Abstract
Adolescence is a developmental period characterized by suboptimal decisions and actions that are associated with an increased incidence of unintentional injuries, violence, substance abuse, unintended pregnancy, and sexually transmitted diseases. Traditional neurobiological and cognitive explanations for adolescent behavior have failed to account for the nonlinear changes in behavior observed during adolescence, relative to both childhood and adulthood. This review provides a biologically plausible model of the neural mechanisms underlying these nonlinear changes in behavior. We provide evidence from recent human brain imaging and animal studies that there is a heightened responsiveness to incentives and socioemotional contexts during this time, when impulse control is still relatively immature. These findings suggest differential development of bottom-up limbic systems, implicated in incentive and emotional processing, to top-down control systems during adolescence as compared to childhood and adulthood. This developmental pattern may be exacerbated in those adolescents prone to emotional reactivity, increasing the likelihood of poor outcomes.
Keywords: adolescence, prefrontal cortex, nucleus accumbens, amygdala, limbic, impulsivity, reward, development, risk taking, emotion
Introduction
Adolescence is the period between childhood and adulthood encompassed by changes in physical, psychological, and social development (Ernst et al. 2006). These alterations make this period a time of vulnerability and adjustment (Steinberg 2005). According to the National Center for Health Statistics, there are over 13,000 adolescent deaths in the United States each year. Approximately 70% of these deaths result from motor vehicle crashes, unintentional injuries, homicide, and suicide (Eaton et al. 2006). Results from the 2005 National Youth Risk Behavior Survey (YRBS) show that adolescents engage in behaviors that increase their likelihood of death or illness by driving a vehicle after drinking or without a seat belt, carrying weapons, using illegal substances, and engaging in unprotected sex resulting in unintended pregnancies and STDs, including HIV infection (Eaton et al. 2006). These statistics underscore the importance of understanding risky choices and behavior in adolescents.
Adolescence is also a time of increased emotional reactivity. During this period, the social environment is changing such that more time is spent with peers versus adults, and more conflicts arise between the adolescent and his/her parents (Csikszentmihalyi et al. 1977; Steinberg 1989). These changes in social interactions may influence the rise of emotional reactivity. In addition, given the increase in risky choices and behavior during adolescence, it appears the value of positive and negative information may be exaggerated. Greater emotional reactivity and sensitivity during adolescence may play a role in the higher incidence of affective disorder onset and addiction during this developmental period (Pine et al. 2001; Silveri et al. 2004; Steinberg 2005).
A number of cognitive and neurobiological hypotheses have been postulated to explain why adolescents engage in suboptimal choice behavior. In a recent review of the literature on human adolescent brain development, Yurgelun-Todd (2007) suggests that cognitive development during adolescence is associated with progressively greater efficiency of cognitive control and affective modulation. An increase in activity in the prefrontal regions as an indication of maturation (Rubia et al. 2000; Rubia et al. 2006; Tamm et al. 2002) and diminished activity in irrelevant brain regions (Brown et al. 2005; Durston et al. 2006; Monk et al. 2003) are described as the neurobiological explanation for the behavioral changes associated with adolescence. This general pattern, of improved cognitive control and emotion regulation with maturation of the prefrontal cortex, suggests a linear increase in development from childhood to adulthood.
As evidenced by the National Center for Health Statistics on adolescent behavior and mortality, suboptimal choices and actions observed during adolescence represent a nonlinear change in behavior, distinct from childhood and adulthood. If immaturity of prefrontal cortex were the basis for suboptimal choice behavior and heightened emotional reactivity in adolescence, then children who have less developed prefrontal cortex and cognitive abilities should look remarkably similar or even worse than adolescents. Thus, immature prefrontal function alone cannot account for adolescent behavior.
This review will provide evidence from developmental animal and human neuroimaging studies that may account for nonlinear changes in behavior and development during adolescence. A model of adolescent brain development is presented in the context of risk factors including suboptimal decision making and heightened emotional reactivity.
Development of Goal-directed Behavior: Risk versus Impulse
An accurate conceptualization of cognitive and neurobiological changes during adolescence must treat adolescence as a transitional developmental period (Spear 2000), rather than a single snapshot in time (Casey et al. 2005). In other words, to understand this developmental period, transitions into and out of adolescence are necessary for distinguishing distinct attributes of this stage of development. Adolescent behavior has been described as impulsive and risky, almost synonymously, yet these behaviors rely on different cognitive and neural processes (Casey et al. in press), which suggest distinct constructs with different developmental trajectories.
A cornerstone of cognitive development is the ability to suppress inappropriate thoughts and actions in favor of goal-directed ones, especially in the presence of compelling incentives (Casey et al. 2005; Casey et al. 2000; Casey et al. 2002). A number of classic developmental studies have shown that this ability develops throughout childhood and adolescence (Case 1972; Flavell et al. 1966; Keating & Bobbitt 1978; Pascual-Leone 1970). Several theorists (e.g., Bjorkland 1985, 1987; Case 1985) have argued that cognitive development is due to increases in processing speed and efficiency and not due to an increase in mental capacity. Other theorists have included the construct of “inhibitory” processes in their account of cognitive development (Harnishfeger & Bjorkland 1993). According to this account, immature cognition is characterized by susceptibility to interference from competing sources that must be suppressed (e.g., Brainerd & Reyna 1993; Dempster 1993) (Casey et al. 2002; Diamond 1985; Munakata & Yerys 2001). Thus goal-directed behavior requires the control of impulses or delay of gratification for optimization of outcomes, and this ability appears to mature across childhood and adolescence.
On a cognitive or behavioral level, the immature cognition of adolescence is characterized as impulsive (i.e., lacking cognitive control) and risk taking, with these constructs used synonymously and without appreciation for distinct developmental trajectories for each. Human imaging and animal studies suggest distinct neurobiological and developmental trajectories for the neural systems that underlie these separate constructs of impulse control and risky decisions. Specifically, a review of the literature suggests that impulsivity diminishes with age across childhood and adolescence (Casey et al. 2005; Casey et al. 2002; Galvan et al. 2007) and is associated with protracted development of the prefrontal cortex (Casey et al. 2005; Casey et al. 2002; Galvan et al. 2007) and is associated with protracted development of the prefrontal cortex (Casey et al. 2005). However, there are individual differences in the degree of impulsivity, regardless of age.
In contrast to the linear increase with age associated with impulse control, risk taking appears greater during adolescence relative to childhood and adulthood and is associated with subcortical systems known to be involved in evaluation of incentives and affective information. Human imaging studies that are reviewed here suggest an increase in subcortical activation (accumbens and amygdala) when making risky choices and processing emotional information (Ernst et al. 2005; Monk et al. 2003; Montague & Berns 2002) (Kuhnen & Knutson 2005; Matthews et al. 2004) that is exaggerated in adolescents, relative to children and adults (Ernst et al. 2005; Galvan et al. 2006).
These findings suggest distinct neurobiological trajectories for impulse versus risk taking behavior. The limbic subcortical systems appear to be developed by adolescence in contrast to control systems that show a protracted and linear developmental course into young adulthood. The prefrontal cortical control systems are necessary for overriding inappropriate choices and actions in favor of goal-directed ones.
