobes do not show OA-immunoreactivity at all [7,86].
In contrast to OA, the distribution of TA in the honeybee brain has not been studied at the cellular level. However, because TA is the precursor of OA during biosynthesis (see below), TA is expected to be present at least in all OA-containing cells. In addition, TA-containing neurons were identified in the CNS of Drosophila larvae, using specific antibodies raised against p-TA [117]. Interestingly, some of these neurons were distinct from OA-containing neurons [117]. Further evidence for the existence of cells that specifically contain TA but not OA comes from the analysis of the enzymes which are required to synthesise TA or OA. In the first step, the amino-acid tyrosine is decarboxylated to TA by tyrosine decarboxylase (TDC; [92]). TA can be hydroxylated on the β-carbon of the side chain by tyramine β-hydroxylase (TβH), thus generating OA [112]. Recently, two TDC genes were cloned from Drosophila melanogaster, dTdc1 and dTdc2 [29]. The gene dTdc1 is expressed non-neuronally, whereas dTdc2 is expressed in neurons. Interestingly, several clusters of cells in the central brain hitherto not known to contain TβH were detected in a genetic screen with dTdc2-GAL4 lines. Recently, TA was shown to modulate transepithelial Cl− conductance in Drosophila Malpighian (renal) tubules [19]. In this non-neuronal tissue, dTdc1expression and TA, but not OA, was detected [19,29]. Since the anti–TA antibody is commercially available [19,117], one may expect that the distribution of tyraminergic cells will soon be described in the honeybee as well.
3.3. Serotonin
The honeybee brain contains approximately 75 serotonin (5-HT)-immunoreactive somata [158] (for a review see [7]). They reside in the optic lobes, in the median and in the dorsal protocerebrum [158]. Immunoreactive fibres are present in all brain regions and in the SOG. A stratified staining was observed in the optic lobes, with the highest intensity in the lobula [158]. A net of 5-HT-immunoreactive fibres innervates the MBs, the central body and the ALs. Only the MB calyces, the protocerebral bridge and a small region of the central body are devoid of 5-HT [158].
3.4. Histamine
The brain of the honeybee contains about 150 histaminergic neurons [21]. The axons of these neurons innervate most parts of the protocerebrum except the mushroom bodies [21]. Photoreceptor fibers terminating either in the lamina or in the medulla as well as axons emanating from ocellar photoreceptors also contain HA [21].
4. GENERAL STRUCTURAL AND FUNCTIONAL PROPERTIES OF AMINE RECEPTORS
Apart from HA-gated ion channels [54–56,188] which will not be covered in this review, all biogenic amine receptors characterised in invertebrates so far belong to the superfamily of G-protein coupled receptors (GPCRs, for reviews see [10,11,126,180]). GPCRs possess a conserved topology. Each polypeptide has an extracellular N-terminus followed by seven transmembrane (TM) segments and an intracellular C-terminus. The N-terminus often contains consensus motives for N-linked glycosylation [128,168]. Cysteine residues in the cytoplasmic tail of the proteins may become posttranslationally palmitoylated. The X-ray structure of bovine rhodopsin was recently solved, which strongly supports the general bauplan of GPCRs [124] (for a review see [52]). GPCR binding to biogenic amines occurs in a binding pocket formed by the TM bundle [74]. Specific residues in different TM segments interact with functional groups of the biogenic amines. In particular, an aspartic-acid residue (D) in TM3, serine residues (S) in TM5 and a phenylalanine residue (F) in TM6 contribute to ligand binding [168,178]. Ligand binding induces changes in the core structure of the receptor protein. Particularly TM3 is thought to participate in receptor activation by changing the relative orientation to TM6 [20]. In addition, a sequence motif at the cytoplasmic interface of TM3 ([D,E]R[Y,W]) is involved in receptor activation. Receptor desensitisation occurs when serine or threonine residues of the protein are phosphorylated and β-arrestins bind to the phosphorylated receptor [28,89,96,123].
Once activated, the receptors interact with heterotrimeric G-proteins [22,113]. The high specificity of the receptors for particular subtypes of G-proteins is decisive for controlling downstream effectors. It has been attempted to identify characteristic sequence motives in GPCRs to predict which G-proteins may couple to a particular receptor [11,22,71,111, 182]. This, however, is a complex task, because a single GPCR may couple to different G-proteins and may show agonist-dependent changes in coupling to G-proteins [129,130].
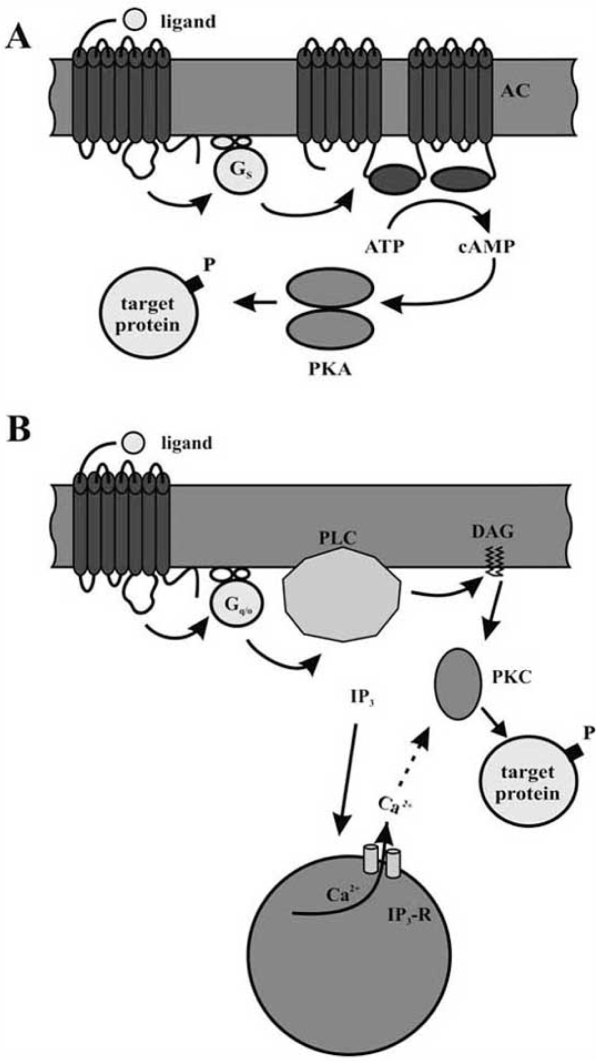
As a result of GPCR activation, the concentrations of intracellular second messengers, especially [cAMP]i or [Ca2+]i, are altered. When the activated GPCR binds to a Gstype protein, the Gs subunit stimulates adenylyl cyclase activity (Fig. (2A)). This leads to the production of cAMP from ATP. The rise in [cAMP]i activates cAMP-dependent protein kinase (protein kinase A, PKA). PKA phosphorylates serine and threonine residues of target proteins and thereby modifies the properties of various cytosolic or membrane-bound proteins. In contrast to adenylyl cyclase stimulation, several neuroactive substances are known to inhibit the activity of the enzyme. This effect is mediated by interaction of GPCRs with inhibitory G-proteins (Gi).
Fig. (2).
Signalling cascades following activation of a G-protein coupled receptor (GPCR). Biogenic amine receptors are activated after binding of the respective ligand. The activated GPCR in turn can activate a stimulatory G-protein (Gs) and thus initiate a cAMP signalling pathway (A) or it can activate a G-protein of the Gq/o family (Gq/o), which couples the amine receptor to IP3/DAG signalling pathways (B).
A. The activated G-protein stimulates an adenylyl cyclase (AC), which catalyses the production of cAMP from ATP. The increasing intracellular concentration of cAMP activates cAMP-dependent protein kinase (PKA). This kinase can phosphorylate a number of target proteins on serine and threonine residues.
B. Members of the Gq/o-protein-family activate phospholipase C (PLC). This enzyme hydrolyses phosphatidylinositol 4,5-bisphosphate into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 binds to specific receptors (IP3-R), which form ligand-gated ion channels in the membrane of the endoplasmatic reticulum. Opening of IP3-Rs causes an efflux of Ca2+ from the endoplasmic reticulum into the cytoplasm. DAG and the increased intracellular Ca2+ levels lead to activation of protein kinase C (PKC) which, like PKA, can phosphorylate different target proteins.
Another pathway activated by GPCRs leads to an increase in [Ca2+]i. Such receptors stimulate Gq/o proteins. Subsequently, phospholipase C (PLC) is activated (Fig. (2B)). PLC hydrolyses membrane-bound phosphatidylinositol 4,5bisphosphate to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). The IP3 freely diffuses and binds to IP3 receptors on the membrane of intracellular Ca2+ stores. These receptors are ligand-gated Ca2+ channels, which release Ca2+ after binding of IP3. In contrast to IP3, DAG remains associated with the membrane, where it activates protein kinase C (PKC). Full enzymatic activity of PKC, however, requires the presence of DAG and Ca2+. Similar to PKA, PKC phosphorylates a variety of proteins on serine and threonine residues, which alters the functional properties of these proteins.
In summary, GPCR activation by biogenic amines generates graded cellular responses depending on the second messenger pathways involved. The different second messenger pathways may also be activated in parallel within the same cell when the respective receptors and coupling partners are present. Such co-activation potentially leads to either amplification or diminishment of cellular responses to a given stimulus and provide a molecular basis for “coincidence detection” [90,187].
4.1. Dopamine Receptors in the Honeybee
In vertebrates, two classes of DA receptors have been defined based on sequence similarity, functional characteristics and pharmacological profiles: D1- and D2-(like) receptors (for reviews see [26,78,105,120]. D1 and D5 receptors constitute the D1 subfamily and activate adenylyl cyclase. Members of the D2 subfamily (i.e., D2, D3, and D4 receptors) either inhibit adenylyl cyclase and/or modulate Ca2+ and K+ channel activity.
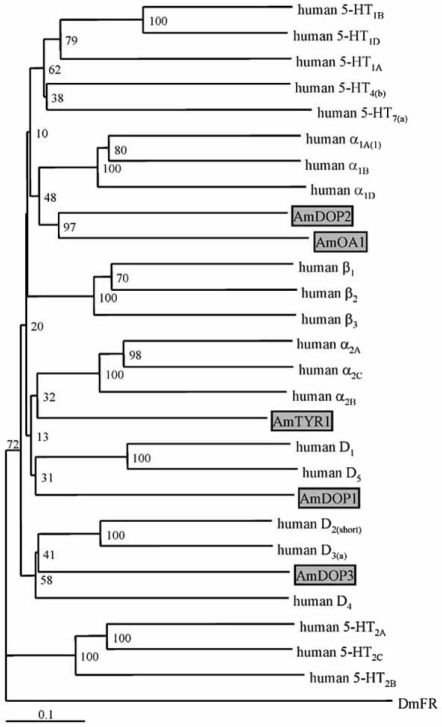
In the honeybee, three DA receptors have been characterised so far: AmDOP1 (Apis mellifera dopamine receptor 1, [14,115]), AmDOP2 [73,114] and AmDOP3 [4]. Sequence comparison showed that AmDOP1 is closely related to vertebrate D1 receptors (Fig. (3), [14,115]). Activation of AmDOP1 expressed in HEK 293 or insect Sf21 cells by DA or 6,7-ADTN leads to the production of cAMP. The benzazepines SCH 23390 and SKF 38393, which bind to mammalian D1 receptors with nanomolar affinity, are much less potent at the AmDOP1 receptor (Table (1), [14]). The AmDOP2 receptor and its Drosophila orthologue, DAMB [50,65], are more similar to mammalian α1-adrenergic- and insect OA-receptors than to DA receptors (Fig. (3), Table (2), [73,114, 115]). They thus form a distinct group of “invertebrate type” DA receptors (INDR) [73,115]. Functionally, however, AmDOP2 and DAMB resemble D1-type receptors, because they up regulate [cAMP]I [73,114].
Fig. (3).
Phylogenetic relationship between biogenic amine receptors of the honeybee and human aminergic receptors. Alignments were performed with the complete amino-acid sequence of each receptor. The receptor sequences, followed by their GenBank accession numbers (#), are listed below in the order illustrated: human 5-HT1B (#NP_000854), human 5-HT1D (#NP_000855), human 5-HT1A (#NP_000515), human 5-HT4 isoform b (#NP_000861), human 5-HT7 isoform a(#NP_000863),human α1A-adrenergic isoform 1 (#NP_000671), human α1B-adrenergic (#NP_000670), human α1D-adrenergic (#NP_000669), Apis mellifera dopamine 2 (AmDOP2; #NP_001011567), A. mellifera octopamine 1 (AmOA1; #NP_001011565), human β1-adrenergic (#NP_000675), human β2-adrenergic (#NP_000015), human β3-adrenergic (#NP_000016), human β2A-adrenergic (#NP_ 000672), human β2C-adrenergic (#NP_000674), human β2B-adrenergic (#NP_000673), A. mellifera tyramine 1 (AmTYR1; #NP_001011594), human D1 (#NP_000785), human D5 (#NP_000789), A. mellifera dopamine 1 (AmDOP1; #NP_001011595), human D2 isoform short (#NP_057658), human D3 isoform a (#NP_000787), A. mellifera D2likedopamine (AmDOP3;#NP_001014983),human D4 (#NP_000788), human 5-HT2A (#NP_000612), human 5-HT2C (#NP_000859), human 5-HT2B (#NP_000858), and Drosophila melanogaster FMRFamide (DmFR; CG2114-PA; #AAF47700). The genetic distance between sequences was calculated with ClustalX (version 1.81). A neighbor-joining tree was constructed with ClustalX by using 1000-fold boot-strap re-sampling. The resulting tree was displayed graphically by TreeView using the divergent D. melanogaster FMRFamide receptor as an outgroup. The numbers at the nodes of the branches represent the percentage bootstrap support for each branch. The scale bar allows conversion of branch lengths in the dendrogram to genetic distance between clades (0.1 = 10% genetic distance).
Table 1A.
Pharmacology of Dopamine Receptors in Apis mellifera: Binding to Brain Membrane Homogenate
| Specificity in vertebrates | Brain membrane homogenate: DA-sensitive [3H]LSD binding site (Ki [nM]) | Brain membrane homogenate: [3H]SCH 23390 binding site (Ki [nM]) | Brain membrane homogenate: [3H]spiperone binding site (Ki [nM]) | |
|---|---|---|---|---|
| Biogenic amines | ||||
| DA | 22 | 30,800 | 300 | |
| Norepinephrine | +/− | n.d. | n.d. | |
| 5-HT | 7,000 | 548,000 | 18,300 | |
| TA | +/− | α1,000,000 | 45 | |
| OA | +/− | 892,000 | 18 | |
| Dopamine receptor agonists | ||||
| 6,7-ADTN | DA receptor agonist | 78 | n.d | n.d. |
| R(+)-SKF 38393 | D1 agonist | − | 3,200 | n.d. |
| Dopamine receptor antagonists | ||||
| R(+)−Lisuride | D2 agonist, D1 antagonist | 4.7 | n.d. | n.d. |
| Chlorpromazine | D2 antagonist | 48 | 208 | n.d. |
| cis(Z)-Flupentixol | DA receptor antagonist | 150 | 218 | n.d. |
| Spiperone | selective D2 antagonist | +/− | 25,400 | 0.17 |
| R(+)− SCH 23390 | selective D1 antagonist | +/− | 9.5 | 456 |
| S(+)−Butaclamol | DA receptor antagonist | 89 | 13,800 | 55.5 |
| Haloperidol | D2, D3, and D4 antagonist | +/− | n.d. | n.d. |
Table 2.
Comparison of honeybee receptors with vertebrate receptors and characteristics of these honeybee receptors.
| Honeybee receptor (endogenous agonist) | Most closely related vertebrate receptor class | Effect of activation | Occurrence in the brain | References |
|---|---|---|---|---|
| AmDOP1 (DA) | DA D1/D5 receptors | cAMP↑ | ubiquitously | [12, 88, 114] |
| AmDOP2 (DA) | 1-adrenergic receptors | cAMP↑ | restricted to the mushroom bodies | [73, 88, 114] |
| AmDOP3 (DA) | DA D2 receptors | cAMP↓ | ubiquitously | [4] |
| AmTYR1 (TA) | α2-adrenergic receptors/5-HT1 receptors | cAMP↓ | ubiquitously | [13, 116] |
| AmOA1 (OA) | α1-adrenergic receptors | Ca2+↑ | ubiquitously | [48, 58] |
The expression patterns of Amdop1 and Amdop2 receptor genes were analysed by in situ hybridisation. Amdop1 mRNA was detected in many neurons of the adult worker honeybee brain, including mushroom body intrinsic neurons, neurons of the central complex, the ALs and dorsal lobes, and neurons of the optic lobes [14,88]. During pupal development, Amdop1 is highly expressed in newly born Kenyon cells of the MBs [88]. In workers and drones, Amdop2 mRNA is expressed in the inner and outer compact cells of the MBs (Fig. (1B)), whereas expression levels in non-compact cells were variable and increased with age [73]. During pupal development, Amdop2 is expressed in the compact cells [88]. Interestingly, the differential expression pattern of Amdop2 correlates with age-dependent changes in worker-bee behaviour [73].
Recently, a cDNA encoding a D2-like receptor (AmDOP3) was cloned (Fig. (3), [4,84,115]). Activation of AmDOP3 receptors results in the downregulation of [cAMP]i, a property characteristic of D2-like receptors [4]. In situ hybridisation revealed that Amdop3 mRNA is widely expressed in the brain [4]. The cellular expression patterns of the three honeybee DA receptors are overlapping but not completely identical. This suggests that D1 : D2 receptor interactions are possible in some neurons, including subpopulations of MB neurons [4].
Consistent with the widespread projections of dopaminergic neurons, in situ hybridisation experiments and radioligand-binding studies confirmed that DA receptors are widely distributed in the honeybee brain and that receptor densities vary with age [4,14,15,73,82–85,88]. More specifically, the density of tritiated benzazepine (e.g. [3H]SCH 23390) binding sites increases dramatically during the first two days of adult life [83]. This is the time when multiple changes occur both in the brain and in the behaviour of honeybees [1,95,131,185]. When interpreting these data, however, one should keep in mind that the benzazepine R(+)−SCH 23390, although being a specific vertebrate D1-receptor antagonist, does not bind with high affinity to AmDOP1 (Ki = 250 nM, [12]). Furthermore, SCH 23390 is not particularly potent in inhibiting DA-induced cAMP elevation via either AmDOP1 (IC50 = 8.1 μM, [114]) or AmDOP2 (IC50 = 17 μM, [114]). This makes it difficult to decide which type of receptor was actually labelled in the binding studies performed on bee-brain tissue.
Taken together, the expression of DA receptors in the MBs, important centres of sensory integration, suggests a major role of this transmitter in honeybee behaviour. The presence of DA receptors in the central complex, in the ALs and in the optic lobes implies that the dopaminergic system affects sensory perception and is involved in the control of motor patterns.
4.2. Tyramine Receptors in the Honeybee
The function of TA in the honeybee is still unclear, but since a honeybee TA receptor was cloned (AmTYR1, Apis mellifera tyramine receptor 1, Table (2), [13]), this question has received special attention. AmTYR1 shares high homology with TA receptors from Drosophila (DmTYR, Drosophila melanogaster tyramine receptor [146]), Locusta (Loc-TYR, Locusta tyramine receptor, [179]), and Bombyx mori [121]. Activation of heterologously expressed AmTYR1 and of TA receptors in membrane homogenates of honeybee brains inhibits forskolin-induced cAMP synthesis [13,116].
Amtyr1 mRNA is expressed in the somata of most neuropils in the honeybee brain. Particularly the MB intrinsic neurons, the somata surrounding the ALs and the DLs, and the first and second optic chiasmata express Amtyr1 mRNA [13]. Because the Amtyr1 gene is expressed in the ALs and MBs, it can be assumed that TA is involved in sensory integration and possibly in learning and memory processes. This hypothesis is supported by recent behavioural and pharmacological experiments (see below).
Expression of Amtyr1 mRNA changes during bee development. The mRNA level increases in the late pupal stages (P6 to P8) and then remains stable until the bees emerge. During adult life, Amtyr1 expression increases in foragers compared to newly emerged bees [116].
4.3. Octopamine Receptors in the Honeybee
The first OA receptor from the honeybee was cloned a few years ago (AmOA1, Apis mellifera octopamine receptor 1, [58] below). This receptor shares a high degree of amino-acid sequence-similarity to OA receptors cloned from other insects and molluscs [58]. Originally, insect OA receptors were classified on the basis of second messenger changes induced in a variety of intact tissue preparations. OCT-1 (octopamine 1) receptors cause an increase in [Ca2+]i, whereas OCT-2A, OCT-2B and OCT-3 receptors activate adenylyl cyclase [41–44,47,134,136,138]. Such a classification system, however, is problematic when more than one receptor subtype is present in the same tissue preparation. Therefore, Evans and co-workers proposed a new classification system for insect OA receptors into “α-adrenergic-like OA receptors (OctαRs)” and “β-adrenergic-like OA receptors (OctαRs)” based on their similarities in primary structure and in signalling properties with vertebrate adrenergic receptors [46,94].
The functional properties of the AmOA1 receptor were investigated after its expression in HEK 293 cells. Low concentrations of OA (α10 nM) induced oscillation of [Ca2+]i. At high ligand concentrations (α1 μM), single, slowly declining Ca2+ responses were observed [58]. In addition to Ca2+ signalling, high concentrations of OA caused a rather moderate production of cAMP in AmOA1-expressing cells. Thus, based on its amino acid sequence as well as on its cellular signalling capabilities, AmOA1 is a member of the OctαR (or former OCT-1) receptor subfamily [11,46] (Table 2). It is, however, unlikely that AmOA1 is the only neuronal octopamine receptor expressed in the bee [137], since orthologues of the Drosophila OctβRs can be identified in the completely sequenced honeybee genome.
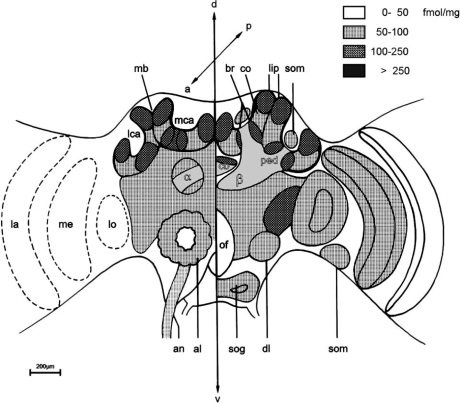
In situ hybridisation to sections of the honeybee brain showed that the Amoa1-gene is expressed in intrinsic MB neurons, in somata of the ALs and optic lobes, and in somata of the SOG [58]. The distribution of binding sites for [3H] OA in tissue sections of the honeybee brain has been ana-lysed with autoradiographic methods (Fig. (4), [37]). Specific and high labelling densities were observed in the MBs, especially in the pedunculus and in the α- and β-lobes. Interestingly, these brain regions are not innervated by octopaminergic neurons (see above) [7,36,37,86]. There are several possible explanations for this observation. (1) OA may act over long distances in a neuromodulatory way [37]; (2) the immunocytochemical staining did not reveal all of the finer processes of octopaminergic neurons; and (3) not all of the [3H]OA binding sites are functionally relevant. The OA receptor antagonist phenolamine displaced ∼93% of [3H]OA binding in all brain areas except the MBs (∼70% displacement). This result suggests that OA receptors in the mushroom bodies are pharmacologically different from those in the rest of the brain [37] and thus provides further evidence for the existence of more than one OA receptor in the bee brain. Radioligand binding studies to membrane preparations of different brain regions have also been performed [31]. A high-affinity OA receptor agonist, [3H]-NC-5Z, binds to membrane fractions from MBs and optic lobes. In samples prepared from other parts of the brain and the SOG, less binding was observed [31].