Animal Studies of Adolescent Brain Development
Until recently, much of our understanding of the adolescent brain has come from animal studies. These experiments have been critical for obtaining information about the neurochemical and cellular changes that occur as a function of age. The validity of animal models to study adolescence has been questioned, since it is argued that only humans undergo the psychological stress of adolescence (e.g., Bogin 1994). However, animals including rodents and nonhuman primates exhibit increased social interactions during adolescence (Primus & Kellogg 1989) as well as novelty-seeking and risk-taking behaviors (Adriani et al. 1998; Spear 2000). These behavioral findings suggest that animal models are appropriate for studying neurobiological changes during adolescence.
Studies in rodents have shown at the cellular level that there are distinct changes in limbic and prefrontal regions during adolescence. During early puberty, there is an overproduction of axons and synapses, followed by rapid pruning in later adolescence (Crews et al. 2007). Specifically, there is dendritic pruning in the amygdala (Zehr et al. 2006), nucleus accumbens (Teicher et al. 1995), and prefrontal cortex (Andersen & Teicher 2004; Andersen et al. 2000) and continual growth in the density of the fibers connecting the amygdala and prefrontal cortex into early adulthood (Cunningham et al. 2002). There is more prolonged pruning throughout adolescence in the prefrontal cortex versus the accumbens (Andersen et al. 2000; Teicher et al. 1995). These differences in pruning in rodents are consistent with our model suggesting that the accumbens matures earlier than the prefrontal cortex.
Consistent with the cellular changes in animals, there are alterations in neurotransmission in these subcortical and cortical areas. Animal studies have shown that dopamine is crucial for communication between the accumbens, amygdala, and prefrontal cortex and that signaling between these regions relies upon the fine balance between excitatory and inhibitory dopamine transmission (Floresco & Tse 2007; Grace et al. 2007; Jackson et al. 2001). There are significant peaks in dopamine expression during adolescence. Dopamine projections to the prefrontal cortex continue to develop into early adulthood, with dopamine levels peaking in the prefrontal cortex during adolescence versus earlier or later in life in nonhuman primates (Rosenberg & Lewis 1994, 1995) and in rats (Kalsbeek et al. 1988). Dopamine receptor expression is highest in the accumbens during early adolescence (Tarazi et al. 1998). These findings in rodents suggest that there are specific regions undergoing structural changes, and therefore, connections and communication between subcortical and cortical regions are in transition and in flux during adolescence. Significant evidence suggests that the neuroanatomical changes described above are also occurring during adolescence in humans, but our methods for studying humans only provide an approximate index of such changes.
Neuroimaging Studies of Human Brain Development
Our current understanding of the human adolescent brain has come from advances in neuroimaging methodologies that can be used with developing human populations. These methods depend on magnetic resonance imaging (MRI) methods (see Fig. 1) and include structural MRI, which is used to measure the size and shape of structures; diffusion tensor imaging (DTI), which is used to index connectivity of white matter fiber tracts; and functional MRI which is used to measure patterns of brain activity. These methods have furthered our understanding of the neurobiological basis and development of reward or incentive behavior relative to goal-directed behavior.
FIGURE 1.

Illustrations of the most common magnetic resonance methods used in the study of human development. (A) Structural magnetic resonance imaging (MRI) to produce structural images of the brain useful for anatomical and morphometric studies, (B) diffusion tensor imaging (DTI) measures myelination and directionality of fiber tracts between anatomical structures, and (C) functional MRI (fMRI) measures patterns of brain activity within those structures (from Casey et al. 2005).
MRI Studies of Human Brain Development
Several studies have used structural MRI to map the developmental course of the normal brain (for review, see Durston et al. 2001). Although the brain reaches approximately 90% of its adult size by age six, the gray and white matter subcomponents of the brain continue to undergo dynamic changes throughout adolescence. Data from recent longitudinal MRI studies indicate that the change in gray matter volume over time has an inverted U-shape pattern and has greater regional variation than white matter (Giedd 2004; Gogtay et al. 2004, Sowell et al. 2003, 2004). In general, regions that involve primary functions, such as motor and sensory systems, mature earliest compared to the higher-order association areas that integrate these primary functions (Gogtay et al. 2004; Sowell et al. 2004). MRI studies show loss of cortical gray matter first in primary sensorimotor areas, followed by that in the dorsolateral prefrontal and lateral temporal cortices (Gogtay et al. 2004) (see Fig. 2). This pattern of change is consistent with nonhuman primate (Bourgeois et al. 1994) and human postmortem studies (Huttenlocher 1979) indicating that the prefrontal cortex is one of the last brain regions to mature. In contrast to gray matter, white matter volume increases in a roughly linear pattern throughout development and into adulthood (Gogtay et al. 2004). These changes most likely reflect ongoing myelination of axons by oligodendrocytes enhancing neuronal conduction and communication.
FIGURE 2.
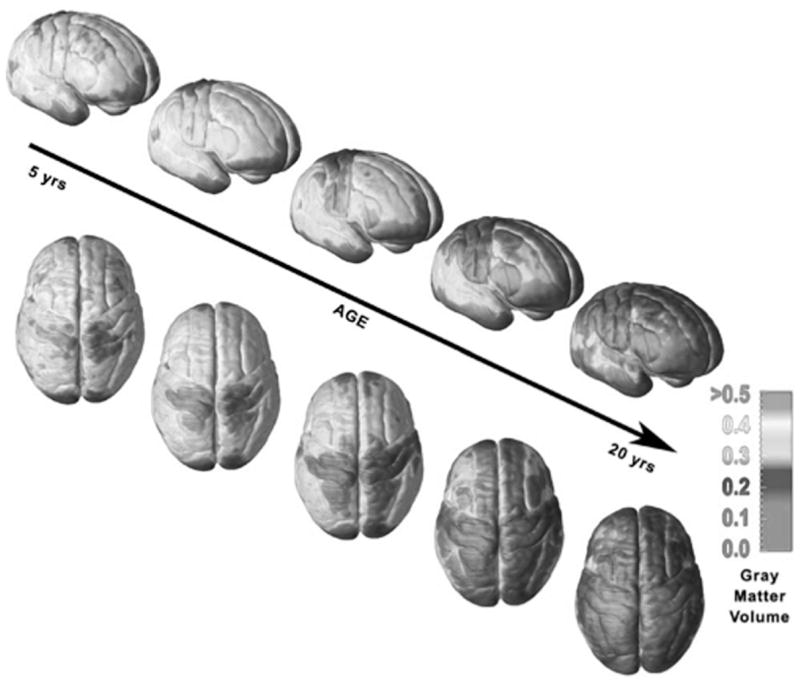
Illustration of gray matter volume maturation over the cortical surface from 5 to 20 years of age (from Lenroot & Giedd 2006).
When examining neuroanatomical changes across development, the subcortical regions are often overlooked, however, it is important to note that these areas have some of the largest changes during development in the brain, particularly in the basal ganglia (Sowell et al. 1999) and specifically in males (Caviness et al. 1996; Giedd et al. 1996; Reiss et al. 1996). Developmental changes in structural volume within basal ganglia and prefrontal regions are interesting in light of the previously mentioned animal work showing pruning in these regions during adolescence. These processes allow for the fine tuning and strengthening of connections between prefrontal and subcortical regions during development and learning that may correspond to greater cognitive control.