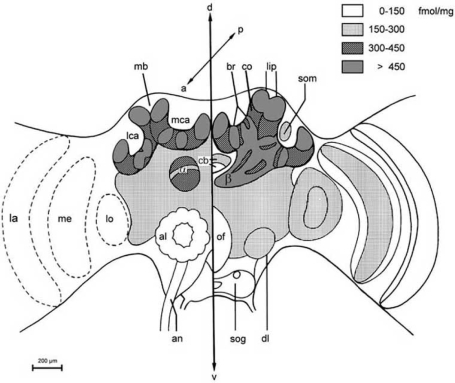
Fig. (4).
Schematic drawing of [3H]OA binding-site distribution in the main neuropils of the bee brain. The brains were incubated with 9 nM [3H]OA and the densities of radioactive labelling are indicated by the degree of shading. The left part of the figure shows anterior parts of the brain whereas the right part shows the posterior parts of the bee brain. Abbreviations: α alpha lobe, β beta lobe of the mushroom bodies. a anterior axis, al antennal lobe, an antennal nerve, br basal ring of the calyx, cb central body, co collar of the calyx, d dorsal axis, dl dorsal lobe, la lamina, lca lateral calyx, lip lip of the calyx, lo lobula, mb, mushroom body, mca median calyx, me medulla, of oesophageal foramen, p posterior axis, ped pedunculus of the mushroom body, sog subesophageal ganglion, som layer of somata, v ventral axis. The figure was taken from [122] with friendly permission of the publisher, © Springer-Verlag 2002.
Recently, Farooqui and co-workers applied the RNAi technique to examine the function of AmOA1 in the ALs [48]. Injection of AmOA1 dsRNA into the tissue led to approximately 80% inhibition of the olfactory acquisition responses and to approximately 50% reduction in memory recall [48].
In conclusion, phylogenetic analyses performed by different authors (Fig. (3), [46,73,137]) suggest that the insect OctαRs (including AmOA1) are closely related to vertebrate α1-adrenergic receptors, whereas insect OctβRs (no honeybee orthologue characterised so far) are most closely related to vertebrate β-adrenergic receptors. In contrast, the AmTYR1 receptor is not related to the classical OA receptors mentioned above. Instead, AmTYR1 and other insect TA receptors seem to form a sub-group which is related to the vertebrate α2-adrenergic receptors (Fig. (3), [73,137]).
4.4. Serotonin Receptors in the Honeybee
In mammals, the effects of 5-HT are mediated by 13 distinct GPCRs and a ligand-gated ion channel (for reviews see [72,87]). The receptors are divided into seven groups, based on their sequence homologies and signalling properties. The 5-HT1 receptors couple preferentially to Gi/o and inhibit cAMP synthesis. 5-HT2 receptors couple preferentially to Gq/11 and activate PLC. The rise in [IP3]i subsequently elevates [Ca2+]i. 5-HT3 receptors are ligand-gated ion channels. 5-HT4, 5-HT6, and 5-HT7 receptors couple to Gs and stimulate adenylyl cyclase activity.
In insects, the serotonergic system seems to be similarly complex (for reviews see[145,177]). Phylogenetic analysis of the receptor sequences suggests that most of 5-HTreceptor subtypes evolved before the divergence of invertebrate and vertebrate branches. Unfortunately, no honeybee 5HT receptor has been described at the molecular level so far.
However, 5-HT-sensitive binding sites have been characterised in membrane preparations of honeybee brains [16]. The pharmacological properties of these potential 5-HT receptors differ considerably from those of mammalian receptors [16]. Radioligand binding studies revealed a relatively uniform distribution of [3H]5-HT binding-sites in each of the three optic ganglia (Fig. (5), [36]). As has been described for OA, there is an interesting mismatch between the distribution of 5-HT-immunoreactivity and [3H]5-HT binding sites. The lips of the MB calyces are strongly labelled with [3H]5-HT [36], but no 5-HT-immunoreactivity was observed (for a review see [7]). These findings suggest that 5-HT can bind to receptors expressed in brain regions which are remote from its release sites [36].
Fig. (5).
Schematic drawing of [3H]5-HT binding-site distribution in the bee brain. The brains were incubated with 10 nM [3H]5-HT and the densities of radioactive labelling are indicated by the degree of shading. The left part of the figure shows anterior parts whereas the right part shows the posterior parts of the bee brain. Abbreviations as in Fig. (4). The figure was taken from [122] with friendly permission of the publisher, © Springer-Verlag 2002.
5. MODULATION OF HONEYBEE LEARNING AND MEMORY BY BIOGENIC AMINES
From the previous chapters, it is obvious that biogenic amines exert a multitude of effects in insects. They can act on different levels: at the sensory periphery, at the level of interneurons and brain compartments, and at the level of motor output and muscles. In the honeybee, biogenic amines are important modulators of learning and division of labour. Both forms of behaviour can be tested under laboratory conditions or in the field. The impact of biogenic amines has been studied in honeybees either by systemic application, i.e. feeding, injection into the haemolymph or onto the brain, and by local microinjections into defined brain compartments.
Each of these methods has advantages and disadvantages. The advantage of feeding experiments is that the animal does not have to be wounded for application of the substances. However, the fed compounds enter the digestive tract where they are eventually metabolically modified. In addition, it is not clear whether the substances reach the respective brain areas where they should induce modulatory effects. An advantage of microinjecting compounds is that the amines can be applied to a particular brain area where they are supposed to act.
Generally, some caution is appropriate when interpreting data obtained from injection or feeding experiments for the following reasons. 1) Most experiments have tested the effect of a drug which was injected in excess of intrinsic drug titres. Under such conditions, it is difficult to conclude that a particular substance serves to modulate or mediate a specific function, because an excessive amount of a substance may disturb a balanced system such that even opposing effects may be initiated [102]. 2) We often find complex dose-response relationships for biogenic amines, such as U-shaped dose-response functions. An excess of a substance may therefore antagonise its effect under physiological concentrations. 3) In most cases, the injected substances are not specific for a single receptor subtype. Therefore, it is difficult to assess which receptor subtypes are actually affected. Even a cross-talk between different aminergic systems (e.g., TA vs. OA) seems possible when the injected ligand concentrations are high enough. 4) Another problem is that there is little knowledge on the diffusion rates, half lives or the bioavailability of the injected substances in the bee. 5) Finally, the drug effects may depend on the condition of the bee and factors such as arousal, satiation level, age and gustatory responsiveness may influence the drug action.
Learning and memory are very general forms of plasticity in the nervous system. They have been conserved across distant animal species. Thus, certain features of the cellular and molecular processes underlying learning and memory are similar in vertebrates and invertebrates. Because learning and memory are rather difficult to study in complex vertebrate brains, invertebrates, such as the honeybee, offer a promising alternative for studying brain functions at the cellular and molecular level.
Learning is an essential part of honeybee life. Foraging bees have to remember the location of their hive and different food sources. Once they arrive at a flower, they have to remember how they find their way to nectar or pollen. Honeybees learn conditioned stimuli of different modalities very fast and establish long-lasting memories under free-flying conditions and in the laboratory (for reviews see [9,57,99]). Different established paradigms for testing non-associative and associative learning in honeybees use the proboscis extension response (PER, for a review see [98]). When the antennae of a bee are stimulated with sucrose solutions of sufficient concentration, the animal reflexively extends its proboscis in expectation of food (Fig. (6)). While some bees are very sensitive to gustatory stimuli and even show the PER after stimulation with water or low sucrose concentrations, other bees are rather insensitive and only display the PER when their antennae are stimulated with 30% sucrose [149].
Fig. (6).
Proboscis extension response (PER) in the honeybee. When the antennae of a fixed bee are stimulated with a droplet of sucrose solution above the individual response threshold, the bee reflexively extends its proboscis in expectation of food. This behaviour is employed for different non-associative and associative learning paradigms.
5.1. Non-Associative Learning
Repeated stimulation of the antennae with a low sucrose concentration leads to habituation of the PER [24,64,148]. Habituated bees can be dishabituated by stimulating the antennae with a high sucrose concentration, such as 30% sucrose. There is convincing evidence that some biogenic amines are involved in these processes. TA appears to increase the rate of habituation, when fed 12 h before testing the bees. Animals fed with TA needed fewer trials than controls to achieve complete habituation of the PER [24]. Interestingly, the OA receptor agonist chlordimeform had the same effect as TA [24], suggesting a similar role for both amines in habituation of the PER. This would contradict the general assumption that TA and OA are “antagonistic modulators of behaviour” [140]. Originally, it was assumed that the effects of TA were mediated by the activation of OA receptors either after biochemical conversion of TA to OA or by direct binding of TA to OA receptors [24]. Considering the existence of specific TA receptors, however, some experiments need to be repeated using ligands binding specifically to either TA or OA receptors.
Sensitisation can be tested as PER to antennal stimulation with odours [101,102]], water vapour [12] or gustatory stimuli [105]. Normally, bees do not show proboscis extension in response to antennal stimulation with an odour or water vapour. When bees are stimulated with a high sucrose concentration, such as 30% sucrose, shortly before the odour is presented to their antennae, they can be sensitised to the odour. Successful sensitisation leads to a PER after subsequent antennal stimulation with an odour [12,101]. Similarly, bees can be sensitised to antennal stimulation with water [105]. Application of OA, either into the haemolymph or into the dorsal lobe, increased responsiveness to water and thus mimicked the effect of sucrose stimulation [103]. In contrast, sensitisation to odours was neither affected by OA nor by DA in honeybees whose nervous system had been depleted of biogenic amines by reserpine [102]. In addition to their effects on non-associative learning, biogenic amines exert complex effects on associative learning, which has been studied more extensively than non-associative learning in the bee.
5.2. Associative Learning
To examine associative learning under laboratory conditions, olfactory and tactile conditioning of the PER have been employed (Fig. (7)). In both paradigms, the conditioned stimulus (CS, which could be an odour or a flat object that can be touched by the antennae) is paired with an unconditioned stimulus (US, which is sucrose in this case). First, spontaneous responsiveness to the CS is measured by applying the odour to the antennae of a bee or by moving the tactile stimulus into the scanning range of the bee antennae. The conditioning trial begins when the bee can smell the odour or when it scans the tactile object. While the bee experiences the CS, the PER is elicited by applying a small droplet of sucrose solution to its antennae (US). When the bee extends its proboscis, it is allowed to drink a small volume of sucrose solution as reward. Usually, a few pairings of CS and US suffice for the formation of a memory, which lasts for days (Fig. (7), [9,35], for a review see [99]).
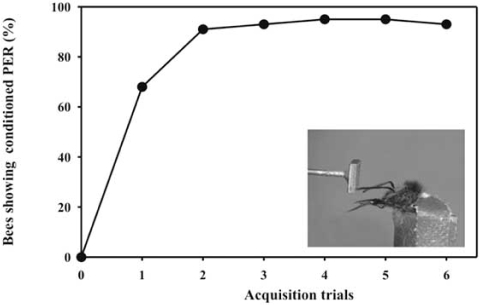
Fig. (7).
Acquisition curve in tactile antennal learning. The x-axis shows the number of acquisition trials. The y-axis displays the percentage of bees showing the conditioned proboscis extension response (PER). After only two conditioning trials, the bees reach a stable plateau in their learning performance. The inlet displays a bee showing conditioned proboscis extension while it scans the conditioned stimulus, a small metal plate.
Using such tests, it has been demonstrated that DA selectively inhibits the retrieval of learned information without affecting the storage process. Injection of DA into the brain or brain compartments after conditioning led to a reversible decrease in conditioned responses (CRs). In contrast, DA injections prior to conditioning had no effects on memory [8,93,100,102–105,107]. Serotonin can reduce both memory storage and retrieval when injected prior to conditioning [8,100,104,105]. When 5-HT is injected after conditioning, responses are only slightly reduced. The role of TA in associative learning has not been studied.
OA appears to play a decisive role in acquisition and memory formation in bees. Application of OA improves olfactory acquisition, memory formation and retrieval. Satiated bees normally do not display any associative PER learning. OA injections into the haemolymph of satiated bees can restore the “motivation” to learn. In bees whose nervous system had been depleted of biogenic amines by reserpine, OA significantly improved the acquisition performance but not retention [102]. Local injections of OA into the calyx or the α-lobe of the MBs enhanced memory formation [104]. When the OA-receptor antagonist mianserin was injected into the AL, it strongly reduced acquisition and retrieval [48]. A similar but irreversible effect was observed when the expression of the AmOA1 receptor was down-regulated by injection of Amoa1 dsRNA [48].
Evidence for the role of OA in memory formation at the cellular level originates from the analysis of the VUMmx1 neuron (ventral unpaired median neuron 1 of the maxillary neuromere [60]). The VUMmx1 neuron depolarises in response to the presentation of sucrose rewards to antennae and proboscis. Current injection into the VUMmx1 neuron can substitute for the sucrose reward during olfactory conditioning [60,63]. VUMmx1 belongs to a group of OA-immunoreactive neurons, the cell bodies of which are located ventrally in the SOG [86,163]. It is assumed that VUM neurons release OA, which could then mediate the US in PER conditioning (for reviews see [25,61,63,140]). Whether or not TA is coreleased with OA from activated VUM neurons can currently not be assessed.
Besides these striking effects on learning behaviour, the different biogenic amines also act on division of labour, which is a central feature of social insects.
6. BIOGENIC AMINES AND DIVISION OF LABOUR
Division of labour is a key feature of honeybee social life. Worker honeybees show a complex age-dependent division of labour, which is also referred to as “age polyethism” [161,184,186]. Young bees work in the centre of the nest and care for the brood and the queen. Bees in their second week of life engage in the processing of nectar and pollen and in comb building. In their third week of life, bees begin to forage after spending a couple of days as guarding bees at the hive entrance. A small number of bees are concerned with the removal of dead brood from the hive, before they start foraging; this is referred to as “undertaking behaviour”. The performance of most of these tasks depends on the age of the worker bee [132,161,186]. Division of labour, however, also occurs among bees of similar age. Foragers are a good example of this phenomenon. While some bees collect pollen, other bees of the same age collect nectar and again other bees collect both pollen and nectar. A small number of bees collect water, which is necessary to cool the hive and to maintain the climatic conditions of the nest. A few bees collect propolis, a resinous substance which is used to repair the hive [161,186]. Thus, the division of labour in a honeybee colony is very plastic. Small changes in the amount of brood, in the foraging resources or in the weather conditions may greatly change the pattern of division of labour in a colony [186].
6.1. Age-Dependent Division of Labour
To gain insight into the contribution of biogenic amines in age-dependent division of labour, amine titres have been determined in the brains of bees performing different tasks inside and outside the hive and of bees which differ in age. DA, OA and 5-HT are present in the brains of larvae, pupae and adult honeybees [53,68,81,83,106,175]. During the transition from larval to pupal stage, the levels of all three biogenic amines generally increase [175]. In adults, biogenic-amine titres increase with age, with the highest concentrations being found in foragers [68,152,156,181]. Whether the differences in biogenic-amine titres between bees of different ages are related to age differences or whether they are related to the different tasks the bees perform is difficult to test. Single-cohort colonies, which only consist of same-aged bees, are very helpful for distinguishing between these alternatives. Schulz and Robinson [152] showed that differences in the titres of DA, OA and 5-HT in MBs of foragers and nurse bees were related to age, whereas in the AL, the differences were related to different tasks. Unfortunately, TA titres have not yet been measured in single-cohort colonies. Another way of studying the role of biogenic amines in division of labour is to manipulate amine titres and to determine the behavioural effects. Thus it was shown that OA induced bees to forage precociously, whereas TA had the opposite effect [153].
6.2. Octopamine and Juvenile Hormone
Juvenile hormone (JH) and OA are decisive factors in regulating the onset of foraging behaviour [133]. Typically, JH titres increase with age in adult bees, so that foragers have higher levels of JH than younger bees. Treating younger bees with methoprene, a JH analogue, accelerates the onset of foraging [18]. Removal of the corpora allata, the organs synthesising JH, delays the initiation of foraging [173]. This effect can be reversed by methoprene [173]. These findings suggest that JH controls the pace at which bees become foragers.
It is generally assumed that OA and JH regulate each other and modulate the onset of foraging behaviour and the initiation of other tasks [77,154] (for a review see [155]). Foragers have high titres of both JH and OA, particularly in the ALs [152,155]. Treating 1-day-old bees with methoprene causes increased levels of OA in the ALs 12 days later compared to controls, and leads to precocious foraging [154]. When bees whose corpora allata had been removed were fed OA, they became normal foragers. These experiments suggest that OA probably acts downstream of JH. On the other hand, OA was shown to increase JH release from the copora allata in vitro in a dose-dependent manner [77]. The details of the complex interaction pattern of JH and OA remain to be resolved. Usually, a close interaction of both neuroactive substances is required for adequate initiation of foraging behaviour.
6.3. Age-Independent Division of Labour
Whereas DA, OA, and 5-HT were shown to be decisive regulators of age-dependent division of labour, the role of these amines in age-independent division of labour is less clear. Božič and Woodring [23] described that the levels of the three amines differed between foraging-aged bees when they performed different tasks. These bees are usually foragers, “dancers” or “followers”. After returning from a foraging bout, “dancers” perform waggle dances to inform other foragers about the location of a food source. Some foraging-aged bees in the hive follow the dancers and beg for small food samples. These bees are “followers”. Throughout the season, the titres of DA, OA and 5-HT were higher in dancers than in followers. This may be related to richer sources of sensory input they collect from the environment or to the locomotor activity they perform during foraging. To confirm these initial results on age-independent division of labour, additional measurements of biogenic-amine titres in same-aged bees are necessary.
7. HOW DO BIOGENIC AMINES AFFECT DIVISION OF LABOUR?
There are different hypotheses on how division of labour in honeybee colonies is organised. The most widely accepted hypothesis is that division of labour is achieved by differences in response thresholds of individual bees to stimuli that are associated with specific tasks [5,6,122,132,176]. Individuals with a low response threshold for a task-related stimulus are the first to start the respective job, once their response threshold is exceeded. Individuals with higher response thresholds perform the task only if the stimulus intensity increases even further. Recent experiments support this hypothesis and demonstrate how biogenic amines can modulate response thresholds for certain stimuli.
7.1. Response Thresholds for Gustatory and Olfactory Stimuli
Foragers differ not only in the material they collect but also in their response thresholds to gustatory stimuli presented to their antennae [125]. One week-old bees which have a high sensitivity for sucrose are most likely to later collect water or pollen, whereas bees with lower gustatory sensitivity collect nectar. Biogenic amines alter these gustatory response-thresholds. Both OA and TA significantly decrease the threshold [150]. This is another example of a similar action of OA and TA, which is in contrast to the assumption that OA and TA are “antagonistic modulators of behaviour” [140].
The opposite effect was observed for DA [150], whereas 5-HT had no effect (Scheiner, personal observation). Thus it is conceivable that response thresholds for foraging-related stimuli are adapted to the conditions in the hive and in the environment by changing levels of biogenic amines.
The spontaneous PER to antennal stimulation with an odour is often used as an indicator of olfactory sensitivity [100,101,105]. OA injection into the brain or brain compartments was shown to decrease olfactory response thresholds. DA and 5-HT injections had no effect on odour responsiveness [101,103,105] but increased response thresholds to water vapour [12,100,103]. The role of TA in honeybee olfaction is currently being analysed.
OA also affects response thresholds for the odour of brood (brood pheromone) [3]. When hive bees were fed with OA for several days, their response thresholds for brood pheromone decreased. The animals subsequently increased their foraging activity [3], but did not change the rate of corpse removal, another flight-related task [2]. Interestingly, OA treatment also did not increase attendance in the queen’s retinue [2]. Since retinue behaviour is mediated by queen mandibular pheromone, elevated brain levels of OA apparently did not cause a general increase in responsiveness to odour stimuli. Although OA treatment enhanced the foraging response to brood pheromone, it decreased the cell capping response, a component of brood care [2]. This selective modulation of different responses to brood pheromone suggests that OA does not generally increase sensitivity to brood pheromone and demonstrates a rather specific octopaminergic modulation of pheromone-mediated behaviour. In honeybee strains differing in their hygienic behaviour [167], OA affects the sensitivity to the odour of diseased brood. Bees of the hygienic strain discover diseased brood quickly and remove it from the hive. In contrast, animals of non-hygienic strains need more time and higher stimulus intensities to perform the task. OA treatment of bees from the unhygienic strain resulted in increased electroantennogram (EAG) responses to odours originating from diseased brood. In bees of the hygienic strain, however, OA did not change the sensitivity of the bees. They most likely had reached satiation in their sensitivity, because the OA-receptor antagonist epinastine [139] led to a reduction of EAG amplitude in these bees. In contrast, epinastine had no effect on unhygienic bees. This is a good example of how strongly the impact of a biogenic amine depends on the state of a bee.
7.2. Visual Sensitivity
Honeybees rely heavily on their visual senses, particularly at foraging age. They can recognise the flight paths to a foraging source, they look for potential food sources they have been informed about by their hive mates, and they have to discover the way to the pollen or nectar sources once they have arrived at a flower.
The direction-specific visual antennal reflex is a useful tool to study neuromodulation in the visual system of the bee. A stripe pattern which is moved upwards in front of a fixed bee induces downward antennal movements, whereas a downward moving pattern induces antennal movements in the opposite direction. The difference in the antennal angles for the two directions of the stimulus can be used as a measure of the direction specificity of the response [34,36,37]. This antennal reflex, when induced under controlled laboratory conditions, is very similar to the antennal movements of bees under free-flying conditions [33,39]. OA injections into the lobula can enhance the direction-specificity, whereas injections of 5-HT reduce this specificity [34,36, 37]. These experiments suggest that 5-HT and OA modulate motion-sensitive neurons in the lobula in an opposite way. The physiological changes of movement-sensitive neurons in the lobula during application of transmitters were measured by intracellular electrical recordings. Whereas 5-HT injections reduced spontaneous activity in many movement-sensitive neurons, OA application did not affect these responses [36]. DA appears not to be involved in the modulation of honeybee vision. The role of TA has yet to be tested.
7.3. Motor Activity and Locomotion
A decisive component of foraging behaviour consists of active scanning movements of the bee to analyse the surface of a flower and to use tactile information to identify a food source [79]. Antennal scanning behaviour can be characterised by the frequency of antennal contacts with an object, which can be measured electronically [127]. Comparable to the visual system, both 5-HT and OA acted antagonistically in the dorsal lobe, the sensory motor-centre of the antenna. OA stimulated antennal scanning activity, whereas 5-HT reduced it [127].
Another parameter of motor activity is the latency of PER after sucrose stimulation. Biogenic amines are most likely involved in PER, because the latency of PER was increased when bees were depleted of biogenic amines. Interestingly, DA restored the latency of the response to normal levels whereas injection of OA had no rescuing effect [102].
CONCLUSION
The four biogenic amines DA, TA, OA and 5-HT are present in different quantities in the bee brain and differ widely in their behavioural roles. Although OA is only present in small amounts in the honeybee brain, it modulates numerous behaviours. Together with JH, OA is involved in the initiation of foraging behaviour. OA also plays a decisive role in other forms of division of labour by modulating the sensitivity for specific stimulus modalities. In addition, OA can increase both acquisition and retrieval of information and thus controls learning and memory formation. DA and 5HT, both of which are present in high amounts in the honeybee, often have inhibitory effects on behaviour. In associative olfactory learning, DA reduces the retrieval of learned information. 5-HT inhibits both storage and retrieval of information. The role of TA is less clear, probably because TA has mainly been considered as the biochemical precursor of OA rather than being a neurotransmitter itself.
In contrast to the general assumption that OA and TA act antagonistically [140], we presented data showing that both amines have similar functions. This finding can be explained either by the fact that the behavioural changes which were observed after TA application are a result of OA receptor activation or that TA may have both agonistic and antagonistic effects to those of OA depending on the behaviour investigated. More experiments, particularly with ligands highly specific for either OA or TA receptors will help to unequivocally unravel the functions of the two amines.