How do these changes in structure relate to differences in cognition? A number of studies have related frontal lobe structural maturation and cognitive function using neuropsychological and cognitive measures (e.g., Sowell et al. 2003). Specifically, these studies showed associations between MRI-based regional volumes of the prefrontal cortex and basal ganglia with measures of cognitive control (i.e., ability to override an inappropriate response in favor of another or to suppress attention toward irrelevant stimulus attribute in favor of relevant stimulus attribute) (Casey et al. 1997, 1997). These findings suggest that cognitive changes are reflected in structural brain changes and underscore the importance of subcortical (basal ganglia) as well as cortical (e.g., prefrontal cortex) development. While these findings showed associations between structure and function, a more in-depth discussion of functional imaging evidence for changes in activity that more directly coincide with behavior across development is presented in the fMRI section.
DTI Studies of Human Brain Development
The MRI-based morphometry studies previously reviewed suggest that during development, cortical connections are fine tuned via elimination of an over-abundance of synapses and by strengthening of relevant connections, although these measures do not have the resolution to visualize or measure synapses. Recent advances in MRI technology, like DTI, provide a potential tool for examining the role of specific white matter tracts in the development of the brain and behavior (for review, see Cascio et al. 2007). Examining white matter tracts can provide knowledge about pathways of connectivity in the brain, and presumably it is via these pathways that information is able to travel from one region of the brain to another (Cascio et al. 2007). Relevant to this paper are the neuroimaging studies that have linked the development of white matter fiber tracts with improvements in cognitive ability with age.
Recently, associations have been shown between DTI-based measures of prefrontal white matter development and cognition in children. Nagy and colleagues showed a positive correlation between maturation of prefrontal–parietal fiber tracts and working memory in children (Nagy et al. 2004), which is consistent with functional neuroimaging studies showing differential recruitment of these regions in children relative to adults. Using a similar approach, Liston and colleagues (2006) have shown that white matter tracts between prefrontal–basal ganglia and posterior fiber tracts continue to develop across childhood into adulthood, but only tracts between the prefrontal cortex and basal ganglia are correlated with impulse control, as measured by performance on a go/no-go task. The prefrontal fiber tracts were defined by regions of interests, which were identified in an fMRI study using the go/no-go task. In developmental DTI studies, fiber tract measures were correlated with age, but specificity of particular fiber tracts with cognitive performance were shown by dissociating the particular tract (Liston et al. 2006) or cognitive ability (Nagy et al. 2004). These findings highlight the importance of examining not only regional, but also related circuitry changes, when making inferences about neural changes in cognition across development.
Functional MRI Studies of Human Brain Development
Compared to MRI and DTI, fMRI is a more direct approach for examining behavior changes during development and for establishing structure–function relationships. Using fMRI to measure functional changes in the developing brain has significant potential for the field of developmental science and provides a means for constraining interpretations of adolescent behavior.
As stated previously, the development of the prefrontal cortex is believed to play an important role in the maturation of higher cognitive abilities such as decision making and cognitive control (Casey et al. 2002; Casey et al. 1997; Hare & Casey 2005). Many behavioral paradigms, together with fMRI, have assessed the neurobiological basis of these abilities, including flanker, Stroop, and go/no-go tasks (Casey et al. 1997; Casey et al. 2000; Durston et al. 2003). Collectively, these studies show that children recruit distinct but often larger and more diffuse prefrontal regions when performing these tasks than do adults. The patterns of brain activity that are important for task performance, such as those regions that correlate with cognitive performance, become more fine tuned with age. Regions that are not correlated with task performance diminish in activity with age. This pattern has been observed across both cross-sectional (Brown et al. 2005) and longitudinal studies (Durston et al. 2006) and across a variety of paradigms. Neuroimaging studies cannot definitively characterize the mechanism of such developmental changes as dendritic arborization or synaptic pruning. However, these studies suggest that change over a period of time results in both refinement within brain regions as well as fine tuning of projections from these regions (Brown et al. 2005; Bunge et al. 2002; Casey et al. 1997; Casey et al. 2002; Luna et al. 2001; Moses et al. 2002; Schlaggar et al. 2002; Tamm et al. 2002; Thomas et al. 2004; Turkeltaub et al. 2003).
Functional MRI Studies of Behavior during Adolescence
The question remains how can fMRI studies help explain whether adolescents, compared to children or adults, are 1) lacking sufficient cognitive control (impulsive), 2) risky in their choices and actions, and 3) more sensitive to affective information when required to exert cognitive control than children or adults.
Impulse control, as measured by cognitive control tasks like the go/no-go task, shows a linear pattern of development across childhood and adolescence, as described above. However, we were interested in understanding changes across development in top-down control regions and subcortical reward-seeking regions. It is only recently that risk taking in adolescents has been examined with neuroimaging techniques (Ernst et al. 2005; May et al. 2004). These studies have focused primarily on the region of the accumbens, a portion of the basal ganglia involved in predicting reward outcomes. Although two recent reports showed less ventral prefrontal activity (Eshel et al. 2007) and posterior mesofrontal activity (Bjork et al. 2007) in adolescents versus adults on risk-taking behavior, the goal of our studies was to characterize the development of limbic subcortical regions involved in motivation and emotional reactivity in conjunction with top-down control regions (prefrontal cortex). Many studies have examined the neural response in children and adolescents to affective information (e.g., emotional faces) (Baird et al. 1999; Killgore et al. 2001; Monk et al. 2003; Thomas et al. 2001b; Yurgelun-Todd & Killgore 2006) but typically have used passive viewing or attention tasks (Monk et al. 2003) unrelated to processing of the affective information. Our studies examine how affect influences cognitive control across development and characterizes the activation of the subcortical systems (amygdala) involved in affect regulation relative to the cortical (prefrontal) regions associated with cognitive control.
A Neurobiological Model of Adolescence
How do neural changes in subcortical regions (e.g., accumbens and amygdala) associated with reward-seeking and emotion coincide with development of the prefrontal regions and do they relate to impulsivity and risk-taking behaviors? We have developed a neurobiological model of adolescent development within this framework that builds on rodent models (Laviola et al. 1999; Spear 2000) and recent imaging studies of children, adolescents, and adults (Ernst et al. 2005; Galvan et al. 2007; Galvan et al. 2006; Hare & Casey, in press). Figure 3 depicts this model illustrating how bottom-up limbic and prefrontal top-down control regions should be considered together. The graph shows different developmental trajectories for these systems, with limbic systems developing earlier than prefrontal control regions. According to this model, the individual is biased more by functionally mature limbic regions during adolescence (i.e., imbalance of limbic relative to prefrontal control), compared to children, for whom these systems are both still developing, and compared to adults, for whom these systems are fully mature. This perspective provides a basis for nonlinear shifts in behavior across development, due to earlier maturation of this limbic system relative to the less mature top-down prefrontal control region. Furthermore, with development and experience, the functional connectivity between these regions provides a mechanism for top-down control of these regions (Hare & Casey, in press). Our model reconciles the contradiction between health statistics of risky behavior during adolescence and the astute observation by Reyna and Farley (2006) that adolescents are able to reason and understand risks of behaviors in which they engage.
FIGURE 3.