The signals mediated by biogenic amines are transformed into cellular responses by signalling cascades triggered by amine-specific GPCRs. Hitherto, a couple of these receptors have been molecularly identified and functionally characterised after heterologous expression. At present, it can be speculated that honeybees express a multitude of receptor genes with a complexity comparable to that of vertebrate systems. Forthcoming studies should deal with the comprehensive molecular identification, biochemical and pharmacological analyses, and the determination of the cellular expression patterns of these receptors. Such studies are urgently awaited and will help to further unravel the contribution of amine-induced signalling cascades to honeybee behaviour. We are convinced that results can be expected in reasonable time, because the availability of the honeybee genome sequence facilitates the molecular analysis of the system significantly. Biogenic-amine research in the honeybee will thus provide important new insights into various fields in the near future. Among these, the control of learning behaviour and division of labour are the most challenging ones. The DNA microarray technology [27,59,183] and molecular knock-down technologies, such as the antisense technique [51] and the RNA interference (RNAi) technique [48], are attractive developments with promising perspectives for honeybee molecular genetic studies. These techniques in combination with behavioural experiments will help to elucidate the physiological functions of the various biogenic-amine receptor subtypes in the bee. Thus, the study of aminergic signalling in the honeybee will shed further light on the role of amine modulators in complex behaviours of animals of all phyla.
Table 1B.
Pharmacology of Dopamine Receptors in Apis mellifera: Binding in Cell Cultures
| Specificity in vertebrates | AmDOP1 in HEK 293: [3H]LSD binding (Ki [nM]) | AmDOP1 in Sf9: cAMP production (EC50 or IC50 [nM]) | AmDOP2 in Sf9: amp production (EC50 or IC50 [nM]) | |
|---|---|---|---|---|
| Biogenic amines | ||||
| DA | 56 | 360 | 2,200 | |
| Norepinephrine | 3,100 | n.d. | 58,000 | |
| 5-HT | 3,600 | n.d. | − | |
| TA | 9,900 | n.d. | − | |
| OA | 110,000 | n.d. | n.d. | |
| Dopamine receptor agonists | ||||
| 6,7-ADTN | DA receptor agonist | 93 | 650 | 5,100 |
| R(+)−SKF 38393 | D1 agonist | 4,200 | n.d. | − |
| Dopamine receptor antagonists | ||||
| R(+)−Lisuride | D2 agonist, D1 antagonist | 4.3 | +/− | +/− |
| Chlorpromazine | D2 antagonist | 15 | n.d. | + |
| cis(Z)-Flupentixol | DA receptor antagonist | 17 | 200 | 3.8 |
| Spiperone | selective D2 antagonist | 64 | 2,200 | 8,500 |
| R(+)− SCH 23390 | selective D1 antagonist | 250 | 8,100 | 17,000 |
| S(+)−Butaclamol | DA receptor antagonist | 77 | 540 | 81 |
| Haloperidol | D2, D3, and D4 antagonist | 390 | n.d. | + |
ACKNOWLEDGEMENTS
We would like to thank J. Erber for his helpful comments on our manuscript. This work was supported by grants from the German Research Foundation awarded to A. Baumann (BA 1541/4), W. Blenau (BL 469/4, GRK837) and R. Scheiner (SFB 515).
REFERENCES
- 1.Allan SA, Slessor KN, Winston ML, King GGS. The influence of age and task specialization on the production and perception of honey bee pheromones. J Insect Physiol. 1987;33:917–922. [Google Scholar]
- 2.Barron AB, Robinson GE. Selective modulation of task performance by octopamine in honey bee (Apis mellifera) division of labour. J Comp Physiol [A] 2005;191:659–668. doi: 10.1007/s00359-005-0619-7. [DOI] [PubMed] [Google Scholar]
- 3.Barron AB, Schulz DJ, Robinson GE. Octopamine modulates responsiveness to foraging-related stimuli in honey bees (Apis mellifera) J Comp Physiol [A] 2002;188:603–610. doi: 10.1007/s00359-002-0335-5. [DOI] [PubMed] [Google Scholar]
- 4.Beggs KT, Hamilton IS, Kurshan PT, Mustard JA, Mercer AR. Characterization of a D2-like dopamine receptor (AmDOP3) in honey bee, Apis mellifera. Insect Biochem. Mol. Biol. 2005;35:873–882. doi: 10.1016/j.ibmb.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Beshers SN, Fewell JH. Models of division of labor in social insects. Annu Rev Entomol. 2001;46:413–440. doi: 10.1146/annurev.ento.46.1.413. [DOI] [PubMed] [Google Scholar]
- 6.Beshers SN, Robinson GE, Mittenthal JE. Response thresholds and division of labor in insect colonies. In: Detrain C, Deneubourg JL, Pasteels JM, editors. Information processing in social insects. Basel, Switzerland: Birkhäuser Verlag; 1999. pp. 115–139. [Google Scholar]
- 7.Bicker G. Biogenic amines in the brain of the honeybee: Cellular distribution, development, and behavioral functions. Microsc Res Tech. 1999;44:166–178. doi: 10.1002/(SICI)1097-0029(19990115/01)44:2/3<166::AID-JEMT8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 8.Bicker G, Menzel R. Chemical codes for the control of behaviour in arthropods. Nature. 1989;337:33–39. doi: 10.1038/337033a0. [DOI] [PubMed] [Google Scholar]
- 9.Bitterman ME, Menzel R, Fietz A, Schäfer S. Classical conditioning of proboscis extension in honeybees (Apis mellifera) J Comp Psychol. 1983;97:107–119. [PubMed] [Google Scholar]
- 10.Blenau W, Baumann A. Molecular and pharmacological properties of insect biogenic amine receptors: Lessons from Drosophila melanogaster and Apis mellifera. Arch Insect Biochem Physiol. 2001;48:13–38. doi: 10.1002/arch.1055. [DOI] [PubMed] [Google Scholar]
- 11.Blenau W, Baumann A. Aminergic signal transduction in invertebrates: focus on tyramine and octopamine receptors. Recent Res Devel Neurochem. 2003;6:225–240. [Google Scholar]
- 12.Blenau W, Erber J. Behavioural pharmacology of dopamine, serotonin and putative aminergic ligands in the mushroom bodies of the honeybee (Apis mellifera) Behav Brain Res. 1998;96:115–124. doi: 10.1016/s0166-4328(97)00201-5. [DOI] [PubMed] [Google Scholar]
- 13.Blenau W, Balfanz S, Baumann A. Amtyr1: Characterization of a gene from honeybee (Apis mellifera) brain encoding a functional tyramine receptor. J Neurochem. 2000;74:900–908. doi: 10.1046/j.1471-4159.2000.0740900.x. [DOI] [PubMed] [Google Scholar]
- 14.Blenau W, Erber J, Baumann A. Characterization of a dopamine D1 receptor from Apis mellifera: Cloning, functional expression, pharmacology, and mRNA localization in the brain. J Neurochem. 1998;70:15–23. doi: 10.1046/j.1471-4159.1998.70010015.x. [DOI] [PubMed] [Google Scholar]
- 15.Blenau W, May T, Erber J. Characterization of a dopamine-sensitive [3H]LSD binding site in honeybee (Apis mellifera) brain. Comp Biochem Physiol [C] 1995a;110:197–205. [Google Scholar]
- 16.Blenau W, May T, Erber J. Characterization of [3H]LSD binding to a serotonin-sensitive site in honeybee (Apis mellifera) brain. Comp Biochem Physiol [B] 1995b;112:377–384. [Google Scholar]
- 17.Blenau W, Schmidt M, Faensen D, Schürmann F-W. Neurons with dopamine-like immunoreactivity target mushroom body Kenyon cell somata in the brain of some hymenopteran insects. Int J Insect Morphol Embryol. 1999;28:203–210. [Google Scholar]
- 18.Bloch G, Wheeler DE, Robinson GE. In: Endocrine influences on the organization of insect societies Hormones, Brain and behavior. Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Vol. 3. San Diego, CA: Academic Press; 2002. pp. 195–235. [Google Scholar]
- 19.Blumenthal EM. Regulation of chloride permeability by endogenously produced tyramine in the Drosophila Malpighian tubule. Am J Physiol Cell Physiol. 2002;284:718–728. doi: 10.1152/ajpcell.00359.2002. [DOI] [PubMed] [Google Scholar]
- 20.Bockaert J, Pin JP. Molecular tinkering of G-proteincoupled receptors: An evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bornhauser BC, Meyer EP. Histamine-like immunoreactivity in the visual system and brain of an orthopteran and a hymenopteran insect. Cell Tissue Res. 1997;287:211–221. doi: 10.1007/s004410050747. [DOI] [PubMed] [Google Scholar]
- 22.Bourne HR. How receptors talk to trimeric G-proteins. Curr Opin Cell Biol. 1997;9:134–142. doi: 10.1016/s0955-0674(97)80054-3. [DOI] [PubMed] [Google Scholar]
- 23.Božič J, Woodring J. Variations of brain biogenic amines in mature honeybees and induction of recruitment behavior. Comp Biochem Physiol [A] 1998;120:737–744. [Google Scholar]
- 24.Braun G, Bicker G. Habituation of an appetitive reflex in the honeybee. J Neurophysiol. 1992;67:588–598. doi: 10.1152/jn.1992.67.3.588. [DOI] [PubMed] [Google Scholar]
- 25.Bräunig P, Pflüger H-J. The unpaired median neurons of insects. Adv Insect Physiol. 2001;28:185–266. [Google Scholar]
- 26.Callier S, Snapyan M, Le Crom S, Prou D, Vincent JD, Vernier P. Evolution and cell biology of dopamine receptors in vertebrates. Biol Cell. 2003;95:489–502. doi: 10.1016/s0248-4900(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 27.Cash AC, Whitfield CW, Ismail N, Robinson GE. Behavior and the limits of genomic plasticity: power and replicability in microarray analysis of honeybee brains. Genes Brain Behav. 2005;4:267–271. doi: 10.1111/j.1601-183X.2005.00131.x. [DOI] [PubMed] [Google Scholar]
- 28.Chuang TT, Iacovelli L, Sallese M, De Blasi A. G-protein-coupled receptors: Heterologous regulation of homologous desensitization and its implications. Trends Pharmacol Sci. 1996;17:416–421. doi: 10.1016/s0165-6147(96)10048-1. [DOI] [PubMed] [Google Scholar]
- 29.Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- 30.David J-C, Coulon J-F. Octopamine in invertebrates and vertebrates. A review. Prog Neurobiol. 1985;24:141–185. doi: 10.1016/0301-0082(85)90009-7. [DOI] [PubMed] [Google Scholar]
- 31.Degen J, Gewecke M, Roeder T. Octopamine receptors in the honey bee and locust nervous system: Pharmacological similarities between homologous receptors of distantly related species. Br J Pharmacol. 2000;130:587–594. doi: 10.1038/sj.bjp.0703338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehmer B, Gronenberg W. Segregation of visual input to the mushroom bodies in the honeybee (Apis mellifera) J Comp Neurol. 2002;451:362–373. doi: 10.1002/cne.10355. [DOI] [PubMed] [Google Scholar]
- 33.Erber J. Neural correlates of learning in the honeybee. Trends Neurosci. 1981;4:270–273. [Google Scholar]
- 34.Erber J, Kloppenburg P. The modulatory effects of serotonin and octopamine in the visual system of the honey bee (Apis mellifera L.): I. Behavioral analysis of the motion-sensitive antennal reflex. J Comp Physiol [A] 1995;176:111–118. [Google Scholar]
- 35.Erber J, Kierzek S, Sander E, Grandy K. Tactile learning in the honeybee. J Comp Physiol [A] 1998;183:737–744. [Google Scholar]