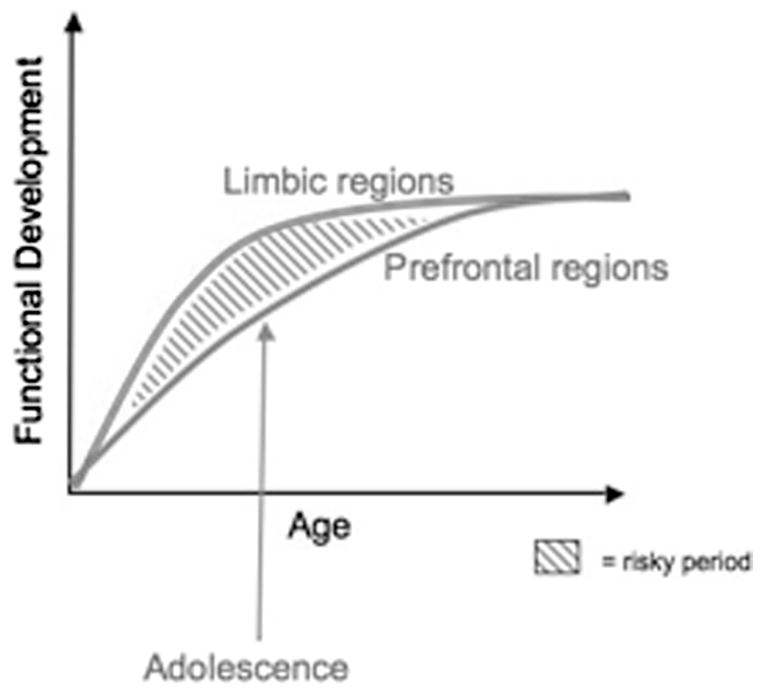
The traditional explanation of adolescent behavior has been that it is due to the protracted development of the prefrontal cortex. Our model takes into consideration the development of the prefrontal cortex together with subcortical limbic regions (e.g., nucleus accumbens and amygdala) that have been implicated in risky choices and emotional reactivity.
According to our model, in emotionally salient situations, the more mature limbic system will win over the prefrontal control system. In other words, when a poor decision is made in an emotional context, the adolescent may know better, but the salience of the emotional context biases his or her behavior in opposite direction of the optimal action.
Our neurobiological model proposes that the combination of heightened responsiveness to rewards and immaturity in behavioral control areas may bias adolescents to seek immediate rather than long-term gains, perhaps explaining their increase in risky decision making and emotional reactivity. Tracking subcortical (e.g., accumbens and amygdala) and cortical (e.g., prefrontal) development of decision making and emotional reactivity across childhood and through adulthood provides additional clarification on whether changes reported in adolescence are specific to this period of development or, rather, reflect maturation that is steadily occurring in a somewhat linear pattern from childhood to adulthood.
Two recent fMRI studies spanning from childhood to adulthood provide empirical evidence consistent with our neurobiological model. In the first study (Galvan et al. 2006), we examined behavioral and neural responses to reward manipulations across development, focusing on brain regions implicated in reward-related learning and behavior in animal (Hikosaka & Watanabe 2000; Pecina et al. 2003; Schultz 2006) and adult imaging studies (e.g., Knutson et al. 2001; O’Doherty et al. 2001; Zald et al. 2004) and in studies of addiction (Hyman & Malenka 2001; Volkow & Li 2004). Based on rodent models (Laviola et al. 1999; Spear 2000) and previous imaging work (Ernst et al. 2005), we hypothesized that relative to children and adults, adolescents would show exaggerated responses to reward as indexed by elevated accumbens activity in concert with less mature recruitment of top-down prefrontal control regions.
Our findings were consistent with rodent models (Laviola et al. 2003) and previous imaging studies during adolescence (Ernst et al. 2005), which show enhanced accumbens activity to rewards. Adolescents, as compared to children and adults, showed an exaggerated accumbens response in anticipation of reward. However, both children and adolescents showed a less mature response in prefrontal control regions than adults. These findings suggest that there are different developmental trajectories for these regions. The enhancement in accumbens activity during adolescence may relate to the increase in impulsive and risky behaviors observed during this period of development (see Fig. 4).
FIGURE 4.
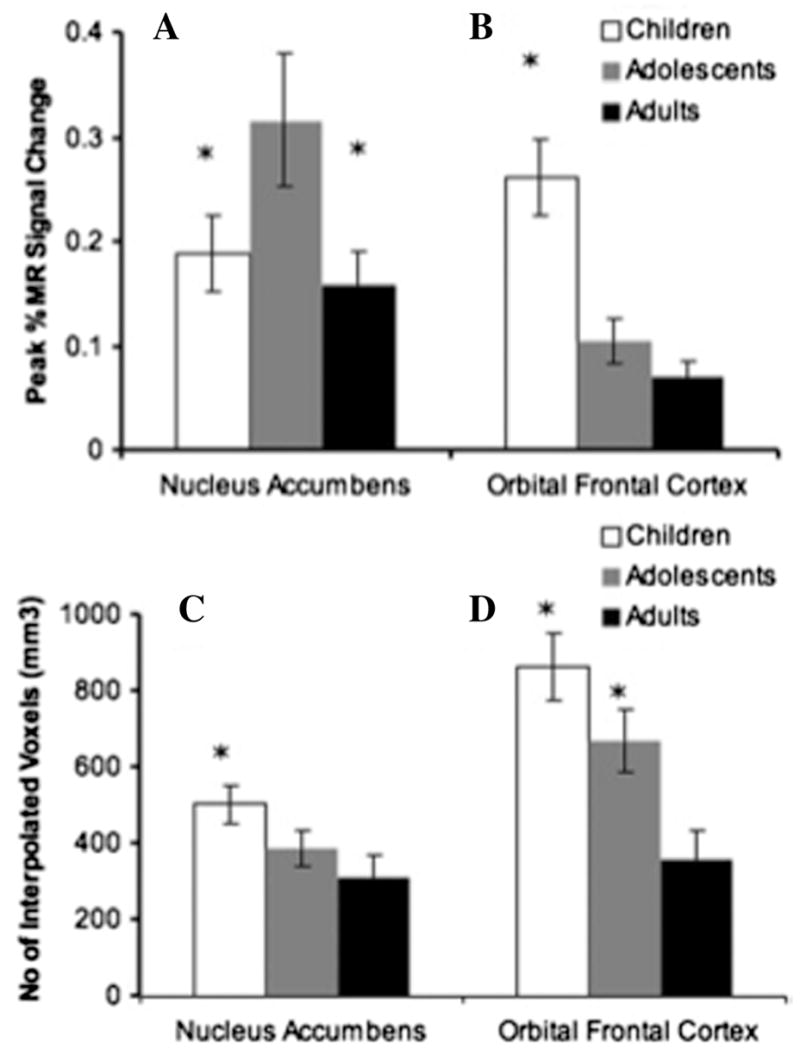
Magnitude and extent of accumbens and OFC activity to reward. Adolescents (13–17 years) showed greater percent signal change to large rewards than either children (aged 7–11 years) or adults (23–29 years) in the accumbens (A). Children had the greatest percent signal change in the OFC compared to adolescents and adults (B). Children had the greatest volume of activity in the accumbens relative to adolescents and adults (C) Children and adolescents showed greater volume of activity in the OFC than adults (D). Adapted from Galvan et al. (2006).
In the second study, we examined the development of behavioral and neural responses in performance of an emotional go/no-go paradigm (Hare & Casey, in press; Hare et al. 2005). During the experiment, participants were presented with two emotional facial expressions (fearful, neutral, or happy) and were asked to respond to one of the emotions (e.g., fear) and suppress their response to the other emotion (e.g., neutral). In the context of negative emotional information (fearful faces), reaction times improved with age but were longer when detecting fearful faces relative to a neutral or happy face. This slowing in reaction time was correlated with greater amygdala activity (Hare & Casey, in press). Activity in the orbital frontal cortex increased with age, and greater orbital frontal activity relative to amygdala was associated with more efficiency in suppressing emotional reactivity (longer reaction times and greater amygdala activity). These findings are in accordance with animal studies (Baxter et al. 2000) which show connectivity between the amgydala and orbital frontal cortex are important for assessing changes in emotional value of an object and adapting behavior accordingly.
Differential recruitment of prefrontal and subcortical regions has been reported across a number of developmental fMRI studies (Casey et al. 2002; Monk et al. 2003; Thomas et al. 2004). These findings were typically interpreted in terms of immature prefrontal regions rather than as an imbalance between prefrontal and subcortical regional development. Given evidence of prefrontal regions in guiding appropriate actions in different contexts (Miller & Cohen 2001), immature prefrontal activity might hinder appropriate estimation of future outcomes, especially when making a decision within an emotional context (i.e., heat of the moment). This interpretation is consistent with previous research showing elevated subcortical, relative to cortical, activity when decisions are biased by immediate versus long-term gains (McClure et al. 2004). Further, fMRI studies have shown limbic subcortical activity positively correlates with suboptimal choice behaviors (Kuhnen & Knutson 2005).
In sum, during adolescence, relative to childhood or adulthood, an immature ventral prefrontal cortex may not provide sufficient top-down control of robustly activated reward and affect processing regions (e.g., accumbens and amygdala). This imbalance in development of these regions and relative top-down control results in less influence of prefrontal systems (orbitofrontal cortex) relative to the accumbens and amygdala in reward valuation and emotional reactivity.
Why Would the Adolescent Brain Be Programmed This Way?
Adolescence can be described as a progressive transition from childhood into adulthood with an indefinable ontogenetic time course (Spear 2000) yet often co-occurring with puberty, which is defined by specific biological markers. The significant neuroendocrinological changes associated with puberty, such as increases in adrenal and gonadal hormones, are correlated with the development of secondary sexual characteristics and can influence brain function (for a review see Spear 2000). The onset and hormone fluctuations of puberty may provide an explanation for the observed functional differences in subcortical activity between children and adolescents, versus activity in the prefrontal region, which reflects a linear change with age.
From an evolutionary perspective, adolescence is the period in which independence skills are acquired in order to increase the success of separating from the protective influence of the family. It is also a period when there is an increase in the likelihood of harm such as injury, depression, anxiety, drug use, and addiction (Kelley et al. 2004). However, our neurobiological model suggests that risky behavior and emotional reactivity are the products of a biologically driven imbalance between increased novelty and positive sensation seeking in conjunction with immature “self-regulatory competence” (Steinberg 2004).
As previously mentioned, during adolescence, independence-seeking behaviors are prevalent across species, such as increases in peer-directed social interactions and intensifications in novelty seeking and risk-taking behaviors. In other species such as rodents, nonhuman primates, and birds, behaviors like seeking out same-age peers and fighting with parents are also observed and may be important adaptive skills to remove the adolescent from the home territory in order to mate (Spear 2000). Relative to adults, periadolescent rats show increased novelty-seeking behaviors in a free-choice novelty paradigm (Laviola et al. 1999). Neurochemical evidence indicates that the balance in the adolescent brain between cortical and subcortical dopamine systems begins to shift toward greater cortical dopamine levels during adolescence (Spear 2000). And as previously described, in the nonhuman primate there is an increase in dopamine enervation of the prefrontal cortex into early adulthood (Rosenberg & Lewis 1995). Thus this elevated risk-taking behavior appears to occur across species and have important adaptive functions.
It is possible that this developmental pattern is an evolutionary feature. One needs to engage in high-risk behavior in order to leave the family and village to find a mate. This risk behavior occurs simultaneously with an increase in sexual hormones, resulting in adolescents seeking sexual partners and is seen in other species. In conjunction with this novelty-seeking behavior, there would need to be some mechanism for detecting cues of safety or danger. The increase in emotional reactivity during this period may allow adolescents to be more vigilant and aware of threat, to ensure their survival as they move from a safe environment to a novel one. In today’s society when adolescence may extend indefinitely—with individuals well into their 20s living with their parents, remaining financially dependent, and choosing mates later in life—these behaviors may be deemed inappropriate.
Biological Predispositions, Development, and Risky Behavior
The recognition of individual differences in impulse control and taking risks is not new in the field of psychology (Benthin et al. 1993). Perhaps one of the classic examples of individual differences in the social, cognitive, and developmental psychology literatures is delay of gratification (Mischel et al. 1989). Delay of gratification is typically assessed in 3- to 4-year-old toddlers. A toddler is seated in a room with two cookies and a bell. The child is then told that the experimenter will leave the room in order to prepare for upcoming activities and explains to the child that if she remains in her seat and does not eat a cookie, she will receive the large reward (2 cookies). If the child cannot wait, she should ring a bell to summon the experimenter and thereby receive the smaller reward (1 cookie). Distractions in the room are minimized, with no toys, books, or pictures. The experimenter returns after 15 minutes or after the child has rung the bell, eaten the rewards, or shown any signs of distress. Mischel (1989) showed that children typically behave in one of two ways: 1) either they ring the bell almost immediately in order to have the cookie, which means they only get one; or 2) they wait and optimize their gains to receive both cookies. This observation suggests that some individuals are better than others in their ability to control impulses in the presence of highly salient incentives, and this bias can be detected in early childhood (Mischel et al. 1989). This differential in impulse control appears to remain throughout adolescence and young adulthood (Eigsti et al. 2006).
What might explain individual differences in decision making and behavior? Some theorists have postulated that the dopaminergic mesolimbic circuitry, implicated in reward processing, underlies risky behavior (Blum et al. 2000), and that individual differences in this circuitry might relate to the propensity to engage in risky behavior (O’Doherty 2004). A number of studies have shown increases in activity in the nucleus accumbens immediately prior to making risky choices on monetary-risk paradigms (Kuhnen & Knutson 2005; Matthews et al. 2004; Montague & Berns 2002), and as described previously, adolescents show exaggerated accumbens activity to rewarding outcomes relative to children or adults (Ernst et al. 2005; Galvan et al. 2006). Collectively, these data suggest that as a group adolescents may be more likely to engage in risky choices (Gardener & Steinberg 2005). However, some adolescents will be more prone than others to engage in risky behaviors, putting them at potentially greater risk for negative outcomes. Therefore it is important to consider individual variability when examining complex brain–behavior relationships related to risk taking and reward processing in developmental populations.
To explore individual differences in risk-taking behavior, Galvan and colleagues (2007) recently examined the association between activity in reward-related neural circuitry in anticipation of a large monetary reward with behavioral measures of risk taking and impulsivity in adolescence. Specifically, Galvan and colleagues used functional magnetic resonance imaging and anonymous self-report rating scales of risky behavior, risk perception, and impulsivity in individuals between the ages of 7 and 29 years (see Fig. 6). There was a positive association between accumbens activity and the likelihood of engaging in risky behavior across development. In other words, those individuals, who perceived risky behaviors as leading to dire consequences, activated the accumbens less to reward. Impulsivity ratings were not associated with accumbens activity, but rather with age, further dissociating impulse control from incentive-based risky behaviors. These findings suggest that during adolescence, some individuals have a predisposition to engage in risky behaviors due to developmental neural changes.