- 36.Erber J, Kloppenburg P, Scheidler A. Neuromodulation in the honeybee: Autoradiography, behaviour and electrophysiology. In: Goodman LJ, Fisher RC, editors. The Behaviour and Physiology of Bees. Wallingford, UK: CAB International; 1991. pp. 272–287. [Google Scholar]
- 37.Erber J, Kloppenburg P, Scheidler A. Neuromodulation by serotonin and octopamine in the honeybee: Behaviour, neuroanatomy and electrophysiology. Experientia. 1993a;49:1073–1083. [Google Scholar]
- 38.Erber J, Masuhr T, Menzel R. Localization of short-term memory in the brain of the bee, Apis mellifera. Physiol Entomol. 1980;5:343–358. [Google Scholar]
- 39.Erber J, Pribbenow B, Bauer A, Kloppenburg P. Antennal reflexes in the honeybee: Tools for studying the nervous system. Apidologie. 1993b;24:283–296. [Google Scholar]
- 40.Evans PD. Biogenic amines in the insect nervous system. Adv Insect Physiol. 1980;15:317–473. [Google Scholar]
- 41.Evans PD. Multiple receptor types for octopamine in the locust. J Physiol. 1981;318:99–122. doi: 10.1113/jphysiol.1981.sp013853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans PD. The role of cyclic nucleotides and calcium in the mediation of the modulatory effects of octopamine on locust skeletal muscle. J Physiol. 1984a;348:325–340. doi: 10.1113/jphysiol.1984.sp015113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evans PD. Studies on the mode of action of octopamine, 5-hydroxytryptamine and proctolin on a myogenic rhythm in the locust. J Exp Biol. 1984b;110:231–251. doi: 10.1242/jeb.110.1.231. [DOI] [PubMed] [Google Scholar]
- 44.Evans PD. Octopamine. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry, and Pharmacology. Vol. 11. Pharmacology: Oxford, Pergamon Press; 1985. pp. 499–530. [Google Scholar]
- 45.Evans PD. Borkovec AB, Gelman DB. Insect Neurochemistry and Neurophysiology. Clifton, NJ: Humana Press; 1986. Biogenic amine receptors and their mode of action; pp. 117–141. [Google Scholar]
- 46.Evans PD, Maqueira B. Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert Neurosci. 2005;5:111–118. doi: 10.1007/s10158-005-0001-z. [DOI] [PubMed] [Google Scholar]
- 47.Evans PD, Robb S. Octopamine receptor subtypes and their mode of action. Neurochem Res. 1993;18:869–874. doi: 10.1007/BF00998270. [DOI] [PubMed] [Google Scholar]
- 48.Farooqui T, Robinson K, Vaessin H, Smith BH. Modulation of early olfactory processing by an octopaminergic reinforcement pathway in the honeybee. J Neurosci. 2003;23:5370–5380. doi: 10.1523/JNEUROSCI.23-12-05370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farris SM, Robinson GE, Davis RL, Fahrbach SE. Larval and pupal development of the mushroom bodies in the honey bee, Apis mellifera. J Comp Neurol. 1999;414:97–113. doi: 10.1002/(sici)1096-9861(19991108)414:1<97::aid-cne8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 50.Feng G, Hannan F, Reale V, Hon YY, Kousky CT, Evans PD, Hall LM. Cloning and functional characterization of a novel dopamine receptor from Drosophila melanogaster. J. Neu-rosci. 1996;16:3925–3933. doi: 10.1523/JNEUROSCI.16-12-03925.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fiala A, Müller U, Menzel R. Reversible downregulation of protein kinase A during olfactory learning using antisense tech-nique impairs long-term memory formation in the honeybee, Apis mellifera. J Neurosci. 1999;19:10125–10134. doi: 10.1523/JNEUROSCI.19-22-10125.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Filipek S, Teller DC, Palczewski K, Stenkamp R. The crystallographic model of rhodopsin and its use in studies of other G-protein-coupled receptors. Annu Rev Biophys Biomol Struct. 2003;32:375–397. doi: 10.1146/annurev.biophys.32.110601.142520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuchs E, Dustmann J-H, Stadler H, Schürmann F-W. Neuroactive compounds in the brain of the honeybee during imaginal life. Comp Biochem Physiol [C] 1989;92:337–342. [Google Scholar]
- 54.Gengs C, Leung HT, Skingsley DR, Iovchev MI, Yin Z, Semenov EP, Burg MG, Hardie RC, Pak WL. The target of Drosophila photoreceptor synaptic transmission is a histamine-gated chloride channel encoded by ort (hclA) J Biol Chem. 2002;277:42113–42120. doi: 10.1074/jbc.M207133200. [DOI] [PubMed] [Google Scholar]
- 55.Gisselmann G, Pusch H, Hovemann BT, Hatt H. Two cDNAs coding for histamine-gated ion channels in D. melanogaster. Nat Neurosci. 2002;5:11–12. doi: 10.1038/nn787. [DOI] [PubMed] [Google Scholar]
- 56.Gisselmann G, Plonka J, Pusch H, Hatt H. Unusual functional properties of homo-and heteromultimeric histaminegated chloride channels of Drosophila melanogaster: spontaneous currents and dual gating by GABA and histamine. Neurosi Lett. 2004;372:151–156. doi: 10.1016/j.neulet.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 57.Giurfa M. The amazing mini-brain: Lessons from a honey bee. Bee World. 2003;84:5–18. [Google Scholar]
- 58.Grohmann L, Blenau W, Erber J, Ebert PR, Strünker T, Baumann A. Molecular and functional characterization of an octopamine receptor from honeybee (Apis mellifera) brain. J Neurochem. 2003;86:725–735. doi: 10.1046/j.1471-4159.2003.01876.x. [DOI] [PubMed] [Google Scholar]
- 59.Grozinger CM, Sharabash NM, Whitfield CW, Robinson GE. Pheromone-mediated gene expression in the honey bee brain. Proc Natl Acad Sci USA. 2003;100:14519–14525. doi: 10.1073/pnas.2335884100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hammer M. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature. 1993;366:59–63. doi: 10.1038/366059a0. [DOI] [PubMed] [Google Scholar]
- 61.Hammer M. The neural basis of associative reward learning in honeybees. Trends Neurosci. 1997;20:245–252. doi: 10.1016/s0166-2236(96)01019-3. [DOI] [PubMed] [Google Scholar]
- 62.Hammer M, Menzel R. Learning and memory in the honeybee. J Neurosci. 1995;15:1617–1630. doi: 10.1523/JNEUROSCI.15-03-01617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hammer M, Menzel R. Multiple sites of associative odor learning as revealed by local brain microinjections of octopamine in honeybees. Learn Mem. 1998;5:146–156. [PMC free article] [PubMed] [Google Scholar]
- 64.Hammer M, Braun G, Mauelshagen J. Food-induced arousal and nonassociative learning in honeybees: Dependence of sensitization on the application site and duration of food stimulation. Behav Neural Biol. 1994;62:210–223. doi: 10.1016/s0163-1047(05)80019-6. [DOI] [PubMed] [Google Scholar]
- 65.Han KA, Millar NS, Grotewiel MS, Davis RL. DAMB, a novel dopamine receptor expressed specifically in Dro-sophila mushroom bodies. Neuron. 1996;16:1127–35. doi: 10.1016/s0896-6273(00)80139-7. [DOI] [PubMed] [Google Scholar]
- 66.Hardie RC. Is histamine a neurotransmitter in insect photoreceptors? J. Comp. Physiol. [A] 1987;161:201–213. doi: 10.1007/BF00615241. [DOI] [PubMed] [Google Scholar]
- 67.Hardie RC. A histamine-activated chloride channel involved in neurotransmission at a photoreceptor synapse. Nature. 1989;339:704–706. doi: 10.1038/339704a0. [DOI] [PubMed] [Google Scholar]
- 68.Harris JW, Woodring J. Effects of stress, age, season, and source colony on levels of octopamine, dopamine and serotonin in the honey bee (Apis mellifera) brain. J Insect Physiol. 1992;38:29–35. [Google Scholar]
- 69.Heisenberg M. Mushroom body memoir: From maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 70.Homberg U. Progress in Zoology. Vol. 40. Stuttgart: Fischer Verlag; 1994. Distribution of neurotransmitters in the insect brain. [Google Scholar]
- 71.Horn F, van der Wenden EM, Oliveira L, IJzerman AP, Vriend G. Receptors coupling to G-proteins: Is there a signal behind the sequence? Proteins. 2000;41:448–459. doi: 10.1002/1097-0134(20001201)41:4<448::aid-prot30>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 72.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 73.Humphries MA, Mustard JA, Hunter SJ, Mercer A, Ward V, Ebert PR. Invertebrate D2 type dopamine receptor ex-hibits age-based plasticity of expression in the mushroom bodies of the honeybee brain. J Neurobiol. 2003;55:315–330. doi: 10.1002/neu.10209. [DOI] [PubMed] [Google Scholar]
- 74.Ji TH, Grossmann M, Ji I. G protein-coupled receptors. I. Diversity of receptor-ligand interactions. J Biol Chem. 1998;273:17299–17302. doi: 10.1074/jbc.273.28.17299. [DOI] [PubMed] [Google Scholar]
- 75.Jones BJ, Blackburn TP. The medical benefit of 5-HT research. Pharmacol Biochem Behav. 2002;71:555–568. doi: 10.1016/s0091-3057(01)00745-6. [DOI] [PubMed] [Google Scholar]
- 76.Jutel M, Blaser K, Akdis CA. Histamine in allergic inflammation and immune modulation. Int Arch Allergy Immunol. 2005;137:82–92. doi: 10.1159/000085108. [DOI] [PubMed] [Google Scholar]
- 77.Kaatz H, Eichmüller S, Kreissl S. Stimulatory effect of octopamine on juvenile hormone biosynthesis in honey bees (Apis mellifera): Physiological and immunocytochemical evidence. J Insect Physiol. 1994;40:865–872. [Google Scholar]
- 78.Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 79.Kevan PG, Lane MA. Flower petal microtexture is a tactile cue for bees. Proc. Natl. Acad. Sci. USA. 1985;82:4750–4752. doi: 10.1073/pnas.82.14.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kenyon FC. The brain of the bee. J Comp Neurol. 1896;6:133–210. [Google Scholar]
- 81.Kirchhof BS, Homberg U, Mercer AR. Development of dopamine-immunoreactive neurons associated with the antennal lobes of the honey bee, Apis mellifera. J Comp Neurol. 1999;411:643–653. [PubMed] [Google Scholar]
- 82.Kokay IC, Mercer AR. Characterisation of dopamine receptors in insect (Apis mellifera) brain. Brain Res. 1996;706:47–56. doi: 10.1016/0006-8993(95)01179-x. [DOI] [PubMed] [Google Scholar]
- 83.Kokay IC, Mercer AR. Age-related changes in dopamine receptor densities in the brain of the honey bee, Apis mellifera. J Comp Physiol [A] 1997;181:415–423. [Google Scholar]
- 84.Kokay IC, Ebert PR, Kirchhof BS, Mercer AR. Distribution of dopamine receptors and dopamine receptor homologs in the brain of the honey bee, Apis mellifera L. Microsc Res Tech. 1999;44:179–189. doi: 10.1002/(SICI)1097-0029(19990115/01)44:2/3<179::AID-JEMT9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 85.Kokay I, McEwan J, Mercer A. Autoradiographic local-isation of [3H]-SCH23390 and [3H]-spiperone binding sites in honey bee brain. J Comp Neurol. 1998;394:29–37. doi: 10.1002/(sici)1096-9861(19980427)394:1<29::aid-cne3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 86.Kreissl S, Eichmüller S, Bicker G, Rapus J, Eckert M. Octopamine-like immunoreactivity in the brain and subesophageal ganglion of the honeybee. J Comp Neurol. 1994;348:583–595. doi: 10.1002/cne.903480408. [DOI] [PubMed] [Google Scholar]