FIGURE 6.

Activity in the nucleus accumbens in anticipation of reward (A). Percent change in fMRI signal in the accumbens in anticipation of reward as a function of age (B). The association between accumbens activity to reward and the likelihood of engaging in risky behavior in three age groups (C) (Adapted from Galvan et al. 2007).
Adolescent behavior is repeatedly characterized as impulsive and risky, yet this review of the imaging literature suggests different neurobiological substrates and developmental trajectories for these two types of behavior. Specifically, impulsivity is associated with immature ventral prefrontal development and gradually diminishes from childhood to adulthood (Casey et al. 2005). The negative correlation between impulsivity ratings and age in the study by Galvan and colleagues (2007) further supports this notion. In contrast, risk taking is associated with an increase in accumbens activity (Kuhnen & Knutson 2005; Matthews et al. 2004; Montague & Berns 2002) that is exaggerated in adolescents, relative to both children and adults (Ernst et al. 2005; Galvan et al. 2006). Thus adolescent choices and behavior cannot be explained by impulsivity or protracted development of the prefrontal cortex alone, as children would then be predicted to be greater risk takers. The findings provide a neural basis for why some adolescents are at greater risk than others, but also demonstrate a basis for why adolescent risk-taking behavior in general is different from risk taking in children and adults.
Adolescence, Individual Differences, and Affective Disorders
Adolescence is a time of greater emotional reactivity and a period when symptoms of many psychiatric disorders (e.g., schizophrenia, depression, anxiety) manifest. Normal adolescent development can be interpreted as the coordination of emotions and behavior in the social and intellectual environment, and the development of psychopathology during adolescence can be seen as resulting from a difficulty in balancing these factors (Steinberg 2005). We have previously described enhanced bottom-up emotional processing in subcortical regions relative to less effective top-down modulation in prefrontal regions to affective information during adolescence. It is possible that this imbalance may play a role in the increased risk for affective disorders during adolescence (Steinberg 2005). Clearly, not all adolescents develop psychopathology; there must be individual variability in emotional reactivity and the ability to modulate these behaviors. Individual differences may predispose a person to be at greater risk for poorer outcomes.
The amygdala has been implicated as a key neural region in emotional dysregulation in psychiatric disorders. This region is essential to learning the emotional significance of cues in the environment (see Maren & Quirk 2004 for review). In animal studies, amygdala lesions result in a reduction of fear behavior (Anglada-Figueroa & Quirk 2005; Davis & Whalen 2001; Kalin et al. 2004), and human neuroimaging studies have shown increases in activity in the amygdala to fearful stimuli in adults (Breiter et al. 1996; Morris et al. 1998) and in children (Thomas et al. 2001b). There is evidence for dysregulation of amygdala activity in anxious and depressed children (Thomas et al. 2001a) and adults (Leppanen 2006; Rauch et al. 2003; Thomas et al. 2001a).
In a recent study, we examined individual differences in anxiety levels as measured by the Spielberger State Trait Anxiety Index and neural responses to affective information in adolescents and adults during an emotional go/no-go task (Hare et al. in press). Adolescents showed greater initial amygdala activity than adults, and sustained amygdala activity was correlated with trait anxiety. Increased activity in orbitofrontal regions correlated with a decrease in amygdala activity over time (i.e., repeated presentation of a fearful face, see Fig. 7), suggesting dampening of emotional reactivity due to top-down control from prefrontal regions. These findings are consistent with animal studies showing the importance of the orbitofrontal cortex (OFC) in extinction of fear conditioning in animals with repeated exposure to empty threat (Gallagher et al. 1999).
FIGURE 7.
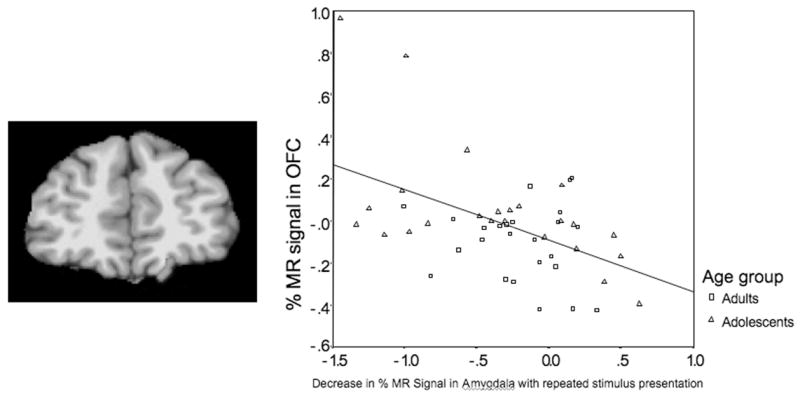
Picture depicts left orbitofrontal activity. Graph illustrates correlation of activity in the OFC and in the amygdala in both adults and adolescents (adapted from Hare et al. in press).
Our results are consistent with previous fMRI studies in clinically anxious adults and children that have shown unregulated amygdala activity to negative emotional information and less activity in the prefrontal cortex (McClure et al. 2007; Shin et al. 2004; Thomas et al. 2001b) in adolescents at risk for anxiety disorders (Perez-Edgar et al. 2007). Together these findings suggest that prefrontal regions serve to regulate emotional reactivity and that individual differences in emotion regulation may be due to an imbalance in activity between these regions that is exacerbated during adolescence.
Conclusions
Human imaging studies show structural and functional changes in frontolimbic regions (Jernigan et al. 1991; Giedd et al. 1999; Giedd et al. 1996; Sowell et al. 1999; for review, Casey et al. 2005; Jernigan et al. 1991; Giedd et al. 1999; Giedd et al. 1996; Sowell et al. 1999) for review, (Casey et al. 2005) that seem to parallel increases in cognitive control and self-regulation (Casey et al. 1997; Luna & Sweeney 2004; Luna et al. 2001; Rubia et al. 2000; Steinberg 2004). These changes appear to show a shift in activation of prefrontal regions from diffuse to more focal recruitment over time (Brown et al. 2005; Bunge et al. 2002; Casey et al. 1997; Durston et al. 2006; Moses et al. 2002) and elevated recruitment of subcortical regions during adolescence (Casey et al. 2002; Durston et al. 2006; Luna et al. 2001). Although neuroimaging studies cannot definitively characterize the mechanism of such developmental changes, these changes in volume and structure may reflect development within, and refinement of, projections to and from these brain regions during maturation suggestive of fine tuning of the system with development.
We have discussed the importance of considering individual variability when examining complex brain–behavior relationships related to risk taking, reward processing, and emotional reactivity in developmental populations. Using an approach that looks at developmental trajectories, rather than snapshots in time, allows one to comprehensively study these behaviors during development and examine individual differences. It is not possible to fully explain the emergence of affective disorders or atypical development by simply examining one time point. Longitudinal studies across development would be the best methodology to address these issues.
Taken together, the findings synthesized here indicate that increased risk-taking behavior and greater emotional reactivity in adolescence are associated with different developmental trajectories of subcortical limbic regions relative cortical control regions. These developmental changes can be exacerbated by individual differences (e.g., genetic risk) in baseline activity of limbic systems.
This model of development reconciles a number of contradictions and myths about adolescence. First, there have been many reports that suggest that adolescent behavior is due to protracted development of prefrontal cortex. However, if this were the case, then children would engage in similar or worse behavior than adolescents. The National Center for Health Statistics on adolescent behavior and mortality shows that suboptimal choices and actions observed during adolescence represent a nonlinear change in behavior, distinct from childhood and adulthood. Adolescents, unlike children, may be in situations (e.g., driving a car) that may put them at greater risk for mortality, but even when taking these conditions into account, there is still a significant elevation of risky behavior in adolescents in comparison to children. Furthermore, experimental studies have shown that when risk is held constant, such as in the appraisal of risky vignettes, children perceive greater risk in hypothetical scenarios than do adolescents (reviewed in Furby & Beyth-Marom 1992). Our neurodevelopmental model provides an explanation for these nonlinear changes in behavior.
Reyna and Farley (2006) have reconciled the second myth by showing that adolescents are able to reason and understand risks of behaviors in which they engage and do not consider themselves invincible. Prior research has also shown that adolescents knowingly engage in risky behavior, and this is often due to influences of feelings, emotions, and peers (Gardener & Steinberg 2005; Steinberg 2004, 2005). The observation that adolescents know that they are engaging in risky behavior is not supported by the sole explanation of a less developed prefrontal cortex. In this context, our model suggests that the adolescent is capable of making rational decisions, but in emotionally charged situations the more mature limbic system will win over the prefrontal control system.
When faced with an immediate personal decision, adolescents will rely less on intellectual capabilities and more on feelings. Nevertheless, when reasoning about a hypothetical, moral dilemma, the adolescent will rely more on logical information (Steinberg 2005). In other words, when a poor decision is made in the heat of the moment, the adolescent may know better, but the salience of the emotional context biases his or her behavior in opposite direction of the optimal action. This work coincides with studies of social cognition showing that adolescents make more rational decisions about hypothetical scenarios versus real-life situations (Sobesky 1983). The environmental context and emotional significance of the decision greatly influence the adolescent (Steinberg 2005).
Our findings and model have significant implications for heated debates on public policy and the treatment of minors in our judicial system. Adolescents show adult levels of intellectual capability earlier than they show evidence of adult levels of impulse control (Reyna & Farley 2006). As such, adolescents may be capable of making informed choices about their future (e.g., terminating a pregnancy) but do not yet have full capacity to override impulses in emotionally charged situations that require decisions in the heat of the moment. Unfortunately, judges, politicians, advocates, and journalists are biased toward drawing a single line between adolescence and adulthood for different purposes under the law that is at odds with developmental cognitive neuroscience (Steinberg et al. in press). Our neurodevelopmental model of adolescence will hopefully help to make strides in moving this single line to multiple lines that consider developmental changes across both context (emotionally charged or not) and time (in the moment or in the future).
FIGURE 5.
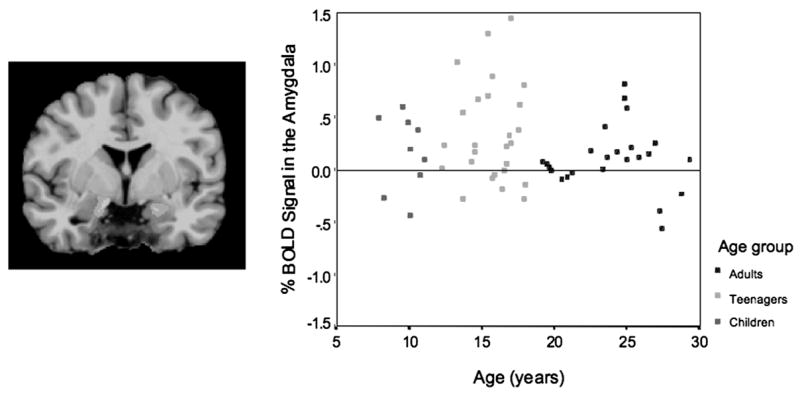
Bilateral amygdala activation (left). Graph depicts amygdala activity in adults, teenagers, and children. Adapted from Hare et al. (in press).
Acknowledgments
This work was supported in part by grants from the National Institute of Drug Abuse R01 DA18879 and the National Institute of Mental Health R01 MH73175 and P50 MH62196 to BJC.
Footnotes
Conflict of Interests
The authors declare no conflicts of interest.
References
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112(5):1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29(11):1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37(2):167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25(42):9680–9685. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird AA, Gruber SA, Fein DA, Maas LC, Steingard RJ, Renshaw PF, et al. Functional magnetic resonance imaging of facial affect recognition in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1999;38(2):195–199. doi: 10.1097/00004583-199902000-00019. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20(11):4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benthin A, Slovic P, Severson H. A psychometric study of adolescent risk perception. J Adolesc. 1993;16(2):153–168. doi: 10.1006/jado.1993.1014. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. J Neurosci. 2007;27(18):4839–4849. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkland DF. The role of conceptual knowledge in the development of organization in children’s memory. In: Brainerd CJ, Pressley M, editors. Basic Processes in Memory Development: Progress in Cognitive Development Research. New York: Springer-Verlag; 1985. pp. 103–142. [Google Scholar]
- Bjorkland DF. How age changes in knowledge base contribute to the development of children’s memory: An interpretive review. Developmental Review. 1987;7:93–130. [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000;32(Suppl i–iv):1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Bogin B. Adolescence in evolutionary perspective. Acta Paediatr Suppl. 1994;406:29–35. doi: 10.1111/j.1651-2227.1994.tb13418.x. discussion 36. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4(1):78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF. Memory independence and memory interference in cognitive development. Psychol Rev. 1993;100(1):42–67. doi: 10.1037/0033-295x.100.1.42. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15(3):275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Gerig G, Piven J. Diffusion tensor imaging: Application to the study of the developing brain. J Am Acad Child Adolesc Psychiatry. 2007;46(2):213–223. doi: 10.1097/01.chi.0000246064.93200.e8. [DOI] [PubMed] [Google Scholar]
- Case R. Balidation of a neo-Piagetian capacity construct. Journal of Experimental Child Psychology. 1972;14:287–302. [Google Scholar]
- Case R. Intellectual Development Birth to Adulthood. New York: Academic Press; 1985. [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997a;36(3):374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Curr Opin Neurobiol. 2005a;15(2):239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Casey B, Getz S, Galvan A. Developmental Review in press. [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000a;54(1–3):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Davidson MC, Kunz K, Franzen PL. Dissociating striatal and hippocampal function developmentally with a stimulus-response compatibility task. J Neurosci. 2002a;22(19):8647–8652. doi: 10.1523/JNEUROSCI.22-19-08647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, et al. Dissocaiation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci, USA. 2000b;97(18):8728–8733. doi: 10.1073/pnas.97.15.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Fossella J. Clinical, imaging, lesion, and genetic approaches toward a model of cognitive control. Dev Psychobiol. 2002b;40(3):237–254. doi: 10.1002/dev.10030. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Science. 2005b;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997b;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Caviness V, Kennedy D, Richelme C, Rademacher J, Filipek P. The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex. 1996;6(5):726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86(2):189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R, Prescott S. The ecology of adolescent activity and experience. Journal of Youth and Adolescence. 1977;6:281–294. doi: 10.1007/BF02138940. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dempster FN, editor. Resistance to Interference: Developmental Changes in a Basic Processing Mechanism. Vol. 1. New York: Springer-Verlag; 1993. [Google Scholar]
- Diamond A. Development of the ability to use recall to guide action, as indicated by infants’ performance on AB. Child Development. 1985;56:868–883. [PubMed] [Google Scholar]
- Durston S, Davidson MC, Thomas KM, Worden MS, Tottenham N, Martinez A, et al. Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. Neuroimage. 2003;20(4):2135–2141. doi: 10.1016/j.neuroimage.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff HE, Casey BJ, Giedd JN, Buitelaar JK, Van Engeland H. Anatomical MRI of the developing human brain: What have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40(9):1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Eaton LK, Kinchen S, Ross J, Hawkins J, Harris WA, Lowry R, et al. Youth risk behavior surveillance-United States, 2005, surveillance summaries. Morbidity and Mortality Weekly Report. 2006;55(SS5):1–108. [PubMed] [Google Scholar]