- 87.Kroeze WK, Kristiansen K, Roth BL. Molecular biology of serotonin receptors structure and function at the molecular level. Curr Top Med Chem. 2002;2:507–528. doi: 10.2174/1568026023393796. [DOI] [PubMed] [Google Scholar]
- 88.Kurshan PT, Hamilton IS, Mustard JA, Mercer AR. Developmental changes in expression patterns of two dopamine receptor genes in mushroom bodies of the honeybee, Apis mellifera. J Comp Neurol. 2003;466:91–103. doi: 10.1002/cne.10864. [DOI] [PubMed] [Google Scholar]
- 89.Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 90.Levin LR, Han PL, Hwang PM, Feinstein PG, Davis RL, Reed RR. The Drosophila learning and memory gene rutabaga encodes a Ca2+/Calmodulin-responsive adenylyl cyclase. Cell. 1992;68:479–489. doi: 10.1016/0092-8674(92)90185-f. [DOI] [PubMed] [Google Scholar]
- 91.Littner MR, Kushida C, Anderson WM, Bailey D, Berry RB, Hirshkowitz M, Kapen S, Kramer M, Lee-Chiong T, Li KK, Loube DL, Morgenthaler T, Wise M. Practice parameters for the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 2004;27:557–559. doi: 10.1093/sleep/27.3.557. [DOI] [PubMed] [Google Scholar]
- 92.Livingstone MS, Tempel BL. Genetic dissection of monoamine neurotransmitter synthesis in Drosophila. Nature. 1983;303:67–70. doi: 10.1038/303067a0. [DOI] [PubMed] [Google Scholar]
- 93.Macmillan CS, Mercer AR. An investigation of the role of dopamine in the antennal lobes of the honeybee, Apis mellifera. J Comp Physiol [A] 1987; 160:359–366. [Google Scholar]
- 94.Maqueira B, Chatwin H, Evans PD. Identification and characterization of a novel family of Drosophilaβ-adrenergic-like octopamine G-protein coupled receptors. J Neurochem. 2005;94(2):547–560. doi: 10.1111/j.1471-4159.2005.03251.x. [DOI] [PubMed] [Google Scholar]
- 95.Masson C, Arnold G. Ontogeny, maturation and plasticity of the olfactory system in the workerbee. J Insect Physiol. 1984;30:7–14. [Google Scholar]
- 96.McDonald PH, Lefkowitz RJ. βArrestins: New roles in regulating heptahelical receptors’ functions. Cell Signal. 2001;13:683–689. doi: 10.1016/s0898-6568(01)00203-0. [DOI] [PubMed] [Google Scholar]
- 97.Melzig J, Buchner S, Wiebel F, Wolf R, Burg M, Pak WL, Buchner E. Genetic depletion of histamine from the nervous system of Drosophila eliminates specific visual and mechanosensory behavior. J Comp Physiol [A] 1996;179:763–773. doi: 10.1007/BF00207355. [DOI] [PubMed] [Google Scholar]
- 98.Menzel R, Erber J. Learning and memory in bees. Sci Am. 1978;239:102–110. [Google Scholar]
- 99.Menzel R, Müller U. Learning and memory in honeybees: From behavior to neural substrates. Annu Rev Neurosci. 1996;19:379–404. doi: 10.1146/annurev.ne.19.030196.002115. [DOI] [PubMed] [Google Scholar]
- 100.Menzel R, Durst C, Erber J, Eichmüller S, Hammer M, Hildebrandt H, Mauelshagen J, Müller U, Rosenboom H, Rybak J, Schäfer S, Scheidler A. The mushroom bodies in the honeybee: From molecules to behaviour. Fortschr Zool. 1994;39:81–103. [Google Scholar]
- 101.Menzel R, Hammer M, Braun G, Mauelshagen J, Sugawa M. Neurobiology of learning and memory in honeybees. In: Goodman LJ, Fisher RC, editors. The behaviour and physiology of bees. Wallingford, Oxon: CAB International; 1991. pp. 323–353. [Google Scholar]
- 102.Menzel R, Heyne A, Kinzel C, Gerber B, Fiala A. Pharmacological dissociation between the reinforcing, sensitizing, and response-releasing functions of reward in honeybee classical conditioning. Behav Neurosci. 1999;113:744–754. [PubMed] [Google Scholar]
- 103.Menzel R, Michelsen B, Rüffer P, Sugawa M. Neuropharmacology of learning and memory in honey bees. In: Hertting G, Spatz H-C, editors. Modulation of synaptic transmission and plasticity in nervous systems. Vol. H19. Berlin, Heidelberg: Springer-Verlag; 1988. pp. 333–350. NATO ASI Series. [Google Scholar]
- 104.Menzel R, Wittstock S, Sugawa M. Chemical codes of learning and memory in honey bees. In: Squire L, Lindenlaub K, editors. The biology of memory. Stuttgart: Schattauer; 1990. pp. 335–360. [Google Scholar]
- 105.Mercer AR, Menzel R. The effects of biogenic amines on conditioned and unconditioned responses to olfactory stimuli in the honeybee Apis mellifera. J Comp Physiol. 1982;145:363–368. [Google Scholar]
- 106.Mercer AR, Mobbs PG, Davenport AP, Evans PD. Biogenic amines in the brain of the honey bee, Apis mellifera. Cell Tissue Res. 1983;234:655–677. doi: 10.1007/BF00218658. [DOI] [PubMed] [Google Scholar]
- 107.Michelsen DB. Catecholamines affect storage and retrieval of conditioned odor stimuli in honey bees. Comp Biochem Physiol [C] 1988;91:479–482. [Google Scholar]
- 108.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: From structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 109.Mobbs P. Neural networks in the mushroom bodies of the honeybee. J Insect Physiol. 1984;30:43–58. [Google Scholar]
- 110.Mobbs PG. Brain structure. In: Kerkut GA, Gilbert LI, editors. Comparative Insect Physiology, Biochemistry and Pharmacology. Vol. 5. Oxford: Pergamon Press; 1985. pp. 299–370. Nervous system: Structure and motor function. [Google Scholar]
- 111.Moller S, Vilo J, Croning MD. Prediction of the coupling specificity of G protein coupled receptors to their G proteins. Bioinformatics. 2001;17:174–181. doi: 10.1093/bioinformatics/17.suppl_1.s174. [DOI] [PubMed] [Google Scholar]
- 112.Monastirioti M, Linn CE, Jr, White K. Characterization of Drosophila tyramineβ-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci. 1996;16:3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morris AJ, Malbon CC. Physiological regulation of G protein-linked signaling. Physiol Rev. 1999;79:1373–1430. doi: 10.1152/physrev.1999.79.4.1373. [DOI] [PubMed] [Google Scholar]
- 114.Mustard JA, Blenau W, Hamilton IS, Ward VK, Ebert PR, Mercer AR. Analysis of two D1-like dopamine receptors from the honey bee Apis mellifera reveals agonist-independent activity. Mol Brain Res. 2003;113:67–77. doi: 10.1016/s0169-328x(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 115.Mustard JA, Beggs KT, Mercer AR. Molecular biol-ogy of invertebrate dopamine receptors. Arch Insect Biochem Physiol. 2005a;59:103–117. doi: 10.1002/arch.20065. [DOI] [PubMed] [Google Scholar]
- 116.Mustard JA, Kurshan PT, Hamilton IS, Blenau W, Mercer AR. Developmental expression of a tyramine receptor gene in the brain of the honey bee, Apis mellifera. J Comp Neurol. 2005b;483:66–75. doi: 10.1002/cne.20420. [DOI] [PubMed] [Google Scholar]
- 117.Nagaya Y, Kutsukake M, Chigusa SI, Komatsu A. A trace amine, tyramine, functions as a neuromodulator in Drosophila melanogaster. Neurosi Lett. 2002;329:324–328. doi: 10.1016/s0304-3940(02)00596-7. [DOI] [PubMed] [Google Scholar]
- 118.Nässel DR. Neurotransmitters and neuromodulators in the insect visual system. Prog Neurobiol. 1991;37:179–254. doi: 10.1016/0301-0082(91)90027-x. [DOI] [PubMed] [Google Scholar]
- 119.Nässel DR. Histamine in the brain of insects: a review. Microsc Res Techn. 1999;44:121–136. doi: 10.1002/(SICI)1097-0029(19990115/01)44:2/3<121::AID-JEMT6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 120.Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- 121.Ohta H, Utsumi T, Ozoe Y. B96Bom encodes a Bombyx mori tyramine receptor negatively coupled to adenylate cyclase. Insect Mol Biol. 2003;12:217–223. doi: 10.1046/j.1365-2583.2003.00404.x. [DOI] [PubMed] [Google Scholar]
- 122.Page RE, Erber J. Levels of behavioral organization and the evolution of division of labor. Naturwissenschaften. 2002;89:91–106. doi: 10.1007/s00114-002-0299-x. [DOI] [PubMed] [Google Scholar]
- 123.Palczewski K. GTP-binding-protein-coupled receptor kinases--two mechanistic models. Eur J Biochem. 1997;248:261–269. doi: 10.1111/j.1432-1033.1997.00261.x. [DOI] [PubMed] [Google Scholar]
- 124.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 125.Pankiw T, Page RE. Response thresholds to sucrose predict foraging behavior in the honey bee (Apis mellifera L.) Behav Ecol Sociobiol. 2000;47:265–267. [Google Scholar]
- 126.Park Y, Adams ME. Insect G protein-coupled receptors: Recent discoveries and implications. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive molecular insect science. Vol. 5. Pharmacology: Oxford, Elsevier; 2005. pp. 143–171. [Google Scholar]
- 127.Pribbenow B, Erber J. Modulation of antennal scanning in the honeybee by sucrose stimuli, serotonin, and octopamine: Behavior and electrophysiology. Neurobiol Learn Mem. 1996;66:109–120. doi: 10.1006/nlme.1996.0052. [DOI] [PubMed] [Google Scholar]
- 128.Probst WC, Snyder LA, Schuster DI, Brosius J, Sealfon SC. Sequence alignment of the G-protein coupled receptor superfamily. DNA Cell Biol. 1992;11:1–20. doi: 10.1089/dna.1992.11.1. [DOI] [PubMed] [Google Scholar]
- 129.Reale V, Hannan F, Hall LM, Evans PD. Agonist-specific coupling of a cloned Drosophila melanogaster D1-like do-pamine receptor to multiple second messenger pathways by synthetic agonists. J Neurosci. 1997;17:6545–6553. doi: 10.1523/JNEUROSCI.17-17-06545.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Robb S, Cheek TR, Hannan FL, Hall LM, Midgley JM, Evans PD. Agonist-specific coupling of a cloned Drosophila octopamine/tyramine receptor to multiple second messenger systems. EMBO J. 1994;13:1325–1330. doi: 10.1002/j.1460-2075.1994.tb06385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Robinson GE. Regulation of honey bee age polyethism by juvenile hormone. Behav Ecol Sociobiol. 1987;20:329–338. [Google Scholar]
- 132.Robinson GE. Regulation of division of labor in insect societies. Annu Rev Entomol. 1992;37:637–665. doi: 10.1146/annurev.en.37.010192.003225. [DOI] [PubMed] [Google Scholar]
- 133.Robinson GE. Genomics and integrative analyses of division of labor in honey bee colonies. Am Nat. 2002;160:160–172. doi: 10.1086/342901. [DOI] [PubMed] [Google Scholar]
- 134.Roeder T. A new octopamine receptor class in locust nervous tissue, the octopamine 3 (OA3) receptor. Life Sci. 1992;50:21–28. doi: 10.1016/0024-3205(92)90193-s. [DOI] [PubMed] [Google Scholar]
- 135.Roeder T. Biogenic amines and their receptors in insects. Comp Biochem Physiol [C] 1994;107:1–12. [Google Scholar]
- 136.Roeder T. Octopamine in invertebrates. Prog Neurobiol. 1999;59:533–561. doi: 10.1016/s0301-0082(99)00016-7. [DOI] [PubMed] [Google Scholar]