- Eigsti IM, Zayas V, Mischel W, Shoda Y, Ayduk O, Dadlani MB, et al. Predicting cognitive control from preschool to late adolescence and young adulthood. Psychol Sci. 2006;17(6):478–484. doi: 10.1111/j.1467-9280.2006.01732.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45(6):1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell JH, Feach DR, Chinsky JM. Spontaneous verbal rehearsal in a memory task as a function of age. Child Development. 1966;37:283–299. [PubMed] [Google Scholar]
- Floresco SB, Tse MT. Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdala-prefrontal cortical pathway. J Neurosci. 2007;27(8):2045–2057. doi: 10.1523/JNEUROSCI.5474-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furby L, Beyth-Marom R. Risk taking in adolescence: A decision-making perspective. Developmental Review. 1992;12(1):1–44. [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19(15):6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ. Risk-taking and the adolescent brain: who is at risk? Dev Sci. 2007;10(2):F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardener M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology. 2005;41:625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kaysen D, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Hare T, Casey B. Biological Psychiatry in press. [Google Scholar]
- Hare TA, Casey BJ. The neurobiology and development of cognitive and affective control. Cognition, Brain, Behavior. 2005;9(3):273–286. [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry. 2005;57(6):624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Voss HU, Glover GH, Casey BJ. The adolescent brain and potential risk for anxiety and depression. Biological Psychiatry in press. [Google Scholar]
- Harnishfeger KK, Bjorkland F. The ontogeny of inhibition mechanisms: A renewed approach to cognitive development. In: Howe ML, Pasnek R, editors. Emgering Themes in Cognitive Development. Vol. 1. New York: Springer-Verlag; 1993. pp. 28–49. [Google Scholar]
- Hikosaka K, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cereb Cortex. 2000;10(3):263–271. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2(10):695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Frost AS, Moghaddam B. Stimulation of prefrontal cortex at physiologically relevant frequencies inhibits dopamine release in the nucleus accumbens. J Neurochem. 2001;78(4):920–923. doi: 10.1046/j.1471-4159.2001.00499.x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Zisook S, Heaton RK, Moranville JT, Hesselink JR, Braff DL. Magnetic resonance imaging abnormalities in lenticular nuclei and cerebral cortex in schizophrenia. Arch Gen Psychiatry. 1991;48:881–890. doi: 10.1001/archpsyc.1991.01810340013002. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24(24):5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269(1):58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Keating DP, Bobbitt BL. Individual and developmental differences in cognitive processing components of mental ability. Child Development. 1978;49:155–167. [Google Scholar]
- Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Ann N Y Acad Sci. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Oki M, Yurgelun-Todd DA. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport. 2001;12(2):427–433. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47(5):763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23(7):993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27(1–2):19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Leppanen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry. 2006;19(1):34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex. 2006;16(4):553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Luna B, Sweeney JA. The emergence of collaborative brain function: FMRI studies of the development of response inhibition. Ann N Y Acad Sci. 2004;1021:296–309. doi: 10.1196/annals.1308.035. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5(11):844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Simmons AN, Lane SD, Paulus MP. Selective activation of the nucleus accumbens during risk-taking decision making. Neuroreport. 2004;15(13):2123–2127. doi: 10.1097/00001756-200409150-00025. [DOI] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, et al. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol Psychiatry. 2004;55(4):359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244(4907):933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20(1):420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36(2):265–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(Pt 1):47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Moses P, Roe K, Buxton RB, Wong EC, Frank LR, Stiles J. Functional MRI of global and local processing in children. Neuroimage. 2002;16(2):415–424. doi: 10.1006/nimg.2002.1064. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Yerys BE. All together now: when dissociations between knowledge and action disappear. Psychol Sci. 2001;12(4):335–337. doi: 10.1111/1467-9280.00361. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone JA. A mathematical model for transition in Piaget’s developmental stages. Acta Psychologica. 1970;32:301–345. [Google Scholar]
- Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J Neurosci. 2003;23(28):9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35(4):1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Brook JS. Emotional reactivity and risk for psychopathology among adolescents. CNS Spectr. 2001;6(1):27–35. doi: 10.1017/s1092852900022860. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22(6):633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Reiss A, Abrams M, Singer H, Ross J, Denckla M. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Reyna V, Farley F. Risk and rationality in adolescent decision making: implications for theory, practice, and public policy. Psychological Science in the Public Interest. 2006;7(1):1–44. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biol Psychiatry. 1994;36(4):272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358(3):383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24(1):13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296(5572):1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annual Reviews of Psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61(2):168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Tzilos GK, Pimentel PJ, Yurgelun-Todd DA. Trajectories of adolescent emotional and cognitive development: effects of sex and risk for drug use. Ann N Y Acad Sci. 2004;1021:363–370. doi: 10.1196/annals.1308.046. [DOI] [PubMed] [Google Scholar]
- Sobesky W. The effects of situational factors on moral judgements. Child Development. 1983;54:575–584. [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10(4):372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Pubertal maturation and parent-adolescent distance: an evolutionary perspective. In: Adams G, Montemayor R, Gullotta T, editors. Advances in Adolescent Behavior and Development. Newbury Park, CA: Sage Publications; 1989. pp. 71–97. [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Ann N Y Acad Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9(2):69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Cauffman E, Woolard J, Graham S, Banich M. Are adolescents less mature than adults? Minors’ access to abortion, the juvenile death penalty, and the alleged APA “Flip-Flop”. doi: 10.1037/a0014763. in press. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neurosci Lett. 1998;254(1):21–24. doi: 10.1016/s0304-3940(98)00644-2. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89(2):167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, et al. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001a;58(11):1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, et al. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001b;49(4):309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Hunt RH, Vizueta N, Sommer T, Durston S, Yang Y, et al. Evidence of developmental differences in implicit sequence learning: an FMRI study of children and adults. J Cogn Neurosci. 2004;16(8):1339–1351. doi: 10.1162/0898929042304688. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6(7):767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci. 2004;5(12):963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. 2007;17(2):251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Killgore WD. Fear-related activity in the prefrontal cortex increases with age during adolescence: a preliminary fMRI study. Neurosci Lett. 2006;406(3):194–199. doi: 10.1016/j.neulet.2006.07.046. [DOI] [PubMed] [Google Scholar]
- Zald DH, Boileau I, El-Dearedy W, Gunn R, McGlone F, Dichter GS, et al. Dopamine transmission in the human striatum during monetary reward tasks. J Neurosci. 2004;24(17):4105–4112. doi: 10.1523/JNEUROSCI.4643-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. J Neurobiol. 2006;66(6):578–590. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]