- 137.Roeder T. Tyramine and octopamine: ruling behavior and metabolism. Annu Rev Entomol. 2005;50:447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- 138.Roeder T, Nathanson JA. Characterization of insect neu-ronal octopamine receptors (OA3 receptors) Neurochem Res. 1993;18:921–925. doi: 10.1007/BF00998278. [DOI] [PubMed] [Google Scholar]
- 139.Roeder T, Degen J, Gewecke M. Epinastine, a highly specific antagonist of insect neuronal octopamine receptors. Eur J Pharmacol. 1998;349:171–177. doi: 10.1016/s0014-2999(98)00192-7. [DOI] [PubMed] [Google Scholar]
- 140.Roeder T, Seifert M, Kähler C, Gewecke M. Tyramine and octopamine: Antagonistic modulators of behavior and metabo-lism. Arch Insect Biochem. Physiol. 2003;54:1–13. doi: 10.1002/arch.10102. [DOI] [PubMed] [Google Scholar]
- 141.Rybak J, Menzel R. Anatomy of the mushroom bodies in the honey bee brain: The neuronal connections of the alpha-lobe. J Comp Neurol. 1993;334:444–465. doi: 10.1002/cne.903340309. [DOI] [PubMed] [Google Scholar]
- 142.Sachse S, Galizia CG. Role of inhibition for temporal and spatial odor representation in olfactory output neurons: a calcium imaging study. J Neurophysiol. 2002;87:1106–1117. doi: 10.1152/jn.00325.2001. [DOI] [PubMed] [Google Scholar]
- 143.Sasaki K, Nagao T. Distribution and levels of dopamine and its metabolites in brains of reproductive workers in honeybees. J Insect Physiol. 2001;47:1205–1216. doi: 10.1016/s0022-1910(01)00105-6. [DOI] [PubMed] [Google Scholar]
- 144.Sasaki K, Nagao T. Brain tyramine and reproductive states of workers in honeybees. J Insect Physiol. 2002;48:1075–1085. doi: 10.1016/s0022-1910(02)00200-7. [DOI] [PubMed] [Google Scholar]
- 145.Saudou F, Hen R. 5-Hydroxytryptamine receptor subtypes in vertebrates and invertebrates. Neurochem Int. 1994;25:503–532. doi: 10.1016/0197-0186(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 146.Saudou F, Amlaiky N, Plassat J-L, Borrelli E, Hen R. Cloning and characterization of a Drosophila tyramine receptor. EMBO J. 1990;9:3611–3617. doi: 10.1002/j.1460-2075.1990.tb07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schäfer S, Rehder V. Dopamine-like immunoreactivity in the brain and suboesophageal ganglion of the honeybee. J Comp Neurol. 1989;280:43–58. doi: 10.1002/cne.902800105. [DOI] [PubMed] [Google Scholar]
- 148.Scheiner R. Responsiveness to sucrose and habituation of the proboscis extension response in honey bees. J Comp Physiol [A] 2004;190:727–733. doi: 10.1007/s00359-004-0531-6. [DOI] [PubMed] [Google Scholar]
- 149.Scheiner R, Page RE, Erber J. Sucrose responsiveness and behavioral plasticity in honey bees (Apis mellifera) Apidologie. 2004;35:133–142. [Google Scholar]
- 150.Scheiner R, Plückhahn S, Öney B, Blenau W, Erber J. Behavioural pharmacology of octopamine, tyramine and dopamine in honey bees. Behav Brain Res. 2002;136:545–553. doi: 10.1016/s0166-4328(02)00205-x. [DOI] [PubMed] [Google Scholar]
- 151.Schröter U, Menzel R. A new ascending sensory tract to the calyces of the honeybee mushroom body, the subesophagealcalycal tract. J Comp Neurol. 2003;465:168–178. doi: 10.1002/cne.10843. [DOI] [PubMed] [Google Scholar]
- 152.Schulz DJ, Robinson GE. Biogenic amines and division of labor in honey bee colonies: Behaviorally related changes in the antennal lobes and age-related changes in the mushroom bodies. J Comp Physiol [A] 1999;184:481–488. doi: 10.1007/s003590050348. [DOI] [PubMed] [Google Scholar]
- 153.Schulz DJ, Robinson GE. Octopamine influences division of labor in honey bee colonies. J Comp Physiol [A] 2001;187:53–61. doi: 10.1007/s003590000177. [DOI] [PubMed] [Google Scholar]
- 154.Schulz DJ, Barron AB, Robinson GE. A role for octo-pamine in honey bee division of labor. Brain Behav. Evol. 2002a;60:350–359. doi: 10.1159/000067788. [DOI] [PubMed] [Google Scholar]
- 155.Schulz DJ, Sullivan JP, Robinson GE. Juvenile hormone and octopamine in the regulation of division of labor in honey bee colonies. Horm Behav. 2002b;42:222–231. doi: 10.1006/hbeh.2002.1806. [DOI] [PubMed] [Google Scholar]
- 156.Schulz DJ, Pankiw T, Fondrk MK, Robinson GE, Page RE. Comparison of juvenile hormone hemolymph and octopamine brain titers in honey bees (Hymenoptera: Apidae) selected for high and low pollen hoarding. Ann Entomol Soc Am. 2004;97:1313–1319. [Google Scholar]
- 157.Schürmann FW. Bemerkungen zur Funktion der corpora pedunculata im Gehirn der Insekten aus morphologischer Sicht. Exp Brain Res. 1974;19:406–432. doi: 10.1007/BF00234464. [DOI] [PubMed] [Google Scholar]
- 158.Schürmann FW, Klemm N. Serotonin-immunoreactive neurons in the brain of the honeybee. J Comp Neurol. 1984;225:570–580. doi: 10.1002/cne.902250407. [DOI] [PubMed] [Google Scholar]
- 159.Schürmann FW, Elekes K, Geffard M. Dopamine-like immunoreactivity in the bee brain. Cell Tissue Res. 1989;256,:399–410. [Google Scholar]
- 160.Schwartz JC, Arrang JM, Garbarg M, Pollard H, Ruat M. Histaminergic transmission in the mammalian brain. Physiol Rev. 1991;71:1–51. doi: 10.1152/physrev.1991.71.1.1. [DOI] [PubMed] [Google Scholar]
- 161.Seeley TD. The wisdom of the hive. Cambridge, MA: Harvard University Press; 1995. [Google Scholar]
- 162.Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA. The role of dopamine in cognitive sequence learning: Evidence from Parkinson’s disease. Behav Brain Res. 2005;156:191–199. doi: 10.1016/j.bbr.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 163.Sinakevitch I, Niwa M, Strausfeld NJ. Octopamine-like immunoreactivity in the hony bee and cockroach: comparable organization in the brain and subesophageal ganglion. J Comp Neurol. 2005;488:233–254. doi: 10.1002/cne.20572. [DOI] [PubMed] [Google Scholar]
- 164.Skingsley DR, Laughlin SB, Hardie RC. Properties of histamine-activated chloride channels in the large monopolar cells of the dipteran compound eye: A comparative study. J Comp Physiol [A] 1995;176:611–623. [Google Scholar]
- 165.Snodgrass RE. London: Cornell University Press; 1984. Anatomy of the honey bee (4th edition) [Google Scholar]
- 166.Spanagel R, Weiss F. The dopamine hypothesis of reward: Past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- 167.Spivak M, Masterman R, Ross R, Mesce KA. Hygienic behavior in the honey bee (Apis mellifera L.) and the modulatory role of octopamine. J Neurobiol. 2003;55:341–354. doi: 10.1002/neu.10219. [DOI] [PubMed] [Google Scholar]
- 168.Strader CD, Fong TM, Graziano MP, Tota MR. The family of G-protein-coupled receptors. FASEB J. 1995;9:745–754. [PubMed] [Google Scholar]
- 169.Strange PG. Antipsychotic drugs: Importance of dopamine receptors for mechanisms of therapeutic actions and side effects. Pharmacol Rev. 2001;53:119–133. [PubMed] [Google Scholar]
- 170.Strausfeld NJ. Organization of the honey bee mushroom body: Representation of the calyx within the vertical and gamma lobes. J Comp Neurol. 2002;450:4–33. doi: 10.1002/cne.10285. [DOI] [PubMed] [Google Scholar]
- 171.Strauss R. The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol. 2002;12:633–638. doi: 10.1016/s0959-4388(02)00385-9. [DOI] [PubMed] [Google Scholar]
- 172.Stuart AE. From fruit flies to barnacles, histamine is the neurotransmitter of arthropod photoreceptors. Neuron. 1999;22:431–433. doi: 10.1016/s0896-6273(00)80699-6. [DOI] [PubMed] [Google Scholar]
- 173.Sullivan JP, Fahrbach SE, Robinson GE. Juvenile hormone paces behavioral development in the adult worker honey bee. Horm Behav. 2000;37:1–14. doi: 10.1006/hbeh.1999.1552. [DOI] [PubMed] [Google Scholar]
- 174.Suzuki H. Antennal movements induced by odour and central projection of the antennal neurones in the honey-bee. J Insect Physiol. 1975;21:831–847. [Google Scholar]
- 175.Taylor DJ, Robinson GE, Logan BJ, Laverty R, Mercer AR. Changes in brain amine levels associated with the morphological and behavioural development of the worker honeybee. J Comp Physiol [A] 1992;170:715–721. doi: 10.1007/BF00198982. [DOI] [PubMed] [Google Scholar]
- 176.Theraulaz G, Bonabeau E, Deneubourg J-L. Response threshold reinforcement and division of labour in insect societies. Proc R Soc Lond B. 1998;265:327–332. [Google Scholar]
- 177.Tierney AJ. Structure and function of invertebrate 5-HT receptors: A review. Comp Biochem Physiol [A] 2001;128:791–804. doi: 10.1016/s1095-6433(00)00320-2. [DOI] [PubMed] [Google Scholar]
- 178.Valdenaire O, Vernier P. G protein coupled receptors as modules of interacting proteins: A family meeting. Prog Drug Res. 1997;49:173–218. doi: 10.1007/978-3-0348-8863-9_6. [DOI] [PubMed] [Google Scholar]
- 179.Vanden Broeck J, Vulsteke V, Huybrechts R, De Loof A. Characterization of a cloned locust tyramine receptor cDNA by functional expression in permanently transformed Drosophila S2 cells. J Neurochem. 1995;64:2387–2395. doi: 10.1046/j.1471-4159.1995.64062387.x. [DOI] [PubMed] [Google Scholar]
- 180.Vernier P, Cardinaud B, Valdenaire O, Philippe H, Vincent JD. An evolutionary view of drug-receptor interaction: The bioamine receptor family. Trends Pharmacol. Sci. 1995;16:375–381. doi: 10.1016/s0165-6147(00)89078-1. [DOI] [PubMed] [Google Scholar]
- 181.Wagener-Hulme C, Kuehn JC, Schulz DJ, Robinson GE. Biogenic amines and division of labor in honey bee colonies. J Comp Physiol [A] 1999;184:471–479. doi: 10.1007/s003590050347. [DOI] [PubMed] [Google Scholar]
- 182.Wess J. Molecular basis of receptor/G-protein-coupling selectivity. Pharmacol Ther. 1998;80:231–264. doi: 10.1016/s0163-7258(98)00030-8. [DOI] [PubMed] [Google Scholar]
- 183.Whitfield CW, Cziko AM, Robinson GE. Gene expression profiles in the brain predict behavior in individual honey bees. Science. 2003;302:296–299. doi: 10.1126/science.1086807. [DOI] [PubMed] [Google Scholar]
- 184.Wilson EO. The insect societies. Cambridge, MA: Harvard University Press; 1971. [Google Scholar]
- 185.Winnington AP, Napper RM, Mercer AR. Structural plasticity of identified glomeruli in the antennal lobes of the adult worker honey bee. J Comp Neurol. 1996;365:479–490. doi: 10.1002/(SICI)1096-9861(19960212)365:3<479::AID-CNE10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 186.Winston ML. The biology of the honey bee. Cambridge MA, London: Harvard University Press; 1987. [Google Scholar]
- 187.Xia Z, Storm DR. Calmodulin-regulated adenylyl cyclases and neuromodulation. Curr Opin Neurobiol. 1997;7:391–396. doi: 10.1016/s0959-4388(97)80068-2. [DOI] [PubMed] [Google Scholar]
- 188.Zheng Y, Hirschberg B, Yuan J, Wang AP, Hunt DC, Ludmerer SW, Schmatz DM, Cully DF. Identification of two novel Drosophila melanogaster histamine-gated chloride channel subunits expressed in the eye. J Biol Chem. 2002;277:2000–2005. doi: 10.1074/jbc.M107635200. [DOI] [PubMed] [Google Scholar]